Abstract
The Global Burden of Disease assessment estimates that 20% of global type 2 diabetes cases are related to chronic exposure to particulate matter (PM) with a diameter of 2·5 μm or less (PM2·5). With 99% of the global population residing in areas where air pollution levels are above current WHO air quality guidelines, and increasing concern in regard to the common drivers of air pollution and climate change, there is a compelling need to understand the connection between air pollution and cardiometabolic disease, and pathways to address this preventable risk factor. This Review provides an up to date summary of the epidemiological evidence and mechanistic underpinnings linking air pollution with cardiometabolic risk. We also outline approaches to improve awareness, and discuss personal-level, community, governmental, and policy interventions to help mitigate the growing global public health risk of air pollution exposure.
Introduction
A changing environment and factors such as sedentary habits, seismic shifts in agrarian practices, and widespread availability of nutrient-dense foods have irrevocably altered the balance between energy intake and expenditure.1 The International Diabetes Federation reports that 10·5% of adults worldwide, or 537 million individuals, have type 2 diabetes, with almost half of these individuals unaware that they have the condition.2 By 2045, projections show that 783 million individuals will be living with type 2 diabetes, with 75% of these cases occurring in low-income and middle-income countries.2 There is strong accruing evidence linking chronic exposure to pollutants in air, soil, and water with cardiometabolic health.3,4 Air pollution is by far the most dominant environmental risk factor for health in general, and is responsible for over 9 million annual deaths globally, most of which are due to cardiovascular causes.3,5,6 A recent Global Burden of Disease study estimated that a fifth of worldwide type 2 diabetes is attributable to chronic fine particulate matter (PM2·5) exposure.7 People with type 2 diabetes are likely to be more susceptible to adverse health effects from PM2·5, which further amplifies pollution-related cardiovascular risk.3,7 This Review aims to provide an up to date summary of the epidemiological and mechanistic evidence linking air pollution with diabetes, outline approaches to improve awareness, and review much-needed personal-level and policy interventions to help mitigate this enormous and growing global public health risk. Unless otherwise stated in this Review, PM2·5 exposure in animals almost always means inhalational exposure to concentrated ambient PM2·5. Recently, preliminary evidence linking air pollution and type 1 diabetes has also emerged.8 However, given the strength of evidence for the association between type 2 diabetes and air pollution, as well as its dominant global prevalence and relevance to cardiovascular disease, the focus in this Review is type 2 diabetes.
Environmental underpinnings of type 2 diabetes
Type 2 diabetes is not a single disease, but rather a group of conditions broadly categorised by a single diagnostic criterion—hyperglycaemia. Subtypes of type 2 diabetes that are phenotypically and genetically distinct have been noted, with differential susceptibility to target organ complications such as cardiovascular disease, nephropathy, neuropathy, and retinopathy.9 Phenotypic manifestations of type 2 diabetes can include individuals with excess visceral adiposity, lean individuals, and individuals with predominant liver and skeletal muscle deposition of fat. This phenotypic diversity complicates genetic attribution of type 2 diabetes, as each feature might have differential genetic susceptibility, and also complicates the interpretation of environmental studies, including those on air pollution. The preponderance of studies nevertheless suggests a major contribution of the environment in shaping susceptibility to type 2 diabetes, defined by glycaemic criteria, and its complications.10 External factors in the built and natural environment, lifestyle and behaviours, social environment, chemical exposures, and internal factors (such as the microbiome, stress, and sleep duration), which collectively constitute the exposome, have also been shown to affect susceptibility to type 2 diabetes.11–15
Air pollution source components and relationship to metabolic disease
Air pollution continues to be the world’s most pronounced environmental health threat. Worldwide, poor air quality accounts for 93 billion days lived with illness and more than 6 million deaths each year, although some estimates put the number of deaths well above 9 million.3 The highest burdens of air pollution mortality are often in large, urban cities.16 The total economic cost of air pollution exceeds US$8 trillion, or roughly 6·1% of global annual gross domestic product.17 The major sources of modern-day air pollution in high-income countries include fossil fuel combustion. However, globally, other sources (eg, desert dust and volcanic ash) play a major role in air pollution, with growing concern related to wildfire-associated air pollution, which is markedly increasing in the USA and globally due to the effects of climate change.5 Air pollution chemistry and sources have been reviewed extensively in previous reports, but most health effects are associated with particulate matter fraction, with the particles being categorised by size fraction into ultrafine particles (<0·1 μm [PM0·1]), fine particles (diameter ≥0·1 μm to 2·5 μm [PM2·5]), and coarse particles (diameter >2·5 μm to 10 μm [PM10]).18 Coarse particles originate from crustal materials and agricultural and industrial practices, and can dominate air particulate composition in certain environments. PM2·5 exposure occurs primarily from fossil fuel combustion from power generation and transportation, although exposures from wildfire smoke and other climate events are increasingly common and can dominate many environments for months.19–21
Air pollution is a complex mixture of gasses, particles, and liquids that are continually changing and interacting with each other and natural atmospheric gasses. Although PM2·5 mass has rightfully attracted attention as a target for regulation and study, more than 98% of the mass of air pollution is from gases or vapour-phase compounds, such as CO, non-methane hydrocarbons or volatile organic carbons, NO2, NO, O3, and SO2.3,5,22 In addition to primary pollutants formed directly by combustion, many other pollutants are produced primarily through chemical reactions in the atmosphere and are known as secondary pollutants. Examples include PM-associated sulphate, nitrate, and ammonium, and many of the organic compounds within PM2·5, including various inorganic and organic acids and volatile organic compounds. However, the current understanding of each individual component’s effect on human health is insufficient. Fresh combustion PM, such as from traffic-related pollutants, is largely composed of ultrafine particles. Although there is some evidence linking ultrafine particles with cardiometabolic diseases, definitive data are difficult to acquire due to the short-lived nature of ultrafine particles, their high spatial variability, the focal locations of exposures (such as near roadways), and their high correlation to other environmental exposures (eg, noise).
Global PM2.5 concentrations and burden of disease
Over 99% of the global population live in areas with PM2·5 exposure exceeding WHO air quality guidelines (<5 μg/m3 annually) (figure 1A).23 Pollution-attributed health effects disproportionately affect heavily populated countries, especially China and India.3,4 In 2019, only 0·18% of the global land area and 0·001% of the global population had an annual exposure to PM2·5 that was lower than the WHO limit of 5 μg/m3.23 The mean global annual population-weighted PM2·5 concentration for 2000–19 was estimated at 32·8 μg/m3.23 Areas with higher than average Black, Asian, and Hispanic or Latino populations are routinely exposed to higher than average PM2·5 concentrations, with evidence of significant health effects even at lower PM2·5 concentrations.24,25
Figure 1: Global PM2·5 and diabetes data for 2019.
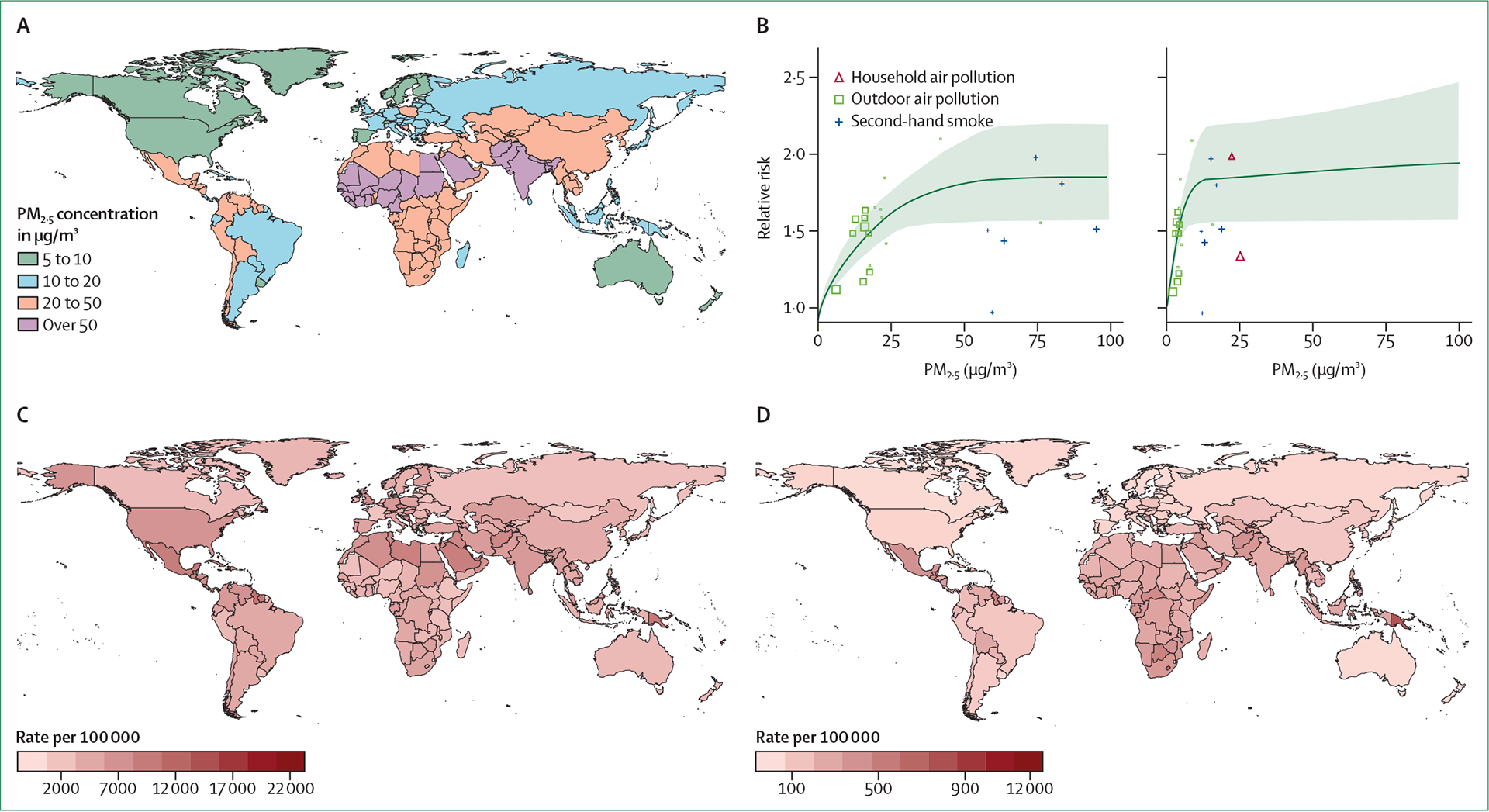
From Global Burden of Disease data.7 (A) Ambient PM2·5 concentrations. (B) Exposure–response curve of PM2.5 and diabetes, shown across PM2·5 0–100 μg/m3 and 0–500 μg/m3. (C) Age-standardised prevalence of type 2 diabetes. (D) Type 2 diabetes age-standardised disability-adjusted life years associated with particulate matter pollution. PM2·5=particulate matter smaller than 2·5 μm.
Dose response of air pollution and incident type 2 diabetes
A study evaluating PM2·5 exposure and the development of diabetes in a group of 1 729 108 US veterans with no history of diabetes was one of the first to use an integrated exposure–response function to estimate risk.26 An increase of 10 μg/m3 in long-term PM2·5 exposure was associated with an increased risk of diabetes (hazard ratio [HR] 1·15, 95% CI 1·08–1·22) after adjusting for 57 variables comprising individual-level health, sociodemographic factors, physical environment, and health behaviours. At levels of exposure typical for the USA, ambient PM2·5 was calculated to contribute to about 3·2 million incident cases of diabetes and 206 105 deaths.26 In an updated analysis, using data from ambient air pollution, household air pollution, and second-hand smoking, the Global Burden of Disease investigators extended the range of their analysis to all known global concentrations of PM2·5 (figure 1B).7 The shape of the exposure–response curve was curvilinear, with a more robust association at lower PM2·5 levels and an attenuation of the relationship above 50 μg/m3 (HR approximately 1·5–1·7). Applying the exposure–response curve to the global population in 2019, the study estimated that 20% of all diabetes-related deaths and disability-adjusted life years (DALYs) globally were attributable to PM2·5 (13·5% to ambient air pollution and 6·5% to household air pollution).27 The shape of the exposure–response curve has helped provide a credible explanation for why even low doses of second-hand smoke or air pollution are deleterious from a cardiometabolic perspective. The global type 2 diabetes age-standardised prevalence is illustrated in figure 1C, and figure 1D depicts the PM2·5-associated type 2 diabetes DALY rates in 2019, obtained from the Global Burden of Disease study.28
Epidemiological evidence linking PM2·5 and cardiometabolic disease
Insulin resistance
Several studies (appendix p 2–4) have shown that short-term exposure (days) to air pollution is associated with insulin resistance, a primary predisposing condition to type 2 diabetes across various concentrations and age groups. The development timeline in humans is rapid, occurring within hours to days. This association has been noted in children and adults across a broad range of ages for PM2·5 and traffic-related pollutants (such as NO2). However, it is important to note that air pollutants often correlate spatiotemporally; thus, it might be challenging to isolate the effects of one pollutant in epidemiological studies.
Air pollution and diabetes
Several meta-analyses (appendix p 5–9) have reviewed the effect of air pollution exposure on diabetes incidence and prevalence; in general, air pollution exposure was associated with an average increase in incident diabetes (type 2 diabetes) that generally ranged between 10–25%. The association between air pollution and diabetes is higher in men, groups with low socioeconomic status, and individuals with other comorbidities.29 A meta-analysis of 31 eligible cohort studies demonstrated an increased risk for gestational diabetes associated with increased PM2·5, PM10, SO2, and NO2 concentrations.30 The presence of obesity might also modify the risk for type 2 diabetes.31 Diabetes is a complex disease that takes years to develop, with an additional period of delayed diagnosis. Given these facts, epidemiological findings that have categorised type 2 diabetes at the time of clinical recognition are underestimates, with the real burden of disease probably substantially higher.
Air pollution and obesity
In a meta-analysis to estimate the effects of childhood exposure to air pollutants on weight, 15 studies were included.32 Both obesity and BMI were significantly associated with annual PM10, PM2·5, and PM0·1 exposure. The estimate was strongest for PM2·5, with an odds ratio (OR) of 1·28 for weight (95% CI 1·13–1·45) and 0·11 for BMI (0·05–0·17) per 10 μg/m3 increment in exposure.32 In a cohort consisting of male US veterans, correlations were significant between PM2·5 exposure and obesity (HR 1·08, 95% CI 1·06–1·11) and weight (1·07, 1·06–1·08).33 Several nationwide cohort studies in adults demonstrated a link between air pollution exposure and obesity and other metabolic syndrome components at higher levels of air pollutants.34,35 Longitudinal studies have been largely negative at lower annual PM2·5 concentrations (median ≤20 μg/m3).36–38 Given that air pollution concentrations have been decreasing over time owing to regulation, even when PM2·5 exposure was used as a time-dependent variable to adjust for decreasing PM2·5 concentrations, levels higher than 25 μg/m3 were associated with higher risks of obesity and components of metabolic syndrome in at least two large national cohorts.38,39
Air pollution and diabetes-related mortality
Several studies have explored the association between PM2·5 and diabetes-related mortality at high and low air pollution levels. In a study of 2·1 million adults living in Canada, at low PM2·5 exposure levels (8·7 μg/m3; SD 3·9), there was an increase in the risk of diabetes-related mortality per 10 μg/m3 of PM2·5 (HR 1·49, 95% CI 1·37–1·62), which persisted well below levels of 5 μg/m3.40 A study of 52 061 participants in the Danish Diet, Cancer and Health cohort found an association between increased risk for diabetes-related mortality and long-term exposure to NO2 (mortality rate ratio 1·31, 95% CI 0·98–1·76 for 10 μg/m3 increase after adjustment for confounders).41 Positive associations between diabetes-related mortality and exposure to short-term NO2, CO, and SO2 concentrations have been reported in a time series study conducted in Montreal.42 In the American Cancer Society, Cancer Prevention Study II cohort, a 10-μg/m3 increase in PM2·5 was associated with an 18% increase in diabetes-related mortality.43
Susceptibility and vulnerability
The health effects of air pollution from a cardiometabolic perspective are pronounced among low-income and minoritised racial and ethnic groups populations, partly due to increased exposures.24,25 Evidence also shows that the benefit of lowering PM2·5 levels, even to well below the current US National Ambient Air Quality Standards standard of 12 mg/m3, might also be larger in these populations.44,45 Although some studies have shown that people with diabetes might have greater adverse health effects compared with those without diabetes at similar PM2·5 exposure levels, this is not a consistent finding because people with diabetes show substantial variation in health (eg, duration of disease and risk factor profile) and medication treatment.45
Cardiovascular risk
The evidence that links acute and chronic PM2·5 exposure with fatal and non-fatal cardiovascular events, including stroke and heart failure, has been reviewed extensively.3,5 A small number of studies have investigated PM2·5 and cardiovascular risk in type 2 diabetes. In a subset of the 2001 Canadian Census Health and Environment Cohort, association between PM2·5 and cardiovascular mortality was stronger in people with diabetes (HR 1·51, 95% CI 1·39–1·65, per 10 μg/m3) compared with those without (1·20, 1·16–1·25), suggesting increased PM2·5 susceptibility in patients with diabetes.46 In a study from China including 15 477 individuals with multipollutant ascertainment (eg, PM1·0, PM2·5, PM10, SO2, NO2, and O3), diabetes status potentiated the relationship between pollutants and cardiovascular disease in all exposures.47
Renal and ophthalmic risk
Emerging evidence suggests that PM2·5 is associated with chronic kidney disease in populations both with and without diabetes. Bowe and colleagues described a linear relationship between long-term PM2·5 exposure, incident chronic kidney disease, and progression to end-stage renal disease that was independent of the presence of diabetes.48 In another cohort study of more than 2·4 million US veterans with median 8·5 years of follow-up, Bowe and colleagues found that a 10 μg/m3 increase in PM2·5 was associated with increased odds of diabetes and chronic kidney disease.49 Diabetes mediated 4·7% (4·3–5·7) of the association of PM2·5 with incident estimated glomerular filtration rate (eGFR) less than 60 mL/min per 1·73 m2, 4·8% (95% CI 4·2–5·8) with incident chronic kidney disease, 5·8% (5·0–7·0) with 30% or greater decline in eGFR, and 17·0% (13·1–20·4) with end-stage renal disease or 50% or greater decline in eGFR.49 A longitudinal study of people with diabetes in Taiwan associated urinary albumin-to-creatinine ratio measurements with PM2·5 and CO exposure.50 In an analysis of 469 933 deaths due to chronic kidney disease across 2304 counties in the USA, the association between PM2·5 and chronic kidney disease mortality was strongest among counties with the highest social deprivation index (β0·70, 95% CI 0·49–0·92I), versus counties with the lowest social deprivation index (β0·49, 0·41–0·56).51
Few studies have examined the association between PM2·5 and diabetic retinopathy risk. In a study of newly diagnosed diabetes in Taiwan (2003–12), which included 579 patients, every 10 μg/m3 increase in PM2·5 was associated with a 29% increase in the odds of retinopathy (OR 1·29, 95% CI 1·11–1·50), whereas every 10 μg/m3 increase in PM10 was associated with a 37% increased risk in developing retinopathy (1·37, 1·17–1·61).52
Non-alcoholic fatty liver disease
In a nationwide cross-sectional analysis in the USA in patients with non-alcoholic fatty liver disease (n=269 705) there was a positive association between ambient PM2·5 exposure and the odds of non-alcoholic fatty liver disease (adjusted OR 1·24 per 10 μg/m3 increase, 95% CI 1·15–1·33) among hospitalised patients.53 Despite the PM2·5 exposure levels being much higher than those in the USA and Europe, similar results were also demonstrated using nationwide data from China.54
Air pollution exposure during pregnancy and diabetes
A recent meta-analysis investigated the association between exposure to PM2·5 and gestational diabetes at various stages of pregnancy.55 For the full pregnancy, the pooled relative risk (RR) was 1·074 (95% CI 1·001–1·152). Moreover, during the first and second trimesters, RRs of 1·015 (1·000–1·031) and 1∙021 (1·006–1·035), respectively, were observed. However, no significant association was found between preconception exposure to PM2·5 and gestational diabetes (1·013, 0·990–1·038). In another study from Taiwan that explored the potential effect-modifying capacity of PM2·5 exposure on development of type 2 diabetes in women with and without gestational diabetes, the odds ratio of diabetes per interquartile range increase in PM2·5 was 1·31 (1·22–1·41), with the risks of developing type 2 diabetes with exposure to increased PM2·5 levels being significantly higher in the gestational diabetes group than the non-gestational diabetes group.56 Despite this evidence, there are still substantial knowledge gaps that require further investigations to explore the ramifications of PM2·5 exposure during different gestational periods and the potential long-term cardiometabolic effects.
Towards mechanistic understanding of air pollution-mediated metabolic risk
Understanding of the pathways by which air pollution might contribute to the risk of heart and metabolic problems, while still evolving, have been refined over the past decade.5 The generally understood pathways by which air pollution might potentiate cardiometabolic disease are depicted in figure 2. Conceptually, these pathways could be viewed as follows: primary initiating pathways that are localised in the airways and lungs, and might comprise oxidative stress, ion channel or receptor activation, and inflammation; systemic transduction of metabolic effects through release of biological intermediates (eg, oxidised lipids, cytokines, activated immune cells, microparticles, microRNA, endothelins); autonomic imbalance; hypothalamic–pituitary–axis activation; nanoparticles or pollutants reaching the circulation or transmitted via neurological pathways; and effector mechanisms in insulin responsive organs. These pathways could include one or multiple mechanisms, such as endothelial barrier disruption; adipose and hepatic inflammation; endoplasmic reticulum stress; brown adipose tissue and mitochondrial dysfunction; end-organ responses to autonomic imbalance and CNS activation, including hypothalamic inflammation or dysfunction, and hypothalamic–pituitary–axis activation (eg, vasoconstriction, increased blood pressure, and hypercortisolaemia); and organ responses to circadian disruption.
Figure 2: Mechanisms of PM2·5-mediated metabolic and cardiovascular effects.
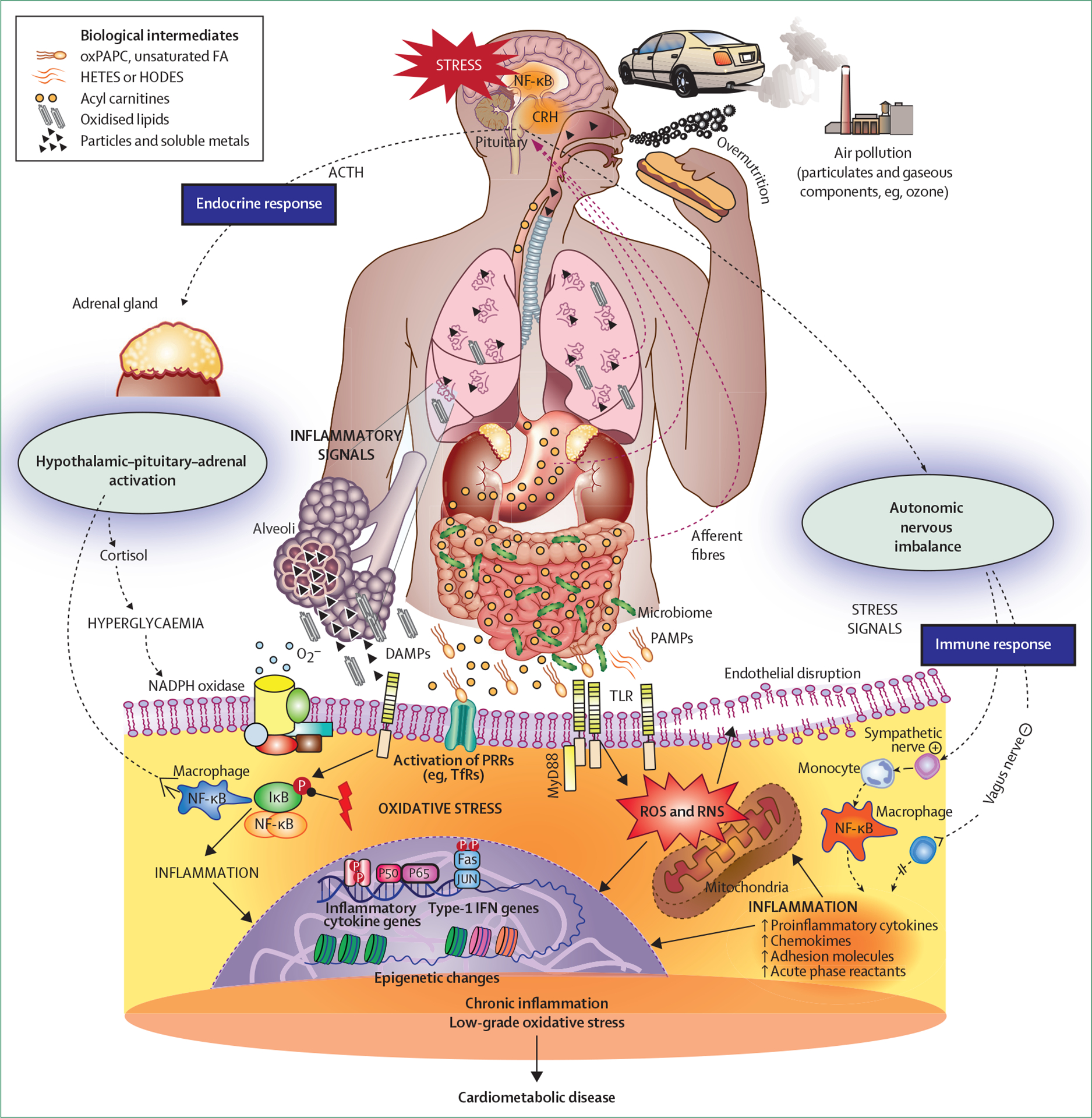
ACTH=adrenocorticotropic hormone. AMP=activated molecular patterns. CRH=corticotropin releasing hormone. DAMPs=disease-activated molecular patterns. FA=fatty acids. HETES=hydroxyeicosatetraenoic acids. HODES=hydroxyoctadecadienoates. IFN=interferon. IκB=IκB kinase. MyD88=myeloid differentiation primary response protein 88. NADPH=nicotinamide adenine dinucleotide phosphate. NF-κB=nuclear factor κB. oxPAPC=oxidised phospholipid 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine. PAMPs=pathogen. PRRs=pattern recognition receptors. RNS=reactive nitrogen species. ROS=reactive oxygen species. TfR=transferrin receptor. TLR=toll-like receptors.
Specific pathways relevant to insulin resistance are depicted in figure 3. In reality, the pathways can be much more complex and mutually dependent than what is conceptualised in figure 2 and figure 3, with temporally overlapping timescales. Also, biological effects might vary based on individual predilection and the composition of air pollution.
Figure 3: Organ-specific mechanisms of air pollution-related insulin resistance.
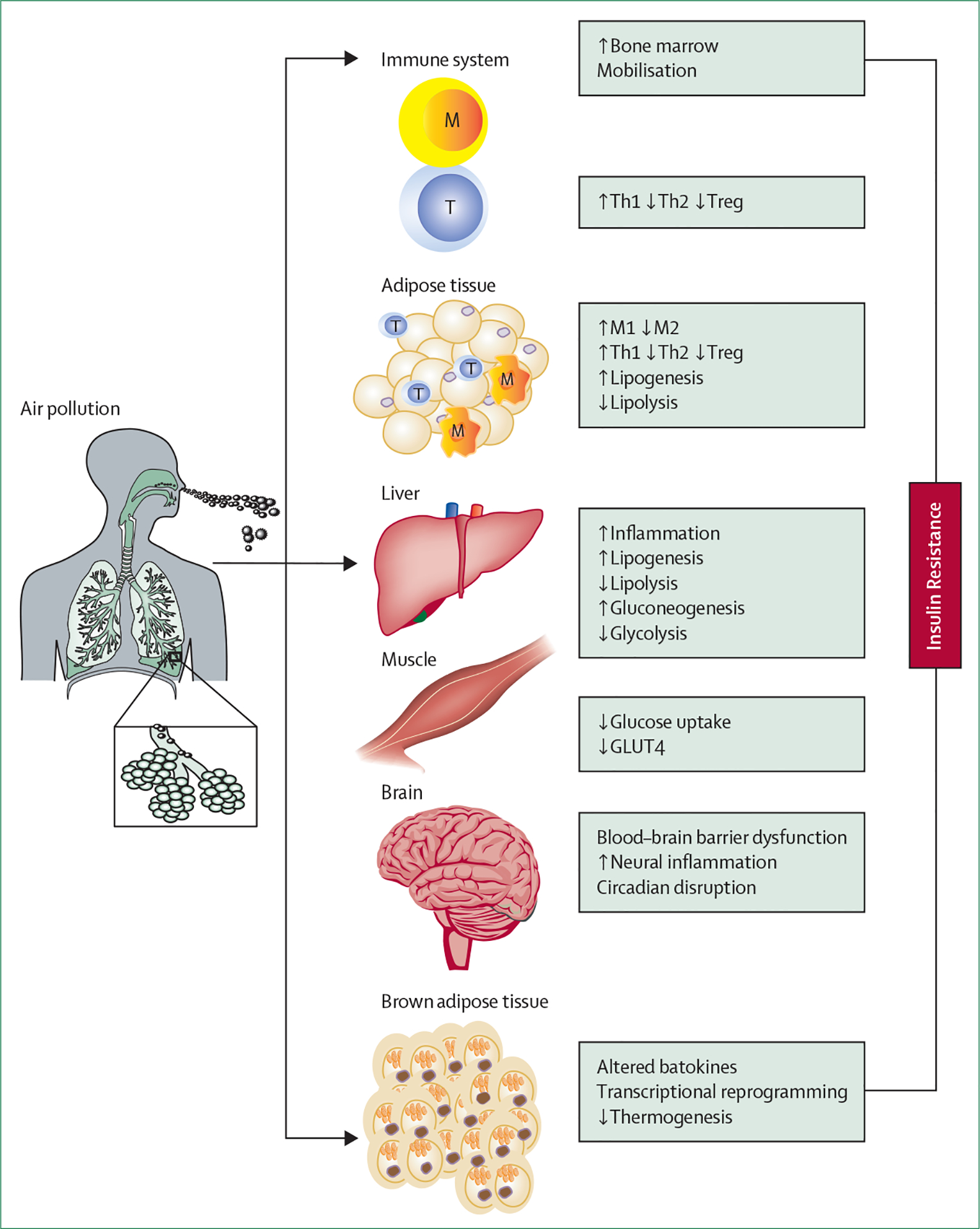
GLUT4=glucose transporter type 4. M=macrophages. T=T cells. Th1=type 1 helper T cell. Th2=type 2 helper T cell. Treg=regulatory T cell.
Primary initiating pathways
Oxidative stress is the first hierarchical response in reaction to air pollution exposure, occurring initially in the respiratory tract and lungs, and playing a key role in many of the subsequent pathways.57 Chemical transformation of endogenous thiols, fatty acids, and lipids—resulting in the additional generation of reactive products—can occur, and has been implicated in translating systemic responses.58 This transformation is evidenced by markers and mediators such as 8-iso-prostaglandin2α, malondialdehyde, oxidised-LDL, oxidised HDL species, and lipid peroxidation biomarkers, which have all been noted to be associated with higher levels of PM2·5 and other air pollutant exposure in humans.58–60 Conversely, in reducing air pollution levels with filtration and treatment with antioxidants, fish oils have been shown to mitigate markers of oxidant stress in response to PM exposure.61
Systemic transduction of air pollution effects
The direct translocation of pollutant particles and other leachable components is now well established in humans.3,5,10 The formation and transmission of reactive biological intermediates, including oxidant stress-generated signal transduction molecules, might mediate systemic metabolic effects.10 Oxidative stress produced in the lung is important in transducing systemic insulin resistance and inflammation. Inhalational exposure to concentrated PM2·5 for 10 days in mice has been shown to increase circulating saturated fatty acids (eg, palmitate), unsaturated fatty acids (eg, palmitoleate and myristoleate), acyl carnitines, and bile acid metabolites. Overexpression of extracellular superoxide dismutase in the lung prevented not only the increase in circulating biomarkers, but vascular inflammation in response to concentrated PM2·5 exposure.62 A 2-week exposure to ultrafine particles (diesel exhaust) led to increased lipid peroxidation and reduced plasma HDL protective capacities. Malondialdehyde and products of arachidonate metabolism, such as hydroxyeicosatetraenoic acids and hydroxyoctadecadienoates, occurred preferentially in the plasma and liver, and less so in the lung.63 In a double-blind, randomised trial in China, air filtration lowered participants’ blood pressure and various metabolic mediators, including inflammatory and oxidant stress measures.64 The effect of inflammation resolution measures in mitigating responses to PM exposure needs to be better understood.
Air pollution components or biological intermediates can be sensed by multiple families of sensing receptors, including toll-like receptors (TLR2 and TLR4), nucleotide-binding domain leucine-rich repeats of nucleotide oligomerisation domain-like receptors, and transient receptor potential (TRPA1 and TRPV1) channels.10,57 A few human panel studies and double-blind interventional trials of air-purifiers have shown an association between PM2·5 exposure and several plasma inflammatory markers (eg, C-reactive protein, IL-6, and tumour necrosis factor-α). Reduction in some of these markers with air filtration that suggests a cause and effect relationship, but the results of these studies have not always been consistent.61,64–66
Experimental studies with chronic inhalation exposure to concentrated ambient PM2·5 have implicated infiltration of monocytes as a cause of inflammation and an M1–M2 switch in adipose tissue. TLR4 appears to be crucial in the effects of PM2·5, including haematopoietic efflux and tissue infiltration.67 C–C chemokine receptor type 2 (CCR2) and chemokine (C–X–C motif) receptor 3 also appear to be crucial in the inflammatory response to PM2·5 through distinct monocyte and T-cell mediated pathways.68 CCR2-knockout mice, fed high-fat chow, show reduced adipose inflammation, improved whole-body insulin resistance, and reduced hepatic lipid accumulation.69 Chemokine (C–X–C motif) receptor 3 knockout (CXCR3) plays a role in the migration of activated T-cell populations (CD44+, CD62L−, and CD4+) into adipose tissue and lungs.68 The overall effects of chronic exposure to concentrated airborne particulate matter in the animal model appears to be consistent with systemic type 1 immunity (type 1 helper T cells [Th1]) dominant immune responses, with a skew from M2 towards M1 and from Th2 towards Th1 in tissue depots.
Dysregulation of the autonomic nervous system and sympathetic activation have been noted acutely in humans and animal models with inhalational exposure to air pollution.58,70,71 Given the well known relationship between sympathetic activation and insulin resistance, activation of this pathway in response to PM2·5 and ultrafine particle exposure (commonly inferred by an acute reduction in heart rate variability or increase in blood pressure and elevation in catecholamines) in both panel studies and with direct inhalational exposure in humans is supportive of the relevance of this pathway in insulin resistance with air pollution exposure.72 Randomised filtration interventions to lower air pollution levels also provide supportive evidence for the causal involvement of sympathetic nervous system and hypothalamic–pituitary–adrenal axis activation.64,73 Acute experiments in canine models and studies in chronically cannulated mice exposed to concentrated ambient PM2·5 have documented increased blood pressure, with evidence of CNS and sympathetic nervous system activation in response to PM2·5 exposure.74,75 Abnormalities in autonomic function or blood pressure responses are, however, not consistently observed, and might relate to compositional differences of pollution exposures and study design.76,77 Inhalation of diesel exhaust ultrafine particles caused a significant elevation within 30 min in muscle sympathetic nerve activity metrics compared with filtered air. Heart rate significantly increased, and there were trends for systolic and diastolic blood pressure elevations, probably haemodynamic consequences of sympathetic nervous system activation. Other components of air pollution, such as volatile organic compounds (eg, acrolein and 1,3-butadiene), might contribute to elevated blood pressure in participants with increased sympathetic tone.78
Effector mechanisms in insulin-responsive organs
Previous reviews have extensively examined the effect of air pollution on conduit artery and resistance vessel function.58,70 Alterations in endothelial function might translate into muscle insulin resistance and hypertension. Endothelial barrier dysfunction has been noted in sites such as the blood–brain barrier in response to PM2·5 and ultrafine particle exposure. Endothelial barrier dysfunction might provide a potential mechanism by which PM2·5 can predispose individuals to peripheral effects that include brown adipose tissue dysfunction, white adipose tissue inflammation, and insulin resistance.79,80
Adipose and hepatic inflammation
Inhalational exposure to PM2·5 hastens the development of insulin resistance and abnormal glucose tolerance in both diet-induced and genetic models of obesity.80 Genetic ablation of CCR2 and neutrophil cytosol factor 1 (p47phox) improves concentrated airborne particulate-induced insulin resistance and adipose inflammation, and is indeed consistent with the important role of CCR2 and oxidant stress pathways in inflammatory monocyte and macrophage recruitment into adipose tissue and insulin resistance.69,81 Chronic concentrated ambient PM2·5 exposure has been associated with a multitude of abnormalities in the liver involving glucose transport, metabolism, lipolysis–lipogenesis balance, altered insulin signalling, inflammation, and findings consistent with steatohepatitis.69,82,83
Endoplasmic reticulum stress
Experimental studies provide substantial evidence that supports activation of all three components of the evolutionarily conserved unfolded protein response or endoplasmic reticulum stress with PM2·5 exposure.84,85 Endoplasmic reticulum stress is an essential regulator of adipocyte lipid metabolism and adipose tissue inflammation. Concentrated airborne particulate exposure in C57BL/6J mice for 10 months resulted in increased adipocyte size and lipid deposition in white adipose tissue, together with increased endoplasmic reticulum stress genes (such as BiP/GRP78, Xbp-1, and Edem1) in white adipose tissue, along with genes involved in lipogenesis (Acaca), transport (CD36), triglyceride synthesis (Dgat2), and adipocyte differentiation or lipid droplet formation (Smaf1, Ceacam1, Fsp27, Plin1, and Fit2).86
Brown adipose tissue effects
In experiments performed in metabolic cages in C57BL/6J mice, concentrated airborne particulate exposure resulted in reduced VO2 and VCO2 levels and decreased thermogenesis, consistent with a reduction in metabolism.71,87,88 These effects were associated with a reduction in uncoupled protein-1 expression in brown adipose tissue and dysregulation of multiple transcriptional regulators of brown adipose tissue metabolism and function.88
CNS inflammation in key energy regulatory centres
A considerable amount of human data suggest that hypothalamic inflammation and dysregulation of energy balance and glucose homoeostasis might be at the core of type 2 diabetes.89 Both short-term and long-term exposure to air pollutants have been shown to induce hypothalamic inflammation and reduce metabolism, with reversal noted in response to inhibition of inflammatory mediators in the brain.88,90 Energy balance is also impacted through the aforementioned brown adipose tissue mechanisms and through alterations in the hypothalamic–pituitary–adrenal axis. Increased secretion of corticosterone and catecholamines has been demonstrated in animal models and humans in response to both PM2·5 and ozone exposure.64,71,90–92 In a post-hoc analysis of the MESA cohort, higher levels of annual NO2 and PM2·5 were associated with higher epinephrine and dopamine levels.93
Circadian disruption
Previous experimental studies have demonstrated that circadian genes are some of the most commonly expressed genes in response to air pollution exposure.94 C57BL/6 mice exposed to concentrated ambient PM2·5 showed insulin resistance, reduced energy expenditure, decreased thermogenesis, decreased metabolism, and dysregulated circadian genes that reversed with cessation of exposure.94 A study comparing exposure to PM2·5 with light at night exposure showed that, although PM2·5 and light at night induced an identical phenotype of insulin resistance and metabolic dysfunction, the transcriptional and epigenetic pathways (including differentially expressed circadian genes) seemed to differ. In humans, metabolic syndrome parameters, sleep deprivation, and depression (circadian syndrome) were highly correlated with PM2·5 exposure.71
Epigenomic changes
Epigenetic reprogramming in response to environmental exposures might represent a crucial buffer against an adverse health response by regulating gene expression and chromosome integrity.95 Chronic PM2·5 exposure promoted substantial chromatin remodelling, especially at promoter and enhancer sites that were pliable, with cessation of exposure resulting in a reversal of changes in chromatin accessibility and of expression of transcripts—notably those involved in insulin action, circadian rhythm, and inflammation.71 Methylation changes and epigenomic changes in networks enriched for pathways related to inflammation, thrombosis, insulin resistance, and lipid metabolism have all been noted.95
Opportunities for mitigating air pollution cardiometabolic health effects
One of the most important first steps in preventing air pollution-mediated metabolic diseases worldwide is to acknowledge the impact of environmental factors such as air pollution on these conditions. Studies on pollution inequity or the differences between environmental health exposures have shown that disproportionally high direct exposure to pollution and climate-related risk factors results in substantial life expectancy disparities, even between adjacent post-codes.44,96 There is a direct link between climate change and air pollution, and both can have direct and indirect effects on health.97 Overall, climate change might worsen air quality by altering meteorological air pollution removal processes and intensifying responses in atmospheric chemistry from both man-made and natural sources.98 Although strategies to reduce climate change can have co-benefits in reducing air pollution, there might also be short-term unintended responses that could affect climate. For instance, a reduction of sulphate emissions from ships has been linked to reduced cloud cover and global warming.99,100 There is a real need for dissemination of the health impacts of climate, and dire need for integrating the health impact of climate in all policy decisions.
Integrated mitigation strategies to address cardiometabolic health that might help improve physical activity and healthy nutrition, but also curb air pollution, ultimately advancing health, climate, and equity goals, are illustrated in figure 4. Policies that endorse clean energy transition, enforcement of pollution control standards, and incentives for reducing processed foods and increasing consumption of plant-based foods, will substantially reduce the burden of type 2 diabetes.3,101 The simultaneous promotion of clean energy modes of public transportation and active transport, such as walking and biking, can help reduce air pollution and improve physical activity. Health impact studies indicate that the health and economic benefits of increasing active transportation simultaneously with clean energy transportation vastly exceed those divined by policies promoting electric mobility transition alone.102 Thus, increasing active transport must be integral to clean energy policy, and new innovative urban design and planning, such as the 15-minute City in Paris, superblocks in Barcelona, and car-free neighbourhoods in Freiburg, are greatly needed.103 Similarly, a shift to locally sourced plant-based diets, even partly, might considerably reduce greenhouse gas emissions and PM2·5 pollution, and improve metabolic health.104 Urban redesign with low-carbon sustainable construction materials, green infrastructure, and walking trails might help increase physical activity while reducing embodied carbon and air pollution from the manufacturing, transportation, installation, and disposal of building materials.104
Figure 4:
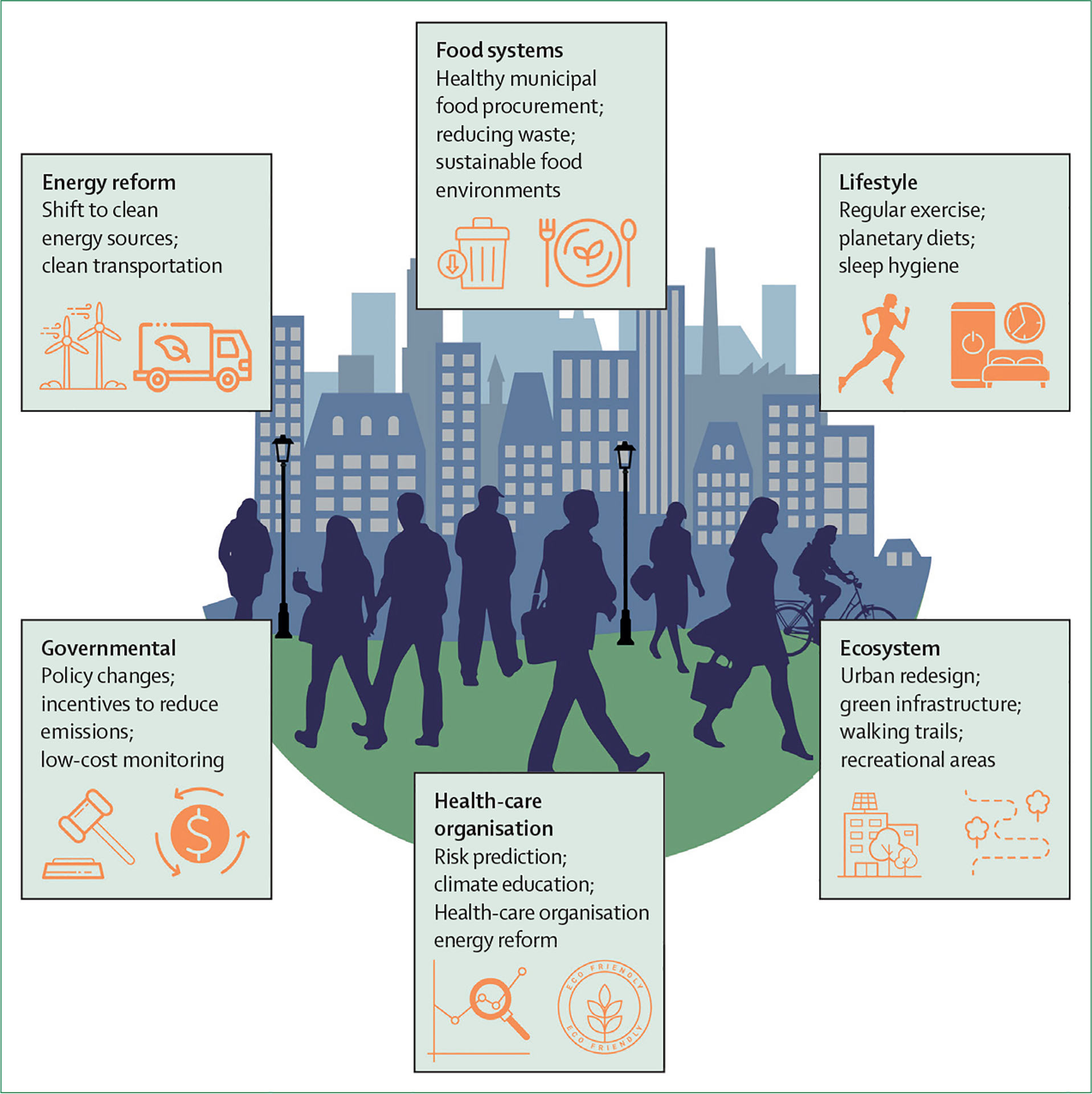
Mitigation strategies to control air pollution-related cardiometabolic disease
The American Heart Association statement on personal protective actions against air pollution, which considers both exposure risks and individual susceptibility, provides a useful framework for individuals at high risk for air pollution-mediated health effects.61 Portable air cleaners are practical and inexpensive in-home strategies suited for at-risk populations, and can help acutely reduce PM2·5 exposures by as much as 60%.61 Several small, short-term, randomised studies in humans have provided proof of concept that reductions in PM2·5 exposures with portable air cleaners can result in rapid, albeit small, reductions in blood pressure and other markers of adverse cardiometabolic risk effects, including inflammation (eg, lower C-reactive protein and IL-6), which are well established risk factors for type 2 diabetes.61 In areas facing very high levels of PM2·5, or during extreme air pollution events (such as occurs regionally for days to weeks from wildfires), indoor portable air cleaners with high-efficiency particulate air filtration, in addition to N95 face masks when travelling outdoors, might be warranted for individuals at high risk of cardiometabolic effects. Some small studies have shown the potential for health protection from wearing face masks; however, more work is needed in this regard.21,61
Health-care organisations and non-profit organisations are well positioned to not only urge governments to take action to prevent pollution-related cardiovascular disease by setting targets and timetables for pollution reduction, but to lead the way in attaining net zero emission targets. The economic impact of the pollution derived from the US health-care system is comparable to the economic impact attributable to medical errors.105 Importantly, the economic impact of pollution is primarily driven by air pollution.105 By mandating sustainability standards in hospitals and health-care organisations, an immediate impact on emissions can be seen, together with cost savings. Including planetary diets in hospitals will allow much-needed alignment of health benefits with planetary goals.106
Air pollution and exercise
Acute elevations in air pollution might discourage physical activity and increase sedentary behaviour in adults and children. Several regulatory authorities, such as the UK Department of Environment and the US Environmental Protection Agency, recommend that the general population should restrict outdoor physical activity on days when PM2·5 levels exceed predefined thresholds. Based on health impact modelling studies, even in areas with very high PM2·5 concentrations (annual average 120 μg/m3), the overall favourable effects of outdoor exercise—either in the form of cycling or walking—obviates the negative effects of inhaling high levels of air pollution, with a reduction in mortality risk with cycling of 15% in Beijing and 14% in New Delhi, assuming annual average PM2·5 concentrations of 95 μg/m3 in Beijing and 120 μg/m3 in New Delhi.107 It is important to note that these reported reductions in mortality risk are in healthy individuals, and whether these results are applicable to high-risk individuals (eg, individuals with type 2 diabetes and cardiovascular or renal disease) is unknown. Overall, the health benefits of regular exercise appear to outweigh the risks of pollution exposure in all but the most extreme settings. Nevertheless, it is a reasonable precautionary approach to exercise indoors or outdoors away from pollution sources (eg, roadways) or postpone the timing of exercise, if possible, to less polluted periods.
Challenges and opportunities for the future: air pollution and beyond
Given the importance of air pollution as a relevant risk factor for cardiometabolic disease, progressive reduction in fossil fuel emissions through the transition to clean energy is expected to decrease the incidence of cardiometabolic disease. Although air pollution emissions are decreasing, worsening trends in physical activity, diet, and exposures to chemical pollution could outweigh these improvements.
The creation of sustainable environments with attention to urban spatial design to encourage walking and active transportation, and with attention to the key provisioning systems of food supply, energy, transportation, buildings, sanitation, water, and green infrastructure, provide the greatest opportunity to reduce cardiometabolic disease while ensuring climate and equity goals. Currently, inadequate and antiquated provisioning systems are responsible for multiple adverse health exposures, such as regional air pollution, local soil or water pollution, adverse nutritional exposures, and climate risks. Policy, appropriate governance, and legal frameworks enable execution of urban planning decisions and regulation of risk exposures. The shortage of policy levers and governance of urban decisions is at the core of unregulated urban proliferation and acceleration of adverse cardiometabolic outcomes in many urban environments worldwide. The economic valuation of the negative externalities of air pollution, including its impact on health, can help justify clean energy initiatives with rapid recoupment of initial investments. The Organization for Economic Co-operation and Development, using such a model, estimated that global air pollution-related health-care costs will increase from US$21 billion in 2015 to $176 billion in 2060.108 Furthermore, the market effects of outdoor air pollution, due to impacts on labour productivity, health expenditure, and agricultural crop yields, might reach 1% of the global gross domestic product by 2060. Importantly, air pollution continues to be a negative externality that is not paid for by either the consumer or the polluter. The use of financial instruments to regulate pollution (polluter pays) could help catalyse a shift to cleaner solutions.
Although global PM2·5 guidelines are important for alignment between country policies, and a step in the right direction, national, state, and local strategies must also be identified and developed to reduce PM2·5. Sector-specific plans might also help with optimising the cost-effectiveness of PM2·5 interventions and improving industry accountability.108
A revolution in personal devices that quantify human health and provide exposure information to a range of chemicals and entities, in addition to PM2·5, provides an opportunity to develop better predictors of human health. Air pollution coexists with enduring elements in the built environment and other key provisioning systems that foster sedentary habits, poor nutritional choices, and exposures to chemicals implicated in type 2 diabetes. The exposome concept strives to capture the diversity and range of internal and external exposures in the environment, such as pollution, synthetic chemicals, dietary constituents, psychosocial stressors, and climate related factors, and their corresponding biological responses. Technological advances, such as unbiased high-throughput genomic and epigenomic approaches, high-resolution metabolomics, and network science, have allowed us to take the first steps toward a comprehensive assessment of the exposome and its effects on cardiometabolic health.109,110 Linking exposomic signatures with biological surrogates and endpoints might provide new insights into the pathophysiology of cardiometabolic disease.111
Research gaps
There are still substantial research gaps in our knowledge of air pollution and its effect on cardiometabolic disease. A greater mechanistic understanding of the impact of the specific subcomponents of PM2·5 is required. Furthermore, interventional studies evaluating the effect of PM2·5 mitigation approaches, such as personal air filtration devices, and the effect of PM2·5 mitigation approaches on cardiometabolic outcomes, could provide valuable evidence for development of individual-level interventions. The effect of air pollution needs to be understood in the context of multiple social and environmental exposures that might co-segregate with air pollution and drive risk associations with cardiometabolic health. Given the increased recognition of the dominant role that non-genetic factors play in type 2 diabetes, an effort to characterise the exposome at a scale similar to that of the human genome is warranted.
Conclusion
Air pollution is a major determinant of cardiometabolic disease globally, and a major contributor to climate change. Efforts to reduce air pollution exposure and mitigate its health effects involve raising individual awareness, appropriate policy levers to reduce sources of air pollution, and measures to reduce personal health impact.
Supplementary Material
Search strategy and selection criteria.
References for this Review were identified through searches of PubMed for articles published from Jan 01, 2006, to June 30, 2023, using the search terms “diabetes”, “insulin resistance”, “exercise“, “obesity”, “cardiovascular disease”, “health impact”, and “cardiometabolic”, in combination with the term “air pollution”. Articles resulting from these searches, and relevant references cited in those articles, were reviewed and included when appropriate. Articles were required to have a full-text English version. Both human and animal studies were considered. No additional search restriction or selection criteria was applied.
Acknowledgments
This study was partly funded by NIH grants (R35ES031702, R01ES019616, R01HL141846).
Footnotes
Declaration of interests
We declare no competing interests.
See Online for appendix
References
- 1.Tuomisto HL, Scheelbeek PFD, Chalabi Z, et al. Effects of environmental change on population nutrition and health: a comprehensive framework with a focus on fruits and vegetables. Wellcome Open Res 2017; 2: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Diabetes Federation. IDF Diabetes Atlas 2021. 2021. https://diabetesatlas.org/atlas/tenth-edition (accessed July 21, 2023). [PubMed] [Google Scholar]
- 3.Rajagopalan S, Landrigan PJ. Pollution and the heart. N Engl J Med 2021; 385: 1881–92. [DOI] [PubMed] [Google Scholar]
- 4.Fuller R, Landrigan PJ, Balakrishnan K, et al. Pollution and health: a progress update. Lancet Planet Health 2022; 6: e535–47. [DOI] [PubMed] [Google Scholar]
- 5.Al-Kindi SG, Brook RD, Biswal S, Rajagopalan S. Environmental determinants of cardiovascular disease: lessons learned from air pollution. Nat Rev Cardiol 2020; 17: 656–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lelieveld J, Klingmüller K, Pozzer A, et al. Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur Heart J 2019; 40: 1590–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.GBD 2019 Diabetes and Air Pollution Collaborators. Estimates, trends, and drivers of the global burden of type 2 diabetes attributable to PM2·5 air pollution, 1990–2019: an analysis of data from the Global Burden of Disease Study 2019. Lancet Planet Health 2022; 6: e586–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mozafarian N, Hashemipour M, Yazdi M, et al. The association between exposure to air pollution and type 1 diabetes mellitus:a systematic review and meta-analysis. Adv Biomed Res 2022; 11: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nair ATN, Wesolowska-Andersen A, Brorsson C, et al. Heterogeneity in phenotype, disease progression, and drug response in type 2 diabetes. Nat Med 2022; 28: 982–88. [DOI] [PubMed] [Google Scholar]
- 10.Rajagopalan S, Al-Kindi SG, Brook RD. Air pollution and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol 2018; 72: 2054–70. [DOI] [PubMed] [Google Scholar]
- 11.Bunker A, Wildenhain J, Vandenbergh A, et al. Effects of air temperature on climate-sensitive mortality and morbidity outcomes in the elderly; a systematic review and meta-analysis of epidemiological evidence. EBioMedicine 2016; 6: 258–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De la Fuente F, Saldías MA, Cubillos C, et al. Green space exposure association with type 2 diabetes mellitus, physical activity, and obesity: a systematic review. Int J Environ Res Public Health 2020; 18: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dzhambov AM. Long-term noise exposure and the risk for type 2 diabetes: a meta-analysis. Noise Health 2015; 17: 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Motairek I, Lee EK, Janus S, et al. Historical neighbourhood redlining and contemporary cardiometabolic risk. J Am Coll Cardiol 2022; 80: 171–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Münzel T, Sørensen M, Hahad O, Nieuwenhuijsen M, Daiber A. The contribution of the exposome to the burden of cardiovascular disease. Nat Rev Cardiol 2023; 20: 651–69. [DOI] [PubMed] [Google Scholar]
- 16.Khomenko S, Cirach M, Pereira-Barboza E, et al. Premature mortality due to air pollution in European cities: a health impact assessment. Lancet Planet Health 2021; 5: e121–34. [DOI] [PubMed] [Google Scholar]
- 17.Awe YA, Larsen BK, Sanchez-Triana E. The global health cost of PM2·5 air pollution: a case for action beyond 2021. International development in focus. 2022. https://documents.worldbank.org/en/publication/documents-reports/documentdetail/455211643691938459/the-global-health-cost-of-pm2-5-air-pollution-a-case-for-action-beyond-2021 (accessed July 23, 2023). [Google Scholar]
- 18.Al-Kindi SG, Brook RD, Biswal S, Rajagopalan S. Environmental determinants of cardiovascular disease: lessons learned from air pollution. Nat Rev Cardiol 2020; 17: 656–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeFlorio-Barker S, Crooks J, Reyes J, Rappold AG. Cardiopulmonary effects of fine particulate matter exposure among older adults, during wildfire and non-wildfire periods, in the USA 2008–10. Environ Health Perspect 2019; 127: 37006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wettstein ZS, Hoshiko S, Fahimi J, Harrison RJ, Cascio WE, Rappold AG. Cardiovascular and cerebrovascular emergency department visits associated with wildfire smoke exposure in California in 2015. J Am Heart Assoc 2018; 7: e007492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hadley MB, Henderson SB, Brauer M, Vedanthan R. Protecting cardiovascular health from wildfire smoke. Circulation 2022; 146: 788–801. [DOI] [PubMed] [Google Scholar]
- 22.Brook RD, Rajagopalan S, Pope CA 3rd, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 2010; 121: 2331–78. [DOI] [PubMed] [Google Scholar]
- 23.Yu W, Ye T, Zhang Y, et al. Global estimates of daily ambient fine particulate matter concentrations and unequal spatiotemporal distribution of population exposure: a machine learning modelling study. Lancet Planet Health 2023; 7: e209–18. [DOI] [PubMed] [Google Scholar]
- 24.Jbaily A, Zhou X, Liu J, et al. Air pollution exposure disparities across USA population and income groups. Nature 2022; 601: 228–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yazdi MD, Wang Y, Di Q, et al. Long-term effect of exposure to lower concentrations of air pollution on mortality among US Medicare participants and vulnerable subgroups: a doubly-robust approach. Lancet Planet Health 2021; 5: e689–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bowe B, Xie Y, Li T, Yan Y, Xian H, Al-Aly Z. The 2016 global and national burden of diabetes mellitus attributable to PM2·5 air pollution. Lancet Planet Health 2018; 2: e301–12. [DOI] [PubMed] [Google Scholar]
- 27.Landrigan PJ, Fuller R, Acosta NJR, et al. The Lancet Commission on pollution and health. Lancet 2018; 391: 462–512. [DOI] [PubMed] [Google Scholar]
- 28.Institute for Health Metrics and Evaluation. GBD results. 2020. https://vizhub.healthdata.org/gbd-results/ (accessed Jan 13, 2024). [Google Scholar]
- 29.Sørensen M, Poulsen AH, Hvidtfeldt UA, et al. Effects of sociodemographic characteristics, comorbidity, and co-exposures on the association between air pollution and type 2 diabetes: a nationwide cohort study. Environ Health Perspect 2023; 131: 27008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang W, Zhu H, Xu J, et al. Ambient air pollution and gestational diabetes mellitus: an updated systematic review and meta-analysis. Ecotoxicol Environ Saf 2023; 255: 114802. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Wang M, Song Y, et al. Obesity and the relation between joint exposure to ambient air pollutants and incident type 2 diabetes: a cohort study in UK Biobank. PLoS Med 2021; 18: e1003767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang C, Li C, Zhao F, Zhu J, Wang S, Sun G. The association between childhood exposure to ambient air pollution and obesity: a systematic review and meta-analysis. Int J Environ Res Public Health 2022; 19: 4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bowe B, Gibson AK, Xie Y, et al. Ambient fine particulate matter air pollution and risk of weight gain and obesity in US veterans: an observational cohort study. Environ Health Perspect 2021; 129: 47003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han W, Xu Z, Hu X, et al. Air pollution, greenness, and risk of overweight among middle-aged and older adults: a cohort study in China. Environ Res 2023; 216: 114372. [DOI] [PubMed] [Google Scholar]
- 35.Zheng X, Tang S, Liu T, et al. Effects of long-term PM2·5 exposure on metabolic syndrome among adults and elderly in Guangdong, China. Environ Health 2022; 21: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matthiessen C, Lucht S, Hennig F, et al. Long-term exposure to airborne particulate matter and NO2 and prevalent and incident metabolic syndrome—results from the Heinz Nixdorf Recall Study. Environ Int 2018; 116: 74–82. [DOI] [PubMed] [Google Scholar]
- 37.Kim JS, Chen Z, Alderete TL, et al. Associations of air pollution, obesity and cardiometabolic health in young adults: the Meta-AIR study. Environ Int 2019; 133(Pt A): 105180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voss S, Schneider A, Huth C, et al. ENVINT-D-20–01309: long-term exposure to air pollution, road traffic noise, residential greenness, and prevalent and incident metabolic syndrome: results from the population-based KORA F4/FF4 cohort in Augsburg, Germany. Environ Int 2021; 147: 106364. [DOI] [PubMed] [Google Scholar]
- 39.Chen YC, Chin WS, Pan SC, Wu CD, Guo YL. Long-term exposure to air pollution and the occurrence of metabolic syndrome and its components in Taiwan. Environ Health Perspect 2023; 131: 17001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brook RD, Cakmak S, Turner MC, et al. Long-term fine particulate matter exposure and mortality from diabetes in Canada. Diabetes Care 2013; 36: 3313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raaschou-Nielsen O, Sørensen M, Ketzel M, et al. Long-term exposure to traffic-related air pollution and diabetes-associated mortality: a cohort study. Diabetologia 2013; 56: 36–46. [DOI] [PubMed] [Google Scholar]
- 42.Goldberg MS, Burnett RT, Yale JF, Valois MF, Brook JR. Associations between ambient air pollution and daily mortality among persons with diabetes and cardiovascular disease. Environ Res 2006; 100: 255–67. [DOI] [PubMed] [Google Scholar]
- 43.Pope CA 3rd, Turner MC, Burnett RT, et al. Relationships between fine particulate air pollution, cardiometabolic disorders, and cardiovascular mortality. Circ Res 2015; 116: 108–15. [DOI] [PubMed] [Google Scholar]
- 44.Josey KP, Delaney SW, Wu X, et al. Air pollution and mortality at the intersection of race and social class. N Engl J Med 2023; 388: 1396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kan H, Jia J, Chen B. The association of daily diabetes mortality and outdoor air pollution in Shanghai, China. J Environ Health 2004; 67: 21–26. [PubMed] [Google Scholar]
- 46.Pinault L, Brauer M, Crouse DL, et al. Diabetes status and susceptibility to the effects of PM2·5 exposure on cardiovascular mortality in a national Canadian cohort. Epidemiology 2018; 29: 784–94. [DOI] [PubMed] [Google Scholar]
- 47.Yang B-Y, Guo Y, Markevych I, et al. Association of long-term exposure to ambient air pollutants with risk factors for cardiovascular disease in China. JAMA Netw Open 2019; 2: e190318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bowe B, Xie Y, Li T, Yan Y, Xian H, Al-Aly Z. Particulate matter air pollution and the risk of incident CKD and progression to ESRD. J Am Soc Nephrol 2018; 29: 218–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bowe B, Xie Y, Yan Y, Xian H, Al-Aly Z. Diabetes minimally mediated the association between PM2·5 air pollution and kidney outcomes. Sci Rep 2020; 10: 4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chin W-S, Chang Y-K, Huang L-F, Tsui H-C, Hsu C-C, Guo Y-LL. Effects of long-term exposure to CO and PM2.5 on microalbuminuria in type 2 diabetes. Int J Hyg Environ Health 2018; 221: 602–08. [DOI] [PubMed] [Google Scholar]
- 51.Motairek I, Sharara J, Makhlouf MHE, et al. Association between particulate matter pollution and CKD mortality by social deprivation. Am J Kidney Dis 2023; 81: 497–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan S-C, Huang C-C, Chin W-S, Chen B-Y, Chan C-C, Guo YL. Association between air pollution exposure and diabetic retinopathy among diabetics. Environ Res 2020; 181: 108960. [DOI] [PubMed] [Google Scholar]
- 53.VoPham T, Kim NJ, Berry K, Mendoza JA, Kaufman JD, Ioannou GN. PM2·5 air pollution exposure and non-alcoholic fatty liver disease in the Nationwide Inpatient Sample. Environ Res 2022; 213: 113611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo B, Guo Y, Nima Q, et al. Exposure to air pollution is associated with an increased risk of metabolic dysfunction-associated fatty liver disease. J Hepatol 2022; 76: 518–25. [DOI] [PubMed] [Google Scholar]
- 55.Zhou X, Li C, Cheng H, et al. Association between ambient air pollution exposure during pregnancy and gestational diabetes mellitus: a meta-analysis of cohort studies. Environ Sci Pollut Res Int 2022; 29: 68615–35. [DOI] [PubMed] [Google Scholar]
- 56.Pan SC, Huang CC, Chen BY, Chin WS, Guo YL. Risk of type 2 diabetes after diagnosed gestational diabetes is enhanced by exposure to PM2·5. Int J Epidemiol 2023; 52: 1414–23. [DOI] [PubMed] [Google Scholar]
- 57.Gangwar RS, Bevan GH, Palanivel R, Das L, Rajagopalan S. Oxidative stress pathways of air pollution mediated toxicity: recent insights. Redox Biol 2020; 34: 101545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Münzel T, Gori T, Al-Kindi S, et al. Effects of gaseous and solid constituents of air pollution on endothelial function. Eur Heart J 2018; 39: 3543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin Y, Ramanathan G, Zhu Y, et al. Pro-oxidative and proinflammatory effects after travelling from Los Angeles to Beijing: a biomarker-based natural experiment. Circulation 2019; 140: 1995–2004. [DOI] [PubMed] [Google Scholar]
- 60.Li J, Zhou C, Xu H, et al. Ambient air pollution is associated with HDL (high-density lipoprotein) dysfunction in healthy adults. Arterioscler Thromb Vasc Biol 2019; 39: 513–22. [DOI] [PubMed] [Google Scholar]
- 61.Rajagopalan S, Brauer M, Bhatnagar A, et al. Personal-level protective actions against particulate matter air pollution exposure: a scientific statement from the American Heart Association. Circulation 2020; 142: e411–31. [DOI] [PubMed] [Google Scholar]
- 62.Hill BG, Rood B, Ribble A, Haberzettl P. Fine particulate matter (PM2·5) inhalation-induced alterations in the plasma lipidome as promoters of vascular inflammation and insulin resistance. Am J Physiol Heart Circ Physiol 2021; 320: H1836–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yin F, Lawal A, Ricks J, et al. Diesel exhaust induces systemic lipid peroxidation and development of dysfunctional pro-oxidant and pro-inflammatory high-density lipoprotein. Arterioscler Thromb Vasc Biol 2013; 33: 1153–61. [DOI] [PubMed] [Google Scholar]
- 64.Li H, Cai J, Chen R, et al. Particulate matter exposure and stress hormone levels: a randomised, double-blind, crossover trial of air purification. Circulation 2017; 136: 618–27. [DOI] [PubMed] [Google Scholar]
- 65.Pope CA 3rd, Hansen ML, Long RW, et al. Ambient particulate air pollution, heart rate variability, and blood markers of inflammation in a panel of elderly subjects. Environ Health Perspect 2004; 112: 339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brook RD, Urch B, Dvonch JT, et al. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension 2009; 54: 659–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kampfrath T, Maiseyeu A, Ying Z, et al. Chronic fine particulate matter exposure induces systemic vascular dysfunction via NADPH oxidase and TLR4 pathways. Circ Res 2011; 108: 716–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deiuliis JA, Kampfrath T, Zhong J, et al. Pulmonary T cell activation in response to chronic particulate air pollution. Am J Physiol Lung Cell Mol Physiol 2012; 302: L399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu C, Xu X, Bai Y, et al. Air pollution-mediated susceptibility to inflammation and insulin resistance: influence of CCR2 pathways in mice. Environ Health Perspect 2014; 122: 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hahad O, Rajagopalan S, Lelieveld J, et al. Noise and air pollution as risk factors for hypertension: part II-pathophysiologic insight. Hypertension 2023; 80: 1384–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Palanivel R, Vinayachandran V, Biswal S, et al. exposure to air pollution disrupts circadian rhythm through alterations in chromatin dynamics. iScience 2020; 23: 101728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brook RD, Xu X, Bard RL, et al. Reduced metabolic insulin sensitivity following sub-acute exposures to low levels of ambient fine particulate matter air pollution. Sci Total Environ 2013; 448: 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Faridi S, Brook RD, Yousefian F, et al. Effects of respirators to reduce fine particulate matter exposures on blood pressure and heart rate variability: a systematic review and meta-analysis. Environ Pollut 2022; 303: 119109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bartoli CR, Wellenius GA, Coull BA, et al. Concentrated ambient particles alter myocardial blood flow during acute ischemia in conscious canines. Environ Health Perspect 2009; 117: 333–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ying Z, Xu X, Bai Y, et al. Long-term exposure to concentrated ambient PM2·5 increases mouse blood pressure through abnormal activation of the sympathetic nervous system: a role for hypothalamic inflammation. Environ Health Perspect 2014; 122: 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sullivan JH, Schreuder AB, Trenga CA, et al. Association between short term exposure to fine particulate matter and heart rate variability in older subjects with and without heart disease. Thorax 2005; 60: 462–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peretz A, Kaufman JD, Trenga CA, et al. Effects of diesel exhaust inhalation on heart rate variability in human volunteers. Environ Res 2008; 107: 178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McGraw KE, Riggs DW, Rai S, et al. Exposure to volatile organic compounds—acrolein, 1,3-butadiene, and crotonaldehyde—is associated with vascular dysfunction. Environ Res 2021; 196: 110903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Münzel T, Sørensen M, Gori T, et al. Environmental stressors and cardio-metabolic disease: part II-mechanistic insights. Eur Heart J 2017; 38: 557–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rao X, Montresor-Lopez J, Puett R, Rajagopalan S, Brook RD. Ambient air pollution: an emerging risk factor for diabetes mellitus. Curr Diab Rep 2015; 15: 603. [DOI] [PubMed] [Google Scholar]
- 81.Weisberg SP, Hunter D, Huber R, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest 2006; 116: 115–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun Q, Yue P, Deiuliis JA, et al. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation 2009; 119: 538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zheng Z, Xu X, Zhang X, et al. Exposure to ambient particulate matter induces a NASH-like phenotype and impairs hepatic glucose metabolism in an animal model. J Hepatol 2013; 58: 148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu C, Ying Z, Harkema J, Sun Q, Rajagopalan S. Epidemiological and experimental links between air pollution and type 2 diabetes. Toxicol Pathol 2013; 41: 361–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Laing S, Wang G, Briazova T, et al. Airborne particulate matter selectively activates endoplasmic reticulum stress response in the lung and liver tissues. Am J Physiol Cell Physiol 2010; 299: C736–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mendez R, Zheng Z, Fan Z, Rajagopalan S, Sun Q, Zhang K. Exposure to fine airborne particulate matter induces macrophage infiltration, unfolded protein response, and lipid deposition in white adipose tissue. Am J Transl Res 2013; 5: 224–34. [PMC free article] [PubMed] [Google Scholar]
- 87.Liu C, Bai Y, Xu X, et al. Exaggerated effects of particulate matter air pollution in genetic type II diabetes mellitus. Part Fibre Toxicol 2014; 11: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu C, Fonken LK, Wang A, et al. Central IKKβ inhibition prevents air pollution mediated peripheral inflammation and exaggeration of type II diabetes. Part Fibre Toxicol 2014; 11: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Myers MG Jr, Affinati AH, Richardson N, Schwartz MW. Central nervous system regulation of organismal energy and glucose homeostasis. Nat Metab 2021; 3: 737–50. [DOI] [PubMed] [Google Scholar]
- 90.Ying Z, Xu X, Bai Y, et al. Long-term exposure to concentrated ambient PM2·5 increases mouse blood pressure through abnormal activation of the sympathetic nervous system: a role for hypothalamic inflammation. Environ Health Perspect 2014; 122: 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miller DB, Ghio AJ, Karoly ED, et al. Ozone exposure increases circulating stress hormones and lipid metabolites in humans. Am J Respir Crit Care Med 2016; 193: 1382–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kodavanti UP. Stretching the stress boundary: linking air pollution health effects to a neurohormonal stress response. Biochim Biophys Acta 2016; 1860: 2880–90. [DOI] [PubMed] [Google Scholar]
- 93.Hajat A, Diez Roux AV, Castro-Diehl C, et al. The association between long-term air pollution and urinary catecholamines: evidence from the multi-ethnic study of atherosclerosis. Environ Health Perspect 2019; 127: 57007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rajagopalan S, Park B, Palanivel R, et al. Metabolic effects of air pollution exposure and reversibility. J Clin Invest 2020; 130: 6034–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu H, Eckhardt CM, Baccarelli AA. Molecular mechanisms of environmental exposures and human disease. Nat Rev Genet 2023; 24: 332–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Clark LP, Tabory S, Tong K, et al. A data framework for assessing social inequality and equity in multi-sector social, ecological, infrastructural urban systems: focus on fine-spatial scales. J Ind Ecol 2022; 26: 145–63. [Google Scholar]
- 97.Orru H, Ebi KL, Forsberg B. The interplay of climate change and air pollution on health. Curr Environ Health Rep 2017; 4: 504–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fiore AM, Naik V, Leibensperger EM. Air quality and climate connections. J Air Waste Manag Assoc 2015; 65: 645–85. [DOI] [PubMed] [Google Scholar]
- 99.Watson-Parris D, Christensen MW, Laurenson A, Clewley D, Gryspeerdt E, Stier P. Shipping regulations lead to large reduction in cloud perturbations. Proc Natl Acad Sci USA 2022; 119: e2206885119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Scovronick N, Budolfson M, Dennig F, et al. The impact of human health co-benefits on evaluations of global climate policy. Nat Commun 2019; 10: 2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kaufman JD, Elkind MSV, Bhatnagar A, et al. Guidance to reduce the cardiovascular burden of ambient air pollutants: a policy statement from the American Heart Association. Circulation 2020; 142: e432–47. [DOI] [PubMed] [Google Scholar]
- 102.Maizlish N, Rudolph L, Jiang C. Health benefits of strategies for carbon mitigation in US transportation, 2017–2050. Am J Public Health 2022; 112: 426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nieuwenhuijsen MJ. New urban models for more sustainable, liveable and healthier cities post Covid 19; reducing air pollution, noise and heat island effects and increasing green space and physical activity. Environ Int 2021; 157: 106850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rajagopalan S, Vergara-Martel A, Zhong Z, et al. The Urban Environment and Cardiometabolic Health. Circulation 2024. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rabin AS, Pinsky EG. Reducing health care’s climate impact—mission critical or extra credit? N Engl J Med 2023; 389: 583–85. [DOI] [PubMed] [Google Scholar]
- 106.Willett W, Rockström J, Loken B, et al. Food in the anthropocene: the EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019; 393: 447–92. [DOI] [PubMed] [Google Scholar]
- 107.Giallouros G, Kouis P, Papatheodorou SI, Woodcock J, Tainio M. The long-term impact of restricting cycling and walking during high air pollution days on all-cause mortality: health impact assessment study. Environ Int 2020; 140: 105679. [DOI] [PubMed] [Google Scholar]
- 108.Development Organisation for Economic Co-operation and Development. The economic consequences of outdoor air pollution. 2016. https://www.oecd.org/environment/indicators-modelling-outlooks/Policy-Highlights-Economic-consequences-of-outdoor-air-pollution-web.pdf (accessed Jan 13, 2024). [Google Scholar]
- 109.Vermeulen R, Schymanski EL, Barabási AL, Miller GW. The exposome and health: where chemistry meets biology. Science 2020; 367: 392–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang P, Carlsten C, Chaleckis R, et al. Defining the scope of exposome studies and research needs from a multidisciplinary perspective. Environ Sci Technol Lett 2021; 8: 839–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Motairek I, Makhlouf MHE, Rajagopalan S, Al-Kindi S. The exposome and cardiovascular health. Can J Cardiol 2023; 39: 1191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


