Abstract

Cannabis sativa L. is a multipurpose crop with high value for food, textiles, and other industries. Its secondary metabolites, including cannabidiol (CBD), have potential for broad application in medicine. With the CBD market expanding, traditional production may not be sufficient. Here we review the potential for the production of CBD using biotechnology. We describe the chemical and biological synthesis of cannabinoids, the associated enzymes, and the application of metabolic engineering, synthetic biology, and heterologous expression to increasing production of CBD.
Keywords: Cannabidiol (CBD), cannabinoid, biosynthesis, metabolic engineering, synthetic biology
1. Introduction
Cannabis sativa L. is one of the earliest domesticated crops. It has a cultivation history of at least 8500 years.1 Utilization of Cannabis in China dates back to about 5000 years ago.2 For thousands of years, Cannabis has had a profound impact on human life in areas of materials,3 textiles,4 food5 and medicine.6Cannabis was used to treat rheumatic pain, malaria, intestinal disorders, and nervous disorders as early as the ancient Chinese and Indian period.7 Cannabinoid, or phytocannabinoid, is a general term for a series of terpenoid phenolic compounds originally identified in Cannabis. Currently, more than 130 cannabinoids that have been identified in Cannabis,(8,9) of which tetrahydrocannabinol (THC), cannabidiol (CBD), cannabigerol (CBG), and cannabichromene (CBC) are the most abundant.10 THC is the major psychoactive cannabinoid, and can cause high euphoria and hallucinations.11 Industrial Cannabis (hemp) is distinct from drug-type (marijuana) Cannabis. Most countries legally define Cannabis varieties with <0.3% THC as hemp, while those with >0.3% THC as marijuana.12 CBD is another unique cannabinoid, and has reported effects against cancer, inflammation, oxidative damage, and cardiovascular, cerebrovascular, and nervous system diseases.13 The size and value of the CBD market has increased significantly, which has stimulated research in its use and production. It is estimated that by 2025, CBD alone will have a global market value of $ 16 billion.14 With the expansion of the research on the health benefits of CBD, the international market is expected to expand further.
At present, the main source of cannabinoids such as CBD is Cannabis flowers. Cannabis varieties with very high CBD content and low THC content are uncommon, which limits commercial availability of CBD. Therefore, there is motivation to increase the production of CBD through biological, chemical, or biotechnological means to meet market demand.15 In this article, research on the natural and artificial mechanisms of CBD synthesis is reviewed, to provide a reference for further improvements in CBD production.
2. Endocannabinoid System
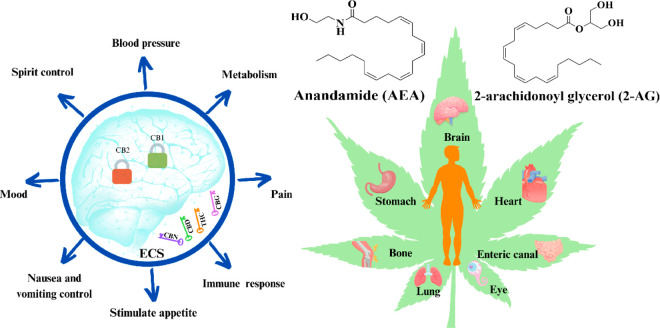
Endocannabinoid system (ECS) is an important molecular system to maintain homeostasis in humans and mammals. It is composed of endogenous cannabinoid receptors, endogenous cannabinoid ligands and enzymes responsible for the synthesis and degradation of endogenous cannabinoids(Figure 1).13,16 ECS components are distributed in both the central and peripheral nervous system and respond to different developmental, physiological, and pathological conditions.17 The ECS has important functions in regulating emotion and cognition, and is a drug target for the treatment of various diseases including some mental disorders.18 The two main endocannabinoid receptors, CB1 and CB2, are G-protein-coupled receptors (GPCRs).19,20 The receptors are expressed in the nervous system, such as neocortex, basal ganglia, cerebellum and brainstem.11,21 However, CB1 and CB2 have different affinity for different cannabinoids. For example, CB1 has higher affinity for THC than CBD.22 At the same time, CB1 is the most abundant G-protein-coupled receptor in the central nervous system,23 so THC is more prone to creating mental dependence than other Cannabis secondary metabolites.19 The expression pattern of CB2 within the brain is more defined, and it is also found on cells of the immune system,23 It plays a role in the treatment of diseases such as neuroprotection and inflammation.24 CBD has much lower affinity than THC to CB1 or CB2, and has beneficial effects including anxiolytic, anti-inflammation, antibacterial, antiallergy, antinausea and regulating gastrointestinal motility.25−29
Figure 1.
Endocannabinoid system. Anandamide (AEA) and 2-arachidonoyl glycerol (2-AG) are two major endogenous cannabinoids. ECS is distributed throughout the human body and regulates the energy balance of the human central nervous system and peripheral nerve metabolism. It is related to many physiological functions such as appetite digestion, emotion, inflammation and pain response, immune function, temperature regulation and so on.
3. Discovery and Application of Cannabidiol
In 1940, Adams et al.30 isolated CBD from Cannabis sativa, but the correct CBD structure could not be determined at that time. In 1963, Mechoulam et al.31 proposed a chemical structure of CBD. Subsequently, in 1977, Jones et al.32 reported two chiral structures of CBD, the main difference being the conformation of the pentyl side chain (Figure 2).33 In plants, this cannabinoid exists in the form of (−)-CBD.
Figure 2.
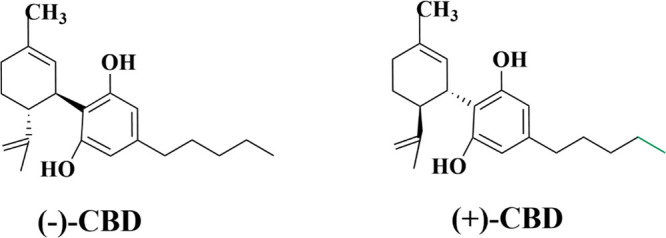
(−)-CBD and (+)-CBD molecular structures
As noted above, CBD plays a role in antianxiety, antipsychosis, anti-inflammation,33,34 and treatment of diseases such as epilepsy and schizophrenia.35−37 But access to CBD has been limited due to is co-occurrence with THC. In 2018, the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) successively approved the CBD-based drug Epidiolex as an adjuvant treatment for seizures associated with Lennox–Gastaut syndrome and Dravet syndrome.38−40
CBD also has uses outside of the pharmaceutical industry. A portion of CBD production is marketed as cigarettes or e-cigarettes.41 CBD-containing e-cigarettes, in a variety of flavors, are popular especially with young people.42 Although CBD is not psychoactive, it is possible that CBD may be converted to some THC-like compounds during acidic or heated evaporation.43−45 In the textile industry, the phenolic acids contained in hemp fibers give them antioxidant and antibacterial properties. CBD can be applied to the surface of textiles to possibly functionalize them with antioxidant, antiaging and skin improvement effects.46 In addition, CBD and its products have been added to consumer products including beverages such as cola, beer, coffee, and apple vinegar, as well as daily necessities such as skin creams, soaps, and lotions.47,48 Recently, CBD was approved for use in the cosmetics industry, providing new opportunities for the market development of Cannabis products.49,50 With the increasing market demand, it is urgent to improve the production of cannabinoids.
4. Biosynthesis of Cannabinoids
4.1. Biosynthesis of Cannabinoids in Cannabis
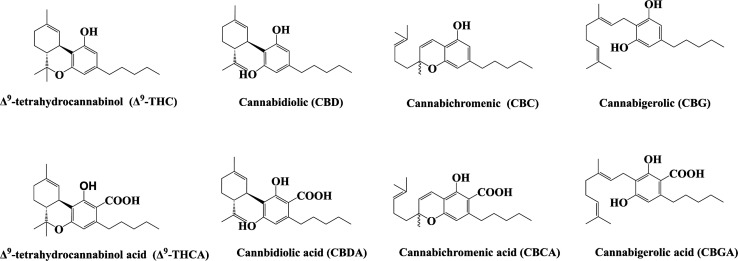
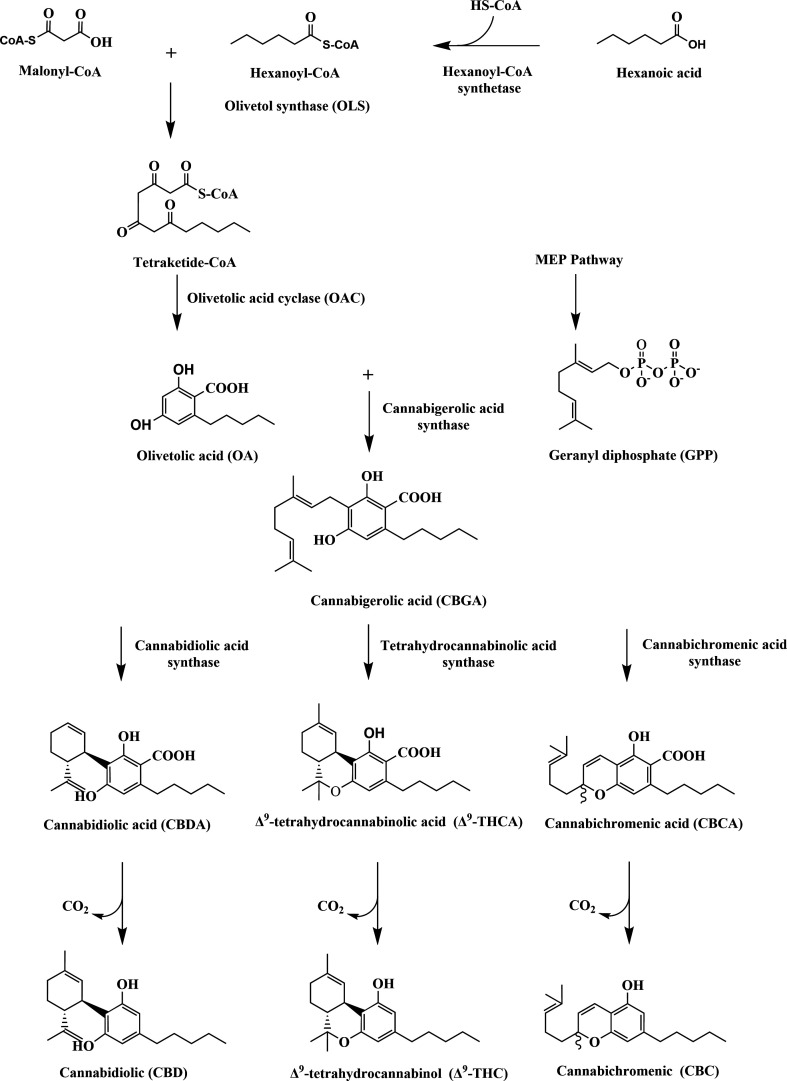
At the molecular level, the biosynthesis of cannabinoids in Cannabis is catalyzed in three main steps. It is first catalyzed by type III polyketide synthase (PKS)51 and olivetolic acid cyclase (OAC) which convert caproyl Co-A and three malonyl Co-A into olivetolic acid (OLA).52,53 Second, the aromatic isoprenyl transferase (cannabigerolic acid synthase, GBGAS)54 catalyzes the isoprenylation of geranyl diphosphate (GPP) and OLA to produce cannabigerolic acid (CBGA).55 Finally, various cannabinoid synthases catalyze the partial oxidative cyclization of CBGA monoterpenes, which are converted into corresponding cannabinoid acids, such as Δ9-THCA, CBDA and CBCA, respectively (Figure 3). Cannabinoids such as Δ9-THC, CBD and CBC are generated by nonenzymatic decarboxylation (Figure 4).56
Figure 3.
Molecular formulas of several important cannabinoids and their acids.
Figure 4.
Biosynthesis pathway of cannabinoids.
Olivetolic acid synthesis is catalyzed by polyketide synthases. In 2004, Raharjo et al.57 tested PKS with hexanoyl-Co-A and malonyl-Co-A as substrates, but there was no production of olivetolic acid or olivetol. In 2009, Taura et al.58 found that extracts from Cannabis encoding olivetol synthase (OLS) did not catalyze olivetolic acid biosynthesis and only produced decarboxylated olivetol. Therefore, it was speculated that there might be another enzyme involved in olivetol biosynthesis. In 2012, Gagne et al.59 through transcriptome analysis of cannabis glandular hairs, demonstrated that the process of the polyketide pathway also requires the involvement of olivetolic acid cyclase (OAC), which carries out the intramolecular C2 → C7 hydroxyl aldol condensation and catalyzes the intermediates that are formed, which are subsequently converted to olivetolic acid by OAC. In 2019, Kearsey et al.51 also confirmed that in the absence of OAC, the nonenzymatic C2 → C7 decarboxylative aldehyde condensation of the tetraketone intermediate produces olivetol rather than olivetolic acid.52
Aromatic prenyltransferase (APT) transfers geranyl pyrophosphate (GPP) to olivetolic acid to produce the cannabigerolic acid (CBGA).55 CBGA is considered to be the central precursor of cannabinoid biosynthesis, and different cyclization of its pentyl moiety leads to the formation of either THCA or its isomers cannabichromenic acid (CBCA) and CBDA.60,61 In 1998, Fellermeier et al.54 found that cannabigerolic acid synthase (GBGAS) used GPP and NPP as donors and olivetolic acid as a specific isopentenyl receptor. In 2011, Page et al. found that isoprenyl transferase (Cannabis GOT, CsPT1) can catalyze the condensation of OLA and GPP to form CBGA. Due to the nonspecificity of its substrate, CsPT1 is considered to be an uncertain link in the cannabinoid synthesis pathway and has not been applied to the production of CBGA.62,63 Therefore, scholars can only find candidate prenyltransferase genes in Cannabis and other organisms. NphB is a soluble aromatic pentenyl transferase from Streptomyces (strain CL190),64−66 which catalyzes the transfer of geranyl to various aromatic receptor molecules to replace CBGAS. In 2017, Zirpel et al. first proved that NphB could catalyze the formation of CBGA.67 In addition, in 2019, the Keasling team proved that the transferase encoded by CsPT4 produced the most CBGA.62 CBGA is the first cannabinoid produced in the process of cannabinoid biosynthesis. It is also the synthetic precursor of cannabinoids such as CBD, THC and CBC. The discovery and application of CsPT4 and NphB is an important progress in cannabinoid metabolic engineering and heterologous expression engineering.
Tetrahydrocannabinol and cannabidiol were first isolated from Cannabis plants beginning in the 1940s.30,68 At that time, it was generally believed that THCA is based on CBDA closed-loop synthesis,69 until Taura proved that THCA is actually derived from CBGA, but whether CBDA is also derived from CBGA is still unknown.70 In 1996, the discovery of cannabidiolic acid (CBDA) synthase directly proved that CBDA was synthesized by oxidative cyclization of CBGA by cannabidiol acid synthase.71 Both tetrahydrocannabinol and cannabidiolic acid are cannabinoids that are synthesized under the catalysis of tetrahydrocannabinolic acid synthase (THCAS) and cannabidiolic acid synthase (CBDAS), respectively, and are the key steps in cannabinoid synthesis.72 At present, THCAS and CBDAS are two types of cannabinoid synthases that are most studied.73 They have great similarities in structure and function, encoding polypeptides of 545 and 544 amino acids, respectively. The first 28 amino acids form a signal peptide sequence, and the translated THCAS has 8 aspartic glycosylation sites, which are easily modified by glycosylation.72,74 The primary structure of the peptide chain of CBDAS and THCAS has 83.9% homology, both of which are FAD-dependent oxidoreductases and have a common sequence of flavin protein.52 In addition, their mechanisms of action are very similar. The only difference is that CBDAS extracts a proton from the terminal methyl group of CBGA rather than the benzyl group of CBGA when catalyzing the formation of CBD.60,75 Evolutionarily, THCAS may have evolved from the CBDAS group.76,77
4.2. Cannabinoid Biosynthesis in Non-Cannabis Plants
The discovery of cannabinoid synthase in plants is a breakthrough in the study of Cannabis. For a long time, cannabinoids have been considered to exist only in Cannabis plants. However, cannabinoid synthase is not only limited to Cannabis plants, but also widely distributed in other plants. The amino acid sequences of cannabinoid synthase MnCBDAS-like from Morus notabilis were compared with those of CsTHCAS and CsCBDAS in Cannabis, and it was found that all three proteins contained FAD_PCMH (PCMH type FAD-BINDING) domain.78 In addition, CBG and CBGA were isolated from the aerial parts of Helichrysum umbraculigerum (H. umbraculigerum) growing in southern Africa.79 Recent studies have reported that H. umbraculigerum has similar biochemical steps to the biosynthesis of cannabinoids in Cannabis plants, and specific enzymes involved in each step are recruited from similar enzyme family members at different times. The convergence of synthetic pathway development between the two species is obvious.80,81 In addition, some terpenoids with a cannabinoid backbone have been found in Rhododendron.82−85 In New Caledonian liverworts, compounds with a bibenzyl backbone structure were detected.86 Bibenzylcis-tetrahydrocannabinol (perrottetinene) was found in the liverwort Radula genus in northern New Zealand, which has the potential of THC analogues.87,88
5. Artificial Chemical Synthesis of CBD and Its Research Progress
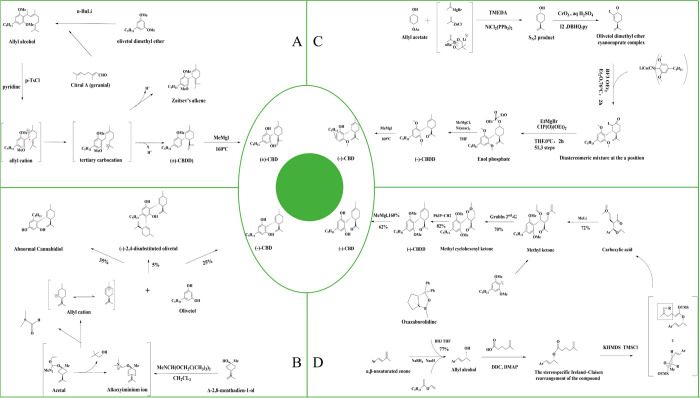
As early as 1965, Mechoulam and Gaoni89,90 proposed a method for the chemical synthesis of racemic (±)-CBD. This scheme gave the first access to racemic (±)-CBD from citral A by demethylation (Figure 5A). Subsequently, in 1967, Petrzilka et al.91 proposed a method based on the retrosynthetic disconnection of a part of the C–C bond between resorcinol Chinese limonene, which allowed the direct stereoselective synthesis of (−)-CBD from olivetol and optically pure Δ-2,8-menthadien-1-ol) (Figure 5B). However, this method suffered from poor regioselectivity, so in 1984, Rickards and Ronneberg92 used BF3-catalyzed regioselective and diastereoselective synthesis of (−)-CBDD. In 2006, Kobayashi et al.93 synthesized (−) −CBD by the coupling reaction of a-iodoketone and olivine dimethyl ether cyanide copper salt complex. On the other hand, (−)-CBD can be better synthesized by adding various olefinic metal reagents in cyclohexenyl monoacetate and using Ni as a catalyst (Figure 5C). In 2018, Leahy et al.94 showed the reduction of ketones by Corey-Bakshi-Shibata (CBS) reduction of ketones, stereospecific Ireland-Claisen rearrangement (SCR) and the combination of three recognized reactions, Ru-catalyzed ring-closing metathesis (RCM), to produce the enantiomer (−)-CBD (Figure 5D). In addition, Nguyen et al.95 have provided chemical synthesis strategies for other rare cannabinoids in Cannabis, such as THC, CBG, cannabinol (CBN), and cannabicyclol (CBL).
Figure 5.
CBD chemical synthesis scheme. (A) p-TsCl = para-toluenesulfonyl chloride. (B) Stereoselective synthesis of (−)-CBD. (C) Kobayashi ’s CBD synthesis scheme. TMEDA = tetramethylethylenediamine, DBHQ = 2,5-di-tert-butylhydroquinone, py = pyridine, and THF = tetrahydrofuran. (D) DCC = N,N′-dicyclohexylcarbodiimide, DMAP = 4-dimethylaminopyridine, KHMDS = potassium bis(trimethylsilyl) amide, and TMSCl = chlorotrimethylsilane.
6. Research Progress of CBD Synthesis by Biotechnology
In order to meet the rapidly growing demand for plant cannabinoids, especially in the absence of high CBD varieties, the use of biotechnology to synthesize cannabinoids in vitro is being explored.96 The market demand for cannabinoid products, including CBD, is increasing year by year, so it is urgent to expand the production of cannabidiol. At present, the main source of cannabidiol is still directly extracted from Cannabis, but it is restricted by the varieties, tissues, different growth periods and growth conditions of Cannabis plants.97,98 It is a long process to meet the market demand of cannabidiol through traditional cultivation and breeding technology, which cannot meet the market demand. Therefore, it is necessary to use biotechnology to achieve rapid production of cannabinoids.
6.1. Application of Hairy Roots in Cannabinoid Production
Metabolic engineering is one means to enhance biosynthetic pathways of plants,99,100 either by increasing flux through desired pathways or by introducing new pathways.101 In the 1980s, it was found that hairy root culture could be used to produce various bioactive substances and secondary metabolites.15 These include, artemisinin,102 aconitine,103 and rosmarinic acid.104
Hairy roots are a plant disease caused by a Gram-negative soil bacterium (Agrobacterium rhizogenes, A. rhizogenes).105 When these bacteria infect plants, hairy roots will be produced at or near the infected site during the transformation process.106 Hairy roots grow rapidly, have good genetic stability, and are highly branched on media without plant hormones. The transformed roots are highly differentiated and can efficiently and stably synthesize secondary metabolites under in vitro culture conditions.104,107 In addition, hairy roots can also be used to synthesize substances that cannot be synthesized by normal plants and suspension cell cultures, and the yield of products is relatively high.108
The use of A. rhizogenes as a gene vector for genetic engineering in higher plants has been widely documented.109 A tobacco hairy root expression system has been used to produce recombinant bovine lactoferrin-derived antimicrobial peptide,107 dermaseptin peptide,110 stilbene compounds111 and other substances. In 2004, Taura et al.72 reported the use of tobacco hairy roots for the heterologous production of THC using tobacco hairy roots, by expressing THCAS and exogenously feeding CBGA. Despite the growing sophistication of tobacco trichome root transformation systems, cannabinoid synthesis precursors are ultimately absent from tobacco, requiring the development of marijuana-derived, trichome-bearing roots. In 2013, Wahby et al.112 first reported that Cannabis can also be transformed into hairy roots. The transformed hairy root inducers showed rapid growth, high incidence of lateral branches, abundant root hairs, and no dependence on hormones. Subsequently, Berahmand et al.113 explored the induced growth of four different strains on trichome roots from stem and leaf explants of Cannabis, and found that A. rhizogenes strain MSU440 and stem segments were the best strains and explants for trichome root induced culture. Despite the development of research on Cannabis hairy cultures, the efficiency of using hairy roots to produce cannabinoid-like substances is still limiting. In 2015, Kayser et al.114 found that induced transformed hairy root grown in shake flasks using callus showed a cyclical increase in growth rate over 35 days, but low cannabinoid content. Hairy root culture has the potential to produce secondary metabolites such as THC and CBD, but further optimization is required. Several studies have attempted large-scale production of cannabinoids like CBD using hairy root cultures, and while the biological yield for CBD production is still minimal, the potential and prospects for production remain promising. The application of transcriptomics, metabolomics, and modeling of metabolic fluxes will inevitably enhance hairy root culture as a more powerful system for plant metabolite production including CBD.
6.2. Synthetic Biology
Reconstruction of biosynthetic pathways to synthesize biochemicals in heterologous species is a common strategy in the biotechnology industry.115 The synthesis of cannabinoids using synthetic biology requires not only a source of isoprene, but also coordination and expression of genes encoding enzymes related to the cannabinoid biosynthesis pathway. Optimizing the polyketide pathway and isoprenoid metabolic pathway to provide sufficient initial precursors for cannabinoid biosynthesis is challenging in heterologous bioproduction systems.
Secondary metabolites such as cannabinoids are usually produced in plant, fungal, or microbial hosts. Tobacco is widely used for heterologous expression of proteins and biologically active substances.20 Among microbes, yeast is a potential biofactory for cannabinoid synthesis.116 The aromatic pentenyltransferases, CsPT4 has CBGAS activity in both Saccharomyces cerevisiae (S. cerevisiae) and Nicotiana benthamiana (N. benthamiana) produces olivetolic acid glucoside and cannabinoid terpene phenolic acid glucoside.117 In 2015, Zirpel et al.118 reported that Pasteur Picot yeast cells have the ability to synthesize medicinal THCA, converting 1 mM of CBGA to THCA (0.36 g·L–1THCA) before loss of enzymatic activity. Furthermore, in 2015, it was found for the first time that, isoprenyltransferase (NphB) and THCAS originating from Streptomyces sp. can be simultaneously expressed in both S. cerevisiae and Komagataella phaffii (K. phaffi) at the same time, and the synthesis of THC from olivetolic acid and geranyl diphosphate was realized in K. phaffii.67 Subsequently, Zirpel et al.119 investigated the functional impact of coexpression of 12 auxiliary proteins in K. phaffii on THCAS expressed in heterologs, with the most significant impact on Hac1s coexpression, and isolated optimized strains that efficiently expressed Hac1S, FAD1, and CNE1 showed a 20-fold increase in THCAS activity compared to the control original strain. In 2019, Luo et al.62 realized the synthesis of cannabinoids from monosaccharide galactose in S. cerevisiae. Both the production of cannabinoids from simple sugars and the production of cannabinoids from cannabinoid synthase substrates demonstrate the promise of heterologous biological production of cannabinoids. Yarrowia lipolytica (Y. lipolytica) is a safe and lipid-rich yeast used to transform and produce various valuable metaboloites.120,121 In 2022, Ma et al.122 reported a method for the production of OA by Y. lipolytica, and the titer of OA reached 9.18 mg/L in shake flask culture. In addition, Escherichia coli is widely considered to be an ideal host for microbial production due to its simple operation and short reproductive cycle.123 In 2018, Tan et al.124 achieved OA synthesis in Escherichia coli (E. coli) by coexpressing olivetol synthase (OLS) and olivetolic acid cyclase (OAC). It marks an important mileage for E. coli to produce cannabinoids. In 2023, Kearsey et al.125 realized the production of CBG in bacteria for the first time, demonstrating the potential of the microbial pathway to functional cannabinoids (Table 1). Although significant progress has been made in the biosynthesis of cannabinoids in microorganisms such as S. cerevisiae, achieving high CBD production is still a major challenge. Simultaneously, H. umbraculigerum has been found to accumulate cannabinoids through parallel evolution, potentially offering a suite of alternative enzymes for cannabinoid synthetic biology and thereby advancing the development of CBD synthetic biology.126
Table 1. Summary of CBD and Other Cannabinoid Biosynthesis Research Progress.
| products | transgenic | host | substrate | titer | meaning | refs |
|---|---|---|---|---|---|---|
| THC | CsTHCAS | Tobacco hairy roots | CBGA | THCA (82 μg, 8.2% conversion from CBGA (1 mg)) | CsTHCAS can control THCA production in other. | (72) |
| Cannabis Hairy root | GUS | Cannabis | The first reported protocol for the establishment of Cannabis hairy root cultures. | (112) | ||
| Cannabinoids | Cannabis Hairy root | Cannabinoids (below 2.0 μg/g dry weight) | The first report on the induction of hairy roots and cannabinoids production of Cannabis. | (114) | ||
| Cannabis Hairy root | Cannabis | A reliable protocol for hairy roots induction of Cannabis was established. | (113) | |||
| CBGA; THCA | CsPT4, CsTHCAS | N. benthamiana and S. cerevisiae | Hexanoic acid or olivetolic acid or GPP | S. cerevisiae: OA (0.1 mM) + CsPT4 = CBGA (1.0 mg/L); N. benthamiana: OA (1 mM) + GPP (1 mM) + CsPT4 = CBGA; CBGA (1 mM) + CsTHCAS = THCAS | CsPT4 has CBGAS activity in N. benthamiana and S. cerevisiae | (117) |
| THCA | CsTHCAS | S. cerevisiae and K. phaffii | CBGA | CBGA (1 mM) + CsTHCAS = THCAS (0.36 g/LTHCA) | Whole cells of P. pastoris offer the capability of synthesizing pharmaceutical THCA production | (118) |
| THCA | NphB, CsTHCAS | S. cerevisiae and K. phaffii | GPP and olivetolic acid | GPP (1 mM) + OA (1 mM) + NphB + CsTHCAS = CBGA (82 ± 4.6 pmol L –1 OD–1 h–1 THCA) (K. phaffii) | It was found for the first time that NphB could produce CBGA. | (67) |
| THCA | CsTHCAS and 12 helper protein | K. phaffii | CBGA | After 8 h, the cells produced 3.05 g/L | The possibility of cannabinoid production was highlighted. | (119) |
| Cannabinoids | CsAAE1, CsTKS, CsOAC, CsPT4, CsCBDAS, CsTHCAS | S. cerevisiae | Galactose or hexanoic acid | 1 mM hexanoic acid can product CBGA (7.2 mg/L) or CBDA (4.3 μg/L) or THCA (1.1 mg/L); CBGA (1.4 mg/L), CBDA (4.2 μg/L) and CBDVA (6.0 ug/L) or THCA (2.3 mg/L) and THCVA (1.2 mg/L) from galactose; THCA (8.0 mg/L) and THCVA (4.8 mg/L) from OA (1 mM) | The synthesis of cannabinoids and related derivatives from galactose in microorganisms was reported for the first time. | (62) |
| OA | CsOLS, CsOAC, CsAAE1, CsAAE3, PpLvaE, SeACSL641P and McMAE2 | Y. lipolytica | Hexanoic acid | OA (9.18 mg/L) | It provides a good microbial chassis for the high-throughput production of cannabinoids. | (122) |
| OA | olivetol synthase (OLS) and olivetolic acid cyclase (OAC) | E. coli | Hexanoyl-CoA | OA (80 mg/L) | The production of OA in E. coli was first reported. | (124) |
| CBG, CBGA | AtaPT (Aspergillus terreus), TKS, GPP synthase (GPPS) | E. coli | Hexanoyl-CoA, malonyl-CoA, OA and/or GPP | Highest titer of CBG (32.9 ± 31.5 μg/L) | This is the first time that CBG has been produced in a bacterial whole cell system. | (125) |
7. Conclusion and Future Perspectives
The traditional method for obtaining secondary metabolites primarily involves extraction from plants or chemical synthesis. Plant extraction is often constrained by factors such as plant growth cycle and conditions. Generally, the complex chemical structure of secondary metabolites makes chemical synthesis costly and inefficient, rendering it an ineffective means of synthesizing cannabinoids like CBD. However, with the rapid development of plant genomics, the biosynthetic mechanism, chemical structure, and metabolic pathway of cannabinoids such as CBD have been elucidated one after another, and high-throughput biosynthesis will become the main means of CBD production. Researchers have been studying and modifying pathways within organisms like yeast or bacteria to create a biosynthetic route for CBD production. By understanding the biosynthetic pathways involved in CBD production, the specific genes responsible for synthesis are introduced into microorganisms. Scientists have been able to manipulate these pathways to increase yield and efficiency. Furthermore, precise genome editing can also be performed using biotechnology tools such as CRISPR/Cas9, enabling scientists to tailor microbes to enhance CBD production and provide more reliable and sustainable resources. In the future, commercial CBD production may be achieved through optimizing heterologous synthesis pathways, regulating relationships between intermediates, and establishing a stable and efficient cell factory. This increases availability of pure CBD free from psychoactive substances like THC.
Author Contributions
▽ Fu Wang and Zhenyun Zang contributed equally to this work. Fu Wang: Conceived and wrote the manuscript. Zhenyun Zang: Conceived and approved the manuscript. Qiao Zhao: Conceived and revised the manuscript. Yangchunxiao Xiao: Wrote part of the manuscript. Xiujuan Lei: Modification and validation, Yingping Wang: Reading, revision and validation. Yiqiao Ma: Read and provide advice. Rongan Cao: Approval and modification. Xixia Song: Read and supervision. Lili Tang: Modification revised the manuscript. Michael K. Deyholos: Edited, revised and wrote the manuscript. Jian Zhang: Conceived idea, Supervision, Funding support, read, revised, and wrote the manuscript. All authors read and approved the manuscript.
This work was funded by a Jilin Agricultural University high-level researcher grant (JLAUHLRG20102006).
The authors declare no competing financial interest.
References
- Fike J. Industrial Hemp: Renewed Opportunities for an Ancient Crop. Crit Rev. Plant Sci. 2016, 35 (5–6), 406–424. 10.1080/07352689.2016.1257842. [DOI] [Google Scholar]
- Valizadehderakhshan M.; Shahbazi A.; Kazem-Rostami M.; Todd M. S.; Bhowmik A.; Wang L. J. Extraction of Cannabinoids from Cannabis sativa L. (Hemp)-Review. Agriculture 2021, 11 (5), 384. 10.3390/agriculture11050384. [DOI] [Google Scholar]
- Karche T.; Singh M. R. The Application of Hemp (Cannabis Sativa L.) for a Green Economy: a Review. Turk J. Bot 2019, 43 (6), 710–723. 10.3906/bot-1907-15. [DOI] [Google Scholar]
- Schluttenhofer C.; Yuan L. Challenges Towards Revitalizing Hemp: A Multifaceted Crop. Trends Plant Sci. 2017, 22 (11), 917–929. 10.1016/j.tplants.2017.08.004. [DOI] [PubMed] [Google Scholar]
- Krüger M.; Van eeden T.; Beswa D. Cannabinoids as Functional Ingredients in Snack Foods-Historical and Developmental Aspects. Plants-Basel 2022, 11 (23), 3330. 10.3390/plants11233330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini S. A.; Premoli M.; Tambaro S.; Kumar A.; Maccarinelli G.; Memo M.; Mastinu A. Cannabis sativa: A Comprehensive Ethnopharmacological Review of a Medicinal Plant with a Long History. J. Ethnopharmacol 2018, 227, 300–315. 10.1016/j.jep.2018.09.004. [DOI] [PubMed] [Google Scholar]
- Daris B.; Verboten M. T.; Knez Z.; Ferk P. Cannabinoids in Cancer Treatment: Therapeutic Potential and Legislation. Bosnian J. Basic Med. 2019, 19 (1), 14–23. 10.17305/bjbms.2018.3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalchuk I.; Pellino M.; Rigault P.; Velzen R. V.; Ebersbach J.; Ashnest J. R.; Mau M.; Schranz M. E.; Alcorn J.; Laprairie R. B.; et al. The Genomics of Cannabis and Its Close Relatives. Annu. Rev. Plant Biol. 2020, 71, 713–739. 10.1146/annurev-arplant-081519-040203. [DOI] [PubMed] [Google Scholar]
- Sirangelo T. M.; Ludlow R. A.; Spadafora N. D. Multi-Omics Approaches to Study Molecular Mechanisms in Cannabis sativa. Plants 2022, 11 (16), 2182. 10.3390/plants11162182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Sanchez I. J.; Verpoorte R. Secondary Metabolism in Cannabis. Phytochem Rev. 2008, 7, 615–639. 10.1007/s11101-008-9094-4. [DOI] [Google Scholar]
- Bisogno T.; Di Marzo V. Cannabinoid Receptors and Endocannabinoids: Role in Neuroinflammatory and Neurodegenerative Disorders. Cns Neurol Disord-Dr 2010, 9 (5), 564–573. 10.2174/187152710793361568. [DOI] [PubMed] [Google Scholar]
- Cherney J. H.; Small E. Industrial Hemp in North America: Production, Politics and Potential. Agronomy 2016, 6 (4), 58. 10.3390/agronomy6040058. [DOI] [Google Scholar]
- Yuan J.; Yang B.; Hou G.; Xie X. Q.; Feng Z. Targeting The Endocannabinoid System: Structural Determinants and Molecular Mechanism of Allosteric Modulation. Drug Discov Today 2023, 28 (7), 103615 10.1016/j.drudis.2023.103615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valleriani J. Identity Narratives in The Face of Market Competition: The Emerging Legal Medical Cannabis Market in Canada. Drug-Educ Prev Polic 2020, 27 (1), 37–48. 10.1080/09687637.2018.1531828. [DOI] [Google Scholar]
- Hesami M.; Baiton A.; Alizadeh M.; Pepe M.; Torkamaneh D.; Jones A. M. P. Advances and Perspectives in Tissue Culture and Genetic Engineering of Cannabis. Int. J. Mol. Sci. 2021, 22 (11), 5671. 10.3390/ijms22115671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V.; Piscitelli F. The Endocannabinoid System and Its Modulation by Phytocannabinoids. Neurotherapeutics 2015, 12 (4), 692–698. 10.1007/s13311-015-0374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P.; Bátkai S.; Kunos G. The Endocannabinoid System as an Emerging Target of Pharmacotherapy. Pharmacol Rev. 2006, 58 (3), 389–462. 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocq M. A. History of Cannabis and The Endocannabinoid System. Dialogues Clin Neuro 2020, 22 (3), 223–228. 10.31887/DCNS.2020.22.3/mcrocq. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E.; Cavic M.; Krivokuca A.; Casadó V.; Canela E. The Endocannabinoid System as a Target in Cancer Diseases: Are We There Yet?. Front Pharmacol 2019, 10, 339. 10.3389/fphar.2019.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gülck T.; Møller B. L. Phytocannabinoids: Origins and Biosynthesis. Trends Plant Sci. 2020, 25 (10), 985–1004. 10.1016/j.tplants.2020.05.005. [DOI] [PubMed] [Google Scholar]
- Svíženská I.; Dubový P.; Šulcová A. Cannabinoid Receptors 1 and 2 (CB1 and CB2), Their Distribution, Ligands and Functional Involvement in Nervous System Structures — a Short Review. Pharmacol Biochem Be 2008, 90 (4), 501–511. 10.1016/j.pbb.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Anand U.; Pacchetti B.; Anand P.; Sodergren M. H. Cannabis-Based Medicines and Pain: A Review of Potential Synergistic and Entourage Effects. Pain Manag 2021, 11 (4), 395–403. 10.2217/pmt-2020-0110. [DOI] [PubMed] [Google Scholar]
- Kendall D. A.; Yudowski G. A. Cannabinoid Receptors in The Central Nervous System: Their Signaling and Roles in Disease. Front Cell Neurosci 2017, 10, 294. 10.3389/fncel.2016.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazuelos J.; Aguado T.; Pazos M. R.; Julien B.; Carrasco C.; Resel E.; Sagredo O.; Benito C.; Romero J.; et al. Azcoitia, I. Microglial CB2 Cannabinoid Receptors are Neuroprotective in Huntington’s Disease Excitotoxicity. Brain 2009, 132 (11), 3152–3164. 10.1093/brain/awp239. [DOI] [PubMed] [Google Scholar]
- Shinjyo N.; Di Marzo V. The Effect of Cannabichromene on Adult Neural Stem/Progenitor Cells. Neurochem. Int. 2013, 63 (5), 432–437. 10.1016/j.neuint.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Delong G. T.; Wolf C. E.; Poklis A.; Lichtman A. H. Pharmacological Evaluation of The Natural Constituent of, Cannabichromene and Its Modulation By Δ-Tetrahydrocannabinol. Drug Alcohol Depen 2010, 112 (1–2), 126–133. 10.1016/j.drugalcdep.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubaro A.; Giangaspero A.; Sosa S.; Negri R.; Grassi G.; Casano S.; Loggia R. D.; Appendino G. Comparative Topical Anti-Inflammatory Activity of Cannabinoids and Cannabivarins. Fitoterapia 2010, 81 (7), 816–819. 10.1016/j.fitote.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Izzo A. A.; Capasso R.; Aviello G.; Borrelli F.; Romano B.; Piscitelli F.; Gallo L.; Capasso F.; Orlando P.; Di Marzo V. Inhibitory Effect of Cannabichromene, A Major Non-Psychotropic Cannabinoid Extracted from Cannabis Sativa, On Inflammation-Induced Hypermotility in Mice. Br. J. Pharmacol. 2012, 166 (4), 1444–1460. 10.1111/j.1476-5381.2012.01879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano B.; Borrelli F.; Fasolino I.; Capasso R.; Piscitelli F.; Cascio M. G.; Pertwee R. G.; Coppola D.; Vassallo L.; Orlando P.; et al. The Cannabinoid TRPA1 Agonist Cannabichromene Inhibits Nitric Oxide Production in Macrophages and Ameliorates Murine Colitis. Br. J. Pharmacol. 2013, 169 (1), 213–229. 10.1111/bph.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams R.; Hunt M.; Clark J. H. Structure of Cannabidiol. III. Reduction and Cleavage. J. Am. Chem. Soc. 1940, 62, 735–737. 10.1021/ja01861a011. [DOI] [Google Scholar]
- Mechoulam R.; Shvo Y. Hashish—I: The Structure of Cannabidiol. Tetrahedron 1963, 19 (12), 2073–2078. 10.1016/0040-4020(63)85022-X. [DOI] [PubMed] [Google Scholar]
- Mechoulam R.; Hanuš L. Cannabidiol: An Overview of Some Chemical and Pharmacological Aspects. Part I: Chemical Aspects. Chem. Phys. Lipids 2002, 121 (1–2), 35–43. 10.1016/S0009-3084(02)00144-5. [DOI] [PubMed] [Google Scholar]
- Govindarajan R. K.; Mishra A. K.; Cho K. H.; Kim K. H.; Yoon K. M.; Baek K. H. Biosynthesis of Phytocannabinoids and Structural Insights: A Review. Metabolites 2023, 13 (3), 442. 10.3390/metabo13030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein S. Cannabidiol (CBD) and Its Analogs: A Review of Their Effects on Inflammation. Bioorg. Med. Chem. 2015, 23 (7), 1377–1385. 10.1016/j.bmc.2015.01.059. [DOI] [PubMed] [Google Scholar]
- Hill A. J.; Williams C. M.; Whalley B. J.; Stephens G. J. Phytocannabinoids as Novel Therapeutic Agents in CNS Disorders. Pharmacol Ther 2012, 133 (1), 79–97. 10.1016/j.pharmthera.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Cristino L.; Bisogno T.; Di Marzo V. Cannabinoids and The Expanded Endocannabinoid System in Neurological Disorders. Nat. Rev. Neurol 2020, 16 (1), 9–29. 10.1038/s41582-019-0284-z. [DOI] [PubMed] [Google Scholar]
- Reddy D. S. Therapeutic and Clinical Foundations of Cannabidiol Therapy for Difficult-To-Treat Seizures in Children and Adults with Refractory Epilepsies. Exp. Neurol. 2023, 359, 114237 10.1016/j.expneurol.2022.114237. [DOI] [PubMed] [Google Scholar]
- Dijk S.; Lok P. NICE Rejects Cannabidiol for Two Types of Treatment Resistant Epilepsy In Children. BMJ. 2019, 366, l5280 10.1136/bmj.l5280. [DOI] [PubMed] [Google Scholar]
- Friedman D.; French J. A.; Maccarrone M. Safety, Efficacy, and Mechanisms of Action of Cannabinoids in Neurological Disorders. Lancet Neurol 2019, 18 (5), 504–512. 10.1016/S1474-4422(19)30032-8. [DOI] [PubMed] [Google Scholar]
- MacCallum C. A.; Russo E. B. Practical Considerations in Medical Cannabis Administration and Dosing. Eur. J. Intern Med. 2018, 49, 12–19. 10.1016/j.ejim.2018.01.004. [DOI] [PubMed] [Google Scholar]
- Bertholet N.; Marmet S.; Wicki M.; Gmel G.; Studer J. Prevalence, Modes of Administration and Motives for Cannabidiol Use in Young Swiss Men. Swiss Med. Wkly 2021, 151, w30054 10.4414/SMW.2021.w30054. [DOI] [PubMed] [Google Scholar]
- Leigh N. J.; Goniewicz M. L. Acute Effect of Electronic Cigarette-Generated Aerosol from Flavored CBD-Containing Refill Solutions on Human Bronchial Epithelial Cells. Front Physiol 2020, 11, 592321 10.3389/fphys.2020.592321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czégény Z.; Nagy G.; Babinszki B.; Bajtel Á.; Sebestyén Z.; Kiss T.; Csupor-Löffler B.; Toth B.; Csupor D. CBD, a Precursor of THC in E-Cigarettes. Sci. Rep 2021, 11 (1), 8951. 10.1038/s41598-021-88389-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N. P.; Aditya A.; Kang H. J.; Park H. D. Unique Approach of a Telemedicine System for CBD-Infused Foods. Processes 2021, 9 (6), 936. 10.3390/pr9060936. [DOI] [Google Scholar]
- Leas E. C.; Moy N.; McMenamin S. B.; Shi Y.; Benmarhnia T.; Stone M. D.; Trinidad D. R.; White M. Availability and Promotion of Cannabidiol (CBD) Products in Online Vape Shops. Int. J. Environ. Res. Public Health 2021, 18 (13), 6719. 10.3390/ijerph18136719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimniewska M.; Pawlaczyk M.; Romanowska B.; Gryszczyńska A.; Kwiatkowska E.; Przybylska P. Bioactive Hemp Clothing Modified with Cannabidiol (CBD) Cannabis Sativa L. Extract. Materials 2021, 14 (20), 6031. 10.3390/ma14206031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenson S.; Chambers L. Cannabidiol: A Budding Industry!. Nutr Bull. 2019, 44 (3), 228–240. 10.1111/nbu.12395. [DOI] [Google Scholar]
- Leas E. C.; Nobles A. L.; Caputi T. L.; Dredze M.; Smith D. M.; Ayers J. W. Trends in Internet Searches for Cannabidiol (CBD) in The United States. JAMA Netw Open 2019, 2 (10), e1913853 10.1001/jamanetworkopen.2019.13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzara E.; Torresi J.; Fico G.; Papini A.; Kulbaka N.; Dall’Acqua S.; Sut S.; Garzoli S.; Mustafa A. M.; et al. A Comprehensive Phytochemical Analysis of Terpenes, Polyphenols and Cannabinoids, And Micromorphological Characterization of 9 Commercial Varieties of Cannabis sativa L. Plants 2022, 11 (7), 891. 10.3390/plants11070891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mnekin L.; Ripoll L. Topical Use of Cannabis sativa L. Biochemicals. Cosmetics-Basel 2021, 8 (3), 85. 10.3390/cosmetics8030085. [DOI] [Google Scholar]
- Kearsey L. J.; Prandi N.; Karuppiah V.; Yan C.; Leys D.; Toogood H.; Takano E.; Scrutton N. S. Structure of The Cannabis sativa Olivetol-Producing Enzyme Reveals Cyclization Plasticity in Type III Polyketide Synthases. FEBS J. 2020, 287 (8), 1511–1524. 10.1111/febs.15089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taura F.; Sirikantaramas S.; Shoyama Y.; Yoshikai K.; Shoyama Y.; Morimoto S. Cannabidiolic-Acid Synthase, The Chemotype-Determining Enzyme in The Fiber-Type Cannabis sativa. FEBS Lett. 2007, 581 (16), 2929–2934. 10.1016/j.febslet.2007.05.043. [DOI] [PubMed] [Google Scholar]
- Tan Z. G.; Clomburg J. M.; Gonzalez R. Synthetic Pathway for The Production of Olivetolic Acid in Escherichia coli. ACS Synth. Biol. 2018, 7 (8), 1886–96. 10.1021/acssynbio.8b00075. [DOI] [PubMed] [Google Scholar]
- Fellermeier M.; Zenk M. H. Prenylation of Olivetolate by a Hemp Transferase Yields Cannabigerolic Acid, The Precursor of Tetrahydrocannabinol. FEBS Lett. 1998, 427 (2), 283–285. 10.1016/S0014-5793(98)00450-5. [DOI] [PubMed] [Google Scholar]
- Singh A.; Bilichak A.; Kovalchuk I. The Genetics of Cannabis—Genomic Variations of Key Synthases and Their Effect on Cannabinoid Content. Genome 2021, 64 (4), 490–501. 10.1139/gen-2020-0087. [DOI] [PubMed] [Google Scholar]
- Hanuš L. O.; Meyer S. M.; Muñoz E.; Taglialatela-Scafati O.; Appendino G. Phytocannabinoids: A Unified Critical Inventory. Nat. Prod Rep 2016, 33 (12), 1357–92. 10.1039/C6NP00074F. [DOI] [PubMed] [Google Scholar]
- Raharjo T. J.; Chang W. T.; Verberne M. C.; Peltenburg-Looman A. M. G.; Linthorst H. J. M.; Verpoorte R. Cloning and Over-Expression of a cDNA Encoding a Polyketide Synthase from Cannabis sativa. Plant Physiol Biochem 2004, 42 (4), 291–297. 10.1016/j.plaphy.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Taura F.; Tanaka S.; Taguchi C.; Fukamizu T.; Tanaka H.; Shoyama Y.; Morimoto S. Characterization of Olivetol Synthase, a Polyketide Synthase Putatively Involved in Cannabinoid Biosynthetic Pathway. FEBS Lett. 2009, 583 (12), 2061–2066. 10.1016/j.febslet.2009.05.024. [DOI] [PubMed] [Google Scholar]
- Gagne S. J.; Stout J. M.; Liu E.; Boubakir Z.; Clark S. M.; Page J. E. Identification of Olivetolic Acid Cyclase from Cannabis Sativa Reveals a Unique Catalytic Route to Plant Polyketides. Proc. Natl. Acad. Sci. 2012, 109 (31), 12811–12816. 10.1073/pnas.1200330109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyama Y.; Tamada T.; Kurihara K.; Takeuchi A.; Taura F.; Arai S.; Blaber M.; Shoyama Y.; Morimoto S.; Kuroki R. Structure and Function of Δ1-Tetrahydrocannabinolic Acid (THCA) Synthase, The Enzyme Controlling The Psychoactivity of Cannabis sativa. J. Mol. Biol. 2012, 423 (1), 96–105. 10.1016/j.jmb.2012.06.030. [DOI] [PubMed] [Google Scholar]
- Apicella P. V.; Sands L. B.; Ma Y.; Berkowitz G. A. Delineating Genetic Regulation of Cannabinoid Biosynthesis During Female Flower Development in Cannabis sativa. Plant Direct 2022, 6 (6), e412 10.1002/pld3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X. Z.; Reiter M. A.; d’Espaux L.; Wong J.; Denby C. M.; Lechner A.; Zhang Y. F.; Grzybowski A. T.; Harth S.; Lin W. Y.; et al. Complete Biosynthesis of Cannabinoids and Their Unnatural Analogues in Yeast. Nature 2019, 567 (7746), 123–126. 10.1038/s41586-019-0978-9. [DOI] [PubMed] [Google Scholar]
- Sands L. B.; Haiden S. R.; Ma Y.; Berkowitz G. A. Hormonal Control of Promoter Activities of Cannabis sativa Prenyltransferase 1 and 4 and Salicylic Acid Mediated Regulation of Cannabinoid Biosynthesis. Sci. Rep 2023, 13 (1), 8620. 10.1038/s41598-023-35303-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzuyama T.; Noel J. P.; Richard S. B. Structural Basis for The Promiscuous Biosynthetic Prenylation of Aromatic Natural Products. Nature 2005, 435 (7044), 983–987. 10.1038/nature03668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonitz T.; Alva V.; Saleh O.; Lupas A. N.; Heide L. Evolutionary Relationships of Microbial Aromatic Prenyltransferases. PLoS One 2011, 6 (11), e27336 10.1371/journal.pone.0027336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahir M. N.; Shahbazi F.; Rondeau-Gagné S.; Trant J. F. The Biosynthesis of The Cannabinoids. J. Cannabis Res. 2021, 3 (1), 7. 10.1186/s42238-021-00062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirpel B.; Degenhardt F.; Martin C.; Kayser O.; Stehle F. Engineering Yeasts as Platform Organisms for Cannabinoid Biosynthesis. J. Biotechnol. 2017, 259, 204–212. 10.1016/j.jbiotec.2017.07.008. [DOI] [PubMed] [Google Scholar]
- Mechoulam R.; Gaoni Y. A Total Synthesis of Dl-Δ1-Tetrahydrocannabinol, The Active Constituent of Hashish. J. Am. Chem. Soc. 1965, 87, 3273–5. 10.1021/ja01092a065. [DOI] [PubMed] [Google Scholar]
- Mechoulam R. Marihuana, Chemistry. Science 1970, 168 (3936), 1159–1166. 10.1126/science.168.3936.1159. [DOI] [PubMed] [Google Scholar]
- Taura F.; Morimoto S.; Shoyama Y.; Mechoulam R. First Direct Evidence for The Mechanism of. Δ1-Tetrahydrocannabinolic Acid Biosynthesis. J. Am. Chem. Soc. 1995, 117 (38), 9766–9767. 10.1021/ja00143a024. [DOI] [Google Scholar]
- Taura F.; Morimoto S.; Shoyama Y. Purification and Characterization of Cannabidiolic-Acid Synthase from Cannabis sativa L.: Biochemical Analysis of a Novel Enzyme that Catalyzes the Oxidocyclization of Cannabigerolic Acid to Cannabidiolic Acid. J. Biol. Chem. 1996, 271 (29), 17411–17416. 10.1074/jbc.271.29.17411. [DOI] [PubMed] [Google Scholar]
- Sirikantaramas S.; Morimoto S.; Shoyama Y.; Ishikawa Y.; Wada Y.; Shoyama Y.; Taura F. The Gene Controlling Marijuana Psychoactivity: Molecular Cloning and Heterologous Expression of Δ1-Tetrahydrocannabinolic Acid Synthase from Cannabis sativa L. J. Biol. Chem. 2004, 279 (38), 39767–39774. 10.1074/jbc.M403693200. [DOI] [PubMed] [Google Scholar]
- Lim K. J. H.; Lim Y. P.; Hartono Y. D.; Go M. K.; Fan H.; Yew W. S. Biosynthesis of Nature-Inspired Unnatural Cannabinoids. Molecules 2021, 26 (10), 2914. 10.3390/molecules26102914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supaart S.; Futoshi T.; Satoshi M.; Yukihiro S. Recent Advances in Cannabis Sativa Research: Biosynthetic Studies and Its Potential in Biotechnology. Curr. Pharm. Biotechnol 2007, 8 (4), 237–243. 10.2174/138920107781387456. [DOI] [PubMed] [Google Scholar]
- Zirpel B.; Kayser O.; Stehle F. Elucidation of Structure-Function Relationship of THCA and CBDA Synthase from Cannabis sativa L. J. Biotechnol. 2018, 284, 17–26. 10.1016/j.jbiotec.2018.07.031. [DOI] [PubMed] [Google Scholar]
- Onofri C.; de Meijer E. P. M.; Mandolino G. Sequence Heterogeneity of Cannabidiolic- and Tetrahydrocannabinolic Acid-Synthase in Cannabis sativa L. and Its Relationship with Chemical Phenotype. Phytochemistry 2015, 116, 57–68. 10.1016/j.phytochem.2015.03.006. [DOI] [PubMed] [Google Scholar]
- Ren G. P.; Zhang X.; Li Y.; Ridout K.; Serrano-Serrano M. L.; et al. Large-Scale Whole-Genome Resequencing Unravels The Domestication History of Cannabis sativa. Sci. Adv. 2021, 7 (29), eabg2286 10.1126/sciadv.abg2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryal N.; Orellana D. F.; Bouie J. Distribution of Cannabinoid Synthase Genes in Non-Cannabis Organisms. J. Cannabis Res. 2019, 1 (1), 8. 10.1186/s42238-019-0008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho Â.; Hansen E. H.; Kayser O.; Carlsen S.; Stehle F. Designing Microorganisms for Heterologous Biosynthesis of Cannabinoids. FEMS Yeast Res. 2017, 17 (4), fox037 10.1093/femsyr/fox037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman P.; de Haro L. A.; Jozwiak A.; Panda S.; Pinkas Z.; Dong Y.; Cveticanin J.; Barbole R.; Livne R.; Scherf T.; et al. Parallel Evolution of Cannabinoid Biosynthesis. Nat. Plants 2023, 9 (5), 817–831. 10.1038/s41477-023-01402-3. [DOI] [PubMed] [Google Scholar]
- Chavez B. G.; D’Auria J. C. Turning a new Leaf on Cannabinoids. Nat. Plants 2023, 9 (5), 687–688. 10.1038/s41477-023-01415-y. [DOI] [PubMed] [Google Scholar]
- Yang Y. X.; Wang J. X.; Wang Q.; Li H. L.; Tao M.; Luo Q.; Liu H. New Chromane and Chromene Meroterpenoids from Flowers of Rhododendron rubiginosum Franch. var. rubiginosum. Fitoterapia 2018, 127, 396–401. 10.1016/j.fitote.2018.03.017. [DOI] [PubMed] [Google Scholar]
- Iwata N.; Kitanaka S. Tetracyclic Chromane Derivatives from Rhododendron anthopogonoides. J. Nat. Prod 2010, 73 (7), 1203–1206. 10.1021/np900543r. [DOI] [PubMed] [Google Scholar]
- Taura F.; Iijima M.; Kurosaki F. Daurichromenic Acid and Grifolic Acid: Phytotoxic Meroterpenoids that Induce Cell Death in Cell Culture of Their Producer Rhododendron dauricum. Plant Signal Behav 2018, 13 (1), e1422463 10.1080/15592324.2017.1422463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata N.; Kitanaka S. New Cannabinoid-Like Chromane and Chromene Derivatives from Rhododendron anthopogonoides. Chem. Pharm. Bull. 2011, 59 (11), 1409–1412. 10.1248/cpb.59.1409. [DOI] [PubMed] [Google Scholar]
- Métoyer B.; Lebouvier N.; Hnawia E.; Herbette G.; Thouvenot L.; Asakawa Y.; Nour M.; Raharivelomanana P. Chemotypes and Biomarkers of Seven Species of New Caledonian Liverworts from The Bazzanioideae Subfamily. Molecules 2018, 23 (6), 1353. 10.3390/molecules23061353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain T.; Espley R. V.; Gertsch J.; Whare T.; Stehle F.; Kayser O. Demystifying The Liverwort Radula Marginata, a Critical Review on Its Taxonomy, Genetics, Cannabinoid Phytochemistry and Pharmacology. Phytochem Rev. 2019, 18, 953–965. 10.1007/s11101-019-09638-8. [DOI] [Google Scholar]
- Chicca A.; Schafroth M. A.; Reynoso-Moreno I.; Erni R.; Petrucci V.; Carreira E. M.; Gertsch J. Uncovering The Psychoactivity of a Cannabinoid from Liverworts Associated with a Legal High. Sci. Adv. 2018, 4 (10), eaat2166 10.1126/sciadv.aat2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R.; Gaoni Y. Hashish—IV: The Isolation and Structure of Cannabinolic Cannabidiolic and Cannabigerolic Acids. Tetrahedron 1965, 21 (5), 1223–1229. 10.1016/0040-4020(65)80064-3. [DOI] [PubMed] [Google Scholar]
- Jung B.; Lee J. K.; Kim J.; Kang E. K.; Han S. Y.; Lee H. Y.; Choi I. S. Synthetic Strategies for (−)-Cannabidiol and Its Structural Analogs. Chem. Asian J. 2019, 14 (21), 3749–3762. 10.1002/asia.201901179. [DOI] [PubMed] [Google Scholar]
- Petrzilka T.; Sikemeier C. ÜBer. Inhaltsstoffe Des Haschisch. 3., Vorläufige Mitteilung. Umwandlung Von (−)-Δ6,1-3,4-Trans-Tetrahydrocannabinol In (−)-Δ1,2-3,4-Trans Tetrahydrocannabinol. Helv. Chim. Acta 1967, 50 (7), 2111–2113. 10.1002/hlca.19670500749. [DOI] [PubMed] [Google Scholar]
- Rickards R. W.; Roenneberg H. Synthesis of (−)-. Δ9-6a,10a-Trans-Tetrahydrocannabinol. Boron Trifluoride Catalyzed Arylation by a Homocuprate. J. Org. Chem. 1984, 49 (3), 572–573. 10.1021/jo00177a044. [DOI] [Google Scholar]
- Kobayashi Y.; Takeuchi A.; Wang Y. G. Synthesis of Cannabidiols Via Alkenylation of Cyclohexenyl Monoacetate. Org. Lett. 2006, 8 (13), 2699–2702. 10.1021/ol060692h. [DOI] [PubMed] [Google Scholar]
- Hoffmann G.; Studer A. Short and Protecting-Group-Free Approach to The (−)-Δ8-THC-Motif: Synthesis of THC-Analogues, (−)-machaeriol B and (−)-machaeriol D. Org. Lett. 2018, 20 (10), 2964–2966. 10.1021/acs.orglett.8b01005. [DOI] [PubMed] [Google Scholar]
- Nguyen G. N.; Jordan E. N.; Kayser O. Synthetic Strategies for Rare Cannabinoids Derived from Cannabis sativa. J. Nat. Prod 2022, 85 (6), 1555–1568. 10.1021/acs.jnatprod.2c00155. [DOI] [PubMed] [Google Scholar]
- Hesami M.; Pepe M.; Baiton A.; Jones A. M. P. Current Status and Future Prospects in Cannabinoid Production Through In Vitro Culture and Synthetic Biology. Biotechnol Adv. 2023, 62, 108074 10.1016/j.biotechadv.2022.108074. [DOI] [PubMed] [Google Scholar]
- Żuk-Gołaszewska K.; Żuk-Gołaszewska K.; Gołaszewski J. Cannabis sativa L. – Cultivation and Quality of Raw Material. J. Elem 2018, 23, 1500 10.5601/jelem.2017.22.3.1500. [DOI] [Google Scholar]
- Chandra S.; Lata H.; ElSohly M. A.; Walker L. A.; Potter D. Cannabis Cultivation: Methodological Issues for Obtaining Medical-Grade Product. Epilepsy Behav 2017, 70, 302–312. 10.1016/j.yebeh.2016.11.029. [DOI] [PubMed] [Google Scholar]
- Capell T.; Bassie L.; Christou P. Modulation of The Polyamine Biosynthetic Pathway in Transgenic Rice Confers Tolerance to Drought Stress. Proc. Natl. Acad. Sci. 2004, 101 (26), 9909–9914. 10.1073/pnas.0306974101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capell T.; Christou P. Progress in Plant Metabolic Engineering. Curr. Opin Biotechnol 2004, 15 (2), 148–154. 10.1016/j.copbio.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Chandra S.; Chandra R. Engineering Secondary Metabolite Production in Hairy Roots. Phytochem Rev. 2011, 10 (3), 371–395. 10.1007/s11101-011-9210-8. [DOI] [Google Scholar]
- Pandey P.; Singh S.; Tewari N.; Srinivas K. V. N. S.; Shukla A.; Gupta N.; Vasudev P. G.; Khan F.; Pal A.; et al. Hairy Root Mediated Functional Derivatization of Artemisinin and Their Bioactivity Analysis. J. Mol. Catal. B: Enzym 2015, 113, 95–103. 10.1016/j.molcatb.2015.01.007. [DOI] [Google Scholar]
- Rawat J. M.; Rawat B.; Bhandari A.; Yadav S.; Mishra S.; Chandra A.; Mishra S. N. Aconitum Biotechnology: Recent Trends and Emerging Perspectives. Acta Physiol Plant 2016, 38 (12), 280. 10.1007/s11738-016-2295-3. [DOI] [Google Scholar]
- Kwon D. Y.; Kim Y. B.; Kim J. K.; Park S. U. Production of Rosmarinic Acid and Correlated Gene Expression in Hairy Root Cultures of Green and Purple Basil (Ocimum basilicum L.). Prep Biochem Biotechnol 2021, 51 (1), 35–43. 10.1080/10826068.2020.1789990. [DOI] [PubMed] [Google Scholar]
- Flores-Félix J. D.; Menéndez E.; Peix A.; García-Fraile P.; Velázquez E. History and Current Taxonomic Status of Genus Agrobacterium. Syst. Appl. Microbiol 2020, 43 (1), 126046 10.1016/j.syapm.2019.126046. [DOI] [PubMed] [Google Scholar]
- Chilton M. D.; Tepfer D. A.; Petit A.; David C.; Casse-Delbart F.; Tempé J. Agrobacterium rhizogenes Inserts T-DNA Into The Genomes of The Host Plant Root Cells. Nature 1982, 295 (5848), 432–434. 10.1038/295432a0. [DOI] [Google Scholar]
- Chahardoli M.; Fazeli A.; Ghabooli M. Recombinant Production of Bovine Lactoferrin-Derived Antimicrobial Peptide in Tobacco Hairy Roots Expression System. Plant Physiol Biochem 2018, 123, 414–421. 10.1016/j.plaphy.2017.12.037. [DOI] [PubMed] [Google Scholar]
- Zhou M. L.; Zhu X.; Shao M.; Wu J. R. Y. M.; Tang Y. X. Transcriptional Response of The Catharanthine Biosynthesis Pathway to Methyl Jasmonate/Nitric Oxide Elicitation in Catharanthus roseus hairy root culture. Appl. Microbiol. Biotechnol. 2010, 88 (3), 737–750. 10.1007/s00253-010-2822-x. [DOI] [PubMed] [Google Scholar]
- Hu Z. B.; Du M. Hairy Root and Its Application in Plant Genetic Engineering. J. Integr Plant Biol. 2006, 48 (2), 121–127. 10.1111/j.1744-7909.2006.00121.x. [DOI] [Google Scholar]
- Varasteh-Shams M.; Nazarian-Firouzabadi F.; Ismaili A. The Direct and Indirect Transformation Methods on Expressing a Recombinant Dermaseptin Peptide in Tobacco Transgenic Hairy Root Clones. Curr. Plant Biol. 2020, 24, 100177 10.1016/j.cpb.2020.100177. [DOI] [Google Scholar]
- Hidalgo D.; Georgiev M.; Marchev A.; Bru-Martínez R.; Cusido R. M.; Corchete P.; Palazon J. Tailoring Tobacco Hairy Root Metabolism for The Production of Stilbenes. Sci. Rep 2017, 7 (1), 17976. 10.1038/s41598-017-18330-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahby I.; Caba J. M.; Ligero F. Agrobacterium Infection of Hemp (Cannabis sativa L.): Establishment of Hairy Root Cultures. J. Plant Interacti 2013, 8 (4), 312–320. 10.1080/17429145.2012.746399. [DOI] [Google Scholar]
- Berahmand F.; Beizaee N.; Dehghan Nayyeri M.; Sharafi A.; Kheiri Manjili H.; Danafar H.; Hashemi Sohi H. Cannabis sativa L. Genetically Transformed Root Based Culture via Agrobacterium rhizogenes. Pharm. Biomed Res. 2016, 2 (3), 13–18. 10.18869/acadpub.pbr.2.3.13. [DOI] [Google Scholar]
- Farag S.; Kayser O. Cannabinoids Production by Hairy Root Cannabis sativa L. American J. Plant Sci. 2015, 6 (11), 1874–1884. 10.4236/ajps.2015.611188. [DOI] [Google Scholar]
- Koukara J.; Papadopoulou K. K. Advances in Plant Synthetic Biology Approaches to Control Expression of Gene Circuits. Biochem. Biophys. Res. Commun. 2023, 654, 55–61. 10.1016/j.bbrc.2023.02.061. [DOI] [PubMed] [Google Scholar]
- Thomas F.; Schmidt C.; Kayser O. Bioengineering Studies and Pathway Modeling of The Heterologous Biosynthesis of Tetrahydrocannabinolic Acid in Yeast. Appl. Microbiol. Biotechnol. 2020, 104 (22), 9551–9563. 10.1007/s00253-020-10798-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gülck T.; Booth J. K.; Carvalho A.; Khakimov B.; Crocoll C.; Motawia M. S.; Møller B. L.; Bohlmann J.; Gallage N. J. Synthetic Biology of Cannabinoids and Cannabinoid Glucosides in Nicotiana benthamiana and Saccharomyces cerevisiae. J. Nat. Prod 2020, 83 (10), 2877–2893. 10.1021/acs.jnatprod.0c00241. [DOI] [PubMed] [Google Scholar]
- Zirpel B.; Stehle F.; Kayser O. Production of Δ9-Tetrahydrocannabinolic Acid from Cannabigerolic Acid by Whole Cells of pichia (komagataella) pastoris Expressing Δ9-Tetrahydrocannabinolic Acid Synthase from Cannabis sativa L. Biotechnol. Lett. 2015, 37 (9), 1869–1875. 10.1007/s10529-015-1853-x. [DOI] [PubMed] [Google Scholar]
- Zirpel B.; Degenhardt F.; Zammarelli C.; Wibberg D.; Kalinowski J.; Stehle F.; Kayser O. Optimization of Δ9-Tetrahydrocannabinolic Acid Synthase Production in Komagataella phaffii via Post-Translational Bottleneck Identification. J. Biotechnol. 2018, 272–273, 40–47. 10.1016/j.jbiotec.2018.03.008. [DOI] [PubMed] [Google Scholar]
- Ma Y.; Li J.; Huang S.; Stephanopoulos G. Targeting Pathway Expression to Subcellular Organelles Improves Astaxanthin Synthesis in Yarrowia lipolytica. Metab Eng. 2021, 68, 152–161. 10.1016/j.ymben.2021.10.004. [DOI] [PubMed] [Google Scholar]
- Luo Z.; Liu N.; Lazar Z.; Chatzivasileiou A.; Ward V.; Chen J.; Zhou J. W.; Stephanopoulos G. Enhancing Isoprenoid Synthesis in Yarrowia lipolytica by Expressing The Isopentenol Utilization Pathway and Modulating Intracellular Hydrophobicity. Metab Eng. 2020, 61, 344–351. 10.1016/j.ymben.2020.07.010. [DOI] [PubMed] [Google Scholar]
- Ma J.; Gu Y.; Xu P. Biosynthesis of Cannabinoid Precursor Olivetolic Acid in Genetically Engineered Yarrowia lipolytica. Commun. Biol. 2022, 5 (1), 1239. 10.1038/s42003-022-04202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D.; Park S. Y.; Park Y. S.; Eun H.; Lee S. Y. Metabolic engineering of Escherichia coli for Natural Product Biosynthesis. Trends Biotechnol. 2020, 38 (7), 745–765. 10.1016/j.tibtech.2019.11.007. [DOI] [PubMed] [Google Scholar]
- Tan Z.; Clomburg J. M.; Gonzalez R. Synthetic Pathway for The Production of Olivetolic Acid in Escherichia coli. ACS Synth Biol. 2018, 7 (8), 1886–1896. 10.1021/acssynbio.8b00075. [DOI] [PubMed] [Google Scholar]
- Kearsey L. J.; Yan C.; Prandi N.; Toogood H. S.; Takano E.; Scrutton N. S. Biosynthesis of Cannabigerol and Cannabigerolic Acid: The Gateways to Further Cannabinoid Production. Synth Biol. (Oxf). 2023, 8 (1), ysad010 10.1093/synbio/ysad010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z. Y.; Mi Y. L.; Kong L. Z.; Gao M. L.; Chen S. S.; Chen W. Q.; Meng X. X.; Sun W.; Chen S. L.; Xu Z. C. Cannabis sativa: Origin and History, Glandular Trichome Development, and Cannabinoid Biosynthesis. Hortic Res. 2023, 10 (9), uhad150 10.1093/hr/uhad150. [DOI] [PMC free article] [PubMed] [Google Scholar]