Abstract
Background
Recurrent aphthous stomatitis (RAS) is a common chronic inflammatory oral disease that negatively impacts the quality of life. Current therapies aim to reduce pain and healing process yet challenges such as rapid loss due to salivary flushing in topical drugs and adverse effects due to prolonged use of systemic medications require further notice. Low-level laser therapy is reported with immediate pain relief and faster healing thus preserving the potential for optimal treatment modalities. This review critically analyses and summarizes the effectiveness of LLLT in reducing pain scores and healing time of RAS.
Methods
A systematic search was conducted in ScienceDirect, PubMed, and Scopus using keywords of low-level laser therapy, photo-biomodulation therapy, and recurrent aphthous stomatitis. RCTs between 1967 to June 2022, presenting characteristics of the laser and reporting pain score and/or healing time of RAS after irradiation were included. Animal studies and recurrent aphthous ulcers with a history of systemic conditions were excluded. Studies were critically appraised using the RoB 2 tool. A meta-analysis was performed using inverse variance random effects.
Results
Fourteen trials with a total of 664 patients were included. Reduced pain was reported in 13 studies, while shortened healing time was presented in 4. The pooling of two studies after CO2 irradiation demonstrated faster healing time compared to placebo (MD − 3.72; 95% CI − 4.18, − 3.25).
Conclusion
Pain score and healing time of RAS were reduced after irradiation with LLLT. RoB resulted in “some concerns” urging well-designed RCTs with larger samples to further assess each laser application for comparison.
Systematic review registration
PROSPERO CRD42022355737.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13643-024-02595-0.
Keywords: Healing time, Low-level laser therapy, Pain score, Recurrent aphthous stomatitis
Background
Recurrent aphthous stomatitis (RAS), also known as canker sores, is a common inflammatory oral condition that presents as painful round to oval-shaped ulcers with a well-defined border surrounded by erythematous area and covered by pseudomembranous base affecting non-keratinized oral mucosa [1, 2]. This condition occurs in 20% of the global population ranging from 5 to 60% based on study and population. In Indonesia, national prevalence reported the prevalence of RAS at 8 to 12% including 45.42% among the South Kalimantan population, 48% among prisoners, and 68% among dental students [3–5]. The major symptom of RAS is pain that impairs nutritional and water intake, possibly leading to subsequent debilitating dehydration. Annual reoccurrence can be seven or more episodes, equating to 27% of a given year that a patient may experience discomfort associated with the disease. This emphasizes the need to manage the ulcers before compromising the well-being of a person [6, 7].
Three principles in the treatment of RAS are to decrease symptoms, reduce ulcer size and number, and increase the ulcer-free period [8]. Glucocorticoids and antimicrobial therapy are considered the conventional treatment for RAS. These medications have been applied as topical pastes, mouth rinses, and intralesional injections. However, there are currently many therapeutic challenges, including low drug efficacy and poor retention at the targeted site of action [9]. Severe and constantly recurring ulcerations were indications for systemic therapy. However, such approaches can generate severe side effects ranging from somnolence to nausea and gastrointestinal symptoms [10]. Several innovative drug delivery systems have been developed for the local treatment and the prevention of various diseases in the oral cavity, yet the efficacy in reducing the size and the number of ulcers was only achieved in 50 to 62% of cases [9]. No improvement after 14 days of treatment was also reported, which urges the need for another remedy unaffected by salivary wash-out and presented systemic side effects with higher efficacy should be evaluated [10, 11].
Low-level laser therapy (LLLT) or cold laser is non-destructive energy that occurs at the periphery of the target tissue with a wavelength from 630 to 1100 nm and a power between 2 and 200 mW [12, 13]. It has bio-stimulating effects, thereby accelerating tissue healing, presenting anti-inflammatory effects on target cells and tissues, and reducing pain from various aetiologies [12, 14]. Several different types of laser (Nd:YAG laser, CO2 laser, and diode laser, etc.) have been applied as the treatment of RAS and they indeed demonstrated superiority in pain relief and faster healing compared to placebo or medical treatment group [14]. The benefit of LLLT includes its short-term local application in contact or non-contact mode thus unaffected by salivary wash-out and not presenting with any systemic side effect [15–17].
Previous reviews summarized the effect of LLLT in treating RAS using a systematic approach, nonetheless, a study to analyze the efficacy and estimate the effect of LLLT compared to other therapies is essential for consideration in utilizing this treatment in clinical settings [18–22]. Based on preliminary searches in databases (PubMed, ScienceDirect, and Scopus) and a registry for systematic review protocol (PROSPERO), this is the first review to study the effects of intervention and the first meta-analysis for low-level laser therapy to assess the reduction of pain and healing time in RAS.
Method
This review follows the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) 2020, the 6.3 version of the Cochrane Handbook for Systematic Review of Interventions [20, 23, 24]. Prospective registration of the review protocol was submitted to PROSPERO with registration number CRD42022355737 to help minimize bias in the conduct and reporting of the review, reduce duplication of effort between groups, and keep the previous systematic reviews updated [25].
Eligibility criteria
The inclusion and exclusion criteria of the studies in this review are detailed using the PICOS (Population; Intervention; Comparator; Outcome(s); Study design) framework [20].
Types of population
This review included studies that examine the effect of low-level laser therapy as the treatment of recurrent aphthous stomatitis in patients of any age. Recurrent aphthous stomatitis is defined as an inflammatory condition of oral mucosal surface in the form of painful small round ulcers that reappear from the mouth from time to time in non-movable/non-keratinizing mucosa without a history of other systemic diseases [26, 27]. A trained individual evaluated participants with recurrent aphthous stomatitis from full history taking and clinical examination based on the 1972 Stanley Classification of Recurrent Aphthous Stomatitis [28, 29]. There are three clinical presentations of RAS: Minor RAS, major RAS, and herpetiform ulceration. Minor RAS is superficial, usually < 1 cm in diameter, and their size is approximately 4–5 mm in diameter. Major RAS are similar in appearance to those of minor RAS; however, they are larger than 10 mm in diameter, are deeper, often scarred, and can last for weeks to months. Herpetiform ulcers are small (1–2 mm), and multiple ulcers (5–100) may be present at the same time. Animal studies and recurrent aphthous ulcers with a history of systemic conditions were excluded.
Types of intervention
Low-level laser therapy uses coherent light sources (lasers) or non-coherent light sources consisting of filtered lamps, light emitting diodes (LED) or a combination of both with power ranges from 1 to 500 mW with a wavelength range from 300 to 10,600 nm. Laser parameters are mainly reported effective at a wavelength from 630 to 1100 nm and with a power between 2 and 200 mW [12, 13, 30, 31].
Types of comparators
The comparator in this review included other therapies, namely placebo and conventional therapy. Placebo included passive laser, which was applied at the same procedure in the treatment group but without any power about which the patient was not aware or with no intervention. Meanwhile, conventional therapy included topical or systemic application of pharmacotherapy such as antimicrobial agents, anesthesia, anti-inflammatory drugs, immunomodulators, or corticosteroids [8, 11].
Types of outcomes measured
Articles presenting pain intensity (reported in pain scale or score) immediately and 1 day after therapy as well as the healing time of the ulcer (reported in days) were included in this review if present.
Types of study
Randomized controlled trials (RCTs) were included, comprising quasi-RCTs (when the allocation of participants may be based on alternation, date of birth, or case record number) and RCTs with an open-label study design where investigators and patients are aware of the intervention given. No language restriction was imposed in this review.
Search methods for identification of studies
In the search strategy, a combination of Medical Subject Headings (MeSH) terms and keywords was applied: (low-level laser therapy OR phototherapy laser OR biostimulation laser OR photobiomodulation therapy) AND recurrent aphthous stomatitis. A validated RCT filter was used to exclude studies with different designs. A literature search was performed in the following electronic databases from their inception date from 1 January 1967 to 30 June 2022: ScienceDirect, PubMed, and Scopus.
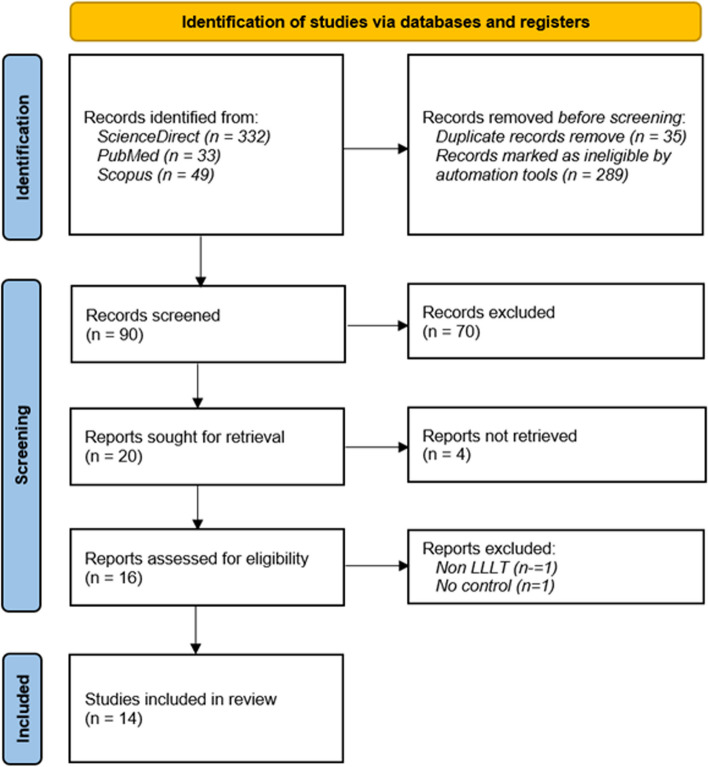
Figure 1 illustrates the search and selection process, which includes identification, inclusion, and process of exclusion. In the identification stage, automation tools removed articles after screening for duplication and ineligibility. Records to be screened were excluded based on title relevancy with inclusion criteria. After the exclusion of the irrelevant title, reports were assessed for relevant information in the abstract. Following the exclusion based on irrelevant abstract, records were further assessed for the eligibility criteria.
Fig. 1.
Flow diagram based on PRISMA 2020
Data collection and analysis
Titles and abstract searches were independently performed by three reviewers (SRP/FYM/IGASP) and assessed to obtain relevant full-text articles for the review. Data were further extracted from the included articles using the predefined form, which was previously prepared in a spreadsheet by SRP and FYM after consulting with DR and AEP. Data extraction form includes author, year, characteristics of participants, characteristics of intervention (including low-level laser therapy [type of laser, wavelength, mode, output power, exposure time, total energy, application of gel, application distance] and other therapies namely topical medication [types of medication, times of application], and placebo), outcome measures, and statistical result. All data were double-checked by SLV for accuracy after the initial abstraction. Any disagreements were resolved between reviewers. When consensus was not achieved, reviewers consulted disagreements with DR as appropriate. Additional information was obtained from the original RCTs, other online supplementary material, or by contacting study authors (Supplementary material 1 and 2).
Assessment of methodological quality of included reviews
Risk of bias was independently assessed by three reviewers (SRP/FYM/RKB) for each included trial using the Cochrane 'Risk of bias' 2 tool. We resolved disagreements by discussion between the reviewers. When consensus was not achieved, reviewers consulted disagreements with DR [20]. The overall quality of the evidence for each outcome was evaluated independently using the GRADE approach. The GRADE approach improves reliability in comparison to intuitive judgments about the certainty of a body of evidence [32].
Data synthesis
This review deployed narrative synthesis for pain score as the outcome measures. The diversity of scale used for outcome measures and the absence of standard deviation in study results prevented the input from meta-analysis for pain score.
Studies presenting healing time were assessed using the standard meta-analysis (direct comparisons) performed using Review Manager 5.4 to determine the effectiveness of treatments directly compared to each other. The I2 statistic was used to quantify the heterogeneity of the results in individual studies since heterogeneity is a common issue encountered while performing meta-analyses. The random-effects model was applied as the default option for illustrative purposes in all the forest plots presenting effect measure data per treatment. MD or mean difference was used for continuous outcomes such as healing time. Data analysis was performed by SLV.
Subgroup analyses were considered in reporting the continuous effect of healing time by splitting intervention based on the type of laser wavelength. Sensitivity analysis by excluding studies with a high risk of bias could not be performed as the number of studies is limited while mixing all types of lasers into one estimated effect and splitting based on the laser dosage may help detect changes in the findings [20].
Result
Description of study
Results of the search
A total of 414 papers were identified. Ninety records were screened after removing duplicates (35) and ineligible reports marked by an advance filter for RCTs (289). Seventy-four articles were excluded after title and abstract evaluation, and sixteen reports were retrieved for eligibility assessment. The full-text assessment resulted in two papers discarded and 14 RCTs to be reviewed.
Included studies
Study population
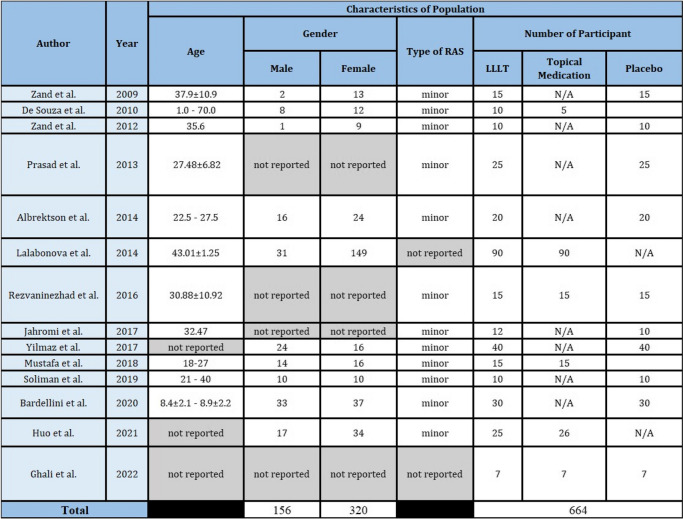
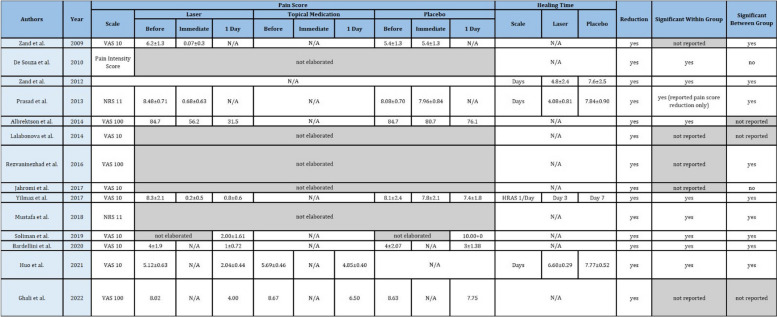
Fourteen studies involving 664 participants, ranging from 15 to 180 [33] participants [33, 34], were included. Only one study enrolled more than 100 patients [33]. Participants included in this review ranged from 1 year old to 70 years old with three studies not reporting the age of participants. Gender distribution demonstrated males with a total of 156 participants and females with a total of 320 participants. Three studies did not report the characteristics of the participants based on age [12, 14, 35] and four studies did not report the characteristics of the participants based on gender [12, 36–38].
Two studies used the history of aphthous ulcer and clinical manifestation in diagnosing RAS followed by laboratory testing and pathergy test to eliminate the presence of any systemic disease [39, 40]. One study reported using history taking, clinical manifestation, and laboratory testing only [36]. Ten studies reported the diagnosis of RAS based on the history of disease and clinical manifestation, yet not mentioning which classification or characteristics of ulcer were used [14, 34, 35, 37, 38, 41–44]. Only one study mentioned the use of the 1972 Stanley Classification of RAS, while the other two did not mention the method to diagnose RAS [12, 33]. Twelve studies reported the use of intervention and control in minor RAS, while the other two did not mention the type of RAS.
Study design and setting
All trials were randomized where five trials were single-blinded [35–37, 39, 41], three trials were double-blinded [38, 40, 44] and six trials not reporting the blinding of participants and evaluator [12, 14, 33, 34, 42, 43]. Based on the study setting, four studies were conducted in Iran [37–40], and the others were each from Brazil [34], India [36], Sweden [41], Bulgaria [33], Turkey [35], Malaysia [42], Egypt [43], Italy [44], China [14], and Iraq [12]. Summary for study population and study design presented is presented in Table 1.
Table 1.
Characteristics of population and study design
N/A not applicable
Intervention and comparators
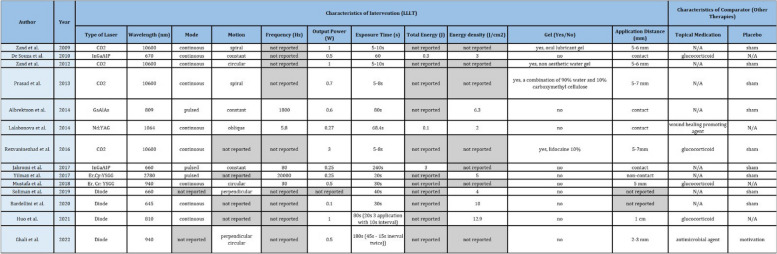
Four studies reported CO2 laser [36, 37, 39, 40], while the other four used diode laser [12, 14, 43, 44] as an intervention. Two studies reported the use of InGaAIP [34, 38], two studies used Er,Cy: YSGG [35, 42], one study used GaAIAs [41], and one study used Nd: YAG [33]. Eight studies were placebo-controlled (sham treatment) [12, 35–41, 43, 44], four were in comparison with topical medication (three with glucocorticoid and one with wound healing promoting agent) [14, 33, 34, 42], and two studies were in comparison with both topical medication and placebo (one with glucocorticoid and sham treatment [37] while the other one with antimicrobial and motivation only [12]). Summary for studies intervention and comparators is presented in Table 2.
Table 2.
Characteristics of intervention and comparators
N/A not applicable
Outcomes
Outcomes varied in terms of measurement and reporting. Pain scores were reported on the scale (VAS 10 in eight studies [14, 33, 35, 38, 39, 43, 44], VAS 100 in three studies [12, 37, 41], NRS 11 in two studies [36, 42], and Pain Intensity Score in one study [34]). Six studies presented the result as mean value with standard deviation (one study presented the description only) [14, 35, 37, 39, 43, 44], three studies presented mean value only [12, 36, 41], one study presented mean difference [42], one study presented percentage of pain-free patient [38], one study presented in percentage of pain reduction [33], and one study not providing pain intensity outcome [40].
Healing time was also reported in various parameter measurements in which three studies reported in days [14, 36, 40] and one study in healing of RAS (HRAS) score [35]. For meta-analysis to estimate the effect of LLLT on healing time, we removed one study due to the absence of mean value and standard deviation in HRAS score.
Excluded studies
Two studies were excluded for the following reasons: they were non-LLLT or presented an ablative and thermogenesis effect [45] and no control [46].
Risk of bias in included studies
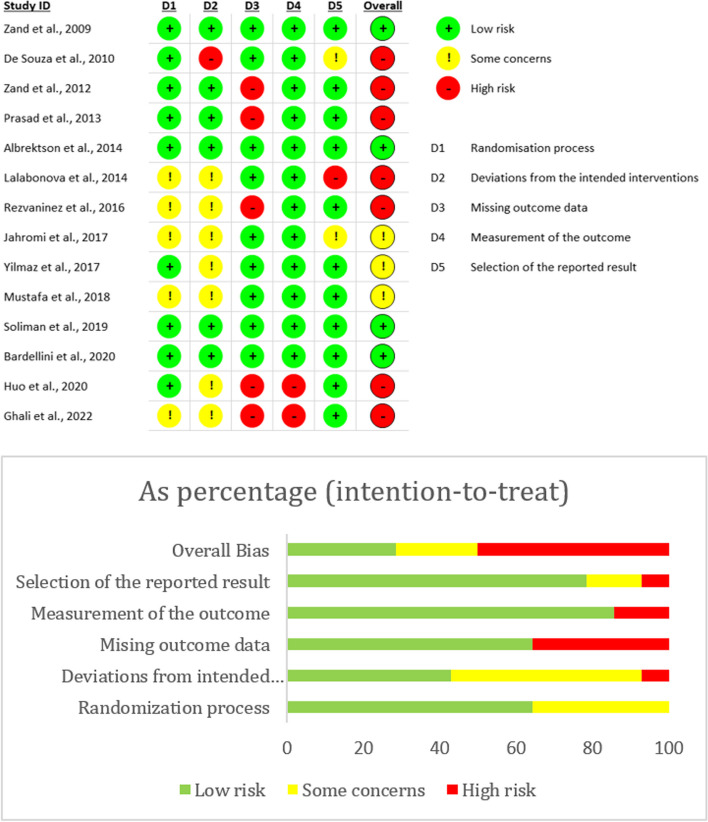
The risk of bias in all included studies was summarized in Fig. 2. Four studies scored low risk across all domains [39, 41, 43, 44], three studies scored some concerns [35, 38, 42], and seven studies scored high risk (five in one domain and two in multiple domains) [33, 34, 36, 37, 40]. However, the overall risk for all studies was considered to be in “some concerns” because susceptibility to bias resulted from no explicit report of the domain, and no differences were observed in baseline results between intervention and comparator groups [20].
Fig. 2.
Risk of bias summary of each included study and risk of bias in percentage across all included studies
Effect of intervention
Based on Table 3, it can be seen that 13 studies described the reduction in pain score [12, 14, 33–39, 41–44]. Eight studies reported a significant reduction in pain scores within the group after applying LLLT [14, 34–36, 41–44], and five studies did not report statistical analysis within the group [12, 33, 37–39]. Statistical analysis within the group presented significant pain reduction immediately after LLLT application in two studies [36, 39] and 1 day after LLLT application in four studies [14, 42–44]. Significant pain reduction both immediately and 1 day after the application of LLLT was reported in two studies [35, 41].
Table 3.
Characteristics of outcomes and “summary of findings”
N/A not applicable
Compared to topical medication, three studies reported a significant reduction in pain score [14, 37, 42], and two studies did not report statistical analysis for a significant reduction between groups [12, 33]. Compared to placebo, six studies reported significant pain reduction between laser and placebo groups [35–37, 39, 43, 44], while one study reported no significant reduction [38]. Two studies reported no statistical analysis between groups [12, 41].
Shortened healing time is reported in four studies where significant differences between the laser group and the control group were reported statistically [14, 35, 36, 40]. Three studies resulted in significant differences in healing time when compared to placebo [35, 36, 40]. One study reported a significant difference when compared to topical medication [14].
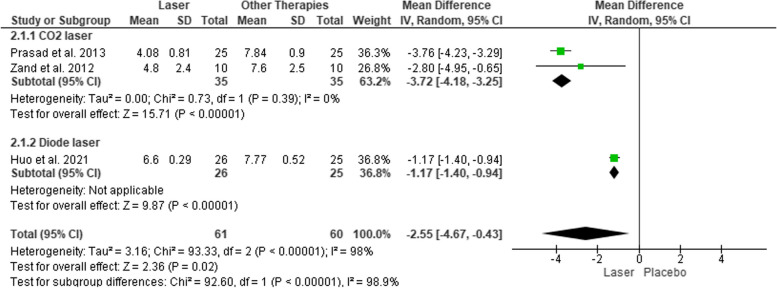
Meta-analysis was performed in three studies [35, 36, 40] with an inverse variance random effect because healing time is continuous data (changing over time), and a mean difference was obtained considering that all studies used the same parameter to measure healing time in days [20]. The overall analysis presented a reduction in healing time after the application of LLLT compared to topical medication and placebo (− 2.55 [CI 95% − 4.67, − 0.43]) with high heterogeneity (I2 = 98%). Subgroup analysis reported a reduction of healing time after application of CO2 laser compared to placebo (− 3.72 [CI 95% − 4.67, − 0.43]) with low heterogeneity (I2 = 0%). Subgroup analysis for diode laser cannot be performed due to a limited number of trials but a single MD also showed a reduction in healing time (− 1.17 [CI 95% − 1.40, − 0.94]). Estimated effect of LLLT on healing time is presented in Fig. 3.
Fig. 3.
Forest plot for estimating the effect of LLLT on healing time
The overall quality of evidence for reduced healing time was assessed using GRADE, as presented in Table 4. The evidence was initially graded as “high” since all studies were randomized controlled trials. Due to crucial limitations for one or multiple risks of bias domains that are sufficient to lower confidence in the estimate of effect in comparison to topical medication and placebo, such as not explicitly reporting randomization process, deviations from the intended interventions, measurement of the outcome and selection of the reported result, the quality was downgraded two levels to “low”. Evidence of CO2 laser application in reducing healing time was downgraded one level to “moderate” because there is a crucial limitation in not explicitly reporting any missing outcome data.
Table 4.
Summary of findings for the main comparison: healing time
| Comparisons | Number of participants (studies) | Quality of the evidence (GRADE) | Anticipated mean difference (95% CI) |
|---|---|---|---|
| Low-level laser therapy (LLLT) vs conventional therapy (topical medication and placebo) |
121 (3 studies) |
⊕⊕◯◯ Low |
− 2.55 [95% CI − 4.67, − 0.43]; I2 = 98% |
| CO2 vs placebo |
70 (2 studies) |
⊕⊕⊕◯ Moderate |
− 3.72 [95% CI − 4.18, − 3.25]; I2 = 0% |
High quality further research is very unlikely to change our confidence in the estimate of effect, Moderate quality further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate, Low quality further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate, Very low quality we are very uncertain about the estimate
Discussion
Recurrent aphthous stomatitis (RAS) is a mucosal lesion resulting from a T cell-mediated immunologic reaction that may develop from an erythematous macule to round well-defined ulcers surrounded by an erythematous border [1, 2]. The mechanism of ulcer formation in RAS includes activating T helper cells after heat shock protein presentation by antigen-presenting cells in lymph nodes. This antigen will be cross-presented to T cytotoxic cells via IL-2 production from activated T helper cells and MHC class I presentation in APC after CD40-CD40 ligand binding. T cell cytotoxic will migrate to the affected site and cause mucosal destruction via the synthesis of perforin and granzyme. Endogenous mediators released from the damaged tissues increase the extravasation of the vessels and attract the immune cells, including mast cells, macrophages, neutrophils, and platelets, to the injured site for the inflammatory response [47, 48]. Inflammatory mediators produced from this response can directly activate the nociceptors, evoking pain or modulating the sensitivity of the primary nociceptors, thus causing a hyperreactive reaction to stimuli [49]. Focusing on the pathogenesis of RAS, pain becomes the primary symptom reported in this review using VAS scores ranging from 4 to 8 [12, 14, 33–39, 41–44]. This condition usually lasts 7 to 21 days before healing spontaneously and may recur up to more than seven episodes in a year, thus affecting individual well-being [6, 7, 26]. Treatments are frequently investigated to reduce pain, decrease the size of the lesion, and prevent reoccurrence [8]. Accelerated wound healing may also target tissue repair, preventing direct contact of external stimuli to nerve endings in lamina propria due to ulcer formation [50].
This review included 14 studies investigating the effect of low-level laser therapy on RAS, mainly minor RAS. Patients with minor RAS should be reassured about disease development; medication is only prescribed when required [51]. Reassurance or motivation is typically provided in the form of accurate or potentially corrective verbal information that intends to reduce the threat value of disease, such as thoughts and beliefs about pain, that can reduce pain intensity [52]. When there is a symptom of exacerbation, topical glucocorticoids (GCs) are the first-line drug for minor RAS. They are used for local treatment through their anti-inflammatory and immunosuppressive effects [9, 53, 54]. Glucocorticoids suppress pain intensity by directly binding glucocorticoid receptor (GR) complex in the gene promoter region or by interaction with other transcription factors, particularly activating protein-1 or nuclear factor kappa-β, in the cell nucleus. GR binding to Nf-Kβ loci results in the repression of target genes, thus down-streaming inflammatory signaling [55, 56]. Antimicrobial agents are another topical medication used for the treatment of RAS that protects the ulcer from bacterial infection, yet they exhibit no anti-inflammatory or wound healing promoter effect [2]. There are also wound-healing-promoting agents that neutralize tissue damage from oxidative stress and promote cell proliferation [33]. Liu et al. (2022) recommend using a laser as a short-term alternative intervention during the exacerbation phase of RAS due to its positive effect in accelerating tissue repair and relieving pain [54].
Low-level laser therapy (LLLT) or cold laser is a term used to describe laser applied at an intensity that stimulates biological processes instead of producing ablative or thermal effects. This biological process may produce analgesic and anti-inflammatory effects for pain relief in various diseases [57]. In this review, all 13 trials reporting pain intensity of RAS described reduction after the application of LLLT [12, 14, 33–39, 41–44]. LLLT applied with sufficient intensity inhibits action potentials that cause approximately 30% neural blockade within 10 to 20 min of application and may reverse within about 24 h [58]. Neural blockage results from produced photons that will be absorbed by chromophores in the mitochondria membrane, increasing the production of adenosine triphosphate (ATP) [15, 58]. Adenosine triphosphate (ATP) is the energy source for all cells, and in neurons, this ATP is synthesized by mitochondria located in the dorsal root ganglion. These mitochondria are then transported along the cytoskeleton of the nerve by a monorail system of molecular motors. LLLT has been shown to disrupt the cytoskeleton for hours temporarily, as evidenced by the increase of ATP synthesis, which causes hyperpolarization and stimuli obstruction, thus decreasing pain stimuli induction [58, 59]. The result of this review also synthesized that there is significant difference between group when compared to placebo (sham treatment and motivation only) and topical medication such as glucocorticoid (triamcinolone acetonide 0.1% and betamethasone), antimicrobial agents (dequalinium chloride), wound healing promoting agent (deproteinized calves blood extract 5%), and placebo [12, 14, 33–39, 41–44]. Despite their beneficial effects, topical medication showed limited retention on the targeted site due to salivary flush; therefore, it may contribute to reducing its efficacy for the healing process of an ulcer [2, 10, 54]. Meanwhile, LLLT demonstrates short-term local application in contact or non-contact mode, thus unaffected by salivary wash-out and not presenting with any systemic side effect [15–17].
Four studies investigating the effectivity of LLLT also demonstrated shortened healing time compared to topical medication and placebo. A meta-analysis demonstrated healing time with a mean difference of 2.55 days faster than other therapies (topical medication and placebo) with high heterogeneity (I2 = 98%) that may result from variability in the type of laser and comparators [20]. In subgroup analysis, the estimated effect of CO2 laser on the reduction of healing time presented a result of 3.72 days faster than placebo (I2 = 0%). There is strong evidence that LLLT affects the mitochondria of the cells, resulting in the induction of transcription factors that increase the release of growth factors [15]. Low doses of LLLT have been reported to promote cell proliferation of fibroblasts [60, 61] and keratinocytes [62]. Apart from a direct influence on cell proliferation and mitochondrial activity, LLLT also promotes angiogenesis. There is an upregulation of angiogenesis markers VEGF and hypoxia-inducible factor-1α (HIF-1α) with downregulation of tissue remodeling marker matrix metalloproteinase-2 (MMP-2) [63], thus enhancing the proliferation of endothelial cells [64]. Newly formed blood vessels participate in provisional granulation tissue formation, providing nutrition and oxygen to growing tissues [65].
The effectiveness of LLLT depends on many treatment parameters, including wavelength, depth of penetration, size of dose, time of application, level of power density, pulse repetition rate, and treatment protocol [15, 58]. These different parameters all affect the delivered dosage. Dosage measures the energy entering the body and is equal to average power (watts) over treatment time (seconds). The power emitted from the laser probe is determined by the machine's output and is measured in watts. Longer treatment time is associated with a larger dosage of laser administered to the patient [58]. The type of laser included in this review comprised of solid lasers such as Nd: YAG (1064 nm), inert gas lasers such as carbon dioxide (CO2; 10,600 nm), semiconductor laser diodes such as gallium aluminum arsenide (GaAlAs; 809 nm) and InGaAIP (660–670 nm), as well as hydrokinetic laser (Er, Cr: YSGG; 940–2780) [30, 66]. The output power ranged from 0.25 to 1 W, whereas lower output power mostly presented with longer exposure time (varied from 5 to 240 s). The fluency of laser light is mostly in continuous mode at 5–7 mm distance and often pulsed when the laser is in contact with oral mucosa. No agreement has been reported on which continuous wave or pulsed light is better or which factors govern the choice of pulse parameters. Unlike continuous-wave lasers, pulsed lasers dissipate the thermal effect. The pulsed mode has a reduced time of application, but energy levels are still obtained in deeper tissue [58].
Limitations of evidence in this review include reports that resulted in “some concerns” and “high risk” of individual literature bias for not explicitly reporting the randomization process, deviations from the intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. This affects the confidence of the estimated effect of LLLT in reducing the pain score and healing time of RAS. The limited number of studies also complicates data synthesis because subgroup analysis for each type of laser therapy has yet to be conducted. All studies remained included in data synthesis, considering that the vulnerability to bias resulted from not explicitly reporting the study method instead of bias in study outcomes. Including all eligible studies despite the bias may also provide information for future studies to minimize biases when designing a trial [20, 67].
Despite of reduction in pain score and healing time of RAS after the application of LLLT, clinical applications are inconsistent possibly owing to a lack of comprehension of how dosage is affected by physical and anatomic penetration characteristics [58]. Generalizability in human study is also strenuous as it may be affected by a greater variety of situations and external environments. Future studies may be directed to determine the standardized parameter for low-level laser therapy in recurrent aphthous stomatitis and the type of laser recommended to produce optimum pain reduction and healing in the laboratory setting as it presents greater control of irrelevant variables that might otherwise influence the results of the study [68].
Supplementary Information
Supplementary Material 2. Search Strategy.
Acknowledgements
The authors sincerely thank the Department of Oral Medicine, Faculty of Dental Medicine Universitas Airlangga for the support on the writing of this review.
Authors’ contributions
SRP contributed to performing data collection, analysis, synthesis, and writing the manuscript. FYM contributed to performing data collection, analysis, synthesis, and writing the manuscript. DR contributed to performing data collection, analysis, synthesis, and writing the manuscript. RKB contributed to performing data analysis, synthesis, and writing the manuscript. AEP contributed to performing data collection, analysis, synthesis, and writing the manuscript. AS contributed to performing data analysis, synthesis, and writing the manuscript. IGASP contributed to performing data collection, analysis, and writing the manuscript.
Funding
This project was funded by Universitas Airlangga, under the scheme Airlangga Research Fund (ARF) Batch 2 Year 2023 Grant Decree Number 1532/UN3.LPPM/PT.01.02/2023.
Availability of data and materials
All data generated or analyzed during this review are included in this published article and its additional files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fatma Yasmin Mahdani, Reiska Kumala Bakti, Adiastuti Endah Parmadiati, Ajiravudh Subarnbhesaj, Selviana Rizky Pramitha, and I Gusti Agung Sri Pradnyani contributed equally to this work.
References
- 1.Sanchez-Bernal J, Conejero C, Conejero R. Recurrent aphthous stomatitis. Actas Dermosifiliogr. 2020;111(6):471–80. [DOI] [PubMed] [Google Scholar]
- 2.Chiang CP, Chang JY, Wang YP, Wu YH, Wu YC, Sun A. Recurrent aphthous stomatitis - etiology, serum autoantibodies, anemia, hematinic deficiencies, and management. J Formos Med Assoc. 2019;118:1279–89. 10.1016/j.jfma.2018.10.023 [DOI] [PubMed] [Google Scholar]
- 3.Hatta I, Firdaus IWAK, Apriasari ML. The prevalence of oral mucosa disease of Gusti Hasan Aman Dental Hospital in Banjarmasin, South Kalimantan. Dentino J Kedokt Gigi. 2018;2(2):211–4. [Google Scholar]
- 4.Hariyani N, Bramantoro T, Nair R, Singh A, Sengupta K. Depression symptoms and recurrent aphthous stomatitis—Evidence from a population-based study in Indonesia. Oral Dis. 2020;26(5):948–54. 10.1111/odi.13303 [DOI] [PubMed] [Google Scholar]
- 5.Purnama T, Sofian R, Sasongko BG, Sabilillah MF, Miko H, Heriyanto Y. Academic stress on the incidence of recurrent aphthous stomatitis: a cross sectional study. J Drug Deliv Ther. 2021;11(3):61–4. 10.22270/jddt.v11i3.4854 [DOI] [Google Scholar]
- 6.Kürklü-Gürleyen E, Öğüt-Erişen M, Çakır O, Uysal Ö, Ak G. Quality of life in patients with recurrent aphthous stomatitis treated with a mucoadhesive patch containing citrus essential oil. Patient Prefer Adherence. 2016;10:967–73. 10.2147/PPA.S106530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rivera C, Munoz-Pasten M, Nunez-Munoz E, Hernández-Olivos R. Recurrent aphthous stomatitis affects quality of life. A case-control study. Clin Cosmet Investig Dent. 2022;14(July):217–23. 10.2147/CCIDE.S369481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarakji B, Gazal G, Al-Maweri SA, Azzeghaiby SN, Alaizari N. Guidelines for diagnosis and treatment of recurrent aphthous stomatitis for dental practitioners. J Int Oral Heal. 2015;7(5):74–80. [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen S, Hiorth M. Advanced drug delivery systems for local treatment of the oral cavity. Ther Deliv. 2015;6(5):197–210. 10.4155/tde.15.5 [DOI] [PubMed] [Google Scholar]
- 10.Dalessandri D, Zotti F, Laffranchi L, Migliorati M, Isola G, Bonetti S, et al. Treatment of recurrent aphthous stomatitis (RAS; Aphthae; canker sores) with a barrier forming mouth rinse or topical gel formulation containing hyaluronic acid: a retrospective clinical study. BMC Oral Health. 2019;19(1):1–10. 10.1186/s12903-019-0850-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suharyani I, Mohammed AFA, Muchtaridi M, Wathoni N, Abdassah M. Evolution of drug delivery systems for recurrent aphthous stomatitis. Drug Des Devel Ther. 2021;15:4071–89. 10.2147/DDDT.S328371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghali HGH, Abdulhamed BS. Treatment of recurrent minor aphthous stomatitis using diode laser (940 nm). J Popul Ther Clin Pharmacol. 2022;28(2):e99–112. [DOI] [PubMed] [Google Scholar]
- 13.Ślebioda Z, Dorocka-Bobkowska B. Systemic and environmental risk factors for recurrent aphthous stomatitis in a Polish cohort of patients. Postep Dermatol Alergol. 2019;36(2):196–2011. 10.5114/ada.2018.74638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huo X, Han N, Liu L. Effect of different treatments on recurrent aphthous stomatitis: laser versus medication. Lasers Med Sci. 2021;36(5):1095–100. 10.1007/s10103-020-03166-0 [DOI] [PubMed] [Google Scholar]
- 15.Cotler HB, Chow RT, Hamblin MR, Carroll J. The use of low level laser therapy (LLLT) for musculoskeletal pain. MOJ Orthop Rheumatol. 2015;2(5):1–17. 10.15406/mojor.2015.02.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan MAN, Alam N. Low level laser therapy for non-invasive dental applications: a review. Int J Eng Res Technol. 2020;9(07):1556–62. [Google Scholar]
- 17.Nascimento UDA, Berria K, Grando LJ, Freygang S, Pilati M. Evaluation of analgesic effect of laser therapy in patients with oral ulcers with inflammatory origin and knowledge of patients about their lesions. 2022;7(1):1–4.
- 18.Karada E, Studies E. Leadership and organizational outcomes. 2015. [Google Scholar]
- 19.Najeeb S, Khurshid Z, Zohaib S, Najeeb B, Qasim SB, Zafar MS. Management of recurrent aphthous ulcers using low-level lasers: a systematic review. Med. 2016;52(5):263–8. 10.1016/j.medici.2016.07.006. 10.1016/j.medici.2016.07.006 [DOI] [PubMed] [Google Scholar]
- 20.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. Cochrane Handbook for Systematic Reviews of Interventions. 2019. p. 1–694. [DOI] [PMC free article] [PubMed]
- 21.Ahmed MK, Jafer M, Nayeem M, Moafa IH, Quadri MFA, Gopalaiah H, Ali Quadri MF. Low-level laser therapy and topical medications for treating aphthous ulcers: a systematic review. J Multidiscip Healthc. 2020;13:1595–605. 10.2147/JMDH.S281495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vale FA, Moreira MS, De Almeida FCS, Ramalho KM. Low-level laser therapy in the treatment of recurrent aphthous ulcers: a systematic review. Sci World J. 2015;2015:150412. 10.1155/2015/150412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, Hartmann-Boyce J, Ryan R, Shepperd S, Thomas J, Welch V, Thomson H. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. 2020;368:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:2020–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The PLoS Medicine Editors. Best practice in systematic reviews: The importance of protocols and registration. PLoS Med. 2011;8(2):1–2. 10.1371/journal.pmed.1001009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Challacombe SJ, Alsahaf S, Tappuni A. Recurrent aphthous stomatitis: towards evidence-based treatment? Curr Oral Heal Reports. 2015;2(3):158–67. 10.1007/s40496-015-0054-y [DOI] [Google Scholar]
- 27.Glick M, Greenberg MS, Lockhart PB, CSJ. Burket’s Oral Medicine. 13th ed. India: Wiley Blackwell; 2021. p. 37–72. https://www.researchgate.net/publication/269107473_What_is_governance/link/548173090cf22525dcb61443/download%0Ahttp://www.econ.upf.edu/~reynal/Civilwars_12December2010.pdf%0Ahttps://think-asia.org/handle/11540/8282%0Ahttps://www.jstor.org/stable/41857625. [Google Scholar]
- 28.Preeti L, Magesh KT, Rajkumar K, Karthik R. Recurrent aphthous stomatitis. J Oral Maxillofac Pathol. 2011;15(3):252–6. 10.4103/0973-029X.86669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neville B. W., Damm D. D., Allen C. M. CAC. Oral and maxillofacial pathology. 4th ed. Canada: Elsevier Inc.; 2016. p. 305–6. [Google Scholar]
- 30.Vuievi V, Vidovi D, Andabak A, Gabri D, Verzak eljko, Brailo V. Applications of low level laser therapy. A Textb Adv Oral Maxillofac Surg. 2013;
- 31.Kohale BR, Agrawal AA, Raut CP. Effect of low-level laser therapy on wound healing and patients’ response after scalpel gingivectomy: a randomized clinical split-mouth study. J Indian Soc Periodontol. 2018;22:419–26. 10.4103/jisp.jisp_239_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Handbook GH, October U, Sch H, Bro J, Guyatt G, Oxman A. GRADE Handbook. 2017;(October 2013).
- 33.Lalabonova H, Daskalov H. Clinical assessment of the therapeutic effect of low-level laser therapy on chronic recurrent aphthous stomatitis. Biotechnol Biotechnol Equip. 2014;28(5):929–33. 10.1080/13102818.2014.966526. 10.1080/13102818.2014.966526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Souza TOF, Martins MAT, Bussadori SK, Fernandes KPS, Tanji EY, Mesquita-Ferrari RA, et al. Clinical evaluation of low-level laser treatment for recurring aphthous stomatitis. Photomed Laser Surg. 2010;28(SUPPL. 2):10–3. [DOI] [PubMed] [Google Scholar]
- 35.Yilmaz HG, Albaba MR, Caygur A, Cengiz E, Boke-Karacaoglu F, Tumer H. Treatment of recurrent aphthous stomatitis with Er, Cr:YSGG laser irradiation: a randomized controlled split mouth clinical study. J Photochem Photobiol B Biol. 2017;170:1–5. 10.1016/j.jphotobiol.2017.03.011. 10.1016/j.jphotobiol.2017.03.011 [DOI] [PubMed] [Google Scholar]
- 36.Prasad RS, Pai A. Assessment of immediate pain relief with laser treatment in recurrent aphthous stomatitis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(2):189–93. 10.1016/j.oooo.2013.02.011. 10.1016/j.oooo.2013.02.011 [DOI] [PubMed] [Google Scholar]
- 37.Rezvaninezhad RS, Navabi N, Atai Z, Shahravan A. The effect Co2 laser on reducing pain associated with aphthous stomatitis. J Babol Univ Med Sci. 2016;18(10):20–5. [Google Scholar]
- 38.Jahromi NZ, Ghapanchi J, Pourshahidi S, Zahed M, Ebrahimi H. Clinical evaluation of high and low-level laser treatment (Co2vsInGaAlP Diode Laser) for recurrent aphthous stomatitis. J Dent Shiraz Univ Med Sci. 2017;18(1):17–23. [PMC free article] [PubMed] [Google Scholar]
- 39.Zand N, Ataie-Fashtami L, Djavid GE, Fateh M, Alinaghizadeh MR, Fatemi SM, et al. Relieving pain in minor aphthous stomatitis by a single session of non-thermal carbon dioxide laser irradiation. Lasers Med Sci. 2009;24(4):515–20. 10.1007/s10103-008-0555-1 [DOI] [PubMed] [Google Scholar]
- 40.Zand N, Fateh M, Ataie-Fashtami L, Djavid GE, Fatemi SM, Shirkavand A. Promoting wound healing in minor recurrent aphthous stomatitis by non-thermal, non-ablative CO2 laser therapy: a pilot study. Photomed Laser Surg. 2012;30(12):719–23. 10.1089/pho.2012.3301 [DOI] [PubMed] [Google Scholar]
- 41.Albrektson M, Hedström L, Bergh H. Recurrent aphthous stomatitis and pain management with low-level laser therapy: a randomized controlled trial. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117(5):590–4. 10.1016/j.oooo.2014.01.228. 10.1016/j.oooo.2014.01.228 [DOI] [PubMed] [Google Scholar]
- 42.Mustafa NS, Kashmoola MA, ZulhelmiBaharudin M, Hashim HI, Jabbar OA, Alahmad BEM. A pilot study on the use of biolase in the treatment of recurrent aphthous ulcer. Brazilian J Oral Sci. 2018;17:1–10. [Google Scholar]
- 43.Soliman HA, Mostafaa D. Clinical evaluation of 660 nm diode laser therapy on the pain, size and functional disorders of recurrent aphthous stomatitis. Open Access Maced J Med Sci. 2019;7(9):1516–22. 10.3889/oamjms.2019.268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bardellini E, Veneri F, Amadori F, Conti G, Majorana A. Photobiomodulation therapy for the management of recurrent aphthous stomatitis in children: clinical effectiveness and parental satisfaction. Med Oral Patol Oral y Cir Bucal. 2020;25(4):e549–53. 10.4317/medoral.23573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tezel A, Kara C, Balkaya V, Orbak R. An evaluation of different treatments for recurrent aphthous stomatitis and patient perceptions: Nd:YAG laser versus medication. Photomed Laser Surg. 2009;27(1):101–6. 10.1089/pho.2008.2274 [DOI] [PubMed] [Google Scholar]
- 46.Rocca JP, Zhao M, Fornaini C, Tan L, Zhao Z, Merigo E. Effect of laser irradiation on aphthae pain management: A four different wavelengths comparison. J Photochem Photobiol B Biol. 2018;189:1–4. 10.1016/j.jphotobiol.2018.09.016. 10.1016/j.jphotobiol.2018.09.016 [DOI] [PubMed] [Google Scholar]
- 47.Campos TM, Costa R, Passos S, Carvalho LP. Cytotoxic activity in cutaneous leishmaniasis. Mem Inst Oswaldo Cruz. 2017;112(11):733–40. 10.1590/0074-02760170109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abbas AK, Lichtman AH, Pillai S. Basic immunology : fungtion and disorders of the immune system. 2007. [Google Scholar]
- 49.Su Y., Sun W. CC. Molecular mechanism of inflammatory pain. World J Anesthesiol. 2014;3(1):71. 10.5313/wja.v3.i1.71 [DOI] [Google Scholar]
- 50.Anand V, Gulati M, Govila V, Anand B. Low level laser therapy in the treatment of aphthous ulcer. Indian J Dent Res. 2013;24(2):267–70. 10.4103/0970-9290.116691 [DOI] [PubMed] [Google Scholar]
- 51.Farah CS, Balasubramaniam R, McCullough MJ. Contemporary oral medicine: a comprehensive approach to clinical practice. contemporary oral medicine. Australia: Springer Nature Switzerland AG; 2019. [Google Scholar]
- 52.Traeger AC, O’Hagan ET, Cashin A, McAuley JH. Reassurance for patients with non-specific conditions - a user’s guide. Braz J Phys Ther. 2017;21(1):1–6. 10.1016/j.bjpt.2016.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akintoye SO, Greenberg MS. Recurrent aphthous stomatitis. Dent Clin North Am. 2014;58(2):1–25. 10.1016/j.cden.2013.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu H, Tan L, Fu G, Chen L, Tan H. Efficacy of topical intervention for recurrent aphthous stomatitis: a network meta-analysis. Medicina (B Aires). 2022;58(6):771. 10.3390/medicina58060771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carolina E, Kato T, Khanh VC, Moriguchi K, Yamashita T, Takeuchi K, et al. Glucocorticoid impaired the wound healing ability of endothelial progenitor cells by reducing the expression of CXCR4 in the PGE2 pathway. Front Med. 2019;5(SEP):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bekhbat M, Rowson SA, Neigh GN. Check and balances: the glucocorticoid receptor and NFkB in good times and bad. Front Neuroendocr. 2017;46:15–31. 10.1016/j.yfrne.2017.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Farivar S, Malekshahabi T, Shiari R. Biological effects of low level laser therapy. J Lasers Med Sci. 2014;5(2):58–62. [PMC free article] [PubMed] [Google Scholar]
- 58.Taylor DN, Wienfield T, Wynd S. Low-level laser light therapy dosage variables vs treatment efficacy of neuromusculoskeletal conditions: a scoping review. J Chiropr Med. 2020;19:119–27. 10.1016/j.jcm.2020.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Akerzoul N, Chbicheb S. Low laser therapy as an effective treatment of recurrent aphtous ulcers: A clinical case reporting two locations. Pan Afr Med J. 2018;30:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shingyochi Y, Kanazawa S, Tajima S, Tanaka R, Mizuno H, Tobita M. A low-level carbon dioxide laser promotes fibroblast proliferation and migration through activation of Akt, ERK, and JNK. PLoS ONE. 2017;12(1):1–13. 10.1371/journal.pone.0168937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Frigo L, Fávero GM, Lima HJ, Maria DA, Bjordal JM, Joensen J, Iversen VV, Marcos RL, Parizzoto NA, Lopes-Martins RA. Low-level laser irradiation (InGaAlP-660 nm) increases fibroblast cell proliferation and reduces cell death. Photomed Laser Surg. 2010;28(1):1–6. [DOI] [PubMed] [Google Scholar]
- 62.Basso FG, Oliveira CF, Kurachi C, Hebling J, Costa CADS. Biostimulatory effect of low-level laser therapy on keratinocytes in vitro. Lasers Med Sci. 2013;28(2):367–74. 10.1007/s10103-012-1057-8 [DOI] [PubMed] [Google Scholar]
- 63.Tam SY, Tam VCW, Ramkumar S, Khaw ML, Law HKW, Lee SWY. Review on the cellular mechanisms of low-level laser therapy use in oncology. Front Oncol. 2020;10(July):1–10. [DOI] [PMC free article] [PubMed]
- 64.Szymanska J, Goralczyk K, Klawe JJ, Lukowicz M, Michalska M, Goralczyk B, Zalewski P, Newton JL, Gryko L, Zajac A, Rosc D. Phototherapy with low-level laser influences the proliferation of endothelial cells and vascular endothelial growth factor and transforming growth factor-beta secretion. J Physiol Pharmacol. 2013;64(3):387–91. [PubMed] [Google Scholar]
- 65.Kumar P, Kumar S, Udupa EP, Kumar U, Rao P, Honnegowda T. Role of angiogenesis and angiogenic factors in acute and chronic wound healing. Plast Aesthetic Res. 2015;2(5):243. 10.4103/2347-9264.165438 [DOI] [Google Scholar]
- 66.Kamani E, Patel DN, Kamani Z, KA. Medical lasers. J Ophthalmol Clin Res. 2019;3(2):1–3. [Google Scholar]
- 67.Harvey LA, DMP. Should trials that are highly vulnerable to bias be excluded from systematic reviews? Spinal Cord. 2019;57:715–6. 10.1038/s41393-019-0340-y [DOI] [PubMed] [Google Scholar]
- 68.Aziz HA. Comparison between field research and controlled laboratory research. Arch Clin Biomed Res. 2017;01(02):101–4. 10.26502/acbr.50170011 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 2. Search Strategy.
Data Availability Statement
All data generated or analyzed during this review are included in this published article and its additional files.