Abstract
Worldwide, human immunodeficiency virus (HIV) is transmitted predominantly by heterosexual contact. Here, we investigate for the first time, by examining mononuclear cells obtained from cervicovaginal tissue, the mechanisms whereby HIV type 1 (HIV-1) directly targets cells from the human genital tract. In contrast to earlier findings in mucosal models such as human skin, we demonstrate that the majority of T cells and macrophages but none or few dendritic cells (DC) express the HIV-1 coreceptor CCR5 in normal human cervicovaginal mucosa, whereas all three cell types express the coreceptor CXCR4. To understand the role of coreceptor expression on infectivity, mucosal mononuclear cells were infected with various HIV-1 isolates, using either CCR5 or CXCR4. Unstimulated T cells become rapidly, albeit nonproductively, infected with R5- and X4-tropic variants. However, DC and T cells form stable conjugates which permit productive infection by viruses of both coreceptor specificities. These results indicate that HIV-1 can exploit T-cell–DC synergism in the human genital tract to overcome potential coreceptor restrictions on DC and postentry blocks of viral replication in unactivated T cells. Thus, mononuclear cells infiltrating the genital mucosa are permissive for transmission of both R5- and X4-tropic HIV-1 variants, and selection of virus variants does not occur by differential expression of HIV-1 coreceptors on genital mononuclear cells.
As human immunodeficiency virus type 1 (HIV-1) infection continues to spread globally and predominantly by heterosexual contact, strategies to control the epidemic rely upon understanding the cellular and molecular events that favor infection after sexual exposure. Thus far, the inability to examine the relevant mucosal tissue in sufficient quantity and viability has impeded progress. Two key elements yet to be elucidated are the mechanisms whereby some HIV-1 strains are selected in preference to others and the identification of the cell types in the human genital tract that are the earliest targets for infection.
Virus variants isolated shortly after sexual transmission of HIV-1 are preferentially macrophage-tropic (M-tropic) rather than T-cell line-tropic (T-tropic) and exhibit the non-syncytium-inducing rather than the syncytium-inducing phenotype (27, 33, 42). The mechanism for this selection is unknown, but many believe it can be explained by the selective expression of CCR5, the coreceptor used by M-tropic strains (1, 6, 10–12, 15), rather than other coreceptors on target cells in the genital mucosa (19, 38, 41). The cells first attacked by HIV-1 after exposure to mucous membranes are not known, although once infection is established both macrophages and lymphocytes appear susceptible (21, 22). Moreover, several models implicate conjugates of dendritic cells and T cells as efficient propagators of HIV-1 in the mucosa (3, 16, 23, 24, 30), although this has not been documented in the human genital mucosa.
To clarify the impact of target cell type and its coreceptor repertoire on sexual transmission and variant selection of HIV-1, we developed a procedure to isolate pure viable populations of T cells, dendritic cells (DC), and macrophages from the female lower genital tract. We analyzed the fresh mucosal mononuclear cells for HIV-1 coreceptor expression and the infectivity of M-tropic and T-tropic mucosal and laboratory-adapted HIV-1 strains. Our findings provide new insights into mechanisms of HIV-1 transmission not gleaned from mucosal models of HIV or SIV infection and analysis of tissue sections and indicate that differential expression of HIV-1 coreceptors on genital mononuclear cells cannot account for selection of R5-tropic viruses.
MATERIALS AND METHODS
Isolation of mucosal and blood mononuclear cells.
Blood and tissue blocks containing portions of ectocervix or vagina were obtained from women undergoing hysterectomy or vaginal repair operation at the University of Washington Medical Center and affiliated hospitals. Subjects were either at low risk for HIV-1 infection or tested HIV-1 negative and had no cervicovaginal inflammation when examined preoperatively. The study was approved by the University of Washington Human Subjects Committee, and volunteers provided written consent prior to the procedure.
The tissue was processed within 2 h, washed extensively, and incubated 10 min at 37°C in culture medium (RPMI 1640 supplemented with 100 U of penicillin per ml, 100 μg of streptomycin per ml, 2.5 μg of amphotericin B per ml, 2 mM l-glutamine [BioWhittaker, Walkersville, Md.], and 10% fetal bovine serum [Gemini, Calibasas, Calif.] containing 1 μM dithiothreitol (Sigma, St. Louis, Mo.) to dissolve mucus and cleave off loose epithelial cells. The mucosa was placed in sterile water for 30 s to lyse contaminating blood cells, extensively washed, and cut into small pieces. Tissue pieces were transferred to T75 flasks and washed to remove remaining contaminating blood cells, and they were incubated for 36 h at 37°C and 5% CO2. Collagenase D (2 mg/ml; Boehringer Mannheim, Indianapolis, Ind.) was gently added for 10 min at 37°C to reduce trapping of emigrated cells within collagenous debris. The emigrant cells were carefully harvested, placed over Ficoll-Hypaque (Pharmacia, Uppsala, Sweden), and centrifuged for 20 min at 895 × g. The cells collected at the interface were washed three times and counted by trypan blue exclusion. Lymphocyte yields were 0.3 × 106 to 10 × 106 with >95% viability and <5% contamination with epithelial cells. Peripheral blood mononuclear cells (PBMC) from the same donors were isolated by Ficoll-Hypaque density centrifugation and washed and counted as above. Peripheral blood DC were obtained from unrelated donors by differentiation of precursor cells with granulocyte-macrophage colony-stimulating factor GM-CSF and interleukin-4 (Genzyme Transgenics, Cambridge, Mass.) (26).
Flow cytometry analysis and cell sorting.
To define phenotypic markers of mucosal mononuclear cells (MMC), cells were reacted with combinations of the following fluorescent-conjugated monoclonal antibodies (MAbs): anti-CD1a fluorescein isothiocyanate (FITC) (Ortho, Raritan, N.J.); anti-CD3 phycoerythrin (PE)-Cy5, anti-CD4 FITC, and anti-CD8 PE-Cy5 (Sigma); anti-CD3 FITC, anti-CD45RO FITC, anti-CD45RA PE, and anti-CD103 (αEβ7 integrin) (Dako, Carpinteria, Calif.); anti-CD14 FITC, anti-HLA-DQ FITC, anti-TCRαβ FITC, and anti-TCRγδ PE (Becton Dickinson, Bedford, Mass.); anti-CD14 PE, anti-CD14 PE-Cy5, and anti-CD83 PE (Coulter, Miami, Fla.); anti-CXCR4 FITC (Research & Diagnostics, Minneapolis, Minn.); and anti-CXCR4 PE (Pharmingen, San Diego, Calif.). For CCR5 detection, anti-CCR5 MAb 2D7 (National Institutes of Health AIDS Research and Reference Reagent Program) recognizing the second extracellular domain and anti-CCR5 MAb 227R (ICOS Corp., Seattle, Wash.) recognizing the N-terminal portion was used. Unconjugated antibodies were subsequently reacted with FITC- or PE-labeled goat anti-mouse immunoglobulin G (Fc) F(ab′)2 (Coulter). Cells were analyzed on a Calibur flow cytometer (Becton Dickinson). Cells were reacted with anti-HLA-DQ FITC, anti-CD14 PE, and anti-CD3 PE-Cy5 and sorted on a Vantage flow cytometer (Becton Dickinson).
Chemokine receptor mRNA expression.
Total RNA was extracted from sorted MMC by using tRNA (0.1 mg/sample) (Sigma) as a carrier, and cDNA was synthesized by using random primers (Pharmacia) and Moloney murine leukemia virus M-MLV reverse transcriptase (Gibco-BRL, Gaithersburg, Md.). PCR was performed with primers for 35 cycles as previously described (17). Similarly, sequences encoding hypoxanthine phosphoribosyl transferase (HPRT) from the same sorted MMC were amplified as a control gene with specific primers (Clontech). PCR products were electrophoresed on a 2.5% agarose gel and visualized by ethidium bromide staining. Copy numbers of CCR5 transcripts in MMC were estimated by comparison with serial dilutions of CCR5-transduced Jurkat cells expressing one to two copies of CCR5 mRNA per cell (13).
HIV-1 isolates.
Two primary mucosal HIV-1 isolates (HIV-1M1 and HIV-1M2) were derived from cervical mononuclear cells of two HIV-1-infected women. Both isolates were R5-tropic as evidenced by infection of MAGI indicator cell lines (34). M-tropic HIV-1Ba-L and T-tropic HIV-1SF-2 and HIV-1LAI were obtained from the National Institutes of Health AIDS Research and Reference Reagent Program.
HIV-1 infection of cell suspensions.
Cells purified by cell sorting (for virus detection in culture supernatants and for nested-PCR experiments) or bulk MMC (for electron microscopy) were cultured in 96-well plates at 37°C and 5% CO2. Viruses (treated with 30 U of RNase-free DNase I per ml [Boehringer Mannheim] for 15 min at 37°C in PCR experiments) were added to cell cultures at a multiplicity of infection (MOI) of 0.5 for quantitative PCR and electron microscopy and an MOI of 0.1 for all other experiments. For PCR, cells were harvested after 24 h (nested PCR) or 48 h (quantitative PCR), 3 × 105 Jurkat cells were added as fillers, and cells were washed three times before DNA extraction. To test for residual HIV-1 DNA after DNase treatment, treated virus supernatants were incubated in parallel with the infected cell cultures and then mixed with 3 × 105 Jurkat cells at MOIs matching the inocula used for mucosal cells, washed three times immediately, and also prepared for PCR. Copy numbers at time zero were subtracted from copy numbers after 48 h of culture, and these were always less than 15% of the measurement obtained at 48 h. The supernatants were aspirated after 24 h, fresh medium was added, and cells were cultured for up to 14 days. For electron microscopy, cells were harvested after 5 days, washed twice, and fixed.
Measurements of proviral copy numbers.
Proviral DNA copy numbers were measured by using quantitative-competitive PCR enzyme immunoassay (7). An internal quantitation standard was constructed containing a chimeric 251-bp HIV-1 gag gene fragment (positions 1291 to 1542) in which positions 1403 to 1435 corresponding to the SK102 gag probe region were replaced by an equal length sequence from the Drosophila white locus (37). Biotin-labeled primers SK462 and SK431 (20) were used, and PCR conditions were 10 min at 25°C, 5 cycles of 95°C for 10 s, 55°C for 10 s, and 72°C for 10 s; 35 cycles of 90°C for 10 s, 60°C for 10 s, and 72°C for 10 s; and then 5 min at 72°C and 2.5 min at 95°C. Amplified products were hybridized at 42°C for 1 h with either the gag-specific GAGP1 probe (5′-GAGGAAGCTGCAGAATGGGA-3′ digoxigenin) or the Drosophila insert-specific Fly-C probe (5′-GTCCTACCAACTACAATCCGG-3′ digoxigenin) (Genosys Biotechnologies, The Woodlands, Tex.). Bound probe was detected by anti-digoxigenin F(ab′)2 conjugated to alkaline phosphatase (Boehringer Mannheim) with p-nitrophenyl phosphate (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) as a substrate. The absorbance was read at 405 nm, and copy numbers per amount input DNA were determined by linear regression from a plot of the log of the absorbance of HIV-1 gag divided by the IQS versus the log of the input IQS copy number and adjusted for copies per 104 cells by assuming a maximum DNA yield of 2 μg per isolation (104 mucosal cells plus 3 × 105 filler Jurkat cells).
Reverse transcriptase assay and p24 ELISA.
Reverse transcriptase activity and p24 antigen production were monitored in culture supernatants at various time points after exposure as described previously (35) and with a commercially available p24 enzyme-linked immunosorbent assay (ELISA; Abbott Laboratories, Abbott Park, Ill.).
Electron microscopy.
HIV-1Ba-L-infected and uninfected MMC were fixed in half-strength Karnovsky’s fixative and then stained, embedded, cut by using standard electron microscopy techniques, and viewed and photographed on a JEOL 100 SX transmission electron microscope.
Nested LTR-gag PCR.
A previously published nested-PCR method was used to detect late RT intermediates (5), primer pairs long terminal repeat 1 (LTR1)-gag2 (round 1) or LTR2-gag4 (round 2). PCR conditions for both amplifications were 35 cycles of 95°C for 1 min, 60°C for 1 min, and 72°C for 2 min, followed by a 2-min extension. HLA-DQ sequences were amplified with the primers GH26 and GH27.
RESULTS
Phenotype of cervicovaginal mononuclear cells.
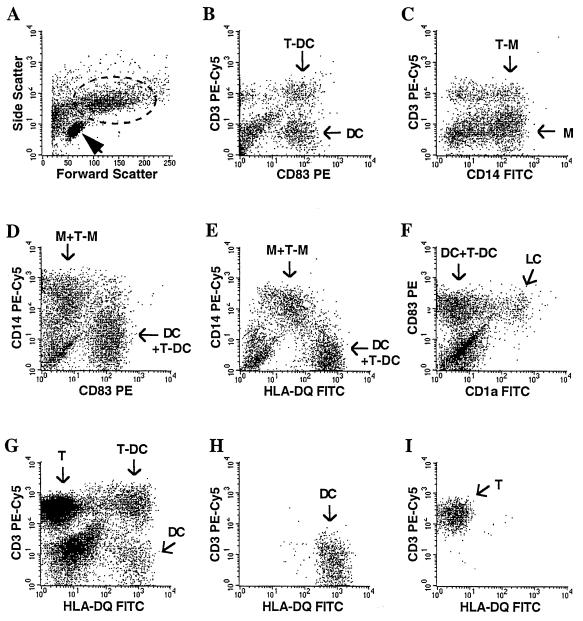
Adapting a method used in skin organ cultures (23), we established an isolation procedure whereby human vaginal or cervical mononuclear cells selectively emigrate from mucosal explants over a 36-h period. Three subpopulations were distinguished by light microscopy: lymphocytes, larger cells often with dendritic morphology, and conjugates of usually one to four lymphocytes surrounding one larger cell. Five major cell populations were identified by flow cytometry by using phenotypic markers (Fig. 1): small cells with low granularity (Fig. 1A) that were >90% CD3+ T lymphocytes (not shown), large cells with higher granularity (Fig. 1A) containing CD83+ DC (Fig. 1B), clusters of CD83+ DC with CD3+ T cells (Fig. 1B), CD14+ macrophages (Fig. 1C), and clusters of CD14+ macrophages with CD3+ T cells (Fig. 1C). Populations expressing CD14+ and CD83+ cells were largely mutually exclusive (Fig. 1D). Moreover, macrophages expressed intermediate levels of HLA-DQ with CD14 (Fig. 1E), whereas DC, and virtually all CD83+ cells, demonstrated high levels of HLA-DQ in the absence of CD14 (Fig. 1E and data not shown). The CD83+ DC contained a subpopulation coexpressing CD1a+ cells, a characteristic typical of Langerhans cells (Fig. 1F). The majority of CD3+ T cells were T-cell receptor α+β+ and CD45R0+, and more than 50% also expressed CD103, the αEβ7 integrin characteristic of mucosal intraepithelial lymphocytes (not shown). In 10 samples the mean CD4+/CD8+ ratio was 0.76 (range, 0.2 to 2.1). Based upon phenotypic markers, MMC could be sorted with >98% purity into the following distinct single cell populations: HLA-DQhigh CD14− CD3− DC (Fig. 1G and H), CD3+ HLA-DQ− CD14− T cells (Fig. 1G and I), and CD14+ CD3− macrophages (not shown).
FIG. 1.
Phenotype and sorting by flow cytometry of MMC. (A) Typical scatterplot distinguishing small cells with low granularity (>95% lymphocytes) (arrowhead) and large cells with high granularity (dotted circle). The large cells identified in panel A (dotted circle) were gated and contained four major subpopulations, as shown in panels B to F. (B) Staining with anti-CD3 and anti-CD83 MAbs distinguish CD83+ DC (DC) and CD3+ CD83+ T-cell–DC clusters (T-DC). The CD3+ CD83− cells within the gated large MMC are mostly clusters of CD3+ T cells with CD83− macrophages, and the CD3− CD83− population includes residual epithelial cells. (C) Staining with anti-CD3 and anti-CD14 MAbs identifies CD14+ macrophages (M) and CD3+ CD14+ T-cell–macrophage clusters (T-M). CD3+ CD14− cells within the gated large MMC are mostly clusters of CD3+ T cells with CD14− DC. (D) Two distinct cell populations, CD83+ DC and CD14+ macrophages, are shown. Positive populations in panels D to F contain single cells and clusters (DC+T-DC and M+T-M). (E) Staining with anti-CD14 and anti-HLA-DQ MAbs distinguishes between CD14+ HLA-DQlow macrophages and CD14− HLA-DQhigh DC. (F) CD83+ DC contain a subpopulation of CD1a+ Langerhans cells (LC). (G to I) To sort for individual MMC populations (with a different donor from that in panels A to F), all live cells were gated (G) and isolated with >98% purity into HLA-DQhigh CD3− CD14− DC (H), CD3+ HLA-DQ− CD14− T cells (I) and CD14+ CD3− macrophages (CD14 versus HLA-DQ or CD3 not shown).
HIV-1 coreceptor expression.
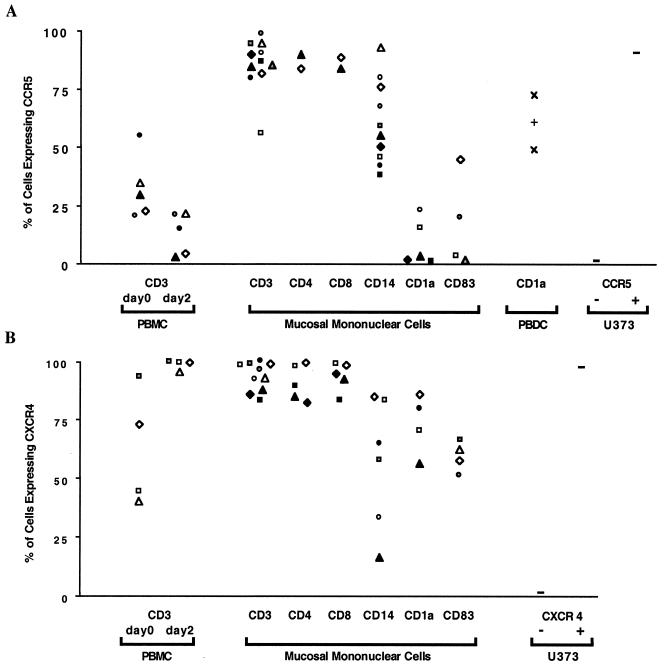
To determine whether MMC that are potentially susceptible to HIV-1 infection express the predominant HIV-1 coreceptors, we analyzed by flow cytometry the surface expression of CCR5 (Fig. 2A) and CXCR4 (Fig. 2B) among individual MMC subpopulations. CCR5 was demonstrated in more than 80% of CD3+ T cells in 10 of 11 donors, and in the majority (median, 55%) of the CD14+ macrophages in all 10 donors examined (Fig. 2A). However, CCR5 expression was either absent or expressed in only a minority (<23%) of DC (defined as either CD1a+ or CD83+ cells) in seven of eight donors examined (Fig. 2A). Although we cannot exclude the possibility of in vitro downregulation during the 36-h emigration period, we believe this is unlikely because (i) expression was not downregulated in differentiated DC from peripheral blood in three donors (Fig. 2A), (ii) comparable studies in other mucosal mononuclear cells yielded high CCR5 levels (Fig. 2A), (iii) no significant changes in the percentage of skin Langerhans cells expressing CCR5 were observed after in vitro culture in a recent report (41), and (iv) cervicovaginal DC in situ did not express CCR5 as determined by immunohistologic evaluation.
FIG. 2.
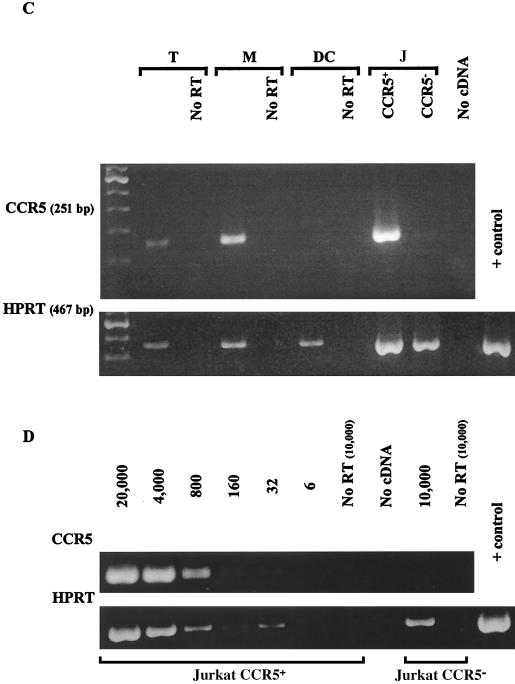
Expression of HIV-1 coreceptors CCR5 and CXCR4 by individual MMC subpopulations. (A and B) Surface expression was detected by flow cytometry after simultaneous labeling of MMC with MAbs conjugated to three distinct fluorochromes recognizing CD3; either CCR5 or CXCR4; and either CD14, CD83, or CD1a. Clusters of T cells with DC or macrophages were excluded from the analysis. The percentages of mononuclear cells are shown which express CCR5 (A) and CXCR4 (B). Cells examined include MMC and peripheral blood CD3+ T cells from all donors studied (each symbol represents one donor), in comparison to controls, peripheral blood dendritic cells in three donors (PBDC) (A), and native and CCR5-transduced (A) or CXCR4-transduced (B) U373 cells. (C) Detection of CCR5 mRNA by RT-PCR in MMC from one donor sorted into >98% pure T cells (T), macrophages (M), and DC as described in Fig. 1G to I. Equal amounts of cDNA corresponding to 104 cells in each subpopulation were amplified for CCR5, and the 251-bp reaction products are demonstrated after ethidium bromide staining. cDNAs corresponding to 2 × 104 CCR5-transduced (J CCR5+) and nontransduced (J CCR5−) Jurkat cells were included as positive and negative controls, respectively. cDNAs corresponding to 4 × 103, 6 × 103, 2.5 × 103, and 2 × 104 (T, M, DC, and J, respectively) were also amplified by PCR for the HPRT gene as a control to demonstrate the presence of cDNA in all samples. Control reactions in which RNA was not reverse transcribed (No RT) and in which no cDNA was added are shown. (D) Serial dilutions of CCR5-transduced Jurkat cells containing 1-2 CCR5 copies per cell were individually subjected to RT-PCR for CCR5 and HPRT to demonstrate the sensitivity of detecting these mRNAs by the PCR assay. The approximate copy number is indicated above the lanes, and control reactions omitting RT (No RT) and cDNA are included.
With the same purified mucosal cell populations, more than 85% of the mucosal CD3+ T cells exhibited strong CXCR4 staining (Fig. 2B). These findings were evident in both CD4+ and CD8+ T cells in the five donors examined. The majority (median, 61%) of mucosal CD14+ macrophages expressed CXCR4 (Fig. 2B), although in two donors the percentage of CXCR4+ cells was lower (16 and 34%). In contrast to the low level of CCR5 expression, CXCR4 was demonstrated in most of the mucosal DC (median, 65%; range, 51 to 80%) in the seven donors examined (Fig. 2B).
High levels of CCR5 expression in the fresh mucosal T cells were unexpected, particularly since lower levels were previously reported in unstimulated peripheral blood T cells. To address this further, we compared receptor expression on peripheral blood T cells immediately after isolation and after 36 h in culture (comparable to the time period necessary for the MMC to emigrate from the organ cultures) in five of the mucosal donors. CCR5 was expressed on 30% (median; range, 21 to 55%) of peripheral blood CD3+ T cells and decreased to 15% (median; range, 4 to 21%) of cells after 36 h of culture (Fig. 2A). By contrast, CCR5 expression on mucosal T cells derived from the same individuals was >81% (Fig. 2A). Expression of CXCR4 on peripheral blood CD3+ T cells was 58% (median; range 41 to 94%) in four individuals and was upregulated to >99% after 36 h of in vitro culture (Fig. 2B). CXCR4 expression on mucosal T cells of the same individuals was >94% (Fig. 2B). These results indicate that the proportion of T cells derived from the female genital mucosa expressing CCR5 is markedly greater than those from peripheral blood and that the observed differences are probably not a consequence of in vitro events.
To determine whether the levels of CCR5 mRNA expression were similar to the surface expression in the MMC, cellular RNA isolated from purified MMC populations was reverse transcribed to cDNA and amplified by PCR with CCR5-specific primers, and the intensities of the ethidium bromide-stained gel bands were compared with similarly amplified CCR5-transduced Jurkat cells (Fig. 2C and D). As shown in one donor in Fig. 2C, CCR5 mRNA was easily recognized in purified mucosal CD3+ T cells (∼16,000 copies/106 cells) and CD14+ macrophages (∼80,000 copies/106 cells). However, low levels of CCR5 mRNA (<3,000 copies/106 cells) were found in HLA-DQhigh CD14− mucosal DC (Fig. 2C). Similarly, CCR5 mRNA could not be detected in HLA-DQhigh CD14− mucosal DC from a second donor, whereas an equal number of mucosal CD3+ T cells again clearly demonstrated the presence of CCR5 mRNA (not shown). Thus, the patterns of CCR5 mRNA expression confirmed our studies of CCR5 surface expression. Based on these observations, the high expression of CCR5 detected on mucosal T cells and macrophages in comparison to the low or absent expression on DC suggests that T cells and macrophages may be primary targets for uptake of M-tropic HIV-1 strains in the human female genital tract. Furthermore, expression of CXCR4 on all subpopulations studied suggests that selective exclusion of T-tropic HIV-1 strains during sexual HIV-1 transmission is unlikely to occur at the level of virus-receptor binding to mononuclear cells in the mucosal compartment.
HIV-1 infection of mucosal cells.
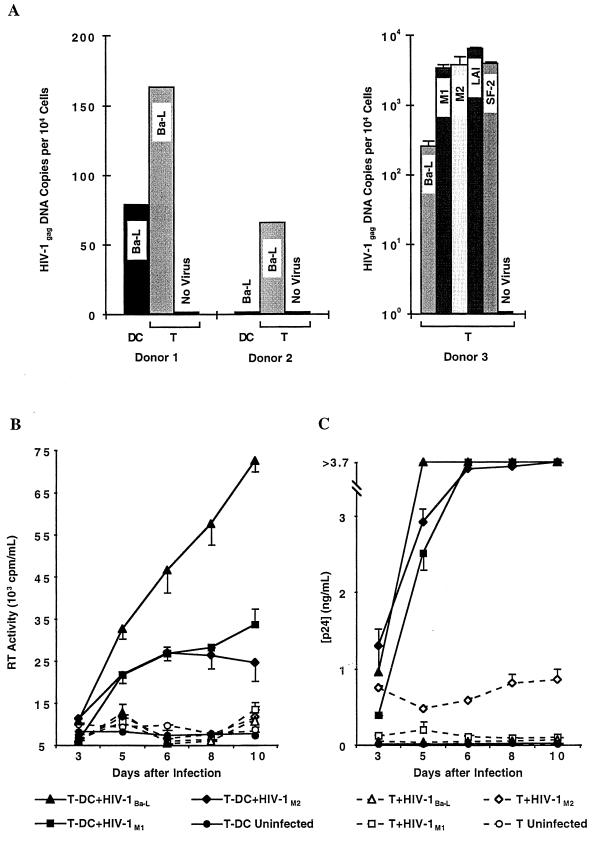
To test whether CCR5 expression of MMC correlates with infectivity by R5-tropic HIV-1 strains, we pulsed sorted unstimulated vaginal T cells and DC from two donors for 48 h with R5-tropic HIV-1Ba-L and then examined cellular DNA for HIV-1 gag transcripts by quantitative PCR (Fig. 3A). To further investigate whether mucosal T cells become infected with both R5- and X4-tropic strains, T cells from a third donor were infected in duplicates with a panel of five different virus strains, HIV-1Ba-L, two primary R5-tropic mucosal isolates derived from the cervix of two HIV-1 infected women (HIV-1M1 and HIV-1M2), and two X4-tropic strains (HIV-1SF-2 and HIV-1LAI). Whereas vaginal T cells always contained gag transcripts for HIV-1Ba-L, no transcripts were detected in vaginal DC from one donor, and copy numbers were lower than with T cells from another donor. In addition, both the two R5-tropic mucosal and the two X4-tropic HIV-1 isolates infected mucosal T cells in the third donor. These findings suggest that genital DC, having low or absent CCR5 expression, may be less susceptible to R5-tropic HIV-1 strains than mucosal T cells, which display markedly higher frequencies of CCR5 expression. We cannot exclude other cellular factors that DC lack which may also contribute to their nonreplicative nature. However, genital T cells, which express both CCR5 and CXCR4, are susceptible to infection with both R5- and X4-tropic HIV-1.
FIG. 3.
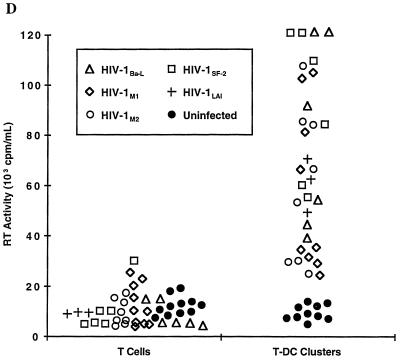
HIV-1 infection of mucosal mononuclear cells I. (A) Detection of proviral HIV-1 DNA in sorted CD3+ T cells (T) and HLA-DQhigh CD14− CD3− DC. At 48 h after inoculation with DNase-treated HIV-1Ba-L (donors 1 and 2) or HIV-1Ba-L, HIV-1M1, HIV-1M2, HIV-1LAI, and HIV-1SF-2 (donor 3), DNA was isolated from equal numbers (104) of cells and HIV-1 gag DNA copy numbers were measured by using a quantitative-competitive PCR enzyme immunoassay. Residual HIV-1 gag DNA copy numbers detected in the DNase-treated inocula (donor 1: Ba-L, 112 copies; donor 2: Ba-L, 0 copies; donor 3: Ba-L, 25 copies, M1, 0 copies, M2, 36 copies, LAI, 274 copies, and SF-2, 26 copies) were subtracted from the sample copy numbers. Error bars indicate standard deviations for duplicate infections in the third donor. (B) Reverse transcriptase (RT) activity in supernatants taken at various time points after infection of sorted CD3+ T cells (T, dashed lines) and CD3+ HLA-DQhigh T-cell–DC clusters (T-DC, solid lines) with either HIV-1Ba-L or two primary genital mucosal HIV-1 isolates (HIV-1M1 and HIV-1M2). Infections were performed in duplicate, and the error bars indicate standard deviations. (C) HIV-1 p24 antigen concentrations measured in the same culture supernatants as in panel B. The upper limit of the assay was 3.7 ng/ml. (D) Comparison of reverse transcriptase activities in the supernatants of T and T-DC cultures from five mucosal donors after 5 to 6 days of infection with five different HIV-1 isolates.
These data raise the possibility that mucosal DC are more apt to enhance CCR5-dependent virus replication through interactions with T cells than serve as initial targets of infection. To explore this issue, we sorted vaginal MMC into CD3+ T cells and CD3+ HLA-DQhigh T-cell–DC clusters and inoculated the populations with various HIV-1 isolates. Productive infection, as measured by reverse transcriptase activity and p24 production, was undetectable or at a very low level when T cells alone were infected with either HIV-1Ba-L or the two R5-tropic primary isolates HIV-1M1 and HIV-1M2 (Fig. 3B and C). In comparison, coculture of mucosal T cells with DC led to substantially higher levels of replication with all three viral strains (Fig. 3B and C). Similar findings were observed in five donors and for the X4-tropic strains HIV-1SF-2 (three donors) and HIV-1LAI (one donor) and are summarized in Fig. 3D.
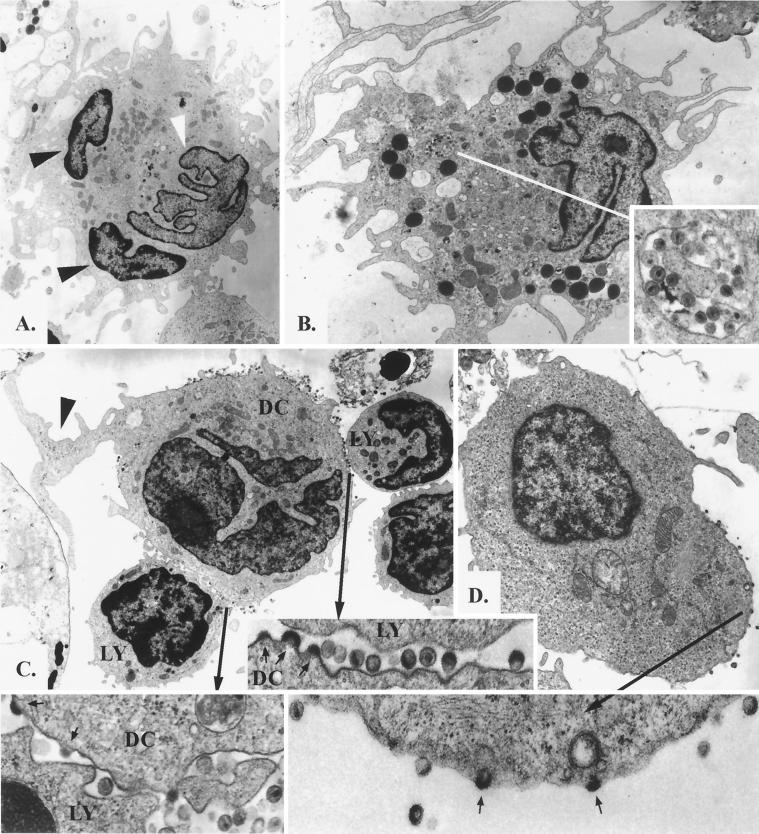
To identify which cell type in the conjugates produces HIV-1, we challenged vaginal MMC with R5-tropic HIV-1Ba-L for 5 days and then examined the cells by electron microscopy. T-cell–DC conjugates were common among the MMC. The cell membranes of both cell types fused to various extents, and in some instances complete syncyta of two or three cells were observed (Fig. 4A). However, syncytium formation seemed to occur independently from HIV-1 infection because membrane fusion was also observed in HIV-negative control samples, and classical HIV-induced syncytia larger than three cells were extremely rare (not shown). HIV-1 appeared in vaginal DC in two forms: within intracytoplasmic vesicles in single DC (Fig. 4B) and budding from membranes of mostly conjugated DC (Fig. 4C). Lymphocytes demonstrated virus budding only when morphologically activated (Fig. 4D). These results indicate that, like in the resting T cells at other sites (23, 31, 32, 40), HIV-1 enters but does not replicate well in purified T cells isolated from the vaginal mucosa. However, T cells and DC form stable conjugates in which the postentry block of viral replication is efficiently overcome by viruses of both coreceptor specificities. Moreover, the successful propagation of R5-tropic strains in DC within mucosal T-cell–DC conjugates despite meager CCR5 expression on DC indicates that HIV-1 coreceptor restriction may be bypassed by conjugation with CCR5-positive T cells. Finally, production of virus in activated but not resting mucosal lymphocytes is evidence that interactions with antigen-presenting cells such as DC are necessary for completion of the virus life cycle in T cells. These results underscore that the efficient productive infection supported by DC–T-cell interactions is not solely dependent on coreceptor expression of the individual cell types, and thus selection of virus variants is unlikely to occur via coreceptor restriction on genital mononuclear cells.
FIG. 4.
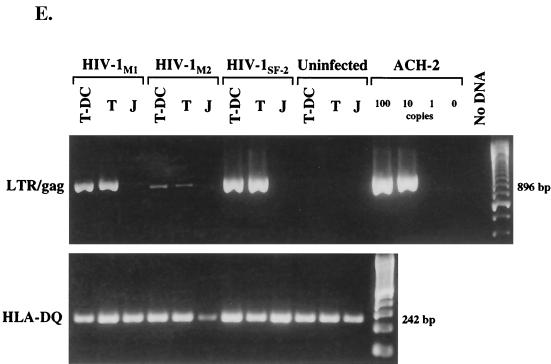
HIV-1 infection of MMC. (A to D) Electron photomicrographs of MMC infected with HIV-1Ba-L. (A) Fused conjugate of two lymphocytes and one DC (magnification, ×3,200). Two nuclei exhibit the darker, more condensed chromatin of lymphocytes (black arrowheads), and one nucleus has the typical veiled shape and less-condensed chromatin of a DC (white arrowhead). The cytoplasm contains many large mitochondria and a well-developed Golgi apparatus, which is typical for DC. Many cytoplasmic processes are also seen. (B) Single DC demonstrating a veiled nucleus with a small nucleolus, large mitochondria, electron-dense lysosome-like granules, and pinocytic vesicles (magnification, ×4,000). One vesicle contains multiple complete HIV-1 virions (inset, bottom right; magnification, ×28,000); however, no budding was seen. (C) Conjugate of one DC with two lymphocytes demonstrating productive HIV-1 infection of the DC (magnification, ×3,200). The DC contains the typical veiled nucleus as well as multiple large mitochondria, and one large cytoplasmic process is formed at the top right (black arrow). Virions and virus budding can be seen along the surface of the DC. The two contact zones with the lymphocytes are further magnified (×32,000), displaying virus budding (small arrows) from the DC but not from the lymphocytes. No virus production was visualized from the morphologically unactivated lymphocytes. (D) HIV-1 infection of an activated lymphocyte (magnification, ×5,600). The cell contains an eccentric round nucleus and some mitochondria and rough endoplasmic reticulum, and virus budding (small arrows) is apparent in the enlarged area (×28,000). (E) Detection of HIV-1 late RT products by nested PCR for the LTR-gag region (23) in sorted CD3+ T cells (T) and CD3+ HLA-DQhigh T-cell–DC clusters (T-DC). At 24 h after inoculation with DNase-treated HIV-1M1, HIV-1M2, or HIV-1SF-2, DNA was isolated from equal numbers (5 × 104) of cells, and an 896-bp LTR-gag fragment was amplified. Jurkat cells mixed with the DNase-treated HIV-1 were included to test for residual HIV-1 DNA after DNase treatment (J), and serial dilutions of latently infected ACH-2 cells served as positive control. A 242-bp HLA-DQ fragment was also amplified to demonstrate the presence of DNA in all mucosal samples.
Block in virus replication.
To examine whether the postentry block of virus replication in vaginal T cells occurs during or after reverse transcription (RT), mucosal T cells were infected with HIV-1 and after 24 h late HIV-1 transcripts generated during second-strand synthesis were amplified by nested PCR within the LTR-gag region. The presence of late RT products in mucosal T cells was clearly demonstrated after exposure to the R5-tropic strains HIV-1M1 and HIV-1M2 and the X4-tropic strain HIV-1SF-2 (Fig. 4E). Furthermore, late RT products in T cells were comparable to products in T-cell–DC conjugates (Fig. 4E). These results confirm the above findings that mucosal T cells, largely expressing early (CD69) but not late (CD38, CD25, and HLA-DR) activation markers (19a) are susceptible to infection with HIV-1 of both coreceptor specificities. Moreover, RT of viral RNA by R5- and X4-tropic strains occurs equally well in unstimulated mucosal T cells and T-cell–DC conjugates, but completion of the virus life cycle requires interaction of mucosal T cells with DC, a finding which is consistent with those of others examining resting T cells from peripheral blood (31, 32, 40). Our experiments do not distinguish whether the DC–T-cell interaction facilitates viral integration or later steps in virus expression and/or maturation.
DISCUSSION
The efficient and reproducible recovery of mononuclear cells from the human genital mucosa, as reported here for the first time, has enabled us to explore the cellular targets and interactions associated with initial HIV-1 infection and its propagation after heterosexual exposure. Our findings indicate that HIV-1 coreceptor expression on target cells is not sufficient to determine which viral strains are preferentially transmitted. Clearly, the use of mucosal models such as skin has led to sentinel observations concerning the amplification of HIV-1 infection through DC–T-cell conjugates (3, 16, 23, 24, 30), but these models appear to be misleading in defining HIV-1 coreceptors in the local genital tract. Thus, we argue that advances toward understanding mechanisms to interrupt HIV-1 transmission will emerge foremost from the relevant site of HIV-1 exposure, either the human genitourinary or gastrointestinal tract.
Based on our findings, we propose revision of the widely accepted view from skin and peripheral blood models that coreceptor expression on genital DC is responsible for the preferential transmission of M-tropic HIV-1 strains (2, 14, 19, 38, 41). We demonstrate that the HIV-1 coreceptor expression on cervical or vaginal DC is the opposite of that observed on skin Langerhans cells (41) and that coreceptor expression of PBMC (4, 38) does not predict the expression of the corresponding cells in the genital tract. Thus, the majority of genital DC express CXCR4 but not CCR5; by contrast, the majority of T cells and macrophages express both CXCR4 and CCR5.
Our data suggest that mucosal macrophages and T cells, rather than DC, may be the early targets for R5-tropic HIV-1 entry following sexual exposure. This is underscored by the finding that purified genital DC are less susceptible to infection with R5-tropic HIV-1 strains than are mucosal T cells (Fig. 3A). Since X4-tropic strains are also capable of infecting mucosal T cells and CXCR4 is abundantly expressed on genital T cells, macrophages, and DC, why R5-tropic rather than X4-tropic viruses are preferentially transmitted remains unclear. Thus, while CCR5 and CXCR4 permit cell entry of HIV-1 and can influence later stages of viral replication (5), additional events must be important in the selective amplification of R5-tropic strains. Outgrowth of R5-tropic NSI strains may occur during or after the rapid expansion of HIV-1 as it spreads systemically, a possibility which is supported by the fact that selection of this viral phenotype is not limited to transmission by sexual routes (8, 28, 33). The mechanisms contributing to this may include the induction of a specific immune response (8), the availability of target cells expressing specific HIV-1 coreceptors, and postentry differences in nuclear importation of the viral preintegrated complexes (29). Potentially, both R5 and X4 viruses are equally transmitted but only one dominates as a result of the immune responses of the host. Alternatively, expression of HIV-1 coreceptors on genital epithelial cells as well as release of chemokines locally (9, 39), particularly SDF-1, may influence selection of virus variants prior to and upon contact with mucosal leukocytes.
As predicted from skin and adenoid tissues, the interaction of T cells with DC in the genital tract must be key to the propagation of HIV-1 from the mucosa to the lymph nodes and circulation. Formation of DC–T-cell conjugates overcomes potential HIV-1 coreceptor restriction on DC and postentry block of viral replication, resulting in efficient propagation of both R5- and X4-tropic HIV-1 strains. As such, DC are unlikely to be involved in virus variant selection during sexual transmission. Unexpectedly, we also noted clusters of T cells with resident CD14+ macrophages. Conceivably, macrophages serve as long-term reservoirs for local HIV-1 production, and this process may be enhanced through interactions with local CD4+ T cells. The efficiency of HIV-1 propagation is additionally influenced by the maturation state of cells within the monocyte-DC lineage (18, 25, 36, 37). Further studies are under way to understand the impact of cellular differentiation within the macrophage-DC lineage on the efficiency of HIV-1 targeting in the genital mucosa, as well as to define the role of DC in helping T cells complete the reverse transcriptase process leading to integration.
Finally, we now have an approach to examine the dynamics of HIV-1 replication in the female genital tract that will provide insight into antigen processing and presentation of HIV-1 epitopes to mucosal T cells, which is likely to shape the repertoire of the immune response after infection. These studies will have obvious implications in the design of future vaccines and may be easily extended to other pathogens causing sexually transmitted diseases of public health importance.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants R37 AI35605 and RO1 38518 from the National Institutes of Health and Fonds zur Förderung der Wissenschaftlichen Forschung.
We thank David Eschenbach, Linda Eckert, and Julie Smith (Department of Obstetrics and Gynecology, University of Washington) and Jack Lamey (Swedish Medical Center, Seattle, Wash.) for assistance and support in procuring surgical specimens; Vicky Schweickart at ICOS Corporation (Bothell, Wash.) for providing anti-CCR5 MAb 227R; Mike Emerman and Wei-Chun Goh for determination of HIV-1 receptor usage and for kindly providing CCR5- and CXCR4-transfected cell lines; Robert Coombs for providing the quantitative HIV-1 gag PCR system; Christopher Alef for preparation of the figures; and Julie Overbaugh, Lawrence Corey, James Mullins, and Stanley Riddell for helpful discussions.
REFERENCES
- 1.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 2.Ayehunie S, Garcia Zepeda E A, Hoxie J A, Horuk R, Kupper T S, Luster A D, Ruprecht R M. Human immunodeficiency virus-1 entry into purified blood dendritic cells through CC and CXC chemokine coreceptors. Blood. 1997;90:1379–1386. [PubMed] [Google Scholar]
- 3.Ayehunie S, Groves R W, Bruzzese A M, Ruprecht R M, Kupper T S, Langhoff E. Acutely infected Langerhans cells are more efficient than T cells in disseminating HIV type 1 to activated T cells following a short cell-cell contact. AIDS Res Hum Retroviruses. 1995;11:877–884. doi: 10.1089/aid.1995.11.877. [DOI] [PubMed] [Google Scholar]
- 4.Bleul C C, Wu L, Hoxie J A, Springer T A, Mackay C R. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chackerian B, Long E M, Luciw P A, Overbaugh J. Human immunodeficiency virus type 1 coreceptors participate in postentry stages in the virus replication cycle and function in simian immunodeficiency virus infection. J Virol. 1997;71:3932–3939. doi: 10.1128/jvi.71.5.3932-3939.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 7.Coombs R W, Peterson G. Estimates of HIV-1 proviral DNA copy number using a novel quantitative-competitive PCR enzyme immunoassay (QC-PCR-EIA). Keystone Symposium, HIV Pathogenesis and Treatment, Park City, Utah. 1998. [Google Scholar]
- 8.Cornelissen M, Mulder Kampinga G, Veenstra J, Zorgdrager F, Kuiken C, Hartman S, Dekker J, van der Hoek L, Sol C, Coutinho R, et al. Syncytium-inducing (SI) phenotype suppression at seroconversion after intramuscular inoculation of a non-syncytium-inducing/SI phenotypically mixed human immunodeficiency virus population. J Virol. 1995;69:1810–1818. doi: 10.1128/jvi.69.3.1810-1818.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delézay O, Koch N, Yahi N, Hammache D, Tourres C, Tamalet C, Fantini J. Co-expression of CXCR4/fusin and galactosylceramide in the human intestinal epithelial cell line HT-29. AIDS. 1997;11:1311–1318. doi: 10.1097/00002030-199711000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 11.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 12.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 13.Emerman, M. 1997. Personal communication.
- 14.Fauci A S. Host factors and the pathogenesis of HIV-induced disease. Nature. 1996;384:529–534. doi: 10.1038/384529a0. [DOI] [PubMed] [Google Scholar]
- 15.Feng Y, Broder C B, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 16.Frankel S S, Wenig B M, Burke A P, Mannan P, Thompson L D, Abbondanzo S L, Nelson A M, Pope M, Steinman R M. Replication of HIV-1 in dendritic cell-derived syncytia at the mucosal surface of the adenoid. Science. 1996;272:115–117. doi: 10.1126/science.272.5258.115. [DOI] [PubMed] [Google Scholar]
- 17.Goh W C, Markee J, Akridge R, Meldorf M, Musey L, Karchmer T, Krone M, Collier A, Corey L, Emerman M, McElrath M J. Protection against human immunodeficiency virus type 1 infection in persons with repeated exposure: evidence for T cell immunity in the absence of inherited CCR5 coreceptor defects. J Infect Dis. 1999;179:548–557. doi: 10.1086/314632. [DOI] [PubMed] [Google Scholar]
- 18.Granelli-Piperno A, Delgado E, Finkel V, Paxton W, Steinman R M. Immature dendritic cells selectively replicate macrophagetropic (M-tropic) human immunodeficiency virus type 1, while mature cells efficiently transmit both M- and T-tropic virus to T cells. J Virol. 1998;72:2733–2737. doi: 10.1128/jvi.72.4.2733-2737.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Granelli-Piperno A, Moser B, Pope M, Chen D, Wei Y, Isdell F, O’Doherty U, Paxton W, Koup R, Mojsov S, Bhardwaj N, Lewis I C, Baggiolini M, Steinman R M. Efficient interaction of HIV-1 with purified dendritic cells via multiple chemokine coreceptors. J Exp Med. 1996;184:2433–2438. doi: 10.1084/jem.184.6.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19a.Hladik, F., G. Lentz, E. Delpit, A. McElroy, and M. J. McElrath. Co-expression of CCR5 and IL-2 in human genital but not blood T cells: implications for the ontogeny of the CCR5+ TAI phenotype. Submitted for publication. [PubMed]
- 20.Kwok S, Sninsky J J. PCR detection of human immunodeficiency virus type 1 proviral DNA sequences. Washington, D.C: ASM Press; 1993. [Google Scholar]
- 21.Orenstein J M, Fox C, Wahl S M. Macrophages as a source of HIV during opportunistic infections. Science. 1997;276:1857–1861. doi: 10.1126/science.276.5320.1857. [DOI] [PubMed] [Google Scholar]
- 22.Pomerantz R J, de la Monte S M, Donegan S P, Rota T R, Vogt M W, Craven D E, Hirsch M S. Human immunodeficiency virus (HIV) infection of the uterine cervix. Ann Intern Med. 1988;108:321–327. doi: 10.7326/0003-4819-108-3-321. [DOI] [PubMed] [Google Scholar]
- 23.Pope M, Betjes M G, Romani N, Hirmand H, Cameron P U, Hoffman L, Gezelter S, Schuler G, Steinman R M. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell. 1994;78:389–398. doi: 10.1016/0092-8674(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 24.Pope M, Gezelter S, Gallo N, Hoffman L, Steinman R M. Low levels of HIV-1 infection in cutaneous dendritic cells promote extensive viral replication upon binding to memory CD4+ T cells. J Exp Med. 1995;182:2045–2056. doi: 10.1084/jem.182.6.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reece J C, Handley A J, Anstee E J, Morrison W A, Crowe S M, Cameron P U. HIV-1 selection by epidermal dendritic cells during transmission across human skin. J Exp Med. 1998;187:1623–1631. doi: 10.1084/jem.187.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romani N, Gruner S, Brang D, Kampgen E, Lenz A, Trockenbacher B, Konwalinka G, Fritsch P O, Steinman R M, Schuler G. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roos M T, Lange J M, de Goede R E, Coutinho R A, Schellekens P T, Miedema F, Tersmette M. Viral phenotype and immune response in primary human immunodeficiency virus type 1 infection. J Infect Dis. 1992;165:427–432. doi: 10.1093/infdis/165.3.427. [DOI] [PubMed] [Google Scholar]
- 28.Scarlatti G, Tresoldi E, Bjorndal A, Fredriksson R, Colognesi C, Deng H K, Malnati M S, Plebani A, Siccardi A G, Littman D R, Fenyo E M, Lusso P. In vivo evolution of HIV-1 co-receptor usage and sensitivity to chemokine-mediated suppression. Nat Med. 1997;3:1259–1265. doi: 10.1038/nm1197-1259. [DOI] [PubMed] [Google Scholar]
- 29.Schmidtmayerova H, Alfano M, Nuovo G, Bukrinsky M. Human immunodeficiency virus type 1 T-lymphotropic strains enter macrophages via a CD4- and CXCR4-mediated pathway: replication is restricted at a postentry level. J Virol. 1998;72:4633–4642. doi: 10.1128/jvi.72.6.4633-4642.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spira A I, Marx P A, Patterson B K, Mahoney J, Koup R A, Wolinsky S M, Ho D D. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J Exp Med. 1996;183:215–225. doi: 10.1084/jem.183.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevenson M, Stanwick T L, Dempsey M F, Lamonica C A. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 1990;9:1551–1560. doi: 10.1002/j.1460-2075.1990.tb08274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang S, Patterson B, Levy J A. Highly purified quiescent human peripheral blood CD4+ T cells are infectible by human immunodeficiency virus but do not release virus after activation. J Virol. 1995;69:5659–5665. doi: 10.1128/jvi.69.9.5659-5665.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van’t Wout A B, Kootstra N A, Mulder Kampinga G A, Albrecht van Lent N, Scherpbier H J, Veenstra J, Boer K, Coutinho R A, Miedema F, Schuitemaker H. Macrophage-tropic variants initiate human immunodeficiency virus type 1 infection after sexual, parenteral, and vertical transmission. J Clin Invest. 1994;94:2060–2067. doi: 10.1172/JCI117560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vodicka M A, Goh W C, Wu L I, Rogel M E, Bartz S R, Schweickart V L, Raport C J, Emerman M. Indicator cell lines for detection of primary strains of human and simian immunodeficiency viruses. Virology. 1997;233:193–198. doi: 10.1006/viro.1997.8606. [DOI] [PubMed] [Google Scholar]
- 35.Wahl S M, Allen J B, Gartner S, Orenstein J M, Popovic M, Chenoweth D E, Arthur L O, Farrar W L, Wahl L M. HIV-1 and its envelope glycoprotein downregulate chemotactic ligand receptors and chemotactic function of peripheral blood monocytes. J Immunol. 1989;142:3553–3559. [PubMed] [Google Scholar]
- 36.Warren M K, Rose W L, Cone J L, Rice W G, Turpin J A. Differential infection of CD34+ cell-derived dendritic cells and monocytes with lymphocyte-tropic and monocyte-tropic HIV-1 strains. J Immunol. 1997;158:5035–5042. [PubMed] [Google Scholar]
- 37.Weissman D, Li Y, Ananworanich J, Zhou L J, Adelsberger J, Tedder T F, Baseler M, Fauci A S. Three populations of cells with dendritic morphology exist in peripheral blood, only one of which is infectable with human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1995;92:826–830. doi: 10.1073/pnas.92.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu L, Paxton W A, Kassam N, Ruffing N, Rottman J B, Sullivan N, Choe H, Sodroski J, Newman W, Koup R A, Mackay C R. CCR5 levels and expression patterns correlate with infectability by macrophage-tropic HIV-1 in vitro. J Exp Med. 1997;185:1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang S K, Eckmann L, Panja A, Kagnoff M F. Differential and regulated expression of C-X-C, C-C, and C-chemokines by human colon epithelial cells. Gastroenterology. 1997;113:1214–1223. doi: 10.1053/gast.1997.v113.pm9322516. [DOI] [PubMed] [Google Scholar]
- 40.Zack J A, Arrigo S J, Weitsman S R, Go A S, Haislip A, Chen I S. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 41.Zaitseva M, Blauvelt A, Lee S, Lapham C K, Klaus-Kovtan V, Mostowski H, Manischewitz J, Golding H. Expression and function of CCR5 and CXCR4 on human Langerhans cells and macrophages: implications for HIV primary infection. Nat Med. 1997;3:1369–1375. doi: 10.1038/nm1297-1369. [DOI] [PubMed] [Google Scholar]
- 42.Zhu T, Mo H, Wang N, Nam D S, Cao Y, Koup R A, Ho D D. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]