Abstract
The orexinergic system and its receptors are involved in many physiological processes. Their functions in energy homeostasis, arousal, cognition, stress processing, endocrine functions, and pain modulation have been investigated. Many studies have shown that the orexinergic system cooperates with the dopaminergic system in the addiction process. Emerging evidence suggests that the orexinergic system can be effective in the induction of drug dependence and tolerance. Therefore, several researches have been conducted on the effect of orexin receptor (OXR) antagonists on reducing tolerance and dependence caused by drug abuse. Due to the significant growth of the studies on the orexinergic system, the current literature was conducted to collect the findings of previous studies on orexin and its receptors in the induction of drug addiction. In addition, cellular and molecular mechanisms of the possible role of orexin in drug tolerance and dependence are discussed. The findings indicate that the administration of OXR antagonists reduces drug dependence. OXR blockers seem to counteract the addictive effects of drugs through multiple mechanisms, such as preventing neuronal adaptation. This review proposes the potential clinical use of OXR antagonists in the treatment of drug dependence.
Keywords: Orexin, Hypocretin, Tolerance, Dependence, Withdrawal, Addiction
Introduction
Drug addiction is a reversible chronic disorder. Among the characteristics of drug addiction are the occurrence of emotional states (when access to drugs is prevented), the compulsion to use drugs, and lack of control during deprivation.1 One of the major problems in the chronic use of drugs (e.g., morphine) is the formation of tolerance and dependence, which limits their clinical use.2,3 Treatment for drug addiction is provided in several approaches, including pharmacological and behavioral therapies or their combination. Various medications are prescribed to manage withdrawal symptoms, treat co-occurring disorders, and prevent relapse.1,4-6
Orexin (hypocretin/OX) is a sleep-wake cycle regulating neuropeptide secreted from the lateral hypothalamus (LH). Orexin A (OXA) and OXB are two orexin neuropeptides.7 Orexins are peptide products from the processing of the hypocretin (HCRT) gene, which is responsible for encoding the prepro-orexin peptide with 130 amino acids.8-10 Studies have revealed that OXA has the same affinity for both orexin receptors, while OXB selectively prefers the OX type 2 receptor (OX2R).11 Although less than 100 000 orexin-secreting neurons are located in the LH, orexinergic receptors are expressed in various brain areas.7,11 Research on orexinergic neurons has shown that OX neurons have different functions at different locations in the hypothalamus. For example, OX neurons (in LH) are more involved in regulating the reward cycle for addictive behaviors.12,13 Also, evidence suggests that OX1R and OX2R have different functions. For example, OX1Rs are involved in the induction of dependence on morphine and cocaine.14-16
Today, various antagonists for OX receptors have been developed with the aim of therapeutic application. Some antagonists can block both orexin receptors (dual orexin receptor antagonists/DORAs), but others selectively block one of the receptors (selective orexin receptor antagonists/SORAs).17-19
The role of OX in the physiological functions of the body
Orexin has been detected in various tissues such as cerebrospinal fluid, hypothalamus, sensory ganglia, pituitary, spinal cord, enteric nervous system, adrenal, salivary and lacrimal glands, vestibular gland, testis and skin.20 In general, the neuropeptide orexin is important in regulating and modulating many functions, including arousal, sleep, food and fluid intake, pain, memory, smell perception, and sexual activity.7,11,20 Findings of different studies have demonstrated that the level of OXA has an inverse relationship with body mass index and is lower in severely obese patients.21 Therefore, the function of orexin is not only limited to the central nervous system (CNS).22 Also, orexin is involved in regulating the sleep/wake cycle.23 In fact, neurons that are effective in stimulating wakefulness (such as LC noradrenergic neurons) interact with orexin.24-26 Orexin also affects maternal behavior. Therefore, it can be said that orexin affects a range of motivated behaviors.12,27 Interestingly, according to the available evidence, the orexinergic system is related to pathological processes in neurological diseases such as depression, narcolepsy, Alzheimer’s disease, ischemic stroke, and addiction.28 Currently, there is emerging evidence that implicates the role of OX in the induction of tolerance and dependence to opioids and other drugs.12,13
The role of OX in drug dependence
Glutamate is known as the most abundant excitatory neurotransmitter in the CNS, which is involved in about 70% of synaptic transmissions.29,30 Reports indicate the key role of glutamate in drug addiction.29 In fact, the glutamatergic system is related to drug addiction through specific releases.31-33 Another factor involved in drug addiction is dopamine (key neurotransmitter in drug addiction). Studies have shown that drugs facilitate dopamine signaling. For example, morphine increases the release of dopamine in the brain.29 Interestingly, reports indicate the existence of axon-axon synapses between glutamate and dopamine neurons, providing morphological evidence for their interaction in the addiction process.34,35
It has also been revealed that dopamine (in ventral tegmental area/VTA) and OX neurons are under gamma-aminobutyric acid (GABA) inhibition.36 Morphine inhibits GABA release in the nucleus accumbens (NAc), which in turn increases the release of orexin and dopamine in downstream targets (this effect persists even after stopping morphine intake).37,38
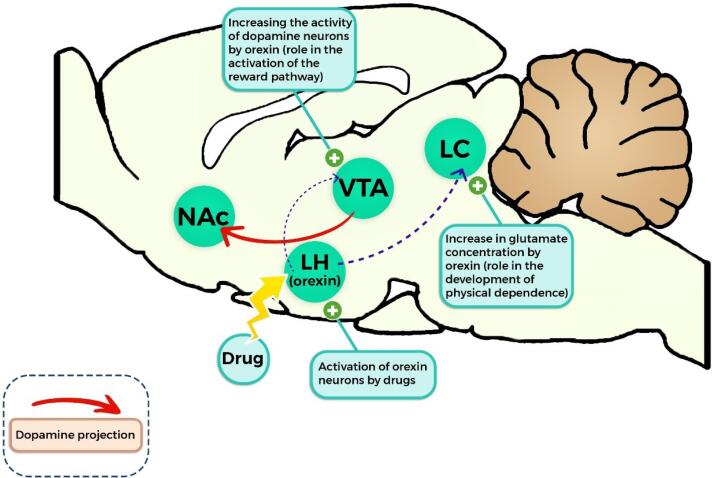
Georgescu et al in 2003 demonstrated the role of OX neurons located in the LH in the induction of morphine tolerance and morphine-induced withdrawal syndrome.39 Studies have indicated that OX neurons are activated by drugs. In fact, about 50% of orexinergic neurons can directly respond to opioids through the expression of µ opioid receptors.39-41 According to the results of studies, systemic injection of orexin can elevate the concentration of glutamate in locus coeruleus (LC). Also, orexin causes the release of dopamine in downstream targets by increasing the output and activity of dopamine neurons (in VTA).42-45 In fact, orexin is a potent stimulatory transmitter for dopamine neurons and can cause addictive behaviors46 (Figure 1).
Figure 1.
The role of orexin on the reward pathway and the development of dependence caused by drug abuse (indicated based on rodent brain). NAc: nucleus accumbens, LH: lateral hypothalamus, VTA: ventral tegmental area, LC: locus coeruleus
Although there is currently little evidence of a link between orexin and addiction in humans, animal models have well demonstrated that OX neurons are important for the induction of addiction.47 The studies conducted to date show a strong connection between orexin and the occurrence of addictive behaviors, which we will discuss further.
Orexin in cocaine and amphetamine dependence
Cocaine addiction is characterized by loss of control over use, cocaine acquisition, and compulsive patterns of use.48 After administration, cocaine enters all parts of the brain and leads to activation in the VTA, NAc and caudate nucleus, which are known as reward regions.49 Interestingly, cocaine increases the number and activity of OX neurons (in LH).50 According to the findings, orexin not only plays a role in maintaining hyperarousal states for cocaine seeking, but also contributes to the compulsive motivation to take cocaine.48,51,52 In fact, inhibition of orexin signaling reduces several addictive effects of cocaine.39,45 For example, OX1R antagonists attenuate amphetamine and cocaine-induced conditioned place preference (CPP) expression.53,54 Also, evidence suggests that dual OXR antagonists (DORAs) may reduce cocaine-dependent behaviors by lowering increased dopamine levels in the ventral striatal.55 One study found that suvorexant (DORA) administration could reduce the number of cocaine injections, prevent CPP in rats and reduce initial positive hedonic reactivity to cocaine. Their findings showed that suvorexant diminished the amount of extracellular dopamine in the ventral striatal.56 Similarly, other studies have revealed that administration of OX1R antagonists reduces cocaine self-administration.54,57,58
Similarly, chronic administration of amphetamine induces behavioral sensitization and thus synaptic and structural adaptations in the VTA, dorsal raphe (DR) and ventrolateral preoptic area (VLPO).2,59 Also, administration of methamphetamine or acute amphetamine increases Fos protein level (an indicator of neuron activation) in dorsomedial hypothalamus (DMH)/perifornical area (PFA) OX neurons.60–62 These findings support the relevance of orexin signaling in drug-induced adaptation.2 The results of a study showed that almorexant (DORA) does not produce conditional reward effects but interferes with CPP expression and drug-induced locomotor sensitization. In fact, almorexant was able to attenuate the expression of CPP in high doses of amphetamines.63 In another study, administration of SB-334867 (OX1R antagonist) dramatically decreased the effect of amphetamine on dopamine levels (in NAc shell/rat brain).64 Overall, studies show that OXR antagonists are associated with decreased cocaine intake, CPP induced by cocaine or amphetamines and cocaine demand48,63,65,66 (Tables 1 and 2).
Table 1. Cocaine-based experiments .
| Addictive drug | Manipulation | Target | Subjects | Main findings/References |
| Cocaine | Suvorexant | dual OX1/2R | R (rat) | Attenuation of cocaine-induced impulsive behaviors (systematic or direct injection in VTA)67 |
| Suvorexant | dual OX1/2R | R | Attenuation of the hedonic and motivational effect induced by cocaine56 | |
| SB-334867 | OX1R | R | Counteracts the development of cocaine self-administration and attenuates the induction of amphetamine-induced CPP54 | |
| SB-334867 | OX1R | R | Administration of SB-334867 decreased cocaine intake (in a dose-dependent manner)48 | |
| SB-334867 | OX1R | M (mice) | SB-334867 blocks CPP induced by micro-injection of orexin in VTA 65 | |
| SB-334867 | OX1R | M | SB-334867 injections (in VTA) attenuated impulsive-like behavior, LH self-stimulation, and cocaine self-administration57 | |
| SB-334867 | OX1R | R | Diminished the motivation for cocaine50 | |
| SB-334867 | OX1R | Female monkeys (rhesus) |
Reduced cocaine self-administration58 | |
| SB-334867 | OX1R | R | Decreased demand for cocaine (in the presence of cocaine-related cues)66 | |
| SB-334867 | OX1R | R | Blocking OX1R or OX1R and OX2R together reduces the effect of cocaine on dopamine signaling and cocaine motivation, but blocking OX2R alone showed no effect55 | |
| Almorexant | dual OX1/2R | R | Decrease cocaine self-administration and weaken cocaine-induced dopamine uptake inhibition55 | |
| RTIOX-276 | OX1R | R | Attenuation of cocaine-induced inhibition of dopamine uptake68 |
Table 2. Amphetamine/cannabis/nicotine-based experiments .
| Addictive drug | Manipulation | Target | Subjects | Main findings/ References |
| Amphetamine | Almorexant | dual OX1/2R | R (rat) | Decreased cocaine and amphetamine-induced CPP expression but did not affect morphine-induced CPP expression/Interfered with morphine-induced locomotor sensitization but had no effect on cocaine and amphetamine-induced locomotor sensitization63 |
| SB-334867 | OX1R | R | Decreased amphetamine-induced dopamine outflow (in NAc) and reduced amphetamine-induced sensitization64 | |
| Cannabis | SB-334867 | OX1R | M (mice) | SB-334867 reduced the reinforcing and motivational properties of WIN55,212-2 (SORA2-TCS-OX2-29 had no effect)69 |
| Nicotine | TCS 1102 | dual OX1/2R | R | It had no effect on nicotine-seeking behavior70 |
| SB-334867 | OX1R | M | Administration of SB-334867 reduced the somatic signs of nicotine-induced withdrawal but SORA2-TCS-OX2-29 had no effect71 |
Orexin in alcohol dependence
The orexin signaling system has an important function in generating highly salient positive reinforcement motivation.52 Orexin is specifically associated with the drive toward drug-seeking, including alcohol.72-75 In some animal studies orexin peptide increased following alcohol consumption, while in others it decreased.76-78 Studies have revealed that the brain regions of the anterior thalamic paraventricular nucleus (aPVT), NAc and VTA are related with the OX system in alcohol abuse.79-81 The findings indicate that the administration of OXR antagonists has been effective in reducing alcohol self-administration, consumption, and relapse.82-84. Also, the administration of suvorexant in alcohol withdrawal syndrome reduced the latency to rapid eye movement (REM) and slow-wave sleep onset in a dose-dependent manner85 (Table 3).
Table 3. Alcohol-based experiments .
| Addictive drug | Manipulation | Target | Subjects | Main findings/ References |
| Alcohol | Suvorexant | dual OX1/2R | R (rat) | Reduced the latency to REM sleep and sleep and slow-wave-sleep (SWS) onset in a dose-dependent manner/produced REM sleep and SWS fragmentation85 |
| Almorexant | dual OX1/2R | Healthy humans | Almorexant did not affect the pharmacokinetics of ethanol and did not synergize its effects86 | |
| Almorexant | dual OX1/2R | R | Diminished alcohol self-administration (Systemic or VTA administration)79 | |
| Almorexant | dual OX1/2R | R | It did not enhance the sedative effect of alcohol87 | |
| SB-334867 | OX1R | R | Reduced alcohol intake and preference (Intra-NAc infusions)80 | |
| SB-334867 | OX1R | R | Decreased alcohol relapse drinking83 | |
| SB-334867 | OX1R | R | Attenuated ethanol self-administration and the cue-induced reinstatement of ethanol–seeking in highly motivated rats 82 | |
| GSK1059865 | OX1R | M (mice) | Significantly reduced alcohol consumption in ethanol-dependent Mice84 | |
| TCS-OX2-29 | OX2R | R | Microinjections of TCS-OX2-29 (into the aPVT) reduced intermittent-access ethanol drinking81 |
Orexin in opioids dependence
Experiments indicated that OX neurons are activated by morphine (during the CPP acquisition phase/in LH), and administration of the SB-334867 can dramatically inhibit morphine-seeking expression.15,16 Other studies have also indicated that injection of SB-334867 into VTA attenuates morphine-induced CPP in rats.46,74,88,89 Studies in transgenic mice (knockout mice) also confirm that the absence of orexin reduces morphine dependence in mice.39 Hooshmandi et al.’s findings demonstrated that blocking the OX1R receptor before receiving morphine could prevent dependence, but single-dose administration of the SB-334867 antagonist after dependence could not prevent the withdrawal syndrome.90 Evidence suggests that orexin antagonists attenuate naloxone-induced withdrawal syndrome.91,92 For example, Aghajani et al. reported that the administration of SB-334867 prevented hyperactivity of LC neurons following naloxone administration.93 Interestingly, SB-334867 reduces the increased cyclic adenosine monophosphate (cAMP) concentration (in LC neurons/in morphine-dependent rats receiving naloxone).94 Overall, the results propose that the activation of OX1R in the LC nucleus may be involved in the induction of morphine dependence, and OXR antagonists such as SB-334867 attenuate this effect.95
Although studies indicate a crucial role for OX1R receptor signaling in addictive behaviors,8,12,74,96 it can be argued that orexin acts as a mediator in reward and drug addiction through both receptors.88 For example, heroin self-administration (in rat) was dose-dependently attenuated by systemic administration of NBI-80713 (OX2R antagonist).97 Therefore, it seems that DORAs can be considered in the study of drug addiction as a therapeutic potential. In another study by Esmaili-Shahzade-Ali-Akbari et al, the researchers found that suvorexant administration before each morphine injection could significantly prevent morphine dependence and tolerance. This study revealed that suvorexant administration reduces the increased levels of p-ERK and cAMP response element-binding (CREB) proteins.98 In the only clinical study conducted in this field, Huhn et al. found that suvorexant can reduce opioid withdrawal symptoms in opioid use disorder (OUD) patients. Suvorexant also increased the total sleep time of study participants. The results indicate that suvorexant may have therapeutic potential in treating opioid withdrawal99 (Table 4).
Table 4. Opioids-based experiments .
| Addictive drug | Manipulation | Target | Subjects | Main findings/ References |
| Opioids | Suvorexant | Dual OX1/2R | M (mice) | Decreased morphine tolerance and dependence / decreased increased levels of CREB and p-ERK proteins98 |
| SB-334867 | OX1R | M | Administration of SB-334867 prevented morphine-induced sensitivity to locomotor activity in mice100 | |
| SB-334867 | OX1R | R (rat) | Significantly reduced naloxone-induced withdrawal syndrome physical symptoms in morphine-dependent rats92 | |
| SB-334867 | OX1R | R | Microinjection of SB-334867 into LC dramatically suppresses glutamate-induced morphine withdrawal101 | |
| SB-334867 | OX1R | M | Attenuated the symptoms of naloxone-induced withdrawal91 | |
| SB-334867 | OX1R | R | Attenuation of morphine-induced CPP (acquisition and expression/micro-injection into VTA)89 | |
| SB-334867 | OX1R | R | Intra-DG (dentate gyrus) administration dose-dependently reduced morphine priming-induced reinstatement102 | |
| SB-334867 | OX1R | R | Decreased motivation and the cue-induced reinstatement of remifentanil-seeking103 | |
| SB-334867 | OX1R | R | Inhibition of increased activity of LC neurons following naloxone administration in morphine-dependent rats93 | |
| SB-334867 | OX1R | R | Prevention of naloxone-induced neuronal activation within the LC in morphine-dependent rats/ Decreased cAMP concentration in LC neurons94 | |
| SB-334867 | OX1R | R | Significant reduction of physical symptoms of morphine withdrawal syndrome induced by naloxone95 | |
| TCS-OX2-29 | OX2R | R | Intra-DG (dentate gyrus) administration dose-dependently reduced morphine priming-induced reinstatement102 | |
| TCS-OX2-29 | OX2R | R | Attenuation of morphine-induced CPP (acquisition and expression/micro-injection into VTA)89 | |
| NBI-80713 | OX2R | R | Dose-dependently reduced heroin self-administration (systemic administration) 97 |
Orexin in cannabis and nicotine dependence
Studies on the effect of OXR antagonists on cannabinoid and nicotine abuse have yielded different results. For example, administration of SB-334867 attenuated the motivational and reinforcing properties of WIN55,212-2 (synthetic cannabinoid agonist), but administration of TCS-OX2-29 (selective OX2R antagonist, SORA2) had no effect.69 It was also shown that administration of TCS 1102 (DORA) had no effect on nicotine seeking in rats, but administration of SB-334867 could reduce nicotine withdrawal (somatic signs)70,71 (Table 2).
Possible mechanisms of orexin involvement in the induction of drug dependence
Although the role of OX in increasing the levels of neurotransmitters (glutamate and dopamine) involved in addiction has been indicated in many studies,42–45,55,56 the cellular mechanisms related to the involvement of OX in the induction of addictive behaviors have not been fully investigated. Evidence has indicated that cocaine, amphetamines, and other addictive substances strongly activate the ERK (extracellular signal-regulated kinases) pathway through the signaling system initiated by activating either dopamine or glutamate receptors, or both.104-106 Indeed, N-methyl-D-aspartic acid (NMDA) receptors increase ERK phosphorylation by increasing Ca2+ permeability and activating several Ca2+ -dependent kinases.29 Also, OXA and OXB can increase ERK phosphorylation 107,108. In fact, orexin activates the p38-MAPK signaling pathway and increases ERK1/2 phosphorylation by interfering with the Gq/PLC/PKC signaling pathway.28 Since OX neurons respond to opioids,39-41 consumption of opioids increases orexin levels, which can eventually lead to increased ERK phosphorylation. Esmaili-Shahzade-Ali-Akbari et al. demonstrated that suvorexant administration with morphine reduces the level of p-ERK (in mouse brain).98 It is possible that antagonists of orexin receptors, such as suvorexant, may reduce the level of p-ERK by blocking orexin receptors and preventing the activation of the MAPK signaling pathway.
CREB transcription factor has been widely demonstrated in forms of cellular adaptation and it has been reported that its expression changes in drug addiction.109,110 Studies on the pathways involved in morphine dependence have demonstrated that chronic administration of morphine alters the cAMP system. In general, chronic morphine use increases CREB, PKA (cAMP-dependent protein kinase) and cAMP.29 Also, it has been demonstrated that the administration of OXR antagonists leads to a significant reduction in CREB protein levels in morphine-dependent animals.98,111 Therefore, orexin appears to help in the induction of drug addiction by increasing the level of CREB protein, which is inhibited by orexin receptor antagonists.
Conclusions and future perspectives
Addiction is a chronic health-related disorder; research suggests a role for the OX receptors in drug addiction.. Hitherto, most studies have focused on the OX1R, while the OX2R also appears to play a role. OX2R is generally involved in sleep arousal, but in specific regions of the brain, it can play a role in expressing and regulating addiction and negative emotions. There is a relative lack of studies on OX2R in drug dependence in the literature. Although the mechanisms by which OX may be involved in drug addiction are not yet fully understood, it has been reported that orexin can alter dopamine levels and affect the levels of p-ERK and CREB proteins. Animal and clinical studies on the orexinergic system show a valuable therapeutic potential for OXR antagonists in the treatment of drug dependence and the alleviation of withdrawal symptoms. Nevertheless, the development of OXR antagonists with better pharmacological properties (for example better solubility, faster distribution, higher functional power, and more bioavailability) can improve their clinical application.
Acknowledgments
The authors express their gratitude to Maryam Saeedi-Mofrad and the Research Vice-Chancellor of Kashan University of Medical Sciences.
Citation: Esmaili-Shahzade-Ali-Akbari P, Ghaderi A, Sadeghi A, Nejat F, Mehramiz A. The role of orexin receptor antagonists in inhibiting drug addiction: a review article. Addict Health. 2024;16(2):130–139. doi:10.34172/ahj.2024.1491
Footnotes
Authors’ Contribution
Conceptualization: Peyman Esmaili-Shahzade-Ali-Akbari, Amir Ghaderi.
Data curation: Amir Ghaderi.
Funding acquisition: Amir Ghaderi.
Investigation: Peyman Esmaili-Shahzade-Ali-Akbari, Amir Ghaderi.
Methodology: Peyman Esmaili-Shahzade-Ali-Akbari.
Project administration: Peyman Esmaili-Shahzade-Ali-Akbari, Amir Ghaderi.
Resources: Amir Ghaderi.
Software: Peyman Esmaili-Shahzade-Ali-Akbari.
Supervision: Peyman Esmaili-Shahzade-Ali-Akbari, Amir Ghaderi.
Validation: Peyman Esmaili-Shahzade-Ali-Akbari.
Visualization: Peyman Esmaili-Shahzade-Ali-Akbari.
Writing–original draft: Peyman Esmaili-Shahzade-Ali-Akbari, Amir Ghaderi, Atena Sadeghi, Alireza Mehramiz, Fatemeh Nejat.
Writing–review & editing: Peyman Esmaili-Shahzade-Ali-Akbari.
Competing Interests
The authors declare none.
Data Availability Statement
Data are contained within the article and are available from the corresponding authors upon reasonable request.
Ethical Approval
Not applicable.
Funding
This research received no external funding.
References
- 1.Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3(8):760–73. doi: 10.1016/s2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey CP, Connor M. Opioids: cellular mechanisms of tolerance and physical dependence. Curr Opin Pharmacol. 2005;5(1):60–8. doi: 10.1016/j.coph.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Williams JT, Ingram SL, Henderson G, Chavkin C, von Zastrow M, Schulz S, et al. Regulation of μ-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev. 2013;65(1):223–54. doi: 10.1124/pr.112.005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simonovska N, Chibishev A, Babulovska A, Pereska Z, Jurukov I, Glasnovic M. Program of the university clinic of toxicology, skopje, republic of macedonia in treatment of drug addiction (buprenorfin treatment protocol) Mater Sociomed. 2011;23(4):232–4. doi: 10.5455/msm.2011.23.232-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delargy I, Crowley D, Van Hout MC. Twenty years of the methadone treatment protocol in Ireland: reflections on the role of general practice. Harm Reduct J. 2019;16(1):5. doi: 10.1186/s12954-018-0272-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Judd LL, Marston MG, Attkisson C, Berrettini W, Buc NL, Bunney BS, et al. Effective medical treatment of opiate addiction. JAMA. 1998;280(22):1936–43. [PubMed] [Google Scholar]
- 7.Dubey AK, Handu SS, Mediratta PK. Suvorexant: The first orexin receptor antagonist to treat insomnia. J Pharmacol Pharmacother. 2015;6(2):118–21. doi: 10.4103/0976-500x.155496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barson JR, Leibowitz SF. Hypothalamic neuropeptide signaling in alcohol addiction. Prog Neuropsychopharmacol Biol Psychiatry. 2016;65:321–9. doi: 10.1016/j.pnpbp.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95(1):322–7. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebrahim IO, Howard RS, Kopelman MD, Sharief MK, Williams AJ. The hypocretin/orexin system. J R Soc Med. 2002;95(5):227–30. doi: 10.1177/014107680209500503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Razavi BM, Hosseinzadeh H. A review of the role of orexin system in pain modulation. Biomed Pharmacother. 2017;90:187–93. doi: 10.1016/j.biopha.2017.03.053. [DOI] [PubMed] [Google Scholar]
- 12.Mahler SV, Moorman DE, Smith RJ, James MH, Aston-Jones G. Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat Neurosci. 2014;17(10):1298–303. doi: 10.1038/nn.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.James MH, Mahler SV, Moorman DE, Aston-Jones G. A decade of orexin/hypocretin and addiction: where are we now? Curr Top Behav Neurosci. 2017;33:247–81. doi: 10.1007/7854_2016_57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49(4):589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437(7058):556–9. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 16.Harris GC, Wimmer M, Randall-Thompson JF, Aston-Jones G. Lateral hypothalamic orexin neurons are critically involved in learning to associate an environment with morphine reward. Behav Brain Res. 2007;183(1):43–51. doi: 10.1016/j.bbr.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonifazi A, Del Bello F, Giorgioni G, Piergentili A, Saab E, Botticelli L, et al. Targeting orexin receptors: recent advances in the development of subtype selective or dual ligands for the treatment of neuropsychiatric disorders. Med Res Rev. 2023;43(5):1607–67. doi: 10.1002/med.21959. [DOI] [PubMed] [Google Scholar]
- 18.Cao M, Guilleminault C. Hypocretin and its emerging role as a target for treatment of sleep disorders. Curr Neurol Neurosci Rep. 2011;11(2):227–34. doi: 10.1007/s11910-010-0172-9. [DOI] [PubMed] [Google Scholar]
- 19.Scammell TE, Winrow CJ. Orexin receptors: pharmacology and therapeutic opportunities. Annu Rev Pharmacol Toxicol. 2011;51:243–66. doi: 10.1146/annurev-pharmtox-010510-100528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adeghate E. Orexins: tissue localization, functions, and its relation to insulin secretion and diabetes mellitus. Vitam Horm. 2012;89:111–33. doi: 10.1016/b978-0-12-394623-2.00007-x. [DOI] [PubMed] [Google Scholar]
- 21.Adam JA, Menheere PP, van Dielen FM, Soeters PB, Buurman WA, Greve JW. Decreased plasma orexin-A levels in obese individuals. Int J Obes Relat Metab Disord. 2002;26(2):274–6. doi: 10.1038/sj.ijo.0801868. [DOI] [PubMed] [Google Scholar]
- 22.Adamantidis A, de Lecea L. Sleep and metabolism: shared circuits, new connections. Trends Endocrinol Metab. 2008;19(10):362–70. doi: 10.1016/j.tem.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Mieda M, Sakurai T. Overview of orexin/hypocretin system. Prog Brain Res. 2012;198:5–14. doi: 10.1016/b978-0-444-59489-1.00002-1. [DOI] [PubMed] [Google Scholar]
- 24.Horvath TL, Peyron C, Diano S, Ivanov A, Aston-Jones G, Kilduff TS, et al. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol. 1999;415(2):145–59. [PubMed] [Google Scholar]
- 25.van den Pol AN, Ghosh PK, Liu RJ, Li Y, Aghajanian GK, Gao XB. Hypocretin (orexin) enhances neuron activity and cell synchrony in developing mouse GFP-expressing locus coeruleus. J Physiol. 2002;541(Pt 1):169–85. doi: 10.1113/jphysiol.2002.017426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, et al. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A. 1999;96(19):10911–6. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rivas M, Ferreira A, Torterolo P, Benedetto L. Hypocretins, sleep, and maternal behavior. Front Behav Neurosci. 2023;17:1184885. doi: 10.3389/fnbeh.2023.1184885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang C, Wang Q, Ji B, Pan Y, Xu C, Cheng B, et al. The orexin/receptor system: molecular mechanism and therapeutic potential for neurological diseases. Front Mol Neurosci. 2018;11:220. doi: 10.3389/fnmol.2018.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo Y, Wang HL, Xiang XH, Zhao Y. The role of glutamate and its receptors in mesocorticolimbic dopaminergic regions in opioid addiction. Neurosci Biobehav Rev. 2009;33(6):864–73. doi: 10.1016/j.neubiorev.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75(1):218–65. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2003;168(1-2):57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159(10):1642–52. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev. 1997;25(2):192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- 34.Goldman-Rakic PS. The physiological approach: functional architecture of working memory and disordered cognition in schizophrenia. Biol Psychiatry. 1999;46(5):650–61. doi: 10.1016/s0006-3223(99)00130-4. [DOI] [PubMed] [Google Scholar]
- 35.Bouyer JJ, Park DH, Joh TH, Pickel VM. Chemical and structural analysis of the relation between cortical inputs and tyrosine hydroxylase-containing terminals in rat neostriatum. Brain Res. 1984;302(2):267–75. doi: 10.1016/0006-8993(84)90239-7. [DOI] [PubMed] [Google Scholar]
- 36.Xi ZX, Stein EA. Blockade of ionotropic glutamatergic transmission in the ventral tegmental area reduces heroin reinforcement in rat. Psychopharmacology (Berl) 2002;164(2):144–50. doi: 10.1007/s00213-002-1190-3. [DOI] [PubMed] [Google Scholar]
- 37.Acquas E, Di Chiara G. Depression of mesolimbic dopamine transmission and sensitization to morphine during opiate abstinence. J Neurochem. 1992;58(5):1620–5. doi: 10.1111/j.1471-4159.1992.tb10033.x. [DOI] [PubMed] [Google Scholar]
- 38.Alam MN, Kumar S, Bashir T, Suntsova N, Methippara MM, Szymusiak R, et al. GABA-mediated control of hypocretin- but not melanin-concentrating hormone-immunoreactive neurones during sleep in rats. J Physiol. 2005;563(Pt 2):569–82. doi: 10.1113/jphysiol.2004.076927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, et al. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci. 2003;23(8):3106–11. doi: 10.1523/jneurosci.23-08-03106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeoh JW, James MH, Jobling P, Bains JS, Graham BA, Dayas CV. Cocaine potentiates excitatory drive in the perifornical/lateral hypothalamus. J Physiol. 2012;590(16):3677–89. doi: 10.1113/jphysiol.2012.230268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rao Y, Mineur YS, Gan G, Wang AH, Liu ZW, Wu X, et al. Repeated in vivo exposure of cocaine induces long-lasting synaptic plasticity in hypocretin/orexin-producing neurons in the lateral hypothalamus in mice. J Physiol. 2013;591(7):1951–66. doi: 10.1113/jphysiol.2012.246983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23(1):7–11. doi: 10.1523/jneurosci.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vittoz NM, Berridge CW. Hypocretin/orexin selectively increases dopamine efflux within the prefrontal cortex: involvement of the ventral tegmental area. Neuropsychopharmacology. 2006;31(2):384–95. doi: 10.1038/sj.npp.1300807. [DOI] [PubMed] [Google Scholar]
- 44.Ahmadi-Soleimani SM, Azizi H, Gompf HS, Semnanian S. Role of orexin type-1 receptors in paragiganto-coerulear modulation of opioid withdrawal and tolerance: a site specific focus. Neuropharmacology. 2017;126:25–37. doi: 10.1016/j.neuropharm.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 45.Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake M, et al. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26(2):398–405. doi: 10.1523/jneurosci.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baimel C, Borgland SL. Hypocretin modulation of drug-induced synaptic plasticity. Prog Brain Res. 2012;198:123–31. doi: 10.1016/b978-0-444-59489-1.00008-2. [DOI] [PubMed] [Google Scholar]
- 47.Zarrabian S, Riahi E, Karimi S, Razavi Y, Haghparast A. The potential role of the orexin reward system in future treatments for opioid drug abuse. Brain Res. 2020;1731:146028. doi: 10.1016/j.brainres.2018.11.023. [DOI] [PubMed] [Google Scholar]
- 48.Schmeichel BE, Herman MA, Roberto M, Koob GF. Hypocretin neurotransmission within the central amygdala mediates escalated cocaine self-administration and stress-induced reinstatement in rats. Biol Psychiatry. 2017;81(7):606–15. doi: 10.1016/j.biopsych.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harvey JA. Cocaine effects on the developing brain: current status. Neurosci Biobehav Rev. 2004;27(8):751–64. doi: 10.1016/j.neubiorev.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 50.James MH, Stopper CM, Zimmer BA, Koll NE, Bowrey HE, Aston-Jones G. Increased number and activity of a lateral subpopulation of hypothalamic orexin/hypocretin neurons underlies the expression of an addicted state in rats. Biol Psychiatry. 2019;85(11):925–35. doi: 10.1016/j.biopsych.2018.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.España RA, Oleson EB, Locke JL, Brookshire BR, Roberts DC, Jones SR. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci. 2010;31(2):336–48. doi: 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, et al. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29(36):11215–25. doi: 10.1523/jneurosci.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gozzi A, Turrini G, Piccoli L, Massagrande M, Amantini D, Antolini M, et al. Functional magnetic resonance imaging reveals different neural substrates for the effects of orexin-1 and orexin-2 receptor antagonists. PLoS One. 2011;6(1):e16406. doi: 10.1371/journal.pone.0016406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hutcheson DM, Quarta D, Halbout B, Rigal A, Valerio E, Heidbreder C. Orexin-1 receptor antagonist SB-334867 reduces the acquisition and expression of cocaine-conditioned reinforcement and the expression of amphetamine-conditioned reward. Behav Pharmacol. 2011;22(2):173–81. doi: 10.1097/FBP.0b013e328343d761. [DOI] [PubMed] [Google Scholar]
- 55.Prince CD, Rau AR, Yorgason JT, España RA. Hypocretin/orexin regulation of dopamine signaling and cocaine self-administration is mediated predominantly by hypocretin receptor 1. ACS Chem Neurosci. 2015;6(1):138–46. doi: 10.1021/cn500246j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gentile TA, Simmons SJ, Barker DJ, Shaw JK, España RA, Muschamp JW. Suvorexant, an orexin/hypocretin receptor antagonist, attenuates motivational and hedonic properties of cocaine. Addict Biol. 2018;23(1):247–55. doi: 10.1111/adb.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muschamp JW, Hollander JA, Thompson JL, Voren G, Hassinger LC, Onvani S, et al. Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc Natl Acad Sci U S A. 2014;111(16):E1648–55. doi: 10.1073/pnas.1315542111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Foltin RW, Evans SM. Hypocretin/orexin antagonists decrease cocaine self-administration by female rhesus monkeys. Drug Alcohol Depend. 2018;188:318–27. doi: 10.1016/j.drugalcdep.2018.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111(2):379–87. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- 60.Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111(2):379–87. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- 61.McPherson CS, Featherby T, Krstew E, Lawrence AJ. Quantification of phosphorylated cAMP-response element-binding protein expression throughout the brain of amphetamine-sensitized rats: activation of hypothalamic orexin A-containing neurons. J Pharmacol Exp Ther. 2007;323(3):805–12. doi: 10.1124/jpet.107.125732. [DOI] [PubMed] [Google Scholar]
- 62.Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, et al. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21(5):1656–62. doi: 10.1523/jneurosci.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Quarta D, Valerio E, Hutcheson DM, Hedou G, Heidbreder C. The orexin-1 receptor antagonist SB-334867 reduces amphetamine-evoked dopamine outflow in the shell of the nucleus accumbens and decreases the expression of amphetamine sensitization. Neurochem Int. 2010;56(1):11–5. doi: 10.1016/j.neuint.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 64.Quarta D, Valerio E, Hutcheson DM, Hedou G, Heidbreder C. The orexin-1 receptor antagonist SB-334867 reduces amphetamine-evoked dopamine outflow in the shell of the nucleus accumbens and decreases the expression of amphetamine sensitization. Neurochem Int. 2010;56(1):11–5. doi: 10.1016/j.neuint.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 65.Tung LW, Lu GL, Lee YH, Yu L, Lee HJ, Leishman E, et al. Orexins contribute to restraint stress-induced cocaine relapse by endocannabinoid-mediated disinhibition of dopaminergic neurons. Nat Commun. 2016;7:12199. doi: 10.1038/ncomms12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bentzley BS, Aston-Jones G. Orexin-1 receptor signaling increases motivation for cocaine-associated cues. Eur J Neurosci. 2015;41(9):1149–56. doi: 10.1111/ejn.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gentile TA, Simmons SJ, Watson MN, Connelly KL, Brailoiu E, Zhang Y, et al. Effects of suvorexant, a dual orexin/hypocretin receptor antagonist, on impulsive behavior associated with cocaine. Neuropsychopharmacology. 2018;43(5):1001–9. doi: 10.1038/npp.2017.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Levy KA, Brodnik ZD, Shaw JK, Perrey DA, Zhang Y, España RA. Hypocretin receptor 1 blockade produces bimodal modulation of cocaine-associated mesolimbic dopamine signaling. Psychopharmacology (Berl) 2017;234(18):2761–76. doi: 10.1007/s00213-017-4673-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Flores Á, Maldonado R, Berrendero F. The hypocretin/orexin receptor-1 as a novel target to modulate cannabinoid reward. Biol Psychiatry. 2014;75(6):499–507. doi: 10.1016/j.biopsych.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 70.Khoo SY, McNally GP, Clemens KJ. The dual orexin receptor antagonist TCS1102 does not affect reinstatement of nicotine-seeking. PLoS One. 2017;12(3):e0173967. doi: 10.1371/journal.pone.0173967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Plaza-Zabala A, Flores Á, Maldonado R, Berrendero F. Hypocretin/orexin signaling in the hypothalamic paraventricular nucleus is essential for the expression of nicotine withdrawal. Biol Psychiatry. 2012;71(3):214–23. doi: 10.1016/j.biopsych.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 72.Brown RM, Lawrence AJ. Ascending orexinergic pathways and alcohol-seeking. Curr Opin Neurobiol. 2013;23(4):467–72. doi: 10.1016/j.conb.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 73.Lawrence AJ. Regulation of alcohol-seeking by orexin (hypocretin) neurons. Brain Res. 2010;1314:124–9. doi: 10.1016/j.brainres.2009.07.072. [DOI] [PubMed] [Google Scholar]
- 74.Mahler SV, Smith RJ, Moorman DE, Sartor GC, Aston-Jones G. Multiple roles for orexin/hypocretin in addiction. Prog Brain Res. 2012;198:79–121. doi: 10.1016/b978-0-444-59489-1.00007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baimel C, Bartlett SE, Chiou LC, Lawrence AJ, Muschamp JW, Patkar O, et al. Orexin/hypocretin role in reward: implications for opioid and other addictions. Br J Pharmacol. 2015;172(2):334–48. doi: 10.1111/bph.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Olney JJ, Navarro M, Thiele TE. Binge-like consumption of ethanol and other salient reinforcers is blocked by orexin-1 receptor inhibition and leads to a reduction of hypothalamic orexin immunoreactivity. Alcohol Clin Exp Res. 2015;39(1):21–9. doi: 10.1111/acer.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Morganstern I, Chang GQ, Barson JR, Ye Z, Karatayev O, Leibowitz SF. Differential effects of acute and chronic ethanol exposure on orexin expression in the perifornical lateral hypothalamus. Alcohol Clin Exp Res. 2010;34(5):886–96. doi: 10.1111/j.1530-0277.2010.01161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148(6):752–9. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Srinivasan S, Simms JA, Nielsen CK, Lieske SP, Bito-Onon JJ, Yi H, et al. The dual orexin/hypocretin receptor antagonist, almorexant, in the ventral tegmental area attenuates ethanol self-administration. PLoS One. 2012;7(9):e44726. doi: 10.1371/journal.pone.0044726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mayannavar S, Rashmi KS, Rao YD, Yadav S, Ganaraja B. Effect of Orexin A antagonist (SB-334867) infusion into the nucleus accumbens on consummatory behavior and alcohol preference in Wistar rats. Indian J Pharmacol. 2016;48(1):53–8. doi: 10.4103/0253-7613.174528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barson JR, Ho HT, Leibowitz SF. Anterior thalamic paraventricular nucleus is involved in intermittent access ethanol drinking: role of orexin receptor 2. Addict Biol. 2015;20(3):469–81. doi: 10.1111/adb.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moorman DE, James MH, Kilroy EA, Aston-Jones G. Orexin/hypocretin-1 receptor antagonism reduces ethanol self-administration and reinstatement selectively in highly-motivated rats. Brain Res. 2017;1654(Pt A):34–42. doi: 10.1016/j.brainres.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dhaher R, Hauser SR, Getachew B, Bell RL, McBride WJ, McKinzie DL, et al. The orexin-1 receptor antagonist SB-334867 reduces alcohol relapse drinking, but not alcohol-seeking, in alcohol-preferring (P) rats. J Addict Med. 2010;4(3):153–9. doi: 10.1097/ADM.0b013e3181bd893f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lopez MF, Moorman DE, Aston-Jones G, Becker HC. The highly selective orexin/hypocretin 1 receptor antagonist GSK1059865 potently reduces ethanol drinking in ethanol dependent mice. Brain Res. 2016;1636:74–80. doi: 10.1016/j.brainres.2016.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sanchez-Alavez M, Benedict J, Wills DN, Ehlers CL. Effect of suvorexant on event-related oscillations and EEG sleep in rats exposed to chronic intermittent ethanol vapor and protracted withdrawal. Sleep. 2019;42(4):zsz020. doi: 10.1093/sleep/zsz020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hoch M, Hay JL, Hoever P, de Kam ML, te Beek ET, van Gerven JM, et al. Dual orexin receptor antagonism by almorexant does not potentiate impairing effects of alcohol in humans. Eur Neuropsychopharmacol. 2013;23(2):107–17. doi: 10.1016/j.euroneuro.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 87.Steiner MA, Lecourt H, Strasser DS, Brisbare-Roch C, Jenck F. Differential effects of the dual orexin receptor antagonist almorexant and the GABA(A)-α1 receptor modulator zolpidem, alone or combined with ethanol, on motor performance in the rat. Neuropsychopharmacology. 2011;36(4):848–56. doi: 10.1038/npp.2010.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Calipari ES, España RA. Hypocretin/orexin regulation of dopamine signaling: implications for reward and reinforcement mechanisms. Front Behav Neurosci. 2012;6:54. doi: 10.3389/fnbeh.2012.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Farahimanesh S, Zarrabian S, Haghparast A. Role of orexin receptors in the ventral tegmental area on acquisition and expression of morphine-induced conditioned place preference in the rats. Neuropeptides. 2017;66:45–51. doi: 10.1016/j.npep.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 90.Hooshmandi M, Hosseinmardi N, Janahmadi M, Khakpai F, Rohampour K, Doostmohammadi J. Antagonism of orexin type-1 receptors (OX1Rs) attenuates naloxone-precipitated morphine withdrawal syndrome in rat dorsal hippocampus. Pharmacol Biochem Behav. 2017;158:39–48. doi: 10.1016/j.pbb.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 91.Sharf R, Sarhan M, Dileone RJ. Orexin mediates the expression of precipitated morphine withdrawal and concurrent activation of the nucleus accumbens shell. Biol Psychiatry. 2008;64(3):175–83. doi: 10.1016/j.biopsych.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Davoudi M, Azizi H, Mirnajafi-Zadeh J, Semnanian S. The blockade of GABAA receptors attenuates the inhibitory effect of orexin type 1 receptors antagonist on morphine withdrawal syndrome in rats. Neurosci Lett. 2016;617:201–6. doi: 10.1016/j.neulet.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 93.Aghajani N, Pourhamzeh M, Azizi H, Semnanian S. Central blockade of orexin type 1 receptors reduces naloxone induced activation of locus coeruleus neurons in morphine dependent rats. Neurosci Lett. 2021;755:135909. doi: 10.1016/j.neulet.2021.135909. [DOI] [PubMed] [Google Scholar]
- 94.Fakhari M, Azizi H, Semnanian S. Central antagonism of orexin type-1 receptors attenuates the development of morphine dependence in rat locus coeruleus neurons. Neuroscience. 2017;363:1–10. doi: 10.1016/j.neuroscience.2017.08.054. [DOI] [PubMed] [Google Scholar]
- 95.Mousavi Y, Azizi H, Mirnajafi-Zadeh J, Javan M, Semnanian S. Blockade of orexin type-1 receptors in locus coeruleus nucleus attenuates the development of morphine dependency in rats. Neurosci Lett. 2014;578:90–4. doi: 10.1016/j.neulet.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 96.Boutrel B, Steiner N, Halfon O. The hypocretins and the reward function: what have we learned so far? Front Behav Neurosci. 2013;7:59. doi: 10.3389/fnbeh.2013.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schmeichel BE, Barbier E, Misra KK, Contet C, Schlosburg JE, Grigoriadis D, et al. Hypocretin receptor 2 antagonism dose-dependently reduces escalated heroin self-administration in rats. Neuropsychopharmacology. 2015;40(5):1123–9. doi: 10.1038/npp.2014.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Esmaili-Shahzade-Ali-Akbari P, Hosseinzadeh H, Mehri S. Effect of suvorexant on morphine tolerance and dependence in mice: role of NMDA, AMPA, ERK and CREB proteins. Neurotoxicology. 2021;84:64–72. doi: 10.1016/j.neuro.2021.02.005. [DOI] [PubMed] [Google Scholar]
- 99.Huhn AS, Finan PH, Gamaldo CE, Hammond AS, Umbricht A, Bergeria CL, et al. Suvorexant ameliorated sleep disturbance, opioid withdrawal, and craving during a buprenorphine taper. Sci Transl Med. 2022;14(650):eabn8238. doi: 10.1126/scitranslmed.abn8238. [DOI] [PubMed] [Google Scholar]
- 100.Łupina M, Tarnowski M, Baranowska-Bosiacka I, Talarek S, Listos P, Kotlińska J, et al. SB-334867 (an orexin-1 receptor antagonist) effects on morphine-induced sensitization in mice-a view on receptor mechanisms. Mol Neurobiol. 2018;55(11):8473–85. doi: 10.1007/s12035-018-0993-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hooshmand B, Azizi H, Javan M, Semnanian S. Intra-LC microinjection of orexin type-1 receptor antagonist SB-334867 attenuates the expression of glutamate-induced opiate withdrawal like signs during the active phase in rats. Neurosci Lett. 2017;636:276–81. doi: 10.1016/j.neulet.2016.10.051. [DOI] [PubMed] [Google Scholar]
- 102.Ebrahimian F, Naghavi FS, Yazdi F, Sadeghzadeh F, Taslimi Z, Haghparast A. Differential roles of orexin receptors within the dentate gyrus in stress- and drug priming-induced reinstatement of conditioned place preference in rats. Behav Neurosci. 2016;130(1):91–102. doi: 10.1037/bne0000112. [DOI] [PubMed] [Google Scholar]
- 103.Porter-Stransky KA, Bentzley BS, Aston-Jones G. Individual differences in orexin-I receptor modulation of motivation for the opioid remifentanil. Addict Biol. 2017;22(2):303–17. doi: 10.1111/adb.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Berhow MT, Hiroi N, Nestler EJ. Regulation of ERK (extracellular signal regulated kinase), part of the neurotrophin signal transduction cascade, in the rat mesolimbic dopamine system by chronic exposure to morphine or cocaine. J Neurosci. 1996;16(15):4707–15. doi: 10.1523/jneurosci.16-15-04707.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci. 2000;20(23):8701–9. doi: 10.1523/jneurosci.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, et al. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci U S A. 2005;102(2):491–6. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shin HS, Cho HS, Sung KW, Yoon BJ. Orexin-A increases cell surface expression of AMPA receptors in the striatum. Biochem Biophys Res Commun. 2009;378(3):409–13. doi: 10.1016/j.bbrc.2008.11.051. [DOI] [PubMed] [Google Scholar]
- 108.Guo Y, Feng P. OX2R activation induces PKC-mediated ERK and CREB phosphorylation. Exp Cell Res. 2012;318(16):2004–13. doi: 10.1016/j.yexcr.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Carlezon WA Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28(8):436–45. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 110.Nestler EJ, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997;278(5335):58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- 111.Riahi E, Khodagholi F, Haghparast A. Role of dorsal hippocampal orexin-1 receptors in associating morphine reward with contextual stimuli. Behav Pharmacol. 2013;24(4):237–48. doi: 10.1097/FBP.0b013e3283635ee9. [DOI] [PubMed] [Google Scholar]