Abstract
Introduction
To assess differences in the diagnosis journey and access to care in a large sample of patients with axial spondyloarthritis (axSpA) from around the world, included in the International Map of Axial Spondyloarthritis (IMAS).
Methods
IMAS was a cross-sectional online survey (2017–2022) of 5557 unselected patients with axSpA from 27 countries. Across five worldwide geographic regions, the patient journey until diagnosis and healthcare utilization in the last 12 months prior to survey were evaluated. Univariable and multivariable linear regression was used to analyze factors associated with higher healthcare utilization.
Results
Of 5557 participants in IMAS, the diagnosis took an average of 7.4 years, requiring more than two visits to HCPs (77.7% general practitioner and 51.3% rheumatologist), and more than two diagnostic tests [67.5% performed human leukocyte antigen B27 (HLA-B27), 64.2% x-ray, and 59.1% magnetic resonance imaging (MRI) scans]. North America and Europe were the regions with the highest number of healthcare professional (HCP) visits for diagnosis, while the lowest number of visits was in the Asian region. In the previous 12 months, 94.9% (n = 5272) used at least one healthcare resource, with an average of 29 uses per year. The regions with the highest healthcare utilization were Latin America, Europe, and North America. In the multiple linear regression, factors associated with higher number of healthcare utilization were younger age (b = – 0.311), female gender (b = 7.736), higher disease activity (b = 1.461), poorer mental health (b = 0.624), greater functional limitation (b = 0.300), greater spinal stiffness (b = 1.527), and longer diagnostic delay (b = 0.104).
Conclusion
The diagnosis of axSpA usually takes more than two visits to HCPs and at least 7 years. After diagnosis, axSpA is associated with frequent healthcare resource use. Younger age, female gender, higher disease activity, poorer mental health, greater functional limitation, greater spinal stiffness, and longer diagnostic delay are associated with higher healthcare utilization. Europe and North America use more HCP visits and diagnostic tests before and after diagnosis than the other regions.
Keywords: Axial spondyloarthritis, Diagnosis, Healthcare, Access to care, Worldwide
Key Summary Points
| Why carry out this study? |
| The diagnosis of axial spondyloarthritis (axSpA) is a challenge for clinical specialists, as there is no single test to diagnose it. |
| Patients with axSpA need to visit several specialists and take multiple and diverse tests to confirm their diagnosis. |
| The present study aims to evaluate regional differences in patient journey to diagnosis and to assess whether higher healthcare utilization after diagnosis is associated with worse disease outcomes. |
| What was learned from this study? |
| North America and Europe were the regions with the highest number of healthcare professional (HCP) visits for diagnosis, while the lowest number of visits was in the Asian region. |
| Higher healthcare utilization in diagnosed patients is associated with worse disease outcomes, including younger age, female gender, higher disease activity, poorer mental health, greater functional limitation, greater spinal stiffness, and longer diagnostic delay. |
| The study has shown the long patient journey to diagnosis (7.4 years on average), requiring more than two visits to specialists and different tests. In addition, patients who require a greater number of healthcare utilization after diagnosis present worse disease outcomes. |
Introduction
Diagnosing axial spondyloarthritis (axSpA) can be challenging, requiring first disease suspicion and referral to rheumatologists, and later an accurate evaluation of the patient’s symptoms and signs, as well as laboratory and imaging tests [1], often resulting in a long period [2].
Currently, there is no gold standard test to diagnose axSpA. The most common laboratory tests include blood tests to determine acute phase reactants or genetic testing for human leukocyte antigen B27 (HLA-B27), which is often associated with an increased risk of developing axSpA [3]. In addition, imaging tests, such as x-rays, computed tomography (CT), and especially magnetic resonance imaging (MRI) scans are key tools in the diagnosis of axSpA [4, 5]. Healthcare professionals (HCPs) routinely test their patients to identify the causes of their pain, inflammation, or stiffness. If HCPs are aware of the potential axSpA features, they should refer the patient to a rheumatologist [6].
Once axSpA has been diagnosed, patient follow-up care is essential to ensure adequate disease management and to optimize patient’s quality of life, especially due to the fact that axSpA usually appears before the age of 45 years and its a peak age of onset is between 20 and 30 years [7]. Therefore, patients should have regular visits with a rheumatologist to assess disease status and impact, and adjust the treatment plan as needed [8]. These assessments may require periodic laboratory and imaging tests—x-rays or MRIs—to monitor disease progression, including structural damage development in the sacroiliac joints and the spine [9]. Based on the clinical evaluation and test results, the rheumatologist can adjust the treatment plan, which may include changes in medication, physical therapy, or surgical interventions in severe cases [10–12].
Healthcare follow-up should be comprehensive and include a multidisciplinary approach. Besides the rheumatologist, other HCPs, such as nurses, physiotherapists, and occupational therapists, may be involved in managing symptoms and improving the patient’s quality of life [11, 13]. AxSpA disease can affect patients’ quality of life and emotional well-being and, therefore, psychological support, in the form of either individual or group therapy, can be beneficial in helping patients cope with the emotional challenges associated with the disease [14] or helping patients change behaviors associated with worse health outcomes. such as inactivity, smoking, excess alcohol use. or poor sleep.
The aim of this study is to assess regional differences in the patient’s journey to achieve axSpA diagnosis and in access to care in a large sample of patients worldwide included in the International Map of Axial Spondyloarthritis study (IMAS).
Methods
Survey Design and Development
IMAS is a research initiative collaborated by the Axial Spondyloarthritis International Federation (ASIF), the Health & Territory Research Group (HTR) of the University of Seville and Novartis Pharma, as well as a scientific committee composed of representatives of patients with axSpA, rheumatologists, psychologists, and health researchers. The design and dissemination of the survey were described in detail in the European [15] and international [16] seminal manuscripts.
The present manuscript does not contain any studies with animal subjects and IRB approval was not necessary. All participants were asked to provide explicit opt-in consent prior to participating in the IMAS survey. Furthermore, the participants’ data were anonymized and did not contain confidential, personal. or subject-identifying information. Ethical aspects related to data extracted from patients and their treatment were in accordance with the Declaration of Helsinki.
Sample Selection and Recruitment
The total sample of patients for each of the countries was recruited in two separate paths, carried out by Ipsos through patient panel and local patient organizations between 2017 and 2022 through an online survey, including unselected patients from 27 countries worldwide. Although we do not know the exact proportion of patients who dropped out of the survey, the main reason may have been the length of the questionnaire, although this is a hypothesis to be verified. For Spain, where the first questionnaire was carried out, patients with a completion rate of more than 75% were selected; however, Ipsos did not provide this information for the other countries, although it is likely that, given that the initial study Atlas was carried out in Spain, the same pattern was followed in the other countries. However, the inclusion criteria were the same for all the countries: age ≥ 18 years, being resident in the specified country, and with a self-reported diagnosis of axSpA (either radiographic or non-radiographic (r-axSpA, nr-axSpA).
Furthermore, the patient questionnaire was translated and adapted to 27 countries, considering aspects such as currency to calculate income or the local labor market and job categories to determine the employment status. The sample size for each of the 27 IMAS countries was: Spain (680), France (638), Canada (542), Norway (509), United Kingdom (374), Turkey (289), Russia (233), India (232), US (228), Colombia (164), Brazil (159), South Africa (146), Italy (134), South Korea (128), Philippines (128), Argentina (115), Taiwan (112), Netherlands (107), Slovenia (83), Austria (82), Switzerland (80), Germany (78), Belgium (76), Sweden (71), Mexico (60), Lithuania (59), and Costa Rica (50).
Variables
The description of variable, question and categories or measurement units included in the present analysis are shown in Table 1.
Table 1.
Variables, questions, and categories/measurement units included in this analysis
| Variables | Questions | Categories/measures |
|---|---|---|
| Sociodemographic | ||
| Age | Please specify your age | In years |
| Gender | Please specify your gender | Male, female |
| Patient association membership | Are you a member of any support group or association for spondylitis/spondyloarthritis? | Yes, no |
| Patient journey | ||
| Diagnostic delay | Calculated based on the age at onset of first symptoms | In years |
| HCP seen before diagnosis | Which of the following health professionals did you see for your spondylitis/ spondyloarthritis before it was diagnosed? | General practitioner / rheumatologist / orthopaedic specialist / physiotherapist / osteopath /chiropractor / other |
| HCP making the diagnosis | Which medical professional made the diagnosis of spondylitis/spondyloarthritis? | General practitioner / rheumatologist / orthopaedic specialist / physiotherapist / other |
| First test for diagnosis | What were the tests done to diagnose your spondylitis/spondyloarthritis? | MRI scan / x-rays / HLA-B27 / ultrasound / radionuclide scintigraphy / CT scan / other |
| Healthcare utilization in the last 12 months prior to survey | ||
| Healthcare visits | Please indicate the number of spondylitis/spondyloarthritis-related medical appointments you have had in the past 12 months | Rheumatologist / general practitioner / nurse / orthopaedic specialist / physiotherapist / ophthalmologist or optician / pulmonologist / cardiologist / psychologist or psychiatrist / gastroenterologist / chiropractor |
| Medical test | Please indicate the number of spondylitis/spondyloarthritis-related medical tests you have had in the past 12 months | X-rays / MRI scan / Ultrasound scan /radionuclide scintigraphy / CT scan /blood test / urine test |
| No. of hospital admissions | How many times have you been admitted to hospital in the past 12 months due to your spondylitis/spondyloarthritis? | Numeric |
| No. of emergency visits | How many times have you used an emergency service in the past 12 months due to your spondylitis/spondyloarthritis? | Hospital / outpatient center / home emergency / ambulance |
HCP healthcare professional, MRI magnetic resonance imaging, HLA-B27 human leukocyte antigen (HLA) B27, CT computed tomography
Patient-reported outcomes were collected from the following scales:
Bath Ankylosing Spondylitis Disease Activity Index (BASDAI): a self-administered questionnaire to assess disease activity in patients with axSpA. It includes six questions (0–10 numeric rating scale) related to the following symptoms: fatigue; back pain; inflammation/pain in joints except neck, back and hips; localized tender areas (also called enthesitis or inflammation of tendons and ligaments); and the level and duration of stiffness in the morning. The BASDAI ranges from 0 to 10 and the cut-off of 4 (BASDAI ≥ 4) indicates active disease [17]. For the BASDAI scale, Ipsos requested permission for its application and used the validated adaptation for each country.
12-item General Health Questionnaire (GHQ-12): a screening measure of common mental health disorders in the general population, including symptoms of anxiety, depression, social dysfunction, and loss of confidence. The total GHQ-12 ranges from 0 to 12, with a cutoff score of 3 (GHQ-12 ≥ 3) indicating risk of poor mental health [18, 19]. For the GHQ-12 scale, Ipsos requested permission for its application and used the validated adaptation for each country.
Spinal Stiffness Index: an index developed by the University of Seville specifically for the IMAS survey to assess the degree of spinal stiffness experienced by patients in the spinal column (cervical, dorsal, and lumbar areas). For these three areas, the degree of stiffness was asked: no stiffness (scored as 1), mild (scored as 2), moderate (scored as 3), and severe (scored as 4). The index is obtained as the sum of the scores collected in the three areas, with a range between 3 and 12 points. Higher values of the index indicate greater spinal stiffness [20].
Functional Limitation Index: an index developed by the University of Seville specifically for the IMAS survey to assess the degree of limitation in 18 activities of daily life [20]. For these 18 areas, the functional limitation was asked: no restriction (scored as 0), low (scored as 1), medium (scored as 2), and high (scored as 4). The index is obtained as the sum of the scores obtained in the 18 areas, with a range between 0 and 54 points. Higher values of the index indicate greater functional limitation.
Total healthcare utilization was calculated as the sum of the number of healthcare visits, medical tests, hospital admissions, and emergency visits based on the last 12 months prior to the survey and due to axSpA disease (Fig. 1). The 70 (of 5272; 1.3%) patients, who reported having used healthcare services, had been diagnosed in the same year of the survey and, therefore, their healthcare utilization referred to a period of less than 12 months.
Fig. 1.
Flowchart of healthcare utilization during the last 12 months. MRI magnetic resonance imaging, CT computed tomography
Statistical Analysis
The results are presented as summary statistics, with mean and standard deviation (±SD) for continuous variables, and frequency and percentages for categorical variables. Kruskal–Wallis and Pearson’s correlation tests were used to analyze associations between the patient journey to diagnosis (time since symptoms onset, number and type of HCPs visited before diagnosis, HCPs making the diagnosis, and first test performed for diagnosis) and subsequent healthcare utilization (healthcare visits, tests performed, hospital admissions, and emergency visits, all in the last 12 months) with respect to five global regions (Europe, North America, Latin America, Asia, and South Africa).
Mann–Whitney, Kruskal–Wallis, and Pearson’s correlation (r) tests were used to analyze associations between sociodemographic characteristics (age, gender, educational level, marital status, and patient organization membership) and patient-reported outcomes [disease activity measured by BASDAI (0–10), mental health measured by General Health Questionnaire GHQ-12 (0–12), functional limitation index (0–54), spinal stiffness index (3–12), and diagnostic delay] with respect to total healthcare utilization. Univariable and multivariable linear regression was used to analyze associated factors with total healthcare utilization. The betas (B) and confidence intervals (CI) are shown at the 95% level (p). Data analysis was conducted using SPSS v26.0.
Results
Regional differences have been found with respect to the patient journey until diagnosis and subsequent healthcare utilization (Fig. 2).
Fig. 2.
Mean and standard deviation of number of HCPsa seen before diagnosis by region. aHealthcare professionals (HCPs) including general practitioner, rheumatologist, orthopaedic specialist, physiotherapist, osteopath, chiropractor, and other specialists
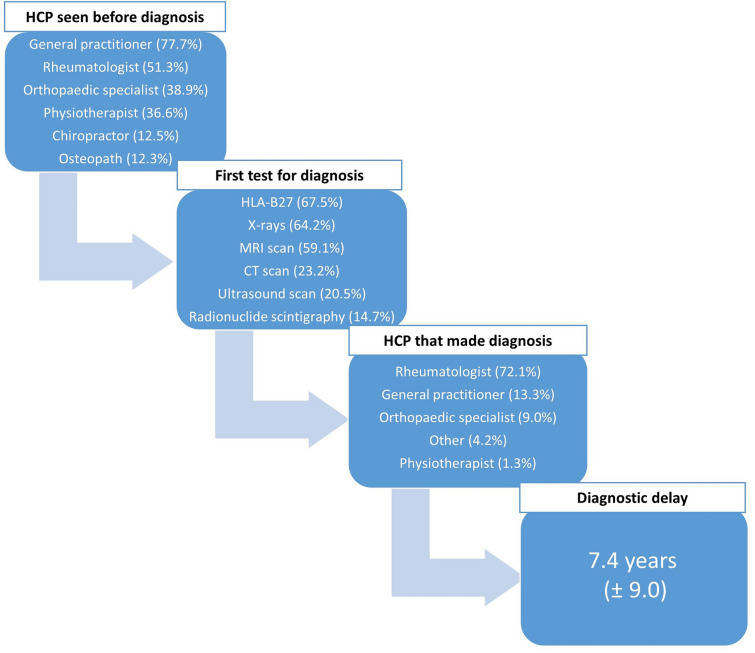
Mean diagnostic delay of patients with axSpA was longer than 7 years. Most of the patients had seen a general practitioner (GP) or rheumatologist prior to diagnosis (77.7% and 51.3%, respectively). The most common tests patients had undergone were HLA-B27, x-rays, and MRI scans (67.5%, 64.2%, and 59.1%, respectively). Rheumatologists diagnosed most of these patients (72.1%), followed by GPs (13.3%) and orthopaedic specialists (9.0%; Fig. 3).
Fig. 3.
The patient journey to axial spondyloarthritis diagnosis.aHealthcare professionals (HCPs) including general practitioner, rheumatologist, orthopaedic specialist, physiotherapist, osteopath, chiropractor, and other specialists. HLA-B27 human leukocyte antigen (HLA) B27, MRI magnetic resonance imaging, CT computed tomography
The longest diagnostic delay was found in South Africa (10.8 years), while the Asian region had the shortest diagnostic delay (4.2 years). More than 80% of patients in Europe, North America, and South Africa had visited the GP before diagnosis, while around 60% of patients in Latin America and Asia had visited an orthopaedic specialist before diagnosis. In Europe and North America, imaging diagnostic tests such as MRI scans and x-rays were more frequent, while, in Latin America, Asia, and South Africa, HLA-B27 testing was the most frequent. Overall, rheumatologists diagnosed most patients, although in Asia 33.9% were diagnosed by orthopaedic specialists (Table 2).
Table 2.
The patient journey to axial spondyloarthritis diagnosis stratified by region
| Mean ± SD or n (%) | p value | |||||
|---|---|---|---|---|---|---|
| Europe | North America | Latin America | Asia | South Africa | ||
| Diagnostic delay, n = 5327 | 7.7 ± 8.8 | 9.0 ± 11.0 | 5.9 ± 8.6 | 4.2 ± 5.4 | 10.8 ± 10.6 | < 0.001 |
| HCP seen before diagnosis, n = 5328 | ||||||
| GP | 2731 (82.5) | 650 (86.1) | 385 (70.5) | 258 (44.9) | 117 (83.0) | < 0.001 |
| Rheumatologist | 1959 (59.2) | 350 (46.4) | 213 (39.0) | 164 (28.5) | 45 (31.9) | |
| Orthopaedic specialist | 1143 (34.5) | 178 (23.6) | 332 (60.8) | 374 (65.0) | 48 (34.0) | |
| Physiotherapist | 1309 (39.5) | 350 (46.4) | 169 (31.0) | 104 (18.1) | 16 (11.3) | |
| Osteopath | 519 (15.7) | 74 (9.8) | 46 (8.4) | 11 (1.9) | 5 (3.5) | |
| Other | 441 (13.3) | 99 (13.1) | 66 (12.1) | 18 (3.1) | 18 (12.8) | |
| Chiropractor | 285 (8.6) | 274 (36.3) | 11 (2.0) | 53 (9.2) | 43 (30.5) | |
| HCP that made diagnosis, n = 5326 | ||||||
| Rheumatologist | 2499 (76.5) | 511 (66.4) | 407 (74.3) | 323 (53.9) | 102 (70.8) | < 0.001 |
| GP | 413 (12.6) | 151 (19.6) | 78 (14.2) | 48 (8.0) | 18 (12.5) | |
| Orthopaedic specialist | 187 (5.7) | 39 (5.1) | 42 (7.7) | 203 (33.9) | 11 (7.6) | |
| Other | 130 (4.0) | 49 (6.4) | 18 (3.3) | 14 (2.3) | 13 (9.0) | |
| Physiotherapist | 36 (1.1) | 20 (2.6) | 3 (0.5) | 11 (1.8) | 0 (0.0) | |
| First test for diagnosis, n = 4619 | ||||||
| X-ray | 2047 (69.5) | 470 (73.6) | 136 (33.3) | 267 (52.7) | 44 (36.7) | < 0.001 |
| HLA-B27 | 2000 (67.9) | 440 (68.9) | 273 (66.9) | 312 (61.5) | 95 (79.2) | |
| MRI scan | 1825 (62.0) | 430 (67.3) | 142 (34.8) | 295 (58.2) | 40 (33.3) | |
| CT scan | 603 (20.3) | 196 (30.7) | 105 (25.7) | 145 (28.6) | 21 (17.5) | |
| Ultrasound | 577 (19.6) | 173 (27.1) | 67 (16.4) | 114 (22.5) | 16 (13.3) | |
| Radionuclide scintigraphy | 430 (14.6) | 100 (15.6) | 78 (19.1) | 61 (12.0) | 10 (8.3) | |
| Other | 125 (4.2) | 61 (9.5) | 20 (4.9) | 11 (2.2) | 12 (10.0) | |
SD standard deviation, HCP healthcare professional, GP general practitioner, HLA-B27 human leukocyte antigen (HLA) B27, MRI magnetic resonance imaging, CT computed tomography
Overall, patients had used healthcare resources almost 30 times in a year, visiting the rheumatologist more than three times, with more than three blood and urine tests, and two x-rays, with an average of two hospitalizations, and visiting the outpatient center almost four times. Regarding the total number of healthcare visits, patients from Europe and Latin America reported above 30, while patients in the Asian region visited 20 times a year. Patients in all regions visited the physiotherapist most frequently, with Europe being the region with the highest number of visits of over 20 times. Except for South Africa, patients in all other regions had more than two hospitalizations on average in the last year. In addition, patients in Latin America were those who had the most frequent emergency visits to both the hospital and the outpatient center (Table 3).
Table 3.
Healthcare utilization in the previous year to the survey overall stratified by region
| Mean ± SD or n (%) | p-value | Total | |||||
|---|---|---|---|---|---|---|---|
| Europe | North America | Latin America | Asia | South Africa | |||
| Healthcare visits in the last 12 months | |||||||
| Physiotherapist, n = 1992 | 21.3 ± 25.3 | 9.0 ± 12.8 | 12.5 ± 16.6 | 9.8 ± 12.4 | 12.2 ± 15.8 | < 0.001 | 17.9 ± 23.0 |
| Chiropractor, n = 308 | 9.7 ± 18.5 | 10.2 ± 12.3 | 3.6 ± 4.3 | 4.6 ± 5.7 | 5.4 ± 4.9 | < 0.001 | 8.6 ± 13.4 |
| Psychologist/psychiatrist, n = 711 | 8.1 ± 14.1 | 5.7 ± 6.7 | 9.2 ± 12.1 | 2.5 ± 1.9 | 8.3 ± 9.4 | 0.008 | 7.7 ± 12.4 |
| GP, n = 3266 | 6.2 ± 8.7 | 4.2 ± 3.9 | 4.8 ± 5.0 | 3.3 ± 3.7 | 4.3 ± 4.5 | < 0.001 | 5.6 ± 7.7 |
| Nurse, n = 979 | 6.1 ± 10.3 | 2.9 ± 3.1 | 3.5 ± 2.8 | 4.3 ± 5.0 | 3.6 ± 2.5 | < 0.001 | 5.5 ± 9.3 |
| Rheumatologist, n = 4503 | 3.5 ± 3.3 | 3.0 ± 2.6 | 4.7 ± 3.4 | 4.2 ± 4.3 | 2.7 ± 1.6 | < 0.001 | 3.6 ± 3.3 |
| Orthopaedic specialist, n = 1024 | 2.7 ± 3.0 | 2.5 ± 2.2 | 3.4 ± 3.1 | 5.7 ± 6.4 | 2.6 ± 3.1 | < 0.001 | 3.3 ± 4.0 |
| Ophthalmologist/optician, n = 1517 | 2.4 ± 3.9 | 2.1 ± 1.5 | 2.1 ± 1.6 | 3.4 ± 4.3 | 1.4 ± 0.7 | < 0.001 | 2.3 ± 3.6 |
| Pulmonologist, n = 534 | 2.1 ± 2.3 | 2.0 ± 1.5 | 1.8 ± 1.2 | 3.4 ± 3.8 | 1.3 ± 0.5 | 0.017 | 2.1 ± 2.2 |
| Gastroenterologist, n = 800 | 2.0 ± 1.9 | 2.3 ± 1.7 | 2.3 ± 1.8 | 2.1 ± 1.4 | 1.6 ± 0.9 | 0.004 | 2.1 ± 1.8 |
| Cardiologist, n = 734 | 1.8 ± 1.5 | 2.2 ± 1.8 | 2.0 ± 1.5 | 2.5 ± 1.7 | 1.5 ± 0.9 | 0.001 | 1.9 ± 1.5 |
| Medical test in the last 12 months | |||||||
| Blood test, n = 4308 | 4.7 ± 4.0 | 4.0 ± 3.6 | 5.1 ± 4.6 | 3.7 ± 3.0 | 4.5 ± 3.4 | < 0.001 | 4.2 ± 3.9 |
| Urine test, n = 3174 | 3.2 ± 3.1 | 2.5 ± 2.6 | 3.8 ± 3.1 | 2.3 ± 2.3 | 1.9 ± 1.5 | < 0.001 | 3.0 ± 2.9 |
| X-rays, n = 3462 | 2.4 ± 2.7 | 2.3 ± 2.1 | 2.8 ± 2.6 | 2.3 ± 2.2 | 2.3 ± 1.8 | 0.005 | 2.4 ± 2.5 |
| MRI scan, n = 2845 | 1.7 ± 1.2 | 1.8 ± 1.8 | 2.1 ± 2.0 | 1.5 ± 1.1 | 1.6 ± 0.9 | < 0.001 | 1.7 ± 1.4 |
| Ultrasound scan, n = 2073 | 1.6 ± 1.3 | 2.1 ± 2.0 | 2.4 ± 2.3 | 1.3 ± 0.8 | 1.3 ± 0.7 | < 0.001 | 1.6 ± 1.5 |
| Radionuclide scintigraphy, n = 863 | 1.6 ± 1.7 | 2.2 ± 2.5 | 1.3 ± 1.6 | 1.3 ± 1.3 | 1.0 ± 0.2 | < 0.001 | 1.5 ± 1.7 |
| CT scan, n = 1790 | 1.4 ± 1.1 | 2.0 ± 1.9 | 2.0 ± 1.8 | 1.2 ± 0.9 | 1.1 ± 0.4 | < 0.001 | 1.5 ± 1.3 |
| No. of hospital admissions in the last 12 months, n = 1049 | 2.2 ± 2.3 | 2.8 ± 4.3 | 2.6 ± 2.3 | 2.6 ± 3.5 | 1.7 ± 0.9 | < 0.001 | 2.3 ± 2.8 |
| No. of emergency visits in the last 12 months | |||||||
| Outpatient centre, n = 813 | 3.9 ± 7.4 | 3.4 ± 4.3 | 4.2 ± 3.4 | 3.5 ± 4.1 | 2.0 ± 1.1 | < 0.001 | 3.8 ± 6.2 |
| Hospital, n = 1121 | 2.8 ± 4.4 | 2.6 ± 5.2 | 4.6 ± 5.5 | 2.5 ± 3.6 | 1.3 ± 0.6 | < 0.001 | 3.0 ± 4.7 |
| Home emergency, n = 250 | 2.0 ± 1.7 | 2.4 ± 2.0 | 2.7 ± 2.2 | 1.2 ± 0.4 | 1.8 ± 1.5 | 0.010 | 2.2 ± 1.8 |
| Ambulance, n = 197 | 1.7 ± 1.3 | 2.7 ± 5.2 | 2.0 ± 2.4 | 1.8 ± 1.2 | 1.0 ± 0.0 | 0.865 | 2.0 ± 3.2 |
| Total healthcare utilization, n = 5272 | 31.8 ± 34.8 | 24.3 ± 25.7 | 30.2 ± 28.1 | 20.3 ± 19.7 | 26.4 ± 20.0 | < 0.001 | 29.2 ± 31.7 |
| % Total healthcare utilization, n = 5272 | 3,312 (94.8) | 762 (99.0) | 515 (94.0) | 542 (90.3) | 141 (96.6) | 5272 (94.6) | |
SD standard deviation, GP general practitioner, MRI magnetic resonance imaging, CT computed tomography
The countries with the highest healthcare utilization were Brazil, France, Spain, and Norway, with values above 30 uses, while the countries with the lowest healthcare utilization were Costa Rica, the United Kingdom, India, Slovenia, and Austria, with less than 20 uses (Fig. 4).
Fig. 4.
Total healthcare utilization in the last 12 months prior to survey, stratified by countries
Younger age (r = – 0.107, p < 0.001), female gender (33.9 vs. 23.2 males, p < 0.001), member of patient organizations (30.2 vs. 28.5 non-member, p = 0.035), higher disease activity (r = 0.264, p < 0.001), poorer mental health (r = 0.221, p < 0.001), greater functional limitation (r = 0.253, p < 0.001), greater spinal stiffness (r = 0.191, p < 0.001), and longer diagnostic delay (r = 0.064, p < 0.001) were associated in the bivariate analysis with a higher healthcare utilization in the last 12 months. In the multiple linear regression, factors associated with higher healthcare utilization were younger age (B = – 0.311), female gender (B = 7.736), longer diagnostic delay (B = 0.104), higher disease activity (B = 1.461), poorer mental health (B = 0.624), greater functional limitation (B = 0.300), and greater spinal stiffness (B = 1.527; Table 4).
Table 4.
Linear regression analysis of socio-demographic and patient-reported outcomes according to total healthcare utilization (n = 5004)
| Simple linear regression | Multiple linear regression | |||
|---|---|---|---|---|
| B | 95% CI | B | 95% CI | |
| Age | – 0.265 | – 0.332, – 0.199 | – 0.311 | – 0.379, – 0.243 |
| Gender. Female | 10.668 | 8.869, 12.368 | 7.736 | 6.079, 9.393 |
| Diagnostic delay | 0.226 | 0.130, 0.322 | 0.104 | 0.010, 0.199 |
| Patient organization membership. Yes | 1.687 | – 0.038, 3.413 | – | – |
| BASDAI (0–10) | 3.887 | 3.500, 4.275 | 1.461 | 0.993, 1.930 |
| GHQ-12 (0–12) | 1.685 | 1.483, 1.887 | 0.624 | 0.402, 0.847 |
| Functional limitation (0–54) | 0.525 | 0.471, 0.579 | 0.300 | 0.241, 0.359 |
| Spinal stiffness (3–12) | 2.451 | 2.109, 2.794 | 1.527 | 1.149, 1.905 |
B beta, CI confidence interval, BASDAI Bath Ankylosing Spondylitis Disease Activity Index, GHQ-12 12-item General Health Questionnaire
Discussion
The present study has evaluated the patients’ journey to diagnosis and their healthcare utilization after diagnosis in a large sample of more than 5000 patients with axSpA from 27 countries worldwide. With respect to the patient journey, patients with axSpA had visited more than two HCPs prior to diagnosis, with a higher frequency of general practitioner (GP) and rheumatologist visits, as well as performing more frequent tests, such as HLA-B27, x-rays, and MRI scans. Finally, the rheumatologist diagnosed seven out of ten of these patients; all this with a diagnostic delay of more than 7 years. Regarding healthcare utilization after diagnosis, 95% of the patients had used at least one healthcare resource during the last year, with an average of 29.2 healthcare resource uses per year, highlighting healthcare visits, medical tests, emergency visits, and hospital admissions. Furthermore, being younger or female, and having longer diagnostic delay, higher disease activity, poorer mental health, greater functional limitation, and greater spinal stiffness, were associated with higher healthcare utilization. In addition, regional differences have been found with respect to patient journey and healthcare utilization.
HCPs Seen Before Diagnosis
In the majority of IMAS regions, patients with axSpA had visited the GP more frequently before diagnosis, except in the Asian region, where patients had visited the orthopaedic specialist more frequently. This may be because chronic back pain is a frequent symptom of patients who visit both GPs and orthopaedic specialists [21], or due to the fact that primary care physicians often overlook the symptomatology of axSpA, as well as the opportunity to diagnose or refer patients with axSpA to rheumatologists, referring them directly to other specialists, such as orthopaedics, spine surgeons, or rehabilitation specialists [22, 23].
In addition, this increased number of medical and orthopedic consultations prior to diagnosis and the fact that patients are not referred to a rheumatologist earlier may be due to the small number of rheumatology specialists in regions such as South Africa, which has only 85 rheumatologists for nearly 56 million people [24]. Although the number of rheumatologists is higher in high-income countries [25], there are inequalities and shortages in the number of rheumatologists worldwide, which represents a serious challenge for the increasing large number of patients with rheumatic diseases.
Test Carried Out Before Diagnosis, HCP That Made Diagnosis and Diagnostic Delay
The HLA-B27 test was the most frequently used test for diagnosis of patients in IMAS, being conducted more frequently in South Africa and less frequently in Asia. While tests such as MRI scans and x-rays were performed more frequently in Europe and North America. This could be due to the fact that higher-income regions, such as Europe and North America, have greater access to imaging tests such as MRI or CT scans, while less expensive tests (e.g., HLA-B27) are more often used in regions such as South Africa. Also, the differences in prevalence or access to B27 carriership could explain different practices from HCPs requesting this. In the case of Asia, perhaps this lower use of diagnostic tests is due to the fact that most of the patients in the Asian region from IMAS were male, who until recently have been thought to be the most likely to suffer from axSpA [26].
Finally, the rheumatologist diagnosed most of these patients, although in the Asian region the orthopaedic specialist also played an important role in the disease diagnosis. This may be because orthopaedists can more easily distinguish axSpA from chronic back pain than other HCPs [21]. However, this could also be because most patients with chronic low back pain are referred to this specialist, given the greater presence of orthopaedics compared to rheumatologists, but also to differences in their qualifications between countries.
Despite, or because of, all of these HCPs visits and diagnostic tests during the patient journey of patients with axSpA, a diagnostic delay of more than 7 years for patients with axSpA in IMAS is evident. This unacceptable diagnostic delay is associated with poor health factors, such as greater disease activity or worse treatment response [27, 28]. Therefore, educating primary care physicians and other HCPs about axSpA is essential, and, together with knowing when to make a referral to a rheumatologist, should reduce the long diagnostic delay in patients with axSpA [29, 30]. Furthermore, these longer diagnostic delays were associated with an increased clinical, economic, and social and psychological burden [31]. Therefore, a generalized approach to the education of professionals and a more accurate calculation of the economic costs of axSpA for healthcare are essential.
Healthcare Utilization After Diagnosis by Region
After diagnosis and during the last 12 months prior to the survey, patients had used on average 29.2 healthcare resources, which included health visits, medical tests, emergency room visits. or hospital admissions, with only 5% of survey respondents not having used any of these resources. The regions with the highest number of healthcare visits and/or tests were Europe and Latin America, while Asia had the patients with the lowest healthcare utilization. Similarly to patients in IMAS, a previous study showed that most patients were seen at least once a year by a rheumatologist [32], although approximately half the patients visited another specialist, with an average of two visits per year [33]. In addition, one out of three patients usually visited a general practitioner [34]. We acknowledge this high amount of healthcare utilization could be due to high levels of the patients’ disease activity, as some studies have shown that patients with more visits to the general practitioner or physiotherapist and more hospital admissions or outpatient visits had greater disease activity [32, 34].
The Role of Physiotherapist
It is worth noting the large number of visits to physiotherapists, especially in Europe, Latin America, and South Africa, after diagnosis (one in three patients) and before diagnosis (21.3, 12.5, and 12.2 visits, respectively). In this regard, physiotherapeutic interventions for axSpA have been shown to be an integral part of the treatment of the disease, and effective in improving spinal mobility in the short term [35]. In addition, a recent study has shown that a high rate of patients with axSpA see a physiotherapist or would like to do so [32], as it is one of the Assessment of SpondyloArthritis international Society (ASAS) recommendations for managing patients with axSpA [36]. Thus, frequent visits to a physiotherapist should be part of the integral management of patients with axSpA.
Healthcare Utilization After Diagnosis and Costs Related to axSpA by Countries
The average healthcare number of visits in the IMAS cohort was 29 times, being higher in countries such as Brazil (50), France (48), Spain (42), and Norway (32), while countries such as Costa Rica, UK, India, Slovenia, Austria, or Taiwan were below 20 visits. All this healthcare utilization before and after axSpA diagnosis involves a significant level of cost to the patient and to the healthcare system. A study of patients with axSpA in the UK showed a total cost during 3 months of £2,802 [34], annual cost per patient of US$6,600 for Colombian patients with axSpA [37], or €11,462.3 for Spanish patients with axSpA [38]. Besides the costs associated with healthcare resources, patients with axSpA suffer significant indirect economic costs associated with self-funded healthcare, work absenteeism, and early retirement [39–41], as well as high drug treatment costs generating a considerable burden on health systems and governments of each country [42, 43]. In this sense, treatment is considered the most important contributor to direct costs of patients with axSpA, especially for patients with higher disease severity and those with a poor response to conventional disease-modifying antirheumatic drugs (cDMARDs) [44].
In our IMAS cohort, younger patients, women, those with higher disease activity, poorer mental health, greater functional limitation, greater spinal stiffness, and longer diagnostic delay experienced higher healthcare utilization. This statement is consistent with the Spanish sub-sample of IMAS [45]. However, these results should be interpreted with caution, as we cannot establish a cause–effect relationship between healthcare utilization and clinical factors of patients with axSpA in the IMAS cohort.
Assessing the healthcare utilization of patients with axSpA is essential to identify the profile of patients who require higher utilization due to their disease, as well as to evaluate how worse disease outcomes lead to higher utilization of healthcare resources, resulting in increased healthcare spending. In addition, optimal disease care, management, and treatment can help reduce the number of visits and tests, with positive consequences on allocated resources. In this regard, although radiography is the most cost-effective imaging test for the initial diagnosis of sacroiliitis in patients with inflammatory low back pain suspected of axSpA [46], MRI of the sacroiliac joints has become the main tool for the early diagnosis of axSpA [47].
Comparing IMAS regions, the diagnostic delay of patients in South Africa and North America was close to 10 years, while in Asia it was 4. Furthermore, South Africa and Latin America were the regions with the highest proportion of patients with physical comorbidities, highest disease activity, and greatest use of NSAIDs and csDMARDs. Finally, spinal stiffness and functional limitation was higher in Europe, North America, and South Africa [16]. The present study has shown that the regions with the highest 1-year healthcare utilization were Europe, Latin America, and South Africa, which is consistent with the disease findings previously mentioned. However, it is important to note that, although it is not possible to establish cause–effect relationships between phenotypic characteristics and healthcare utilization, the results point in the expected direction.
Strengths and Limitations
IMAS is the most extensive survey of patients with axSpA, including 5557 respondents from 27 countries worldwide, making it the largest sample and coverage to date. This study identifies a significant number of variables in the patient journey and healthcare utilization, knowledge of which can help to quantify the cost before and after axSpA diagnosis and can help to inform the patient care pathway. However, IMAS has some limitations. First, the survey was based on self-reported data and the diagnosis of patients could not be confirmed. Secondly, information on healthcare visits and tests carried out were self-reported by patients so can only ever be an estimate. Another limitation is the fact that we only have global information on the complementary tests, but do not know specific information, such as for which anatomical regions the imaging tests were performed or which specific determinations were requested for the laboratory tests. Furthermore, 70 of 5272 (1.3%) patients who reported healthcare utilization may have had the disease for less than 1 year and their assessed healthcare utilization may be lower, although closer to 12 months. In addition, results on disease severity, such as BASDAI scores, GHQ-12, spinal stiffness, and functional limitation should be interpreted with caution, as this information was self-reported and may suffer from self-reporting bias, as respondents often tend to introduce bias as they find it difficult to recall past behaviors [48]. Finally, there is an over-representation of patients from the European region compared to other regions such as Asia or South Africa. The unbalanced samples within each of the countries and regions represent a limitation of the study and, therefore, comparisons between countries and regions should be interpreted with caution.
This IMAS study has shown how the region and country of residence of a patient with axSpA influences the time waited for diagnosis and the management of their disease, although there are other geographical and socioeconomic aspects such as the place of residence (urban-rural), the distance to a rheumatologist, the type of health coverage available, and the economic status of the patient.
Conclusions
Patients with axSpA required more than two medical visits—one in three visited orthopaedic/physiotherapists—and several diagnostic tests—including HLA-B27, x-rays and MRI—to be diagnosed. All this journey of the patient with axSpA—including visits and tests performed—to be diagnosed resulted in an unacceptable diagnostic delay of more than 7 years. In addition, most patients with axSpA had used at least one healthcare resource, with an average of 29.2 healthcare resource uses per year after diagnosis. Europe and North America had greater access to HCPs and diagnostic tests before and after diagnosis than the other regions. After diagnosis, patients in South Africa were less frequently hospitalized, while patients in Latin America were the most frequent users of emergency services. It should be noted that patients in Asia had more frequent visits to orthopaedic specialists before and after diagnosis. Furthermore, patients from countries such as Brazil, France, Spain, and Norway had the highest post-diagnostic healthcare utilization per year. Shortening the patient’s journey to diagnosis, along with regular follow-up care and close collaboration between the patient and the medical team, are essential to effectively manage axSpA and improve the patient’s long-term quality of life.
Acknowledgements
We would like to thank all people with axSpA and patient organizations who participated in the IMAS study.
Author Contributions
All authors (Marco Garrido-Cumbrera, Denis Poddubnyy, Fernando Sommerfleck, Christine Bundy, Souzi Makri, José Correa-Fernández, Shashank Akerkar, Jo Lowe, Elie Karam, Victoria Navarro-Compán) had full access to all study data, participated in the drafting and revision of the article, and approved the final version to be published.
Funding
This study and the journal’s Rapid Service fee was supported by Novartis Pharma AG.
Data Availability
All data generated or analyzed during this study are included in this published article as supplementary information files.
Declarations
Conflict of Interest
Marco Garrido-Cumbrera: Novartis. Denis Poddubnyy: AbbVie, BMS, Celgene, Janssen, Lilly, MSD, Novartis, Pfizer, Roche, UCB, AbbVie, MSD, Novartis, Pfizer. Fernando Sommerfleck: Abbvie, Eli Lilly, Janssen, Novartis, Abbvie, Novartis, Janssen. Christine Bundy: AbbVie, Celgene, Janssen, Lilly, Novartis, Pfizer. Souzi Makri: Novartis, GSK, Bayer. José Correa-Fernández: None declared. Shashank Akerkar: Pfizer, Novartis, Eli Lilly, Jansen. Jo Lowe: No personal funding, but ASIF has received funding from Novartis, UCB, Lilly, Abbvie, Boehringer Ingleheim, Pfizer, Janssen. Elie Karam: None declared. Victoria Navarro-Compán: AbbVie, Eli Lilly, Janssen, MSD, Novartis, Pfizer, UCB Pharma, AbbVie, Eli Lilly, Galapagos, MoonLake, MSD, Novartis, Pfizer, UCB Pharma, AbbVie, Novartis.
Ethical Approval
The present manuscript does not contain any studies with animal subjects and the IRB approval was not necessary. All participants were asked to provide explicit opt-in consent prior to participating in the IMAS survey. Furthermore, the participants’ data were anonymized and did not contain confidential, personal or subject-identifying information. Ethical aspects related to data extracted from patients and their treatment were in accordance with the Declaration of Helsinki.
Footnotes
Prior Presentation: Part of the data of this manuscript has been sent to Annual European Congress of Rheumatology (EULAR) 2024.
References
- 1.Rudwaleit M, Van Der Heijde D, Khan MA, Braun J, Sieper J. How to diagnose axial spondyloarthritis early. Ann Rheum Dis. 2004;63:535–43. 10.1136/ard.2003.011247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao SS, Pittam B, Harrison NL, Ahmed AE, Goodson NJ, Hughes DM. Diagnostic delay in axial spondyloarthritis: a systematic review and meta-analysis. Rheumatology. 2021;60:1620–8. 10.1093/rheumatology/keaa807 [DOI] [PubMed] [Google Scholar]
- 3.Braun J, Sieper J. Fifty years after the discovery of the association of HLA B27 with ankylosing spondylitis. RMD Open. 2023;9:e003102. [DOI] [PMC free article] [PubMed]
- 4.Diekhoff T, Eshed I, Radny F, Ziegeler K, Proft F, Greese J, et al. Choose wisely: Imaging for diagnosis of axial spondyloarthritis. Ann Rheum Dis. 2022;81:237–42. 10.1136/annrheumdis-2021-220136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poddubnyy D, Gaydukova I, Hermann KG, Song IH, Haibel H, Braun J, et al. Magnetic resonance imaging compared to conventional radiographs for detection of chronic structural changes in sacroiliac joints in axial spondyloarthritis. J Rheumatol 2013;40:1557–65. 10.3899/jrheum.130141 [DOI] [PubMed] [Google Scholar]
- 6.Sieper J, Srinivasan S, Zamani O, Mielants H, Choquette D, Pavelka K, et al. Comparison of two referral strategies for diagnosis of axial spondyloarthritis: The Recognising and Diagnosing Ankylosing Spondylitis Reliably (RADAR) study. Ann Rheum Dis. 2013;72:1621–7. 10.1136/annrheumdis-2012-201777 [DOI] [PubMed] [Google Scholar]
- 7.Feldtkeller E, Khan MA, van der Heijde D, van der Linden S, Braun J. Age at disease onset and diagnosis delay in HLA-B27 negative vs. positive patients with ankylosing spondylitis. Rheumatol Int. 2003;23:61–6. [DOI] [PubMed]
- 8.Fernández-Carballido C, Almodóvar R, Cañete JD, Collantes E, de Miguel E, Gratacós J, et al. Resources and strategies for the optimal care of patients with axial spondyloarthritis: The CREA project. Reumatol Clin. 2023;19:82–9. 10.1016/j.reuma.2022.02.008 [DOI] [PubMed] [Google Scholar]
- 9.van der Heijde D, Ramiro S, Landewé R, Baraliakos X, Van Den Bosch F, Sepriano A, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76:978–91. 10.1136/annrheumdis-2016-210770 [DOI] [PubMed] [Google Scholar]
- 10.Zhao SS, Harrison SR, Chan A, Clarke N, Davis C, Eddison J, et al. Treatment of axial spondyloarthritis with biologic and targeted synthetic DMARDs: British Society for Rheumatology guideline scope. Rheumatol Adv Pract. 2023;7(2):rkad039. 10.1093/rap/rkad039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martey C, Sengupta R. Physical therapy in axial spondyloarthritis: Guidelines, evidence and clinical practice. Curr Opin Rheumatol. Lippincott Williams and Wilkins; 2020;32:365–70. 10.1097/BOR.0000000000000714 [DOI] [PubMed] [Google Scholar]
- 12.Braun J, Van Den Berg R, Baraliakos X, Boehm H, Burgos-Vargas R, Collantes-Estevez E, et al. 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis. 2011;70:896–904. 10.1136/ard.2011.151027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barnett R, Sengupta R. The future of axial spondyloarthritis rehabilitation: lessons learned from COVID-19. Arthritis Care Res; 2022;74:44–9. 10.1002/acr.24780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redeker I, Hoffmann F, Callhoff J, Haibel H, Sieper J, Zink A, et al. Determinants of psychological well-being in axial spondyloarthritis: an analysis based on linked claims and patient-reported survey data. Ann Rheum Dis. 2018;77:1017–24. 10.1136/annrheumdis-2017-212629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garrido-Cumbrera M, Poddubnyy D, Gossec L, Gálvez-Ruiz D, Bundy C, Mahapatra R, et al. The European map of axial spondyloarthritis: capturing the patient perspective—an analysis of 2846 patients across 13 countries. Curr Rheumatol Rep. 2019;21:1–9. 10.1007/s11926-019-0819-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poddubnyy D, Sommerfleck F, Navarro-Compán V, Bundy C, Makri S, Akerkar S, et al. Regional differences in clinical phenotype of axial spondyloarthritis: results from the International Map of Axial Spondyloarthritis (IMAS). Rheumatology; 2023; kead665 [DOI] [PMC free article] [PubMed]
- 17.Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the bath ankylosing spondylitis disease activity index. J Rheumatol. 1994;21:2286–91. [PubMed] [Google Scholar]
- 18.Goldberg D. The detection of psychiatric illness by questionnaire; a technique for the identification and assessment of non-psychotic psychiatric illness. London: Oxford University Press; 1972. [Google Scholar]
- 19.Hankins M. The reliability of the twelve-item general health questionnaire (GHQ-12) under realistic assumptions. BMC Public Health. 2008;8:7. 10.1186/1471-2458-8-355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garrido-Cumbrera M, Navarro-Compán V, Zarco P, Collantes-Estévez E, Gálvez-Ruiz D, Braçe O, et al. Atlas of axial spondyloarthritis in Spain 2017: Study design and population. Reumatol Clin. 2019;15:127–32. 10.1016/j.reuma.2018.08.003 [DOI] [PubMed] [Google Scholar]
- 21.Braun A, Saracbasi E, Grifka J, Schnitker J, Braun J. Identifying patients with axial spondyloarthritis in primary care: How useful are items indicative of inflammatory back pain? Ann Rheum Dis. 2011;70:1782–7. 10.1136/ard.2011.151167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyer GS, Templin DW, Bowler A, Lawrence RC, Everett DF, Heyse SP, et al. A comparison of patients with spondyloarthropathy seen in specialty clinics with those identified in a communitywide epidemiologic study: has the classic case misled us? Arch Intern Med; 1997;157:2111–7. 10.1001/archinte.1997.00440390111014 [DOI] [PubMed] [Google Scholar]
- 23.Jois RN, Macgregor AJ, Gaffney K. Recognition of inflammatory back pain and ankylosing spondylitis in primary care. Rheumatology; 2008;47:1364–6. 10.1093/rheumatology/ken224 [DOI] [PubMed] [Google Scholar]
- 24.Mody GM. Rheumatology in Africa-challenges and opportunities. Arthr Res Ther. 2017;19(1):49. 10.1186/s13075-017-1259-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al Maini M, Adelowo F, Al Saleh J, Al Weshahi Y, Burmester GR, Cutolo M, et al. The global challenges and opportunities in the practice of rheumatology: white paper by the world forum on rheumatic and musculoskeletal diseases. Clin Rheumatol. 2015;34:819–29. 10.1007/s10067-014-2841-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kennedy LG, Will R, Calin A. Sex ratio in the spondyloarthropathies and its relationship to phenotypic expression, mode of inheritance and age at onset. J Rheumatol. 1993;20:1900–4. [PubMed] [Google Scholar]
- 27.Fallahi S, Jamshidi AR. Diagnostic delay in ankylosing spondylitis: related factors and prognostic outcomes. Arch Rheumatol. 2016;31:24–30. 10.5606/ArchRheumatol.2016.5562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seo MR, Baek HL, Yoon HH, Ryu HJ, Choi HJ, Baek HJ, Ko KP. Delayed diagnosis is linked to worse outcomes and unfavourable treatment responses in patients with axial spondyloarthritis. Clin Rheumatol. 2015;34:1397–405. 10.1007/s10067-014-2768-y [DOI] [PubMed] [Google Scholar]
- 29.Rudwaleit M, Sieper J. Referral strategies for early diagnosis of axial spondyloarthritis. Nat Rev Rheumatol 2012;8:262–8. [DOI] [PubMed]
- 30.Garrido-Cumbrera M, Navarro-Compán V, Bundy C, Mahapatra R, Makri S, Correa-Fernández J, et al. Identifying parameters associated with delayed diagnosis in axial spondyloarthritis: data from the European Map of axial spondyloarthritis. Rheumatology. 2021;61(2):705–12. 10.1007/978-3-030-97606-4_1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yi E, Ahuja A, Rajput T, George AT, Park Y. Clinical, economic, and humanistic burden associated with delayed diagnosis of axial spondyloarthritis: a systematic review. Rheumatol Ther. 2020;7:65–87. 10.1007/s40744-020-00194-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Derakhshan MH, Pathak H, Cook D, Dickinson S, Siebert S, Gaffney K. Services for spondyloarthritis: a survey of patients and rheumatologists. Rheumatology; 2018;57:987–96. 10.1093/rheumatology/kex518 [DOI] [PubMed] [Google Scholar]
- 33.Ward MM. Functional disability predicts total costs in patients with ankylosing spondylitis. Arthr Rheum. 2002;46:223–31. [DOI] [PubMed] [Google Scholar]
- 34.Rafia R, Ara R, Packham J, Haywood K, Healey E. Healthcare costs and productivity losses directly attributable to ankylosing spondylitis. Clin Exp Rheumatol. 2012;30:246–53. [PubMed] [Google Scholar]
- 35.Tubergen AVAN, Landewe R, Wolter N, Asscher MAX, Falkenbach A, Genth E, et al. Combined spa – exercise therapy is effective in patients with ankylosing spondylitis : a randomized controlled trial. Arthr Care Res. 2001;45:430–8. [DOI] [PubMed] [Google Scholar]
- 36.Ramiro S, Nikiphorou E, Sepriano A, Ortolan A, Webers C, Baraliakos X, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2022;82:19–34. 10.1136/ard-2022-223296 [DOI] [PubMed] [Google Scholar]
- 37.Santos-Moreno P, Parra-Padilla D, Gómez-De la Rosa F, Carrasquilla-Sotomayor M, Villarreal L, Jervis-Jálabe DS, et al. Direct medical costs and healthcare resource utilization of treating patients with two clinical subtypes of axial spondyloarthritis in colombia. Value Heal Reg Issues. 2022;32:88–94. [DOI] [PubMed]
- 38.Merino M, Braçe O, González-Domínguez A, Hidalgo-Vega, Garrido-Cumbrera M, Gratacós J. Social economic costs of ankylosing spondylitis in Spain. Clin Exp Rheumatol 2021;39:357–64. 10.55563/clinexprheumatol/lycdc8 [DOI] [PubMed] [Google Scholar]
- 39.Ara RM, Packham JC, Haywood KL. The direct healthcare costs associated with ankylosing spondylitis patients attending a UK secondary care rheumatology unit. Rheumatology. 2008;47:68–71. 10.1093/rheumatology/kem296 [DOI] [PubMed] [Google Scholar]
- 40.Boonen A. A review of work-participation, cost-of-illness and cost-effectiveness studies in ankylosing spondylitis. Nat Clin Pract Rheumatol. 2006;2(10):546–53. 10.1038/ncprheum0297 [DOI] [PubMed] [Google Scholar]
- 41.Zhu TY, Tam LS, Lee VWY, Hwang WW, Li TK, Lee KK, et al. Costs and quality of life of patients with ankylosing spondylitis in Canada. Rheumatology. 2006;33:289–95. [PubMed] [Google Scholar]
- 42.Strand V, Singh JA. Patient Burden of Axial Spondyloarthritis. J Clin Rheumatol. 2017;23:383–91. 10.1097/RHU.0000000000000589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.López-Medina C, Ramiro S, Van Der Heijde D, Sieper J, Dougados M, Molto A. Characteristics and burden of disease in patients with radiographic and non-radiographic axial Spondyloarthritis: a comparison by systematic literature review and meta-analysis. RMD Open. 2019;5:1–9. 10.1136/rmdopen-2019-001108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reveille JD, Ximenes A, Ward MM. Economic considerations of the treatment of ankylosing spondylitis. Am J Med Sci. 2012;343:371–4. 10.1097/MAJ.0b013e3182514093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garrido-Cumbrera M, Collantes-Estévez E, Navarro-Compán V, Zarco-Montejo P, Sastre C, Correa-Fernández J, et al. Patients with Axial Spondyloarthritis are Great Consumers of Healthcare Resources, especially Young and Women: Results from the Spanish Atlas. Rheumatol Ther. 2023;10:729–39. 10.1007/s40744-023-00543-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gorelik N, Tamizuddin F, Rodrigues TC, Beltran L, Malik F, Reddy S, et al. Comparison between radiography and magnetic resonance imaging for the detection of sacroiliitis in the initial diagnosis of axial spondyloarthritis: a cost-effectiveness study. Skeletal Radiol; 2020;49:1581–8. 10.1007/s00256-020-03444-6 [DOI] [PubMed] [Google Scholar]
- 47.Lee S, Jeon U, Lee JH, Kang S, Kim H, Lee J, et al. Artificial intelligence for the detection of sacroiliitis on magnetic resonance imaging in patients with axial spondyloarthritis. Front Immunol. 2023;14:1–9. 10.3389/fimmu.2023.1278247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Straub D, Limayem M, Karahanna-Evaristo E. Measuring system usage: implications for is theory testing. Management Science 1995;41:1328–42. 10.1287/mnsc.41.8.1328 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article as supplementary information files.