Abstract
Introduction
Despite advances in atopic dermatitis (AD) treatments, many patients face challenges obtaining medications. This study aimed to determine the frequency and causes of insurance coverage delays and denials for AD prescriptions and characterize the associated wait times and extent to which patients understand what to do when faced with a coverage issue.
Methods
This was a cross-sectional, observational study in which adult U.S. residents (aged 18+ years) with AD or caregivers of pediatric U.S. patients with AD (aged 0–17 years) completed an online survey (3 June–16 July 2021).
Results
Respondents (N = 978) were primarily adults with AD (81.8%), female (67.7%), and white (70.2%). There were 645 insurance delays or denials for AD prescriptions, with 48.1% (470/978) of respondents experiencing at least one delay/denial in the past year. Most delays/denials were for topical steroids (39.2%, 253/645), the most highly used prescription treatment class (83.9%, 821/978). However, the highest rate of delay/denials was for biologics, of which 43.6% (109/250) of all prescriptions faced a delay or denial. Denials were caused primarily by step therapy (27.6%) and delays by prior authorization (55.1%). Only 56.0% of respondents said they would know what to do if they faced an issue with AD prescription coverage.
Conclusions
Patients with AD frequently experience insurance-related barriers to obtaining recommended therapies, and many do not know how to respond when these barriers arise. Strategies to improve timely therapeutic access are needed.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-024-01205-0.
Keywords: Atopic dermatitis, Eczema, Prescription access, Prior authorization, Step therapy
Key Summary Points
| Why carry out this study? |
| Atopic dermatitis (AD) is a chronic, inflammatory skin disease associated with multidimensional burdens. Patients can face insurance coverage issues when trying to get prescribed treatments. |
| The administrative cost and burden of coverage delays and denials for dermatology practices have been well documented; however, little is known about the patient perspective on the frequency and impact of insurance coverage delays and denials across all AD medications currently prescribed. |
| Questions asked: What is the patient and caregiver experience with barriers to AD prescription treatment access? Do patients know what to do when faced with a coverage issue, and how long does it take to resolve? |
| What was learned from the study? |
| In this cross-sectional survey study, 978 patients faced 645 insurance coverage delays or denials for AD prescriptions in the past year, with 48% of respondents experiencing at least one delay/denial. Wait times for medications often exceeded the recommended time of 24–72 h (91%), and for many patients there was confusion around the reason for the insurer’s decision or what to do when faced with a coverage issue. |
| Patients with AD frequently experience issues obtaining recommended therapies, which, combined with lack of knowledge about how to address the issues, can create an undue burden. |
Introduction
Atopic dermatitis (AD) is a chronic, inflammatory skin disease affecting over 16.5 million adults in the USA alone [1], and is associated with comorbidities and quality of life burden [2–6]. The variable and often unpredictable disease course of AD can necessitate complex and changing treatment regimens, with patients often using multiple prescription treatments concurrently in efforts to achieve disease control [7, 8].
Prescription treatment access for chronic diseases requires the navigation of insurance barriers, and patients with AD may face high access burdens due to prescription volume, shifting treatment needs over the disease course, and the rate of new AD treatments coming onto market [9–11]. Treatments newly approved by the US Foods and Drug Administration (FDA) can be more costly, and may be more likely to face insurance coverage delays and denials [12–14]. However, utilization management issues, such as prior authorization (PA), are ubiquitous among new and old prescriptions in the dermatology space, suggesting the burden is more widespread [15]. Policies surrounding prescription treatment availability and approval are also discordant across payers, yielding yet more confusion for healthcare providers (HCPs) as well as patients [16].
The administrative cost and burden of coverage delays and denials for dermatology practices has been well documented. It has been reported that dermatology staff can spend up to 3.3 h/day on PAs alone [15, 17], and can incur costs of > 6 USD (US dollars) per PA [18]. The impact on patients has also been documented, with PAs resulting in delayed, discontinued, or less appropriate treatment, as well as decreased health outcomes compared to those who did not face PAs or had them approved [15, 17, 19]. However, to date, the consequences of PAs on patients have been broadly reported by providers, clinics, and academic centers, leaving the disease-specific patient perspective on the impact of insurance coverage issues less clearly defined. Patient uncertainty surrounding the prescription process, and a lack of clear solutions about what to do when faced with coverage delays or denials may exacerbate these issues and the subsequent overall burden on patients with AD.
Little is known about the frequency and impact of insurance delays and denials across all AD medications currently prescribed. We sought to determine the frequency and causes of insurance coverage delays and denials by drug class, characterize the subsequent wait times for prescriptions, and describe the extent to which patients with AD understand what to do when faced with an issue related to insurance prescription coverage.
Methods
A survey study was conducted from 3 June to 16 July 2021. Electronic survey availability was communicated to all National Eczema Association (NEA) members via the NEA website, email, social media, and the NEA EczemaWise app, and distributed more broadly through ads run on Facebook and Google (convenience sample). Adult (aged ≥ 18 years) U.S. residents (including residents of U.S. territories) with a self-reported diagnosis of AD or primary caregivers for U.S. pediatric patients with AD (aged 0–17 years) were included. This study was identified as exempt by the Western Institutional Review Board Copernicus Group under 45 CFR § 46.104(d) (2), because the research only included survey procedures with adequate measures to protect the privacy and confidentiality of participants. Prior to completing the online survey, all respondents provided electronic informed consent. Data were anonymized for analysis and treated confidentially. All methods were carried out according to the Declaration of Helsinki.
Respondents were asked up to 71 questions regarding sociodemographic factors, comorbidities, their current AD prescription use and past treatment history, and experiences with—and impact of— coverage delays and denials. Analysis was performed using R: a language and environment for statistical computing (version 4.1.0) [20]. Descriptive statistics were used to analyze the data.
Results
There were 1291 total survey responses, among which 201 responders did not meet inclusion criteria and 112 did not complete the survey. Therefore, analysis was completed for 978 individuals (978/1291; 75.7%). Patients were primarily adults with AD (81.8%), identified as female (67.7%), white (70.2%), and self-reported moderate AD (43.2%) (Table 1).
Table 1.
Characteristics of the study population
| Study characteristics | Total responders included in analysis (N = 978) |
|---|---|
| Connection to AD, % (n) | |
| Adult patient | 81.8% (800) |
| Caregiver of child | 18.2% (178) |
| Patient age, mean ± SD | |
| Adult patient | 46.3 ± 18.4 |
| Child | 8.5 ± 4.9 |
| Patient gender, % (n) | |
| Female | 67.7% (662) |
| Male | 31.2% (305) |
| Other | 1.1% (11) |
| Respondent income, % (n) | |
| $24,999 or less | 13.1% (128) |
| $25,000–49,999 | 18.7% (183) |
| $50,000–74,999 | 20.4% (200) |
| $75,000–99,999 | 17.1% (167) |
| $100,000–124,999 | 11.4% (112) |
| $125,000–149,999 | 6.2% (61) |
| $150,000 or more | 13.0% (127) |
| Respondent insurance, % (n) | |
| Employer-sponsored | 50.0% (489) |
| Medicare | 22.2% (217) |
| Medicaid | 12.6% (123) |
| TRICARE or VA | 3.3% (32) |
| Policy purchased on commercial market | 4.9% (48) |
| Policy purchased on state/federal exchange | 4.8% (47) |
| Unsure | 2.2% (22) |
| Respondent education, % (n) | |
| Less than high school | 1.1% (11) |
| Completed some high school | 2.1% (21) |
| High school graduate | 9.1% (89) |
| Completed some college | 21.4% (209) |
| Technical post-secondary degree | 6.8% (67) |
| Four-year college degree | 35.0% (342) |
| Master’s degree/doctorate | 24.4% (239) |
| Patient race, % (n) | |
| Asian or Asian American | 9.3% (91) |
| Black or African American | 8.9% (87) |
| Multiracial | 5.7% (56) |
| Native American or Alaskan Native | 1.0% (10) |
| Native Hawaiian or Pacific Islander | 0.5% (5) |
| Other/I don’t know/prefer not to answer | 4.3% (42) |
| White | 70.2% (687) |
| Patient ethnicity, % Hispanic (n) | 13.8% (135) |
| Disease severity, % (n) | |
| Clear | 6.2% (61) |
| Mild | 31.0% (303) |
| Moderate | 43.2% (423) |
| Severe | 19.5% (191) |
AD Atopic dermatitis, SD standard deviation, VA Veterans Affairs
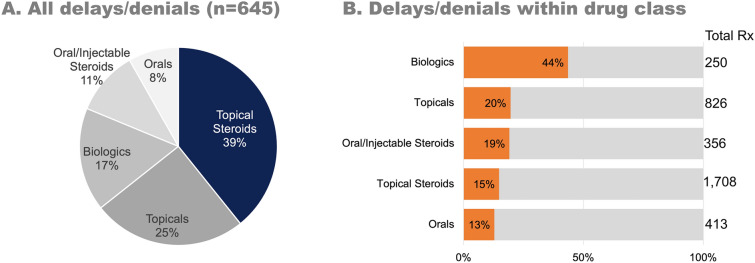
Table 2 shows the AD prescription treatments being used by respondents either currently or in the past 12 months (mutually exclusive answer options). The specific prescriptions included in each drug class can be found in Electronic Supplementary Material Table S1. Around one fourth (25.9%, n = 254/978) of patients were currently using ≥ 3 prescription treatments for AD management; this increased to 51.5% (n = 504/978) when prescriptions used in the past 12 months were also included. Overall, there were 645 insurance delays or denials for AD prescriptions, with 48.1% (470/978) of respondents experiencing at least one delay/denial in the past 12 months. The majority of all delays and denials were for topical steroids (39.2%, 253/645) (Fig. 1a). However, biologics faced the highest burden, as 43.6% (109/250) of prescriptions faced a delay or denial (Fig. 1b). Only 56.0% of respondents said they would know what to do if they faced an issue getting a prescription covered by their insurer.
Table 2.
Prescriptions used by patients for atopic dermatitis (not including over-the-counter products or prescriptions for comorbid conditions)
| Prescriptions used by patients | Percentage (n) of patients using at least 1 prescription in drug category currently or in the past 12 months (N = 978 patients) | Percentage (n) of prescriptions patients are currently using or have used in the past 12 months (N = 3553 prescriptions) |
|---|---|---|
| Steroids (topical) | 83.9% (821) | 48.1% (1708) |
| Steroids (oral/injectable) | 28.8% (282) | 10.0% (356) |
| Non-steroidal topicals | 49.7% (486) | 23.2% (826) |
| Orals | 21.9% (214) | 11.6% (413) |
| Injectable (biologics) | 25.6% (250) | 7.0% (250) |
Fig. 1.
Insurance delays and denials for atopic dermatitis prescriptions. a The proportion of all delays and denials by drug category. Steroids account for the largest number of delays/denials as they are the most commonly prescribed drug. b The proportion of delays and denials compared to the total number of prescriptions used in that category. This demonstrates that, although they are not as commonly prescribed, biologics are most frequently affected by insurance issues
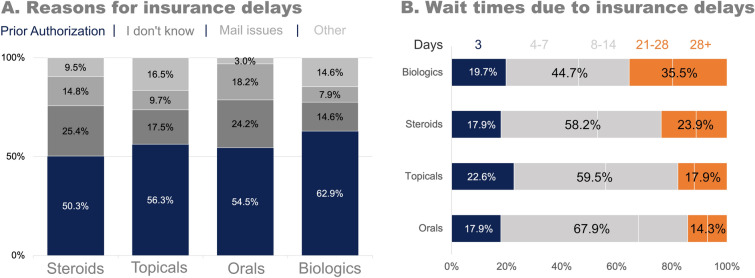
The most commonly reported reason for insurance coverage delays for AD prescriptions, overall, was PA (55.1%, 217/394 reasons). There was a higher proportion of delays due to PA for biologics compared to the other drug classes (Fig. 2a). The mode wait time from initial notification of an insurance delay to ultimately receiving the medication was 4–7 days for all drug categories; however, 35.5% (27/76) of those who faced delays for biologics reported waiting 3 weeks or longer to receive their medication (Fig. 2b).
Fig. 2.
Insurance delays for atopic dermatitis prescriptions in the past 12 months. a The reasons for insurance delays by drug category. b The amount of time that patients had to wait, on average, for a medication due to insurance delays. The gold standard wait time is 24–72 h (in blue)
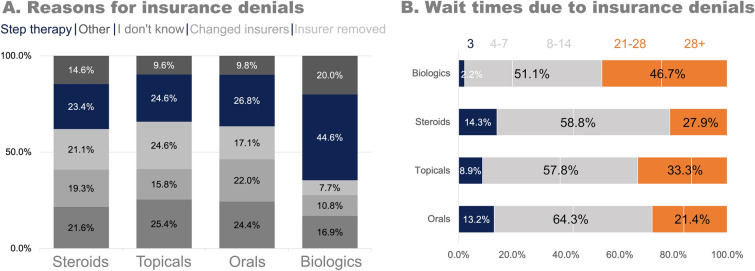
The most commonly reported reason for insurance coverage denials, overall, was step therapy (27.6%, 108/391 reasons). However, out of all reasons for denials indicated by respondents, 22.3% were unknown. There was a higher proportion of denials due to step therapy for biologics than for the other classes of AD drugs (Fig. 3a). The mode wait time from initial notification of an insurance denial to the final decision of the appeal was 8 days to 2 weeks, and 46.7% (21/45) of those who faced a denial for biologics waited 3 weeks or more for their appeal decision (Fig. 3b).
Fig. 3.
Insurance denials for atopic dermatitis prescriptions in the past 12 months. a The reasons for insurance denials by drug category. b The average appeals process time from initial notification of the coverage denial to the final decision of the appeal
Some patients and caregivers responded to coverage denials by simply accepting the reason (20.7%), while others appealed either personally (15.4%), through a HCP (35.3%), or through an advocate (10.4%). Patients were more likely to have a prescription approved when a healthcare professional appealed on their behalf than if they appealed for themselves (69.9% vs. 49.0%; p = 0.009). Finally, 18.2% reported that they did not accept the reason for denial, yet they did not appeal. For 62.8% (115/183) of those who appealed by any means, their prescription was eventually approved, while 22.4% were denied again and 14.8% were still waiting. In total, 35.7% of respondents were not even aware they could appeal an insurer’s decision to deny coverage.
Discussion
Using a survey to characterize the frequency and impact of insurance delays and denials from the perspective of patients with AD and caregivers, we discovered a high degree of prescription polypharmacy coupled with frequent insurance delays and denials across all currently prescribed drug categories. Most respondents reported using multiple prescriptions in different drug classes, with one fourth of respondents currently using three or more prescriptions. Topical steroids accounted for the highest proportion of total prescriptions used and were also associated with the most insurance coverage delays and denials. However, insurance coverage issues were reported more often for biologics relative to their frequency of use, and also led to longer wait times than for other drug classes. While step therapy and prior authorization were commonly reported reasons for coverage issues, many patients and caregivers were uncertain about the reason or about next steps to take if faced with a delay or denial for their AD prescription treatments.
These data show that half of patients were currently using ≥ 3 AD prescriptions, or had done so in the past 12 months. This is consistent with previously published work which showed that patients of all ages with AD face an increased burden of out-of-pocket costs due to prescription polypharmacy [8]. The majority of patients were taking topical steroids (83.9%), which aligns with previously reported prescription patterns for AD in the USA [7, 21]. This similar cohort also reported high oral/injectable steroid use (21.9% of patients using at least 1 topical steroid currently or had done so in the past 12 months), despite systemic steroids no longer being a preferred treatment for AD [22, 23]. Our data show the additional overall burden of insurance coverage issues for all classes of prescriptions, with 645 total delays and denials in the past 12 months alone. Topical steroids accounted for the highest proportion of these coverage issues (39.2%), which is likely due to the abovementioned high rate of use (1708 prescriptions currently or in the past 12 months). As this category accounts for topical steroids of all potencies, it is possible that certain potencies contributed to this finding more than others. However, biologics faced a comparatively higher frequency and burden of delays and denials, with 43.6% of all prescriptions (109/250) encountering an insurance coverage issue. This finding aligns with the reported patient experience for other chronic conditions, such as psoriasis and psoriatic arthritis, and inflammatory bowel disease [24–26].
Wait times for approval varied widely and tended to be longer for biologics and higher cost drugs. By proposed U.S. legislation, after receiving an initial exception request, insurers must provide notice of their decision within 24 h for emergencies, or within 72 h for standard requests [27, 28]. The American Medical Association has stricter guidelines, with recommendations that insurers respond within 48 h for non-urgent requests [29]. In this study, patients reported that 90.7% of insurers’ decisions took longer than 3 days. Even when coverage for AD prescriptions is delayed or denied and eventually granted, long wait times can cause breaks in treatment [30]. Prescriptions that are denied by insurance companies may also have to be paid for in full or in part by the patient, and high out-of-pocket costs that are financially untenable can lead to unfilled prescriptions. In a 2019 survey, 40% of patients with AD reported paying out-of-pocket for prescriptions not covered by insurance [31], and in a 2016 survey, 14% of Americans said they either skipped doses or did not fill prescriptions due to the cost [32]. A recent meta-analysis from the National Pharmaceutical Council (NPC) also showed that increased patient cost-sharing was associated with worse medication initiation (67% of studies) and adherence (84% of studies), all without overall health care savings [33]. Further, challenges with timely acquisition of new or ongoing AD prescription treatments may contribute additional iatrogenic burden for patients already experiencing significant morbidity and negative impacts to quality of life [6, 34].
The most frequently cited reason for insurance delays for all AD prescriptions was prior authorization. PAs have been reported to cause a high administrative burden in dermatology clinics, with dermatologists spending multiple hours per day or hiring full-time staff to address them [17, 18]. Ultimately, this consumption of clinical resources negatively affects patient care outcomes, and can work against shared treatment decision-making efforts by HCPs and patients [15, 17, 19, 35], with patients with AD most often reporting step therapy as the reason for insurance coverage denials (27.6%). This is in line with a recent study of commercial insurance plans that found nearly 40% of prescriptions were subject to step therapy and that protocols varied greatly from plan to plan, even for the same condition [36]. While the majority (61.1%) of patients in the current study appealed insurance denials themselves, through a provider, or through an advocate, there remains a cohort of patients who either accepted the reason (20.7%) or did not accept the reason but still did not appeal (18.2%). Insurers have an opportunity to work with HCPs and patient advocacy organizations to understand the impact of delays and denials on patient care and outcomes, as well as put in place mutually beneficial policies, such as ‘gold-carding’ and offering a transparent step therapy exceptions process accessible to patients and providers. The establishment of medically reasonable circumstances for when a health plan should grant an exception request or of additional electronic processes that can expedite information sharing and review between HCPs and insurance medical directors is warranted.
Insurance coverage issues can be challenging for patients even when they know what to do or are able to work with their provider to address them. However, these difficulties are exacerbated when patients are uncertain about what to do and are perhaps unable or unwilling to communicate with their HCP or their insurer. We found that many respondents did not know the reason their prescriptions were delayed (20.8%) or denied (22.3%). In total, 44.0% said they would not know what to do if they faced an issue getting a prescription covered by their insurer, and 35.7% said they were not aware you could appeal an insurer’s decision to deny a prescription. While HCPs are not able to predict prescription medication coverage issues for all patients, they can further the desired care outcomes by advising patients with AD/caregivers on what to do if a prescription is delayed or denied. Similarly, these findings signal an opportunity for patient advocacy organizations to create educational resources for the AD community on navigating health insurance issues related to prescription treatment access.
Strengths of this study include a large cohort of patients with AD and caregivers from across the USA providing real-world assessments of their experience obtaining prescription AD treatment. The inclusion of all forms of currently available AD prescription drugs allowed for a simultaneous and accurate estimate of polypharmacy and the frequency of utilization management approaches according to AD drug class. The limitations of this study include patient recall bias, its cross-sectional nature, and inability to assess comparable information related to FDA-approved AD therapies since the time of survey administration. It is also important to note that in addition to the potential influence of recall bias, confusion around the appeal process could have contributed to slower communication between the patients and doctors, and therefore to longer reported wait times. Survey recruitment (via a convenience sample) may have contributed to selection bias since patients who have experienced coverage issues may have been more likely to respond; however, overall respondent demographics indicate a diversity of individuals related to insurance status, household income, AD disease severity, race, and ethnicity. As members of a patient advocacy organization, respondents may be more informed and aware of mechanisms to address insurance coverage issues. Consequently, the wider AD community may face an even higher prescription treatment access burden than the current data suggest.
In conclusion, these data both highlight and emphasize the unmet need for transparency and timeliness around access to topical, oral, and injectable AD prescription treatments. Additional studies are needed to better understand longitudinal changes in the patient lived experience as the AD treatment landscape continues to expand, to assess associations of delays/denials by different patient and clinical characteristics, and to further elucidate the specific impacts of prescription treatment-related access issues on AD patient clinical course and quality of life.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the participants of this study.
Author Contributions
Allison Loiselle, Raj Chovatiya, Isabelle J. Thibau, Jessica K. Johnson, Michele Guadalupe, and Wendy Smith Begolka contributed to the study conception and design. Material preparation and data collection and analysis were performed by Isabelle Thibau and Allison Loiselle. The first draft of the manuscript was written by Allison Loiselle, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Funding for this study was provided by the National Eczema Association (NEA). The National Eczema Association will also pay the Rapid Service Fee upon publication.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of Interest
Allison Loiselle, Isabelle Thibau, Jessica J Johnson, and Michele Guadalupe work for the NEA, but have no other relevant disclosures. Raj Chovatiya has served as an advisory board member, consultant, and/or speaker with personal fees for AbbVie, Apogee, Arcutis, Argenx, ASLAN, Beiersdorf, Boehringer Ingelheim, Bristol Myers Squibb, Cara Therapeutics, Dermavant, Eli Lilly and Company, Galderma, Genentech, Incyte, LEO Pharma, L’Oréal, Novan, Inc., Pfizer Inc., Regeneron, Sanofi, and UCB. Wendy Smith Begolka has served as an advisory board member for Pfizer and Amgen, received honoraria and research grants, and served as a Pfizer investigator.
Ethical Approval
This study was identified as exempt by the Western Institutional Review Board Copernicus Group under 45 CFR § 46.104(d) (2), because the research only included survey procedures with adequate measures to protect the privacy and confidentiality of participants. Prior to completing the online survey, all respondents provided electronic informed consent. Data were anonymized for analysis and treated confidentially. All methods were carried out according to the Declaration of Helsinki.
Footnotes
Prior Publication: Findings in this study were presented at the American Academy of Dermatology Conference in Boston, MA (25 March 2022) and in New Orleans, LA (17 March 2023).
References
- 1.Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Investig Dermatol. 2019;139(3):583–90. 10.1016/j.jid.2018.08.028 [DOI] [PubMed] [Google Scholar]
- 2.Yew YW, Thyssen JP, Silverberg JI. A systematic review and meta-analysis of the regional and age-related differences in atopic dermatitis clinical characteristics. J Am Acad Dermatol. 2019;80(2):390–401. 10.1016/j.jaad.2018.09.035 [DOI] [PubMed] [Google Scholar]
- 3.Vakharia PP, Chopra R, Sacotte R, et al. Burden of skin pain in atopic dermatitis. Ann Allergy Asthma Immunol. 2017;119(6):548-52.e3. 10.1016/j.anai.2017.09.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silverberg JI, Margolis DJ, Boguniewicz M, et al. Distribution of atopic dermatitis lesions in United States adults. J Eur Acad Dermatol Venereol. 2019;33(7):1341–8. 10.1111/jdv.15574 [DOI] [PubMed] [Google Scholar]
- 5.Silverberg JI, Gelfand JM, Margolis DJ, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol. 2018;121(3):340–7. 10.1016/j.anai.2018.07.006 [DOI] [PubMed] [Google Scholar]
- 6.Elsawi R, Dainty K, Smith Begolka W, et al. The multidimensional burden of atopic dermatitis among adults: results from a large national survey. JAMA Dermatol. 2022;158(8):887–92. 10.1001/jamadermatol.2022.1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh P, Silverberg J. Real-world outpatient prescription patterns for atopic dermatitis in the United States. Dermatitis. 2019;30(5):294–9. 10.1097/DER.0000000000000520 [DOI] [PubMed] [Google Scholar]
- 8.Chovatiya R, Begolka WS, Thibau I, Silverberg J. Atopic dermatitis polypharmacy and out-of-pocket healthcare expenses. J Drugs Dermatol. 2023;22:154–64. 10.36849/jdd.7038. 10.36849/jdd.7038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simpson E, Udkoff J, Borok J, Tom W, Beck L, Eichenfield L. Atopic dermatitis: emerging therapies. Semin Cutan Med Surg. 2017;36:124–30. 10.12788/j.sder.2017.0137. 10.12788/j.sder.2017.0137 [DOI] [PubMed] [Google Scholar]
- 10.Newsom M, Bashyam AM, Balogh EA, Feldman SR, Strowd LC. New and emerging systemic treatments for atopic dermatitis. Drugs. 2020;80:1041–52. 10.1007/s40265-020-01335-7. 10.1007/s40265-020-01335-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bieber T. Atopic dermatitis: an expanding therapeutic pipeline for a complex disease. Nat Rev Drug Discov. 2022;21(1):21–40. 10.1038/s41573-021-00266-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Regulations.gov. Choice (Middletown):46(06):46-2985.
- 13.ICER (Institute for Clinical and Economic Review). Fair access: coverage policies in 2022. https://icer.org/policy-papers/fair-access-2022/. Accessed 13 Apr 2024.
- 14.ICER (Institute for Clinical and Economic Review). Atopic dermatitis. 2020. https://icer.org/assessment/atopic-dermatitis-2021/. Accessed 13 Apr 2024.
- 15.Guo LN, Nambudiri VE. Impact of prior authorizations on dermatology patients: a cross-sectional analysis. J Am Acad Dermatol. 2021;85(1):217–20. 10.1016/j.jaad.2020.07.095 [DOI] [PubMed] [Google Scholar]
- 16.Beinfeld M, Emond SK, Pearson SD. P3 an assessment of barriers to fair access to cost-effective drugs. Value Health. 2022;25:S287. 10.1016/j.jval.2022.04.011. 10.1016/j.jval.2022.04.011 [DOI] [Google Scholar]
- 17.Petitt CE, Kiracofe E, Adamson A, Barbieri JS. Prior authorizations in dermatology and impact on patient care: an updated survey of US dermatology providers and staff by the American Academy of Dermatology. Dermatol Online J. 2021. 10.5070/D3271052021. 10.5070/D3271052021 [DOI] [PubMed] [Google Scholar]
- 18.Carlisle RP, Flint ND, Hopkins ZH, Eliason MJ, Duffin KC, Secrest AM. Administrative burden and costs of prior authorizations in a dermatology department. JAMA Dermatol. 2020;156:1074. 10.1001/jamadermatol.2020.1852. 10.1001/jamadermatol.2020.1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Popatia S, Flood KS, Golbari NM, et al. Examining the prior authorization process, patient outcomes, and the impact of a pharmacy intervention: a single-center review. J Am Acad Dermatol. 2019;81(6):1308–18. 10.1016/j.jaad.2019.05.024 [DOI] [PubMed] [Google Scholar]
- 20.Ripley BD. The R project in statistical computing. MSOR connections. 2001. https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.449.6899&rep=rep1&type=pdf. Accessed 13 Apr 2024.
- 21.McGregor SP, Farhangian ME, Huang KE, Feldman SR. Treatment of atopic dermatitis in the United States: analysis of data from the national ambulatory medical care survey. J Drugs Dermatol. 2017;16(3):250–5. [PubMed] [Google Scholar]
- 22.Yu SH, Drucker AM, Lebwohl M, Silverberg JI. A systematic review of the safety and efficacy of systemic corticosteroids in atopic dermatitis. J Am Acad Dermatol. 2018;78(4):733-40.e11. 10.1016/j.jaad.2017.09.074 [DOI] [PubMed] [Google Scholar]
- 23.Sidbury R, Davis DM, Cohen DE, Cordoro KM, Berger TG, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71(2):327–49. 10.1016/j.jaad.2014.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vazquez T, Forouzandeh M, Lin D, et al. Insurance delays in the approval of biologic medications for patients with psoriasis and psoriatic arthritis. Arch Dermatol Res. 2023;315(5):1401-3. 10.1007/s00403-022-02457-6. [DOI] [PubMed]
- 25.Dudiak GJ, Popyack J, Grimm C, Tyson S, Solic J, Ishmael FT. Prior authorization delays biologic initiation and is associated with a risk of asthma exacerbations. Allergy Asthma Proc. 2021;42(1):65–71. 10.2500/aap.2021.42.200101 [DOI] [PubMed] [Google Scholar]
- 26.Choi DK, Cohen NA, Choden T, Cohen RD, Rubin DT. Delays in therapy associated with current prior authorization process for the treatment of inflammatory bowel disease. Inflamm Bowel Dis. 2023;29(10):1658-61. 10.1093/ibd/izad012. [DOI] [PubMed]
- 27.Exceptions. https://www.cms.gov/Medicare/Appeals-and-Grievances/MedPrescriptDrugApplGriev/Exceptions
- 28.Congress.Gov. H.R. 2630 bill. https://www.congress.gov/118/bills/hr2630/BILLS-118hr2630ih.pdf. Accessed 13 Apr 2024.
- 29.Congress.Gov. Prior authorization and utilization management reform principles. https://www.ama-assn.org/system/files/principles-with-signatory-page-for-slsc.pdf. Accessed 13 Apr 2024.
- 30.Kesselheim AS, Huybrechts KF, Choudhry NK, et al. Prescription drug insurance coverage and patient health outcomes: a systematic review. Am J Public Health. 2015;105(2):e17-30. 10.2105/AJPH.2014.302240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chovatiya R, Begolka WS, Thibau IJ, Silverberg JI. Impact and associations of atopic dermatitis out-of-pocket health care expenses in the United States. Dermatitis. 2022;33(6S):S43-51. 10.1097/DER.0000000000000795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osborn R, Squires D, Doty MM, Sarnak DO, Schneider EC. In new survey of eleven countries, US adults still struggle with access to and affordability of health care. Health Aff. 2016;35:2327–36. 10.1377/hlthaff.2016.1088. 10.1377/hlthaff.2016.1088 [DOI] [PubMed] [Google Scholar]
- 33.Fusco N, Sils B, Graff JS, Kistler K, Ruiz K. Cost-sharing and adherence, clinical outcomes, health care utilization, and costs: a systematic literature review. J Manag Care Spec Pharm. 2022;7:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chovatiya R, Silverberg JI. Iatrogenic burden of atopic dermatitis. Dermatitis. 2022;33(6S):S17-23. 10.1097/DER.0000000000000799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khan DA. The impact of prior authorization in allergy/immunology: a position statement of the american academy of allergy, asthma & immunology. J Allergy Clin Immunol Pract. 2023;11(4):1087–8. 10.1016/j.jaip.2023.02.014. 10.1016/j.jaip.2023.02.014 [DOI] [PubMed] [Google Scholar]
- 36.Lenahan KL, Nichols DE, Gertler RM, Chambers JD. Variation in use and content of prescription drug step therapy protocols, within and across health plans. Health Aff. 2021;40(11):1749–57. 10.1377/hlthaff.2021.00822 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.