Abstract
Introduction
Patients with systemic lupus erythematosus (SLE) have variable treatment pathways, including antimalarials, glucocorticoids, immunosuppressants, and/or biologics. This study describes differences in clinical outcomes when initiating belimumab (BEL) before and after immunosuppressant use.
Methods
This real-world, retrospective cohort study (GSK Study 217536) used de-identified administrative claims data from January 2015 to December 2022 in the Komodo Health Database. Adults with moderate/severe SLE initiating BEL (index date) were identified from January 2017 to May 2022, allowing a ≥ 24-month baseline period. Patients were stratified into those initiating BEL before immunosuppressant use (no immunosuppressant use within 24 months before index) and those initiating BEL after immunosuppressant use (one immunosuppressant used within 24 months before index). Oral glucocorticoid (OGC) use, SLE flares, new organ damage, and all-cause healthcare resource utilization (HCRU) were analyzed descriptively over a 24-month follow-up.
Results
Baseline SLE severity was similar for patients initiating BEL before (n = 2295) versus after (n = 4114) immunosuppressant use (moderate, 83.1% vs 79.0%; severe, 16.8% vs 21.0%). Patients initiating BEL before versus after immunosuppressant use had lower SLE flare rates and OGC use. Post-index, patients initiating BEL before versus after immunosuppressant use discontinued their OGC sooner (moderate baseline SLE, 4.5 vs 8.9 months; severe baseline SLE, 6.2 vs 11.6 months). Patients initiating BEL before versus after immunosuppressant use had lower SLE flare rates per person-year at all time points (especially severe flare rates in patients with severe baseline SLE, 0.70 vs 1.48 through 24 months post-index). Median time to new organ damage occurrence was longer in patients initiating BEL before versus after immunosuppressant use (moderate baseline SLE, 32.1 vs 26.7 months; severe baseline SLE, 22.7 vs 21.6 months). All-cause HCRU was similar between cohorts.
Conclusions
These results suggest that patients initiating BEL before versus after immunosuppressant use had more favorable outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40744-024-00675-0.
Keywords: Belimumab, Early, Healthcare resource utilization, Immunosuppressant, Systemic lupus erythematosus
Key Summary Points
| Why carry out this study? |
| Belimumab, a human IgG1λ monoclonal antibody, has shown robust efficacy and tolerability in treating systemic lupus erythematosus (SLE) in clinical trials and real-world data. |
| The 2023 EULAR SLE treatment recommendations suggest early consideration of belimumab, with or without immunosuppressants, after inadequate response to antimalarials and oral glucocorticoids. |
| However, limited data are available on outcomes for patients initiating belimumab before or after immunosuppressant use. |
| This study described clinical outcomes in patients initiating belimumab before and after immunosuppressant use within the 24 months before belimumab initiation in a real-world setting. |
| What was learned from this study? |
| Patients initiating belimumab before immunosuppressant use had earlier oral glucocorticoid tapering, fewer flares, and longer time to new organ damage than those initiating belimumab after immunosuppressant use. |
| These results suggest that initiating belimumab before immunosuppressant use may have more favorable outcomes compared with initiating belimumab after immunosuppressant use, supporting its earlier use in the SLE treatment pathway. |
| Future research should compare the effectiveness of belimumab when initiated at different stages of the treatment pathway, considering potential differences in patient characteristics at initiating therapy. |
Introduction
Systemic lupus erythematosus (SLE) is a chronic, autoimmune disease associated with organ damage, decreased quality of life, and increased mortality [1]. While SLE can impact any organ, it affects the kidneys in approximately 50% of patients, leading to the development of lupus nephritis and, if untreated, to chronic kidney disease or end-stage kidney disease [2]. Patients with SLE are typically treated with antimalarials, glucocorticoids (GCs), and immunosuppressants (IS); however, long-term cumulative GC use and use of non-targeted IS are associated with adverse events, increased organ damage accrual, and premature death [3–8]. Therefore, SLE treatment with deleterious side effects, most notably GCs, should be used as a bridge therapy, maintained with the lowest dose for the shortest duration possible, and withdrawn when feasible [9, 10].
Belimumab, a human IgG1λ monoclonal antibody that selectively binds to and inhibits soluble B lymphocyte stimulator (BLyS), is approved as an add-on to standard therapy for treating SLE and active lupus nephritis [11]. The efficacy and tolerability of belimumab in patients with SLE have been demonstrated in various clinical and real-world studies globally, showing reduced disease activity and risk of SLE flares, which, in turn, are associated with reduced organ damage accrual and GC use, as well as an acceptable safety profile similar to that of standard therapy [12–17].
As per the European Alliance of Associations for Rheumatology (EULAR) 2023 recommendations, addition of IS and/or an approved biologic should be considered in patients not responding to first-line hydroxychloroquine (alone or in combination with GCs) or in patients unable to reduce GC dose for chronic use [10]. Prior IS use is not mandatory for initiating biologics [10]. Despite these recommendations, real-world treatment pathways in patients with SLE may not align with treatment recommendations as a result of factors such as patient characteristics, inadequate specialist care, accessibility to specific treatments due to associated high acquisition costs, and the dynamic nature of the disease [18]. Thus, as guidelines evolve, studies help to elucidate the consequences of various recommendations and their impact on patient outcomes.
A previous retrospective study, encompassing data from 466 patients across 24 centers in Italy, demonstrated that patients with SLE treated with belimumab early in the disease course experienced favorable outcomes [19]. Notably, a disease duration ≤ 2 years at belimumab initiation was a predictor of positive treatment response at 6 and 24 months. Additionally, a baseline Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (SDI) score of 0 was an independent predictor of remission in ≥ 25% of the total follow-up period. These findings suggest that the lower the disease duration and baseline damage, the greater the probability that initiating belimumab early in the disease may lead to favorable outcomes in real-life settings [19].
However, real-world data with extensive follow-up on health outcomes (including the GC use) in patients with SLE in the USA prescribed belimumab earlier in the treatment pathway are limited, necessitating further research assessing the impact of early belimumab treatment (i.e., as an alternative to, or in addition to, conventional IS). Claims databases are a viable option capturing sequential use of different therapies, but often patients do not have enrollment within a particular health plan for more than a few years. Consequently, there is a lack of data capturing the original SLE diagnosis date (and initial treatments) to identify patients with true naivety to IS and, thereby, the definitive position of belimumab within the treatment pathway. Thus, evaluating outcomes before and after IS use over a specified time period (e.g., 24 months) relative to therapy initiation is an alternative method of capturing patients’ course of treatment.
This hypothesis-generating study aimed to provide a comprehensive descriptive overview of the real-world clinical outcomes in patients in the USA with moderate or severe SLE who initiated belimumab before or after IS use during the 24 months before belimumab initiation.
Methods
Study Design
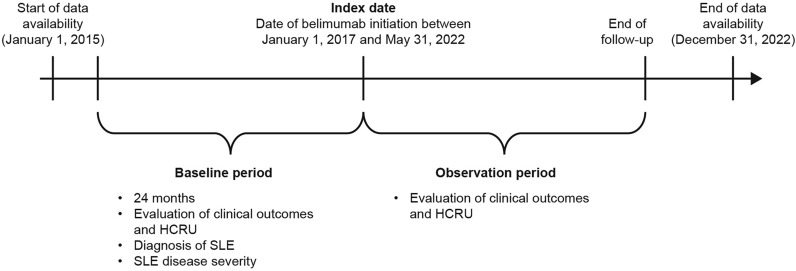
This was a retrospective, longitudinal cohort study (GSK Study 217536) in the USA using de-identified, patient-level administrative claims data from the Komodo Health database between January 2015 and December 2022 (Fig. 1). The Komodo Health database includes data of > 65 billion claims from > 325 million patients enrolled in healthcare plans in the USA with comprehensive information regarding patient demographics, diagnoses, and drugs/prescriptions [20].
Fig. 1.
Study design schematic. HCRU healthcare resource utilization; SLE systemic lupus erythematosus
Patients with SLE were identified from January 2017 until May 2022, allowing for ≥ 24 months of continuous enrollment before belimumab initiation. The index date was defined as the first claim for belimumab, and the baseline period was defined as the 24-month period prior to the index. The observation period spanned from the index date to the end of continuous eligibility or the end of data availability.
Study Population
Eligible patients were ≥ 18 years of age on the index date and filled ≥ 1 prescription for belimumab from January 2017 to May 2022 (including belimumab initiations as part of a clinical trial); diagnosis of SLE (defined on the basis of previous research [21–23]: International Classification of Diseases, 9th Revision, Clinical Modification [ICD-9-CM] 710.0x and ICD-10-CM: M32.1x, M32.8, M32.9, based on ≥ 2 outpatient medical claims or ≥ 1 inpatient/emergency department claim) before or on the index date; and ≥ 24 months continuous enrollment before the index date. Patients were excluded if they had drug-induced lupus (ICD-10-CM: M32.0; based on ≥ 1 outpatient, inpatient, or emergency department claim) any time before the index date; or prescription of belimumab or a Janus kinase inhibitor (i.e., baricitinib, tofacitinib, upadacitinib, ruxolitinib, and fedratinib) during the baseline period (other biologic treatments were permitted). Patients who had received ≥ 2 prior IS during the baseline period were not included in this study.
Cohorts
Patients were stratified into two cohorts: those initiating belimumab before IS use (i.e., patients with no IS use during the baseline period) and those initiating belimumab after IS use (i.e., patients with use of one IS during the baseline period). The IS of interest comprised azathioprine, cyclophosphamide, cyclosporine, leflunomide, methotrexate, mycophenolate mofetil/mycophenolic acid, tacrolimus, and voclosporin. Patients were further stratified by their baseline SLE disease severity, defined using a previously published algorithm based on measures of SLE disease activity and expert clinical opinion (Tables S1 and S2) [24–26]. This paper focuses on patients with moderate and severe baseline SLE, not including patients with mild baseline SLE.
Outcomes
Patient demographics (e.g., age, gender, US geographic region) and clinical characteristics (e.g., organ damage, SLE flares, GC use) were assessed during the 24-month baseline period.
Patient follow-up was over a maximum of 60 months post-index, with the current analysis focusing on the first 24 months post-index. There was no minimum follow-up period, measuring outcomes in the pre-specified patient cohorts including SLE flares, time to new organ damage, and oral GC (OGC) dose (at 6, 12, and 24 months post-index).
The average daily dose (ADD) of OGCs (prednisone equivalent) was measured as the cumulative average at each time point, calculated as the total dosage (prednisolone equivalent dose multiplied by quantity prescribed) divided by the numbers of days supplied (such as the average from days 1 to 183 for the ADD at 6 months, 1 to 365 for the ADD at 12 months). The median time to OGC discontinuation and the median time to reduction of OGC ADD to < 5 mg were measured during the follow-up period for patients with an ADD > 0 mg and ≥ 10 mg over the 3-month period prior to the index date, respectively. Discontinuation was defined as a patient with ≥ 1 OGC prescription who did not fill another OGC prescription for 120 days after the previous prescription ran out according to the fill date and days supplied on the previous prescription.
Rate and severity of SLE flares were measured using both a pre-developed, “original” definition of flare [26], and a modified definition (specifically affecting classification of moderate flares, Table S3). The modified definition was developed to reduce the risk of misclassification since IS refills could erroneously be counted as a new prescription indicative of a flare. Flare rates at 6, 12, and 24 months post-index are described.
Time to organ damage occurrence over the follow-up period was defined as the date of first post-index occurrence of an ICD-9-CM or ICD-10-CM diagnosis code indicative of new organ damage in the inpatient or outpatient setting, namely diagnosis of any of the following classes of medical conditions associated with organ damage [27]: cardiovascular, diabetes, gastrointestinal, malignancy (excluding dysplasia), musculoskeletal, neuropsychiatric, and ocular (Table S4).
All-cause healthcare resource utilization (HCRU) following belimumab initiation, comprising rates of inpatient stays and emergency department visits, was measured over the follow-up period.
Data Analysis
Patient baseline characteristics were described using frequencies and percentages for categorical variables and means, standard deviation (SD), and medians for continuous variables. For calculating rates per person-year (PPY), the sum of the incidences of the measure being evaluated (i.e., SLE flares, inpatient stays, emergency department visits) was divided by the number of patient-years at risk during each specified time point during the follow-up period (e.g., from baseline to 6 months post-index for the 6-month follow-up or from baseline to 12 months post-index for the 12-month follow-up) to account for difference in length of follow-up between patients. Time to occurrence of organ damage and time to OGC dose discontinuation or reduction were assessed using the Kaplan–Meier methodology. All analyses were carried out using SAS software (version 9.4; SAS Institute, Cary, North Carolina) and/or R software (version 3.6.3; the R Foundation). For baseline variables with missing data, the number of patients with missing data was reported in Table 1. Missing data may have led to misclassification of study outcomes and comorbidities; however, it was unlikely that this would differentially affect patients initiating belimumab before IS use and patients initiating belimumab after IS use. Therefore, no further actions (such as multiple imputation) were taken to address the missing data, as the impact of such non-differential misclassification is biased toward the null.
Table 1.
Patient baseline characteristics in patients initiating belimumab before and after IS use stratified by baseline SLE disease severity over the 24-month baseline period
| Moderate SLE at baselinea | Severe SLE at baselinea | |||
|---|---|---|---|---|
| Patients initiating belimumab before IS use (N = 1909) |
Patients initiating belimumab after IS use (N = 3252) |
Patients initiating belimumab before IS use (N = 386) |
Patients initiating belimumab after IS use (N = 862) |
|
| Age at index, years, mean ± SD [median] | 45.2 ± 12.9 [45.2] | 43.5 ± 12.9 [43.6] | 44.8 ± 13.4 [43.7] | 42.2 ± 13.8 [41.3] |
| Gender, n (%) | ||||
| Female | 1808 (94.7) | 3042 (93.5) | 352 (91.2) | 783 (90.8) |
| Male | 101 (5.3) | 208 (6.4) | 34 (8.8) | 78 (9.0) |
| Missing | 0 (0) | 2 (0.1) | 0 (0) | 1 (0.1) |
| US geographic region, n (%) | ||||
| Midwest | 277 (14.5) | 482 (14.8) | 55 (14.2) | 129 (15.0) |
| Northeast | 463 (24.3) | 704 (21.6) | 84 (21.8) | 185 (21.5) |
| South | 803 (42.1) | 1417 (43.6) | 178 (46.1) | 364 (42.2) |
| West | 349 (18.3) | 628 (19.3) | 66 (17.1) | 175 (20.3) |
| Unknown | 17 (0.9) | 21 (0.6) | 3 (0.8) | 9 (1.0) |
| Insurance, n (%) | ||||
| Commercial | 1284 (67.3) | 2081 (64.0) | 224 (58.0) | 475 (55.1) |
| Medicare/Medicaid | 578 (30.3) | 1091 (33.5) | 153 (39.6) | 369 (42.8) |
| Other | 47 (2.5) | 80 (2.5) | 9 (2.3) | 18 (2.1) |
| Index year, n (%) | ||||
| 2017 | 214 (11.2) | 333 (10.2) | 33 (8.5) | 87 (10.1) |
| 2018 | 297 (15.6) | 568 (17.5) | 74 (19.2) | 157 (18.2) |
| 2019 | 358 (18.8) | 608 (18.7) | 65 (16.8) | 142 (16.5) |
| 2020 | 366 (19.2) | 568 (17.5) | 77 (19.9) | 145 (16.8) |
| 2021 | 486 (25.5) | 869 (26.7) | 99 (25.6) | 251 (29.1) |
| 2022 | 188 (9.8) | 306 (9.4) | 38 (9.8) | 80 (9.3) |
| Proportion of patients with organ damage, n (%)b,c | ||||
| Any | 1290 (67.6) | 2223 (68.4) | 324 (83.9) | 713 (82.7) |
| Neuropsychiatric | 573 (30.0) | 862 (26.5) | 188 (48.7) | 351 (40.7) |
| Ocular | 390 (20.4) | 618 (19.0) | 83 (21.5) | 189 (21.9) |
| Diabetes | 319 (16.7) | 514 (15.8) | 81 (21.0) | 201 (23.3) |
| Cardiovascular | 355 (18.6) | 631 (19.4) | 128 (33.2) | 323 (37.5) |
| Musculoskeletal | 256 (13.4) | 401 (12.3) | 79 (20.5) | 169 (19.6) |
| Renal | 123 (6.4) | 292 (9.0) | 50 (13.0) | 176 (20.4) |
| SLE flaresc,d | ||||
| Proportion of patients with ≥ 1 flare, n (%) | ||||
| Mild | 1727 (90.5) | 3127 (96.2) | 365 (94.6) | 834 (96.8) |
| Moderate | 1886 (98.8) | 3252 (100.0) | 361 (93.5) | 860 (99.8) |
| Severe | 254 (13.3) | 490 (15.1) | 301 (78.0) | 724 (84.0) |
| Number of flares by modified definition, mean ± SD [median] | 10.3 ± 6.2 [10.0] | 14.2 ± 6.9 [13.0] | 13.1 ± 8.2 [12.0] | 19.1 ± 11.7 [17.0] |
| OGC use at indexe,f | ||||
| ADD at index, mg, mean ± SD [median] | 3.1 ± 5.2 [1.1] | 3.9 ± 6.4 [1.5] | 6.7 ± 9.6 [3.1] | 9.5 ± 12.5 [5.0] |
| Proportion of patients with ADD > 0 mg at index, n (%) | 1031 (54.0) | 1916 (58.9) | 264 (68.4) | 632 (73.3) |
| Proportion of patients with ADD > 10 mg at index, n (%) | 168 (8.8) | 378 (11.6) | 94 (24.4) | 290 (33.6) |
| All-cause HCRU, mean ± SD [median]c | ||||
| Inpatient stays | 0.6 ± 1.4 [0.0] | 0.6 ± 1.3 [0.0] | 1.6 ± 2.6 [1.0] | 1.9 ± 2.8 [1.0] |
| Emergency department visits | 1.1 ± 2.4 [0.0] | 1.2 ± 2.6 [0.0] | 2.5 ± 4.5 [1.0] | 2.5 ± 4.8 [1.0] |
ADD average daily dose, HCRU healthcare resource utilization, IS immunosuppressant, OGC oral glucocorticoid, SD standard deviation, SLE systemic lupus erythematosus
aPatients were classified into mutually exclusive groups of mild, moderate, or severe disease using a previously published algorithm, which is based on validated measures of SLE activity and the consensus of expert clinical opinion and considers medications of interest and HCRU associated with an SLE-related condition
bOrgan damage values are presented where ≥ 1 cohort had a proportion ≥ 20.0%
cEvaluated during the 24-month baseline period excluded the index date
dSLE flare episodes were identified and classified by severity on the basis of a previously published claims-based algorithm, which considers medications of interest and HCRU associated with an SLE diagnosis or SLE-related condition (also featured in the definition of moderate/severe baseline SLE)
ePrednisone equivalent dosage
fAverage daily dose of OGC at index is calculated as the average daily dose of OGC over the 3 month period prior to the index date
Ethical Approval
Permission was granted for this specific use of the Komodo Health Database. This study complied with all applicable laws regarding participant privacy and no participant contact or primary collection of individual data occurred. As this study utilized secondary data that was de-identified, it was deemed that the study did not require review/approval from an institutional review board/ethics committee or collection of informed consent.
Results
All results presented here are based on descriptive analyses and numerical differences are presented without statistical hypothesis testing.
Patient Baseline Characteristics
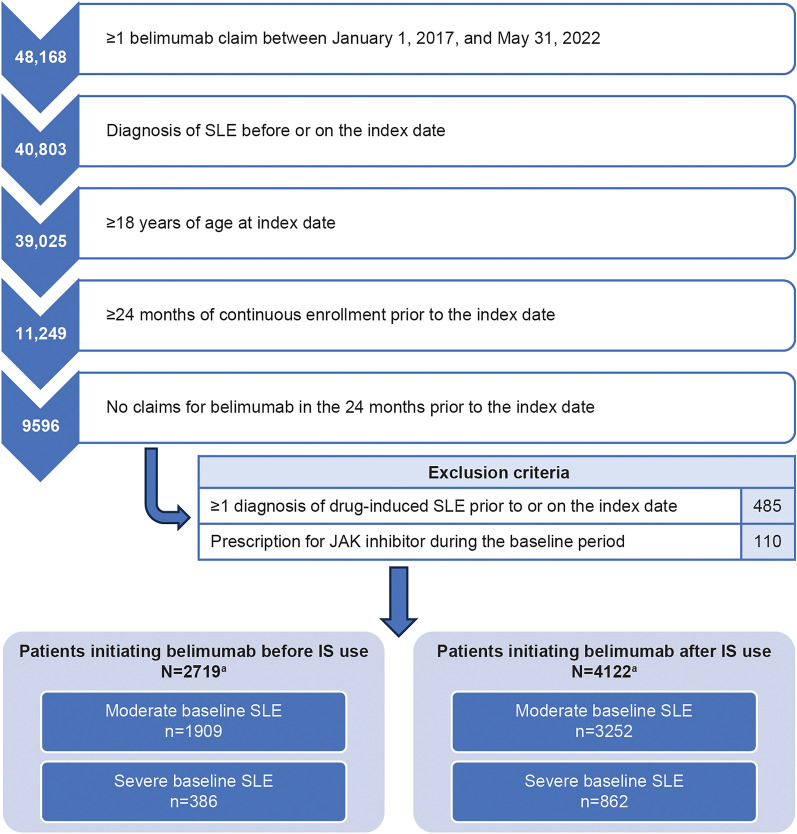
Overall, 2295 patients with moderate/severe baseline SLE were included in the cohort of patients initiating belimumab before IS use and 4114 patients with moderate/severe baseline SLE in the cohort of patients initiating belimumab after IS use (Fig. 2). The sample size of patients with mild baseline SLE among patients initiating belimumab after IS use was too small to be meaningful (n = 8) and, thus, was not evaluated.
Fig. 2.
Patient attrition. aIncluding patients with mild, moderate, and severe baseline SLE. Patients with mild SLE, as well as 2160 patients with the use of ≥ 2 IS during the baseline period, were excluded from this analysis. IS immunosuppressant, JAK Janus kinase inhibitor, SLE systemic lupus erythematosus
Both cohorts had similar proportions of patients with moderate/severe baseline SLE. Among patients initiating belimumab before IS use, 1909 patients (83.2%) had moderate and 386 (16.8%) had severe baseline SLE; among patients initiating belimumab after IS use, 3252 patients (79.0%) had moderate and 862 (21.0%) had severe baseline SLE. A higher proportion of patients with severe baseline SLE had organ damage at baseline (83.9% of patients initiating belimumab before IS use; 82.7% of patients initiating belimumab after IS use) than those with moderate baseline SLE (67.6% of patients initiating belimumab before IS use; 68.4% of patients initiating belimumab after IS use; Table 1), possibly due to an overlap in the diagnosis codes for disease severity and organ damage.
Among patients with moderate baseline SLE, fewer patients initiating belimumab before versus after IS use had a prior mild SLE flare during the baseline period; the proportions of patients with moderate or severe SLE flares were similar across both cohorts (Table 1). Among patients with severe baseline SLE, the proportion of patients with mild flares during the baseline period was similar across both cohorts. In patients with severe baseline SLE, fewer patients initiating belimumab before versus after IS use had moderate or severe SLE flares during the baseline period (Table 1). The mean number of baseline SLE flares was also lower in patients initiating belimumab before versus after IS use, regardless of the baseline SLE severity (e.g., in patients with moderate baseline SLE, 10.3 vs 14.2; in patients with severe baseline SLE, 13.1 vs 19.1). Patients initiating belimumab before IS use also had a lower OGC ADD than those initiating belimumab after IS use, regardless of the baseline SLE severity, though the difference was more prominent in patients with severe versus moderate baseline SLE; the mean (SD) ADD for patients initiating belimumab before versus after IS use was 3.1 (5.2) versus 3.9 (6.4) among those with moderate baseline SLE, and 6.7 (9.6) versus 9.5 (12.5) among those with severe baseline SLE.
With respect to all-cause HCRU, the mean number of all-cause inpatient stays and emergency department visits at baseline was consistent between both cohorts, irrespective of the baseline SLE severity. However, the mean number of visits was more than twofold greater among patients with severe baseline SLE than those with moderate baseline SLE.
Clinical Outcomes
OGC Use
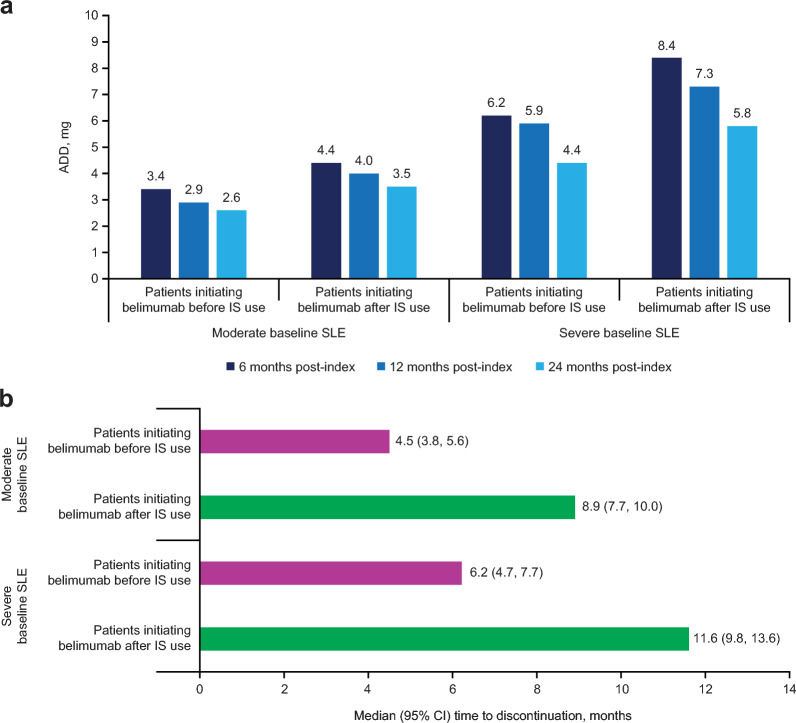
A decrease in OGC ADD was observed at each time point from 6 months in both cohorts of patients initiating belimumab before and after IS use, with a more notable reduction in patients with severe versus moderate baseline SLE (Fig. 3a). Among patients with OGC ≥10 mg/day at index, patients initiating belimumab before versus after IS use were able to discontinue or reduce their OGC ADD to <5 mg earlier, with a greater difference observed in patients with moderate baseline SLE (Table 2). Among patients with OGC ADD ≥10 mg at index, the cumulative incidence of OGC ADD reduction to <5 mg or discontinuation increased more in patients with moderate baseline SLE than those with severe baseline SLE (Table 2). In patients with OGC ADD > 0 mg at index, patients initiating belimumab before versus after IS use had a shorter median time to OGC discontinuation, with slightly more pronounced difference observed in patients with severe baseline SLE (Fig. 3b).
Fig. 3.
ADD of OGC during the follow-up period (a), and time to OGC discontinuation in patients with ADD >0 mg at index (b). ADD average daily dose, CI confidence interval, IS immunosuppressant, OGC oral glucocorticoid, SLE systemic lupus erythematosus
Table 2.
OGC discontinuation or reduction following belimumab initiation before and after IS use stratified by baseline SLE disease activity
| n (%) | Moderate baseline SLE | Severe baseline SLE | ||
|---|---|---|---|---|
| Patients initiating belimumab before IS use (N = 1909) |
Patients initiating belimumab after IS use (N = 3252) |
Patients initiating belimumab before IS use (N = 386) |
Patients initiating belimumab after IS use (N = 862) |
|
| Median (95% CI) time to OGC discontinuation or reduction to ADD < 5 mg in patients with OGC ADD ≥ 10 mg at index, months | 5.9 (4.7, 7.7) | 9.0 (7.1, 11.1) | 6.8 (4.6, 8.4) | 8.7 (6.6, 11.2) |
| Proportion of patients with cumulative incidence of OGC discontinuation or reduction of OGC ADD to < 5 mg in patients with OGC ADD ≥ 10 mg at index, n (%) | ||||
| 6 months | 83 (51.0) | 150 (40.9) | 43 (47.5) | 117 (42.2) |
| 12 months | 109 (69.9) | 206 (58.5) | 57 (64.9) | 155 (57.6) |
| 24 months | 128 (90.6) | 248 (76.2) | 66 (79.2) | 186 (75.2) |
Note: OGC dose = prednisone equivalent dose
ADD average daily dose, CI confidence interval, IS immunosuppressant, OGC oral glucocorticoid, SLE systemic lupus erythematosus
SLE Flares
Patients initiating belimumab before versus after IS use had lower rates of all severity SLE flares (modified definition) at all follow-up time points up to 24 months, regardless of baseline SLE severity (Table 3). The difference was more notable with moderate and severe SLE flares, especially in patients with severe baseline SLE. There was at least a twofold difference in the rate of severe flares between patients with severe baseline SLE initiating belimumab before IS use and those initiating belimumab after IS use at 6 and 24 months of follow-up (0.99 vs 2.07 PPY and 0.70 vs 1.48 PPY, respectively). The difference in the rate of severe flares in patients initiating belimumab before versus after IS use was smaller in patients with moderate baseline SLE (e.g., 0.35 vs 0.50 at 6 months).
Table 3.
Rates of SLE flares, as measured by modified definition, following initiation of belimumab before and after IS use stratified by baseline SLE disease severity
| Modified definition of SLE flaresa,b | ||||
|---|---|---|---|---|
| Moderate baseline SLE | Severe baseline SLE | |||
| Patients initiating belimumab before IS use (N = 1909) |
Patients initiating belimumab after IS use (N = 3252) |
Patients initiating belimumab before IS use (N = 386) |
Patients initiating belimumab after IS use (N = 862) |
|
| Rate of mild flares, PPYc, mean ± SEd | ||||
| 6 months | 4.63 ± 0.071 | 5.20 ± 0.058 | 4.91 ± 0.163 | 5.27 ± 0.114 |
| 12 months | 4.33 ± 0.050 | 4.87 ± 0.041 | 4.56 ± 0.115 | 5.09 ± 0.081 |
| 24 months | 4.27 ± 0.038 | 4.76 ± 0.031 | 4.40 ± 0.087 | 5.02 ± 0.063 |
| Rate of moderate flares, PPYc, mean ± SEd | ||||
| 6 months | 2.12 ± 0.048 | 3.51 ± 0.047 | 2.74 ± 0.122 | 4.75 ± 0.108 |
| 12 months | 1.96 ± 0.033 | 2.87 ± 0.031 | 2.42 ± 0.084 | 3.85 ± 0.071 |
| 24 months | 1.84 ± 0.025 | 2.56 ± 0.023 | 2.29 ± 0.063 | 3.45 ± 0.052 |
| Rate of severe flares, PPYc, mean ± SEd | ||||
| 6 months | 0.35 ± 0.019 | 0.50 ± 0.018 | 0.99 ± 0.073 | 2.07 ± 0.071 |
| 12 months | 0.32 ± 0.013 | 0.44 ± 0.012 | 0.89 ± 0.051 | 1.71 ± 0.047 |
| 24 months | 0.28 ± 0.010 | 0.40 ± 0.009 | 0.70 ± 0.035 | 1.48 ± 0.034 |
HCRU healthcare resource utilization, IS immunosuppressant, PPY per person-year, SE standard error, SLE systemic lupus erythematosus
aSLE flare episodes were identified and classified by severity on the basis of a previously published claims-based algorithm, which considers medications of interest and HCRU associated with an SLE diagnosis or SLE-related condition flare [26]
bThe modified definition categorized initiation of IS as filling a prescription for an immunosuppressant > 60 days after the days’ supply of the former prescription ended
cRate of PPY is calculated as the total number of visits across all patients divided by the total person-time of observation
dSE of the population incidence rate is calculated as √(total number of events)/(total follow-up time)
Similarly, the rate of moderate flares was lower in patients initiating belimumab before versus after IS use, particularly in patients with severe baseline SLE with at least a 1.5-fold difference between groups at 6, 12, and 24 months (Table 3). This trend of a lower mean rate of moderate flares in patients initiating belimumab before versus after IS use was also observed using the original definition of flare (patients with moderate baseline SLE, 2.22 vs 4.71 PPY at 6 months, 2.16 vs 4.31 PPY at 12 months, 2.12 vs 4.08 PPY at 24 months; patients with severe baseline SLE, 2.85 vs 6.13 PPY at 6 months, 2.67 vs 5.47 PPY at 12 months, 2.63 vs 5.19 PPY at 24 months). The rates of moderate flare were lower using the modified definition compared with the original definition.
Time to Organ Damage
Among patients with moderate baseline SLE, 67.6% and 68.4% of patients initiating belimumab before and after IS use, respectively, had organ damage during the baseline period. Among patients with severe baseline SLE, 83.9% and 82.7% of patients initiating belimumab before and after IS, respectively, had organ damage during the baseline period (Table 1). These patients were excluded from the post-index organ damage analysis. Among patients with moderate baseline SLE, the median (95% confidence interval [CI]) time to organ damage was > 5 months longer in patients initiating belimumab before IS use (32.1 [26.8, 37.5] months) than in those initiating belimumab after IS use (26.7 [24.7, 29.9] months). Among patients with severe baseline SLE, the median (95% CI) time to new organ damage accrual was 1 month longer for patients initiating belimumab before versus after IS use (22.7 [14.4, not evaluable] vs 21.6 [11.9, 34.8]). The cumulative incidence of organ damage was similar between both cohorts, though patients with severe versus moderate baseline SLE had a higher cumulative incidence of organ damage at 24 months. The cumulative incidence of organ damage for patients initiating belimumab before and after IS use was 42.7% and 45.4% among patients with moderate baseline SLE, and 53.6% and 51.4% among patients with severe baseline SLE.
All-Cause HCRU
Over the follow-up period, among patients with moderate baseline SLE, the mean rates PPY of all-cause inpatient stays and emergency department visits were generally similar across both cohorts. The rate of inpatient stays was between 0.34 and 0.37 over the 24-month period for patients initiating belimumab before IS use, and 0.31 and 0.32 for patients initiating belimumab after IS use. For emergency department visits, rates were 0.55–0.60 and 0.62–0.64 for patients initiating belimumab before and after IS use, respectively. Among patients with severe baseline SLE, the mean rates of inpatient stays PPY were slightly lower in patients initiating belimumab before versus after IS use (0.63–0.68 vs 0.80–0.94). The mean rates of emergency department visits appeared to be similar across both cohorts, ranging from 1.11 to 1.25 for patients initiating belimumab before IS use and 1.10 to 1.22 for patients initiating belimumab after IS use.
Discussion
To our knowledge, this is the first real-world study to describe clinical outcomes and all-cause HCRU in patients with SLE initiating belimumab before and after IS use during the baseline period. Following belimumab initiation, improvements in clinical outcomes were observed in both cohorts, though the results suggest earlier use of belimumab in the treatment pathway may yield greater benefits on clinical outcomes. Specifically, patients initiating belimumab before IS use were able to discontinue or reduce their OGC dose sooner than patients initiating belimumab after IS use. Furthermore, patients initiating belimumab before versus after IS use had notably lower SLE flare rates and later median time to organ damage occurrence.
The 2023 EULAR recommendations supporting the earlier use of biologics (i.e., following non-response to hydroxychloroquine with or without GCs, and with no requirement for prior IS use) were proposed following the demonstrated efficacy of biologics in randomized clinical trials, in the absence of robust evidence supporting the use of conventional IS [10]. The results of this study support these recommendations, presenting novel evidence for the early use of belimumab in the treatment pathway. Without data on diagnosis in many databases to establish disease duration, assessing position in treatment pathways can provide complementary evidence. Although we did not analyze the disease duration data because of the inability to establish the date of SLE diagnosis, our results are in line with those reported by Gatto et al. (2020), demonstrating that earlier placement of belimumab in the treatment pathway may lead to favorable outcomes in the real-life setting [19].
Per EULAR 2023 recommendations for SLE treatment and management, GCs should be minimized to ≤ 5 mg/day and withdrawn when possible [10], since there are dose-dependent adverse effects associated with long-term cumulative GC use, including organ damage, with 2.8% increased risk for each 1 mg of prednisone equivalent per day [28]. In the current study, while most patients were able to discontinue or reduce their OGC use within 12 months post-index, patients initiating belimumab before IS use were able to discontinue or reduce their OGC dose to < 5 mg/day in a shorter time than those initiating belimumab after IS use. This may translate to a lower risk of side effects, including new organ damage accrual, in patients who initiate belimumab earlier in the treatment pathway. Consistently, a longer median time to incident organ damage was observed in patients initiating belimumab before IS use compared with those initiating belimumab after IS use.
The rates of SLE flares PPY were lower at all time points for patients initiating belimumab before versus after IS use, especially in patients with severe baseline SLE. The definition of SLE flares used in this study was modified from the original definition [26]. For the original definition of flare, IS initiation was categorized as filling of a prescription > 60 days after the previous prescription fill date. In the case of a prescription for 60 days of treatment, for example, if the patient received a new prescription at 61 days, the IS would be described as “initiated” although there was no break in treatment. In the modified definition, this concern is mitigated as a gap of > 60 days between medication supply is required rather than between the prescriptions itself, leading to distinction between initiation and repeat prescriptions. This is evidenced by the fact that fewer moderate flares were identified when comparing the modified versus original flare definitions. The flare rates (both modified and original definitions) were notably lower in patients initiating belimumab before versus after IS use. However, some differences were observed between cohorts during the baseline period, so descriptive comparisons in the observation period should not be interpreted inferentially.
Despite the suggested clinical benefits of early belimumab initiation, the rates of all-cause HCRU were similar across both cohorts. As this was all-cause utilization, it could be reflective of continued treatment of co-morbid conditions among both cohorts. Future research could evaluate the potential economic benefits of the earlier placement of belimumab in the treatment pathway in relation to SLE-specific HCRU and confounding factors such as organ damage and types of therapy before/after IS use.
Limitations
Some of the limitations specific to this study suggest the potential for improvement in further research, notably regarding outcome measures and cohort matching. While patients were stratified by disease severity, this does not eradicate differences in baseline clinical characteristics between patients initiating belimumab before and after IS use. Indeed, patients initiating belimumab before versus after IS use appeared to have slightly lower SLE disease activity, evidenced by fewer SLE flares and lower OGC doses at baseline. Furthermore, selection bias may have influenced results, as physicians might have channelled patients with high SLE disease activity to IS therapy before belimumab initiation. Thus, more robust analyses are needed to explore outcomes further while attempting to rigorously adjust for baseline differences between the cohorts.
In terms of outcome measures, the definition of organ damage was broad: 68% of patients with moderate baseline SLE and 83–84% of patients with severe baseline SLE met the criteria for organ damage at baseline, resulting in their exclusion from the organ damage analysis, which could present a potential for selection bias. The definition of SLE flare was also a potential study limitation; IS use in the moderate flare definition may have resulted in an upward bias in patients initiating belimumab after IS use due to the requirement that they have previously used IS. The modified definition helps to mitigate this concern but does not eliminate this bias. In addition, the flare algorithm does not consider parenteral GCs, possibly resulting in an underestimation of flares.
Assumptions regarding medication usage are common in studies using pharmacy claims to examine medication utilization [29]. The presence of a dispensed medication does not guarantee that the medication was taken as prescribed, which could impact the evaluated outcomes. Furthermore, medications not recorded in the claims data, such as medications received during inpatient stays, were not accounted for in the analysis. Both factors may have resulted in inaccurate study cohort assignment and underestimation of measures that rely on observed medications, such as SLE flares. Further to the point of medication usage, adherence to belimumab was not measured in this study, so long-term outcomes may be impacted by changes in treatment regimens over time.
The study was also subject to limitations inherent to administrative claims data. Variables such as clinical biomarkers and data on patient-centric variables (e.g., disease activity) are unavailable and claims-based proxies for SLE diagnosis, flares, and disease severity might have introduced measurement error. For example, the diagnosis of SLE relied on ICD-9/ICD-10 codes, which may have led to misclassification or underrepresentation of patients. Furthermore, the identification of organ damage in this study also relied on the presence of the ICD-9/ICD-10 codes, as organ damage measures such as SDI are not routinely captured in real-world databases or administrative claims data. Thus, it is possible that inaccuracies or misclassification bias may have occurred.
Conclusions
This hypothesis-generating descriptive analysis indicates a potential benefit of early belimumab use in facilitating OGC tapering or discontinuation at a faster rate than patients receiving belimumab later in the treatment pathway (i.e., after IS use), which could have benefits in reducing GC-related adverse events, including organ damage, as well as reducing rate of flares. This study adds to real-world evidence supporting belimumab’s use in clinical practice. However, there also is an opportunity for future research for more extensively evaluating the causal relationship between early belimumab use and clinical improvement, using both controlled trials and real-world observational studies.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Medical Writing, Editorial, and Other Assistance
Medical writing support was provided by Tasmin Long, PGCert, Fishawack Indicia Ltd., UK, part of Avalere Health, and was funded by GSK.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published.
Author Contributions
B Rubin, YC, KW, RC, and MD contributed to the conception or design of the study. YC, B Rabideau, BW and MD contributed to the acquisition of the data. All authors contributed to the analysis and/or interpretation of the data.
Funding
This study (GSK Study 217536) was funded by GSK. The journal’s Rapid Service and Open Access Fees were funded by GSK.
Data Availability
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Declarations
Conflict of Interests
B Rubin was an employee of GSK at the time of the study and holds stocks and shares in GSK. YC, B Rabideau, BW, RC and MD are employees of the Analysis Group, which received grant/research support from GSK. KW is an employee of GSK and holds stocks and shares in GSK.
Ethical Approval
Permission was granted for this specific use of the Komodo Health Database. This study complied with all applicable laws regarding participant privacy and no participant contact or primary collection of individual data occurred. As this study utilized secondary data that was de-identified, it was deemed that the study did not require review/approval from an institutional review board/ethics committee or collection of informed consent.
Footnotes
Prior Presentation: Elements of this research were previously presented at the American College of Rheumatology Convergence on November 10–15, 2023, in San Diego, California, USA: Chen Y, et al. Arthritis Rheumatol. 2023;75 (suppl 9).
Affiliation for Bernard Rubin was correct at the time of the study.
References
- 1.Bruce IN, O’Keeffe AG, Farewell V, et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort. Ann Rheum Dis. 2015;74(9):1706–13. 10.1136/annrheumdis-2013-205171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almaani S, Meara A, Rovin BH. Update on lupus nephritis. Clin J Am Soc Nephrol. 2017;12(5):825–35. 10.2215/CJN.05780616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apostolopoulos D, Kandane-Rathnayake R, Louthrenoo W, et al. Factors associated with damage accrual in patients with systemic lupus erythematosus with no clinical or serological disease activity: a multicentre cohort study. Lancet Rheumatol. 2020;2(1):e24–30. 10.1016/S2665-9913(19)30105-5 [DOI] [PubMed] [Google Scholar]
- 4.Gladman DD, Urowitz MB, Rahman P, Ibañez D, Tam LS. Accrual of organ damage over time in patients with systemic lupus erythematosus. J Rheumatol. 2003;30(9):1955–9. [PubMed] [Google Scholar]
- 5.Magder LS, Petri M. Incidence of and risk factors for adverse cardiovascular events among patients with systemic lupus erythematosus. Am J Epidemiol. 2012;176(8):708–19. 10.1093/aje/kws130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez R, Davidson JE, Beeby MD, Egger PJ, Isenberg DA. Lupus disease activity and the risk of subsequent organ damage and mortality in a large lupus cohort. Rheumatology (Oxford). 2012;51(3):491–8. 10.1093/rheumatology/ker368 [DOI] [PubMed] [Google Scholar]
- 7.Segura BT, Bernstein BS, McDonnell T, et al. Damage accrual and mortality over long-term follow-up in 300 patients with systemic lupus erythematosus in a multi-ethnic British cohort. Rheumatology (Oxford). 2020;59(3):524–33. 10.1093/rheumatology/kez516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheane BJ, Gladman DD, Su J, Urowitz MB. Disease outcomes in glucocorticosteroid-naive patients with systemic lupus erythematosus. Arthritis Care Res. 2017;69(2):252–6. 10.1002/acr.22938 [DOI] [PubMed] [Google Scholar]
- 9.van Vollenhoven RF, Mosca M, Bertsias G, et al. Treat-to-target in systemic lupus erythematosus: recommendations from an international task force. Ann Rheum Dis. 2014;73(6):958–67. 10.1136/annrheumdis-2013-205139 [DOI] [PubMed] [Google Scholar]
- 10.Fanouriakis A, Kostopoulou M, Andersen J, et al. EULAR recommendations for the management of systemic lupus erythematosus: 2023 update. Ann Rheum Dis. 2024;83(1):15–29. 10.1136/ard-2023-224762 [DOI] [PubMed] [Google Scholar]
- 11.GSK. Belimumab (Benlysta). Highlights of prescribing information. 2023. https://gskpro.com/content/dam/global/hcpportal/en_US/Prescribing_Information/Benlysta/pdf/BENLYSTA-PI-MG-IFU.PDF. Accessed 12 Sept 2023.
- 12.Furie R, Petri M, Zamani O, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011;63(12):3918–30. 10.1002/art.30613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Navarra SV, Guzman RM, Gallacher AE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9767):721–31. 10.1016/S0140-6736(10)61354-2 [DOI] [PubMed] [Google Scholar]
- 14.Collins CE, Dall’Era M, Kan H, et al. Response to belimumab among patients with systemic lupus erythematosus in clinical practice settings: 24-month results from the OBSErve study in the USA. Lupus Sci Med. 2016;3(1):e000118. 10.1136/lupus-2015-000118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins CE, Cortes-Hernández J, Garcia MA, et al. Real-world effectiveness of belimumab in the treatment of systemic lupus erythematosus: pooled analysis of multi-country data from the OBSErve studies. Rheumat Ther. 2020;7(4):949–65. 10.1007/s40744-020-00243-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Kempis J, Duetsch S, Reuschling N, et al. Clinical outcomes in patients with systemic lupus erythematosus treated with belimumab in clinical practice settings: a retrospective analysis of results from the OBSErve study in Switzerland. Swiss Med Wkly. 2019;149:w20022. [DOI] [PubMed] [Google Scholar]
- 17.Levy RA, Gonzalez-Rivera T, Khamashta M, et al. 10 Years of belimumab experience: what have we learnt? Lupus. 2021;30(11):1705–21. 10.1177/09612033211028653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kariburyo F, Xie L, Sah J, Li N, Lofland JH. Real-world medication use and economic outcomes in incident systemic lupus erythematosus patients in the United States. J Med Econ. 2020;23(1):1–9. 10.1080/13696998.2019.1678170 [DOI] [PubMed] [Google Scholar]
- 19.Gatto M, Saccon F, Zen M, et al. Early disease and low baseline damage as predictors of response to belimumab in patients with systemic lupus erythematosus in a real-life setting. Arthritis Rheumatol. 2020;72(8):1314–24. 10.1002/art.41253 [DOI] [PubMed] [Google Scholar]
- 20.Komodo Health, Inc. Perspectives and insights from Komodo Health about the real-world evidence and technology innovations that can reduce the burden of disease. 2023. https://www.komodohealth.com/insights. Accessed Oct 2023.
- 21.Huang SP, DerSarkissian M, Gu YM, et al. Prolonged oral corticosteroid treatment in patients with systemic lupus erythematosus: an evaluation of 12-month economic and clinical burden. J Manag Care Spec Pharm. 2023;29(4):365–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang SP, DerSarkissian M, Gu YM, et al. Health care costs with sustained oral corticosteroid use in systemic lupus erythematosus. Clin Ther. 2023;45(7):619–26. 10.1016/j.clinthera.2023.04.013 [DOI] [PubMed] [Google Scholar]
- 23.DerSarkissian M, Gu YM, Duh MS, et al. Clinical and economic burden in patients with systemic lupus erythematosus during the first year after initiating oral corticosteroids: a retrospective US database study. ACR Open Rheumatol. 2023;5(6):318–28. 10.1002/acr2.11550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Narayanan S, Wilson K, Ogelsby A, Juneau P, Durden E. Economic burden of systemic lupus erythematosus flares and comorbidities in a commercially insured population in the United States. J Occup Environ Med. 2013;55(11):1262–70. 10.1097/JOM.0000000000000008 [DOI] [PubMed] [Google Scholar]
- 25.Shah M, Chaudhari S, McLaughlin TP, et al. Cumulative burden of oral corticosteroid adverse effects and the economic implications of corticosteroid use in patients with systemic lupus erythematosus. Clin Ther. 2013;35(4):486–97. 10.1016/j.clinthera.2013.03.001 [DOI] [PubMed] [Google Scholar]
- 26.Garris C, Jhingran P, Bass D, Engel-Nitz NM, Riedel A, Dennis G. Healthcare utilization and cost of systemic lupus erythematosus in a US managed care health plan. J Med Econ. 2013;16(5):667–77. 10.3111/13696998.2013.778270 [DOI] [PubMed] [Google Scholar]
- 27.Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996;39(3):363–9. 10.1002/art.1780390303 [DOI] [PubMed] [Google Scholar]
- 28.Al Sawah S, Zhang X, Zhu B, et al. Effect of corticosteroid use by dose on the risk of developing organ damage over time in systemic lupus erythematosus-the Hopkins Lupus Cohort. Lupus Sci Med. 2015;2(1):e000066. 10.1136/lupus-2014-000066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;58(4):323–37. 10.1016/j.jclinepi.2004.10.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.