Abstract
Human immunodeficiency virus type 1 (HIV-1) requires, in addition to CD4, coreceptors of the CC or CXC chemokine families for productive infection of T cells and cells of the monocyte-macrophage lineage. Based on the hypothesis that coreceptor expression on alveolar macrophages (AM) may influence HIV-1 infection of AM in the lung, this study analyzes the expression and utilization of HIV-1 coreceptors on AM of healthy individuals. AM were productively infected with five different primary isolates of HIV-1. Levels of surface expression of CCR5, CXCR4, and CD4 were low compared to those of blood monocytes, but CCR3 was not detectable. mRNA for CCR5, CXCR4, CCR2, and CCR3 were all detectable, but to varying degrees and with variability among donors. Expression of CCR5, CXCR4, and CCR2 mRNA was downregulated following stimulation with lipopolysaccharide (LPS). In contrast, secretion of the chemokines RANTES, MIP-1α, and MIP-1β was upregulated with LPS stimulation. Interestingly, HIV-1 replication was diminished following LPS stimulation. Infection of AM with HIV-1 in the presence of the CC chemokines demonstrated blocking of infection. Together, these studies demonstrate that AM can be infected by a variety of primary HIV-1 isolates, AM express a variety of chemokine receptors, the dominant coreceptor used for HIV entry into AM is CCR5, the expression of these receptors is dependent on the state of activation of AM, and the ability of HIV-1 to infect AM may be modulated by expression of the chemokine receptors and by chemokines per se.
The human immunodeficiency virus type 1 (HIV-1) requires interaction of the viral envelope glycoprotein gp120 with CD4 and a second coreceptor for productive infection of its target cell (4, 5, 9, 19, 34). These recently identified coreceptors include the β-chemokine receptors (CCR5, CCR3, and CCR2b) and the α-chemokine receptor CXCR4 (2, 3, 11, 16, 20, 21, 23, 24, 46–48). HIV-1 tropism and entry cofactor utilization are important determinants of pathogenesis (4, 5, 9, 19, 34). During primary HIV-1 infection and throughout the asymptomatic phase of infection, isolates from blood are predominantly macrophagetropic and CCR5 dependent (7, 15, 52, 53). In contrast, strains that emerge later in many infected individuals can use CXCR4, the main coreceptor for HIV-1 infection of T cells (7, 15, 52, 53, 58).
The focus of the present study is to characterize the pattern and usage of the HIV-1 coreceptors on healthy human alveolar macrophages (AM), the pulmonary representative of the mononuclear phagocyte system. Other than evidence of productive infection of AM in HIV-1-positive individuals (1, 12, 30, 35, 38–40, 45, 50), little is known about the interactions of HIV-1 with this cell type. Pulmonary infections are a major cause of the morbidity and mortality associated with infection with HIV-1, and a majority of individuals with AIDS develop one or more episodes of pulmonary infection during the course of their disease (29, 36). AM represent the major cellular host defense against microorganisms on the respiratory epithelial surface (6, 43). In this context, understanding the mechanisms of HIV-1 infection of AM may be central to understanding the loss of respiratory epithelial surface host defense associated with HIV-1 infection.
Based on the knowledge that AM are differentiated from blood monocytes and that HIV-1 mainly uses CCR5 as a coreceptor on blood monocytes and in vitro monocyte-derived macrophages (6, 9, 34, 42, 61, 64) but that the type and level of coreceptor expression on monocytes can be influenced by differentiation and activation (8, 18, 37, 44, 45), it is reasonable to assume that the coreceptors are expressed on AM. Interestingly, the data demonstrate that the coreceptor expression on healthy human AM generally parallels that of autologous blood monocytes. However, most coreceptor expression on AM is markedly lower and is only mildly influenced by activation. Concomitant production of chemokines such as RANTES, MIP-1α, and MIP-1β may also markedly influence the ability of HIV-1 to infect AM.
MATERIALS AND METHODS
Cells.
Human AM were obtained by bronchoalveolar lavage from healthy volunteers as previously described (49). The lavage fluid was filtered through gauze to remove debris and cells were pelleted, washed with phosphate-buffered saline (PBS) (pH 7.4) and resuspended in RPMI 1640 medium containing 10% fetal bovine serum, 2 mM glutamine, 100 U of penicillin/ml, and 10 μg of streptomycin (GIBCO BRL, Gaithersburg, Md.)/ml. For most experiments, AM were purified by adherence to plastic (2 h, 37°C). For flow cytometry studies, the cells were cultured in Teflon-coated vials (Savillex Corp., Minnetonka, Minn.) until evaluation. Peripheral blood monocytes (PBM) and peripheral blood lymphocytes (PBL) were obtained from the blood of the AM donors and purified by Ficoll gradients. The monocytes were separated from the lymphocytes by adherence and maintained in RPMI 1640 media containing 10% human serum, 100 U of penicillin/ml, and 10 μg of streptomycin/ml for 18 h. For RNA analysis, PBM and PBL were isolated by using immunomagnetic beads (Dynal, Lake Success, N.Y.) coated with anti-CD14 for the isolation of monocytes and with anti-CD3 for the isolation of lymphocytes.
Infection with HIV-1 primary isolates.
AM were cultured in 48-well plates and infected with five different primary HIV-1 isolates with known coreceptor usage (AD2-3 [CCR5], AD2-6 [CCR5 and CXCR4], AD3-3 [CCR5], AD3-7 [CCR5, CCR2b, CCR3, and CXCR4] [15], and JRFL [CCR5]). Twenty-four hours following infection, the cells were washed, and fresh medium was added. Productive infection was determined by measuring HIV-1 p24 antigen in the supernatant by enzyme-linked immunosorbent assay (ELISA) (Abbott Laboratories, North Chicago, Ill.) 5, 8, and 14 days after infection.
Flow cytometry.
To analyze surface HIV-1 coreceptor expression on AM, PBM, and PBL, the cells were incubated with PBS containing 2% bovine serum albumin and 10% human serum (4°C for 15 min), followed by incubation with primary antibodies against CCR5 (2D7), CCR3 (7B11), CXCR4 (12G5) (all antibodies were obtained from the AIDS Research and Reference Reagent Program, National Institutes of Health, Bethesda, Md.). After washing with PBS, the cells were incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin G (IgG) [F(ab′)2] fragments (Boehringer Mannheim, Indianapolis, Ind.) or FITC-conjugated anti-CD4 (Pharmingen, San Diego, Calif.) for 30 min. The cells were then washed and incubated with 10% mouse serum for 15 min, followed by incubation with phycoerythricin (PE)-labeled antibodies against HLA-DR (AM), CD14 (PBM), or CD3 (PBL) (Pharmingen), washed, and then analyzed by flow cytometry. Isotype-matched unlabeled and PE-labeled antibodies served as negative controls. To analyze CCR5 surface expression using antibodies other than clone 2D7, the FITC-conjugated primary antibodies recognizing CCR5 (FAB180F, FAB181F, FAB182F, and FAB183F [all from R&D Systems, Minneapolis, Minn.]) were used to stain AM, PBM, and PBL as described above. FITC-labeled isotype-matched antibody was used as a control.
mRNA analysis.
Two strategies were used to evaluate coreceptor mRNA in the AM in comparison to PBM and PBL, reverse transcription (RT)-PCR and Northern analysis. For RT-PCR, total RNA was extracted from AM, PBM (CD14 purified), or PBL (CD3 purified) by using Trizol reagent (GIBCO BRL) and reverse transcribed (45 min, 48°C; 2 min, 94°C), and the resulting DNA was amplified by PCR (9600 Gene Amp; Perkin-Elmer) by 40 cycles of 94°C for 30 s, 56°C for 1 min, and 68°C for 2 min by using synthetic oligonucleotide primers specific for CCR3 (sense primer, TCCACACTCGAGAATGACCATCT; antisense primer, ACTGGAAGTTTGAAGGACTGTTTT; product size, 578 bp), CCR5 (sense primer, CAGGGCTGTGAGGCTTATCTT; antisense primer, CCCAGGCTGTGTATGAAAACT; product size, 437 bp), CXCR4 (sense primer, TTGTCTGAACCCCATCCTCTAT; antisense primer, ACTCCTGAAAACTGAAAAACCA; product size, 626 bp), CCR2B (sense primer, CCAACGAGAGCGGTGAAGAAGT; antisense primer, GGGAGTCCAGAAGAGAAAGTAAACA; product size, 737 bp), and CD4 (sense primer, AGTTGCATCAGGAAGTGAACCT; antisense primer, CTGAGACATCCGCTCTGCTTGG; product size, 383 bp). Primers for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (sense primer, CCTTCATTGACCTCAACTACA; antisense primer, GGCAGTGATGGCATGGACTGT; product size, 443 bp) served as an internal control. The PCR products were analyzed on a 1.5% agarose gel. DNA contamination was ruled out by pretreatment of the samples with DNase (GIBCO BRL) for 15 min at 37°C and by omitting the reverse transcriptase from the PCR as a control.
To analyze coreceptor expression by Northern analysis, total cellular RNA (10 μg) was transferred to Duralon membranes (Stratagene, La Jolla, Calif.) after electrophoretic separation through a 1% agarose gel under denaturing conditions. Probes for CCR5, CCR3, CCR2B, and CXCR4 (kindly provided by Ned Landau, Aaron Diamond AIDS Research Center, New York, N.Y.) were gel purified and labeled with [32P]dCTP by random priming (Stratagene). Hybridizations were performed in hybridization solution (Quickhyb; Stratagene) for 2 h at 65°C, followed by sequential washes in 1× SSC (0.15 M NaCl plus 0.015 M sodium citrate)–0.1% sodium dodecyl sulfate (SDS) for 30 min and 0.1% SSC–0.1% 2× SDS for 30 min. Following hybridization, the membranes were analyzed by autoradiography or phosphorimaging.
Influence of AM stimulation on HIV-1 coreceptor expression.
To analyze if stimulation of AM influenced the expression of the HIV coreceptors, AM were treated with 100 ng of lipopolysaccharide (LPS)/ml for either 4 or 48 h. Total RNA was extracted and analyzed for coreceptor expression by RT-PCR and Northern analysis as described above. To determine if LPS stimulation resulted in increased secretion of chemokines, the levels of MIP-1α, MIP-1β, RANTES, and eotaxin in the supernatant were quantified by ELISA (R&D Systems).
To evaluate if stimulation with LPS influences HIV-1 infection and replication, AM were inoculated with 200 50% tissue culture infective doses (TCID50) of five different primary isolates as described above after overnight stimulation with 100 ng of LPS/ml. HIV-1 p24 levels were measured in the culture supernatant by ELISA at days 5, 8, and 14 postinfection. To ensure that cell viability was not affected by stimulation with LPS, AM were plated in 96-well plates, and viability was assessed in the presence or absence of 100 ng of LPS/ml after 7 and 14 days by using an MTT-based cytotoxicity assay (Sigma, St. Louis, Mo.).
Chemokine blocking of HIV-1 infection in AM.
To assess the ability of chemokines to block HIV-1 infection of AM, cells were infected with 100 TCID50 of HIV-1 AD2-3, AD2-6, AD3-3, AD3-6, or JRFL in the presence of 250 ng of RANTES, MIP-1α, MIP-1β, or SDF-1/ml, either alone or in combination. After 48 h, the cells were washed, and the appropriate chemokines were added back to the wells. p24 levels in the supernatant were measured by ELISA on day 7 after infection and were compared to those of control cultures infected in the absence of added chemokines. Percent inhibition was calculated as (1 − the mean p24 concentration of duplicate wells with chemokines/mean of control wells) × 100.
RESULTS
Infection of AM with primary HIV-1 isolates.
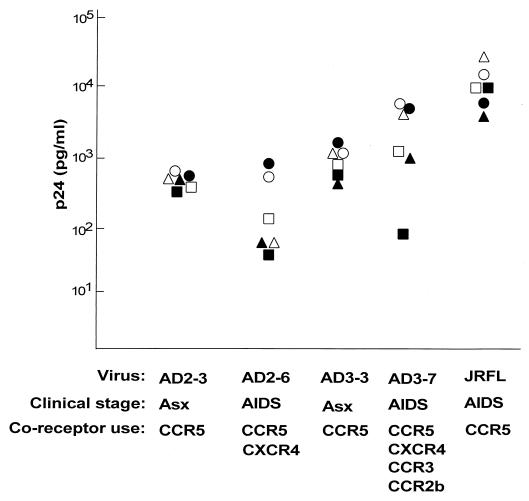
Inoculation of AM from healthy individuals with primary isolates of HIV-1 demonstrated virus replication in AM from all donors for all tested isolates (Fig. 1). Peak p24 levels among the different donors ranged from 313 to 778 pg/ml for AD2-3, 25 to 951 pg/ml for AD2-6, 341 to 1,516 pg/ml for AD3-3, 86 to 4,408 pg/ml for AD3-7, and 604 to 20,000 pg/ml for JRFL.
FIG. 1.
Replication of primary HIV-1 isolates in AM from different donors. AM were obtained by bronchoalveolar lavage of healthy individuals and infected in vitro with five different primary HIV-1 isolates. HIV-1 p24 antigen was measured by ELISA in the supernatant on days 4, 8, and 14. Values are peak p24 levels measured on day 14. The known coreceptor usage of each primary isolate is listed (15), as is the clinical stage of the individual at the time the virus was isolated. Note that AD2-3 and AD2-6 are from the same individual at different stages, as are AD3-3 and AD3-7. Asx, asymptomatic. Each symbol represents data for one individual.
HIV-coreceptor expression on AM.
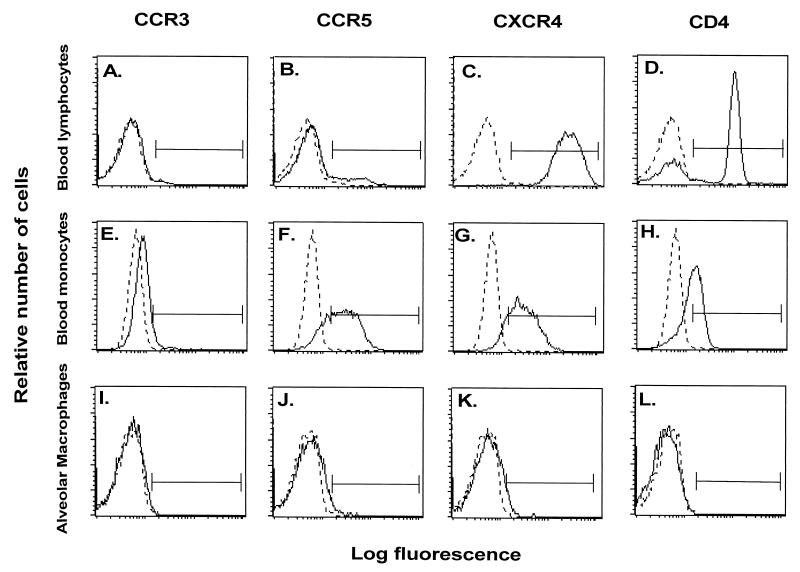
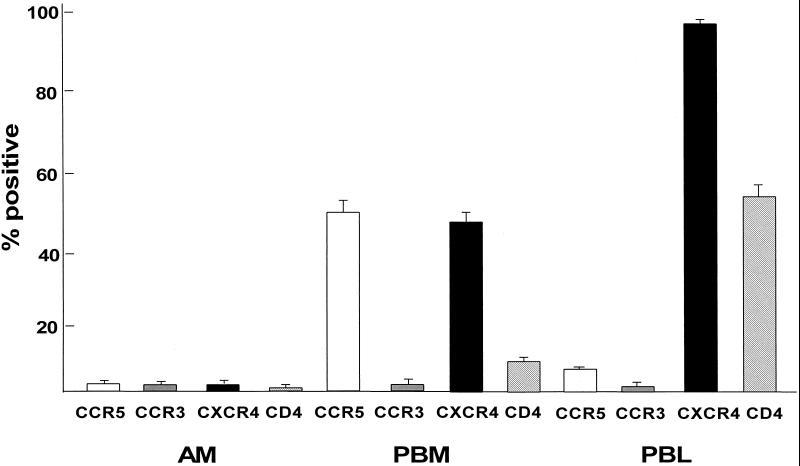
Flow cytometry analysis of the HIV-1 coreceptors CCR5, CCR3, and CXCR4, as well as CD4, on the surface of AM demonstrated very low levels of these receptors (Fig. 2 and 3). In contrast, surface expression of HLA-DR, a marker for AM, was detectable on 93 to 98% of the cells. Higher surface expression of CCR5, CXCR4, and CD4 was detectable on PBM and PBL from the same individuals stained in parallel, while the levels of CCR3 were very low on PBM and PBL. On the average, CCR5 and CXCR4 levels on AM were significantly lower than on autologous PBM and PBL (P < 0.01, all comparisons), while CCR3 levels were similar (P > 0.1, all comparisons). Using antibody clones against CCR5 other than clone 2D7 (Fig. 4), AM demonstrated low-to-undetectable levels of cells positive for FAB180F (1.2% ± 0.9%) and FAB181F (0.2% ± 0.2%), whereas some cells stained positive for FAB182F (8.1% ± 1.0%) and FAB183F (6.2% ± 0.8%). Further testing of these antibodies on PBM and PBL showed staining similar to the CCR5 antibody 2D7 for clone FAB182F (PBM, 38.5% ± 6.1%; PBL, 5.9% ± 0.4%), whereas using the other clones, positive cells were less frequently observed or not detectable (clone FAB180F: PBM, 2.3% ± 0.9%, PBL, 2.0% ± 0.7%; clone FAB181F: PBM 6.5% ± 2.1%, PBL, 1.8% ± 0.5%; clone FAB183F: PBM, 5.3% ± 1.5%, PBL, 2.2% ± 0.3%). These results suggest that CCR5 surface expression levels on AM are low, although a small subpopulation stained positive with the CCR5 clones FAB182F and FAB183F, suggesting that certain epitopes may be masked by using different antibody clones.
FIG. 2.
Flow cytometry evaluation of expression of chemokine receptors on AM. AM were obtained by bronchoalveolar lavage and stained with anti-CCR3, CCR5, or CXCR4 monoclonal antibodies (followed by FITC-labeled anti-IgG) or FITC-conjugated anti-CD4. Blood monocytes and lymphocytes obtained from the same donors were evaluated in parallel. Shown are representative samples from one individual of AM (panels A to D), PBM (panels E to H), and PBL (panels I to M). In addition to coreceptor staining, AM were double stained with PE-labeled HLA-DR, blood monocytes were double stained with PE-labeled CD14, and lymphocytes were double stained with PE-labeled CD3. The histograms shown represent the cells selected by these markers. IgG-irrelevant controls for each antibody are depicted by the dotted lines. Surface expression of CCR3 (panels A, E, and I); CCR5 (panels B, F, and J); CXCR4 (panels C, G, and K); and CD4 (panels D, H, and L) is shown. The solid horizontal line represents the region selected for quantification.
FIG. 3.
Quantitative analysis of chemokine receptor surface expression on AM compared to that on autologous blood monocytes and blood lymphocytes. AM, PBM, and PBL from healthy individuals were labeled with antibodies against CCR5, CCR3, CXCR4, and CD4 and evaluated by flow cytometry (as described in the legend for Fig. 2). Shown are the means ± standard errors of the means for six individuals.
FIG. 4.
Flow cytometry analysis of expression of the chemokine receptor CCR5 using different monoclonal antibodies. AM were obtained by bronchoalveolar lavage and stained with the following FITC-conjugated monoclonal antibodies against CCR5: FAB180F, FAB181F, FAB182F, and FAB183F. AM were double stained with PE-labeled HLA-DR. The histograms represent the cells selected by this marker. IgG-matched control antibody is depicted by the dotted line. Shown is surface expression of representative samples from one individual for FAB180F (A), FAB181F (B), FAB182F (C), and FAB183F (D).
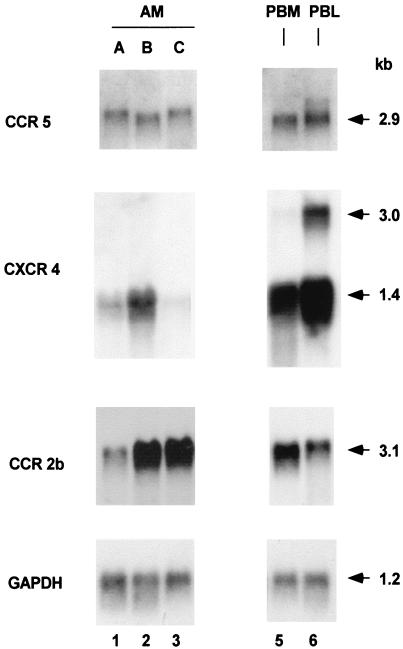
Analysis of CCR5, CCR3, CXCR4, CCR2b, and CD4 expression at the mRNA level using RT-PCR demonstrated detectable expression of each receptor on AM, PBM, and PBL (not shown). Northern analysis demonstrated mRNA transcripts of CCR5, CXCR4, and CCR2b in cells from all AM donors evaluated, although there was variability in the mRNA levels from donor to donor (Fig. 5). In contrast, mRNA expression of the control GAPDH was similar among all individuals. CCR3 transcripts were not detected in AM, PBM, or PBL by Northern analysis from any donor (not shown). The mRNA levels for CCR5 and CXCR4 tended to be lower on the AM than on PBM and PBL, but not with the more variable expression of CCR2b. Interestingly, while PBL (and to a lesser extent PBM) showed two mRNA bands of 1.4 and 3.0 kb for CXCR4 as has been previously reported (25), AM showed expression of the 1.4-kb band only.
FIG. 5.
Chemokine receptor expression in AM assessed by Northern analysis. RNA obtained from AM of three individuals (lanes A, B, and C) and, as a control, from PBM and PBL from individual A. The PBM and PBL were purified with immunomagnetic beads coated with antibodies against CD14 (PBM) and CD3 (PBL), respectively. The cells were analyzed with specific probes for CCR5, CXCR4, and CCR2b. From top to bottom are shown expression of CCR5, CXCR4, CCR2b, and GAPDH. Lanes: 1 to 3, AM for three healthy individuals; 4, PBM from individual A; 5, PBL from individual A. The sizes of the mRNAs are indicated in kilobases (kb).
Influence of AM activation on coreceptor expression, chemokine expression, and HIV-1 replication.
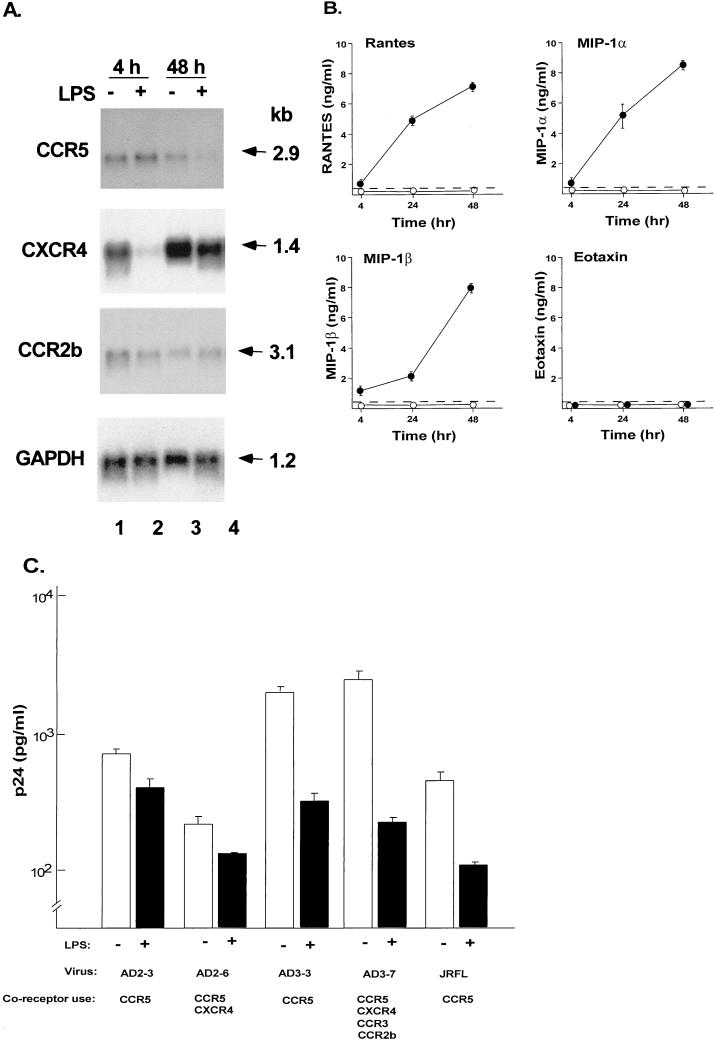
To determine if activation of AM influences the expression of CCR3, CXCR4, CCR5, and CCR2b, the cells were cultured in the presence of LPS for either 4 or 48 h. Northern analysis demonstrated markedly decreased mRNA levels of CXCR4 after 4 h of stimulation and mildly decreased levels of CCR5 mRNA after 48 h of stimulation, (Fig. 6A). CCR3 was not detectable by Northern analysis. GAPDH levels remained unchanged.
FIG. 6.
Consequences of activation of AM on HIV-1 coreceptor expression, chemokine expression, and the ability of AM to be infected by primary isolates of HIV-1. (A) AM were stimulated with LPS (100 ng/ml) for either 4 or 48 h and then analyzed for HIV-1 coreceptor expression by Northern analysis. CCR5, CXCR4, and CCR2b expression analyzed by Northern analysis in unstimulated (−) and LPS-stimulated (+) cells. GAPDH expression is used as a control. The sizes of the mRNAs are indicated in kilobases (kb). This pattern is representative of three different donors analyzed. (B) RANTES, MIP-1α, MIP-1β, and eotaxin secretion in culture supernatant as measured by ELISA following stimulation of AM with LPS (●) or without LPS stimulation (○). Dashed line represents the limit of detection of the assay. (C) Influence of LPS stimulation on HIV-1 replication in AM. AM were stimulated with LPS for 12 h and then infected with five primary HIV-1 isolates. After 14 days, HIV-1 p24 antigen levels in the supernatant were measured by ELISA. Shown are data obtained with (+) and without (−) LPS stimulation. The HIV-1 isolates are the same as in Fig. 1, and the coreceptor use of these isolates is indicated. Data are means ± standard errors of the means of triplicate measurements.
To determine whether activation of AM resulted in increased secretion of chemokines, the levels of RANTES, MIP-1α, MIP-1β, and eotaxin, were measured in the culture supernatant. Increased levels of RANTES, MIP-1α, and MIP-1β, but not eotaxin, were found following stimulation with LPS (Fig. 6B). Although there was some donor-to-donor variability in the response to LPS, there was a marked increase for MIP-1α, MIP-1β, and RANTES following LPS stimulation in all samples (Table 1).
TABLE 1.
Secretion of MIP-1α, MIP-1β, RANTES, and eotaxin in culture supernatants following stimulation with LPSa
| Chemokine and treatment | Amount (ng/ml) secreted by AM from donor:
|
||
|---|---|---|---|
| 1 | 2 | 3 | |
| MIP-1α | |||
| LPS− | 0.0 | 0.1 | 0.1 |
| LPS+ | 5.2 | 100.1 | 638.1 |
| MIP-1β | |||
| LPS− | 0.0 | 0.1 | 0.2 |
| LPS+ | 4.2 | 22.5 | 56.5 |
| RANTES | |||
| LPS− | 0.0 | 0.0 | 0.0 |
| LPS+ | 5.8 | 18.9 | 17.2 |
| Eotaxin | |||
| LPS− | 0.0 | 0.0 | 0.0 |
| LPS+ | 0.0 | 0.0 | 0.0 |
Shown are the means of triplicate samples from the AM from three different individuals at 24 h with (+) or without (−) LPS stimulation.
To analyze if LPS simulation of AM influences infection of AM with HIV-1, LPS-stimulated cells were inoculated with five different primary isolates of HIV-1 in the presence of LPS, and the levels of HIV-1 replication were determined. Strikingly, HIV-1 replication of LPS-stimulated AM was diminished for all of the isolates tested compared to infection of unstimulated cells (Fig. 6C; P < 0.05, all comparisons). The viability of the cells was not affected by LPS stimulation.
Chemokine blocking of HIV-1 replication in AM.
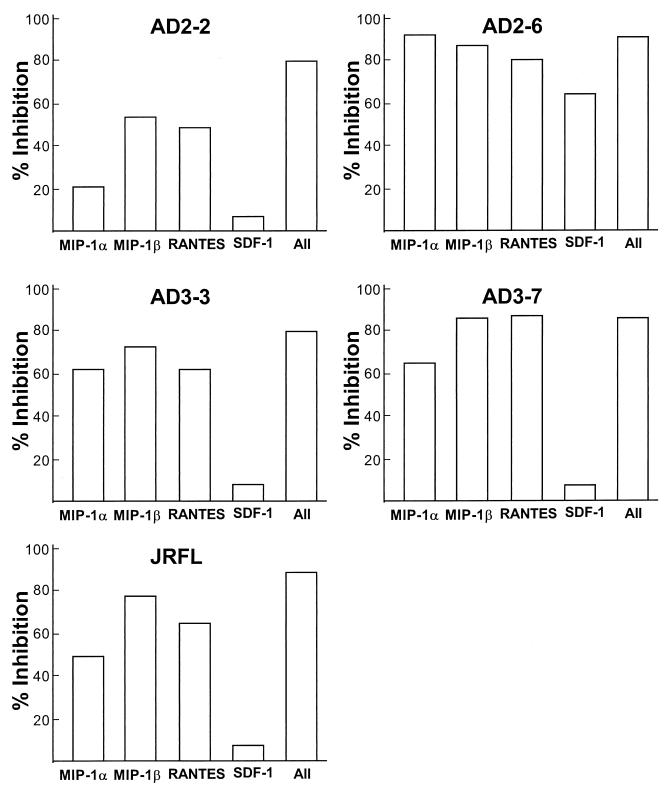
To determine if the ligands for the coreceptors could block HIV-1 replication in AM, cells were infected with the primary isolates AD2-3, AD2-6, AD3-3, AD3-7, or JRFL in the presence of either RANTES, MIP-1α, MIP-1β, SDF-1, or all four combined. HIV-1 replication was inhibited in the presence of RANTES (44 to 84%), MIP-1α (20 to 62%), and MIP-1β (55 to 85%) for all the HIV-1 isolates (Fig. 7). All chemokines combined had a >80% inhibitory effect on HIV-1 replication. Interestingly, for one HIV-1 isolate which, like CCR5, can use CXCR4 (AD2-6), SDF-1 blocked HIV-1 infection to 67%, whereas for all the other isolates SDF-1 did not block HIV-1 infection. However, for AD2-6, the blocking effect of SDF-1 did not exceed the effect seen by MIP-1α (92%), MIP-1β (55%), RANTES (44%), or all chemokines combined (86%).
FIG. 7.
Inhibition of HIV-1 replication in AM by chemokines. AM were infected with primary HIV-1 isolates in the presence of 250 ng of RANTES, MIP-1α, MIP-1β, or SDF-1/ml or all chemokines together. HIV-1 p24 antigen was measured in the culture supernatants on day 10 after virus inoculation. The percent inhibition was calculated based on control cultures infected without added chemokines. Data are means of duplicate wells from one (of three) representative experiment. Shown are results for isolates AD2-2 (coreceptor used, CCR5), AD2-6 (coreceptors used, CCR5 and CXCR4), AD3-3 (coreceptor used, CCR5), AD3-7 (coreceptors used, CCR5, CXCR4, CCR2b, and CCR3), and JRFL (coreceptor used, CCR5).
DISCUSSION
The present study analyzes the expression and utilization of the major chemokine receptors for HIV-1 entry into normal human AM. AM were productively infected with several primary isolates of HIV-1. Expression of the major known HIV-1 coreceptors (CCR5 and CXCR4) was detectable at the RNA level, whereas surface expression of these receptors occurred at lower levels. However, CCR5-specific chemokines were able to significantly inhibit HIV-1 replication in AM as did stimulation of AM with LPS, which leads to increased expression of CCR5-specific chemokines. These data suggest that CCR5 is the predominant coreceptor used by HIV-1 to infect AM, but that coreceptor expression levels are far lower on AM than on blood monocytes. Importantly, the combined observations that activation of normal human AM decreases replication of HIV-1, downregulates CCR5 expression, and increases the release of RANTES, MIP-1α, and MIP-1β together suggest that the interplay of chemokine receptors and chemokine production plays a major role in the susceptibility of AM to HIV-1 infection.
AM and HIV-1 infection.
AM play an important role in the pulmonary host defense and are known targets for HIV-1 infection (1, 6, 12, 30, 35, 38–40, 43, 45, 50). Although pulmonary infections are common in HIV-1-infected individuals and in patients with AIDS, the role of the AM in the progression of HIV-1-related lung disease is not well defined. Importantly, it is not known if the infection of AM takes place within the lung or is secondary to systemic infection of AM precursors or both (1). AM obtained by bronchoalveolar lavage from HIV-1-infected individuals harbor HIV-1 (1, 12, 30, 35, 38–40, 45, 50). In general, the absolute number of HIV-1-infected AM in individuals with AIDS is lower than that of blood monocytes (35, 38, 39), although a significant increase of HIV-1 in AM, but not monocytes, has been reported in some patients as the disease progresses (56).
HIV-1 entry in AM.
From the results of the present study, it is clear that healthy human AM can be productively infected with primary isolates of HIV-1 in vitro. The entry mechanisms for HIV-1 into AM have not been extensively studied. It is known that CD4 is critical for HIV infection of AM, and AM are known to express CD4 (26, 32). The discovery that HIV-1 also requires coreceptors of the CC and CXC chemokine family for entry into lymphocytes and cells of the monocyte-macrophage lineage has shed new light on the pathogenesis of HIV-1 infection (4, 9, 19, 34). Based on a variety of studies of the coreceptors for HIV-1 entry into blood monocytes and monocyte-derived macrophages, it is likely that CCR5 is the main receptor used for entry into these cells (18, 37, 42, 55, 61, 64), although recent data obtained by using CCR5-deficient monocytes demonstrate that CXCR4 can also be used (63). CCR5 seems to play a central role in the transmission of HIV-1 in vivo, as individuals homozygous for a 32-bp deletion in CCR5 have increased resistance to HIV-1 infection (33, 51). HIV-1 strains that use CCR5 are present throughout the course of the disease, whereas in some individuals, variants that use additional coreceptors emerge later in the course of the disease (15, 52).
Current knowledge of the pattern of coreceptor expression on tissue macrophages is limited. Studies with human microglial cells of the brain have demonstrated the expression of CCR3 and CCR5, although recent data suggest a dominant role for CCR5 in infection (28, 54). Human AM are known to express the two orphan seven-transmembrane receptors, GPR-1 and GPR-15, which can be used for simian immunodeficiency virus entry (22). The present study demonstrates that primary HIV-1 isolates which solely use CCR5 can replicate in AM, in addition to isolates which use more than one coreceptor. The potential usage of additional coreceptors, including GPR-1 and/or GPR-15, has not been determined, and thus we cannot rule out the possibility that these receptors play a role in HIV-1 entry into AM.
Coreceptor expression on AM.
While all of the evidence is consistent with the concept that HIV-1 uses predominantly CCR5 to enter AM, the surface expression of CCR5 and the other major chemokine receptors is much lower on AM than on autologous blood monocytes, despite the presence of mRNA. Although there was some variability with different clones of anti-CCR5 antibodies, the overall detectable surface expression of CCR5 was well below 10% of the cells. When using antibodies against CCR5 other than clone 2D7, a small, distinct, positive subpopulation could be seen with the clones FAB182F and FAB183F, suggesting that certain CCR5 epitopes could be masked on AM, but this may represent only a small percentage of the total population. Likewise, as has been previously shown (32), the expression of CD4 on normal AM is low, suggesting that low-level surface expression of CD4 and coreceptors is sufficient for infection of HIV-1. It has been reported that, in retrovirus-modified HeLa cells, CD4 and CCR5 interact in a concentration-dependent manner, i.e., in the presence of low levels of CD4 expression, high levels of CCR5 are sufficient for HIV infection and vice versa (41). In the present study, however, the levels of surface expression of both CD4 and CCR5 were found to be similarly low on AM. Although expression levels of all of the major chemokine receptors are low on healthy AM, the level of expression of CCR3 is by far the lowest, detectable only by RT-PCR. This is similar to that previously noted in blood monocytes (18, 37).
Interestingly, the levels of CXCR4 and CCR2 mRNA in AM vary considerably among individuals. As with blood monocyte-derived macrophages (8, 18, 37, 44, 45), the state of activation of AM influences the expression of some HIV-1 coreceptors. Although dependent on time after activation, expression of CCR2, CXCR4, and CCR5 mRNA is suppressed by activation of AM. CCR2 mRNA levels in monocyte-derived macrophages and monocytic cell lines are diminished by activation, with moderate decreases in CCR5 mRNA levels (55). Stimulation of human endothelial cells with LPS also leads to a decrease in CXCR4 mRNA levels (27).
In addition to receptor mRNA regulation, activation of AM results in the secretion of the CCR5 ligands RANTES, MIP-1α, and MIP-1β. Chemokines, as the natural ligands of the HIV-1 coreceptors, are able to competitively block HIV-1 infection (13, 59). Consistent with these observations, activation of blood-derived monocytes results in decreased replication of HIV-1 (31) and, as shown in the present study, activation of AM results in a similar decrease in replication of HIV-1 isolates which primarily use CCR5 as the coreceptor for infection. This may be due in part to the decreased expression of this receptor following activation and, in part, to the secretion of CCR5 ligands RANTES, MIP-1α, and MIP-1β, which can compete with HIV-1 for the coreceptor. Furthermore the decreased coreceptor expression could have resulted from the secretion of endogenous beta chemokines, which themselves could act to downregulate receptor mRNA. RANTES, MIP-1α, and MIP-1β blocked infection of AM with all the five primary HIV-1 isolates tested in our study, a phenomenon which has been recently described for infection of AM with the HIV-1 strain BAL (14). SDF-1, the ligand for CXCR4, was able to block infection to some extent for only one of two primary isolates utilizing both CCR5 and CXCR4, but the inhibitory effect was less than that seen for the CCR5-specific chemokines. Although it is possible that CXCR4 may be used to some degree for HIV-1 entry, indirect effects of SDF-1 rather than direct blocking may also be an explanation for decreased HIV-1 replication in the presence of SDF-1. For blood monocyte-derived macrophages, HIV-1 replication is inhibited by activation via the release of RANTES, MIP-1α, and MIP-1β (60). Consistent with this concept, MIP-1α has been shown to be produced by AM in increased amounts in HIV-1-infected individuals (17).
Taken as a whole, the present study demonstrates that AM express a variety of chemokine receptors relevant for HIV-1 entry, that HIV-1 likely enters AM mainly through CCR5, and that activation of AM can result in decreased infection of this cell type with HIV-1. Strategies to prevent infection via blockage of chemokine receptors on macrophages, including chemically modified chemokines such as AOP-RANTES (57), are currently being developed. Intracellular blocking of CCR5 receptor expression via “intrakines” may be equally useful to prevent HIV-1 infection of AM (10, 62).
ACKNOWLEDGMENTS
S.W. and R.C. participated equally in this study.
We thank Simon Monard for help with the flow cytometry studies, Barbara Ferris for technical assistance, Philip L. Leopold and Neil R. Hackett for helpful discussions, and N. Mohamed for help in preparing the manuscript.
These studies were supported, in part, by NIH grants P01 HL59312 and R01 HL59861-01; the Will Rogers Memorial Fund, Los Angeles, Calif.; and GenVec, Inc., Rockville, Md. R.C. was also supported, in part, by grant AI 41373 from The Aaron Diamond Foundation, New York, N.Y.
REFERENCES
- 1.Agostini C, Trentin L, Zambello R, Semenzato G. HIV-1 and the lung: infectivity, pathogenic mechanisms, and cellular immune responses taking place in the lower respiratory tract. Am Rev Respir Dis. 1993;147:1038–1049. doi: 10.1164/ajrccm/147.4.1038. [DOI] [PubMed] [Google Scholar]
- 2.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP1α, MIP1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 3.Alkhatib G, Berger E A, Murphy P M, Pease J E. Determinants of HIV-1 co-receptor function on CC chemokine receptor 3. Importance of both extracellular and transmembrane/cytoplasmic regions. J Biol Chem. 1997;272:20420–20426. doi: 10.1074/jbc.272.33.20420. [DOI] [PubMed] [Google Scholar]
- 4.Bazan H A, Alkhatib G, Broder C C, Berger E A. Patterns of CCR5, CXCR4, and CCR3 usage by envelope glycoproteins from human immunodeficiency type 1 isolates. J Virol. 1998;72:4485–4491. doi: 10.1128/jvi.72.5.4485-4491.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger E A. HIV entry and tropism: the chemokine receptor connection. AIDS. 1997;11(Suppl. A):S3–S16. [PubMed] [Google Scholar]
- 6.Bezdicek P, Crystal R G. Pulmonary macrophages. In: Crystal R G, West J B, Weibel E R, Barnes P J, editors. The lung: scientific foundations. Philadelphia, Pa: Lippincott-Raven, Inc.; 1997. pp. 859–875. [Google Scholar]
- 7.Björndal A, Deng H, Jansson M, Fiore J R, Colognesi C, Karlsson A, Albert J, Scarlatti G, Littman D R, Fenyö E M. Coreceptor usage of primary human immunodeficiency virus type 1 isolates varies according to biological phenotype. J Virol. 1997;71:7478–7487. doi: 10.1128/jvi.71.10.7478-7487.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bleul C C, Wu L, Hoxie J A, Springer T A, Mackay C R. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broder C C, Collman R G. Chemokine receptors and HIV. J Leukoc Biol. 1997;62:20–29. doi: 10.1002/jlb.62.1.20. [DOI] [PubMed] [Google Scholar]
- 10.Chen J D, Bai X, Yang A G, Cong Y, Chen S Y. Inactivation of HIV-1 chemokine co-receptor CXCR-4 by a novel intrakine strategy. Nat Med. 1997;3:1110–1116. doi: 10.1038/nm1097-1110. [DOI] [PubMed] [Google Scholar]
- 11.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard G, Sodroski J. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 12.Clarke J R, Krishnan V, Bennett J, Mitchell D, Jeffries D J. Detection of HIV-1 in human lung macrophages using the polymerase chain reaction. AIDS. 1990;4:1133–1136. doi: 10.1097/00002030-199011000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Cocchi F, De Vico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 14.Coffey M J, Woffendin C, Phare S M, Strieter R M, Markovitz D M. Rantes inhibits HIV-1 replication in human peripheral blood monocytes and alveolar macrophages. Am J Physiol. 1997;272:L1025–L1029. doi: 10.1152/ajplung.1997.272.5.L1025. [DOI] [PubMed] [Google Scholar]
- 15.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 17.Denis M, Ghadirian E. Alveolar macrophages from subjects infected with HIV-1 express macrophage inflammatory protein-1 alpha (MIP-1 alpha): contribution to the CD8+ alveolitis. Clin Exp Immunol. 1994;96:187–192. doi: 10.1111/j.1365-2249.1994.tb06540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Marzio P, Tse J, Landau N R. Chemokine receptor regulation and HIV type 1 tropism in monocyte-macrophages. AIDS Res Hum Retroviruses. 1998;14:129–138. doi: 10.1089/aid.1998.14.129. [DOI] [PubMed] [Google Scholar]
- 19.Doms R W, Peiper S C. Unwelcome guests with master keys: how HIV uses chemokine receptors for cellular entry. Virology. 1997;235:179–190. doi: 10.1006/viro.1997.8703. [DOI] [PubMed] [Google Scholar]
- 20.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S, Parmentier M, Collman R G, Doms R W. A dual-tropic, primary HIV-1 isolate that uses fusin and the β-chemokine receptors CKR-5, CKR-3, CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 21.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 22.Farzan M, Choe H, Martin K, Marcon L, Hofmann W, Karlsson G, Sun Y, Barrett P, Marchand N, Sullivan N, Gerard N, Gerard C, Sodroski J. Two orphan seven-transmembrane segment receptors which are expressed in CD4-positive cells support simian immunodeficiency virus infection. J Exp Med. 1997;186:405–411. doi: 10.1084/jem.186.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 24.Frade J M R, Llorente M, Mellado M, Alcami J, Gutierrez-Ramos J C, Zaballos A, del Real G, Martinez A C. The amino-terminal domain of the CCR2 chemokine receptor acts as a coreceptor for HIV-1 infection. J Clin Investig. 1997;100:497–502. doi: 10.1172/JCI119558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Granelli-Piperno A, Moser B, Pope M, Chen D, Wei Y, Isdell F, O’Doherty U, Paxton W, Koup R, Mojsov S, Bhardwaj N, Clark-Lewis I, Baggiolini M, Steinman R M. Efficient interaction of HIV-1 with purified dendritic cells via multiple chemokine coreceptors. J Exp Med. 1996;184:2433–2438. doi: 10.1084/jem.184.6.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guay L A, Sierra-Madero J G, Finegan C K, Rich E A. Mediation of entry of human immunodeficiency virus-1 into alveolar macrophages by CD4 without facilitation by surfactant-associated protein-α. Am J Respir Cell Mol Biol. 1997;16:421–428. doi: 10.1165/ajrcmb.16.4.9115753. [DOI] [PubMed] [Google Scholar]
- 27.Gupta S K, Lysko P G, Pillarisetti K, Ohlstein E, Stadel J M. Chemokine receptors in human endothelial cells. Functional expression of CXCR4 and its transcriptional regulation by inflammatory cytokines. J Biol Chem. 1998;273:4282–4287. doi: 10.1074/jbc.273.7.4282. [DOI] [PubMed] [Google Scholar]
- 28.He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay C R, Sodroski J, Gabuzda D. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 29.Janoff E N, Breiman R F, Daley C L, Hopewell P C. Pneumococcal disease during HIV infection: epidemiologic, clinical, and immunologic perspectives. Ann Intern Med. 1992;117:314–324. doi: 10.7326/0003-4819-117-4-314. [DOI] [PubMed] [Google Scholar]
- 30.Jeffrey A A, Israel-Biet D, Andrieu J M, Even P, Venet A. HIV isolation from pulmonary cells derived from bronchoalveolar lavage. Clin Exp Immunol. 1991;84:488–492. [PMC free article] [PubMed] [Google Scholar]
- 31.Kornbluth R S, Oh P S, Munis J R, Cleveland P H, Richman D D. Interferons and bacterial lipopolysaccharide protect macrophages from productive infection by human immunodeficiency virus in vitro. J Exp Med. 1989;169:1137–1151. doi: 10.1084/jem.169.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewin S R, Sonza S, Irving L B, McDonald C F, Mills J, Crowe S M. Surface CD4 is critical to in vitro HIV infection of human alveolar macrophages. AIDS Res Hum Retroviruses. 1996;12:877–883. doi: 10.1089/aid.1996.12.877. [DOI] [PubMed] [Google Scholar]
- 33.Liu R, Paxton W A, Choe S, Ceradini D, Martin S R, Horuk R, MacDonald M E, Stuhlmann H, Koup R A, Landau N R. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 34.Moore J P, Trkola A, Dragic T. Co-receptors for HIV-1 entry. Curr Opin Immunol. 1997;9:551–562. doi: 10.1016/s0952-7915(97)80110-0. [DOI] [PubMed] [Google Scholar]
- 35.Moreno P, Rebollo M J, Pulido F, Rubio R, Noriega A R, Delgado R. Alveolar macrophages are not an important source of viral production in HIV-1 infected patients. AIDS. 1996;10:682–684. doi: 10.1097/00002030-199606000-00019. [DOI] [PubMed] [Google Scholar]
- 36.Murray J F, Mills J. Pulmonary infectious complications of human immunodeficiency virus infection. Part I. Am Rev Respir Dis. 1990;141:1356–1372. doi: 10.1164/ajrccm/141.5_Pt_1.1356. [DOI] [PubMed] [Google Scholar]
- 37.Naif H M, Li S, Alali M, Sloane A, Wu L, Kelly M, Lynch G, Lloyd A, Cunningham A L. CCR5 expression correlates with susceptibility of maturing monocytes to human immunodeficiency virus type 1 infection. J Virol. 1998;72:830–836. doi: 10.1128/jvi.72.1.830-836.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakata K, Weiden M, Harkin T, Ho D, Rom W N. Low copy number and limited variability of proviral DNA in alveolar macrophages from HIV-1-infected patients: evidence for genetic differences in HIV-1 between lung and blood macrophage populations. Mol Med. 1995;1:744–757. [PMC free article] [PubMed] [Google Scholar]
- 39.Pearce T E, Nowakowski M, Eden E, Huang Z B, Steiner P, Shahabuddin M, Potash M J, Volsky D J. Uniform detection of HIV-1 in alveolar macrophages of pediatric but not adult AIDS patients. J Leukoc Biol. 1993;53:722–726. doi: 10.1002/jlb.53.6.722. [DOI] [PubMed] [Google Scholar]
- 40.Plata F, Garcia-Pons F, Ryter A, Lebargy F, Goodenow M M, Dat M H, Autran B, Mayaud C. HIV-1 infection of lung alveolar fibroblasts and macrophages in humans. AIDS Res Hum Retroviruses. 1990;6:979–986. doi: 10.1089/aid.1990.6.979. [DOI] [PubMed] [Google Scholar]
- 41.Platt E J, Wehrly K, Kuhmann S E, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72:2855–2864. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rana S, Besson G, Cook D G, Rucker J, Smyth R J, Yi Y, Turner J D, Guo H-H, Du J-G, Peiper S C, Lavi E, Samson M, Libert F, Liesnard C, Vassart G, Doms R W, Parmentier M, Collman R G. Role of CCR5 in infection of primary macrophages and lymphocytes by macrophage-tropic strains of human immunodeficiency virus: resistance to patient-derived and prototype isolates resulting from the Δccr5 mutation. J Virol. 1997;71:3219–3227. doi: 10.1128/jvi.71.4.3219-3227.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reynolds H Y. Integrated host defense against infections. In: Crystal R G, West J B, Weibel E R, Barnes P J, editors. The lung: scientific foundations. Philadelphia, Pa: Lippincott-Raven, Inc.; 1997. pp. 2353–2365. [Google Scholar]
- 44.Rich E A, Chen I S, Zack J A, Leonard M L, O’Brien W A. Increased susceptibility of differentiated mononuclear phagocytes to productive infection with human immunodeficiency virus-1 (HIV-1) J Clin Investig. 1992;89:176–183. doi: 10.1172/JCI115559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rose R M, Krivine A, Pinkston P, Gillis J M, Huang A, Hammer S M. Frequent identification of HIV-1 DNA in bronchoalveolar lavage cells obtained from individuals with the acquired immunodeficiency syndrome. Am Rev Respir Dis. 1991;143:850–854. doi: 10.1164/ajrccm/143.4_Pt_1.850. [DOI] [PubMed] [Google Scholar]
- 46.Ross T M, Cullen B R. The ability of HIV type 1 to CCR-3 as a coreceptor is controlled by envelope V1/V2 sequences acting in conjunction with a CCR-5 tropic V3 loop. Proc Natl Acad Sci USA. 1998;95:7682–7686. doi: 10.1073/pnas.95.13.7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rubbet A, Combadiere C, Ostrowski M, Arthos J, Dybul M, Machado E, Cohen M A, Hoxie J A, Murphy P M, Fauchi A S, Weisman D. Dendritic cells express multiple chemokine receptors used as coreceptors for HIV entry. J Immunol. 1998;160:3933–3941. [PubMed] [Google Scholar]
- 48.Rucker J, Edinger A L, Sharron M, Samson M, Lee B, Berson J F, Yi Y, Marguilies B, Collman R G, Doranz B J, Parmentier M, Doms R W. Utilization of chemokine receptors, orphan receptors, and herpesvirus-encoded receptors by diverse human and simian immunodeficiency viruses. J Virol. 1997;71:8999–9007. doi: 10.1128/jvi.71.12.8999-9007.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Russi T J, Crystal R G. Bronchoalveolar lavage. In: Crystal R G, West J B, Weibel E R, Barnes P J, editors. The lung: scientific foundations. Philadelphia, Pa: Lippincott-Raven, Inc.; 1997. p. 371. [Google Scholar]
- 50.Salahuddin S Z, Rose R M, Groopman J E, Markham P D, Gallo R C. Human T lymphotropic virus type III infection of human alveolar macrophages. Blood. 1986;68:281–284. [PubMed] [Google Scholar]
- 51.Samson M, Libert F, Doranz B J, Rucker J, Liesnard C, Farber C M, Saragosti S, Lapoumeroulie C, Cognaux J, Forceille C, Muyldermans G, Verhofstede C, Burtonboy G, Georges M, Imai T, Rana S, Yi Y, Smyth R J, Collman R G, Doms R W, Vassart G, Parmentier M. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 52.Scarlatti G, Tresoldi E, Bjorndal A, Fredriksson R, Colognesi C, Deng H K, Malnati M S, Plebani A, Siccardi A G, Littman D R, Fenyo E M, Lusso P. In vivo evolution of HIV-1 co-receptor usage and sensitivity to chemokine-mediated suppression. Nat Med. 1997;3:1259–1265. doi: 10.1038/nm1197-1259. [DOI] [PubMed] [Google Scholar]
- 53.Schuitemaker H, Koot M, Kootstra N A, Dercksen M W, de Goede R E, van Steenwijk R P, Lange J M, Schattenkerk J K, Miedema F, Tersmette M. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J Virol. 1992;66:1354–1360. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shieh J T, Albright A V, Sharron M, Gartner S, Strizki J, Doms R W, Gonzalez-Scarano F. Chemokine receptor utilization by human immunodeficiency virus type 1 isolates that replicate in microglia. J Virol. 1998;72:4243–4249. doi: 10.1128/jvi.72.5.4243-4249.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sica A, Saccani A, Borsatti A, Power C A, Wells T N, Luini W, Polentarutti N, Sozzani S, Mantovani A. Bacterial lipopolysaccharide rapidly inhibits expression of C-C chemokine receptors in human monocytes. J Exp Med. 1997;185:969–974. doi: 10.1084/jem.185.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sierra-Madero J G, Toossi Z, Hom D L, Finegan C K, Hoenig E, Rich E A. Relationship between load of virus in alveolar macrophages from human immunodeficiency virus type 1-infected persons, production of cytokines, and clinical status. J Infect Dis. 1994;169:18–27. doi: 10.1093/infdis/169.1.18. [DOI] [PubMed] [Google Scholar]
- 57.Simmons G, Clapham P R, Picard L, Offord R E, Rosenkilde M M, Schwartz T W, Buser R, Wells T N C, Proudfoot A E. Potent inhibition of HIV-1 infectivity in macrophages and lymphocytes by a novel CCR5 antagonist. Science. 1997;276:276–279. doi: 10.1126/science.276.5310.276. [DOI] [PubMed] [Google Scholar]
- 58.Simmons G, Wilkinson D, Reeves J D, Dittmar M T, Beddows S, Weber J, Carnegie G, Desselberger U, Gray P W, Weiss R A, Clapham P R. Primary, syncytium-inducing human immunodeficiency virus type 1 isolates are dual-tropic and most can use either LESTR or CCR5 as coreceptors for virus entry. J Virol. 1996;70:8355–8360. doi: 10.1128/jvi.70.12.8355-8360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trkola A, Paxton W A, Monard S P, Hoxie J A, Siani M A, Thompson D A, Wu L, Mackay C R, Horuk R, Moore J P. Genetic subtype-independent inhibition of human immunodeficiency virus type 1 replication by CC and CXC chemokines. J Virol. 1998;72:396–404. doi: 10.1128/jvi.72.1.396-404.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Verani A, Scarlatti G, Comar M, Tresoldi E, Polo S, Giacca M, Lusso P, Siccardi A G, Vercelli D. C-C chemokines released by lipopolysaccharide (LPS)-stimulated human macrophages suppress HIV-1 infection in both macrophages and T cells. J Exp Med. 1997;185:805–816. doi: 10.1084/jem.185.5.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu L, Paxton W A, Kassam N, Ruffing N, Rottman J B, Sullivan N, Choe H, Sodroski J, Newman W, Koup R A, Mackay C R. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med. 1997;185:1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang A G, Bai X, Huang X F, Yao C, Chen S. Phenotypic knockout of HIV type 1 chemokine coreceptor CCR-5 by intrakines as potential therapeutic approach for HIV-1 infection. Proc Natl Acad Sci USA. 1997;94:11567–11572. doi: 10.1073/pnas.94.21.11567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yi Y, Rana S, Turner J D, Gaddis N, Collman R G. CXCR-4 is expressed by primary macrophages and supports CCR5-independent infection by dual-tropic but not T-tropic isolates of human immunodeficiency virus type 1. J Virol. 1998;72:772–777. doi: 10.1128/jvi.72.1.772-777.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zaitseva M, Blauvelt A, Lee S, Lapham C K, Klaus-Kovtun V, Mostowski H, Manischewitz J, Golding H. Expression and function of CCR5 and CXCR4 on human Langerhans cells and macrophages: implications for HIV primary infection. Nat Med. 1997;3:1369–1375. doi: 10.1038/nm1297-1369. [DOI] [PubMed] [Google Scholar]