Abstract
Background
Although cumulative studies have demonstrated a beneficial effect of high-flow nasal cannula oxygen (HFNC) in acute hypercapnic respiratory failure, randomized trials to compare HFNC with non-invasive ventilation (NIV) as initial treatment in acute exacerbations of chronic obstructive pulmonary disease (AECOPD) patients with acute-moderate hypercapnic respiratory failure are limited. The aim of this randomized, open label, non-inferiority trial was to compare treatment failure rates between HFNC and NIV in such patients.
Methods
Patients diagnosed with AECOPD with a baseline arterial blood gas pH between 7.25 and 7.35 and PaCO2 ≥ 50 mmHg admitted to two intensive care units (ICUs) at a large tertiary academic teaching hospital between March 2018 and December 2022 were randomly assigned to HFNC or NIV. The primary endpoint was the rate of treatment failure, defined as endotracheal intubation or a switch to the other study treatment modality. Secondary endpoints were rates of intubation or treatment change, blood gas values, vital signs at one, 12, and 48 h, 28-day mortality, as well as ICU and hospital lengths of stay.
Results
225 total patients (113 in the HFNC group and 112 in the NIV group) were included in the intention-to-treat analysis. The failure rate of the HFNC group was 25.7%, while the NIV group was 14.3%. The failure rate risk difference between the two groups was 11.38% (95% CI 0.25–21.20, P = 0.033), which was higher than the non-inferiority cut-off of 9%. In the per-protocol analysis, treatment failure occurred in 28 of 110 patients (25.5%) in the HFNC group and 15 of 109 patients (13.8%) in the NIV group (risk difference, 11.69%; 95% CI 0.48–22.60). The intubation rate in the HFNC group was higher than in the NIV group (14.2% vs 5.4%, P = 0.026). The treatment switch rate, ICU and hospital length of stay or 28-day mortality in the HFNC group were not statistically different from the NIV group (all P > 0.05).
Conclusion
HFNC was not shown to be non-inferior to NIV and resulted in a higher incidence of treatment failure than NIV when used as the initial respiratory support for AECOPD patients with acute-moderate hypercapnic respiratory failure.
Trial registration: chictr.org (ChiCTR1800014553). Registered 21 January 2018, http://www.chictr.org.cn
Keywords: Chronic obstructive pulmonary diseases, Respiratory failure, High-flow nasal cannula oxygen therapy, Non-invasive ventilation, Randomized controlled trial
Introduction
Chronic obstructive pulmonary diseases (COPD) is a common chronic respiratory disease which continues to have high morbidity and mortality worldwide. Acute exacerbations of COPD (AECOPD) are the leading cause of death in COPD patients, and non-invasive ventilation (NIV) is recommended as standard therapy for AECOPD with moderate hypercapnic acute respiratory failure (ARF) [1]. However, several factors may affect the treatment outcome for NIV, such as the severity of the disease, patient-ventilator interaction, discomfort related to the mask interface, and the skill of the medical team in managing NIV [2, 3].
High-flow nasal cannula oxygen (HFNC) has been shown to have beneficial effects for stable COPD patients [4]. High-flow gas can generate a positive airway pressure that may counterbalance intrinsic positive end-expiratory pressure. Additionally, HFNC can cause a washout effect of the nasopharyngeal dead space to improve ventilatory efficiency and carbon dioxide removal [5]. The warmed and humidified gas can prevent bronchoconstriction in response to otherwise dry air, enhance mucociliary clearance, and decrease inspiratory resistance and diaphragmatic effort [6]. In recent years, the application of HFNC in AECOPD or hypercapnic ARF patients has steadily increased. In a study which enrolled 38 AECOPD patients with pH < 7.38, HFNC was reported to increase pH by 0.052 and decrease the partial pressure of arterial carbon dioxide (PaCO2) by 9.1 mmHg, while also showing that the effect of HFNC was more obvious in patients with pH < 7.35 [7]. Additionally, HFNC was shown to improve patients’ pH and respiratory rate (RR) among 30 patients with moderate hypercapnic ARF who were intolerant to NIV, while the non-response rate was only 13.3% [8]. In two observational studies with larger samples, HFNC was reported to have similar treatment failure rates as NIV in AECOPD patients with moderate hypercapnic ARF, while HFNC had better patient tolerance [9, 10]. The efficacy of HFNC in the treatment of AECOPD needs to be further confirmed by randomized controlled trials (RCTs).
Non-inferiority design has been used in the comparison between HFNC and NIV, however, non-inferiority RCTs comparing treatment failure of HFNC and NIV in AECOPD have so far been rare. We hypothesized that HFNC and NIV had similar treatment failure rates for AECOPD patients with moderate hypercapnic ARF.
Materials and methods
Study design and ethics approval
This was a single center, non-inferiority, unblinded RCT, registered at chictr.org (ChiCTR1800014553). The study was performed in the respiratory intensive care unit (RICU) and the emergency intensive care unit (EICU) of an urban, tertiary care, university hospital in China from March 2018 to December 2022. This study was approved by the hospital’s Institutional Ethics Committee (No. 2017053) and conformed to the Helsinki Declaration guidelines and medical research ethics standards. Informed consent was obtained from all enrolled patients or their relatives.
Patient screening
AECOPD patients with moderate hypercapnic ARF were screened for enrollment. The diagnosis of AECOPD (any worsening of respiratory symptoms that was beyond normal day-to-day variation and led to changes in medication in suspected or confirmed COPD patients) was established using the 2017 GOLD criteria [11]. Moderate hypercapnic ARF was defined as respiratory acidosis with a blood gas pH range between 7.25 and 7.35 and a PaCO2 ≥ 50 mmHg. Exclusion criteria were: age < 18 years old, anyone requiring immediate endotracheal intubation (e.g. those with severe hypoxia such as the ratio of partial pressure of arterial oxygen (PaO2) / the fraction of inspiration oxygen (FiO2) < 150 mmHg without external positive airway pressure, severe respiratory acidosis with a pH < 7.25, RR ≥ 40 breaths/min, or a Glasgow coma score < 8), contraindications to NIV or HFNC (poor sputum excretion ability, oral or facial trauma, significant hemodynamic instability), poor short-term prognosis (those already receiving palliative care or at very high risk of death within seven days due to existing medical/surgical pathology), presence of a tracheostomy, other organ failure, or patients (or their relatives) who could not give informed consent. Patients who withdrew informed consent were secondarily excluded.
Experimental procedure
Patients were divided into HFNC and NIV groups by computer-generated random number sequencing. Using opaque envelopes for covert distribution, with ten envelopes per group, five HFNC and five NIV, so that the number of patients in the two groups were evenly distributed.
For patients in the NIV group, a dedicated NIV (Philips V60 or BiPap Vision) with a standard oral-nasal mask (RT040) were used in S/T mode. The initial NIV settings were as follows: expiratory positive airway pressure was set to 4 cmH2O, the inspiratory positive airway pressure was set to 8 cmH2O, and the both pressures were gradually increased judged to acceptable tolerance by the patient. The expiratory positive airway pressure, inspiratory positive airway pressure and FiO2 were adjusted under the attending physician’s instruction to maintain a 6–8 ml/kg ideal body weight tidal volume, a pulse oxygen saturation (SpO2) of 88–92%, and a RR ≤ 28/min with appropriate inspiratory triggering and effort. The initial use of NIV was targeted to last at least two hours and then continued as needed. NIV could be used intermittently based on patient tolerance. During the intermittent period of NIV, a traditional (low-flow) nasal cannula oxygen could be used. Treatment time was gradually reduced in patients whose blood gas and other respiratory indices sufficiently improved. NIV was discontinued when the total daily treatment duration was less than four hours after the patient's clinical and blood gas values improved past certain cut-offs (pH > 7.35 and PaCO2 < 45 mmHg or > 45 mmHg with RR < 25 breaths/min). NIV could be restarted in case of clinical or blood gas value worsening.
In the HFNC group (AIRVO™ 2, Fisher & Paykel Healthcare, Auckland, New Zealand), subjects received an initial airflow of 40 L/min at a temperature of 37 °C through suitable nasal prongs. The FiO2 was adjusted to maintain a SpO2 between 88 and 92%. The airflow and temperature were adjusted according to patient tolerance. If patients in HFNC group tolerated the apparatus well, the treatment was continued, or it could be applied intermittently. Oxygen therapy during the intermittent period of HFNC was otherwise the same as in the NIV group. FiO2 was gradually reduced to < 35% at first in patients with stable clinical status and blood gas. If the patient had no obvious respiratory distress with a stable PaCO2, airflow was gradually reduced in a step-wise rate of 5–10 L/min per reduction. HFNC was discontinued when the airflow was reduced to 15L/min or less for more than two hours, and could be restarted if clinical status and blood gas deteriorated.
During treatment, if the patient could not tolerate the assigned treatment, or had respiratory distress, hypoxia, or carbon dioxide retention unalleviated by assigned treatment, the patient would be changed to the other study treatment modality. These switches were decided by the patient’s attending physician. The patient’s group classification would not be changed as a result of such a switch in treatment modality (intention to treat analysis), but would be recorded for statistical analysis.
The criteria for invasive mechanical ventilation in our study were: progressively increasing PaCO2 with pH ≤ 7.20, severe hypoxia (defined by as a PaO2 < 50 mmHg despite FiO2 > 0.5), RR > 40 breaths or < 8 breaths per minute, inability to protect their airway, or respiratory or cardiac arrest [11].
Data collection
Sex, age, relevant comorbidities, COPD duration (in years), any respiratory medications, recent pulmonary function tests, time of ICU admission, and severity score including the acute physiological and chronic health status score II (APACHE II), and the Simplified Acute Physiology Score II (SAPS II) for eligible patients were all recorded. Vital signs (heart rate, blood pressure, RR, and SpO2) as well as arterial blood gas analysis results were recorded when entering the ICU (baseline state), and again after one hour, 12 h, then once a day thereafter. The collection of vital signs and blood gas analysis results was stopped if the patient received invasive ventilation.
The initial settings of NIV or HFNC, daily total respiratory support time, and any changes in respiratory support modality (changes from NIV to HFNC or from HFNC to NIV, or a change to invasive ventilation, including specific time and reasons for such changes) were also collected. We recorded the daily total number of nursing airway care interventions (such as correcting unplanned device displacement, assisting in spitting, eating, etc.), Borg dyspnea score, comfort (visual analog scale) score, and adverse reactions to treatment (e.g. excessive air flow, eye irritation, epistaxis, abdominal distension, claustrophobic feelings, or skin breakdown). The 28-day survival of the patients was determined according to electronic medical and follow-up records.
Endpoints
The primary endpoint was treatment failure, defined as invasive ventilation or a switch in respiratory treatment modality. Secondary endpoints included invasive ventilation, treatment switch, vital signs (RR, heart rate, and blood pressure) and arterial blood gas analysis results (pH, PaCO2, and PaO2/FiO2) at one, 12, and 48 h, as well as 28-day mortality, and ICU and hospital lengths of stay. The number of nursing airway care interventions within the first 24 h, Borg dyspnea score and comfort score after 12 h of treatment, the duration of respiratory support, treatment intolerance and the incidence of adverse effects were also analyzed. The definition of nursing airway care interventions, the evaluation method of dyspnea score and comfort score were previously described in additional detail [12].
Statistical analysis
According to previous studies, it was estimated that NIV would fail in 22% of patients with COPD [1]. The absolute difference in treatment failure rates between HFNC and NIV was predicted to fall within a range of 4–12% [12, 13]. In this study, we set the non-inferiority cutoff at 9% after discussions with two senior pulmonologists and one critical care specialist. To assess non-inferiority using an α = 0.05, β = 0.20, and a dropout rate of 5%, each group was estimated to need at least 114 subjects.
The analysis of the main endpoint was performed on both an intention-to-treat and a per-protocol basis. Secondary endpoints were analyzed on an intention-to-treat basis. Kaplan–Meier curves with the log rank test were used to assess patient survival and time to treatment failure. Categorical variables were expressed as frequencies and percentages, using χ2 test or Fisher’s exact probability tests. Continuous data were expressed as means ± standard deviation or medians with interquartile (25–75th) percentiles, and were analyzed with Student’s t-test or the rank-sum test. Repeated measures analysis of variance, or non-parametric tests of multiple correlated samples (Friedman test for heterogeneity of variance or the skewed distributed data) followed by Bonferroni’s test were performed for the data obtained at multiple time points. P-values < 0.05 were considered statistically significant. SPSS 26.0 (IBM Corporation, Armonk, NY, USA) was used for all data analysis.
Results
Patient characteristics
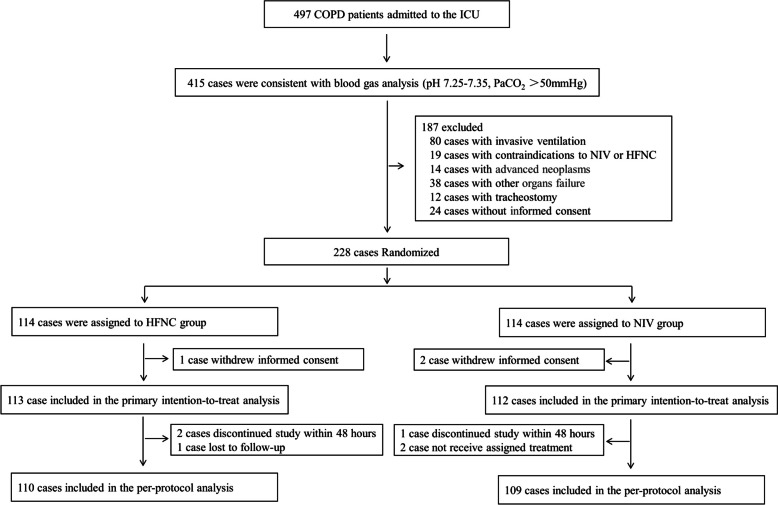
497 COPD patients were admitted to our two ICU units during the study period. 415 patients met the blood gas analysis criteria of hypercapnic ARF in AECOPD. Among these 415 patients, 228 patients were randomized to the HFNC or NIV groups after 187 subjects were excluded for various reasons (see Fig. 1). Three patients withdrew informed consent and were secondarily excluded. Finally, 113 patients in the HFNC group and 112 patients in the NIV group were included in the intention-to-treat analysis. In the two groups, demographic, COPD duration, smoking history, relevant comorbidities, COPD medications, respiratory therapy at home, and pulmonary function tests were similar (all P > 0.05, see Table 1). There were also no significant differences in APACHE II scores, SAPS II scores, vital signs or arterial blood gas analysis results between the two groups (all P > 0.05).
Fig. 1.
Flow chart of patient enrollment. COPD: Chronic obstructive pulmonary disease; ICU: Intensive care unit; HFNC: High-flow nasal cannula oxygen therapy; NIV: Non-invasive ventilation
Table 1.
Baseline characteristics of selected patients
| Characteristics | HFNC (n = 113) | NIV (n = 112) | P value | |
|---|---|---|---|---|
| Male, n (%) | 71 (62.8) | 62 (55.4) | 0.254 | |
| Age, years | 73 (65–78) | 69 (63–76) | 0.082 | |
| History of COPD, years | 8 (6–12) | 8 (6–11) | 0.490 | |
| Smoking history, n (%) | ||||
| Current | 12 (10.6) | 7 (6.3) | 0.239 | |
| Former smoker | 36 (31.9) | 49 (43.8) | 0.066 | |
| Comorbidities, n (%) | ||||
| Diabetes mellitus | 25 (21.1) | 31 (27.7) | 0.335 | |
| Coronary artery disease | 49 (43.4) | 38 (33.9) | 0.146 | |
| Chronic liver disease | 9 (8.0) | 15 (13.4) | 0.187 | |
| Chronic kidney disease | 24 (21.2) | 15 (13.4) | 0.120 | |
| Cerebrovascular disease | 11 (9.7) | 19 (17.0) | 0.111 | |
| Malignancy | 13(11.5) | 16 (14.3) | 0.534 | |
| Medication before exacerbation, n (%) | ||||
| Inhaled corticosteroids | 21 (18.6) | 33 (29.5) | 0.056 | |
| Beta adrenoceptor agonist | 50 (44.2) | 44 (39.3) | 0.451 | |
| Anticholinergics | 23 (20.4) | 32 (28.6) | 0.152 | |
| Home oxygen therapy, n (%) | ||||
| NCO | 23 (20.4) | 18 (16.1) | 0.405 | |
| NIV | 9 (8.0) | 12(10.7) | 0.478 | |
| Pulmonary function class, n (%) | 35 | 44 | ||
| II | 14 (40.0) | 17 (38.6) | 0.902 | |
| III | 19 (54.3) | 24 (54.5) | 0.982 | |
| IV | 2 (5.7) | 3 (6.8) | 0.841 | |
| Mean length from acute attack to ICU admission, days | 5 (3–8) | 4 (3–7) | 0.118 | |
| On admission to ICU | ||||
| APACHE II score | 14 (11–17) | 12 (10–16) | 0.067 | |
| SAPS II score | 32 (26–37) | 29 (26–34) | 0.201 | |
| Heart rate, beats/min | 92 (85–101) | 96 (85–103) | 0.148 | |
| Respiratory frequency, /min | 28 (25–30) | 29 (26–32) | 0.061 | |
| Mean arterial pressure, mmHg | 88 (82–93) | 84 (77–93) | 0.090 | |
| Arterial pH | 7.31(7.29–7.33) | 7.30(7.28–7.32) | 0.342 | |
| PaCO2, mmHg | 63 (59–68) | 61 (58–65) | 0.130 | |
| PaO2/FiO2, mmHg | 175 (167–199) | 184 (167–202) | 0.170 | |
HFNC High flow nasal cannula; NIV Non-invasive ventilation; COPD Chronic obstructive pulmonary disease; NCO Nasal cannula oxygen; ICU Intensive care unit; APACHE II: Acute Physiology and Chronic Health Evaluation II; SAPS II Simplified acute physiology score II: PaCO2 Partial pressure of arterial carbon dioxide; PaO2 Partial pressure of arterial oxygen
Primary endpoint and cause analysis
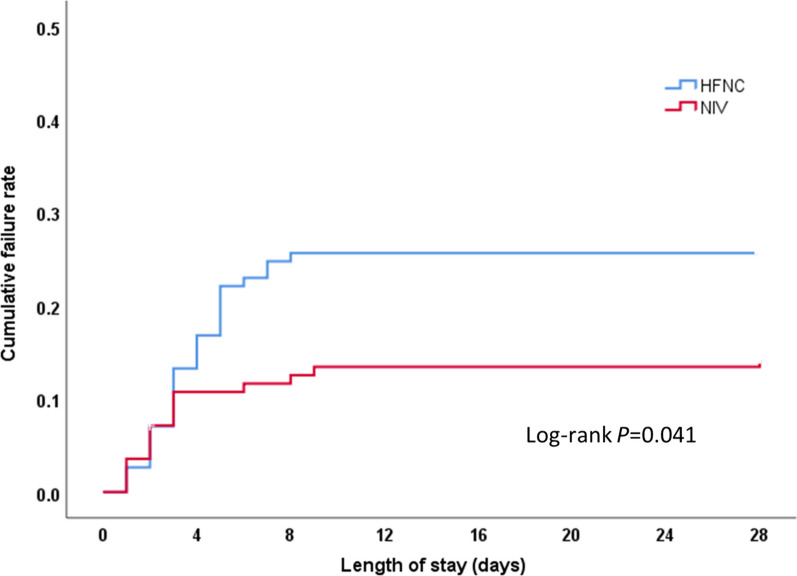
In the intention-to-treat analysis, the treatment failure rate in the HFNC group was 25.7% and 14.3% in the NIV group, with a difference between the two groups of 11.38% (95% CI 0.25–21.20, P = 0.033), which was higher than the non-inferiority threshold of 9%. Kaplan–Meier curve analysis showed that the cumulative failure rate of the HFNC group was significantly higher than that of the NIV group (Log Rank test 4.158, P = 0.041, see Fig. 2). In the per-protocol analysis, treatment failure occurred in 28 of the 110 HFNC patients (25.5%) and in 15 of the 109 NIV patients (13.8%) (risk difference, 11.69%; 95% CI 0.48–22.60).
Fig. 2.
Kaplan–Meier curve analysis for cumulative failure rate. HFNC: High-flow nasal cannula oxygen therapy; NIV: Non-invasive ventilation
Summarizing treatment failures in the HFNC group showed that the most common reasons for failure were carbon dioxide retention and exacerbations of respiratory distress, accounting for 44.8% and 31.0%, respectively. While in the NIV group, the most common reasons for failure were also carbon dioxide retention and exacerbations of respiratory distress, both accounting for 37.5%. (See Table 2).
Table 2.
Primary endpoint and cause analysis
| HFNC | NIV | Risk difference, % (95% CI) | P value | |
|---|---|---|---|---|
| Treatment failure, n (%) | ||||
| Intention-to-treat analysis | 29/113 (25.7) | 16/112 (14.3) | 11.38 (0.25 ~ 21.20) | 0.033 |
| Per-protocol analysis | 28/110 (25.5) | 15/109 (13.8) | 11.69 (0.48 ~ 22.60) | 0.029 |
| Analysis of treatment failure, n (%) | ||||
| Aggravation of respiratory distress | 9/29 (31.0) | 6/16 (37.5) | -6.47 (-37.06 ~ 22.64) | 0.660 |
| Aggravation of hypoxemia | 7/29 (24.1) | 4/16 (25.0) | -0.86 (-31.41 ~ 25.68) | 0.949 |
| Aggravation of carbon dioxide retention | 13/29 (44.8) | 6/16 (37.5) | 7.33 (-24.74 ~ 35.94) | 0.373 |
HFNC High flow nasal cannula oxygen therapy; NIV on-invasive ventilation
Secondary endpoints
The rate of endotracheal intubation in the HFNC group was higher than that in the NIV group (14.2% vs 5.4%, P = 0.026). The treatment switch rate in the HFNC group was 11.5%, which was not statistically different from the NIV group (8.9%, P = 0.505) (see Table 3).
Table 3.
Secondary endpoints in the HFNC and NIV groups
| HFNC (n = 113) | NIV (n = 112) | p value | |
|---|---|---|---|
| Invasive ventilation | 16 (14.2) | 6 (5.4) | 0.026 |
| Treatment switch | 13 (11.5) | 10 (8.9) | 0.524 |
| Length of stay in ICU, days | 7 (6–9) | 9 (6–11) | 0.059 |
| Length of stay in hospital, days | 10 (8–13) | 11 (9–13) | 0.228 |
| 28-day mortality, n (%) | 11 (9.7) | 8 (7.1) | 0.485 |
HFNC High-flow nasal cannula oxygen therapy; NIV Non-invasive ventilation; ICU Intensive care unit
The mean arterial pressure and heart rate after initiating treatment showed no significant difference between the two groups at different time points. In the dynamic observation during the first 48 h, the RR gradually decreased over time in both groups. The RR in the NIV group at 48 h was lower than that in the HFNC group [23 (20–26) breaths per min vs 23 (22–27) breaths per min, P = 0.025, see Table 4)]. However, there was no significant difference in RR between the two groups at one hour and 12 h.
Table 4.
Vital signs and arterial blood gas analysis
| Characteristics | Group | Baseline | 1h | 12h | 48h | P* value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Heart rate (beats/min) | HFNC | 92 (85–101) | 88 (83–100) | 89 (84–98) | 90 (82–100) | 0.210 | |||||
| NIV | 96 (85–103) | 93 (83–104) | 90 (83–102) | 88 (79–99)abc | 0.001 | ||||||
| P^ value | 0.148 | 0.183 | 0.582 | 0.329 | 0.115# | ||||||
| Mean arterial pressure (mmHg) | HFNC | 88 (82–93) | 86 (78–93) | 84 (76–93) | 87 (79–94) | 0.102 | |||||
| NIV | 84 (77–93) | 86 (77–95) | 83 (75–92) | 85 (78–93) | 0.100 | ||||||
| P^ value | 0.090 | 0.754 | 0.713 | 0.296 | 0.558# | ||||||
| Respiratory rate (min) | HFNC | 28 (25–30) | 26 (23–30) | 25 (22–28)a | 23 (22–27)a | < 0.001 | |||||
| NIV | 29 (26–32) | 25 (21–30)a | 23 (21–27)a | 23 (20–26)abc | < 0.001 | ||||||
| P^ value | 0.061 | 0.118 | 0.095 | 0.025 | 0.197# | ||||||
| Arterial pH | HFNC | 7.31(7.29–7.33) | 7.33(7.31–7.36)a | 7.37(7.34–7.39)ab | 7.39(7.35–7.42)abc | < 0.001 | |||||
| NIV | 7.30(7.28–7.32) | 7.34(7.31–7.37)a | 7.37(7.35–7.40)ab | 7.39(7.37–7.42)abc | < 0.001 | ||||||
| P^ value | 0.342 | 0.105 | 0.071 | 0.060 | 0.037# | ||||||
| PaCO2 (mm Hg) | HFNC | 63 (59–68) | 61 (56–64)a | 57 (53–64)ab | 57 (52–60)ab | < 0.001 | |||||
| NIV | 61 (58–65) | 58 (53–64)a | 56 (50–63)ab | 53 (49–59)abc | < 0.001 | ||||||
| P^ value | 0.130 | 0.067 | 0.054 | 0.043 | 0.012# | ||||||
| PaO2/FiO2 (mmHg) | HFNC | 175 (167–199) | 215 (196–242)a | 206 (192–225)ab | 225 (207–253)abc | < 0.001 | |||||
| NIV | 184 (167–202) | 206 (189–232)a | 217 (195–234)ab | 216 (203–238)ab | < 0.001 | ||||||
| P^ value | 0.170 | 0.069 | 0.066 | 0.058 | 0.798# | ||||||
P* for overall comparisons of differences in the same group over time. P# for overall comparisons of differences between the two groups over time. P^ for comparisons of differences between the two groups at the same time point. aCompared with the baseline value in the same group, P < 0.05 after Bonferroni correction. bCompared with the 1h value in the same group, P < 0.05 after Bonferroni correction. cCompared with the 12h value in the same group, P < 0.05 after Bonferroni correction
HFNC High-flow nasal cannula oxygen therapy; NIV Non-invasive ventilation; PaCO2 Partial pressure of arterial carbon dioxide; PaO2 Partial pressure of arterial oxygen; FiO2 Fraction of inspiration oxygen
Arterial blood gas analyses showed that, the pH and the PaO2/FiO2 values were all significantly elevated at one hour, 12 h and 48 h after initiating treatment, but there was no significant difference between the two groups at each time point. The PaCO2 in both groups was decreased at one hour and 12 h after initiating treatment. At 48 h, the PaCO2 in the NIV group continued to decrease and was significantly lower than in the HFNC group [53 (49–59) mmHg vs 57 (52–60) mmHg, P = 0.043, see Table 4].
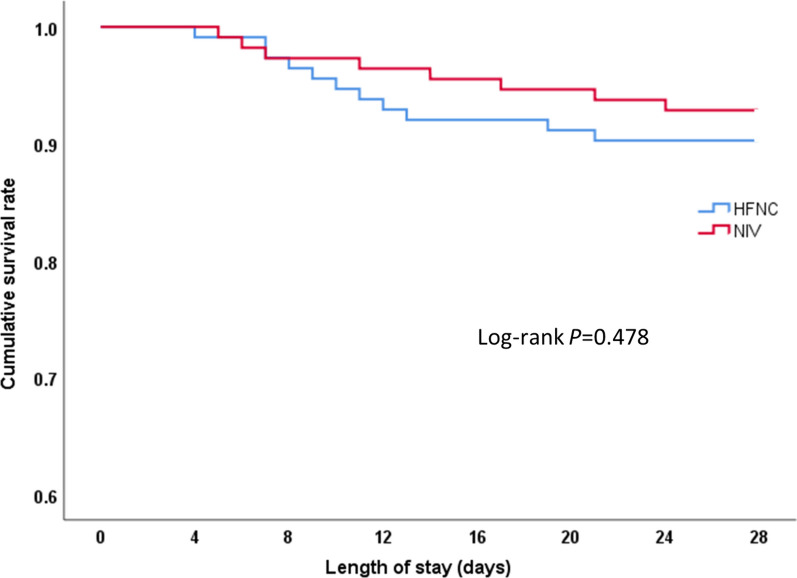
There were no significant differences in ICU or hospital total lengths of stay between the two groups (all P < 0.050, see Table 3). The 28-day mortality in the HFNC group was 9.7%, which was not significantly different from the 7.1% rate in the NIV group (Log Rank test 0.504, P = 0.478, see Fig. 3).
Fig. 3.
Kaplan–Meier curve analysis for cumulative survival rate. HFNC: High-flow nasal cannula oxygen therapy; NIV: Non-invasive ventilation
Other characteristics in the HFNC and NIV groups
There were no significant differences in the total duration of respiratory support (HFNC or NIV), dyspnea scores or treatment intolerance between the two groups (all P > 0.050, see Table 5). In the analysis of respiratory support duration in the first five days, the support duration in the HFNC group was significantly longer than that in the NIV group during the first two days (P < 0.05), but there was no significant difference between the two groups during the last three days. The number of daily airway care interventions and the incidence of nasofacial skin breakdown were significantly lower in the HFNC group than in the NIV group (all P < 0.050, see Table 5). The comfort score in the HFNC group was significantly higher than in the NIV group [7 (6–8) vs 6 (5–7), P < 0.001].
Table 5.
Other characteristics in the HFNC and NIV groups
| HFNC (n = 113) | NIV (n = 112) | P value | |
|---|---|---|---|
| Duration of HFNC or NIV (hours) | 85.9 ± 30.5 | 78.7 ± 33.8 | 0.312 |
| Day 1 (hours) | 13.2 ± 4.5 | 9.9 ± 3.5 | < 0.001 |
| Day 2 (hours) | 16.3 ± 4.1 | 12.8 ± 3.9 | 0.005 |
| Day 3 (hours) | 15.9 ± 6.4 | 14.8 ± 8.6 | 0.068 |
| Day 4 (hours) | 14.9 ± 6.5 | 13.6 ± 8.5 | 0.075 |
| Day 5 (hours) | 12.8 ± 4.9 | 11.4 ± 7.9 | 0.125 |
| Dyspnea score | 2 (2–4) | 2 (1–3) | 0.085 |
| Airway care interventions, per day | 5 (3–7) | 8 (6–10) | < 0.001 |
| Comfort score | 7 (6–8) | 6 (5–7) | < 0.001 |
| Treatment intolerance | 1 (0.9) | 5 (4.5) | 0.210 |
| Nasal facial skin breakdown, n (%) | 3 (2.7) | 10 (8.9) | 0.044 |
HFNC High-flow nasal cannula oxygen therapy; NIV Non-invasive ventilation; ICU Intensive care unit
Discussion
This non-inferiority RCT showed that HFNC was not shown to be non-inferior to NIV for preventing treatment failure for AECOPD patients with moderate hypercapnic ARF in both an intention-to-treat analysis or in a per-protocol analysis. Both analyses suggested that NIV was superior to HFNC in terms of treatment failure rates. The rate of endotracheal intubation in the HFNC group was higher than that in the NIV group. NIV was better than HFNC in reducing PaCO2 at 48 h after initiating respiratory treatment. HFNC had better results compared to NIV in both patient-reported comfort and in the number of nursing airway interventions.
Increasing numbers of studies have explored the efficacy of HFNC for COPD patients. Initially, evidence to support the use of HFNC was mainly limited to stable hypercapnic COPD. Several studies have shown that HFNC can improve exercise tolerance and quality of life in stable COPD patients, while relieving carbon dioxide retention, decreasing RR, and reducing acute attacks and hospitalization times [14–16].
Research focusing on the application of HFNC to AECOPD is now starting to emerge. A recent RCT showed that, in AECOPD patients with acute compensatory hypercapnic respiratory failure (pH ≥ 7.35, PaO2 < 60 mmHg, and PaCO2 > 45 mmHg), HFNC significantly reduced the need for invasive ventilation or NIV compared with conventional oxygen therapy, and PaCO2 after 24 h of treatment was lower in the HFNC group [17]. However, another multicenter RCT by Xia et al. found that HFNC could not reduce the need for intubation compared with conventional oxygen therapy among AECOPD patients with pH ≥ 7.35 and PaCO2 > 45 mmHg, and the authors suggested to explore the effect of HFNC on AECOPD patients with a pH < 7.35 [18].
Few studies comparing HFNC to NIV in AECOPD as the initial treatment choice have yet been published. In an RCT involving 72 AECOPD patients with a PaCO2 > 50 mmHg, HFNC was reported to have lower PaCO2, higher oxygenation and comfort scores than NIV, and showed no significant difference in intubation rates between HFNC and NIV [19]. In our study, however, the treatment failure and intubation rates of HFNC were significantly higher than in NIV. Oxygenation was similar between the two groups, though HFNC had better patient comfort scores than NIV. The aggravation of carbon dioxide retention was the most common reason for treatment failure in the HFNC group in our study.
Several studies have observed the effect of HFNC on reducing PaCO2 in patients with AECOPD. A multicenter observational case series study showed that HFNC treatment for one hour can significantly decrease the RR and PaCO2 when trialed among 40 COPD patients with hypercapnic ARF [20]. A randomized study by Pilcher et al. found that half an hour of HFNC application decreased PaCO2 by 1.4 mmHg and RR by 2 breaths/min compared with conventional oxygen therapy [21]. A cross-over randomized study showed that both HFNC and NIV treatment for one hour can improve respiratory acidosis and relieve dyspnea in AECOPD patients, and HFNC was more effective than NIV for improving both oxygenation and RR [22].
These studies demonstrated that the ability of HFNC to reduce PaCO2 in the short term was better than conventional oxygen therapy, and appeared to be non-inferior to NIV. Regarding the short term results, we found similar outcomes in our study, in which the values of PaCO2 between HFNC and NIV were similar at one hour and 12 h. However, PaCO2 in the NIV group continued to decrease at 48 h and was lower than in the HFNC group in our study. In the RCT by Cortegiani et al. 32% of patients in the HFNC group received NIV within 6 h, though HFNC and NIV had similar effects on reducing PaCO2 after two hours treatment in AECOPD patients with a pH 7.25–7.35 [23]. Although HFNC was found to be superior to NIV in reducing PaCO2 at discharge in an RCT that enrolled 40 patients with hypercapnic ARF (62.5% COPD patients) [24], we still suggest that the effect of HFNC on reducing PaCO2 in AECOPD patients should be treated with caution, especially after several days of initial treatment.
Low levels of positive airway pressure, the wash-out effect of exhaled gas in the upper airways and reduced physiological dead-space are the main physiological bases for HFNC to decrease PaCO2 [25]. During the initial stage of treatment, these effects can effectively remove carbon dioxide. However, the pulmonary function of AECOPD patients with hypercapnic ARF is usually poor, while the inducing factors such as infection have most likely not yet been controlled during the initial treatment stage, so the patient’s ill condition may not yet have reached its peak. With the passage of time, powerful respiratory support may be needed to control respiratory acidosis. Compared with HFNC, NIV has adjustable positive end-expiratory pressure and extra pressure support abilities, which can ensure a patient’s minute ventilation volume more effectively than HFNC. This may be the main reason why the PaCO2 in the NIV group was lower than that in the HFNC group after 48 h of treatment in our study.
Although several meta-analyses have shown that the efficacy of HFNC in hypercapnic ARF was comparable to that of NIV [26, 27], the current body of evidence may not be enough to draw a clear conclusion given study heterogeneity [28]. Non-inferior RCTs comparing HFNC and NIV in AECOPD patients with larger sample sizes have been rarely reported until now. This RCT suggested that the treatment failure rate of HFNC is higher than that of NIV. In other studies, the effectiveness of HFNC in AECOPD with hypercapnic ARF has also been questioned. A retrospective cohort study based on the MIMIC-IV database showed that the length of ICU stay in the HFNC group was significantly longer than that of the NIV group, while the 48-h intubation rate, 28-day intubation rate and 28-day mortality rate in the HFNC group were all higher than in the NIV group [29]. Additionally, it could be that the prolonged length of hospital stay in the HFNC group may be due to delayed or reduced escalation to NIV treatment [30].
As in this study, the better comfort of HFNC over NIV has been confirmed by many studies. This may be the reason why the treatment duration in the HFNC group was longer than in the NIV group on the first and second day. However, the tolerance of NIV can be improved by staff training, patient education and quality improvement [31]. Therefore, there was no difference in the duration of respiratory support between the two groups from the third day to the fifth day. The total endotracheal intubation rate in this study was 9.8%, which was lower than previous studies [1]. This may be related to our center’s many years of experience in using NIV and HFNC, as well as a clear quality improvement framework for both NIV and HFNC, including rapid identification, fast commencement, staff training, escalation protocols and close monitoring in our clinical practice. Moreover, a low endotracheal intubation rate of about 3% was also recently reported in some studies [19, 23].
Our study has some limitations. First, this was a single-center study and multi-center trials would be needed to confirm the external validity of our findings. Second, blinding was not possible to attending physicians or patients due to the treatments utilizing clearly different devices. However, the data analyst was blinded to the study groups and investigators were excluded from clinical decisions to help reduce bias. Third, the initial gas flow rate of HFNC in this study was set to 40 L/min. Whether other flow rates would have a better effect on carbon dioxide removal is worth exploring in future studies. Finally, the switch between HFNC and NIV in this study was decided by each patient’s attending physician, which was influenced by many factors and does introduce a certain subjectivity, despite the study having certain respiratory markers to assist in guiding care. Nevertheless, this study may better reflect the pragmatic application of NIV or HFNC in actual clinical practice.
Conclusions
In AECOPD patients with moderate hypercapnic ARF, HFNC was not shown to be non-inferior to NIV and resulted in a higher incidence of treatment failure than NIV when used as initial respiratory support.
Acknowledgements
None.
Abbreviations
- COPD
Chronic obstructive pulmonary disease
- AECOPD
Acute exacerbations of chronic obstructive pulmonary disease
- ARF
Acute respiratory failure
- NIV
Non-invasive ventilation
- HFNC
High-flow nasal cannula oxygen therapy
- RCT
Randomized controlled trial
- ICU
Intensive care unit
- FiO2
Fraction of inspiration oxygen
- SPO2
Pulse oxygen saturation
- APACHE II
Acute physiological and chronic health status score II
- SAPS II
Simplified acute physiology score II
- PaCO2
Partial pressure of arterial carbon dioxide
- PaO2
Partial pressure of arterial oxygen
- RR
Respiratory rate
Author contributions
TDY and WBX contributed to study conception and design. CP, WYY, SJY, GP, and JHW contributed to data acquisition. TDY, WBX, WYY and SJY contributed to data analysis and interpretation. WCL and WYC contributed to statistical analysis and revision. TDY contributed to acquisition of funding. TDY, WBX, WYY, JHW and SJY contributed to the drafting of the manuscript and its critical revision for important intellectual content. All authors have read and approved the final version of the manuscript.
Funding
This work was supported by the Scientific Research Project of Jiangsu Health and Health Commission (M2020014), Yangzhou Science and Technology Development Plan (YZ2023123), and Social Science Research Project of Universities in Jiangsu Province (2022SJYB2118).
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author in response to reasonable requests.
Declarations
Ethics approval and consent to participate
This study was approved by the hospital’s human subjects ethics committee (No. 2017053), and informed consent was obtained from all enrolled subjects or their relatives.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dingyu Tan and Bingxia Wang have contributed equally to this work.
Contributor Information
Yunyun Wang, Email: lantian_593@163.com.
Jiayan Sun, Email: sunjiayan2006@163.com.
References
- 1.Osadnik CR, Tee VS, Carson-Chahhoud KV, Picot J, Wedzicha JA, Smith BJ. Non-invasive ventilation for the management of acute hypercapnic respiratory failure due to exacerbation of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;7(7):CD004104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cortegiani A, Russotto V, Antonelli M, Azoulay E, Carlucci A, Conti G, et al. Ten important articles on noninvasive ventilation in critically ill patients and insights for the future: a report of expert opinions. BMC Anesthesiol. 2017;17(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruni A, Garofalo E, Pelaia C, Messina A, Cammarota G, Murabito P, et al. Patient-ventilator asynchrony in adult critically ill patients. Minerva Anestesiol Italy. 2019;85(6):676–88. [DOI] [PubMed] [Google Scholar]
- 4.Nagata K, Horie T, Chohnabayashi N, Jinta T, Tsugitomi R, Shiraki A, et al. Home high-flow nasal cannula oxygen therapy for stable hypercapnic COPD: a randomized clinical trial. Am J Respir Crit Care Med. 2022;206(11):1326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spoletini G, Cortegiani A, Gregoretti C. Physiopathological rationale of using high-flow nasal therapy in the acute and chronic setting: a narra tive review. Trends Anaesth Crit Care. 2019;26–27:22–9. [Google Scholar]
- 6.Pantazopoulos I, Daniil Z, Moylan M, Gourgoulianis K, Chalkias A, Zakynthinos S, Ischaki E. Nasal high flow use in COPD patients with hypercapnic respiratory failure: treatment algorithm & review of the literature. COPD. 2020;17(1):101–11. [DOI] [PubMed] [Google Scholar]
- 7.Bräunlich J, Wirtz H. Nasal high-flow in acute hypercapnic exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:3895–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuste ME, Moreno O, Narbona S, Acosta F, Peñas L, Colmenero M. Efficacy and safety of high-flow nasal cannula oxygen therapy in moderate acute hypercapnic respiratory failure. Rev Bras Ter Intensiva. 2019;31(2):156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee MK, Choi J, Park B, Kim B, Lee SJ, Kim SH, et al. High flow nasal cannulae oxygen therapy in acute-moderate hypercapnic respiratory failure. Clin Respir J. 2018;12(6):2046–56. [DOI] [PubMed] [Google Scholar]
- 10.Sun J, Li Y, Ling B, Zhu Q, Hu Y, Tan D, et al. High flow nasal cannula oxygen therapy versus non-invasive ventilation for chronic obstructive pulmonary disease with acute-moderate hypercapnic respiratory failure: an observational cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:1229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Eur Respir J. 2017;49(3):1700214. [DOI] [PubMed] [Google Scholar]
- 12.Tan D, Walline JH, Ling B, Xu Y, Sun J, Wang B, et al. High-flow nasal cannula oxygen therapy versus non-invasive ventilation for chronic obstructive pulmonary disease patients after extubation: a multicenter, randomized controlled trial. Crit Care. 2020;24(1):489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernández G, Vaquero C, Colinas L, Cuena R, González P, Canabal A, et al. Effect of postextubation high-flow nasal cannula vs noninvasive ventilation on reintubation and postextubation respiratory failure in high-risk patients: a randomized clinical trial. JAMA. 2016;316(15):1565–74. [DOI] [PubMed] [Google Scholar]
- 14.Milne RJ, Hockey HU, Garrett J. Hospital cost savings for sequential COPD patients receiving domiciliary nasal high flow therapy. Int J Chron Obstruct Pulmon Dis. 2022;17:1311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Storgaard LH, Hockey HU, Weinreich UM. Development in PaCO(2) over 12 months in patients with COPD with persistent hypercapnic respiratory failure treated with high-flow nasal cannula-post-hoc analysis from a randomised controlled trial. BMJ Open Respir Res. 2020;7(1): e000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlucci A, Rossi V, Cirio S, Piran M, Bettinelli G, Fusar Poli B, et al. Portable high-flow nasal oxygen during walking in patients with severe chronic obstructive pulmonary disease: a randomized controlled trial. Respiration. 2021;100(12):1158–64. [DOI] [PubMed] [Google Scholar]
- 17.Li XY, Tang X, Wang R, Yuan X, Zhao Y, Wang L, et al. High-flow nasal cannula for chronic obstructive pulmonary disease with acute compensated hypercapnic respiratory failure: a randomized, controlled trial. Int J Chron Obstruct Pulmon Dis. 2020;15:3051–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia J, Gu S, Lei W, Zhang J, Wei H, Liu C, et al. High-flow nasal cannula versus conventional oxygen therapy in acute COPD exacerbation with mild hypercapnia: a multicenter randomized controlled trial. Crit Care. 2022;26(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu A, Zhou Y, Pu Z. Effects of high-flow nasal cannula oxygen therapy for patients with acute exacerbation of chronic obstructive pulmonary disease in combination with type II respiratory failure. J Int Med Res. 2023;51(6):3000605231182558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plotnikow GA, Accoce M, Fredes S, Tiribelli N, Setten M, Dorado J, et al. High-flow oxygen therapy application in chronic obstructive pulmonary disease patients with acute hypercapnic respiratory failure: a multicenter study. Crit Care Explor. 2021;3(2): e0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pilcher J, Eastlake L, Richards M, Power S, Cripps T, Bibby S, et al. Physiological effects of titrated oxygen via nasal high-flow cannulae in COPD exacerbations: a randomized controlled cross-over trial. Respirology. 2017;22(6):1149–55. [DOI] [PubMed] [Google Scholar]
- 22.Rezaei A, Fakharian A, Ghorbani F, Idani E, Abedini A, Jamaati H. Comparison of high-flow oxygenation with noninvasive ventilation in COPD exacerbation: a crossover clinical trial. Clin Respir J. 2021;15(4):420–9. [DOI] [PubMed] [Google Scholar]
- 23.Cortegiani A, Longhini F, Madotto F, Groff P, Scala R, Crimi C, et al. High flow nasal therapy versus noninvasive ventilation as initial ventilatory strategy in COPD exacerbation: a multicenter non-inferiority randomized trial. Crit Care. 2020;24(1):692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papachatzakis Y, Nikolaidis PT, Kontogiannis S, Trakada G. High-flow oxygen through nasal cannula vs non-invasive ventilation in hypercapnic respiratory failure: a randomized clinical trial. Int J Environ Res Public Health. 2020;17(16):5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishimura M. High-flow nasal cannula oxygen therapy in adults: physiological benefits, indication, clinical benefits, and adverse effects. Respir Care. 2016;61(4):529–41. [DOI] [PubMed] [Google Scholar]
- 26.Fahey AC, O’Connell M, Cornally N, Saab MM. High flow nasal cannula versus noninvasive ventilation in the treatment of acute hypercapnic respiratory failure: a systematic review and meta-analysis. Clin Respir J. 2023;17(11):1091–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu C, Yang F, Wang Q, Gao W. Comparison of high flow nasal therapy with non-invasive ventilation and conventional oxygen therapy for acute hypercapnic respiratory failure: a meta-analysis of randomized controlled trials. Int J Chron Obstruct Pulmon Dis. 2023;18:955–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ovtcharenko N, Ho E, Alhazzani W, Cortegiani A, Ergan B, Scala R, et al. High-flow nasal cannula versus non-invasive ventilation for acute hypercapnic respiratory failure in adults: a systematic review and meta-analysis of randomized trials. Crit Care. 2022;26(1):348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu T, Zhao Q, Shi Z, Du B. Effect of high-flow nasal cannula oxygen on patients with chronic obstructive pulmonary disease and mild hypercapnia: a retrospective cohort study based on the Medical Information Mart for Intensive Care-IV database. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2021;33(6):686–91. [DOI] [PubMed] [Google Scholar]
- 30.Xia J, Yang H, Zhan Q, Fan Y, Wang C. High-flow nasal cannula may prolong the length of hospital stay in patients with hypercapnic acute COPD exacerbation. Respir Med. 2023;220: 107465. [DOI] [PubMed] [Google Scholar]
- 31.Gavin F, Sanjeevan M, Katharine S, Liam MH. Maintaining the status flow: high-flow nasal cannula is not the right choice for acute hypercapnic respiratory failure. Intern Med J. 2022;52(2):343–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author in response to reasonable requests.