Abstract
Recent developments in menin inhibitors for relapsed or refractory acute myeloid leukemia (AML) were highlighted at the 2023 ASH Annual Meeting. Notably, revumenib showed promising efficacy, achieving a 100% ORR when combined with decitabine/cedazuridine and venetoclax. These findings underscore the potential of menin inhibitors in transforming AML treatment, particularly in genetically defined subgroups, offering hope for improved patient outcomes. Ongoing studies, like KOMET-008, further explore the synergistic potential of menin inhibitors in combination regimens, shaping future AML management strategies.
Keywords: Menin inhibitors, Acute myeloid leukemia, Revumenib, Ziftomenib, Clinical trials
To the editor
Acute myeloid leukemia (AML) remains challenging, particularly subtypes characterized by genomic instability, such as KMT2A rearrangement and NPM1 mutations [1, 2]. Current treatment regimens, including chemotherapy and targeted therapies, often lead to limited long-term success and significant toxicity. Menin inhibitors have emerged as a promising therapeutic approach, particularly for AML subtypes with specific genetic abnormalities such as KMT2A rearrangements and NPM1 mutations (Table 1). Here we synthesize findings from the 2023 ASH Annual Meeting, focusing on the efficacy and safety of Menin inhibitors across various clinical settings.
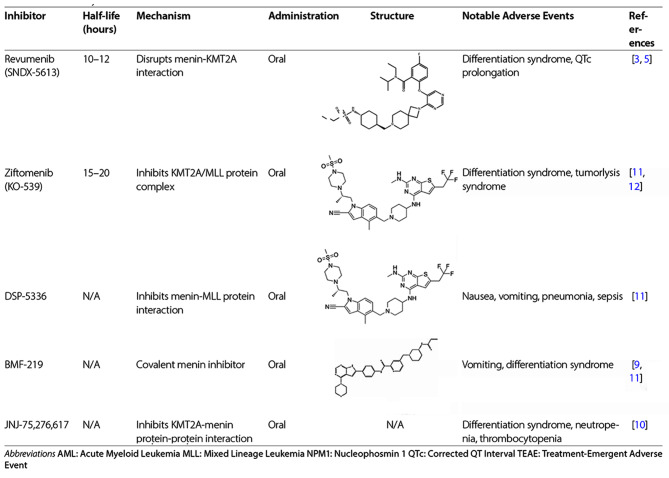
Table 1.
Summary of menin inhibitors
Revumenib (SNDX-5613)
Revumenib, a selective Menin inhibitor, has shown substantial efficacy, particularly for AML patients with KMT2A rearrangements (KMT2Ar). Issa et al. reported significant outcomes in a Phase I/II trial where 7 evaluable patients with relapsed or refractory(r/r) AML characterized by KMT2A rearrangements were assessed. Revumenib combined with decitabine/cedazuridine and venetoclax achieved a 100% overall response rate (ORR), with all evaluated patients attaining morphological remission [3].
Further, Zucenka et al. discussed the outcomes of Revumenib maintenance therapy post-hematopoietic stem cell transplant (HSCT) in the AUGMENT-101 study. Of 131 treated patients, nine resumed Revumenib post-transplant, with treatment durations ranging from 23 to 588 days. Notably, six of the nine patients who resumed Revumenib post-transplant maintained complete response (CR), and five achieved or sustained measurable residual disease (MRD) negativity [4].
Additionally, pivotal Phase 2 results from AUGMENT-101, as reported by Aldoss et al., confirmed Revumenib’s robust clinical profile. Among 94 treated patients with relapsed/refractory KMT2Ar acute leukemia, the CR + CR with partial hematologic recovery (CRh) rate was 22.8%, the composite complete response rate (CRc) was 43.9%, and the ORR was 63.2% [5].
Revumenib’s safety profile was manageable, with differentiation syndrome and QTc prolongation [3–6] (Table 2).
Table 2.
Key clinical outcomes of Menin inhibitors in ASH 2023
| Inhibitor | Study Name | Patient Population | Key Efficacy Outcomes | Safety Profile |
|---|---|---|---|---|
| Revumenib | AUGMENT-101 | Relapsed/refractory KMT2Ar AML | CR + CRh: 22.8% (21/94), CRc: 43.9% (41/94), ORR: 63.2% (59/94) | Manageable; differentiation syndrome (16.0%), febrile neutropenia (13.8%), and QTc prolongation (13.8%); |
| Revumenib | Post-HSCT Study | Post-HSCT patients | CR: 6/9, MRD negativity: 5/9 | Manageable; no new safety signals, common AEs included cytopenias (20%) and infections (10%) |
| Ziftomenib | Komet-008 | Relapsed/refractory AML | Not available | Manageable; common AEs included anemia (25%), pneumonia (20%), thrombocytopenia (15%), neutropenia (15%) |
| DSP-5336 | N/A | MLLr and NPM1 mutated AML | CR with incomplete recovery: 1/6, morphologic leukemia-free state: 1/6 | Not available |
| BMF-219 | COVALENT-101 | Relapsed/refractory acute leukemia | CR: 2/5 | Vomiting 13% (3) and Differentiation Syndrome (DS) 13% (3). No Grade 5 TRAEs were reported. |
| JNJ-75,276,617 | Phase 1 Study | Relapsed/refractory AML | CR + CRh: 22.8% (21/94), CRc: 43.9% (41/94), ORR: 63.2% (59/94) | Differentiation syndrome (8 [14%]), neutropenia (6 [10%]), anemia and thrombocytopenia (4 [7%] each). |
Abbreviations AE: Adverse Event AML: Acute Myeloid Leukemia CR: Complete Remission CRh: Complete Remission with Partial Hematologic Recovery CRc: Composite Complete Remission HSCT: Hematopoietic Stem Cell Transplant KMT2Ar: KMT2A rearrangements MLL: Mixed Lineage Leukemia MRD: Measurable Residual Disease NPM1: Nucleophosmin 1 ORR: Overall Response Rate QTc: Corrected QT Interval TEAE: Treatment-Emergent Adverse Event
Ziftomenib
Ziftomenib, a menin-KMT2A interaction inhibitor, targets NPM1-mutated and KMT2A-rearranged AML. The ongoing phase 1 KOMET-008 trial is investigating the agent plus gilteritinib, FLAG-IDA (fludarabine, cytarabine, granulocyte-colony stimulating factor, and idarubicin), or low-dose cytarabine in patients with r/r NPM1-mutant or KMT2A-rearranged AML [7]. Komet-001 results indicated significant clinical activity, with a 40% complete remission rate and a 45% ORR in 20 patients [8]. These promising outcomes led the FDA to grant breakthrough therapy designation for Ziftomenib in NPM1-mutated AML (Table 2).
DSP-5336
DSP-5336, an investigational oral menin inhibitor, targets the menin and MLL protein interaction. The dose escalation portion of the DSP-5336 study consists of two parallel arms (Arm A: without concomitant anti-fungal azole therapy; Arm B: with concomitant azole therapy). Among six MLLr patients treated, one achieved a five-month CR, another maintained a morphologic leukemia-free state, and one had stable disease. For four NPM1 patients, treatment resulted in stable disease [9] (Table 2).
BMF-219
BMF-219, a pioneering covalent menin inhibitor, is under evaluation in the COVALENT-101 study (NCT05153330), which included adults with r/r acute leukemia ineligible for standard therapy. Among the efficacy evaluable population, two out of five patients achieved complete remission [10] (Table 2).
JNJ-75,276,617
In the Phase 1 study of JNJ-75,276,617(NCT04811560), conducted by Jabbour et al., this menin-KMT2A inhibitor was evaluated in adult patients with r/r AML harboring KMT2A or NPM1 mutations. From the 58 patients enrolled, 97% had r/r AML, and 3% had ALL, adverse events were manageable(Table 2). The clinical efficacy was highlighted by a 63% reduction in bone marrow disease burden. At the highest dose tested, the ORR was 50%, the time to response ranged from 1.0 to 3.3 months [11] (Table 2).
Safety and adverse events
The safety profile of menin inhibitors was manageable. Common adverse events included gastrointestinal symptoms, cytopenias, and, less frequently, differentiation syndrome, which were generally reversible with dose adjustments or supportive care [6] (Table 2).
Traditional AML treatments like intensive chemotherapy and HSCT often lead to significant toxicities and limited efficacy, especially in relapsed/refractory cases. Menin inhibitors have shown in clinical trials to induce remission in heavily pretreated populations with a more manageable safety profile. They present fewer severe adverse events and effective management of differentiation syndrome, making them a significant advancement in AML management.
The 2023 ASH Annual Meeting highlighted the transformative potential of menin inhibitors, especially for genetically defined subgroups. Future research should explore their synergistic potential with other therapeutic agents, such as hypomethylating agents and BCL-2 inhibitors, to enhance treatment efficacy and assess long-term response durability.
Abbreviations
- AML
Acute Myeloid Leukemia
- CR
Complete Remission
- CRh
Complete Remission with Partial Hematologic Recovery
- CRc
Composite Complete Remission
- ORR
Overall Response Rate
- HSCT
Hematopoietic Stem Cell Transplant
- MRD
Measurable Residual Disease
Author contributions
XHZ designed the study. ZYA drafted the manuscript and prepared the tables. All the authors participated in the process of drafting and revising the manuscript. All the authors have read and approved the final manuscript.
Funding
This work was supported by National Key Research and Development Program of China (No. 2021YFC2500304), Key Program of National Natural Science Foundation of China (No. 82230004), National Natural Science Foundation of China (No. 81970113), and the Capital Health Research and Development of Special (No. 2022–1-4082).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.DiNardo CD, et al. Acute myeloid leukaemia. Lancet. 2023;401(10393):2073–86. 10.1016/S0140-6736(23)00108-3 [DOI] [PubMed] [Google Scholar]
- 2.Issa GC, et al. Therapeutic implications of menin inhibition in acute leukemias. Leukemia. 2021;35(9):2482–95. 10.1038/s41375-021-01309-y [DOI] [PubMed] [Google Scholar]
- 3.Issa GC, et al. Early results of the phase I/II study investigating the all-oral combination of the menin inhibitor revumenib (SNDX-5613) with Decitabine/Cedazuridine (ASTX727) and Venetoclax in Acute myeloid leukemia (SAVE). Blood. 2023;142(Supplement 1):58–58. 10.1182/blood-2023-182337 [DOI] [Google Scholar]
- 4.Zucenka A, et al. Revumenib maintenance therapy following Revumenib-Induced Remission and Transplant. Blood. 2023;142(Supplement 1):4950–4950. 10.1182/blood-2023-189036 [DOI] [Google Scholar]
- 5.Aldoss I, et al. Revumenib Monotherapy in patients with Relapsed/Refractory KMT2Ar Acute Leukemia: Topline Efficacy and Safety results from the pivotal Augment-101 phase 2 study. Blood. 2023;142(Supplement 2):pLBA–5. 10.1182/blood-2023-192042 [DOI] [Google Scholar]
- 6.Candoni A, Coppola G. A 2024 update on Menin inhibitors. A New Class of Target agents against KMT2A-Rearranged and NPM1-Mutated Acute myeloid leukemia. Hematol Rep. 2024;16(2):244–54. 10.3390/hematolrep16020024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldberg AD, et al. Komet-008: a phase 1 study to Determine the Safety and Tolerability of Ziftomenib combinations for the treatment of KMT2A-Rearranged or NPM1-Mutant Relapsed/Refractory Acute Myeloid Leukemia. Blood. 2023;142(Supplement 1):1553–1553. 10.1182/blood-2023-190475 [DOI] [Google Scholar]
- 8.Fathi A et al. P504 updated data for ziftomenib in patients with npm1-mutated relapsed or refractory acute myeloid leukemia. HemaSphere, 2023. 7(S3): p. e19161da.
- 9.Daver N, et al. Phase 1/2 first-in-human study of the Menin-MLL inhibitor DSP-5336 in patients with relapsed or refractory Acute Leukemia. Blood. 2023;142(Supplement 1):2911–2911. 10.1182/blood-2023-179252 [DOI] [Google Scholar]
- 10.Lancet J, et al. Covalent menin inhibitor Bmf-219 in patients with relapsed or refractory (R/R) Acute Leukemia (AL): preliminary phase 1 data from the Covalent-101 study. Blood. 2023;142(Supplement 1):2916–2916. 10.1182/blood-2023-173149 [DOI] [Google Scholar]
- 11.Jabbour E, et al. A first-in-human phase 1 study of the Menin-KMT2A (MLL1) inhibitor JNJ-75276617 in adult patients with Relapsed/Refractory Acute Leukemia Harboring KMT2A or NPM1 alterations. Blood. 2023;142(Supplement 1):57–57. 10.1182/blood-2023-172422 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.