Abstract
Idiopathic pulmonary fibrosis is a lethal, progressive, and irreversible condition that has become a significant focus of medical research due to its increasing incidence. This rising trend presents substantial challenges for patients, healthcare providers, and researchers. Despite the escalating burden of pulmonary fibrosis, the available therapeutic options remain limited. Currently, the United States Food and Drug Administration has approved two drugs for the treatment of pulmonary fibrosis—nintedanib and pirfenidone. However, their therapeutic effectiveness is limited, and they cannot reverse the fibrosis process. Additionally, these drugs are associated with significant side effects. Myofibroblasts play a central role in the pathophysiology of pulmonary fibrosis, significantly contributing to its progression. Consequently, strategies aimed at inhibiting myofibroblast differentiation or promoting their dedifferentiation hold promise as effective treatments. This review examines the regulation of myofibroblast dedifferentiation, exploring various signaling pathways, regulatory targets, and potential pharmaceutical interventions that could provide new directions for therapeutic development.
Keywords: Fibroblast, Myofibroblast, Dedifferentiation, Pulmonary fibrosis, TGF-β signaling
Background
Currently, the estimated prevalence rate of pulmonary fibrosis per 10,000 individuals ranges from 0.57 to 4.51 in the Asia-Pacific region, 0.33 to 2.51 in Europe, and 2.40 to 2.98 in North America [1]. This condition imposes significant suffering on patients and places a heavy burden on healthcare systems and financial resources. Despite these challenges, treatment options remain severely limited. To date, only two drugs, nintedanib and pirfenidone, have been approved by the United States Food and Drug Administration for the treatment of pulmonary fibrosis. However, their effectiveness is limited; they cannot reverse fibrotic progression and are associated with significant side effects. [2–4]. Non-pharmacological interventions, such as palliative care, pulmonary rehabilitation, lung transplantation, and the management of complications and acute exacerbations, aim to alleviate symptoms and enhance quality of life but do not reverse pulmonary fibrosis.
There is an urgent need to explore and implement more treatment modalities in clinical practice. The pathogenesis of pulmonary fibrosis primarily involves sustained or repeated pulmonary epithelial injury, subsequent activation of fibroblasts, and differentiation into myofibroblasts. This process results in excessive extracellular matrix (ECM) deposition, distortion of normal lung architecture, and irreversible loss of lung function [5]. Myofibroblast differentiation plays a crucial role in the pathological physiology of pulmonary fibrosis. Although traditionally believed to be terminally differentiated cells, recent research consistently demonstrates the dedifferentiation capacity of myofibroblasts, suggesting that dedifferentiation may contribute to the regression of established fibrosis.
Myofibroblast
What is a myofibroblast
Myofibroblast are a type of fibroblast-like cells that contain myosin and actin, along with other muscle proteins [6]. Additionally, myofibroblasts function as intermediate cells positioned between fibroblasts and smooth muscle cells. Extensively present at wound in healing sites and fibrotic organs with high remodeling capacities, such as the lungs, kidneys, liver, and skin, myofibroblasts are the primary effector cells in the fibrotic processes of various organs [7]. They are capable of producing a substantial amount of extracellular matrix (ECM) proteins, including type I collagen and fibronectin, thereby facilitating wound healing and the organ fibrotic response [8]. Currently, smooth muscle actin (SMA) is the most commonly used biomarker to distinguish myofibroblasts from fibroblasts and other precursors. In addition, vimentin, fibroblast-specific protein 1 (FSP-1) and cadherin-11 are potential markers for fibroblast cells. Angiotensin 1 receptor (AT 1), transforming growth factor-β type II receptor (TβRII), paxillin, tensin, fibronectin extra dominant A splice variant, Frizzled-2, osteopontin, Tenascin C and periostin are highly expressed or exclusively expressed in myofibroblasts, may serve as potential markers for myofibroblasts [9].
Origin of myofibroblasts
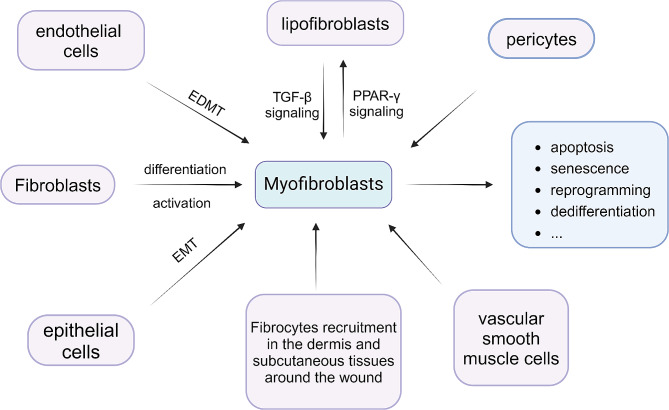
Myofibroblasts have several common origins. Firstly, fibroblasts can differentiate into myofibroblasts induced by the TGF-β/SMAD pathway or platelet-derived growth factor (PDGF) [10]. Secondly, there are epithelial-mesenchymal transition (EMT) and endothelial-mesenchymal transition (EDMT) [11]. Other sources include the recruitment of fibrocytes from the dermis and subcutaneous tissue around wounds, the transformation of perivascular cells and vascular smooth muscle cells into myofibroblasts [12], and the interconversion between lipogenic and myogenic fibroblastic phenotypes [13]. The origins and pathways of elimination of myofibroblasts are depicted in Fig. 1.
Fig. 1.
Several common origins and eliminating ways of myofibroblasts. EMT, epithelial-mesenchymal transition. EDMT, endothelial-mesenchymal transition. TGF-β, Transforming growth factor-β. PPAR-γ, peroxisome proliferator-activated receptor-γ
Myofibroblast dedifferentiation and its therapeutic implications
The treatment of pulmonary fibrosis can be approached through several strategies: eliminating the underlying causes of fibrosis, degrading and removing fibrotic extracellular matrix (ECM), inhibiting the proliferation of fibroblasts and their differentiation into myofibroblasts, and eliminating myofibroblasts, which involves inducing apoptosis, senescence, reprogramming, dedifferentiation, and other processes [7]. Among these, dedifferentiation refers to a process wherein mature cells, which have already undergone differentiation or partial differentiation, regress from their mature state to adopt a less differentiated cellular phenotype. For instance, endothelial cells undergoing dedifferentiation transform into endothelial progenitor cells. Myofibroblast dedifferentiation refers to a significant reduction in ECM production, a decrease in the expression of α-smooth muscle actin (α-SMA) in myofibroblasts, and a lower binding affinity with stress fibers, possibly even restoring the inactive phenotypic characteristics of myofibroblast precursor cells [14, 15]. Because myofibroblast dedifferentiation is a relatively small and new research direction, there is no unified consensus on research methods in previous studies, so the experimental procedures from different articles are different. A frequently utilized experimental procedure involves subjecting fibroblasts to serum-starvation in DMEM for 24 h, followed by TGF-β pretreatment for 24–48 h to induce fibroblast differentiation into myofibroblasts. Subsequently, dedifferentiation factors are applied for a specific duration, culminating in the assessment of molecular expression levels of α-SMA and collagen at protein or RNA levels [16–18]. Generally, a significant decrease in α-SMA and collagen expression is regarded as indicative of dedifferentiation.
Other treatment modalities typically can only restrain the progression of pulmonary fibrosis, alleviate symptoms, and improve the quality of life. By the time fibrosis is diagnosed, tissue damage has already advanced. Therefore, inhibiting the formation of myofibroblasts and impeding fibrotic progression may not achieve a curative effect. In contrast, inducing myofibroblast dedifferentiation is believed to have the potential to reverse established pulmonary fibrosis, thereby achieving a curative outcome. Consequently, an increasing number of studies are focusing on the direction of myofibroblast dedifferentiation.
TGF-β signaling
TGF-β signaling in fibroblast differentiation
Transforming growth factor-β (TGF-β) serves as the prototype within the TGF-β family, which includs factors such as activin, nodal, bone morphogenetic proteins (BMPs), and growth and differentiation factors (GDFs) [19, 20]. In the differentiation process from fibroblasts to myofibroblasts, TGF-β is recognized by TGF-β receptor II (TβRII) which contains the intracellular kinase domain. This domain recruits and phosphorylates TGF-β receptor I (TβRI) through a glycine/serine-rich “GS sequence” undergoing threonine/serine kinase conversion. Subsequently, TβRII and TβRI form a heteromeric complex [21]. Activated TβRI phosphorylates R-Smad (Smad2 and Smad3) proteins, facilitating the formation of a trimeric complex with Co-Smad namely Smad4. This trimeric complex translocates into the cell nucleus, acting as a transcription factor to regulate the expression of fibrosis-related target genes, including fibronectin, collagen, plasminogen activator inhibitor-1 (PAI-1), and connective tissue growth factor (CTGF) [22–24]. This process is modulated by coactivators such as p300, CBP, activating protein-1 (AP-1), and specificity protein 1 (Sp1), or corepressors such as c-Ski, SnoN, transforming growth inhibiting factor, and Smad nuclear-interacting protein-1 [25]. Activation of the Smad signaling pathway induces the expression of transcription factors such as SNAIL, SLUG, ZEB, and TWIST, which act as inhibitors of E-cadherin and mediate desmosome dissociation, contributing to the epithelial-mesenchymal transition (EMT) [26]. Additionally, Smad pathway activation leads to Protein kinase B (Akt) activation and subsequent nuclear translocation of β-catenin, resulting in upregulation of α-SMA [27]. Negative regulators of the Smad pathway, Smad6 and Smad7, counteract TGF-β signaling by binding to type I receptors and competing with activated R-Smads for binding to Co-Smad4. Furthermore, inhibitory Smads recruit E3 ubiquitin-protein ligases Smurf1 and Smurf2, targeting Smad proteins for proteasomal degradation and thereby terminating Smad-mediated signal transduction. Smad6 expression is induced by Smad1 and 5, while Smad7 expression is triggered by Smad3 [28, 29]. IL-7 and IFN-γ induce Smad7 expression through the JAK-STAT pathway, inhibiting TGF-β signal transduction and mitigating bleomycin-induced pulmonary fibrosis [30, 31].
TGF-β can also activate the MAPK family, including extracellular signal-regulated kinase (ERK), p38 mitogen-activated protein kinase (MAPK), and Jun N-terminal kinase (JNK) signals [32]. Additionally, it can activate the phosphoinositide 3-kinase (PI3K)/Akt and Rho GTPase pathways and synergistically interact with the Wnt and Notch signaling cascades [23]. These non-Smad transducers activated by receptors can function as independent pathways or cooperatively mediate signal responses in conjunction with Smads, converging onto Smads to control their activity [33, 34]. For instance, Erk1/2 can phosphorylate transcription factors such as Fos-related antigen 2 (Fra-2), promoting gene transcription [35, 36]. Protein kinase B (Akt) can be activated by TGF-β signaling through phosphoinositide 3-kinase (PI3K), regulating translation reactions via mTOR [33]. Akt can also be activated through TRAF6-mediated Akt lysine-63 chain ubiquitination or miR-216a/217 microRNA cluster-mediated suppression of Smad7 phosphatase and tensin homolog (PTEN) in non-canonical pathways [37, 38].
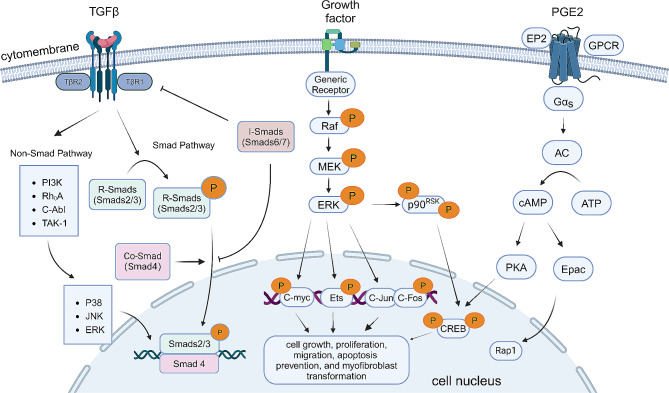
TGF-β signaling and another two signaling pathways involved in myofibroblast dedifferentiation are demonstrated in Fig. 2.
Fig. 2.
Signaling pathways involved in myofibroblast dedifferentiation, including TGF-β signaling, MEK/ERK pathway and cAMP/PKA pathway. TβR, TGF-β receptor. MEK, Mitogen-activated protein kinase kinase. ERK, Extracellular-signal-regulated kinases. GPCR, G-protein-coupled receptors. AC, adenylyl cyclase. PKA, protein kinase A. Epac, exchange protein activated by cAMP. CREB, cAMP-response element binding protein
TGF-β signaling in myofibroblast dedifferentiation
The TGF-β signaling pathway plays a pivotal role in regulating the differentiation of fibroblasts to myofibroblasts and is equally crucial in the regulation of myofibroblast dedifferentiation. Numerous studies on treatment for idiopathic pulmonary fibrosis (IPF) focus on inhibiting the TGF-β pathway, including antagonizing TGF-β, inhibiting Smad2/3 phosphorylation, and activating Smad7 [39]. Research on myofibroblast dedifferentiation also predominantly centers on the regulation of the TGF-β pathway. Nuclear factor erythroid 2-related factor 2 (Nrf2) activators such as arsenic trioxide (ATO) and sulforaphane (SFN) inhibit Smad2/3 phosphorylation, reduce TGF-β-induced reactive oxygen species accumulation (ROS), and restore antioxidant defenses [16, 18]. Regulation of myofibroblast dedifferentiation through Ca2+ modulation is linked to the TGF-β pathway. Inhibitors of store-operated calcium entry (SOCE) can suppress fibrotic marker expression by inhibiting the TGF-β1/Smad3 pathway [40]. Disrupting extracellular Ca2+ or using a selective KCa3.1 blocker hinders Smad2/3 nuclear translocation, inducing myofibroblast dedifferentiation [41]. Cyclosporin A and HIF-1α inhibitors (HIFi) inhibit TGF-β1-induced fibroblast-to-myofibroblast transition, reducing expression levels of α-SMA and fibronectin, and dedifferentiating myofibroblast-like cells (MyoLCs) from pulmonary fibrosis patients [42]. Bromodomain and extra terminal domain (BET) protein inhibitors such as JQ1 can reverse TGF-β-mediated NOX4/SOD2 imbalance and Nrf2 inactivation, attenuating ROS production and reversing myofibroblast differentiation, thus exhibiting therapeutic effects against fibrosis [43, 44]. Bortezomib (BTZ) can inhibit TGF-β1 and key kinases activated by TGF-β1 and FGF, significantly reducing the expression of α-SMA and Collagen in TGF-β-induced myofibroblasts, thus inducing dedifferentiation [45]. Secretome components from mesenchymal stem cells (MSCs) selectively inhibit TGF-β-induced myofibroblast differentiation through the TGF-β Smad2/3 signaling pathway, facilitating reversible dedifferentiation into a fibroblast-like cell subset [46]. Peroxisome proliferator-activated receptor (PPAR) agonists can inhibit the activation of transcription factors STAT3 and EGR1 in TGF-β transgenic mouse kidneys, block TGF signaling and its effects, convert TGF into inactive monomers, and inhibit Smad2/3 phosphorylation to achieve myofibroblast dedifferentiation [47–49].
Nuclear factor erythroid 2-related factor 2 (Nrf2)
Nrf2, encoded by nuclear factor erythroid 2-like 2 (NFE2L2), is a crucial transcription factor in oxidative stress responses. It binds to antioxidant response elements (AREs) in the promoter regions of many cell-protective genes, safeguarding various tissues and organs from oxidative damage and inflammatory stress [50, 51]. Nrf2 regulates the expression of hundreds of genes, including those for antioxidant enzymes, immune and inflammatory response limiters, and genes that restrain tissue remodeling and fibrosis [52]. NFE2L2 can inhibit fibroblast-to-myofibroblast differentiation (FMD) in IPF [53] and promote myofibroblast dedifferentiation, contributing to the reduction of fibrosis. Silencing Nrf2 with siRNA induces oxidative stress and FMD. Activating Nrf2 with keap1 siRNA downregulates the expression of α-SMA and collagen at the RNA level, enhancing antioxidant defense and myofibroblast dedifferentiation in IPF fibroblasts [18]. Arsenic trioxide (ATO), an Nrf2 activator, increases the expression of the antioxidant gene HO-1 in fibroblasts, reducing TGF-β1-induced ROS accumulation. ATO treatment strongly induces the transcription factor NFE2L2, promoting its nuclear translocation and inhibiting Smad2/3 phosphorylation [16]. Sulforaphane (SFN), another Nrf2 activator, promotes myofibroblast dedifferentiation in pulmonary fibrosis by inducing Nrf2 expression. Additionally, SFN inhibits the detrimental pro-fibrotic effects of TGF-β in IPF, controlling fibroblasts and restoring antioxidant defenses [18]. Therefore, both ATO and SFN can concurrently inhibit fibroblast differentiation and promote myofibroblast dedifferentiation, contributing to the therapeutic effects in treating pulmonary fibrosis.
Ca2+
Ca2+ plays a crucial role in pulmonary fibrosis induced by systemic sclerosis (SSc), with most SSc pathological pathways closely associated with Ca2+ signaling [40]. The increased levels of TGF-β1 in SSc are positively correlated with intracellular Ca2+ activity. Store-operated Ca2+ entry (SOCE) represents a vital Ca2+ signal linked to inflammation and carcinogenesis [54]. SOCE channels play a crucial role in promoting fibroblast proliferation, differentiation, and ECM protein production [55]. Inhibitors of SOCE disrupt intracellular Ca2+ activity, leading SSc myofibroblasts to dedifferentiate into normal fibroblasts, resulting in reduced expression of α-SMA and fibronectin [56]. 2-APB, SOCE associated inhibitor, inhibits fibrosis markers such as α-SMA, fibronectin and vimentin, by suppressing TGF-β1/SMAD3 signaling. 2-APB disrupts intracellular Ca2+ regulation, inducing dedifferentiation of TGF-β-induced myofibroblasts. Subcutaneous injection of 2-APB ameliorates bleomycin-induced skin and lung fibrosis [40]. Other SOCE inhibitors, such as SKF96365 and indomethacin, partially achieve the above effects [56].
KCa3.1 is a Ca2+-activated K+ channel that regulates calcium signaling and maintains membrane potential during cell activation [57]. KCa3.1 modulates cell activity by regulating the proliferation, activation, migration, and mediator release of various cells, including fibroblasts. Elevated KCa3.1 expression promotes lung fibroblast proliferation and function. KCa3.1 activity has been shown to enhance Smad phosphorylation, promoting the upregulation of α-SMA in response to TGF-β1 [58–60]. In large animal models of IPF, blocking KCa3.1 channels inhibits the pro-fibrotic effects of primary sheep lung fibroblasts and mitigates bleomycin-induced pulmonary fibrosis in the early stages [61]. In human lung fibroblasts, KCa3.1 activity promotes the functionality of IPF-derived lung fibroblasts. Blocking KCa3.1 reduces TGF-β1-dependent collagen secretion in fibroblasts [59]. Disrupting extracellular Ca2+ or using selective KCa3.1 inhibitors (TRAM-34, ICA-17,043) to block KCa3.1 ion channels inhibits Smad2/3 nuclear translocation, significantly reduces SMA protein expression, and impedes stress fiber formation. This induces dedifferentiation of IPF-derived human lung myofibroblasts (HLMFs) toward a quiescent fibroblast phenotype [41]. Therefore, selective KCa3.1 inhibitors may offer a novel therapeutic approach for IPF, with related drugs awaiting further development.
Hypoxia-inducible factor (HIF)-1α
Hypoxia-inducible factor (HIF)-1α serves as a critical mediator in cellular metabolism, inflammation, and tumorigenesis under hypoxic conditions. In patients with IPF, HIF-1α is highly expressed in the lungs and can induce endoplasmic reticulum (ER) stress in alveolar epithelial cells (AECs) [62]. Additionally, HIF-1α promotes EMT in AECs [63] and participates in TGF-β1-induced FMD [42].
Cyclosporine A (CsA) is a potent immunosuppressive cyclic nonapeptide [64]. Previous studies have demonstrated its efficacy in treating interstitial pneumonia, Sjogren’s syndrome, acute exacerbation of IPF, and other diseases [65–67]. These findings prompted exploration into the inhibitory effects of CsA on pulmonary fibrosis. CsA can inhibit TGF-β1-induced fibroblast-to-myofibroblast transformation by enhancing the degradation of HIF-1α protein. Moreover, CsA and HIF-1α inhibitors (HIFi) can reduce the expression levels of α-SMA and fibronectin, inducing dedifferentiation in myofibroblast-like cells (MyoLCs) derived from pulmonary fibrosis patients [42]. However, the strong toxicity of CsA [64] requires further exploration and evaluation for its clinical use in pulmonary fibrosis treatment.
BET protein
Bromodomain and extra terminal domain (BET) proteins (Brd2, Brd3, Brd4, and BrdT) serve as epigenetic readers by binding to acetylated lysine residues on both histone and non-histone proteins through their conserved bromodomain. They regulate gene expression by recruiting transcriptional activators or repressors [68]. BET proteins have been identified as promising therapeutic targets in cancer and pulmonary fibrosis. In pulmonary fibrosis models, following stimulation by TGF-β1, Brd4 occupies the promoters of interleukin-6 (IL-6), α-smooth muscle actin (α-SMA), and plasminogen activator inhibitor-1 (PAI-1), thereby promoting fibrosis [69]. Additionally, during the pathological progression of pulmonary fibrosis, TGF-β increases NADPH oxidase-4 (NOX4), inhibits superoxide dismutase (SOD2) expression, enhances ROS production, and promotes myofibroblast differentiation. BET protein inhibitors can reverse the TGF-β-mediated imbalance of NOX4/SOD2 and Nrf2 inactivation, attenuate ROS production, and reverse myofibroblast differentiation, thus offering a potential treatment for fibrosis [43, 44].
JQ1, a small molecule inhibitor, disrupts the binding of BET proteins’ bromodomains to acetylated lysine [68]. JQ1 affects the expression of NOX4/SOD2 and Nrf2 activity to restore redox balance, leading to myofibroblast reprogramming or dedifferentiation and exerting anti-fibrotic effects [43]. Research by Kenichi Suzuki and colleagues has provided evidence supporting this notion. They demonstrated that JQ1 significantly reduces the expression levels of α-SMA and ED-A-fibronectin in primary myofibroblasts from severely fibrotic lungs and conducted the first comprehensive analysis of the transcriptome spectrum associated with the dedifferentiation of IPF myofibroblasts. Unfortunately, the exact mechanism of BET inhibition-induced myofibroblast dedifferentiation and the associated gene expression changes remain unclear, and the transcriptional profile related to IPF myofibroblast dedifferentiation remains unknown [70].
MSC extracellular vesicles
Mesenchymal stromal/stem cells (MSCs) are adult stem cells with self-renewal capacity and multipotent differentiation potential. They exhibit robust immunoregulatory, anti-inflammatory, and anti-fibrotic properties. Research has shown that extracellular vesicles (EVs), including exosomes, derived from human bone marrow MSCs can prevent and ameliorate bleomycin-induced pulmonary fibrosis. These EVs achieve systemic regulation of monocyte phenotype, leading to improvements in lung morphology, reduced collagen deposition, and restoration of lung structure [71]. In another study, a non-contact transwell co-culture of MSCs with TGF-β-induced myofibroblasts revealed selective inhibition of the TGF-β-SMAD2/3 signaling pathway in myofibroblasts. This inhibition resulted in their reversible dedifferentiation into a fibroblast-like cell population. These FB-like cells remained sensitive to TGF-β, capable of re-induction into myofibroblasts [46]. MSC-secreted components, particularly MSC-derived extracellular vesicles (MSC-EVs), reduced the number of myofibroblasts and fibroblast activation protein alpha progenitors (FAPa + progenitors), without affecting their apoptosis. This reduction was possibly mediated by intercellular transfer of miR-29c and miR-129 [14].
PPAR
PPAR, a type II nuclear receptor, plays an integral role in fatty acid storage and glucose metabolism in adipose tissue. It enhances lipid uptake and synthesis metabolism by increasing insulin sensitivity and promoting adiponectin release. The insulin-sensitizing effects of PPAR have been widely utilized in glycemic control therapies [72]. Additionally, PPAR exhibits anti-fibrotic properties. In the absence of PPAR-γ, collagen deposition, SMAD2/3 phosphorylation, and α-SMA levels increase in human and murine lung fibroblasts [48]. Research on PPAR-γ agonists, particularly pioglitazone, has revealed pathways that can inhibit or even reverse pulmonary arterial hypertension (PAH) and chronic fibroproliferative kidney diseases [72]. Long-term pioglitazone treatment can inhibit the activation of transcription factors STAT3 and EGR1 in TGFβ transgenic mouse kidneys, preventing TGFβ-induced renal fibrosis [49]. The widely used anti-diabetic drug metformin reduces α-SMA and collagen expression, alleviating bleomycin-induced pulmonary fibrosis, partly through activating PPAR signaling [73–75].
Nitrated fatty acids (NFAs) serve as unique endogenous activators of PPAR [76]. NFAs may promote myofibroblast dedifferentiation through various mechanisms, including upregulating PPAR, blocking TGF signaling and actions, converting TGF into inactive monomers, upregulating the collagen-targeting factor milk fat globule-EGF factor 8 (MFG-E8), and stimulating alveolar macrophages to uptake and degrade collagen. Experimental evidence further demonstrates that NFAs can reverse existing myofibroblast differentiation and collagen deposition in established mouse models of pulmonary fibrosis [48].
Eupatilin, a lipophilic flavonoid derived from Artemisia asiatica, acts as a PPAR-α agonist with anti-apoptotic, antioxidant, and anti-inflammatory effects [77, 78]. It directly targets pathogenic myofibroblasts stimulated by TGF-β, inducing rapid disassembly of myosin. This action leads to the disintegration of latent TGF-β complexes, thereby suppressing the induction of multiple EMT genes. Additionally, Eupatilin promotes myofibroblast dedifferentiation into intermediate cell types by blocking Smad3 phosphorylation, thus, potentially reversing fibrosis [47].
MEK/ERK pathway
The mitogen-activated protein kinase (MAPK)/MAPK kinase (MEK)/extracellular signal-regulated kinase (ERK) signaling cascade, is closely associated with tumorigenesis, and plays a pivotal role in promoting cellular processes related to fibrosis. These processes include cell growth, proliferation, migration, prevention of apoptosis, and myofibroblast transformation [79, 80]. Inhibiting MEK has shown in suppressing lung cell proliferation induced by TGF-α, reducing the expression of matrix genes in vivo, thus preventing TGF-α-induced pulmonary fibrosis, and halting the progression of established pulmonary fibrosis [81]. Growth factors and mitogens transmit signals through this pathway, regulating gene expression in processes vital for cellular function [82]. This signaling cascade is not only implicated in tumorigenesis but also contributes to various cellular events associated with fibrosis, making it a potential therapeutic target for preventing and treating fibrotic disorders.
FGF
Fibroblast Growth Factor (FGF) is a critical growth factor that transmits signals to the cell nucleus by binding to the FGF receptor (FGFR) and activating various signaling pathways. These pathways include Ras, MAPKs, ERKs, Src, p38 MAPKs, phospholipase-Cγ (PLCγ), Crk, jun N-terminal kinases (JNK), and protein kinase C (PKC) [83]. These pathways subsequently activate transcription factors such as ETV4 and ETV5 [84]. TGF-β1 induces the expression and release of FGF-2 in type II alveolar epithelial cells, and FGF-2 specifically counters TGF-β1-stimulated fibroblast proliferation through antibody neutralization [82]. FGFR1 signaling is also essential for fibroblast migration in IPF [85]. Elevated levels of FGF2 have been detected in bronchoalveolar lavage (BAL) from IPF patients, in lung mast cells of IPF, and in lung macrophages and mast cells of bleomycin-treated mice [86–88].Interestingly, while endogenous FGF2 is essential for the production of fibrosis, it does not participate in TGF-β1-induced fibrotic differentiation [82]. Conversely, induced FGF overexpression and exogenous FGF can counteract some pro-fibrotic effects of TGF-ß by downregulating the expression of type I collagen, α-SMA, stress fibers, and Hsp47 chaperone protein, and by increasing the expression of matrix metalloproteinases [89–91]. FGFs play a role in inhibiting TGF-β1 in various organs and cell types, involving multiple mechanisms such as ERK [92–94], focal adhesion kinase [95], Nkx2.5/Csx [96], let-7 miRNA [97], downregulation of TGFβ1 and TGFβR1 [98], and decreased Smad2 phosphorylation [99]. The intricate interplay between FGFs and TGF-β1 underscores the complexity of fibrotic processes and unveils potential avenues for therapeutic interventions.
cAMP/PKA pathway
The cyclic AMP (cAMP)/protein kinase A (PKA) cascade is a pivotal signaling pathway. Various hormones such as prostaglandin E2 (PGE2), β2-adrenergic receptor agonists, and glucagon-like peptide-1 (GLP-1) activate adenylyl cyclase (AC) by binding to G protein-coupled receptors (GPCRs), catalyzing the conversion of ATP to cAMP [100]. cAMP then activates PKA or exchange protein activated by cAMP (Epac). PKA activation leads to the phosphorylation of its key substrates, cAMP-response element binding protein (CREB) [101, 102]. Prostaglandins, particularly prostaglandin E2 (PGE2), play a significant role in regulating fibroblast differentiation and myofibroblast dedifferentiation primarily through the activation of this pathway [89, 103, 104]. The intricate signaling events orchestrated by the cAMP/PKA cascade underscore its importance in cellular processes, providing a molecular basis for understanding the regulatory mechanisms involved in fibroblast dynamics.
PGE
Prostaglandin E2 (PGE2) is a ubiquitous bioactive lipid mediator synthesized from arachidonic acid in the human body. It primarily activates prostaglandin receptor 2 (EP2) receptors, directly inhibiting fibroblast proliferation [105], α-SMA and collagen expression [103, 106], and myofibroblast differentiation [103, 104]. Stimulation of EP2 receptors increases intracellular cAMP production, activating protein kinase A (PKA) or exchange protein activated by cAMP (Epac) [107, 108]. In human lung fibroblasts, Epac activation and subsequent Rap1 activation account for PGE2’s anti-proliferative effects, while PKA activation and subsequent PKC-δ inhibition contribute to PGE2’s suppression of collagen expression and myofibroblast differentiation [107, 109].
Recent studies increasingly highlight PGE2’s critical role in myofibroblast dedifferentiation. Treatment of myofibroblasts with PGE2 induces dedifferentiation through the EP2/cAMP/PKA pathway, suppressing their proliferative capacity and restoring apoptosis sensitivity [89]. A bioinformatics study revealed that PGE2 can reverse the expression of 363 (62%) genes upregulated by TGF-β1 and 345 (50%) genes downregulated by TGF-β1. Genes upregulated by TGF-β1 and reversed by PGE2 are enriched in cell adhesion, contractile fibers, and myosin binding annotations, while those downregulated by TGF-β1 and reversed by PGE2 are enriched in glycoprotein, polysaccharide binding, and cell migration regulation annotations. This demonstrates significant alterations in the transcriptional program of differentiated myofibroblasts induced by PGE2 [110]. Importantly, PGE2-induced myofibroblast dedifferentiation is reversible, as dedifferentiated fibroblasts can redifferentiate into myofibroblasts upon TGF-β induction. This effect of PGE2 is associated with the inhibition of focal adhesion kinase (FAK) signaling [17].
Simultaneously, the synthetic prostaglandin E1 (PGE1) alprostadil has been shown to attenuate or reverse pulmonary fibrosis by activating prostaglandin receptors 2 and 4 (EP2 and EP4) [111].
Other therapeutic targets and drugs
BTZ
Bortezomib (BTZ) is a reversible inhibitor of the chymotrypsin-like activity of the 20 S core proteasome. Previous studies have indicated its ability to prevent fibrosis in the lungs, liver, and kidneys by inhibiting TGF-β1 [112–114]. Recent research has unveiled that BTZ can hinder key kinases activated by TGF-β and FGF-2, thereby suppressing fibroblast proliferation and differentiation induced by TGF-β and FGF2. BTZ also facilitates the dedifferentiation of elicited MyoFibs and IPF Fibs, rendering them more susceptible to FAS-mediated Apoptosis. At both the mRNA and protein levels, a significant decrease is observed in the expression of α-SMA and Col1a2 in MyoFibs and IPF Fibs formed after 48 h of TGF-β pretreatment. Remarkably, these anti-fibrotic effects of BTZ, both in vitro and in vivo, are unrelated to proteasome inhibition but are associated with the induction and activation of dual-specificity protein phosphatase 1 (DUSP1) [45]. It is worth noting that there is limited research on the use of BTZ for pulmonary fibrosis, and further exploration is needed to assess its safety and efficacy in this context.
MyoD
MyoD, a myogenic regulatory factor, plays a crucial role in embryonic and adult skeletal muscle growth and differentiation [115]. Its expression is associated with the presence of myofibroblasts in tissue repair and fibrosis [116]. MyoD mediates TGF-β1-induced myofibroblast differentiation, and its endogenous downregulation regulates myofibroblast dedifferentiation and proliferation. Mitogen-induced downregulation of MyoD is mediated by the ERK1/2 MAPK signaling pathway [117]. In essence, MyoD serves as a critical switch in the differentiation and dedifferentiation of myofibroblasts, and silencing MyoD can be a therapeutic approach for treating pulmonary fibrosis.
Non-senescent lung myofibroblasts retain the ability to dedifferentiate, while aged and IPF myofibroblasts exhibit impaired dedifferentiation. In non-senescent cells, decreased levels of MyoD indicate susceptibility to myofibroblast dedifferentiation and apoptosis. In contrast, aged and IPF myofibroblasts show sustained upregulation of MyoD expression, leading to their inability to dedifferentiate and resistance to apoptosis. Genetic strategies to silence MyoD can restore susceptibility to apoptosis in IPF myofibroblasts, reinstate their ability to dedifferentiate, and partially reverse age-related persistent fibrosis in vivo [118].
SET8
SET8, also known as PR-set7, SETD8, or KMT5A, is the sole lysine methyltransferase capable of specifically catalyzing monomethylation of lysine 20 on histone H4 (H4K20). Positioned on the nuclear chromatin, SET8 interacts not only with histone-modifying proteins but also with various non-histone factors such as p53, Twist, and Wnt. It plays a role in the regulation of crucial physiological processes like the cell cycle, DNA repair, gene transcription, and apoptosis [119]. In the context of BLM-induced lung injury, SET8 predominantly localizes to the nuclei of α-SMA-positive cells co-localized with H4K20me1. Inhibition of SET8 significantly suppresses the expression of α-SMA and ED-A-fibronectin in myofibroblasts [120].
UNC0379, identified through differentiation assays using lung myofibroblasts prepared from end-stage IPF patients, is an epigenetic modulator. By inhibiting SET8, UNC0379 markedly reduces the expression of α-SMA and ED-A-fibronectin in myofibroblasts, inducing their dedifferentiation. This effect contributes to partial alleviation of lung fibrosis without affecting the inflammatory response [120].
Regulation of mechanical forces
The traditional perspective posits that after tissue remodeling and wound healing, myofibroblasts typically undergo apoptosis and clearance [121]. However, recent studies have gradually recognized their potential to terminate their function through phenotypic reversal, namely dedifferentiation, and this is because fibroblasts and myofibroblasts can modify their activity based on the information they receive from the mechanical environment [122]. ECM is both the result and the cause of myofibroblast differentiation. The ECM accumulated by myofibroblasts constitutes a stiff pro-fibroblast environment, promotes the continuous activation and differentiation of fibroblasts, and forms a positive feedback [123, 124]. Enhancement of mechanical stress or matrix stiffness has been shown to induce fibroblast differentiation into myofibroblasts [125, 126], and releasing mechanical stress or reducing stiffness can induce apoptosis, decrease myofibroblast contractility and α-SMA expression [127]. A research altered the ability of the cells to generate tension by altering boundary stiffness and successfully tested the hypothesis that mechanical inhibition of myofibroblast force generation leads to dedifferentiation or apoptosis depending upon the magnitude of the decrease in tension [128]. Omentin-1 can target mechanical signal accelerates fibrosis resolution and reverse established pulmonary fibrosis by promoting mechanically activated myofibroblasts dedifferentiation into lipofibroblasts, which is closely associated with ECM clearance in fibrotic tissue [129]. Paradoxically, another study suggests that pressure therapy - that is, applying appropriate mechanical pressure around the wound – can reverse the differentiation of fibroblasts into a myofibroblast phenotype and maintained fibroblasts in a quiescent state, thereby inhibiting excessive collagen deposition and scar formation [130]. Therefore, the impact of external mechanical stress and matrix stiffness on myofibroblast dedifferentiation need to be further explored, and how to change the mechanical stress and matrix hardness to achieve the cure of pulmonary fibrosis is worthy of further exploration.
Conclusion
As the incidence and mortality rates of pulmonary fibrosis continue to rise annually, research into its pathogenesis and treatment has become increasingly urgent. Myofibroblast dedifferentiation, has emerged as a significant and promising area of study in recent years, offering potential therapeutic benefits in treating pulmonary fibrosis. Many drugs and regulatory approaches discussed in this article not only halt further progression of lung fibrosis but may even reverse established fibrosis, holding crucial implications for improving patient quality of life.
However, many specific molecular mechanisms underlying myofibroblast dedifferentiation remain unclear, particularly which mechanisms are beneficial in treating pulmonary fibrosis remain unclear. Furthermore, most current studies are still at the molecular, cellular, and animal research stages, and the bleomycin-induced lung fibrosis model used in these studies also significantly differs from human pulmonary fibrotic diseases, suggesting a lengthy road ahead for large-scale clinical trials and widespread clinical application of related drugs.
Another excellent review summarizes some effective compounds that promote myofibroblast dedifferentiation, such as amniotic membrane stromal extract, capsaicin, SNAC, GSPs and Cu/Zn SOD [131]. These compounds have shown positive effects in promoting myofibroblast dedifferentiation in other tissues and organs, and their effects in pulmonary fibrosis are awaiting further investigation.
It is anticipated that in the near future, more molecular mechanisms of myofibroblast dedifferentiation will be elucidated, and corresponding drugs will be clinically applied, aiding patients and physicians in overcoming this challenging disease.
Acknowledgements
Figures are created with BioRender.com. We would like to thank Editage (www.editage.cn) for English language editing.
Abbreviations
- IPF
Idiopathic Pulmonary Fibrosis
- ECM
excessive extracellular matrix
- TGF-β
Transforming growth factor-β
- PDGF
Platelet-derived growth factor
- EMT
Epithelial-mesenchymal transition
- EDMT
Endothelial-mesenchymal transition
- BMPs
Bone morphogenetic proteins
- GDFs
Growth and differentiation factors
- TβRII
TGF-β receptor II
- PAI-1
Plasminogen activator inhibitor-1
- CTGF
Connective tissue growth factor
- AP-1
Activating protein-1
- Sp1
Specificity protein 1
- ERK
extracellular-signal-regulated kinases
- MEK
mitogen-activated protein kinase kinase
- AC
Adenylyl cyclase
- PKA
Protein kinase A
- PI3K
The phosphoinositide 3-kinase
- Fra-2
Fos-related antigen 2
- NFE2L2
Nuclear factor erythroid 2-like 2
- Nrf2
Nuclear factor erythroid 2-related factor 2
- ATO
Arsenic trioxide
- SFN
Sulforaphane
- SOCE
store-operated calcium entry
- HIFi
HIF-1α inhibitors
- HIF-1α
hypoxia-inducible factor-1α
- BTZ
Bortezomib
- PPAR
Peroxisome proliferator-activated receptor
- AREs
antioxidant response elements
- ER
Endoplasmic reticulum
- AECs
alveolar epithelial cells
- FMD
Fibroblast-to-myofibroblast differentiation
- CsA
Cyclosporine A
- IL-6
interleukin-6
- α-SMA
α-smooth muscle actin
- PAI-1
plasminogen activator inhibitor-1
- NOX4
NADPH oxidase-4
- ROS
reactive oxygen species
- MSC
Mesenchymal stromal/stem cells
- EVs
Extracellular vesicles
- NFAs
Nitrated fatty acids
- FGF
Fibroblast Growth Factor
- PGE2
prostaglandin E2
- GLP-1
glucagon-like peptide-1
- GPCRs
G protein-coupled receptors
- Epac
exchange protein activated by cAMP
- CREB
cAMP-response element binding protein
- FAK
focal adhesion kinase signaling
- DUSP1
dual-specificity protein phosphatase 1
Author contributions
Conceptualization, Y.W., J.Y.; methodology, C.Z., X.J.; writing—original draft preparation, X.J., C.Z., K.W. and C.W.; writing—review and editing, X.J., K.W., C.Z.; supervision, C.Z., Y.W., C.W. and J.Y.; project administration, Y.W. and J.Y.; funding acquisition, Y.W., J.Y.; All authors read and approved the final manuscript.
Funding
The present study was supported by the National Natural Science Foundation of China (82170078). The Tongji Hospital (HUST) Foundation for Excellent Young Scientists (2020YQ03). Tongji Hospital Foundation for Key project (2022A15), and Wuhan Science and Technology Bureau “Dawn Light Plan Talent Project” (2022020801020450).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Institutional review board statement
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yi Wang, Email: wangyi@tjh.tjmu.edu.cn.
Jun Yu, Email: junyu2018@hotmail.com.
References
- 1.Maher TM, Bendstrup E, Dron L, Langley J, Smith G, Khalid JM, Patel H, Kreuter M. Global incidence and prevalence of idiopathic pulmonary fibrosis. Respir Res. 2021;22:197. 10.1186/s12931-021-01791-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taniguchi H, Ebina M, Kondoh Y, Ogura T, Azuma A, Suga M, Taguchi Y, Takahashi H, Nakata K, Sato A, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35:821–9. 10.1183/09031936.00005209 [DOI] [PubMed] [Google Scholar]
- 3.Flaherty KR, Wells AU, Cottin V, Devaraj A, Walsh SLF, Inoue Y, Richeldi L, Kolb M, Tetzlaff K, Stowasser S, et al. Nintedanib in Progressive Fibrosing interstitial lung diseases. N Engl J Med. 2019;381:1718–27. 10.1056/NEJMoa1908681 [DOI] [PubMed] [Google Scholar]
- 4.The therapy. of idiopathic pulmonary fibrosis: what is next? Vivien Somogyi, Nazia Chaudhuri, Sebastiano Emanuele Torrisi, Eur Respir Rev 2019; 28: 190021. Eur Respir Rev 2019, 28. [DOI] [PMC free article] [PubMed]
- 5.Richeldi L, Collard HR, Jones MG. Idiopathic pulmonary fibrosis. Lancet. 2017;389:1941–52. 10.1016/S0140-6736(17)30866-8 [DOI] [PubMed] [Google Scholar]
- 6.Gabbiani G, Ryan GB, Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971;27:549–50. 10.1007/BF02147594 [DOI] [PubMed] [Google Scholar]
- 7.Jun JI, Lau LF. Resolution of organ fibrosis. J Clin Invest. 2018;128:97–107. 10.1172/JCI93563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Zhang L, Huang T, Wu GR, Zhou Q, Wang FX, Chen LM, Sun F, Lv Y, Xiong F et al. The methyl-CpG-binding domain 2 facilitates pulmonary fibrosis by orchestrating fibroblast to myofibroblast differentiation. Eur Respir J 2022, 60. [DOI] [PMC free article] [PubMed]
- 9.Tarbit E, Singh I, Peart JN, Rose’Meyer RB. Biomarkers for the identification of cardiac fibroblast and myofibroblast cells. Heart Fail Rev. 2019;24:1–15. 10.1007/s10741-018-9720-1 [DOI] [PubMed] [Google Scholar]
- 10.Boutanquoi PM, Burgy O, Beltramo G, Bellaye PS, Dondaine L, Marcion G, Pommerolle L, Vadel A, Spanjaard M, Demidov O et al. TRIM33 prevents pulmonary fibrosis by impairing TGF-beta1 signalling. Eur Respir J 2020, 55. [DOI] [PubMed]
- 11.Moss BJ, Ryter SW, Rosas IO. Pathogenic mechanisms underlying idiopathic pulmonary fibrosis. Annu Rev Pathol. 2022;17:515–46. 10.1146/annurev-pathol-042320-030240 [DOI] [PubMed] [Google Scholar]
- 12.McAnulty RJ. Fibroblasts and myofibroblasts: their source, function and role in disease. Int J Biochem Cell Biol. 2007;39:666–71. 10.1016/j.biocel.2006.11.005 [DOI] [PubMed] [Google Scholar]
- 13.El Agha E, Moiseenko A, Kheirollahi V, De Langhe S, Crnkovic S, Kwapiszewska G, Szibor M, Kosanovic D, Schwind F, Schermuly RT, et al. Two-Way Conversion between Lipogenic and Myogenic Fibroblastic Phenotypes Marks the Progression and Resolution of Lung Fibrosis. Cell Stem Cell. 2017;20:261–e273263. 10.1016/j.stem.2016.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nataliya B, Mikhail A, Vladimir P, Olga G, Maksim V, Ivan Z, Ekaterina N, Georgy S, Natalia D, Pavel M, et al. Mesenchymal stromal cells facilitate resolution of pulmonary fibrosis by miR-29c and miR-129 intercellular transfer. Exp Mol Med. 2023;55:1399–412. 10.1038/s12276-023-01017-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kosla J, Dvorakova M, Dvorak M, Cermak V. Effective myofibroblast dedifferentiation by concomitant inhibition of TGF-beta signaling and perturbation of MAPK signaling. Eur J Cell Biol. 2013;92:363–73. 10.1016/j.ejcb.2013.10.013 [DOI] [PubMed] [Google Scholar]
- 16.Zhong L, Hao H, Chen D, Hou Q, Zhu Z, He W, Sun S, Sun M, Li M, Fu X. Arsenic trioxide inhibits the differentiation of fibroblasts to myofibroblasts through nuclear factor erythroid 2-like 2 (NFE2L2) protein and the Smad2/3 pathway. J Cell Physiol. 2019;234:2606–17. 10.1002/jcp.27073 [DOI] [PubMed] [Google Scholar]
- 17.Garrison G, Huang SK, Okunishi K, Scott JP, Kumar Penke LR, Scruggs AM, Peters-Golden M. Reversal of myofibroblast differentiation by prostaglandin E(2). Am J Respir Cell Mol Biol. 2013;48:550–8. 10.1165/rcmb.2012-0262OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Artaud-Macari E, Goven D, Brayer S, Hamimi A, Besnard V, Marchal-Somme J, Ali ZE, Crestani B, Kerdine-Romer S, Boutten A, Bonay M. Nuclear factor erythroid 2-related factor 2 nuclear translocation induces myofibroblastic dedifferentiation in idiopathic pulmonary fibrosis. Antioxid Redox Signal. 2013;18:66–79. 10.1089/ars.2011.4240 [DOI] [PubMed] [Google Scholar]
- 19.David CJ, Massague J. Contextual determinants of TGFbeta action in development, immunity and cancer. Nat Rev Mol Cell Biol. 2018;19:419–35. 10.1038/s41580-018-0007-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saito A, Horie M, Nagase T. TGF-beta signaling in Lung Health and Disease. Int J Mol Sci 2018, 19. [DOI] [PMC free article] [PubMed]
- 21.Xu P, Liu J, Derynck R. Post-translational regulation of TGF-beta receptor and smad signaling. FEBS Lett. 2012;586:1871–84. 10.1016/j.febslet.2012.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu HH, Chen DQ, Wang YN, Feng YL, Cao G, Vaziri ND, Zhao YY. New insights into TGF-beta/Smad signaling in tissue fibrosis. Chem Biol Interact. 2018;292:76–83. 10.1016/j.cbi.2018.07.008 [DOI] [PubMed] [Google Scholar]
- 23.Derynck R, Zhang YE. Smad-dependent and smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–84. 10.1038/nature02006 [DOI] [PubMed] [Google Scholar]
- 24.Massague J. TGFbeta signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–30. 10.1038/nrm3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–93. 10.1146/annurev.cellbio.21.022404.142018 [DOI] [PubMed] [Google Scholar]
- 26.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–96. 10.1038/nrm3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez IE, Eickelberg O. The impact of TGF-beta on lung fibrosis: from targeting to biomarkers. Proc Am Thorac Soc. 2012;9:111–6. 10.1513/pats.201203-023AW [DOI] [PubMed] [Google Scholar]
- 28.Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-beta signaling in fibrosis. Growth Factors. 2011;29:196–202. 10.3109/08977194.2011.595714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung JY, Chan MK, Li JS, Chan AS, Tang PC, Leung KT, To KF, Lan HY, Tang PM. TGF-beta signaling: from tissue fibrosis to Tumor Microenvironment. Int J Mol Sci 2021, 22. [DOI] [PMC free article] [PubMed]
- 30.Huang M, Sharma S, Zhu LX, Keane MP, Luo J, Zhang L, Burdick MD, Lin YQ, Dohadwala M, Gardner B, et al. IL-7 inhibits fibroblast TGF-beta production and signaling in pulmonary fibrosis. J Clin Invest. 2002;109:931–7. 10.1172/JCI0214685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang CJ, Lin CF, Lee CH, Chuang HC, Shih FC, Wan SW, Tai C, Chen CL. Overcoming interferon (IFN)-gamma resistance ameliorates transforming growth factor (TGF)-beta-mediated lung fibroblast-to-myofibroblast transition and bleomycin-induced pulmonary fibrosis. Biochem Pharmacol. 2021;183:114356. 10.1016/j.bcp.2020.114356 [DOI] [PubMed] [Google Scholar]
- 32.Frangogiannis N. Transforming growth factor-beta in tissue fibrosis. J Exp Med. 2020;217:e20190103. 10.1084/jem.20190103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang YE. Non-smad Signaling pathways of the TGF-beta family. Cold Spring Harb Perspect Biol 2017, 9. [DOI] [PMC free article] [PubMed]
- 34.Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12:325–38. 10.1038/nrneph.2016.48 [DOI] [PubMed] [Google Scholar]
- 35.Finnson KW, Almadani Y, Philip A. Non-canonical (non-SMAD2/3) TGF-beta signaling in fibrosis: mechanisms and targets. Semin Cell Dev Biol. 2020;101:115–22. 10.1016/j.semcdb.2019.11.013 [DOI] [PubMed] [Google Scholar]
- 36.Reich N, Maurer B, Akhmetshina A, Venalis P, Dees C, Zerr P, Palumbo K, Zwerina J, Nevskaya T, Gay S, et al. The transcription factor Fra-2 regulates the production of extracellular matrix in systemic sclerosis. Arthritis Rheum. 2010;62:280–90. 10.1002/art.25056 [DOI] [PubMed] [Google Scholar]
- 37.Xia H, Ooi LL, Hui KM. MicroRNA-216a/217-induced epithelial-mesenchymal transition targets PTEN and SMAD7 to promote drug resistance and recurrence of liver cancer. Hepatology. 2013;58:629–41. 10.1002/hep.26369 [DOI] [PubMed] [Google Scholar]
- 38.Yang WL, Wang J, Chan CH, Lee SW, Campos AD, Lamothe B, Hur L, Grabiner BC, Lin X, Darnay BG, Lin HK. The E3 ligase TRAF6 regulates akt ubiquitination and activation. Science. 2009;325:1134–8. 10.1126/science.1175065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peng D, Fu M, Wang M, Wei Y, Wei X. Targeting TGF-beta signal transduction for fibrosis and cancer therapy. Mol Cancer. 2022;21:104. 10.1186/s12943-022-01569-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsu WL, Hsieh YC, Yu HS, Yoshioka T, Wu CY. 2-Aminoethyl diphenylborinate inhibits bleomycin-induced skin and pulmonary fibrosis via interrupting intracellular ca(2+) regulation. J Dermatol Sci. 2021;103:101–8. 10.1016/j.jdermsci.2021.07.005 [DOI] [PubMed] [Google Scholar]
- 41.Roach KM, Wulff H, Feghali-Bostwick C, Amrani Y, Bradding P. Increased constitutive alphaSMA and Smad2/3 expression in idiopathic pulmonary fibrosis myofibroblasts is KCa3.1-dependent. Respir Res. 2014;15:155. 10.1186/s12931-014-0155-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamazaki R, Kasuya Y, Fujita T, Umezawa H, Yanagihara M, Nakamura H, Yoshino I, Tatsumi K, Murayama T. Antifibrotic effects of cyclosporine A on TGF-beta1-treated lung fibroblasts and lungs from bleomycin-treated mice: role of hypoxia-inducible factor-1alpha. FASEB J. 2017;31:3359–71. 10.1096/fj.201601357R [DOI] [PubMed] [Google Scholar]
- 43.Stock CJW, Michaeloudes C, Leoni P, Durham AL, Mumby S, Wells AU, Chung KF, Adcock IM, Renzoni EA, Lindahl GE. Bromodomain and Extraterminal (BET) Protein Inhibition Restores Redox Balance and Inhibits Myofibroblast Activation. Biomed Res Int 2019, 2019:1484736. [DOI] [PMC free article] [PubMed]
- 44.Tang X, Peng R, Phillips JE, Deguzman J, Ren Y, Apparsundaram S, Luo Q, Bauer CM, Fuentes ME, DeMartino JA, et al. Assessment of Brd4 inhibition in idiopathic pulmonary fibrosis lung fibroblasts and in vivo models of lung fibrosis. Am J Pathol. 2013;183:470–9. 10.1016/j.ajpath.2013.04.020 [DOI] [PubMed] [Google Scholar]
- 45.Penke LRK, Speth J, Wettlaufer S, Draijer C, Peters-Golden M. Bortezomib inhibits lung fibrosis and fibroblast activation without Proteasome Inhibition. Am J Respir Cell Mol Biol. 2022;66:23–37. 10.1165/rcmb.2021-0112OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu R, Wu M, Wang Y, Li C, Zeng L, Wang Y, Xiao M, Chen X, Geng S, Lai P, et al. Mesenchymal stem cells reversibly de-differentiate myofibroblasts to fibroblast-like cells by inhibiting the TGF-beta-SMAD2/3 pathway. Mol Med. 2023;29:59. 10.1186/s10020-023-00630-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim HS, Yoon YM, Meang MK, Park YE, Lee JY, Lee TH, Lee JE, Kim IH, Youn BS. Reversion of in vivo fibrogenesis by novel chromone scaffolds. EBioMedicine. 2019;39:484–96. 10.1016/j.ebiom.2018.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reddy AT, Lakshmi SP, Zhang Y, Reddy RC. Nitrated fatty acids reverse pulmonary fibrosis by dedifferentiating myofibroblasts and promoting collagen uptake by alveolar macrophages. FASEB J. 2014;28:5299–310. 10.1096/fj.14-256263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nemeth A, Mozes MM, Calvier L, Hansmann G, Kokeny G. The PPARgamma agonist pioglitazone prevents TGF-beta induced renal fibrosis by repressing EGR-1 and STAT3. BMC Nephrol. 2019;20:245. 10.1186/s12882-019-1431-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanghvi VR, Leibold J, Mina M, Mohan P, Berishaj M, Li Z, Miele MM, Lailler N, Zhao C, de Stanchina E, et al. The oncogenic action of NRF2 depends on de-glycation by Fructosamine-3-Kinase. Cell. 2019;178:807–e819821. 10.1016/j.cell.2019.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang HC, Nguyen T, Pickett CB. Regulation of the antioxidant response element by protein kinase C-mediated phosphorylation of NF-E2-related factor 2. Proc Natl Acad Sci U S A. 2000;97:12475–80. 10.1073/pnas.220418997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hybertson BM, Gao B, Bose SK, McCord JM. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol Aspects Med. 2011;32:234–46. 10.1016/j.mam.2011.10.006 [DOI] [PubMed] [Google Scholar]
- 53.Hecker L, Logsdon NJ, Kurundkar D, Kurundkar A, Bernard K, Hock T, Meldrum E, Sanders YY, Thannickal VJ. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci Transl Med. 2014;6:231ra247. 10.1126/scitranslmed.3008182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xie J, Pan H, Yao J, Zhou Y, Han W. SOCE and cancer: recent progress and new perspectives. Int J Cancer. 2016;138:2067–77. 10.1002/ijc.29840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yue Z, Zhang Y, Xie J, Jiang J, Yue L. Transient receptor potential (TRP) channels and cardiac fibrosis. Curr Top Med Chem. 2013;13:270–82. 10.2174/1568026611313030005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu CY, Hsu WL, Tsai MH, Chai CY, Yen CJ, Chen CH, Lu JH, Yu HS, Yoshioka T. A potential new approach for treating systemic sclerosis: dedifferentiation of SSc fibroblasts and change in the microenvironment by blocking store-operated Ca2 + entry. PLoS ONE. 2019;14:e0213400. 10.1371/journal.pone.0213400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fanger CM, Rauer H, Neben AL, Miller MJ, Rauer H, Wulff H, Rosa JC, Ganellin CR, Chandy KG, Cahalan MD. Calcium-activated potassium channels sustain calcium signaling in T lymphocytes. Selective blockers and manipulated channel expression levels. J Biol Chem. 2001;276:12249–56. 10.1074/jbc.M011342200 [DOI] [PubMed] [Google Scholar]
- 58.Xie H, Lu J, Zhu Y, Meng X, Wang R. The KCa3.1 blocker TRAM-34 inhibits proliferation of fibroblasts in paraquat-induced pulmonary fibrosis. Toxicol Lett. 2018;295:408–15. 10.1016/j.toxlet.2018.07.020 [DOI] [PubMed] [Google Scholar]
- 59.Roach KM, Duffy SM, Coward W, Feghali-Bostwick C, Wulff H, Bradding P. The K + channel KCa3.1 as a novel target for idiopathic pulmonary fibrosis. PLoS ONE. 2013;8:e85244. 10.1371/journal.pone.0085244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang C, Shen S, Ma Q, Gill A, Pollock CA, Chen XM. KCa3.1 mediates activation of fibroblasts in diabetic renal interstitial fibrosis. Nephrol Dial Transpl. 2014;29:313–24. 10.1093/ndt/gft431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Organ L, Bacci B, Koumoundouros E, Kimpton WG, Samuel CS, Nowell CJ, Bradding P, Roach KM, Westall G, Jaffar J, Snibson KJ. Inhibition of the K(ca)3.1 Channel alleviates established pulmonary fibrosis in a large animal model. Am J Respir Cell Mol Biol. 2017;56:539–50. 10.1165/rcmb.2016-0092OC [DOI] [PubMed] [Google Scholar]
- 62.Delbrel E, Soumare A, Naguez A, Label R, Bernard O, Bruhat A, Fafournoux P, Tremblais G, Marchant D, Gille T, et al. HIF-1alpha triggers ER stress and CHOP-mediated apoptosis in alveolar epithelial cells, a key event in pulmonary fibrosis. Sci Rep. 2018;8:17939. 10.1038/s41598-018-36063-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou G, Dada LA, Wu M, Kelly A, Trejo H, Zhou Q, Varga J, Sznajder JI. Hypoxia-induced alveolar epithelial-mesenchymal transition requires mitochondrial ROS and hypoxia-inducible factor 1. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1120–1130. 10.1152/ajplung.00007.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patocka J, Nepovimova E, Kuca K, Wu W. Cyclosporine A: Chemistry and toxicity - A review. Curr Med Chem. 2021;28:3925–34. 10.2174/0929867327666201006153202 [DOI] [PubMed] [Google Scholar]
- 65.Maeda K, Komuta K, Kuritani T, Igarashi T. Cyclosporine treatment for interstitial pneumonitis associated with dermatomyositis/polymyositis. Intern Med. 1998;37:103. 10.2169/internalmedicine.37.103 [DOI] [PubMed] [Google Scholar]
- 66.Ogasawara H, Murashima A, Kaneko H, Hishikawa T, Tokano Y, Sekigawa I, Iida N, Hashimoto H, Hirose S. Effect of low-dose cyclosporin treatment on interstitial pneumonitis associated with Sjogren’s syndrome. Br J Rheumatol. 1998;37:348–9. 10.1093/rheumatology/37.3.348 [DOI] [PubMed] [Google Scholar]
- 67.Sakamoto S, Homma S, Miyamoto A, Kurosaki A, Fujii T, Yoshimura K. Cyclosporin A in the treatment of acute exacerbation of idiopathic pulmonary fibrosis. Intern Med. 2010;49:109–15. 10.2169/internalmedicine.49.2359 [DOI] [PubMed] [Google Scholar]
- 68.Muller S, Filippakopoulos P, Knapp S. Bromodomains as therapeutic targets. Expert Rev Mol Med. 2011;13:e29. 10.1017/S1462399411001992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tang X, Peng R, Ren Y, Apparsundaram S, Deguzman J, Bauer CM, Hoffman AF, Hamilton S, Liang Z, Zeng H, et al. BET bromodomain proteins mediate downstream signaling events following growth factor stimulation in human lung fibroblasts and are involved in bleomycin-induced pulmonary fibrosis. Mol Pharmacol. 2013;83:283–93. 10.1124/mol.112.081661 [DOI] [PubMed] [Google Scholar]
- 70.Suzuki K, Kim JD, Ugai K, Matsuda S, Mikami H, Yoshioka K, Ikari J, Hatano M, Fukamizu A, Tatsumi K, Kasuya Y. Transcriptomic changes involved in the dedifferentiation of myofibroblasts derived from the lung of a patient with idiopathic pulmonary fibrosis. Mol Med Rep. 2020;22:1518–26. 10.3892/mmr.2020.11218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mansouri N, Willis GR, Fernandez-Gonzalez A, Reis M, Nassiri S, Mitsialis SA, Kourembanas S. Mesenchymal stromal cell exosomes prevent and revert experimental pulmonary fibrosis through modulation of monocyte phenotypes. JCI Insight 2019, 4. [DOI] [PMC free article] [PubMed]
- 72.Kokeny G, Calvier L, Hansmann G. PPARgamma and TGFbeta-Major regulators of metabolism, inflammation, and fibrosis in the lungs and kidneys. Int J Mol Sci 2021, 22. [DOI] [PMC free article] [PubMed]
- 73.Kotarkonda LK, Kulshrestha R, Ravi K. Role of insulin like growth factor axis in the bleomycin induced lung injury in rats. Exp Mol Pathol. 2017;102:86–96. 10.1016/j.yexmp.2017.01.004 [DOI] [PubMed] [Google Scholar]
- 74.Rangarajan S, Bone NB, Zmijewska AA, Jiang S, Park DW, Bernard K, Locy ML, Ravi S, Deshane J, Mannon RB, et al. Metformin reverses established lung fibrosis in a bleomycin model. Nat Med. 2018;24:1121–7. 10.1038/s41591-018-0087-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kheirollahi V, Wasnick RM, Biasin V, Vazquez-Armendariz AI, Chu X, Moiseenko A, Weiss A, Wilhelm J, Zhang JS, Kwapiszewska G, et al. Metformin induces lipogenic differentiation in myofibroblasts to reverse lung fibrosis. Nat Commun. 2019;10:2987. 10.1038/s41467-019-10839-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Panati K, Thimmana LV, Narala VR. Electrophilic nitrated fatty acids are potential therapeutic candidates for inflammatory and fibrotic lung diseases. Nitric Oxide. 2020;102:28–38. 10.1016/j.niox.2020.06.003 [DOI] [PubMed] [Google Scholar]
- 77.Park SJ, Choi H, Kim JH, Kim CS. Antifibrotic effects of eupatilin on TGF-beta1-treated human vocal Fold fibroblasts. PLoS ONE. 2021;16:e0249041. 10.1371/journal.pone.0249041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hu J, Liu Y, Pan Z, Huang X, Wang J, Cao W, Chen Z. Eupatilin ameliorates hepatic fibrosis and hepatic stellate cell activation by suppressing beta-catenin/PAI-1 pathway. Int J Mol Sci 2023, 24. [DOI] [PMC free article] [PubMed]
- 79.McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263–84. 10.1016/j.bbamcr.2006.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu SW, Howat SL, Renzoni EA, Holmes A, Pearson JD, Dashwood MR, Bou-Gharios G, Denton CP, du Bois RM, Black CM, et al. Endothelin-1 induces expression of matrix-associated genes in lung fibroblasts through MEK/ERK. J Biol Chem. 2004;279:23098–103. 10.1074/jbc.M311430200 [DOI] [PubMed] [Google Scholar]
- 81.Madala SK, Schmidt S, Davidson C, Ikegami M, Wert S, Hardie WD. MEK-ERK pathway modulation ameliorates pulmonary fibrosis associated with epidermal growth factor receptor activation. Am J Respir Cell Mol Biol. 2012;46:380–8. 10.1165/rcmb.2011-0237OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xiao L, Du Y, Shen Y, He Y, Zhao H, Li Z. TGF-beta 1 induced fibroblast proliferation is mediated by the FGF-2/ERK pathway. Front Biosci (Landmark Ed). 2012;17:2667–74. 10.2741/4077 [DOI] [PubMed] [Google Scholar]
- 83.Goldfarb M. Signaling by fibroblast growth factors: the inside story. Sci STKE 2001, 2001:pe37. [DOI] [PMC free article] [PubMed]
- 84.Mao J, McGlinn E, Huang P, Tabin CJ, McMahon AP. Fgf-dependent Etv4/5 activity is required for posterior restriction of Sonic hedgehog and promoting outgrowth of the vertebrate limb. Dev Cell. 2009;16:600–6. 10.1016/j.devcel.2009.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.MacKenzie B, Korfei M, Henneke I, Sibinska Z, Tian X, Hezel S, Dilai S, Wasnick R, Schneider B, Wilhelm J, et al. Increased FGF1-FGFRc expression in idiopathic pulmonary fibrosis. Respir Res. 2015;16:83. 10.1186/s12931-015-0242-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liebler JM, Picou MA, Qu Z, Powers MR, Rosenbaum JT. Altered immunohistochemical localization of basic fibroblast growth factor after bleomycin-induced lung injury. Growth Factors. 1997;14:25–38. 10.3109/08977199709021508 [DOI] [PubMed] [Google Scholar]
- 87.Qu Z, Liebler JM, Powers MR, Galey T, Ahmadi P, Huang XN, Ansel JC, Butterfield JH, Planck SR, Rosenbaum JT. Mast cells are a major source of basic fibroblast growth factor in chronic inflammation and cutaneous hemangioma. Am J Pathol. 1995;147:564–73. [PMC free article] [PubMed] [Google Scholar]
- 88.Inoue Y, King TE Jr., Tinkle SS, Dockstader K, Newman LS. Human mast cell basic fibroblast growth factor in pulmonary fibrotic disorders. Am J Pathol. 1996;149:2037–54. [PMC free article] [PubMed] [Google Scholar]
- 89.Fortier SM, Penke LR, King D, Pham TX, Ligresti G, Peters-Golden M. Myofibroblast dedifferentiation proceeds via distinct transcriptomic and phenotypic transitions. JCI Insight 2021, 6. [DOI] [PMC free article] [PubMed]
- 90.Ramos C, Montano M, Becerril C, Cisneros-Lira J, Barrera L, Ruiz V, Pardo A, Selman M. Acidic fibroblast growth factor decreases alpha-smooth muscle actin expression and induces apoptosis in human normal lung fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2006;291:L871–879. 10.1152/ajplung.00019.2006 [DOI] [PubMed] [Google Scholar]
- 91.Koo HY, El-Baz LM, House S, Cilvik SN, Dorry SJ, Shoukry NM, Salem ML, Hafez HS, Dulin NO, Ornitz DM, Guzy RD. Fibroblast growth factor 2 decreases bleomycin-induced pulmonary fibrosis and inhibits fibroblast collagen production and myofibroblast differentiation. J Pathol. 2018;246:54–66. 10.1002/path.5106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kubo E, Shibata S, Shibata T, Kiyokawa E, Sasaki H, Singh DP. FGF2 antagonizes aberrant TGFbeta regulation of tropomyosin: role for posterior capsule opacity. J Cell Mol Med. 2017;21:916–28. 10.1111/jcmm.13030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Papetti M, Shujath J, Riley KN, Herman IM. FGF-2 antagonizes the TGF-beta1-mediated induction of pericyte alpha-smooth muscle actin expression: a role for myf-5 and smad-mediated signaling pathways. Invest Ophthalmol Vis Sci. 2003;44:4994–5005. 10.1167/iovs.03-0291 [DOI] [PubMed] [Google Scholar]
- 94.Kawai-Kowase K, Sato H, Oyama Y, Kanai H, Sato M, Doi H, Kurabayashi M. Basic fibroblast growth factor antagonizes transforming growth factor-beta1-induced smooth muscle gene expression through extracellular signal-regulated kinase 1/2 signaling pathway activation. Arterioscler Thromb Vasc Biol. 2004;24:1384–90. 10.1161/01.ATV.0000136548.17816.07 [DOI] [PubMed] [Google Scholar]
- 95.Greenberg RS, Bernstein AM, Benezra M, Gelman IH, Taliana L, Masur SK. FAK-dependent regulation of myofibroblast differentiation. FASEB J. 2006;20:1006–8. 10.1096/fj.05-4838fje [DOI] [PubMed] [Google Scholar]
- 96.Hu B, Wu YM, Wu Z, Phan SH. Nkx2.5/Csx represses myofibroblast differentiation. Am J Respir Cell Mol Biol. 2010;42:218–26. 10.1165/rcmb.2008-0404OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen PY, Qin L, Li G, Tellides G, Simons M. Fibroblast growth factor (FGF) signaling regulates transforming growth factor beta (TGFbeta)-dependent smooth muscle cell phenotype modulation. Sci Rep. 2016;6:33407. 10.1038/srep33407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen PY, Qin L, Barnes C, Charisse K, Yi T, Zhang X, Ali R, Medina PP, Yu J, Slack FJ, et al. FGF regulates TGF-beta signaling and endothelial-to-mesenchymal transition via control of let-7 miRNA expression. Cell Rep. 2012;2:1684–96. 10.1016/j.celrep.2012.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ramos C, Becerril C, Montano M, Garcia-De-Alba C, Ramirez R, Checa M, Pardo A, Selman M. FGF-1 reverts epithelial-mesenchymal transition induced by TGF-beta1 through MAPK/ERK kinase pathway. Am J Physiol Lung Cell Mol Physiol. 2010;299:L222–231. 10.1152/ajplung.00070.2010 [DOI] [PubMed] [Google Scholar]
- 100.Khannpnavar B, Mehta V, Qi C, Korkhov V. Structure and function of adenylyl cyclases, key enzymes in cellular signaling. Curr Opin Struct Biol. 2020;63:34–41. 10.1016/j.sbi.2020.03.003 [DOI] [PubMed] [Google Scholar]
- 101.Gao F, Yang S, Wang J, Zhu G. cAMP-PKA cascade: an outdated topic for depression? Biomed Pharmacother. 2022;150:113030. 10.1016/j.biopha.2022.113030 [DOI] [PubMed] [Google Scholar]
- 102.Ahmed MB, Alghamdi AAA, Islam SU, Lee JS, Lee YS. cAMP signaling in Cancer: a PKA-CREB and EPAC-Centric Approach. Cells 2022, 11. [DOI] [PMC free article] [PubMed]
- 103.Fine A, Poliks CF, Donahue LP, Smith BD, Goldstein RH. The differential effect of prostaglandin E2 on transforming growth factor-beta and insulin-induced collagen formation in lung fibroblasts. J Biol Chem. 1989;264:16988–91. 10.1016/S0021-9258(18)71448-8 [DOI] [PubMed] [Google Scholar]
- 104.Kolodsick JE, Peters-Golden M, Larios J, Toews GB, Thannickal VJ, Moore BB. Prostaglandin E2 inhibits fibroblast to myofibroblast transition via E. prostanoid receptor 2 signaling and cyclic adenosine monophosphate elevation. Am J Respir Cell Mol Biol. 2003;29:537–44. 10.1165/rcmb.2002-0243OC [DOI] [PubMed] [Google Scholar]
- 105.Elias JA, Rossman MD, Zurier RB, Daniele RP. Human alveolar macrophage inhibition of lung fibroblast growth. A prostaglandin-dependent process. Am Rev Respir Dis. 1985;131:94–9. [DOI] [PubMed] [Google Scholar]
- 106.Huang S, Wettlaufer SH, Hogaboam C, Aronoff DM, Peters-Golden M. Prostaglandin E(2) inhibits collagen expression and proliferation in patient-derived normal lung fibroblasts via E prostanoid 2 receptor and cAMP signaling. Am J Physiol Lung Cell Mol Physiol. 2007;292:L405–413. 10.1152/ajplung.00232.2006 [DOI] [PubMed] [Google Scholar]
- 107.Penke LR, Huang SK, White ES, Peters-Golden M. Prostaglandin E2 inhibits alpha-smooth muscle actin transcription during myofibroblast differentiation via distinct mechanisms of modulation of serum response factor and myocardin-related transcription factor-A. J Biol Chem. 2014;289:17151–62. 10.1074/jbc.M114.558130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wettlaufer SH, Penke LR, Okunishi K, Peters-Golden M. Distinct PKA regulatory subunits mediate PGE(2) inhibition of TGFbeta-1-stimulated collagen I translation and myofibroblast differentiation. Am J Physiol Lung Cell Mol Physiol. 2017;313:L722–31. 10.1152/ajplung.00131.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huang SK, Wettlaufer SH, Chung J, Peters-Golden M. Prostaglandin E2 inhibits specific lung fibroblast functions via selective actions of PKA and Epac-1. Am J Respir Cell Mol Biol. 2008;39:482–9. 10.1165/rcmb.2008-0080OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wettlaufer SH, Scott JP, McEachin RC, Peters-Golden M, Huang SK. Reversal of the Transcriptome by Prostaglandin E2 during Myofibroblast Dedifferentiation. Am J Respir Cell Mol Biol. 2016;54:114–27. 10.1165/rcmb.2014-0468OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sieber P, Schafer A, Lieberherr R, Le Goff F, Stritt M, Welford RWD, Gatfield J, Peter O, Nayler O, Luthi U. Novel high-throughput myofibroblast assays identify agonists with therapeutic potential in pulmonary fibrosis that act via EP2 and EP4 receptors. PLoS ONE. 2018;13:e0207872. 10.1371/journal.pone.0207872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Saeki I, Terai S, Fujisawa K, Takami T, Yamamoto N, Matsumoto T, Hirose Y, Murata Y, Yamasaki T, Sakaida I. Bortezomib induces tumor-specific cell death and growth inhibition in hepatocellular carcinoma and improves liver fibrosis. J Gastroenterol. 2013;48:738–50. 10.1007/s00535-012-0675-z [DOI] [PubMed] [Google Scholar]
- 113.Mutlu GM, Budinger GR, Wu M, Lam AP, Zirk A, Rivera S, Urich D, Chiarella SE, Go LH, Ghosh AK, et al. Proteasomal inhibition after injury prevents fibrosis by modulating TGF-beta(1) signalling. Thorax. 2012;67:139–46. 10.1136/thoraxjnl-2011-200717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zeniya M, Mori T, Yui N, Nomura N, Mandai S, Isobe K, Chiga M, Sohara E, Rai T, Uchida S. The proteasome inhibitor bortezomib attenuates renal fibrosis in mice via the suppression of TGF-beta1. Sci Rep. 2017;7:13086. 10.1038/s41598-017-13486-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Legerlotz K, Smith HK. Role of MyoD in denervated, disused, and exercised muscle. Muscle Nerve. 2008;38:1087–100. 10.1002/mus.21087 [DOI] [PubMed] [Google Scholar]
- 116.Walker JL, Zhai N, Zhang L, Bleaken BM, Wolff I, Gerhart J, George-Weinstein M, Menko AS. Unique precursors for the mesenchymal cells involved in injury response and fibrosis. Proc Natl Acad Sci U S A. 2010;107:13730–5. 10.1073/pnas.0910382107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hecker L, Jagirdar R, Jin T, Thannickal VJ. Reversible differentiation of myofibroblasts by MyoD. Exp Cell Res. 2011;317:1914–21. 10.1016/j.yexcr.2011.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kato K, Logsdon NJ, Shin YJ, Palumbo S, Knox A, Irish JD, Rounseville SP, Rummel SR, Mohamed M, Ahmad K, et al. Impaired myofibroblast dedifferentiation contributes to Nonresolving Fibrosis in Aging. Am J Respir Cell Mol Biol. 2020;62:633–44. 10.1165/rcmb.2019-0092OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang C, Wang K, Zhou Y, Zhang SL. Histone lysine methyltransferase SET8 is a novel therapeutic target for cancer treatment. Drug Discov Today. 2021;26:2423–30. 10.1016/j.drudis.2021.05.004 [DOI] [PubMed] [Google Scholar]
- 120.Ugai K, Matsuda S, Mikami H, Shimada A, Misawa T, Nakamura H, Tatsumi K, Hatano M, Murayama T, Kasuya Y. Inhibition of the SET8 pathway ameliorates lung fibrosis even through fibroblast dedifferentiation. Front Mol Biosci. 2020;7:192. 10.3389/fmolb.2020.00192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Desmouliere A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995;146:56–66. [PMC free article] [PubMed] [Google Scholar]
- 122.Hinz B. The myofibroblast: paradigm for a mechanically active cell. J Biomech. 2010;43:146–55. 10.1016/j.jbiomech.2009.09.020 [DOI] [PubMed] [Google Scholar]
- 123.Liu F, Lagares D, Choi KM, Stopfer L, Marinkovic A, Vrbanac V, Probst CK, Hiemer SE, Sisson TH, Horowitz JC, et al. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am J Physiol Lung Cell Mol Physiol. 2015;308:L344–357. 10.1152/ajplung.00300.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Upagupta C, Shimbori C, Alsilmi R, Kolb M. Matrix abnormalities in pulmonary fibrosis. Eur Respir Rev 2018, 27. [DOI] [PMC free article] [PubMed]
- 125.Aarabi S, Bhatt KA, Shi Y, Paterno J, Chang EI, Loh SA, Holmes JW, Longaker MT, Yee H, Gurtner GC. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J. 2007;21:3250–61. 10.1096/fj.07-8218com [DOI] [PubMed] [Google Scholar]
- 126.Goffin JM, Pittet P, Csucs G, Lussi JW, Meister JJ, Hinz B. Focal adhesion size controls tension-dependent recruitment of alpha-smooth muscle actin to stress fibers. J Cell Biol. 2006;172:259–68. 10.1083/jcb.200506179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol. 2007;179:1311–23. 10.1083/jcb.200704042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kural MH, Billiar KL. Myofibroblast persistence with real-time changes in boundary stiffness. Acta Biomater. 2016;32:223–30. 10.1016/j.actbio.2015.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang Y, Fu J, Li C, Chang Y, Li X, Cheng H, Qiu Y, Shao M, Han Y, Feng D, et al. Omentin-1 induces mechanically activated fibroblasts lipogenic differentiation through pkm2/yap/ppargamma pathway to promote lung fibrosis resolution. Cell Mol Life Sci. 2023;80:308. 10.1007/s00018-023-04961-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhao J, Yang S, Xu Y, Qin S, Bie F, Chen L, Zhou F, Xie J, Liu X, Shu B, Qi S. Mechanical pressure-induced dedifferentiation of myofibroblasts inhibits scarring via SMYD3/ITGBL1 signaling. Dev Cell. 2023;58:1139–e11521136. 10.1016/j.devcel.2023.04.014 [DOI] [PubMed] [Google Scholar]
- 131.Yang X, Chen B, Liu T, Chen X. Reversal of myofibroblast differentiation: a review. Eur J Pharmacol. 2014;734:83–90. 10.1016/j.ejphar.2014.04.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.