Abstract
Background
Proprotein convertase subtilisins/kexin 6 (PCSK6) polymorphisms have been shown to be associated with atherosclerosis progression. This research aimed to evaluate the relationship of PCSK6 rs1531817 polymorphisms with coronary stenosis and the prognosis in premature myocardial infarction (PMI) patients.
Methods
This prospective cohort analysis consecutively included 605 PMI patients who performed emergency percutaneous coronary intervention (PCI) at Tianjin Chest Hospital sequentially between January 2017 and August 2022, with major adverse cardiovascular events (MACEs) as the outcome. Analyses assessed the relationships among PCSK6 rs1531817 polymorphism, Gensini score (GS), triple vessel disease (TVD), and MACEs.
Results
92 (16.8%) patients experienced MACEs with an average follow-up of 25.7 months. Logistic analysis revealed that the PCSK6 rs1531817 CA + AA genotype was an independent protective factor against high GS and TVD. Cox analysis revealed that the PCSK6 rs1531817 CA + AA genotype was an independent protective factor against MACEs. The mediation effect results showed that apolipoprotein A1/apolipoprotein B (ApoA1/ApoB) partially mediated the association between PCSK6 rs1531817 polymorphism and coronary stenosis and that total cholesterol/high-density lipoprotein (TC/HDL) and TVD partially and in parallel mediated the association between the PCSK6 rs1531817 polymorphism and MACEs.
Conclusion
Patients with the PCSK6 CA + AA genotype have milder coronary stenosis and a better long-term prognosis; according to the mediation model, ApoA1/ApoB and TC/HDL partially mediate. These results may provide a new perspective on clinical therapeutic strategy for anti-atherosclerosis and improved prognosis in PMI patients.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12944-024-02206-w.
Keywords: Premature myocardial infarction, Proprotein convertase subtilisins/Kexin 6, Single nucleotide polymorphism, Coronary stenosis, Major adverse cardiovascular events
Introduction
In recent years, an increasing proportion of younger patients are being hospitalized for acute myocardial infarction (AMI) [1–3]. Premature myocardial infarction (PMI), with its high morbidity and mortality, is a significant problem for the health of the general population [4, 5]. This research team has previously conducted a series of long-term studies on coronary stenosis and prognostic risk in PMI patients from a clinical perspective [6, 7]. Given that PMI has 63% heritability, genetics is essential for its development [8]. Therefore, it is important to explore the factors affecting coronary stenosis and prognosis in PMI patients from a genetic perspective for clinical management.
Proprotein convertase subtilisins/kexin 6 (PCSK6) is expressed in liver cells, and its encoding gene is located at q26.3 on the 15th chromosome [9]. It regulates protein structure and function through protein hydrolysis cleavage. Some studies suggest that PCSK6 is associated with atherosclerosis [10, 11]. In a recent study, 3378 individuals with high coronary heart disease risk to investigate the relationship between PCSK6 polymorphisms and the progression of carotid intima-media thickness (cIMT). The results showed that mutations in the PCSK6 rs1531817 (C > A) were significantly associated with maximal progression of internal carotid artery thickness; the patients carrying the A allele showed reduced cIMT progression compared to individuals with CC genotypes (from the AtheroExpress Biospecimen Library) [10]. cIMT is a predictive surrogate for atherosclerosis, suggesting that atherosclerotic disease progression is related to the PCSK6 rs1531817 mutation.
A previous study has shown that PCSK6 polymorphisms are associated with atherosclerosis progression [10]. Cardiac fibroblasts and endothelial cells have been demonstrated to express PCSK6 [12]. However, it is still unknown whether the PCSK6 rs1531817 polymorphism is associated with coronary stenosis and the prognosis in PMI patients. There are no relevant studies available. Therefore, the study aimed to evaluate the relationship between PCSK6 rs1531817 polymorphism, coronary stenosis, and the prognosis in PMI patients.
Methods
Study population
This prospective cohort analysis consecutively included 671 PMI patients who performed emergency percutaneous coronary intervention (PCI) at Tianjin Chest Hospital sequentially between January 2017 and August 2022. There is no standardized age limit for PMI. Current national and international studies generally set the upper age limit for PMI at 45–55 years [13–16]. This study defined PMI as ≤ 50 years for men and ≤ 55 years for women. The inclusion criteria were (1) meeting age inclusion criteria. (2) Fulfilment of AMI diagnosis. (3) Complete coronary angiography and underwent PCI. (4) Informed permission was signed by each patient who was included. The exclusion criteria were (1) severe liver or kidney disease. (2) Having infections or rheumatologic or immunologic diseases. (3) Patients with malignant tumours requiring treatment. (4) Missing data related to PCSK6 rs1531817 genotypes and coronary angiography. This study used the fourth international definition of MI (2018) to diagnose AMI [17]: clinical proof of acute myocardial ischaemia coupled with damage; elevated or reduced cTnT (at least one time above the top 99% threshold), as well as any one of these subsequent circumstances (1) symptoms and signs of myocardial ischaemia; (2) novel ischaemic electrocardiographic alterations; (3) patient characteristics of the Q-wave; (4) imaging evidence indicating that new focal wall motion abnormalities or novel patterns of myocardial inactivation are compatible with pathologic alterations of ischaemia; or (5) coronary thrombosis discovered by autopsy or radiography. After the screening, 605 patients were ultimately included.
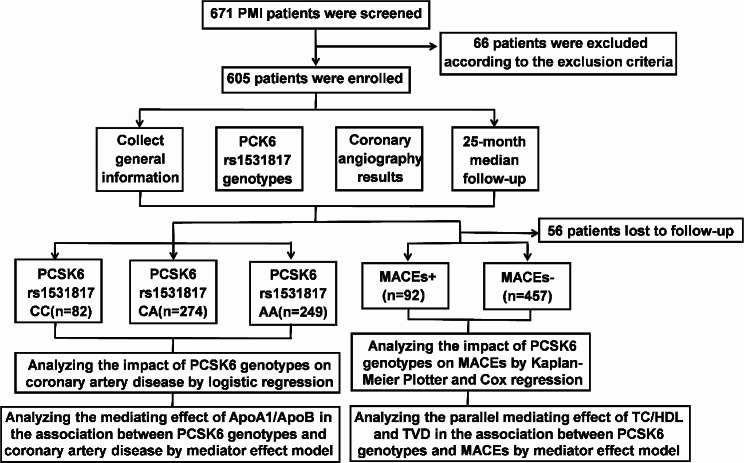
The Tianjin Chest Hospital’s Ethical Commission approved the study (No.2017KY-007-01), and this study received informed consent from each patient. Figure 1 displays the flowchart for the cohort study.
Fig. 1.
Cohort study flowchart
General data collection
Age, sex, medical history, admission blood pressure, heart rate were all recorded as each patient’s clinical baseline data. The patients’ laboratory examination data, echocardiography, and other relevant indicators were also collected. According to the normal reference range of laboratory indicators in Tianjin Chest Hospital, patients with one or more abnormalities of the following indicators were considered dyslipidemia: total cholesterol (TC); triglycerides (TG); low density lipoprotein (LDL); and high density lipoprotein (HDL).
Coronary angiography and intervention
Two cardiologists qualified to diagnose and treat coronary arteries performed coronary angiography and PCI. The GS was computed for each enrolled PMI patient based on the results of their coronary angiography. Each coronary artery stenosis was ranked according to a score of 1 for 1–25%, 2 for 26–50%, 4 for 51–75%, 8 for 76–90%, 16 for 91–99%, and complete arterial occlusion 32. After that, the score was multiplied with a factor. The multiplicand is as follows: the coefficient of multiplication was five for left main cardiac artery, two and a half for left anterior descending and circumflex arteries’ proximities, one and a half for medial left anterior descending branch. The coefficient of multiplication was one for right coronary artery, circumflex’s median and distant segment, and distant segment of left anterior descending; with every other segment, the multiplication factor was 0.5. The total coronary artery score is taken as the GS [18–20].
The present study used MACEs and time-to-MACEs for implicit variables. GSs were stratified into three levels using X-tile software: a low-GS group (< 52.0 points, n = 323), a medium-GS group (52.0–94.0 points, n = 215), and a high-GS group (> 94.0 points, n = 67), which represented mild, moderate, and severe coronary stenosis, respectively. Table 1 shows the estimated cut-off point range of the value region as well as the distributional features of the GS levels.
Table 1.
Cut-off point of GS points by X-tile method
| Group | Range(points) | Patients | Percentage(%) | MACEs(%) |
|---|---|---|---|---|
| Low GS | 5–52 | 286 | 52.09 | 40(13.99) |
| Medium GS | 52–94 | 199 | 36.25 | 33(16.58) |
| High GS | 94–202 | 64 | 11.66 | 19(29.69) |
| Total | 5−202 | 549 | 100.00 | 92(16.76) |
GS Gensini score; MACEs major adverse cardiovascular events
Patients were categorized according to the number of branches involved (≥ 50% stenosis in the major coronary artery) in their coronary lesions into groups of single lesions, double lesions, and triple lesions. Double vessel disease (DVD) was considered when stenosis of the left main lesion involved, regardless of the involvement of the left anterior descending, circumflex, or both; triple vessel disease (TVD) was considered when accompanied by a right coronary arterial lesion.
Genetic analysis
Blood samples were collected from every individual after fasting on the first day after admission. Ten minutes were spent spinning the blood samples at four degrees Celsius and three thousand rotations per minute; moreover, the serum was kept at -80 degrees Celsius in the Chest Hospital Biospecimen Bank. Genomic DNA was isolated and purified from 1 mL EDTA-treated peripheral blood by a blood DNA extraction kit (LaServ, Shanghai, China) and subjected to quality control. For the purpose of testing single nucleotide polymorphism (SNP) sites, MassARRAY Assay Design 3.1 software and genotyping tools from Sequenom were utilized to construct single-base extension primers and polymerase chain reaction (PCR) amplification primers, and the designed primers were submitted to a company for synthesis. The primers were centrifuged under vacuum, and then water was added. After adding water, shaking, and mixing, the primers were diluted with 36 µL water per 1 OD and left at room temperature for thirty min. Next, PCR amplification was carried out, and the PCR amplification system consisted of water (1.8 µl), 10× PCR buffer (0.5 µl), PCR primer combination (1 µl), MgCl2 (0.4 µl), dNTP mixture (0.1 µl), HotStar Taq (5 U/µl) (0.2 µl), and DNA sample (1 µl). The cycling program consisted of 120 s at 95 °C, 45 cycles of 30 s at 56 °C, and 180 s at 72 °C. Subsequently, the PCR products were treated with alkaline phosphatase and subjected to a single-base extension reaction. The PCSK6 rs1531817 genotyping analysis of the included population was performed using the nucleic acid mass spectrometry analysis system of the MassARRAY platform of Beijing COMPASS BIOTECHNOLOGY Co., Ltd. (Sequenom, San Diego, CA, USA) after resin purification and microarray spotting.
Follow-up
Trained nurses or cardiologists monitored every patient involved in the trial for at least 12 months through phone follow-ups, outpatient check-ups, and clinic visits. The follow-up endpoint was defined as the occurrence of a major adverse cardiovascular event (MACE). MACEs consisted of cardiac death, nonfatal stroke, readmission for ischaemic cardiovascular events, target lesion revascularization (TLR), and rehospitalization for cardiac insufficiency. Ischaemic cardiovascular events included acute myocardial infarction and episodes of unstable angina.
Statistical methods
Using the X-tile technique, the GS was split into three categories: low, medium, and high. The cut-off points were selected via an X-tile analysis, which considered covariates and patient survival, to maintain homogeneity and variability within the groups [21].
Categorical variables are stated in numerical and percentage form. All of the continuous variables in this study were nonnormally distributed, and they are expressed as medians and quartiles. Chi-squared tests were applied to evaluate the differences among the categories of categorical data, while nonparametric tests were used to assess the differences among the categories of continuous data. The effect of the PCSK6 rs1531817 genotype on coronary stenosis was examined by logistic regression. The associations between the PCSK6 rs1531817 genotype and MACEs were analyzed by Cox regression and Kaplan‒Meier curves [22]. The mediating effect of lipids associated with the PCSK6 rs1531817 polymorphism, coronary stenosis, and MACEs was investigated via a mediating effect model. The mediating effect analysis was performed using the bootstrap test procedure, and the inverse logarithmic transformation was used to convert the mediating effect values to the corresponding odds ratio.
The data analysis program SPSS 26.0 was used for this research, GraphPad Prism 8.0 was used for all graphs, X-tile analyses were conducted with the X-tile system (Copyright Yale University), and the mediation effects model was analyzed by MPLUS 8.3. P < 0.05 were employed to identify statistically significant differences.
Results
Basic characteristics of various PCSK6 rs1531817 genotypes
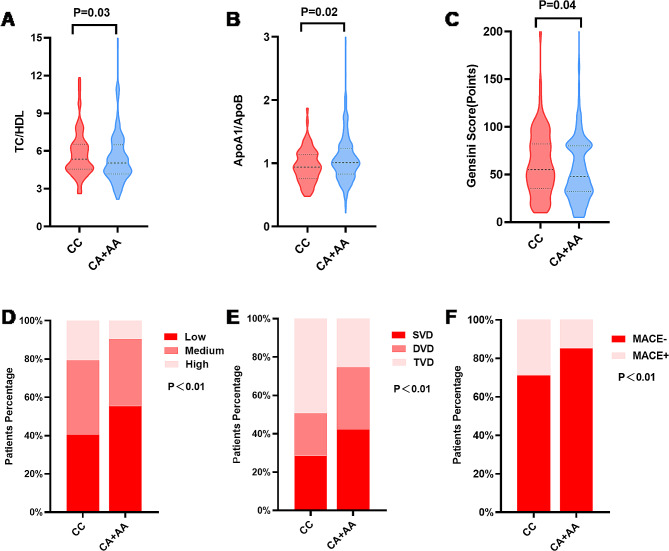
When comparing the PCSK6 rs1531817 genotypes, differences in TC/HDL, ApoA1/ApoB, GS group, number of branches involved in coronary lesions, MACEs, and TLR were statistically significant (P < 0.05). CA + AA genotypes patients had considerably lower TC/HDL and Gensini scores and significantly greater ApoA1/ApoB levels than CC genotype patients. Fewer patients with the PCSK6 rs1531817 CA + AA genotype had a high GS, TVD, and MACEs than patients with the CC genotype (P < 0.05) (Table 2; Fig. 2).
Table 2.
Baseline characteristics of various PCSK6 rs1531817 genotypes
| Characteristics | Wild type CC(n = 82) |
Heterozygous type CA(n = 274) |
Mutant type AA(n = 249) |
P value |
|---|---|---|---|---|
| Male, n(%) | 74(90.24) | 243(88.69) | 220(88.35) | 0.89 |
| Age, years | 42.00(37.00,44.25) | 42.00(38.00,45.00) | 42.00(37.50,45.50) | 0.58 |
| BMI, kg/m2 | 26.12(24.21,28.69) | 26.12(24.09,28.41) | 25.83(23.71,28.37) | 0.59 |
| History, n(%) | ||||
| Smoking | 51(62.20) | 184(67.15) | 164(65.86) | 0.71 |
| Alcohol intake | 27(32.93) | 103(37.59) | 78(31.33) | 0.31 |
| Hypertension | 33(40.24) | 136(49.64) | 110(44.18) | 0.24 |
| Diabetes | 14(17.07) | 45(16.42) | 51(20.48) | 0.47 |
| Dyslipidemia | 71(86.58) | 223(81.98) | 204(81.93) | 0.59 |
| Previous Stroke | 1(1.22) | 7(2.55) | 11(4.42) | 0.25 |
| STEMI, n(%) | 67(81.71) | 234(85.40) | 214(85.94) | 0.64 |
| Systolic pressure, mmHg |
132.00 (125.00,148.00) |
134.00 (120.00,145.00) |
132.00 (120.00,145.00) |
0.46 |
| Diastolic pressure, mmHg | 83.50(72.00,95.25) | 80.00(70.00,91.25) | 80.00(70.00,92.00) | 0.47 |
| Heart rate, bpm | 78.00(70.00,90.00) | 77.00(68.75,88.25) | 76.00(69.00,86.00) | 0.36 |
| Biochemical characteristics | ||||
| WBC,10^9/L | 10.26(8.44,12.10) | 10.59(8.94,12.70) | 10.99(9.06,13.14) | 0.12 |
| CRP, mg/L | 4.93(2.04,10.98) | 5.50(2.44,12.39) | 5.61(2.23,12.23) | 0.84 |
| ALT, U/L | 44.75(30.18,73.23) | 46.35(30.60,71.38) | 46.50(32.15,69.60) | 0.99 |
| Cr, umol/L | 76.00(68.75,84.50) | 73.00(63.75,81.00) | 74.00(65.00,84.00) | 0.11 |
| FBG, mmol/L | 5.85(5.11,7.29) | 6.06(5.30,7.78) | 5.83(5.18,8.69) | 0.71 |
| TC, mmol/L | 4.87(4.28,5.58) | 4.79(4.12,5.42) | 4.76(4.11,5.58) | 0.61 |
| TG, mmol/L | 2.10(1.56,3.09) | 1.93(1.42,2.80) | 2.11(1.48,3.01) | 0.18 |
| HDL, mmol/L | 0.92(0.80,1.01) | 0.95(0.82,1.13) | 0.92(0.80,1.06) | 0.05 |
| LDL, mmol/L | 3.31(2.71,3.76) | 3.22(2.58,3.81) | 3.16(2.50,3.86) | 0.66 |
| TC/HDL | 5.36(4.57,6.55) | 4.92(4.18,5.94) | 5.25(4.17,6.35) | 0.02 |
| ApoA1,g/L | 1.09(0.97,1.23) | 1.14(1.02,1.29) | 1.13(1.01,1.26) | 0.20 |
| ApoB, g/L | 1.17(0.99,1.34) | 1.14(0.92,1.30) | 1.13(0.93,1.35) | 0.36 |
| ApoA1/ApoB | 0.94(0.76,1.14) | 1.01(0.87,1.22) | 1.01(0.79,1.24) | 0.04 |
| cTnT, ng/ml | 2.44(1.19,5.17) | 2.74(1.06,5.67) | 3.14(1.40,5.43) | 0.34 |
| BNP, pg/ml |
213.08 (69.30,524.50) |
234.46 (77.12,671.00) |
267.70 (75.92,839.00) |
0.43 |
| D-Dimer, ug/ml | 0.28(0.21,0.44) | 0.29(0.22,0.47) | 0.31(0.22,0.54) | 0.42 |
| Fg, g/L | 3.33(2.88,3.86) | 3.35(2.88,3.92) | 3.23(2.84,3.82) | 0.52 |
| Echocardiography | ||||
| LVEF,% | 53.00(47.00,56.00) | 52.00(46.00,57.00) | 51.00(45.00,56.00) | 0.41 |
| PAP, mmHg | 30.00(30.00,30.00) | 30.00(30.00,30.00) | 30.00(30.00,30.00) | 0.84 |
| Gensini Score, points | 55.00(35.00,82.00) | 48.00(31.50,80.00) | 46.00(32.00,80.00) | 0.12 |
| Gensini Score Group, n(%) | <0.01 | |||
| <52 | 33(40.24) | 149(54.38) | 141(56.63) | |
| 52–94 | 32(39.02) | 103(37.59) | 80(32.13) | |
| >94 | 17(20.73) | 22(8.03) | 28(11.24) | |
| Coronary vessel disease, n(%) | <0.01 | |||
| SVD | 24(29.27) | 116(42.34) | 111(44.58) | |
| DVD | 18(21.95) | 90(32.8) | 76(30.52) | |
| TVD | 40(48.78) | 68(24.82) | 62(24.90) | |
|
Medication during follow-up, n(%) |
||||
| DAPT | 81(98.78) | 274(100.0) | 247(99.20) | 0.26 |
| Statin | 81(98.78) | 270(98.54) | 244(97.99) | 0.84 |
| Anticoagulant | 74(90.24) | 246(89.78) | 227(91.16) | 0.86 |
| ACEI/ARB | 67(81.71) | 214(78.10) | 199(79.92) | 0.75 |
| Beta-blocker | 71(86.59) | 241(87.96) | 210(84.34) | 0.48 |
| MACEs, n(%) | 22(26.83) | 37(13.50) | 33(13.25) | <0.01 |
| Cardic death | 2(2.44) | 2(0.73) | 1(0.40) | 0.22 |
| Non-fatal stroke | 1(1.22) | 3(1.09) | 0(0.0) | 0.26 |
| Hospital admission for Ischemic cardiovascular events | 13(15.85) | 22(8.03) | 20(8.03) | 0.09 |
| Target lesion revascularization | 10(12.20) | 7(2.55) | 10(4.02) | <0.01 |
| Hospital admission for HF | 3(3.66) | 11(4.01) | 11(4.42) | 0.91 |
PCSK6 proprotein convertase subtilisin/kexin type 6; STEMI ST-segment elevation myocardial infarction; BMI body mass index; WBC white blood cell; ALT alanine transaminase; CRP C-reactive protein; Cr creatinine; FBG fasting blood glucose; TC total cholesterol; TG Triglyceride; HDL high-density lipoprotein; LDL low-density lipoprotein; Apo apolipoprotein; cTnT cardiac troponin T; BNP B type natriuretic peptide; Fg Fibrinogen; LVEF left ventricular ejection fraction; PAP pulmonary artery pressure; SVD single vessel disease; DVD double vessel diseas; TVD triple vessel diseases; DAPT dual antiplatelet therapy; ACEI angiotensin-converting enzyme inhibitors; ARB angiotensin II receptor blockers; MACEs major adverse cardiovascular events
Data are present as mean (inter-quartile range) or number (%)
Fig. 2.
Baseline data were compared between the PCSK6 rs1531817 CC and CA + AA genotypes. A-C Comparison of TC/HDL, ApoA1/ApoB, and GS levels between the PCSK6 rs1531817 CC and CA + AA genotypes. D-F The percentage of patients with different GS subgroups, percentage of patients with different coronary lesions, and incidence of MACEs were compared between the two groups
Correlation of the PCSK6 rs1531817 polymorphism with the Gensini score in PMI patients
Among the various GS groups, hypertension, diabetes, previous stroke, heart rate, white blood cell (WBC), fasting blood glucose (FBG), alanine transaminase (ALT), B type natriuretic peptide (BNP), TC, LDL, ApoB, ApoA1/ApoB, cardiac troponin T (cTnT), fibrinogen (Fg), and PCSK6 rs1531817 genotype (CA + AA vs. CC) were significant differences. Compared to the low and medium GS groups, the percentage of patients with CA + AA genotypes was considerably lower in the high GS group (P < 0.05) (Table S1).
Univariate logistic analysis included indicators with P < 0.05 for comparisons between different GS groups. TC, LDL, and ApoB were not included in the logistic regression since ApoA1/ApoB was significantly correlated with TC (r=-0.62, P < 0.01), LDL (r=-0.65, P < 0.01), and ApoB (r=-0.79, P < 0.01). Univariate logistic regression revealed that diabetes, previous stroke, heart rate, WBC, ALT, FBG, cTnT, and Fg were risk factors for the high GS, ApoA1/ApoB, and PCSK6 rs1531817 CA + AA genotypes were protective factors against the occurrence of high GS (Fig. S1 A). The multivariate logistic regression considered the indicators from the univariate logistic regression with P < 0.05, compared to the low GS, FBG, cTnT, and Fg were risk factors for the high GS. The CA, AA, and CA + AA genotypes were the protective factors (adjOR = 0.30, 95% CI: 0.14–0.63, P < 0.01) (Fig. S1 B, D). Compared to the medium GS group, the risk factor for the high GS group was previous stroke, and the protective factors were the CA and CA + AA genotypes (adjOR = 0.45, 95% CI: 0.21–0.96, P = 0.04) (Fig.S1 C, E).
Correlation of PCSK6 rs1531817 polymorphisms with the number of branches involved in coronary stenosis in individuals with PMI
Age, sex, hypertension status, diabetes, ALT, FBG, TC, LDL, TC/HDL, ApoB, ApoA1/ApoB, cTnT, Fg, and PCSK6rs1531817 genotypes were the variables tested in the comparison among patients with single vessel disease (SVD), DVD, and TVD. Among them, compared to the SVD and DVD groups, the percentage of patients with CA + AA genotypes was decreased in the TVD group (P < 0.05) (Table S2).
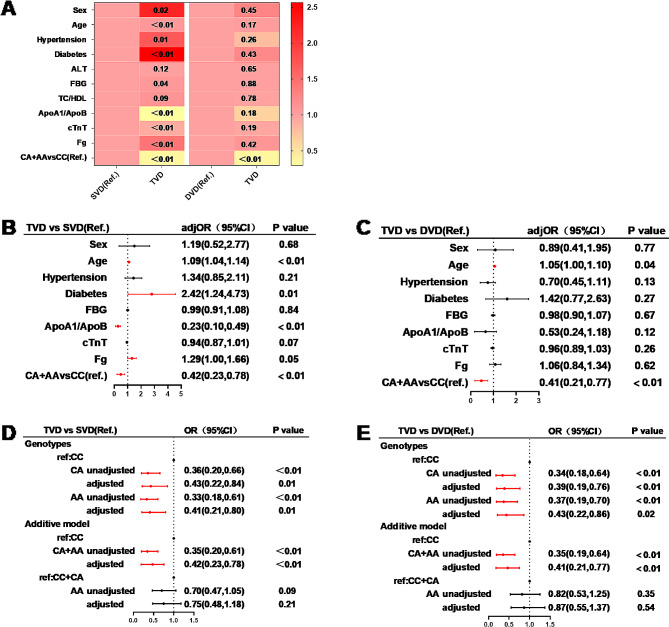
The univariate logistic analysis included indicators with p < 0.05 for comparisons among the SVD, DVD, and TVD patients. TC, LDL, and ApoB were not included in the logistic regression since ApoA1/ApoB was significantly related to TC, LDL, and ApoB. Univariate logistic regression revealed that age, female sex, hypertension status, diabetes status, FBG, and Fg were risk factors for TVD. The ApoA1/ApoB, cTnT, and PCSK6 rs1531817 CA + AA genotypes were protective factors for TVD in patients with PMI (P < 0.05) (Fig. 3A). The multivariate logistic regression considered the indicators from the univariate logistic regression with P < 0.05, which suggested that age and FBG were risk factors for TVD compared with the SVD group, and the protective factors were ApoA1/ApoB and the CA, AA, and CA + AA genotypes (adjOR = 0.42, 95% CI: 0.23–0.78, P < 0.01) (Fig. 3B, D). Age was a risk factor for TVD, and protective factors were the CA, AA, and CA + AA genotypes (adjOR = 0.41, 95% CI: 0.21–0.77, P < 0.01) compared to the DVD group (Fig. 3C, E).
Fig. 3.
Correlation between the number of coronary stenosis branches and PCSK6 rs1531817 polymorphisms in PMI patients. A Heatmap showing the ORs and P values for the risk of TVD according to univariate logistic regression (red and yellow indicate positive and negative correlations, respectively). B, C Multivariate logistic regression for the risk of TVD. D, E Logistic regression of different genotypes of PCSK6 rs1531817 for the risk of TVD (TVD vs. SVD, TVD vs. DVD). Multivariate logistic regression was adjusted for FBG, Fg, ApoA1/ApoB, cTnT, age, sex, hypertension, and diabetes. OR odds ratio; CI confidence intervals
Correlation of the PCSK6 rs1531817 polymorphism with MACEs
The most crucial social genetic component is the Hardy‒Weinberg equilibrium (HWE) [23, 24]; if the population complies with the HWE (P > 0.05), the population is from the same Mendelian stock and the samples are representative of population genetic studies. After testing HWE for both the overall patient group and the loss to follow-up, MACEs-positive and MACEs-negative groups, it was found that the distribution of each genotype group of PCSK6 rs1531817 complied with the HWE (P > 0.05) (Table S3 and Table 3).
Table 3.
Analysis of clinical data of PMI patients with MACEs
| Characteristics | MACEs(n = 92) | Non-MACEs (n = 457) | P value |
|---|---|---|---|
| Male, n(%) | 81(88.04) | 408(89.28) | 0.73 |
| Age, years | 41.00(37.00,45.00) | 42.00(38.00,45.00) | 0.44 |
| BMI, kg/m2 | 26.73(24.77,29.41) | 25.98(23.77,28.36) | 0.02 |
| History, n(%) | |||
| Smoking | 63(68.48) | 300(65.65) | 0.60 |
| Alcohol intake | 35(38.04) | 155(33.92) | 0.45 |
| Hypertension | 45(48.91) | 206(45.08) | 0.50 |
| Diabetes | 20(21.74) | 77(16.85) | 0.26 |
| Previous Stroke | 5(5.43) | 12(2.63) | 0.16 |
| STEMI, n(%) | 71(77.17) | 394(86.21) | 0.03 |
| Systolic pressure, mmHg | 135.00(121.25,150.00) | 132.00(120.00,145.00) | 0.23 |
| Diastolic pressure, mmHg | 82.00(73.00,94.75) | 80.00(70.00,92.50) | 0.81 |
| Heart rate, bpm | 80.00(71.00,90.00) | 76.00(69.00,86.00) | 0.12 |
| Biochemical haracteristics | |||
| WBC,10^9/L | 10.74(8.81,13.33) | 10.58(8.91,12.68) | 0.40 |
| CRP, mg/L | 6.23(3.23,10.81) | 5.36(2.06,12.87) | 0.41 |
| ALT, U/L | 43.40(29.05,67.00) | 47.40(32.20,72.80) | 0.22 |
| Cr, umol/L | 75.50(64.00,89.50) | 74.00(64.50,82.00) | 0.29 |
| FBG, mmol/L | 6.29(5.07,9.26) | 5.86(5.25,7.97) | 0.42 |
| TC, mmol/L | 4.94(4.36,5.67) | 4.78(4.14,5.44) | 0.09 |
| TG, mmol/L | 2.11(1.49,3.13) | 2.05(1.46,2.81) | 0.20 |
| HDL, mmol/L | 0.92(0.77,1.06) | 0.94(0.81,1.10) | 0.12 |
| LDL, mmol/L | 3.37(2.70,3.92) | 3.20(2.53,3.79) | 0.28 |
| TC/HDL | 5.42(4.38,6.49) | 4.98(4.18,6.15) | 0.03 |
| ApoA1,g/L | 1.12(1.01,1.26) | 1.13(1.00,1.27) | 0.90 |
| ApoB, g/L | 1.22(1.05,1.38) | 1.13(0.93,1.32) | 0.05 |
| ApoA1/ApoB | 0.94(0.80,1.15) | 1.02(0.82,1.22) | 0.05 |
| cTnT, ng/ml | 2.95(1.28,5.55) | 2.90(1.15,5.57) | 0.75 |
| BNP, pg/ml | 343.47(75.39,909.13) | 232.79(78.52,650.00) | 0.27 |
| D-Dimer, ug/ml | 0.30(0.21,0.57) | 0.29(0.22,0.48) | 0.96 |
| Fg, g/L | 3.44(3.07,3.85) | 3.24(2.84,3.84) | 0.04 |
| Echocardiography | |||
| LVEF,% | 50.00(42.00,55.75) | 51.00(46.00,56.00) | 0.03 |
| PAP, mmHg | 30.00(30.00,30.00) | 30.00(30.00,30.00) | <0.01 |
| Gensini Score, points | 55.00(32.00,83.75) | 48.00(32.00,80.00) | 0.14 |
| High GS group, n(%) | 19(20.65) | 45(9.85) | <0.01 |
| TVD, n(%) | 39(42.39) | 120(26.26) | <0.01 |
| PCSK6 genotypes | <0.01 | ||
| CC | 22(23.91) | 54(11.82) | |
| CA | 37(40.22) | 216(47.26) | |
| AA | 33(35.87) | 187(40.92) | |
| Additive model | |||
| Dominant model (AA + CAvsCC) | 70(76.09) | 403(88.18) | <0.01 |
| Recessive model (AAvsCA + CC) | 33(35.87) | 187(40.92) | 0.37 |
| Medication during follow-up, n(%) | |||
| DAPT | 91(98.91) | 455(99.56) | 0.44 |
| Statin | 89(96.74) | 451(98.69) | 0.18 |
| Anticoagulant | 82(89.13) | 417(91.25) | 0.52 |
| ACEI/ARB | 74(80.43) | 358(78.34) | 0.65 |
| Beta-blocker | 80(86.96) | 396(86.65) | 0.94 |
| HWE X^2 | 3.11 | 0.49 | |
| HWE P | 0.08 | 0.48 |
PCSK6 proprotein convertase subtilisin/kexin type 6; STEMI ST-segment elevation myocardial infarction; BMI body mass index; WBC white blood cell; ALT alanine transaminase; CRP C-reactive protein; Cr creatinine; FBG fasting blood glucose; TC total cholesterol; TG Triglyceride; HDL high-density lipoprotein; LDL low-density lipoprotein; Apo apolipoprotein; cTnT cardiac troponin T; BNP B type natriuretic peptide; Fg Fibrinogen; LVEF left ventricular ejection fraction; PAP pulmonary artery pressure; TVD triple vessel diseases; DAPT dual antiplatelet therapy; ACEI angiotensin-converting enzyme inhibitors; ARB angiotensin II receptor blockers; MACEs major adverse cardiovascular event; HWE Hardy–Weinberg law of equilibrium
Data are present as mean (inter-quartile range) or number (%)
56 patients (9.26%) were lost to follow-up in total. The baseline information of these 56 patients and overall patients were compared, and we discovered that the clinical markers did not differ between the two cohorts (P>0.05) (Table S3). Therefore, this study excluded these 56 patients from the association analysis between the PCSK6 rs1531817 genotype and MACEs. The follow-up of 92 (16.8%) patients with MACEs revealed five cardiac deaths (0.9%), four nonfatal strokes (0.7%), 55 readmissions for ischaemic cardiovascular events (10.0%), 27 target lesion revascularization (4.9%) and 25 hospitalizations for heart failure (4.6%). Each clinical index was compared between the MACEs and non-MACEs groups; the two groups differed considerably on the basis of body mass index (BMI), STEMI, TC/HDL, Fg, LVD, LVEF, PAP, high GS, TVD, and PCSK6 rs1531817 genotypes (P < 0.05) (Table 3).
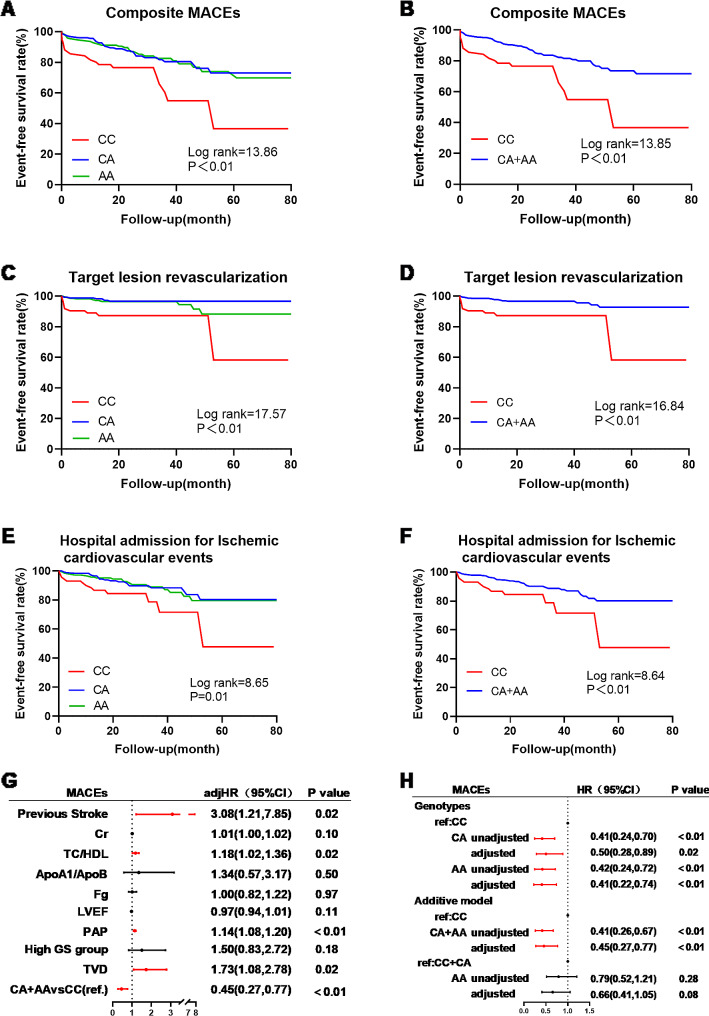
Kaplan-Meier curve analysis showed that composite MACEs, target lesion revascularization, and readmission for ischaemic cardiovascular events were significantly associated with the PCSK6 rs1531817 genotype. Patients with the CC genotype had shorter event-free survival than those with the CA + AA genotype (P < 0.05) (Fig. 4A-F).
Fig. 4.
Correlation of the PCSK6 rs1531817 polymorphism with MACEs. A, B Kaplan–Meier curve for composite MACEs based on the genotype of PCSK6 rs1531817. C, D Kaplan–Meier curve for target lesion revascularization based on the genotype of PCSK6 rs1531817. E, F Kaplan–Meier curve for hospital admission for ischaemic cardiovascular events based on the genotype of PCSK6 rs1531817. G Multivariate Cox regression analysis of MACEs in PMI patients. H Cox regression of the PCSK6 rs1531817 genotype for the occurrence of MACEs. Multivariate Cox regression was adjusted for previous stroke, Cr, TC/HDL, ApoA1/ApoB, Fg, LVEF, PAP, high GS group, and TVD. HR hazard ratio; CI confidence interval
Univariate Cox regression revealed that history of stroke, creatinine (Cr), left ventricular ejection fraction(LVEF), TG, HDL, TC/HDL, ApoB, ApoA1/ApoB, Fg, pulmonary artery pressure (PAP), high GS group, TVD, and PCSK6 rs1531817 genotypes were associated with the occurrence of MACEs (Table S4). Since TC/HDL was significantly related to TG (r = 0.58, P < 0.01) and HDL (r=-0.65, P < 0.01), ApoA1/ApoB was related to ApoB, TG, HDL, and ApoB were not included in the multivariate Cox regression. After adjusting for the above statistically significant variables, multivariate Cox regression analysis revealed an independent association between stroke, TC/HDL, PAP, TVD, and PCSK6 rs1531817 genotype and the occurrence of MACEs (Fig. 4G). Among them, the CA, AA, and CA + AA genotype (adjHR = 0.45, 95% CI: 0.27–0.77, P < 0.01) were protective indicators against MACEs (Fig. 4H).
The mediating role of lipid indicators in the association of PCSK6 rs1531817 genotypes with coronary stenosis and MACEs
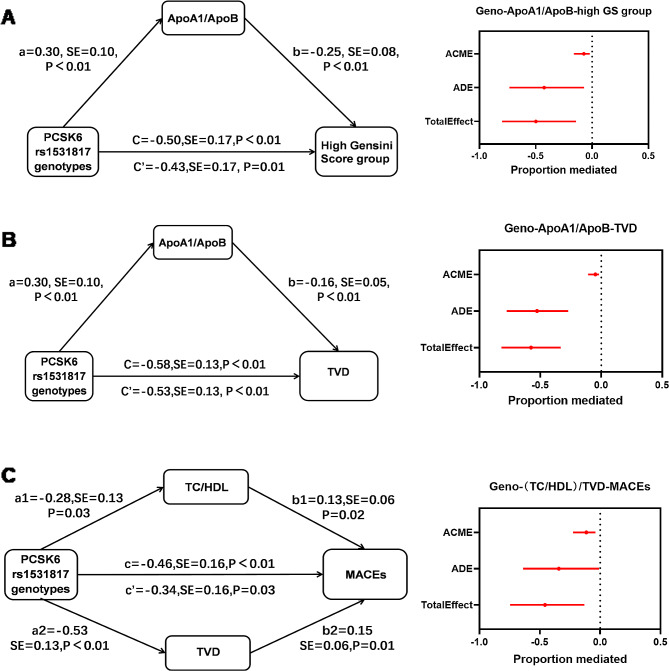
The above results suggested that the PCSK6 rs1531817 genotype was associated with ApoA1/ApoB, high GS and TVD (Fig. 2, Fig.S1, and Fig.S3), and that ApoA1/ApoB was associated with high GS and TVD (Fig.S1 and Fig. 3), which meets the basic requirements of mediation analysis. We used the PCSK6 rs1531817 genotypes (CA + AA vs. CC) as the independent variable, ApoA1/ApoB as the mediator variable, and high GS and TVD as the dependent variables for mediation analysis. The results showed that ApoA1/ApoB partially mediated the association between PCSK6 rs1531817 genotypes (CA + AA vs. CC) and high GS (β=-0.07, 95% CI: -0.16, -0.02), 14.6% of which was mediated; otherwise, we found that ApoA1/ApoB partially mediated the association between the PCSK6 rs1531817 genotypes (CA + AA vs. CC) and TVD in patients with PMI (β=-0.05, 95% CI: -0.11, -0.02), representing an 8.3% difference (Table S5, Fig. 5A, B).
Fig. 5.
The mediating effect of lipid indicators associated with the PCSK6 rs1531817 polymorphism, coronary stenosis, and MACEs. A Association between the PCSK6 rs1531817 genotype and high GS mediated by ApoA1/ApoB. B Association between the PCSK6 rs1531817 genotype and TVD mediated by ApoA1/ApoB. C Association between the PCSK6 rs1531817 genotype and MACEs mediated by TC/HDL and TVD. ADE average direct effect; ACME average causal mediation effect; SE standard error
The PCSK6 rs1531817 genotype was correlated with TC/HDL, TVD, and MACEs (Figs. 2 and 4G), and TC/HDL and TVD were associated with the occurrence of MACEs (Fig. 4G), which meets the basic requirements of parallel mediation analysis. We used the PCSK6 rs1531817 genotype (CA + AA vs. CC) as the independent variable, TC/HDL and TVD as the mediator variables, and MACEs as the dependent variable for parallel mediation analysis. The results showed that TC/HDL (β=-0.04, 95% CI: -0.11, -0.01) and TVD (β=-0.08, 95% CI: -0.17, -0.02) partially and in parallel mediated the association between the PCSK6 rs1531817 genotypes (CA + AA vs. CC), and the MACEs-mediated proportions were 8.1% and 17.1%, respectively (Table S5, Fig. 5C).
Discussion
This prospective cohort study revealed for the first time that the PCSK6 rs1531817 polymorphism was significantly associated with severe coronary stenosis and long-term prognosis. The regression model showed that carrying the PCSK6 rs1531817 mutant A allele was an independent protective factor against severe coronary stenosis and long-term prognosis in PMI patients. To explore the potential underlying mechanisms, the study performed mediating effect analyses, which revealed that ApoA1/ApoB partially mediated the association between the PCSK6 rs1531817 polymorphism and coronary artery stenosis severity, and TC/HDL and TVD partially and in parallel mediated the association between the PCSK6 rs1531817 polymorphism and MACEs.
Endothelial lipase (EL) and lipoprotein lipase (LPL) cleavage are induced by PCSK6 in order to reduce the activity of these enzymes [25, 26]. According to previous studies, plasma ApoB expression is significantly greater in mice deficient in EL than in normal mice, suggesting that EL activity is negatively correlated with ApoB and is not correlated with ApoA [27]. EL levels have also been found to negatively correlate with serum ApoB levels in clinical studies [28]. HDL levels were elevated by LPL activation in rats given the LPL-inducing reagent (NO-1886), indicating a positive correlation between LPL activity and serum HDL concentration [29]; EL plays a crucial role in HDL catabolism by hydrolyzing phospholipids in HDL [30, 31]. The above studies suggest that PCSK6 mediates EL and LPL inactivation to regulate HDL content. In individuals with metabolic syndrome, EL serum levels and TC concentrations were found to be negatively correlated by Klobucar et al. [28]. Decreased PCSK6 expression in patients with PCSK6 rs1531817 mutations in healthy aortas (from the public GTEx resource, n = 197) was shown in a study by Rykaczewska et al. [10]. The differences in the ApoA1/ApoB and TC/HDL ratios among the different PCSK6 genotypes in this study were possibly due to decreased PCSK6 expression caused by the A allele of the PCSK6 rs1531817 mutation, which reduces the cleavage of LPL and EL and increases the levels of LPL and EL, it consequently results in lower TC/HDL levels and higher ApoA1/ApoB levels.
In this study, in addition to the PCSK6 rs1531817 polymorphism, the ApoA1/ApoB polymorphism was identified as an independent protective factor against TVD, TC/HDL was a risk factor for MACEs. ApoA1 reflects the sum of anti-atherosclerotic HDL particulates, and ApoB reflects the sum of atherogenic lipid particulates. In a prospective cohort study, the relation of lipid indices to MI was analyzed in 40,430 patients with atherosclerosis treated with statins at 2.5 years follow-up and in 389,529 patients in the primary prevention group who did not receive lipid-lowering therapy at 11.1 years follow-up. The findings demonstrated that the independent factor influencing the likelihood of having lipid-associated MI was ApoB [32]. In peritoneal dialysis patients, Tianlei Chen et al. analyzed the relation of ApoA1/ApoB to acute coronary syndrome, and the results indicated that ApoA1/ApoB has critical clinical value in the progression of atherosclerotic disease [33]. Fuxue Deng et al. studied 320 atherosclerotic cardiovascular disease (ASCVD) patients treated with PCI to analyze the association between ApoB/ApoA1 and coronary vulnerable plaque; plaque rupture, erosion, and thrombosis were found to be independently predicted by the ApoB/ApoA1 ratio in individuals with ASCVD [34]. The above studies showed that ApoA1/ApoB could more accurately reflect the impact of dyslipidemia on the severity of CHD than traditional lipid indicators, which is in line with the findings of this research. The TC/HDL ratio is a new indicator that has been proposed to predict CHD in recent years. TC and HDL are representative lipid indicators that promote and inhibit atherosclerosis. Calling et al. followed 6,147 women aged 50–59 years for a median time of 17 years and found that the TC/HDL ratio was a robust predictive indicator for AMI [35]. However, TC/HDL was not found to be associated with coronary stenosis in this study. In a survey of 4,957 coronary artery disease individuals, Elshazly et al. assessed the correlation of TC/HDL and the 2-year incidence of MACEs and found that a greater TC/HDL ratio was linked to a worse prognosis [36]; this finding is consistent with this study.
Atherosclerosis is significant for myocardial infarction [37, 38]. PCSK9 serves as a danger marker for atherosclerosis. Its classical pathway regulates circulating LDL levels through degradation of the LDL receptor, the elevated plasma LDL is a risk factor for atherosclerosis [39, 40]. PCSK6, as a member of the PCSK family, has polymorphisms that are associated with atherosclerosis progression [10]. The PCSK6 rs1531817 mutation A allele was shown to be a protective allele for severe coronary stenosis in patients with PMI in this study. Mediation effect analysis showed that ApoA1/ApoB partially mediated the association of PCSK6 genotypes with high GS and TVD. The mutation at PCSK6 rs1531817 increased the ApoA1/ApoB ratio, which in turn protected against severe coronary stenosis. The PCSK6 rs1531817 A allele is a protective allele for long-term prognosis; to date, there are no previous studies in this area. The mediation effect analysis in this study showed that TC/HDL and TVD played a partially parallel mediating role in the association between the PCSK6 genotypes and the occurrence of MACEs. Mutation of PCSK6 rs1531817 decreased the TC/HDL ratio and the incidence of TVD, thereby improving the long-term prognosis.
Study strengths and limitations
Previous studies have mostly investigated the prediction of coronary stenosis and prognostic risk in PMI patients from the clinical perspective. The present study is the first to analyse the relationships among PCSK6 polymorphisms, coronary stenosis, and prognosis in patients with PMI from a genetic point of view. This study found that PCSK6 polymorphisms may play an anti-atherosclerotic and improve prognostic role by affecting lipid levels. which is crucial for guiding the prevention of severe coronary stenosis, better prognostic assessment, and more comprehensive treatment in PMI patients.
Nevertheless, the study has some limitations. First, this prospective cohort research was conducted at a single site and needed to be further validated by multiple centres, more patients, longer follow-up periods, and even different ethnicities and geographical regions. Second, the serum PCSK6 concentration was not detected, and the effect of genotypic mutations in PMI patients on the effect of the PCSK6 concentration and concentration on coronary stenosis and prognosis was not investigated. Finally, this study revealed that the PCSK6 genotype was associated with coronary stenosis and prognosis. Mediated effect analysis revealed that lipid indices play a partial mediating role, and further exploration of the potential mechanisms underlying the effects of PCSK6 on coronary stenosis and prognosis is crucial. Additionally, to confirm whether PCSK6 may serve as an interventional target for preventing severe coronary stenosis and improving long-term prognosis, further clinical and basic studies are required.
Conclusion
This study’s results indicate that coronary stenosis and the prognosis of PMI patients are related to the PCSK6 rs1531817 polymorphism, the presence of the PCSK6 rs1531817 mutant A allele is an independent factor associated with the coronary stenosis and prognosis. PMI patients with the PCSK6 CA + AA genotype have milder coronary stenosis, partially because they have higher ApoA1/ApoB levels; patients with the PCSK6 CA + AA genotype have a better long-term prognosis, partially because they have lower TC/HDL levels and are less likely to have TVD. These results may provide a new perspective on clinical therapeutic strategy for anti-atherosclerosis and improved prognosis in PMI patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Each participant’s data and test results are gratefully acknowledged by the writers, and they are grateful for all of the staff’s outstanding work during specimen collection and clinical data acquisition. The researchers also thank the Chest Hospital Biospecimen Bank for collecting, processing, and storing the blood samples.
Abbreviations
- PCSK
Proprotein convertase subtilisin/kexin
- SNP
Single nucleotide polymorphism
- PMI
Premature myocardial infarction
- GS
Gensini score
- MACEs
Major adverse cardiovascular events
- cIMT
Carotid intima-media thickness
- TLR
Target lesion revascularization
- HWE
Hardy‒Weinberg equilibrium
- WBC
White blood cell
- ALT
Alanine trans aminase
- TC
Total cholesterol
- HDL
High-density lipoprotein
- Apo
Apolipoprotein
- cTnT
Cardiac troponin T
- BNP
B-type natriuretic peptide
- Fg
Fibrinogen
- PAP
Pulmonary artery pressure
- SVD
Single vessel disease
- DVD
Double vessel disease
- TVD
Triple vessel disease
- LVEF
Left ventricular ejection fraction
- EL
Endothelial lipase
- LPL
Lipoprotein lipase
- OR
Odds ratio
- HR
Hazard ratio
- CI
Confidence intervals
Author contributions
SL, Y-HW, A-RJ, M-ML, and LJY participated in data collection and analysis; JM and XZ were responsible for genetic testing; JG, YL, LS, and J-XW wrote the manuscript; and LS designed and supervised the research. Each author checked and authorized the final version.
Funding
The work was got sponsored with the Key Project of Scientific and Technological Support Plan of Tianjin in 2020 (No.20YFZCSY00820), the Key Disciplines of Tianjin Health Science and Technology Project (No.TJWJ2022XK032), and the Key Projects of Tianjin Natural Science Foundation (No. 22JCZDJC00130).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The Ethical Commission of the Tianjin Chest Hospital authorized this study. This research received consent from each patient.
Consent for publication
Each participant provided consent for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yin Liu, Email: liuyin2088@163.com.
Jing Gao, Email: gaojing2088@163.com.
References
- 1.Arora S, Stouffer GA, Kucharska-Newton AM, Qamar A, Vaduganathan M, Pandey A, et al. Twenty Year trends and Sex differences in young adults hospitalized with Acute myocardial infarction. Circulation. 2019;139(8):1047–56. 10.1161/CIRCULATIONAHA.118.037137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu WY, Berman AN, Biery DW, Blankstein R. Recent trends in acute myocardial infarction among the young. Curr Opin Cardiol. 2020;35(5):524–30. 10.1097/HCO.0000000000000781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rallidis LS, Xenogiannis I, Brilakis ES, Bhatt DL, Causes. Angiographic characteristics, and management of premature myocardial infarction: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79(24):2431–49. 10.1016/j.jacc.2022.04.015 [DOI] [PubMed] [Google Scholar]
- 4.Kayikcioglu M, Ozkan HS, Yagmur B. Premature myocardial infarction: a rising threat. Balkan Med J. 2022;39(2):83–95. 10.4274/balkanmedj.galenos.2022-2-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jortveit J, Pripp AH, Langorgen J, Halvorsen S. Incidence, risk factors and outcome of young patients with myocardial infarction. Heart. 2020;106(18):1420–6. 10.1136/heartjnl-2019-316067 [DOI] [PubMed] [Google Scholar]
- 6.Gao J, Yang YN, Cui Z, Feng SY, Ma J, Li CP, et al. Pcsk9 is associated with severity of coronary artery lesions in male patients with premature myocardial infarction. Lipids Health Dis. 2021;20(1):56. 10.1186/s12944-021-01478-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao J, Wang Y, Yang YN, Wu XY, Cui Y, Zou ZH, et al. Impact of metabolic syndrome and its components on clinical severity and long-term prognosis in patients with premature myocardial infarction. Front Endocrinol (Lausanne). 2022;13:920470. 10.3389/fendo.2022.920470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer M, Broeckel U, Holmer S, Baessler A, Hengstenberg C, Mayer B, et al. Distinct heritable patterns of angiographic coronary artery disease in families with myocardial infarction. Circulation. 2005;111(7):855–62. 10.1161/01.CIR.0000155611.41961.BB [DOI] [PubMed] [Google Scholar]
- 9.Wu Q, Chen S. Proprotein Convertase Subtilisin/Kexin 6 in Cardiovascular Biology and Disease. Int J Mol Sci 2022; 23(21). [DOI] [PMC free article] [PubMed]
- 10.Rykaczewska U, Suur BE, Rohl S, Razuvaev A, Lengquist M, Sabater-Lleal M, et al. PCSK6 is a key protease in the control of smooth muscle cell function in vascular remodeling. Circ Res. 2020;126(5):571–85. 10.1161/CIRCRESAHA.119.316063 [DOI] [PubMed] [Google Scholar]
- 11.Testa G, Staurenghi E, Giannelli S, Sottero B, Gargiulo S, Poli G, et al. Up-regulation of PCSK6 by lipid oxidation products: a possible role in atherosclerosis. Biochimie. 2021;181:191–203. 10.1016/j.biochi.2020.12.012 [DOI] [PubMed] [Google Scholar]
- 12.Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419. 10.1126/science.1260419 [DOI] [PubMed] [Google Scholar]
- 13.Gulati R, Behfar A, Narula J, Kanwar A, Lerman A, Cooper L, et al. Acute myocardial infarction in Young individuals. Mayo Clin Proc. 2020;95(1):136–56. 10.1016/j.mayocp.2019.05.001 [DOI] [PubMed] [Google Scholar]
- 14.Tudurachi BS, Anghel L, Tudurachi A, Sascau RA, Statescu C. Assessment of Inflammatory Hematological Ratios (NLR, PLR, MLR, LMR and Monocyte/HDL-Cholesterol Ratio) in Acute Myocardial Infarction and Particularities in Young Patients. Int J Mol Sci. 2023; 24(18). [DOI] [PMC free article] [PubMed]
- 15.Krittanawong C, Khawaja M, Tamis-Holland JE, Girotra S, Rao SV. Acute myocardial infarction: etiologies and mimickers in Young patients. J Am Heart Assoc. 2023;12(18):e029971. 10.1161/JAHA.123.029971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dreyer RP, Sciria C, Spatz ES, Safdar B, D’Onofrio G, Krumholz HM. Young Women with Acute myocardial infarction: current perspectives. Circ Cardiovasc Qual Outcomes. 2017; 10(2). [DOI] [PMC free article] [PubMed]
- 17.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth Universal Definition of Myocardial Infarction (2018). J Am Coll Cardiol. 2018;72(18):2231–64. 10.1016/j.jacc.2018.08.1038 [DOI] [PubMed] [Google Scholar]
- 18.Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. 1983;51(3):606. 10.1016/S0002-9149(83)80105-2 [DOI] [PubMed] [Google Scholar]
- 19.Rampidis GP, Benetos G, Benz DC, Giannopoulos AA, Buechel RR. A guide for Gensini score calculation. Atherosclerosis. 2019;287:181–3. 10.1016/j.atherosclerosis.2019.05.012 [DOI] [PubMed] [Google Scholar]
- 20.Xu Y, Qin Z, Gao J, Yang Y, Lu Y, Yu F et al. Combination of Neutrophil Count and Gensini score as a prognostic marker in patients with ACS and uncontrolled T2DM undergoing PCI. Cardiovasc Innovations Appl. 2023; 8(1).
- 21.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–9. 10.1158/1078-0432.CCR-04-0713 [DOI] [PubMed] [Google Scholar]
- 22.Feng G, Qin G, Zhang T, Chen Z, Zhao Y. Common statistical methods and reporting of results in Medical Research. Cardiovasc Innovations Appl. 2022; 6(3).
- 23.Graffelman J, Weir BS. The transitivity of the hardy-Weinberg law. Forensic Sci Int Genet. 2022;58:102680. 10.1016/j.fsigen.2022.102680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stark AE. Stable populations and Hardy-Weinberg equilibrium. Hereditas. 2023;160(1):19. 10.1186/s41065-023-00284-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi S, Korstanje R. Proprotein convertases in high-density lipoprotein metabolism. Biomark Res. 2013;1(1):27. 10.1186/2050-7771-1-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gauster M, Hrzenjak A, Schick K, Frank S. Endothelial lipase is inactivated upon cleavage by the members of the proprotein convertase family. J Lipid Res. 2005;46(5):977–87. 10.1194/jlr.M400500-JLR200 [DOI] [PubMed] [Google Scholar]
- 27.Brown RJ, Lagor WR, Sankaranaravanan S, Yasuda T, Quertermous T, Rothblat GH, et al. Impact of combined deficiency of hepatic lipase and endothelial lipase on the metabolism of both high-density lipoproteins and apolipoprotein B-containing lipoproteins. Circ Res. 2010;107(3):357–64. 10.1161/CIRCRESAHA.110.219188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klobucar I, Klobucar L, Lechleitner M, Trbusic M, Pregartner G, Berghold A et al. Associations between endothelial lipase and apolipoprotein B-Containing lipoproteins Differ in healthy volunteers and metabolic syndrome patients. Int J Mol Sci 2023; 24(13). [DOI] [PMC free article] [PubMed]
- 29.Kusunoki M, Tsutsumi K, Sato D, Nakamura A, Habu S, Mori Y, et al. Activation of lipoprotein lipase increases serum high density lipoprotein 2 cholesterol and enlarges high density lipoprotein 2 particles in rats. Eur J Pharmacol. 2011;668(1–2):337–9. 10.1016/j.ejphar.2011.06.040 [DOI] [PubMed] [Google Scholar]
- 30.Pisciotta L, Ossoli A, Ronca A, Garuti A, Fresa R, Favari E, et al. Plasma HDL pattern, cholesterol efflux and cholesterol loading capacity of serum in carriers of a novel missense variant (Gly176Trp) of endothelial lipase. J Clin Lipidol. 2022;16(5):694–703. 10.1016/j.jacl.2022.08.002 [DOI] [PubMed] [Google Scholar]
- 31.Khetarpal SA, Vitali C, Levin MG, Klarin D, Park J, Pampana A, et al. Endothelial lipase mediates efficient lipolysis of triglyceride-rich lipoproteins. PLoS Genet. 2021;17(9):e1009802. 10.1371/journal.pgen.1009802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marston NA, Giugliano RP, Melloni GEM, Park JG, Morrill V, Blazing MA, et al. Association of Apolipoprotein B-Containing Lipoproteins and risk of myocardial infarction in individuals with and without atherosclerosis: distinguishing between particle concentration, type, and content. JAMA Cardiol. 2022;7(3):250–6. 10.1001/jamacardio.2021.5083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen T, Yang M. Apo A1/Apo B ratio and acute coronary syndrome among peritoneal dialysis patients. Ren Fail. 2021;43(1):737–42. 10.1080/0886022X.2021.1918556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng F, Li D, Lei L, Yang Q, Li Q, Wang H, et al. Association between apolipoprotein B/A1 ratio and coronary plaque vulnerability in patients with atherosclerotic cardiovascular disease: an intravascular optical coherence tomography study. Cardiovasc Diabetol. 2021;20(1):188. 10.1186/s12933-021-01381-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calling S, Johansson SE, Wolff M, Sundquist J, Sundquist K. The ratio of total cholesterol to high density lipoprotein cholesterol and myocardial infarction in women’s health in the Lund area (WHILA): a 17-year follow-up cohort study. BMC Cardiovasc Disord. 2019;19(1):239. 10.1186/s12872-019-1228-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elshazly MB, Nicholls SJ, Nissen SE, St John J, Martin SS, Jones SR, et al. Implications of total to high-density lipoprotein cholesterol ratio discordance with alternative lipid parameters for Coronary Atheroma Progression and Cardiovascular events. Am J Cardiol. 2016;118(5):647–55. 10.1016/j.amjcard.2016.06.021 [DOI] [PubMed] [Google Scholar]
- 37.Al-Zakwani I, Al Siyabi E, Alrawahi N, Al-Mulla A, Alnaeemi A, Shehab A, et al. Association between Peripheral artery disease and major adverse Cardiovascular events in patients with Acute Coronary Syndrome: findings from the Gulf COAST Registry. Med Princ Pract. 2019;28(5):410–7. 10.1159/000497790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li C, Zong W, Zhang M, Tu Y, Zhou Q, Ni M, et al. Increased ratio of circulating T-Helper 1 to T-Helper 2 cells and severity of coronary artery disease in patients with Acute myocardial infarction: a prospective observational study. Med Sci Monit. 2019;25:6034–42. 10.12659/MSM.913891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang Y, Li SL, Hu JH, Sun KJ, Liu LL, Xu DY. Research progress on alternative non-classical mechanisms of PCSK9 in atherosclerosis in patients with and without diabetes. Cardiovasc Diabetol. 2020;19(1):33. 10.1186/s12933-020-01009-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Punch E, Klein J, Diaba-Nuhoho P, Morawietz H, Garelnabi M. Effects of PCSK9 targeting: alleviating oxidation, inflammation, and atherosclerosis. J Am Heart Assoc. 2022;11(3):e023328. 10.1161/JAHA.121.023328 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.