Abstract
Human herpesvirus 8 (HHV-8) infection is associated with Kaposi’s sarcoma, primary effusion lymphoma (PEL), and multicentric Castleman’s disease. In this study, we used monoclonal antibodies (MAbs) directed against HHV-8 lytic cycle-associated proteins encoded by open reading frame (ORF) 59 (nuclear PF-8 protein) and ORF K8.1 (viral envelope glycoprotein K8.1 [gpK8.1]) to investigate HHV-8 lytic infection in single cells. Lytically infected cells were labeled with MAbs, stained with fluorescently conjugated secondary Abs, and analyzed by flow cytometry. A 3-day stimulation of HHV-8-positive PEL cell lines (BCBL-1 and BC-3) with 12-O-tetradecanoylphorbol-13-acetate (30 nM) or n-butyric acid (0.3 mM) maximized the expression of lytic-phase viral proteins and minimized cell toxicity. The absolute number of expressing cells was inducer and cell line dependent. Expression of PF-8 occurred earlier and more frequently (in up to 20% of cells) than did expression of gpK8.1. A subset of PF-8 positive cells (25%) co-expressed gpK8.1, representing the majority of gpK8.1 expressing cells. Acyclovir, foscarnet, cidofovir, and PMEA reduced the number of cells expressing gpK8.1, but not the number expressing the nonstructural early lytic gene product PF-8. By contrast, alpha interferon (IFN-α) and IFN-β reduced expression of both PF-8 and gpK8.1, implying an overall inhibitory effect on viral gene transcription or translation. In summary, we have characterized and quantified HHV-8 lytic infection in single cells by dual measurement of early- and late-lytic-cycle HHV-8 protein expression. This technique should prove useful for screening of possible antiherpesvirus agents and for detailed phenotypic characterization of HHV-8-infected cells in vitro and in patients with HHV-8-associated diseases.
Human herpesvirus 8 (HHV-8) infection is associated with Kaposi’s sarcoma (KS), primary effusion lymphoma (PEL), and multicentric Castleman’s disease (5, 10, 26, 37). HHV-8 is present in both diseased tissue and peripheral blood mononuclear cells (PBMC) of patients with these conditions (4), a finding which strengthens the possibility that HHV-8 is involved in disease pathogenesis. Unfortunately, detailed characterization and quantification of single cells infected with HHV-8 has been limited and technically difficult. Recently, monoclonal antibodies (MAbs) have been developed against lytic-cycle viral proteins encoded by open reading frame (ORF) 59 and ORF K8.1 (8, 39). ORF 59 protein is a putative viral accessory protein (processivity factor 8 [PF-8]) located in the cell nucleus and expressed in the early lytic phase of HHV-8 replication (8, 21). PF-8 binds HHV-8 DNA polymerase, allowing it to synthesize greatly extended DNA products. By contrast, proteins encoded by ORF K8.1 (glycoprotein K8.1 [gpK8.1], also known as gp35–37) are immunogenic glycoproteins expressed in the late lytic phase and are associated with the viral envelope (9, 30). In this study, we used these new MAbs to assess early nonstructural and late structural viral protein expression during active HHV-8 replication in single cells by flow cytometry. This particular technique should prove useful for developing in vitro HHV-8 infection models and for performing detailed phenotypic characterization of HHV-8-infected cells in vivo, thus providing an important new tool in the study of HHV-8-associated diseases.
Interestingly, past use of certain antiherpesvirus drugs (e.g., foscarnet and cidofovir) correlates with a reduced risk of developing KS (11, 15, 19, 24, 33). These and other potential antiherpesvirus agents may eventually prove to be useful in the prevention of HHV-8-associated disease in HHV-8-infected individuals (4). In concordance with these epidemiologic studies, several investigators have shown that certain antiherpesvirus drugs are capable of blocking HHV-8 replication in chronically infected PEL cell lines (14, 20, 22, 28). These previous in vitro studies, however, used laborious methods and relied on semiquantitative differences in band intensities to assess the efficacy of drugs. In this study, we have also applied our system to characterize the ability of potential antiherpesvirus agents to block the induction of HHV-8 replication. This method is rapid and quantitative and therefore can be easily used for future investigations of novel antiherpesvirus agents.
MATERIALS AND METHODS
Cell lines and reagents.
HHV-8-positive and Epstein-Barr virus (EBV)-negative PEL cell lines BCBL-1 (32) (National Institutes of Health AIDS Research and Reagent Program, Rockville, Md.) and BC-3 (2) (American Type Culture Collection, Rockville, Md.), and the EBV-positive (HHV-8 negative) cell line Daudi (American Type Culture Collection) were grown in RPMI 1640 medium (Gibco BRL, Gaithersburg, Md.) containing 20% fetal calf serum (Gibco), 2 mM l-glutamine (Gibco), 100 U of penicillin (Gibco) per ml, 100 μg of streptomycin (Gibco) per ml, 10 mM HEPES (Gibco), and 5 × 10−5 M 2-mercaptoethanol (Sigma Chemical Co., St. Louis, Mo.). Cells were induced with 12-O-tetradecanoylphorbol-13-acetate (TPA) or n-butyric acid (both from Sigma). Phosphonoacetic acid (foscarnet) and acyclovir were also purchased from Sigma. Cidofovir, 9-2-phosphonyl-methoxypropyl-adenine (PMPA), and 9-2-phosphonyl-methoxyethyl-adenine (PMEA; adefovir) were kind gifts from Gilead Sciences, Inc. (Foster City, Calif.). Gamma interferon (IFN-γ) was purchased from R&D Systems Inc. (Minneapolis, Minn.), IFN-α was purchased from Endogen (Woburn, Mass.), and IFN-β was purchased from Gibco. Indinavir, saquinavir, and ritonavir were kindly donated by M. Hiroaki (National Cancer Institute, Bethesda, Md.).
Protein extraction and Western blot analysis.
Cells were incubated for 10 min on ice in a lysis buffer containing 0.75% (vol/vol) Triton X-100, 0.2% dimethyl sulfoxide, 300 mM NaCl, 50 mM Tris-HCl, 10 μg of leupeptin per ml, 20 μg of aprotinin per ml, 25 μM p-nitrophenyl-p′-guanidinobenzoate, and 10 μM KNI (pH 7.4). The preparations were centrifuged for 10 min at 10,000 rpm to remove cellular debris. Supernatants were analyzed for proteins by using the NuPage System (Novex, San Diego, Calif.). Briefly, proteins were separated on 4 to 12% Bis-Tris-HCl-buffered polyacrylamide gels under reducing conditions with the NuPage morpholinepropanesulfonic acid (MOPS) sodium dodecyl sulfate running buffer. The proteins were transferred to nitrocellulose membranes, which were then incubated overnight in a blocking solution (20 mM Tris-HCl, 150 mM NaCl, 0.05% [vol/vol] Tween 20, 1% bovine serum albumin [BSA]) at 4°C. Membranes were washed twice with blocking solution, labeled for 2 h with MAbs (5 μg/ml) directed against the ORF 59-encoded protein PF-8 (MAb 11D1) or against the ORF K8.1-encoded protein gpK8.1 (MAb 19B4), washed again, incubated for 30 min with alkaline phosphatase-conjugated goat anti-mouse immunoglobulin G IgG, and, finally, washed with Tris-buffered saline. Protein bands were visualized with Western Blue substrate for alkaline phosphatase (Promega, Madison, Wis.).
Analysis of HHV-8 protein expression by flow cytometry.
Cells were seeded into culture flasks (2 × 105 cells/ml) and grown for 24 h. Then inducers were added, and the cells were cultured for another 1 to 4 days. For some experiments, antiviral drugs or IFNs were added 2 h before the inducers. After being harvested, the cells were washed with phosphate-buffered saline (PBS), resuspended in PBS containing 0.1% (wt/vol) BSA and 0.05% (wt/vol) NaN3, and transferred to V-bottom 96-well plates (3 × 105 cells/well). Dead cells were stained with Dead Red (Live/Dead kit; Molecular Probes, Eugene, Oreg.) for 20 min at 4°C, and all of the cells were washed four times. All of the cells were fixed and permeabilized with the Cytofix/Cytoperm kit (Pharmingen, San Diego, Calif.) and washed with PermWash buffer (Pharmingen). For analysis of HHV-8 proteins, cells were resuspended for 30 min at 4°C in PermWash buffer containing mouse MAbs (1 μg/ml) directed against PF-8 and/or gpK8.1 (8, 39). Then the cells were washed twice and incubated for 30 min at 4°C with fluorescein isothiocyanate (FITC)-labeled goat anti-mouse IgG (Caltag Laboratories, Burlingame, Calif.), or with phycoerythrin (PE)-labeled goat anti-mouse IgG2b (Caltag) and FITC-labeled goat anti-mouse IgG1 (Caltag) in experiments where cells were double stained for both PF-8 and gpK8.1. The cells were washed three times, resuspended in PBS containing 0.1% (wt/vol) BSA and 0.05% (wt/vol) NaN3, and analyzed for fluorescence on a FACScan flow cytometer (Becton Dickinson, Mountain View, Calif.). Dead cells were identified by Dead Red fluorescence and excluded from all analyses.
Immunofluorescence assay (IFA).
As described above, cells were harvested, transferred to V-bottom 96-well plates, fixed and permeabilized, and labeled with HHV-8 protein-specific Abs followed by FITC- or PE-conjugated secondary Abs. The cells were cytospun onto clean glass slides, mounted in PermaFluor (Lipshaw Immunon, Pittsburgh, Pa.), and examined under a fluorescence microscope.
TEM.
Cells were fixed overnight in neutral buffered 2.5% (vol/vol) glutaraldehyde and then mixed and pelleted in warm agar, which was then cooled overnight to harden. The cells were postfixed in 1% OsO4, dehydrated in graded ethanol-propylene oxide, and embedded in Spurr’s epoxy. Semithin 1-μm plastic sections were stained with methylene blue, azure II, and basic fuchsin for light microscopic selection of blocks for thinning. Thin sections were stained with uranyl acetate and lead citrate and examined on a Zeiss EM10 electron microscope at 60 kV. Immunogold labeling for transmission electron microscopy (TEM) was carried out at 4°C on permeabilized and nonpermeabilized cells in suspension. The cells were labeled with anti-gpK8.1 MAbs followed by goat anti-mouse IgG bound to 40-nm-diameter gold beads.
RESULTS
Identification of HHV-8 lytic proteins PF-8 and gpK8.1 in HHV-8-infected cells by Western analysis, IFA, and TEM.
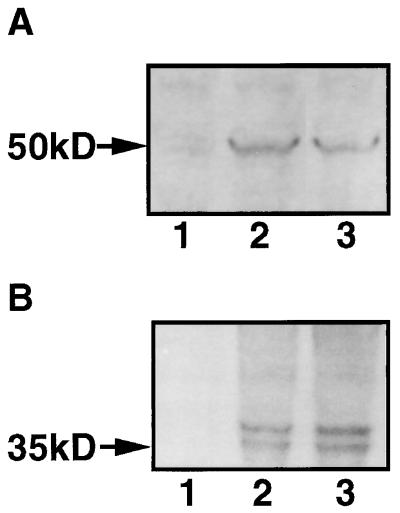
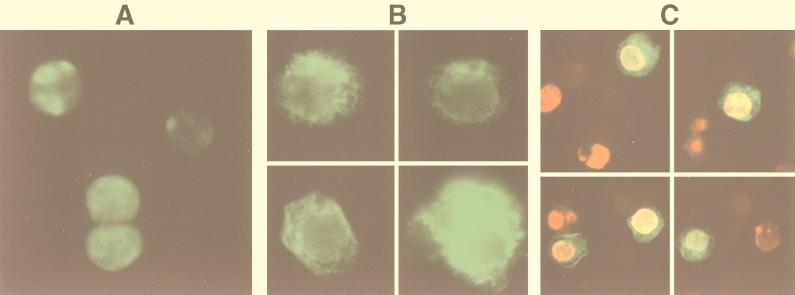
HHV-8 lytic replication was induced in the B-cell lines BCBL-1 and BC-3 by TPA or n-butyric acid. Stimulated B-cell lines were labeled with MAbs against lytic cycle-associated proteins encoded by ORF 59 or ORF K8.1. The 50-kDa ORF 59 protein PF-8 is recognized by MAb 11D1 and localized in the nucleus (8). We confirmed the induction of this 50-kDa protein in 2-day-stimulated BCBL-1 (or BC-3) cells by Western blotting (Fig. 1) and its nuclear localization by IFA (Fig. 2A and C). A new MAb (19B4) directed against the ORF K8.1-encoded protein gpK8.1 identified the two expected bands at 35 and 37 kDa (30, 39) by Western blotting in induced cells (Fig. 1). By IFA, gpK8.1 was expressed in a nonuniform patchy distribution; at times, nuclear and plasma membrane localization was clearly observed (Fig. 2B). Colocalization of PF-8 and gpK8.1 is illustrated by IFA (Fig. 2C). Antigen-expressing cells were rarely found in uninduced cell populations, and no cross-reactivity was detected in HHV-8-negative, EBV-positive B-cell lines (results not shown).
FIG. 1.
Expression of ORF 59 protein (PF-8) and ORF K8.1 protein (gpK8.1) in the BCBL-1 cell line stimulated with TPA or n-butyric acid. Cells were stimulated with 30 nM TPA or 0.3 mM n-butyric acid for 2 days. Cellular protein was isolated, separated by gel electrophoresis, and blotted onto nitrocellulose membranes. PF-8 (A) and gpK8.1 (B) were labeled with protein-specific MAbs, coupled to alkaline phosphatase, and visualized with Western Blue. Lanes: 1, unstimulated cells; 2, TPA-stimulated cells; 3, n-butyric acid stimulated cells. Data shown are representative of five experiments.
FIG. 2.
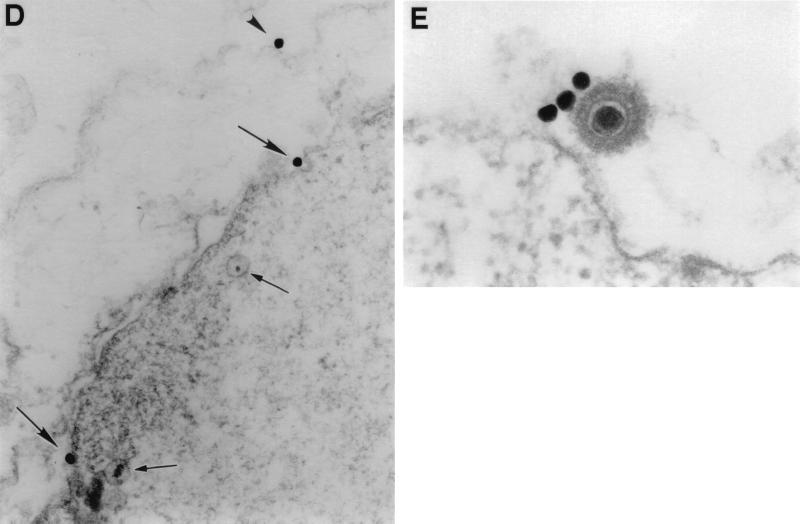
Visualization of PF-8 and gpK8.1 in BCBL-1 cells. Cells stimulated for 2 days with 30 nM TPA were fixed and permeabilized. (A and B) For IFA, cells were labeled with anti-PF-8 or anti-gpK8.1 MAbs followed by FITC-conjugated secondary Abs. The cells were centrifuged onto glass slides and photographed through a fluorescence microscope. Expression of PF-8 (A) and gpK8.1 (B) are shown. Magnification, ×245. (C) For colocalization studies, cells were labeled with both anti-PF-8 and anti-gpK8.1 MAbs followed by PE- and FITC-conjugated isotype-specific secondary Abs. Expression of PF-8 (red), gpK8.1 (green), colocalized PF-8 and gpK8.1 (yellow) are shown. Magnification, ×155. (D and E) For electron microscopy studies, cells were labeled with anti-gpK8.1 MAbs followed by gold-coupled secondary Abs. (D) Three gold beads are seen on a portion of a permeabilized cell, two attached to the nuclear membrane (arrows) and one on the plasma membrane (arrowhead). Note the moderately electron-dense granular material under the nuclear membrane and two nearby nucleoids (small arrows). Magnification, ×52,000. (E) Three 40-nm gold beads are associated with the surface of a typical mature HHV-8 virion. The nearby plasma membrane is free of label. Magnification, ×110,000. Data shown are representative.
Lytically infected cells were distinguished from latently infected cells in TEM by their characteristic nuclear changes (intranuclear inclusions). A band of moderately electron-dense granular material located adjacent to nuclear membranes (which occasionally extended deeper within nuclei) was typically observed (Fig. 2D). This material was detected before the appearance of mature virus particles, and even before classic herpesvirus hexagonal intranuclear nucleoids could be seen. When present, nucleoids were found at the edge of, but not within, this substance (Fig. 2D). Immunogold labeling showed that anti-gpK8.1 MAb localized predominantly to scattered sites on the plasma membrane where no viral morphogenesis was evident and to the surface of mature virions (Fig. 2D and E). gpK8.1 was not detected in the absence intranuclear nucleoids. In permeabilized cells, gold-labeled gpK8.1 could also be seen on the cytoplasmic surface of the nuclear membrane (Fig. 2D) and occasionally on membranes of the endoplasmic reticulum, suggesting sites of protein transit from the nucleus to the plasma membrane (results not shown).
Quantification and characterization of HHV-8 lytically infected cells by flow cytometry.
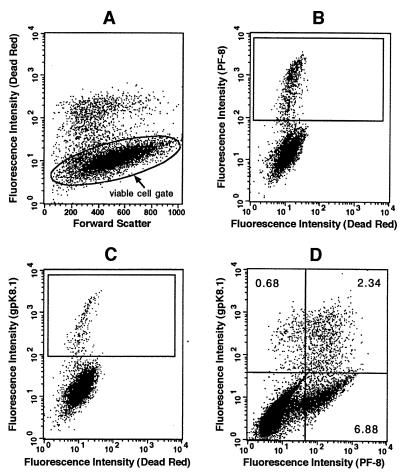
To quantitate HHV-8 lytic infection, the number of cells expressing HHV-8 lytic proteins was determined by flow cytometry. HHV-8-positive B-cell lines were induced with TPA or n-butyric acid at various concentrations. At different time points, uninduced and induced cells were collected, fixed and permeabilized, and labeled with specific MAbs directed against HHV-8 lytic proteins followed by fluorochrome-conjugated secondary Abs. Dead cells were labeled with Dead Red dye before the fixation-permeabilization step. Dead Red-negative cells (i.e., viable cells) were gated and examined for PF-8 or gpK8.1 expression (Fig. 3A to C). Furthermore, coexpression of PF-8 and gpK8.1 is shown after double labeling of 2-day-TPA-stimulated cells with the MAbs against PF-8 and gpK8.1 followed by isotype-specific secondary antibodies (anti-mouse IgG1-FITC for gpK8.1 MAb, anti-mouse IgG2b-PE for PF-8 MAb). Most of the cells expressing HHV-8 proteins expressed only PF-8 (Fig. 3D). A subset (≤25%) of PF-8-expressing cells coexpressed gpK8.1. This particular subset, however, contained the majority (≥75%) of the cells expressing gpK8.1.
FIG. 3.
Detection of PF-8 and/or gpK8.1 in single cells by flow cytometry. (A to C) TPA-stimulated BCBL-1 cells were labeled with Dead Red, washed, fixed, and permeabilized. The cells were labeled with anti-PF-8 or anti-gpK8.1 MAb followed by FITC-conjugated secondary Abs. They were examined for fluorescence by flow cytometry. Dead Red-negative cells (representing the viable cell population) were gated (A) and examined for PF-8 (B) or gpK8.1 (C) expression. The numbers of cells in the defined regions were used for quantification and are shown in further experiments. No PF-8- or gpK8.1-expressing cells were observed when isotype control antibodies or HHV-8-negative cell lines were used (results not shown). (D) For coexpression experiments, cells were double labeled with anti-PF-8 and anti-gpK8.1 MAbs followed by PE- and FITC-conjugated isotype-specific secondary Abs. The numbers of viable cells expressing only PF-8, only gpK8.1, or both PF-8 and gpK8.1 are noted in the relevant quadrants. Data shown are representative of numerous experiments.
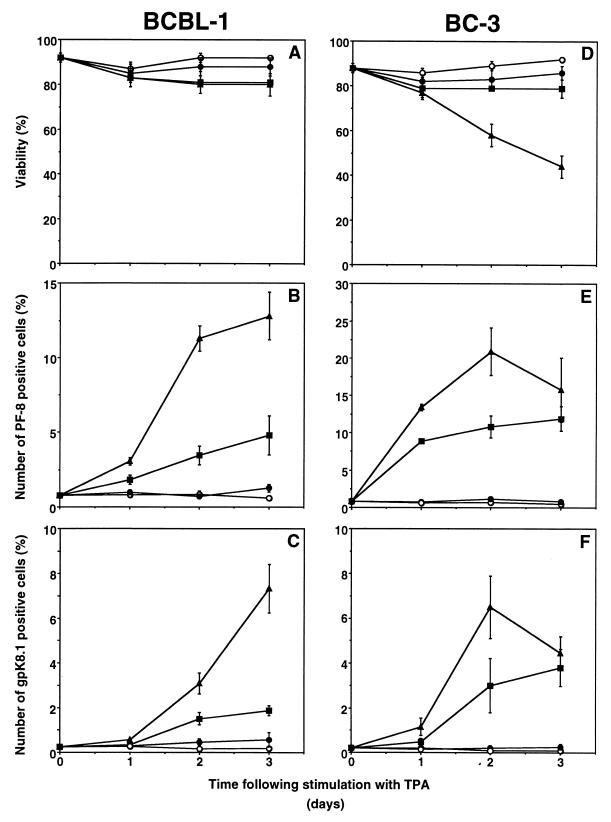
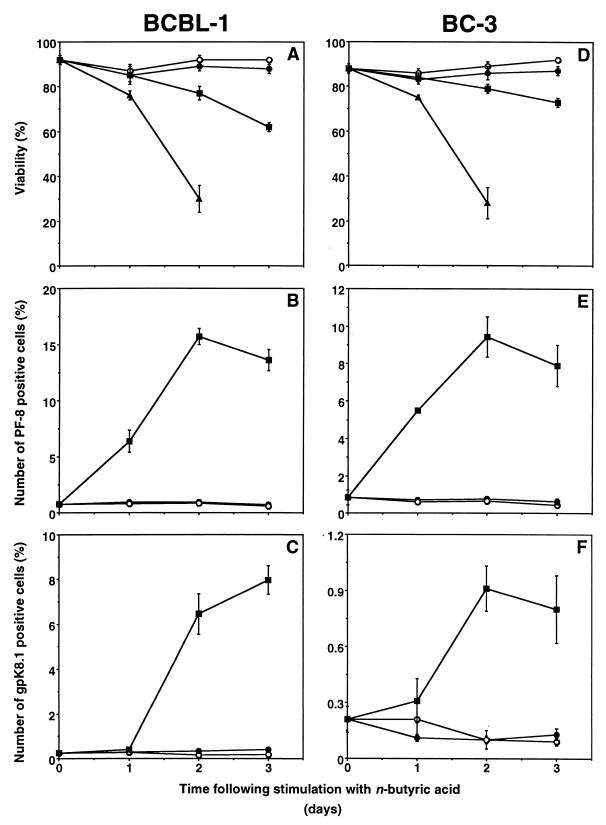
In BCBL-1 cells, TPA induced PF-8 within 24 h followed by gpK8.1 expression, to reach a maximum in 3 days of 12% of cells expressing PF-8 and 7% expressing gpK8.1 (Fig. 4A to C). In general, higher doses of TPA (>30 nM) or longer exposure to TPA (4 days) increased toxicity but not the number of positive cells (results not shown). TPA was more toxic and PF-8 was more rapidly and more frequently expressed (up to 20%) in BC-3 cells than in BCBL-1 cells, whereas gpK8.1 expression was similar (Fig. 4D to F). Compared to TPA, n-butyric acid (0.3 mM) also induced PF-8 within 24 h to reach a maximum in 3 days of 15% (BCBL-1) or 10% (BC-3) (Fig. 5). Expression of gpK8.1 started between 24 and 48 h and maximized at levels of 8% (BCBL-1) or only 1% (BC-3) of cells. Higher concentrations of n-butyric acid (>0.3 mM) increased toxicity and not the number of positive cells. Uninduced BCBL-1 or BC-3 cells contained a very small number of cells expressing gpK8.1 (0.2%) or PF-8 (0.8%) (Fig. 4 and 5). No expression of HHV-8 lytic proteins was found in the HHV-8-negative, EBV-positive B-cell line Daudi in the presence or absence of inducers (results not shown). In summary, the time- and dose-dependent expression patterns of PF-8 or gpK8.1 after TPA or n-butyric acid induction were comparable in the two cell lines. However, less toxicity (with TPA induction) and more gpK8.1 expression (with n-butyric acid induction) favored BCBL-1 over BC-3 cells. Both cell lines were used for further experiments, but only the results of experiments with BCBL-1 cells are shown because the results with BC-3 cells were very similar.
FIG. 4.
Time- and dose-dependent expression of PF-8 and gpK8.1 in TPA-stimulated PEL cell lines. PEL cell lines were stimulated with various concentration of TPA for several days. At various time points, the cells were harvested, labeled with Dead Red, washed, fixed, permeabilized, and labeled with anti-PF-8 or anti-gpK8.1 MAbs followed by FITC-conjugated secondary Abs. The number of cells expressing PF-8 or gpK8.1 was determined as described in the legend to Fig. 3. BCBL-1 (A to C) and BC-3 (D to F) cells were examined for viability (A and D), PF-8 (B and E), and gpK8.1 (C and F). Symbols: ○, unstimulated cells; ●, 0.3 nM TPA; ■, 3 nM TPA; ▴, 30 nM TPA. Data represents the mean and standard error of the mean of at least three separate experiments.
FIG. 5.
Time- and dose-dependent expression of PF-8 and gpK8.1 in n-butyric acid-stimulated PEL cell lines. PEL cell lines were stimulated with various concentrations of n-butyric acid for several days. At various time points, the cells were harvested, labeled with Dead Red, washed, fixed, permeabilized, and labeled with anti-PF-8 or anti-gpK8.1 MAbs followed by FITC-conjugated secondary Abs. BCBL-1 (A to C) and BC-3 (D to F) cells were examined for viability (A and D), PF-8 (B and E) and gpK8.1 (C and F). Symbols: ○, unstimulated cells; ●, 0.03 mM n-butyric acid; ■, 0.3 mM n-butyric acid; ▴, 3 mM n-butyric acid. Data represents the mean ± standard error of the mean of at least three separate experiments.
Characterization of antiherpesvirus agents.
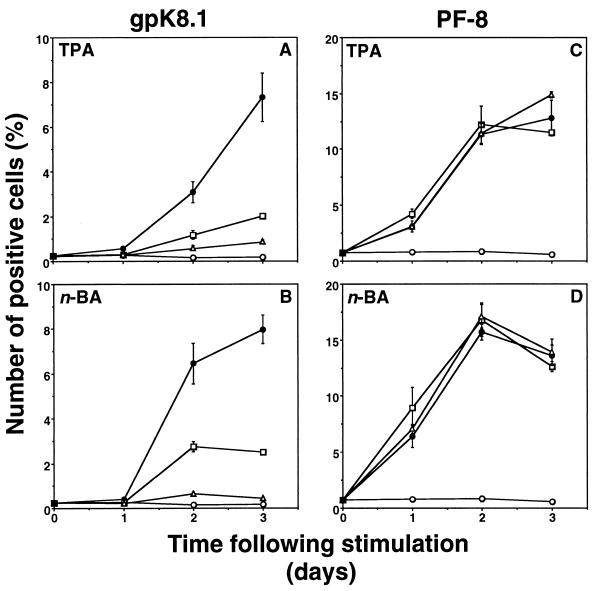
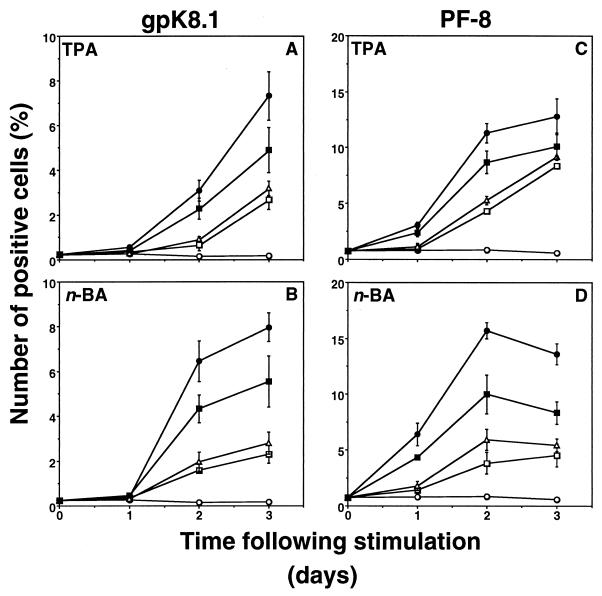
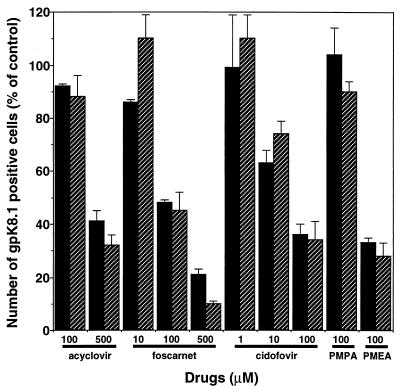
We used our assay to characterize potential antiherpesvirus drugs. BCBL-1 cells were induced with TPA (30 nM) or n-butyric acid (0.3 mM) in the presence or absence of antiviral drugs, and expression of HHV-8 lytic proteins was measured as described above. Acyclovir and foscarnet inhibited the expression of gpK8.1 after induction with TPA or n-butyric acid (Fig. 6A and B). However, expression of the early lytic protein PF-8 was not inhibited by these drugs (Fig. 6C and D). By contrast, the antiviral cytokines IFN-α and IFN-β inhibited the expression of PF-8, as well as gpK8.1, after both TPA and n-butyric acid induction (Fig. 7). IFN-α and IFN-β reduced the number of cells expressing PF-8 by 62 and 53%, respectively, after a 2-day TPA induction and by 76 and 62%, respectively, after a 2-day n-butyric acid induction (all results are statistically significant; P < 0.01 by the two-tailed Student t test). The number of cells expressing gpK8.1 decreased by >70% after treatment with IFN-α or IFN-β. IFN-γ was less effective in inhibiting HHV-8 protein expression. Inhibition of HHV-8 protein synthesis did not correlate with less toxicity or decreased cell numbers (results not shown). Finally, dose-dependent inhibition of gpK8.1 expression of several antiherpesvirus drugs was compared. Cidofovir was the most potent drug, followed by foscarnet and acyclovir in TPA- or n-butyric acid-induced BCBL-1 cells (Fig. 8). The broad-spectrum antiviral drug PMEA (adefovir) also inhibited gpK8.1 expression, while another nucleoside phosphonate analog (PMPA) did not. Again, PF-8 expression was never inhibited by any of these drugs at any concentration. Because of reports of KS regression in patients receiving highly active antiretroviral therapy, we tested several HIV protease inhibitors in our assay for activity against HHV-8. However, indinavir, saquinavir, and ritonavir did not exhibit anti-HHV-8 activity (results not shown).
FIG. 6.
Antiherpesvirus drugs inhibit gpK8.1 but not PF-8 expression. BCBL-1 cells stimulated with 30 nM TPA (A and C) or 0.3 mM n-butyric acid (n-BA) (B and D) in the presence or absence of antiherpesvirus drugs were examined for gpK8.1 (A and B) or PF-8 (C and D) expression. Symbols: ○, unstimulated cells; ●, stimulated cells; □, plus 0.5 mM acyclovir; ▵, plus 0.5 mM foscarnet. Data represents the mean and standard error of the mean of at least three separate experiments.
FIG. 7.
IFNs inhibit gpK8.1 and PF-8 expression. BCBL-1 cells stimulated with 30 nM TPA (A and C) or 0.3 mM n-butyric acid (n-BA) (B and D) in the presence or absence of IFN-α, IFN-β, or IFN-γ were examined for gpK8.1 (A and B) or PF-8 (C and D) expression. Symbols: ○, unstimulated cells; ●, stimulated cells; □, plus 20 ng of IFN-α per ml; ▵, plus 20 ng of IFN-β per ml; ■, plus 20 ng of IFN-γ per ml. Data represents the mean and standard error of the mean of at least three separate experiments.
FIG. 8.
Concentration-dependent inhibition of gpK8.1 expression by several antiherpesvirus drugs. BCBL-1 cells stimulated with 30 nM TPA (solid bars) or 0.3 mM n-butyric acid (hatched bars) in the presence of antiherpesvirus drugs were examined for gpK8.1 expression. The number of gpK8.1-expressing cells is shown as percentage of the control (i.e., cells not treated with antiherpesvirus drugs). Data represents the mean and standard error of the mean of two to four separate experiments.
DISCUSSION
Studies to quantify and characterize productive HHV-8 infection have been technically limited due to the nature of the currently used molecular biologic assays. Particularly, defining the lytic life cycle of HHV-8 in single cells has not been feasible but would be invaluable for the development of in vitro HHV-8 infection models, identification of infected host cells in vivo, and evaluation of potential antiherpesvirus agents. The recent development of MAbs directed against HHV-8 lytic-cycle-associated proteins (8, 39) has provided a new way to monitor HHV-8 active replication. By using these MAbs in a flow cytometry-based detection system, we established a rapid technique for quantification and characterization of HHV-8 lytic infection in single cells.
HHV-8 infection in PEL cell lines is predominantly latent, but active replication can be induced by TPA or n-butyric acid, as shown by the formation of nucleoids and expression of specific viral proteins (6, 29, 32, 34). Viral proteins expressed in lytic HHV-8 infection have been classified as early lytic and late lytic proteins (34). PF-8 is an HHV-8 accessory protein essential for viral replication and expressed in the early lytic phase (8, 13, 31, 34, 41). We show that gpK8.1 is an HHV-8 structural protein and forms part of the viral envelope as demonstrated by electron microscopy. gpK8.1 is also present on the plasma membrane as well as associated with other intracellular membranes. Structural proteins, such as viral envelope glycoproteins, are expressed in the late lytic phase (9, 34). Indeed, in our system, early-lytic-phase PF-8 was expressed more rapidly upon induction than was the late-lytic-phase gpK8.1. Our studies further suggest that cells entering the early lytic phase do not necessarily proceed to expression of late lytic proteins. Curiously, a very small group of cells expressed the late lytic gpK8.1 without detectable expression of the early lytic PF-8. Possibly, PF-8 expression was transient and had been lost in those cells by the time gpK8.1 was detected. Alternatively, expression of the full array of lytic-phase proteins might be subjected to some degree of randomness or errors and therefore may not always proceed in a regimented manner.
Our assay could differentiate between early and late stages of the viral lytic life cycle, and was rapidly quantitative by using flow cytometry. As observed here and by others (7, 25, 27), only a minority of HHV-8-infected cells (<20%) were susceptible to induction of the viral lytic phase. Although the overall patterns of HHV-8 lytic-protein expression in the different PEL cell lines BCBL-1 and BC-3 were quite similar, several quantitative variations were observed between the different cell lines and different inducers (40). For examples, BC-3 cells were less useful than BCBL-1 cells because TPA induction was too toxic and n-butyric acid induction in BC-3 cells resulted in poor expression of viral glycoproteins. Thus, our assay enabled easy quantitative comparison between (i) different cells for their capacity to express HHV-8 lytic-phase proteins and (ii) the time- and concentration-dependent efficacy of different inducers of viral replication.
Expression of late-lytic-phase herpesvirus structural proteins is dependent on the viral DNA polymerase (9). Accordingly, inhibition of DNA polymerase with known antiherpesvirus drugs inhibited the expression of gpK8.1. Inhibition by antiherpesvirus drugs also correlated with reduction in the number of cells containing herpesvirus nucleoids and mature herpesvirus particles as observed by TEM (results not shown). Consistent with other studies (22, 28), cidofovir was the most potent and acyclovir was the weakest inhibitor of HHV-8 replication. However, the effective dose of cidofovir was higher than described previously, which may be due to our short-term culture system or to other differences in experimental conditions. Additionally, the antiretrovirus nucleoside analog PMEA displayed antiherpesvirus activity, again correlating with inhibition of gpK8.1 expression in our system. By contrast, all of the antiretrovirus protease inhibitors we tested were ineffective in blocking HHV-8 replication (results not shown). Our findings also clearly demonstrated that inhibition of the HHV-8 DNA polymerase does not prevent expression of early-lytic-phase antigens. This may be important for the evaluation of potential drugs if early-lytic-phase viral gene products are shown to play a role in HHV-8-induced pathogenesis. If so, IFN-α and IFN-β may be more beneficial because of their capacity to inhibit early-lytic-phase as well as late-lytic-phase protein expression. Taken together, our system enables rapid screening of possible antiherpesvirus agents and contributes information on their possible mechanism of action. Although antiherpesvirus drugs may not benefit patients with established KS (because tumor spindle cells are predominantly latently, not lytically, infected with HHV-8), this therapy may prove useful in blocking the initiation of KS in HHV-8-infected individuals (e.g., in AIDS patients or transplant recipients [4]).
Controversy currently exists about the circulating blood cell type infected with HHV-8 in individuals with KS (4). With DNA from unselected PBMC populations, most studies have detected HHV-8 by PCR in over half of all KS patients (1, 18, 38). One group of investigators, by documenting the presence of linear forms of viral genomes, has shown that active HHV-8 replication occurs in the blood of KS patients (12). In selected or sorted cell populations, HHV-8 has been found by PCR within circulating B cells (1, 16, 17, 23), monocytes (3), CD34+ spindle cells (35), and, rarely, CD8+ T cells (16, 36). These studies, however, were PCR based and were not performed on a single-cell level; therefore, the results are prone to error due to small numbers of contaminating cells in selected populations of cells. Our MAb/fluorescence-activated cell sorter-based system to detect individual cells lytically infected with HHV-8 should prove invaluable in more precise identification and phenotyping of HHV-8-infected cells in vivo.
In summary, we describe HHV-8 lytic-phase infection in single cells by dual measurement of early and late lytic protein expression by using flow cytometry, providing rapid quantitative and qualitative detection of viral activation. Antiherpesvirus agents were characterized for their ability to block complete or partial viral gene transcription and translation. This work should foster future detailed studies on HHV-8-induced pathologic changes and aid in the design and development of new treatments for HHV-8-associated diseases.
ACKNOWLEDGMENTS
We thank Harry Schaefer for preparation of the figures.
This study was supported in part by Public Health Service grants CA75911 and CA82056 to B. Chandran.
REFERENCES
- 1.Ambroziak J A, Blackbourn D J, Herndier B G, Glogau R G, Gullett J H, McDonald A R, Lennette E T, Levy J A. Herpes-like sequences in HIV-infected and uninfected Kaposi’s sarcoma patients. Science. 1995;268:582–583. doi: 10.1126/science.7725108. [DOI] [PubMed] [Google Scholar]
- 2.Arvanitakis L, Mesri E A, Nador R G, Said J W, Asch A S, Knowles D M, Cesarman E. Establishment and characterization of a primary effusion (body cavity-based) lymphoma cell line (BC-3) harboring Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) in the absence of Epstein-Barr virus. Blood. 1996;88:2648–2654. [PubMed] [Google Scholar]
- 3.Blasig C, Zietz C, Haar B, Neipel F, Esser S, Brockmeyer N H, Tschachler E, Colombini S, Ensoli B, Sturzl M. Monocytes in Kaposi’s sarcoma lesions are productively infected by human herpesvirus 8. J Virol. 1997;71:7963–7968. doi: 10.1128/jvi.71.10.7963-7968.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blauvelt A. The role of human herpesvirus 8 in the pathogenesis of Kaposi’s sarcoma. Adv Dermatol. 1999;14:167–207. [PubMed] [Google Scholar]
- 5.Cesarman E, Chang Y, Moore P S, Said J W, Knowles D M. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 6.Cesarman E, Moore P S, Rao P H, Inghirami G, Knowles D M, Chang Y. In vitro establishment and characterization of two acquired immunodeficiency syndrome-related lymphoma cell lines (BC-1 and BC-2) containing Kaposi’s sarcoma-associated herpesvirus-like (KSHV) DNA sequences. Blood. 1995;86:2708–2714. [PubMed] [Google Scholar]
- 7.Cesarman E, Nador R G, Bai F, Bohenzky R A, Russo J J, Moore P S, Chang Y, Knowles D M. Kaposi’s sarcoma-associated herpesvirus contains G protein-coupled receptor and cyclin D homologs which are expressed in Kaposi’s sarcoma and malignant lymphoma. J Virol. 1996;70:8218–8223. doi: 10.1128/jvi.70.11.8218-8223.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan S R, Bloomer C, Chandran B. Identification and characterization of human herpesvirus-8 lytic cycle-associated ORF 59 protein and the encoding cDNA by monoclonal antibody. Virology. 1998;240:118–126. doi: 10.1006/viro.1997.8911. [DOI] [PubMed] [Google Scholar]
- 9.Chandran B, Bloomer C, Chan S R, Zhu L, Goldstein E, Horvat R. Human herpesvirus-8 ORF K8.1 gene encodes immunogenic glycoproteins generated by spliced transcripts. Virology. 1998;249:140–149. doi: 10.1006/viro.1998.9316. [DOI] [PubMed] [Google Scholar]
- 10.Chang Y, Cesarman E, Pessin M S, Lee F, Culpepper J, Knowles D M, Moore P S. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 11.Costagliola D, Mary-Krause M. Can antiviral agents decrease the occurrence of Kaposi’s sarcoma? Lancet. 1995;346:578. doi: 10.1016/s0140-6736(95)91417-x. [DOI] [PubMed] [Google Scholar]
- 12.Decker L L, Shankar P, Khan G, Freeman R B, Dezube B J, Lieberman J, Thorley-Lawson D A. The Kaposi sarcoma-associated herpesvirus (KSHV) is present as an intact latent genome in KS tissue but replicates in the peripheral blood mononuclear cells of KS patients. J Exp Med. 1996;184:283–288. doi: 10.1084/jem.184.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ertl P F, Powell K L. Physical and functional interaction of human cytomegalovirus DNA polymerase and its accessory protein (ICP36) expressed in insect cells. J Virol. 1992;66:4126–4133. doi: 10.1128/jvi.66.7.4126-4133.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flore O, Gao S J. Effect of DNA synthesis inhibitors on Kaposi’s sarcoma-associated herpesvirus cyclin and major capsid protein gene expression. AIDS Res Hum Retroviruses. 1997;13:1229–1233. doi: 10.1089/aid.1997.13.1229. [DOI] [PubMed] [Google Scholar]
- 15.Glesby M J, Hoover D R, Weng S, Graham N M H, Phair J P, Detels R, Ho M, Saah A J. Use of antiherpes drugs and the risk of Kaposi’s sarcoma: data from the multicenter AIDS cohort study. J Infect Dis. 1996;173:1477–1480. doi: 10.1093/infdis/173.6.1477. [DOI] [PubMed] [Google Scholar]
- 16.Harrington W J, Bagasra O, Sosa C E, Bobrowski L E, Baum M, Wen W L, Cabral L, Byrne G E, Pomerantz R J, Wood C. Human herpesvirus type 8 DNA sequences in cell-free plasma and mononuclear cells of Kaposi’s sarcoma patients. J Infect Dis. 1996;174:1101–1105. doi: 10.1093/infdis/174.5.1101. [DOI] [PubMed] [Google Scholar]
- 17.Huang Y Q, Li J J, Poiesz B J, Kaplan M H, Friedman-Kien A E. Detection of herpesvirus-like DNA sequences in matched specimens of semen and blood from patients with AIDS-related Kaposi’s sarcoma by polymerase chain reaction in situ hybridization. Am J Pathol. 1997;150:147–153. [PMC free article] [PubMed] [Google Scholar]
- 18.Humphrey R W, O’Brien T R, Newcomb F M, Nishihara H, Wyvill K M, Ramos G A, Saville M W, Goedert J J, Straus S E, Yarchoan R. Kaposi’s sarcoma (KS)-associated herpesvirus-like DNA sequences in peripheral blood mononuclear cells: association with KS and persistence in patients receiving anti-herpesvirus drugs. Blood. 1996;88:297–301. [PubMed] [Google Scholar]
- 19.Jones J L, Hanson D H, Chu S Y, Ward J W, Jaffe H W. AIDS-associated Kaposi’s sarcoma. Science. 1995;267:1078–1079. doi: 10.1126/science.7855583. [DOI] [PubMed] [Google Scholar]
- 20.Kedes D H, Ganem D. Sensitivity of Kaposi’s sarcoma-associated herpesvirus replication to antiviral drugs. Implications for potential therapy. J Clin Investig. 1997;99:2082–2086. doi: 10.1172/JCI119380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin K, Dai C Y, Ricciardi R P. Cloning and functional analysis of Kaposi’s sarcoma-associated herpesvirus DNA polymerase and its processivity factor. J Virol. 1998;72:6228–6232. doi: 10.1128/jvi.72.7.6228-6232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medveczky M M, Horvath E, Lund T, Medveczky P G. In vitro antiviral drug sensitivity of the Kaposi’s sarcoma-associated herpesvirus. AIDS. 1997;11:1327–1332. doi: 10.1097/00002030-199711000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Mesri E A, Cesarman E, Arvanitakis L, Rafii S, Moore M A S, Posnett D N, Knowles D M, Asch A S. Human herpesvirus-8/Kaposi’s sarcoma-associated herpesvirus is a new transmissible virus that infects B cells. J Exp Med. 1996;183:2385–2390. doi: 10.1084/jem.183.5.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mocroft A, Youle M, Gazzard B, Morcinek J, Halai R, Phillips A N. Anti-herpesvirus treatment and risk of Kaposi’s sarcoma in HIV infection. AIDS. 1996;10:1101–1105. [PubMed] [Google Scholar]
- 25.Moore P S, Boshoff C, Weiss R A, Chang Y. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science. 1996;274:1739–1744. doi: 10.1126/science.274.5293.1739. [DOI] [PubMed] [Google Scholar]
- 26.Moore P S, Chang Y. Detection of herpesvirus-like DNA sequences in Kaposi’s sarcoma in patients with and those without HIV infection. N Engl J Med. 1995;332:1181–1185. doi: 10.1056/NEJM199505043321801. [DOI] [PubMed] [Google Scholar]
- 27.Moore P S, Gao S J, Dominguez G, Cesarman E, Lungu O, Knowles D M, Garber R, Pellett P E, McGeoch D J, Chang Y. Primary characterization of a herpesvirus agent associated with Kaposi’s sarcoma. J Virol. 1996;70:549–558. doi: 10.1128/jvi.70.1.549-558.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neyts J, DeClercq E. Antiviral drug susceptibility of human herpesvirus 8. Antimicrob Agents Chemother. 1997;41:2754–2756. doi: 10.1128/aac.41.12.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orenstein J M, Alkan S, Blauvelt A, Jeang K T, Weinstein M D, Ganem D, Herndier B. Visualization of human herpesvirus type 8 in Kaposi’s sarcoma by light and transmission electron microscopy. AIDS. 1997;11:F35–F45. doi: 10.1097/00002030-199705000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Raab M-S, Albrecht J-C, Birkmann A, Yaguboglu S, Lang D, Fleckenstein B, Neipel F. The immunogenic glycoprotein gp35-37 of human herpesvirus 8 is encoded by open reading frame K8.1. J Virol. 1998;72:6725–6731. doi: 10.1128/jvi.72.8.6725-6731.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reddig P J, Grinstead L A, Monahan S J, Johnson P A, Parris D S. The essential in vivo function of the herpes simplex virus UL42 protein correlates with its ability to stimulate the viral DNA polymerase in vitro. Virology. 1994;200:447–456. doi: 10.1006/viro.1994.1208. [DOI] [PubMed] [Google Scholar]
- 32.Renne R, Zhong W, Herndier B, McGrath M, Abbey N, Kedes D, Ganem D. Lytic growth of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat Med. 1996;2:342–346. doi: 10.1038/nm0396-342. [DOI] [PubMed] [Google Scholar]
- 33.Robles R, Lugo D, Gee L, Jacobson M A. Effect of antiviral drugs used to treat cytomegalovirus end-organ disease on subsequent course of previously diagnosed Kaposi’s sarcoma in patients with AIDS. J Acquired Immune Defic Syndr Hum Retrovirology. 1999;20:34–38. doi: 10.1097/00042560-199901010-00005. [DOI] [PubMed] [Google Scholar]
- 34.Sarid R, Flore O, Bohenzky R A, Chang Y, Moore P S. Transcription mapping of the Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) genome in a body cavity-based lymphoma cell line (BC-1) J Virol. 1998;72:1005–1012. doi: 10.1128/jvi.72.2.1005-1012.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sirianni M C, Uccini S, Angeloni A, Faggioni A, Cottoni F, Ensoli B. Circulating spindle cells: correlation with human herpesvirus-8 (HHV-8) infection and Kaposi’s sarcoma. Lancet. 1997;349:255. doi: 10.1016/s0140-6736(05)64866-0. [DOI] [PubMed] [Google Scholar]
- 36.Sirianni M C, Vincenzi L, Topino S, Scala E, Angeloni A, Gonella R, Uccini S, Faggioni A. Human herpesvirus 8 DNA sequences in CD8+ T cells. J Infect Dis. 1997;176:541. doi: 10.1086/514072. [DOI] [PubMed] [Google Scholar]
- 37.Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d’Agay M F, Clauvel J P, Raphael M, Degos L, Sigaux F. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995;86:1276–1280. [PubMed] [Google Scholar]
- 38.Whitby D, Howard M R, Tenant-Flowers M, Brink N S, Copas A, Boshoff C, Hatzioannou T, Suggett F E A, Aldam D M, Denton A S, Miller R F, Weller I V D, Weiss R A, Tedder R S, Schulz T F. Detection of Kaposi’s sarcoma associated herpesvirus in peripheral blood of HIV-infected individuals and progression to Kaposi’s sarcoma. Lancet. 1995;346:799–802. doi: 10.1016/s0140-6736(95)91619-9. [DOI] [PubMed] [Google Scholar]
- 39.Wu, L., R. Renne, D. Ganem, and B. Forghani. Identification and characterization of human herpesvirus 8 glycoprotein K8.1 in BCBL-1 cells by monoclonal antibody. Submitted for publication. [DOI] [PubMed]
- 40.Yu Y, Black J B, Goldsmith C S, Browning P J, Bhalla K, Offermann M K. Induction of human herpesvirus-8 DNA replication and transcription by butyrate and TPA in BCBL-1 cells. J Gen Virol. 1999;80:83–90. doi: 10.1099/0022-1317-80-1-83. [DOI] [PubMed] [Google Scholar]
- 41.Zhou Y, Chandran B, Wood C. Transcriptional patterns of the pCD41 (U27) locus of human herpesvirus 6. J Virol. 1997;71:3420–3430. doi: 10.1128/jvi.71.5.3420-3430.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]