Abstract
During 1997 in Hong Kong, 18 human cases of respiratory illness, including 6 fatalities, were caused by highly pathogenic avian influenza A (H5N1) viruses. Since H5 viruses had previously been isolated only from avian species, the outbreak raised questions about the ability of these viruses to cause severe disease and death in humans. To better understand the pathogenesis and immunity to these viruses, we have used the BALB/c mouse model. Four H5N1 viruses replicated equally well in the lungs of mice without prior adaptation but differed in lethality for mice. H5N1 viruses that were highly lethal for mice were detected in multiple organs, including the brain. This is the first demonstration of an influenza A virus that replicates systemically in a mammalian species and is neurotropic without prior adaptation. The mouse model was also used to evaluate a strategy of vaccination against the highly pathogenic avian H5N1 viruses, using an inactivated vaccine prepared from nonpathogenic A/Duck/Singapore-Q/F119-3/97 (H5N3) virus that was antigenically related to the human H5N1 viruses. Mice administered vaccine intramuscularly, with or without alum, were completely protected from lethal challenge with H5N1 virus. Protection from infection was also observed in 70% of animals administered vaccine alone and 100% of mice administered vaccine with alum. The protective effect of vaccination correlated with the level of virus-specific serum antibody. These results suggests a strategy of vaccine preparedness for rapid intervention in future influenza pandemics that uses antigenically related nonpathogenic viruses as vaccine candidates.
During May through December 1997, an outbreak of avian influenza A (H5N1) virus in Hong Kong caused 18 human cases (6 fatal) of respiratory illness (7, 11, 30). The H5N1 infections in humans were preceded by the circulation of highly pathogenic H5N1 viruses in birds, first in poultry farms and later in wholesale and retail poultry markets in Hong Kong (5, 8, 28). The H5N1 viruses isolated from both chickens and humans possessed hemagglutinin (HA) molecules with multiple basic amino acids adjacent to the cleavage site between HA1 and HA2 and were lethal for experimentally infected chickens, features characteristic of highly pathogenic avian influenza A viruses (8, 26, 29, 30). The fact that the H5N1 viruses resulted in severe or fatal respiratory disease in the majority of infected persons aged 13 to 60 years was of particular concern since this age group is not normally considered to be at increased risk for death and complications from influenza (6, 31).
The antigenic and genetic analysis of 16 H5N1 viruses isolated from humans identified two closely related but distinguishable groups, represented by A/Hong Kong/156/97 (HK/156) virus (group A) and A/Hong Kong/483/97 (HK/483) virus (group B). Group B viruses share a substitution at residue 156 of HA1, which creates a potential glycosylation site absent in group A viruses (3). An asymmetric cross-reactivity between the groups was demonstrated by postinfection ferret serum in hemagglutination inhibition (HI) assays. Antiserum raised to group B viruses exhibited a greater degree of cross-reactivity with group A viruses compared with the reactivity of group A virus antiserum for group B viruses (3). All internal genes, like those encoding the surface glycoproteins, were of avian origin (8, 27, 29), indicating that the H5N1 viruses that infected humans in Hong Kong had crossed the species barrier without genetic reassortment with a human influenza virus. Neither the HA nor the NA (neuraminidase) genes of the human H5N1 viruses isolated from the outbreak showed evidence of adaptive changes (3). Furthermore, Matrosovich et al. (22) have reported that H5N1 virus isolated from the index case (HK/156) possessed an HA with receptor specificity typical of avian viruses.
Previous studies had demonstrated that humans were not susceptible to infection with a highly pathogenic H5 virus that caused high mortality in poultry in Pennsylvania in 1983 (2). The ability of the H5N1 viruses from Hong Kong to cause severe respiratory illness, multiorgan dysfunction, and a high rate of mortality in humans raised questions concerning the mechanism(s) of pathogenicity and the development of prevention and control measures in preparation for an actual pandemic caused by highly pathogenic avian viruses. In this situation, traditional methods for developing inactivated vaccines for humans would be greatly compromised by the need to prepare and perform safety testing of reassorted vaccine candidates under biosafety level 3-plus (BSL-3+) containment conditions (1).
We investigated the mouse as a mammalian model for the study of H5N1 influenza virus pathogenesis and immunity. We report here on four human H5N1 viruses that replicated efficiently in the lungs, without any adaptation, and have different levels of lethality for BALB/c mice. In addition, we demonstrate the utility of this animal model for evaluating protective immunity to human H5N1 influenza viruses and a strategy for effective vaccination against the pathogenic H5N1 influenza viruses that uses the antigenically related, but nonpathogenic, avian A/duck/Singapore/Q/F119-3/97 (dk/Sing) (H5N3) virus.
MATERIALS AND METHODS
Viruses.
The influenza viruses used in this study were the H5N1 group A viruses HK/156 and A/Hong Kong/486/97 (HK/486); the H5N1 group B viruses HK/483 and A/Hong Kong/485/97 (HK/485); the H5N3 dk/Sing virus; and the reassortment human influenza A virus X-31, which possesses the surface glycoprotein genes of A/Aichi/2/68 (H3N2) and the internal protein genes of A/Puerto Rico/8/34 virus. A U.S. Department of Agriculture permit was obtained before work with avian influenza viruses was begun. Virus stocks were propagated in the allantoic cavities of 10-day-old embryonated hen eggs under conditions that were found to be optimal for virus replication for H5N1 viruses (37°C, 24 h) or dk/Sing and X-31 viruses (34°C, 48 h). Virus stocks were aliquoted and stored at −70°C until use. The passage histories of the viruses are detailed in Table 1. Fifty percent tissue culture infectious dose (TCID50) and 50% egg infectious dose (EID50) titers were determined by serial titration of viruses in Madin-Darby canine kidney (MDCK) cells and eggs, respectively. Titers were calculated by the method of Reed and Muench (24).
TABLE 1.
Characteristics of influenza A H5N1 viruses
| Virus | Groupa | Case description
|
Passage no.
|
Infectivity titer (log10/ml)b
|
MID50c | LD50d | |||
|---|---|---|---|---|---|---|---|---|---|
| Age (yr)/gender | Outcome | MDCK | Eggs | TCID50 | EID50 | ||||
| HK/483 | B | 13/female | Fatal | 1 | 3 | 8.5 | 9.0 | 2.2 | 2.4 |
| HK/485 | B | 24/female | Severe/recovered | 2 | 2 | 8.5 | 8.7 | 1.1 | 2.9 |
| HK/156 | A | 3/male | Fatal | 0 | 3 | 7.7 | 10.0 | 3.2 | 5.9 |
| HD/486 | A | 5/female | Mild/recovered | 2 | 4 | 8.7 | 9.0 | 1.2 | >7 |
| X-31 (H3N2) | NAe | NA | 0 | >10 | 7.3 | 8.5 | 0.7 | >5.2 | |
Antigenic/genetic group of H5N1 viruses isolated from humans (3).
Calculated by the method of Reed and Muench (24) from the results of serial titrations of viruses in MDCK cells and embryonated eggs.
Expressed as the EID50 required to give 1 MID50.
Expressed as the EID50 required to give 1 LD50.
NA, not applicable.
Laboratory facility.
Because of the potential risk to humans and poultry, all experiments using infectious pathogenic avian H5N1 viruses, including work with animals, were conducted using BSL-3+ containment procedures (25). Investigators were required to wear appropriate respirator equipment (RACAL Health and Safety Inc., Frederick, Md.). Work performed with the nonpathogenic virus dk/Sing was conducted under BSL-2 conditions.
Infection of mice and challenge experiments.
Female BALB/c mice, 6 to 8 weeks old (Charles River Laboratories, Wilmington, Mass.), were used in all experiments. Mice were lightly anesthetized with CO2, and 50 μl of infectious virus diluted in phosphate-buffered saline (PBS) was inoculated intranasally (i.n.). Fifty percent mouse infectious dose (MID50) and 50% lethal dose (LD50) titers were determined by inoculating groups of seven mice i.n. with serial 10-fold dilutions of virus. Four days later, three mice from each group were euthanized, and lungs were collected and homogenized in 1 ml of cold PBS. The homogenates were frozen at −70°C and later thawed for ease of handing. Solid debris was pelleted by centrifugation, and tissues were titrated for virus infectivity in eggs. The four remaining mice in each group were checked daily for disease signs and death for 14 days postinfection (p.i.). MID50 and LD50 titers were calculated by the method of Reed and Muench (24) and were expressed as the EID50 value corresponding to 1 MID50 or LD50. Lung virus titers were used for the determination of MID50 because we had previously determined that the H5N1 viruses used in this study replicated to higher titers (≥100-fold) in lung tissue than in upper respiratory tract tissues.
Replication of the H5N1 viruses in mice was examined by determining the virus titers in organs (lung, spleen, liver, kidney, and brain) and blood 4 and 6 days p.i. with 100 MID50 of viruses. Clarified homogenates were titrated for virus infectivity in eggs from initial dilutions of 1:10 (lung) or 1:2 (other tissues and blood). The limits of virus detection were 101.2 EID50/ml for lung and 100.8 EID50/ml for other organs and blood.
To evaluate the degree of protection from a lethal challenge, vaccinated mice were infected i.n. with 50 LD50(=90 MID50) of HK/483 virus and were observed daily for 14 days. To evaluate protection of the lungs and brains from infection, additional mice were euthanized on day 6 p.i., and tissues were collected and titrated for virus infectivity as described above. Virus endpoint titers are expressed as mean log10EID50 per milliliter ± standard deviation (SD).
Immunohistochemistry.
Tissues from infected mice were fixed in formalin and were processed for immunohistochemistry by using a two-step biotin-streptavidin method essentially as previously described (32). The primary polyclonal antibody used was a goat anti-Tern/South Africa/61 (H5N3) (National Institute of Allergy and Infectious Diseases, Bethesda, Md.) antibody which recognizes both surface glycoproteins and internal proteins of the virus.
Antibody assays.
Immune sera from blood samples collected from the orbital plexus were treated with receptor-destroying enzyme from Vibrio cholerae (Denka-Seiken, Tokyo, Japan) as previously described (19) before testing for the presence of HI antibody by standard methods (20). Individual mice were considered to have responded to vaccination if serum HI titers were ≥40. Titers of neutralizing antibody were determined essentially as previously described (16) except that a lower cell concentration (1.5 × 105 MDCK cells/ml) was used. Neutralization titers are expressed as the reciprocal of the highest dilution of serum that gave 50% neutralization of 100 TCID50 of virus.
Vaccine and adjuvant preparations and immunizations.
dk/Sing virus was concentrated from allantoic fluid and purified on a linear sucrose gradient as described previously (10). The HA content of purified dk/Sing virus was estimated to be 30% of the total viral proteins by densitometric analysis of viral protein bands separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Inactivated whole-virus vaccine was prepared by treating purified virus at a concentration of 1 mg/ml with 0.025% formalin at 4°C for >3 days. This treatment resulted in the complete loss of infectivity of the virus. Groups of mice were lightly anesthetized with CO2 and injected intramuscularly (i.m.) with either 10 μg of dk/Sing vaccine (=3 μg of HA) alone, 10 μg of vaccine mixed with alum, or PBS mixed with alum in a volume of 0.1 ml. A 2% alum (Alhydrogel; Superfos Biosectors, Kvistgaard, Denmark) suspension was mixed with an equal volume of vaccine in PBS or PBS only for 6 h at 4°C before immunization. Mice received two inoculations at an interval of 3 weeks.
Statistical analysis.
Statistical significance of the data was determined by using Fisher’s exact or Student’s t tests.
RESULTS
In vitro and in vivo growth characteristics of human H5N1 influenza viruses.
Two group A and two group B H5N1 viruses isolated from patients representing the spectrum of disease severity observed during the outbreak were selected for use in this study. Their origin and growth characteristics in MDCK cells, eggs, and mice are shown in Table 1. For comparison, the characteristics of a human H3N2 reassortant virus X-31 are also shown. HK/156 and HK/486 are group A viruses, and HK/483 and HK/485 are group B viruses. All human H5N1 viruses and X-31 virus had high infectivity titers in MDCK cells and eggs. With the exception of HK/156 virus, the H5N1 viruses had similar MID50 titers determined by the detection of virus in the lungs of mice 4 days p.i. Ten to 100 times more HK/156 virus than the other H5N1 viruses was required to infect mice. The lethality of the four H5N1 viruses for mice varied considerably. HK/483 and HK/485 viruses were the most lethal for mice, whereas HK/156 virus was considerably less lethal. The lethality of HK/486 was substantially lower than that of the other H5N1 viruses. The nonpathogenic avian H5 virus, dk/Sing, could also infect mice (MID50 = 104.2) but was not lethal, even at high doses (106 EID50 [data not shown]).
Lethality of H5N1 viruses in mice.
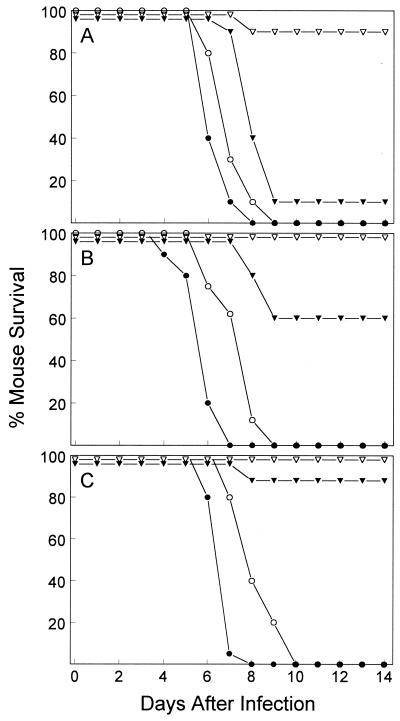
A further experiment compared the time courses of lethal infection for the H5N1 viruses. Mice (10 per group) infected i.n. with 104, 103, or 102 MID50 of virus were observed for 14 days. The percentages of mice surviving the infections are shown in Fig. 1. Mice infected with any dose of HK/483 virus rapidly succumbed 4 to 8 days later. Likewise, 100% of mice infected with any dose of HK/485 virus died 6 to 10 days p.i. Mice infected with 104 MID50 of HK/156 showed significant (90%) mortality by day 9. However, the lethality of HK/156 virus was reduced to 40 and 10% when 103 and 102 MID50 of virus, respectively, were used to infect mice. In contrast to the lethal outcomes with the three H5N1 viruses, none of the mice infected with 103 or 102 MID50 of HK/486 virus died and only 1 of 10 mice infected with 104 MID50 of virus died. These results demonstrated that HK/483 and HK/485 viruses were substantially more lethal for BALB/c mice than were HK/156 and HK/486 viruses.
FIG. 1.
Comparison of lethality of human H5N1 influenza viruses for BALB/c mice. Groups of 10 mice were infected i.n. with 104 (A), 103 (B), or 102 (C) MID50 of HK/483 (●), HK/485 (○), HK/156 (▾), or HK/486 (▿) virus and examined daily for 14 days.
Detection of H5N1 viruses in mouse tissues.
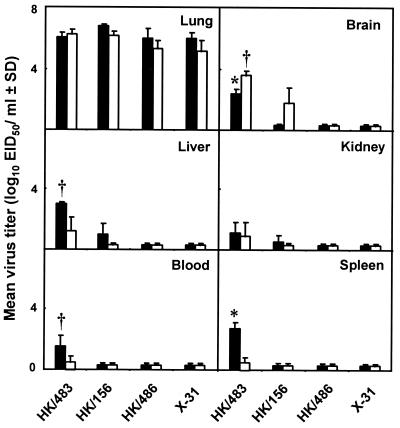
To investigate further the differences in pathogenesis of the human H5N1 viruses in mice, we examined the degree of replication in different tissues. Groups of mice were infected with 100 MID50 of one of three H5N1 viruses or X-31 virus. The mean virus titers (expressed as log10 EID50 per milliliter ± SD) were determined on day 4 p.i., when virus titers in the lungs were maximal, and on day 6 p.i., a time point that immediately preceded the death of mice infected with the highly lethal HK/483 virus (Fig. 2). Infection of mice with each of the H5N1 viruses resulted in high titers of virus in the lungs on days 4 and 6 p.i. Viruses were recovered from the brains of mice infected with HK/483 and HK/156 viruses with titers increasing 15- to 30-fold from days 4 to 6 p.i. The brains of mice infected with HK/483 virus had 50-fold higher titers of virus on day 6 p.i. than those of mice infected with HK/156 virus (P < 0.05). In contrast, mice infected with HK/486 virus had undetectable virus in brains on either day p.i. Virus was also present in the livers, spleens, and blood of mice infected with HK/483 virus on day 4 and 6 p.i., but the titers of virus on day 6 were lower than those on day 4. The kidneys of mice infected with HK/483 virus also yielded substantial titers of virus. A level of virus similar to that of HK/483 was observed in organs and blood of mice infected with HK/485 virus (data not shown). In contrast, mice infected with HK/156 virus had low levels of virus in the livers and kidneys on day 4 and undetectable levels of virus in spleens and blood at either time point. Mice infected with HK/486 or X-31 virus had no detectable virus in these organs on either day p.i.
FIG. 2.
Replication of influenza A (H5N1) viruses in mice. Mice were infected with 100 MID50 of each virus; tissues and blood were collected on days 4 (■) and 6 (□) p.i., and titrated in eggs. The mean virus titers from three mice per group are shown. The limit of virus detection was 101.2 EID50/ml for lungs and 100.8 EID50/ml for blood and other tissues. ∗ and †, P < 0.01 and P < 0.05, respectively, versus groups infected with HK/156 or HK/486 virus.
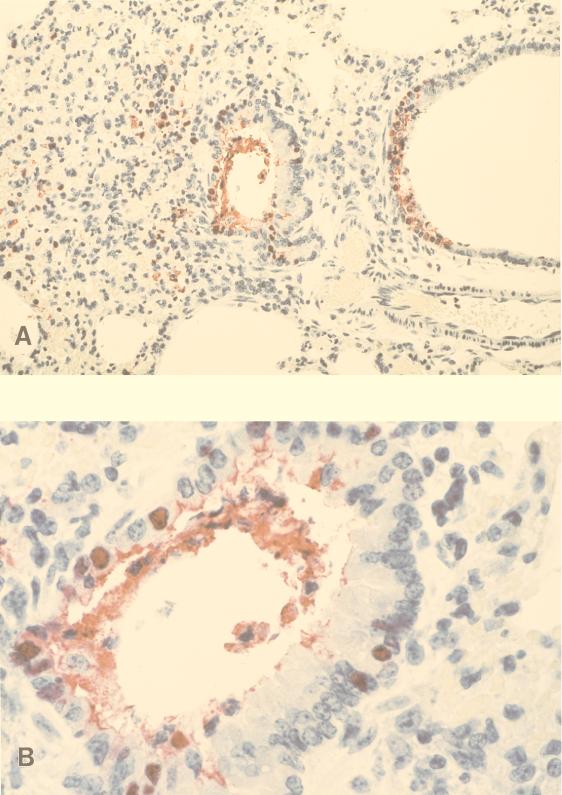
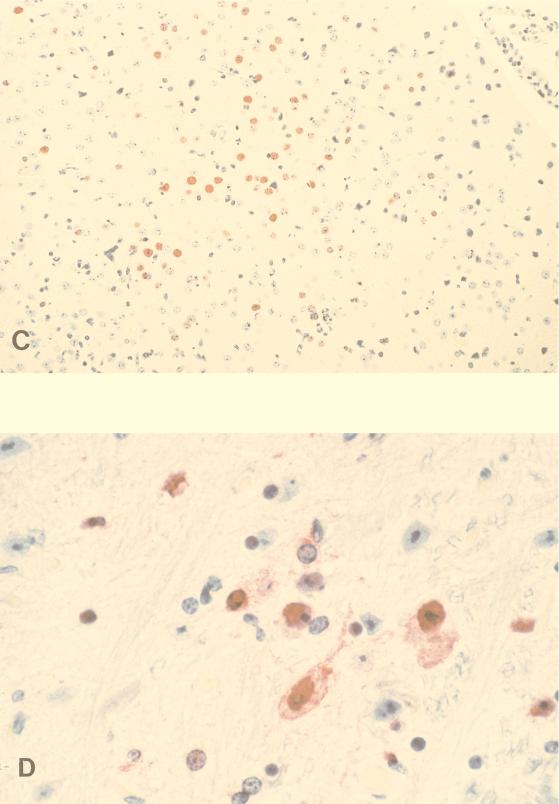
The presence of viral antigens was also detected by immunohistochemistry in the lungs of mice infected with 1,000 MID50 of HK/483 and HK/486 viruses. Antigen-positive cells were more frequent in HK/483 virus-infected lungs than in HK/486 virus-infected lungs (data not shown) and were seen in association with damaged, necrotic bronchi. Immunostaining was observed primarily within epithelial cells lining the bronchi, in detached bronchial cells, and extracellularly within bronchiolar lumens in association with necrotic debris of epithelial cells (Fig. 3A and B). With disease progression, focal immunostaining of inflammatory cells, mainly mononuclear cells, was also observed in subepithelial tissues in the pulmonary interstitium and in association with areas of hemorrhage. Although virus was isolated on days 4 and 6 p.i. in HK/483 virus-infected mice, immunostaining in the brain was observed only 6 or 7 days p.i. Immunostaining in these tissues was focal and seen within both glial cells and neurons (Fig. 3C and D). Taken together, these results demonstrated dissemination of some H5N1 viruses to multiple organs including the brains of mice. An increase of virus titers in the brain over the time course of infection was observed only with the H5N1 viruses that caused lethal infections in mice.
FIG. 3.
Immunostaining of viral antigens in lung and brain from mice infected with 1,000 MID50 of HK/483 virus. (A) Immunostaining in bronchial epithelium and subepithelial tissue from a mouse that succumbed to infection on day 7 p.i. (B) Higher magnification of a bronchus showing mainly nuclear, and to a less extent cytoplasmic, staining of bronchial epithelial cells. Note immunostaining in association with necrotic detached epithelial cells in bronchial lumen. (C) Brain collected on day 6 p.i. showing a focus of antigen-positive cells. (D) High-power magnification showing nuclear and cytoplasmic immunostaining of glial cells and neurons (naphthol-fast red with hematoxylin counterstain; original magnifications, ×50 [A], ×158 [B], ×50 [C], and ×158 [D]).
Immunogenicity of an inactivated whole virus dk/Sing (H5N3) vaccine.
One strategy for developing vaccines against pathogenic avian influenza viruses is the use of a closely related avian virus that is nonpathogenic for poultry and presumably for humans. Such a virus could be handled safely in the laboratory and, on a larger scale, in a vaccine-manufacturing plant. One such virus is dk/Sing virus, which is antigenically related to the human H5N1 viruses and shares 92 to 93% amino acid sequence homology in the HA1 portion of the HA molecule (4).
In preliminary experiments, we had observed that protection from lethal challenge with HK/483 virus in mice previously infected with HK/156, HK/486, or dk/Sing virus correlated with the presence of serum HI antibody against homologous viruses. All mice (n = 37) with serum HI antibody titers of ≥80 were completely protected from death after challenge with HK/483 virus. Ninety-three percent of mice (n = 30) with an HI titer of 40 were protected from death. In contrast, all mice (n = 27) with HI antibody titers of ≤20 died within 7 days of challenge. These results indicated that serum HI antibody titers of ≥40 may be sufficient to protect mice from a lethal challenge with H5N1 virus.
To evaluate the potential of the dk/Sing vaccine candidate and the utility of the mouse model for vaccine efficacy studies, a group of mice (n = 22) was immunized with 10 μg of purified dk/Sing vaccine. A second dose was administered 3 weeks after the first. Because avian viruses have been reported to elicit minimal, if any, serum HI antibody responses in mammalian species (17), another group of mice (n = 20) was immunized with two doses of 10 μg of vaccine mixed with alum, an adjuvant licensed for use in humans. A negative control group of mice (n = 17) received alum alone.
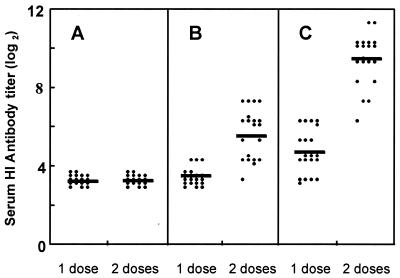
The serum HI antibody response to HK/156 virus measured in individual serum samples collected 3 weeks after the first and second i.m. vaccination is shown in Fig. 4. One dose of vaccine alone failed to elicit a response (HI titer ≥ 40) in mice. A second dose of vaccine was required to induce a substantial HI antibody response in 65% of mice (geometric mean titer [GMT] = 51). Although the GMT of mice receiving one dose of vaccine with alum (GMT = 26) was significantly greater than those of mice receiving one dose of vaccine alone (P < 0.001), only 40% of animals responded. The administration of a second dose of vaccine with alum resulted in 100% of mice responding and greatly elevated HI antibody titers (GMT = 710) compared with mice receiving a second dose of vaccine alone (P < 0.001). These results indicated that two doses of the vaccine, with or without alum, were required to elicit responses in most mice. The addition of alum to the vaccine greatly augmented the HI antibody response after a second dose.
FIG. 4.
Serum HI antibody responses following one or two doses of dk/Sing vaccine. Mice were vaccinated i.m. with alum alone (A), 10 μg of dk/Sing vaccine alone (B), or vaccine with alum (C). Sera from 17 to 20 mice per group were collected 3 weeks after the first and second vaccinations and tested individually for HI antibody against HK/156 virus. HI titers are expressed as a log2 value of the reciprocal of the highest dilution of serum inhibiting agglutination of 0.5% of turkey erythrocytes at 4 HA units of virus. Antibody responses shown in panel C were significantly greater than the corresponding responses shown in panel B (P < 0.001). A log2 value of ≥5.3 represents an HI titer of ≥40. Solid bars represent the GMT.
Because two closely related but antigenically distinguishable groups of human H5N1 viruses cocirculated during the avian influenza outbreak in Hong Kong, we next evaluated the ability of serum from vaccinated mice to cross-react with different representative group A and group B viruses. Serum samples pooled from each group of twice-vaccinated mice were compared in an HI test with the level of antibody in postinfection ferret reference antiserum which distinguished the two groups (Table 2). Serum from mice administered vaccine alone reacted equally well with group A and group B viruses. A twofold difference in the HI test is not considered significant. Likewise, serum from mice administered vaccine in the presence of alum reacted equally well with viruses of both groups. In contrast, the reference postinfection ferret serum raised to dk/Sing or HK/156 virus reacted to higher titers with the group A viruses, while serum raised to the group B HK/483 virus, in general, reacted equally well with both groups of viruses. These results indicated that vaccination with dk/Sing vaccine elicited antibody in mice that was cross-reactive for both groups of human H5N1 viruses.
TABLE 2.
HI reactivity of serum samples from vaccinated mice for influenza A H5 viruses
| Serum | HI antibody titer
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| dk/Sing (H5N3) | H5N1 group A
|
H5N1 group B
|
|||||||
| HK/156 | HK/486 | HK/516 | HK/538 | HK/483 | HK/485 | HK/491 | HK/514 | ||
| Vaccinated micea | |||||||||
| dk/Sing | 80 | 80 | 40 | 40 | 40 | 40 | 40 | 80 | 40 |
| dk/Sing + alum | 320 | 320 | 160 | 320 | 640 | 160 | 80 | 320 | 160 |
| Alum | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Infected ferretsb | |||||||||
| dk/Sing | 160 | 160 | 80 | 160 | 160 | 40 | 40 | 80 | 80 |
| HK/156 | 160 | 160 | 160 | 160 | 160 | 20 | 20 | 80 | 80 |
| HK/483 | 160 | 160 | 80 | 160 | 160 | 160 | 40 | 80 | 80 |
Serum samples from groups of 17 to 22 mice immunized with dk/Sing vaccine with or without alum or alum alone were collected 3 weeks after the second vaccination and were pooled for HI assay against H5N1 group A and group B viruses.
Serum samples from single ferrets infected with dk/Sing, HK/483, or HK/156 virus were concentrated fourfold. The ferret infected with HK/156 virus was also inoculated i.n. with A/Turkey/Wisconsin/68 virus (H5N9).
Protective efficacy of dk/Sing vaccine.
We next investigated the protective efficacy of the dk/Sing vaccine against a lethal challenge with the group B virus HK/483. This virus was chosen for two reasons. First, HK/483 virus was the most pathogenic of the four H5N1 viruses examined in this study (Table 1 and Fig. 1). Second, the group B viruses have been characterized with reference ferret antiserum to have decreased reactivity with antibody raised to group A H5N1 or dk/Sing viruses. We considered challenge with HK/483 virus to be a more stringent test of protective efficacy.
Four months after vaccination, mice were infected i.n. with 50 LD50 of HK/483 virus. Each vaccine group was then divided into mice (n = 10 to 12) that were monitored for signs of disease and death for 14 days or mice (n = 7 to 10) that were euthanized on day 6 p.i. to determine the level of virus replication in the lung and brain. Day 6 was chosen to evaluate protection from infection because unvaccinated mice were shown previously to have substantial titers of virus in both lungs and brains at this time point. As shown in Table 3, control mice receiving alum alone died 6 to 7 days p.i. with high titers of virus in the lungs and brains. In contrast, 100% of mice vaccinated with dk/Sing virus, with or without alum, survived lethal challenge. Thirty percent of mice that received vaccine alone had low levels of virus in the lungs on day 6 p.i. Virus titers in these mice ranged from 103.0 to 104.0 EID50/ml. No virus was detected in the brains of mice from either vaccine group. When considered as a group, protection from infection in mice receiving vaccine alone was highly significant compared with the control group (P < 0.001). Protection from infection was augmented by the addition of alum to the dk/Sing vaccine. All mice receiving dk/Sing vaccine with alum were completely protected from infection in both lungs and brains. These results demonstrated that vaccination of mice with two doses of dk/Sing (H5N3) vaccine induced a high degree of protection from infection and death following challenge with a highly lethal human H5N1 virus.
TABLE 3.
Protective efficacy of dk/Sing (H5N3) vaccine against lethal infection with HK/483 virus
| Vaccine group | Prechallenge antibody titera against:
|
Mean virus titer (log10 EID50/ml) ± SDb
|
No. protected/total no.c
|
|||||
|---|---|---|---|---|---|---|---|---|
| HK/156
|
HK/483
|
|||||||
| HI | Neutralization | HI | Neutralization | Lung | Brain | Infection | Death | |
| dk/Singd | 80 | 400 | 40 | 100 | 2.6 ± 0.6e | ≤0.8e | 7/10f | 12/12e |
| dk/Sing + alum | 1,280 | 8,000 | 320 | 1,600 | ≤1.2e | ≤0.8e | 8/8e | 12/12e |
| Alum | 10 | 25 | 10 | 25 | 6.3 ± 0.9 | 2.5 ± 1.0 | 0/7 | 0/10 |
Serum samples pooled from 10 mice per group were used.
Mean virus titers and protection from infection were determined on day 6 p.i. Titers represent means ± SD of 7 to 10 mice per group.
Mice were monitored for 14 days after challenge. Mice in the alum-vaccinated group died on day 6 or 7.
Mice were vaccinated with two doses of 10 μg of formalin-inactivated dk/Sing vaccine and challenged 4 months later with 50 LD50 of HK/483 virus.
P < 0.001 compared with alum-vaccinated group.
P < 0.01 compared with alum-vaccinated group.
DISCUSSION
The introduction of influenza A (H5N1) viruses into humans served as an important reminder that another influenza pandemic is highly likely, if not inevitable. The H5N1 outbreak highlighted the potential of pandemic strains to arise directly from avian species, the natural reservoir of all 15 known HA subtypes of influenza A viruses. The outbreak also demonstrated that an avian virus could cross the species barrier to replicate in humans and cause severe disease. The H5N1 viruses resulted in a high rate of mortality in an age group not normally considered to be at increased risk for death and complications from influenza. For these reasons, the H5N1 viruses warrant further study in an effort to understand their pathogenesis in humans and to establish prevention and control measures in preparation for an actual pandemic.
Although the ferret model has been widely used for the study of other influenza A viruses, it has not yet been shown to be a suitable animal model for the study of human H5N1 viruses in vivo. In contrast, the BALB/c mouse was found to be a useful model system for investigation of human H5N1 virus pathogenesis and immunity. The H5N1 viruses replicated in mouse lungs to high titers and caused lethal disease without the prior adaptation that is generally required for human influenza A viruses to possess these properties (18). Furthermore, the highly lethal HK/483 and HK/485 viruses which caused severe or fatal disease in humans were also highly lethal in mice. These viruses replicated systemically and were neurotropic in mice, two properties which have not previously been demonstrated for an influenza A virus without prior adaptation to the host. Although the four H5N1 viruses examined in this study replicated to similarly high titers in the lungs of mice, differences in the lethality of the viruses were observed. HK/483 and HK/485 viruses were highly lethal for BALB/c mice; as little as 1 MID50 of HK/483 virus was sufficient to kill mice. HK/156 virus required a substantially higher MID50 to elicit a lethal infection in mice, and the lethality of HK/486 virus was substantially less than that of the HK/483 and HK/485 viruses. Shortridge et al. (28) have also recently reported that high dose (106 EID50) of HK/156 virus was highly lethal for BALB/c mice. However, in contrast to the results presented here, Gubareva et al. (15) reported that HK/156 virus was lethal for BALB/c mice even at low infectious doses. One reason for the differences in the lethality between the HK/156 virus preparations may be that the HK/156 virus used in the Gubareva study had been passaged twice in mouse lungs prior to characterization for lethality. In the present study, the viruses were only passaged in vitro prior to characterization in vivo. The differences in pathogenicity observed among H5N1 viruses did not correlate with passage history. We have tested the lethality of HK/486 and HK/483 virus stocks which had fewer passages in eggs or which had been passaged only in MDCK cells (HK/486). In each case, the viruses exhibited lethality for mice similar to that reported here (data not shown). It is noteworthy that HK/486 virus, which was substantially less lethal for mice, also caused mild disease in the child from whom it was isolated.
A feature of the highly pathogenic HK/483 viruses was the presence of elevated levels of virus in the spleen, liver, and blood at day 4 p.i. Disease progression was associated with the presence of viral antigens in subepithelial tissues in the lungs. More striking still were substantial titers of virus and viral antigens in the brains of mice infected with HK/483 virus, that increased during the infection and peaked on day 6 just before death. Mice infected with the less pathogenic HK/156 virus had significantly less virus in the brain on day 6 p.i. than did mice infected with HK/483 virus (P < 0.05). Mice infected with HK/486 had no detectable virus in the brain at days 4 and 6. Taken together these results suggest that the dissemination of virus to organs other than the lungs may depend on the extent of viremia that occurs early in the infection. In addition, the more pathogenic viruses may replicate more efficiently in other organs, including the brain. In this study, H5N1 group B viruses were highly pathogenic, whereas group A viruses were of intermediate to low pathogenicity. It is not clear from these results whether the additional glycosylation site in HA1, present in group B viruses but not group A viruses, contributes to the pathogenicity of these viruses. Sequence analysis of the HA gene of viruses used in this study confirmed the presence of multiple basic amino acids adjacent to the cleavage site in all viruses, including HK/486 which was substantially less lethal for mice (data not shown). Therefore, further studies are required to determine the molecular basis for the differences in lethality of the H5N1 viruses.
The mouse model also provided a means to evaluate a strategy for vaccination against H5 viruses. Currently, vaccine strains with an optimal antigenic match are achieved by producing high-growth reassortants from a circulating virus that has the desired antigenic characteristics. Preparation of vaccine candidate strains from the highly pathogenic H5N1 viruses by reassortant or by genetic manipulation and gene rescue techniques must be conducted under BSL-3+ conditions. Extensive BSL-3+ safety testing of those candidate strains would be needed and may delay vaccine production in a pandemic situation. An alternative strategy for the rapid and safe development of a vaccine against a highly pathogenic avian virus is to use an antigenically related nonpathogenic virus that can be handled under laboratory (BSL-2) conditions. This study demonstrated that a vaccine prepared from the nonpathogenic dk/Sing (H5N3) virus effectively protected mice from lethal challenge with the antigenically related but highly pathogenic human HK/483 (H5N1) virus. High titers of serum HI or neutralizing antibody and complete protection from infection and death were achieved in mice administered vaccine in the presence of alum. Mice administered vaccine alone had substantially lower serum HI and neutralizing antibody titers. One hundred percent of these animals were protected from death, but only 70% were protected from infection. Vaccine-induced serum HI titers of 40 or greater were sufficient to protect mice from lethal infection with H5N1 virus, whereas higher titers of antibody were required to protect mice completely from infection. Garcia et al. (13) reported that an inactivated vaccine prepared from low pathogenic A/chicken/Hidalgo/232/94 (H5N2) virus effectively protected chickens from lethal infection with a highly pathogenic influenza H5N2 virus. On the other hand, Kodihalli et al. (21) recently reported that a DNA vaccine encoding HK/156 virus HA, but not A/Turkey/Ireland/1/83 (H5N8) HA, protected BALB/c mice from infection with a lethal dose of HK/156 virus. This lack of protection with a heterologous H5 HA may reflect the greater amino acid sequence difference between the viruses used by Kodihalli et al. (12% difference in HA1) compared with the dk/Sing and HK/483 viruses used in the present study (8% difference in HA1). The present study demonstrated that increased levels of protection from infection correlated with increased serum antibody titers. Therefore, differences between the results of the two studies may, alternatively, reflect differences in the ability of the DNA-based vaccine compared with the protein-based vaccine used in the present study to elicit adequate titers of neutralizing antibody.
The protection afforded by the dk/Sing (H5N3) vaccine against lethal challenge with an H5N1 virus suggested that an antigenically related NA was not necessary for the protective effect observed in mice in this study. However, other investigators have demonstrated that vaccines composed of purified NA protected mice from lethal challenge (12), and the presence of NA-specific antibody has been associated with reduction in the magnitude of disease in humans (9). Therefore, it remains desirable to have both major surface glycoproteins of a candidate vaccine strain antigenically matched with the virus causing disease in humans.
Our studies also demonstrated that alum adjuvant substantially augmented the antibody response to H5 vaccine when mice received two doses of vaccine with adjuvant. However, studies in humans demonstrated that alum was ineffective for enhancing HI antibody to a recombinant H3 HA vaccine (23). These studies were performed with adults who would have previously been infected with related H3N2 viruses and therefore would have cellular responses primed to H3 viruses (14). Because a vaccine against a novel HA subtype may not have this advantage, the effectiveness of adjuvants for vaccination against pandemic influenza should be explored further.
The efficacy of the dk/Sing vaccine supports previous proposals to develop a library of vaccine strains representing different influenza A subtypes that could be evaluated in humans in preparation for future pandemic. Improved surveillance in avian and animal reservoirs may distinguish influenza A subtypes that pose a pandemic threat. These candidate vaccines would be available for a rapid intervention strategy following the emergence and spread of a virus with pandemic potential.
ACKNOWLEDGMENTS
We thank Dennis Alexander, Central Veterinary Laboratory, Surrey, United Kingdom, for providing the dk/Sing virus originally isolated by Yueh Lee-Lin, Veterinary Laboratory Branch, Animal Health and Inspection Division, Singapore; Jing Huang for the confirmatory sequencing of H5N1 virus stocks; Thomas Rowe for preparation of figures; and Kanta Subbarao for critical review of the manuscript.
ADDENDUM IN PROOF
A recent study by Gao et al. (P. Gao, S. Watanabe, T. Ito, H. Goto, K. Wells, M. McGregor, A. J. Cooley, and Y. Kawaoka, J. Virol. 73:3184–3189, 1999) has also demonstrated heterogeneity in the pathogenicity for BALB/c mice of influenza A (H5N1) viruses isolated from humans.
REFERENCES
- 1.Anonymous. Code of federal regulations. Washington, D. C: Office of the Federal Register, National Archives and Records Administration; 1997. Animals and animal products; pp. 683–684. [Google Scholar]
- 2.Bean W J, Kawaoka Y, Wood J M, Pearson J E, Webster R G. Characterization of virulent and avirulent A/chicken/Pennsylvania/83 influenza A viruses: potential role of defective interfering RNAs in nature. J Virol. 1985;54:151–160. doi: 10.1128/jvi.54.1.151-160.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bender C, Hall H, Huang J, Klimov A, Cox N, Hay A, Gregory V, Cameron K, Lim W, Subbarao K. Characterization of the surface proteins of influenza A (H5N1) viruses isolated from humans in 1997–1998. Virology. 1999;254:115–123. doi: 10.1006/viro.1998.9529. [DOI] [PubMed] [Google Scholar]
- 4.Bender, C. Personal communication.
- 5.Centers for Disease Control and Prevention. Isolation of avian influenza A (H5N1) viruses from humans—Hong Kong, May–December. Morbid Mortal Weekly Rep. 1997;46:1204–1207. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Prevention and control of influenza. Recommendations of the advisory committee on immunization practices (ACIP) Morbid Mortal Weekly Rep. 1998;47:1–26. [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Update: isolation of avian influenza A (H5N1) viruses from humans—Hong Kong, 1997–1998. Morbid Mortal Weekly Rep. 1998;46:1245–1247. [PubMed] [Google Scholar]
- 8.Claas E C J, Osterhaus A D M E, Van Beek R, De Jong J C, Rimmelzwaan G F, Senne D A, Krauss S, Shortridge K F, Webster R G. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet. 1998;351:472–477. doi: 10.1016/S0140-6736(97)11212-0. [DOI] [PubMed] [Google Scholar]
- 9.Couch R B, Kasel J A, Gerin J L, Schulman J L, Kilbourne E D. Induction of partial immunity to influenza by a neuraminidase-specific vaccine. J Infect Dis. 1974;129:411–420. doi: 10.1093/infdis/129.4.411. [DOI] [PubMed] [Google Scholar]
- 10.Cox N J, Kendal A P. Genetic stability of A/Ann/6/60 cold-mutant (temperature-sensitive) live influenza virus genes: analysis by oligonucleotide mapping of recombinant vaccine strains before and after replication in volunteers. J Infect Dis. 1984;149:194–200. doi: 10.1093/infdis/149.2.194. [DOI] [PubMed] [Google Scholar]
- 11.De Jong J C, Class E C J, Osterhaus A D M E, Webster R G, Lim W L. A pandemic warning? Nature. 1998;389:554. doi: 10.1038/39218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deroo T, Jou W M, Fiers W. Recombinant neuraminidase vaccine protects against lethal influenza. Vaccine. 1996;14:561–569. doi: 10.1016/0264-410x(95)00157-v. [DOI] [PubMed] [Google Scholar]
- 13.Garcia A, Johnson H, Srivastava DK, Jayawardene DA, Wehr DR, Webster R G. Efficacy of inactivated H5N1 influenza vaccines against A/Chicken/Queretaro/19/95 infection. Avian Dis. 1998;42:248–256. [PubMed] [Google Scholar]
- 14.Gelder C M, Welsh K I, Faith A, Lamb J R, Askonas B A. Human CD4+ T-cell repertoire of responses to influenza A virus hemagglutinin after recent natural infection. J Virol. 1995;69:7497–7506. doi: 10.1128/jvi.69.12.7497-7506.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gubareva L V, McCullers J A, Bethell R C, Webster R G. Characterization of influenza A/HongKong/156/97 (H5N1) virus in a mouse model and protective effect of Zanamivir on H5N1 infection in mice. J Infect Dis. 1998;178:1592–1596. doi: 10.1086/314515. [DOI] [PubMed] [Google Scholar]
- 16.Harmon M W, Rota P A, Walls H H, Kendal A P. Antibody response in humans to influenza virus type B host-cell-derived variants after vaccination with standard (egg-derived) vaccine or natural infection. J Clin Microbiol. 1988;26:333–337. doi: 10.1128/jcm.26.2.333-337.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hinshaw V S, Webster R G, Easterday B C, Bean W J., Jr Replication of avian influenza A viruses in mammals. Infect Immun. 1981;34:354–361. doi: 10.1128/iai.34.2.354-361.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoyle L. Adaptation of virus to mice. In: Gard S, Hallauer C, Meyer K F, editors. The influenza viruses. New York, N.Y: Springer-Verlag; 1968. pp. 170–171. [Google Scholar]
- 19.Katz J M, Lu X, Young S A, Galphin J C. Adjuvant activity of the heat-labile enterotoxin from enterotoxigenic Escherichia coli for oral administration of inactivated influenza virus vaccine. J Infect Dis. 1997;175:352–363. doi: 10.1093/infdis/175.2.352. [DOI] [PubMed] [Google Scholar]
- 20.Kendal A P, Skehel J J, Pereira M S. Concepts and procedures for laboratory-based influenza surveillance. Atlanta, Ga: Centers for Disease Control; 1982. pp. B17–B35. [Google Scholar]
- 21.Kodihalli S, Goto H, Kobasa D L, Krauss S, Kawaoka Y, Webster R G. DNA vaccine encoding hemagglutinin provides protective immunity against H5N1 influenza virus infection in mice. J Virol. 1999;73:2094–2098. doi: 10.1128/jvi.73.3.2094-2098.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matrosovich M, Zhou N, Kawaoka Y, Webster R. The surface glycoproteins of H5 influenza viruses isolated from humans, chickens, and wild aquatic birds have distinguishable properties. J Virol. 1999;73:1146–1155. doi: 10.1128/jvi.73.2.1146-1155.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Powers D C, Smith G E, Anderson E L, Kennedy D J, Hackett C S, Wilkinson B E, Volvovitz F, Belshe R B, Treanor J J. Influenza A virus vaccines contained purified recombinants H3 hemagglutinin are well tolerated and induced protective immune responses in healthy adults. J Infect Dis. 1995;171:1595–1599. doi: 10.1093/infdis/171.6.1595. [DOI] [PubMed] [Google Scholar]
- 24.Reed L J, Muench H. A simple method of estimating fifty per cent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 25.Richmond J Y, Mckinney R W. Laboratory biosafety level criteria. In: Richmond J Y, Mckinney R W, editors. Biosafety in microbiological and biomedical laboratories. 3rd ed. Atlanta, Ga: Centers for Disease Control; 1993. pp. 16–43. [Google Scholar]
- 26.Senne D A, Panigrapy B, Kawaoka Y, Pearson J E, Suss J, Lipkind M, Kida H, Webster R G. Survey of the hemagglutinin (HA) cleavage site sequence of H5 and H7 avian influenza viruses: amino acid sequence at the HA cleavage site as a marker of pathogenicity potential. Avian Dis. 1996;40:425–437. [PubMed] [Google Scholar]
- 27.Shaw, M. Personal communication.
- 28.Shortridge K F, Zhou N N, Guan Y, Gao P, Ito T, Kawaoka Y, Kodihalli S, Krauss S, Markwell D, Murti K G, Norwood M, Senne D, Sims L, Takada A, Webster R G. Characterization of avian H5N1 influenza viruses from poultry in Hong Kong. Virology. 1998;252:331–342. doi: 10.1006/viro.1998.9488. [DOI] [PubMed] [Google Scholar]
- 29.Suarez D L, Perdue M L, Cox N J, Rowe T, Bender C, Huang J, Swayne D E. Comparisons of highly virulent H5N1 influenza A viruses isolated from humans and chickens from Hong Kong. J Virol. 1998;72:6678–6688. doi: 10.1128/jvi.72.8.6678-6688.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subbarao K, Klimov A, Katz J, Regnery H, Lim W, Hall H, Perdue M, Swayne D, Bender C, Huang J, Hemphill M, Rowe T, Shaw M, Xu X, Fukuda K, Cox N. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998;279:393–396. doi: 10.1126/science.279.5349.393. [DOI] [PubMed] [Google Scholar]
- 31.Yuen K Y, Chan P K, Peiris M, Tsang D N C, Que T L, Shortridge K F, Cheung P T, To W K, Ho E T F, Sung R, Cheng A F B Members of the H5N1 Study Group. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet. 1998;351:467–471. doi: 10.1016/s0140-6736(98)01182-9. [DOI] [PubMed] [Google Scholar]
- 32.Zaki S R, Greer P W, Coffield L M, Goldsmith C S, Nolte K B, Foucar K, Feddersen R M, Zumwalt R E, Miller G L, Khan A S, Rollin P E, Ksizaek T G, Nichol S T, Mahy B W, Peters C J. Hantavirus pulmonary syndrome: pathogenesis of an emerging infectious disease. Am J Pathol. 1995;146:552–579. [PMC free article] [PubMed] [Google Scholar]