Abstract
Background
Insulin-like growth factor (IGF)-1 and its binding proteins are important in cancer growth, especially in prostate cancer. Observational studies suggest that protein restriction can lower IGF-1 levels. However, it is unclear whether an isocaloric protein-restricted diet affects IGF-1 and IGFBPs in men with prostate cancer.
Methods
In this academic, single-center, parallel-group, prospective, randomized, open-label, blinded end-point trial, 38 consenting overweight (BMI 30.5 ± 5.5 kg/m2) men with localized prostate cancer, aged 43–72 years, were randomized (1:1) with permuted blocks to 4–6 weeks of customized isocaloric PR diets (0.8 g protein/kg lean body mass) or their usual diet. Biomarkers influencing cancer biology, including serum IGF-1 and its binding proteins were measured longitudinally.
Results
Contrary to our hypothesis, feeding individuals an isocaloric protein-restricted diet did not result in a significant reduction in serum IGF-1. Moreover, there was no observed increase in serum IGFBP-1 or IGFBP-3 concentration.
Conclusion
These findings demonstrate that protein restriction without calorie restriction does not reduce serum IGF-1 concentration or increase IGFBP-1 and IGFBP-3 in men with localized prostate cancer. Further research is needed to identify dietary interventions for safely and effectively reducing IGF-1 in this patient group.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40364-024-00613-w.
To the editor,
The insulin-like growth factor 1 (IGF-1) axis plays a crucial role in the biology of prevalent cancers, including prostate, breast, and colon cancers [1, 2]. In rodents, calorie restriction (CR) lowers plasma IGF-1 concentration by ~ 40%, a reduction that may contribute to its powerful anti-cancer and anti-aging effects [3]. Unlike in rodents, data from observational and randomized trials show that CR with adequate protein intake significantly lowers insulin and increases IGFBP-1, but does not change IGF-1 levels in humans, unless protein intake is also reduced [4, 5]. However, to the best of our knowledge, no randomized feeding trial so far has formally tested the effects of isocaloric protein restriction (PR) on circulating levels of IGF-1 and IGF binding proteins in men with localized prostate cancer who might benefit the most from a sustained reduction in IGF-1 levels.
In this trial, we evaluated the impact of feeding a customized PR diet on serum IGF-1, IGFBP-1 and IGFBP-3 concentrations independent of calorie intake. Otherwise healthy men (aged 43–72 y) diagnosed with localized prostate cancer, scheduled for a radical prostatectomy in four to six weeks, were randomly assigned to a PR diet or their usual ad-libitum Western-like diet. The prescribed protein intake was ~ 0.8 g/kg of lean body mass measured by dual-energy X-ray absorptiometry. PR participants received customized isocaloric diets to maintain body weight.
A protein restricted diet without calorie restriction does not affect the IGF-1 axis
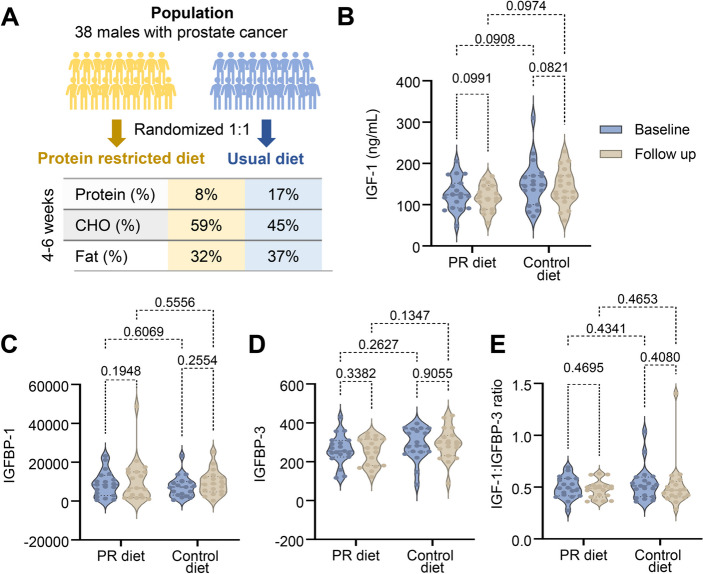
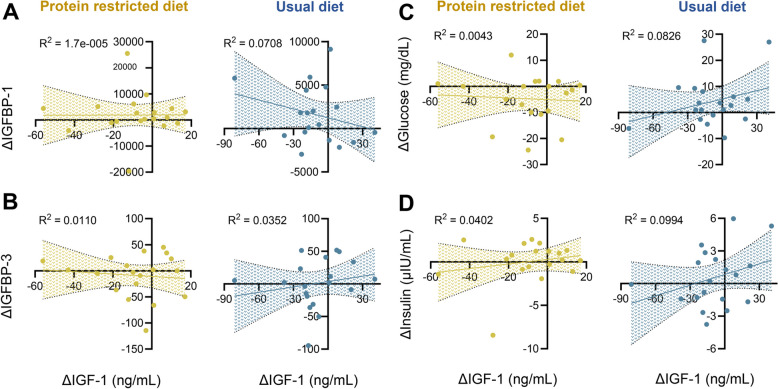
To assess the impact of an isocaloric PR diet on serum IGF-1 and IGFBPs levels, a total of 38 overweight (BMI 30.5 ± 5.5 kg/m2) adult males (59 ± 7 years old) adhered to either a calorie-customized 8% protein diet (n = 19) or a control diet (n = 19) for 43 ± 11 days (Fig. 1a). All meals were provided to maximize compliance. Energy density and macronutrient composition are provided in Supplementary Table 2. Participants in the intervention group consumed 2621 ± 411 kcal/day at baseline and 2856 ± 199 kcal/day during PR (p = 0.06), resulting in slight weight loss (101.5 ± 18.8 kg to 98.8 ± 18.6 kg, p < 0.0001) despite isocaloric intake, as previously reported [6]. Control participants consumed 2664 ± 611 kcal/day at baseline and 2367 ± 445 kcal/day at follow up (p = 0.03), maintaining their body weight (93.2 ± 17.3 kg to 93.0 ± 16.9 kg, p = 0.52). At baseline, average serum IGF-1 levels were 126 ± 37 ng/ml in the PR group and 151 ± 55 ng/ml in the control group (between groups p-value = 0.1295). At follow up, IGF-1 levels were 118 ± 30 ng/ml and 141 ± 44 ng/ml, respectively (between group p = 0.9465), showing no evidence of statistical significance and a clinically negligible reduction of 8 ng/ml in the intervention group (Fig. 1b and Supplementary Fig. 1). Additionally, the levels of IGFBP-1, IGBP-3 and IGF-1 to IGFBP-3 ratio did not show evidence of change with isocaloric PR (Fig. 1c-e and Supplementary Fig. 1 and Table 1). We also examined whether fluctuations in glucose and insulin influenced individual IGF-1 outcomes (Fig. 2). PR led to reduced fasting glucose (113 ± 36 to 106 ± 31 mg/dL, p = 0.02), unlike the control diet (103 ± 16 to 106 ± 20 mg/dL, p = 0.23), whereas insulin remained unchanged in both groups (PR: 7.3 ± 5.3 to 7.4 ± 5.2 µU/mL, p = 0.94; control: 6.8 ± 4.2 to 7.3 ± 4.6 µU/mL, p = 0.4), as previously reported [6]. None of these variables alone explained the changes in IGF-1 levels in either group (p > 0.05 for all; Fig. 2a-d).
Fig. 1.
A protein restricted diet without calorie restriction does not affect the IGF-1 axis. A Schematic representation of the study. The average macronutrient composition (% of energy) was calculated from provided customized meals (intervention = PR diet) and food diary assessments (control = usual diet). B-E Fasting blood measurements of IGF-1, IGFBP-1, IGFBP-3 (absolute) and IGF-1:IGFBP-3 ratio (relative) at baseline and at 4–6 weeks follow up. Each dot represents an individual (n = 19 per group). Violin plots represent the distribution of the variables. Statistical significance is indicated by p-values calculated using 2-way ANOVA for repeated measurements (baseline and follow-up) and multiple comparisons
Fig. 2.
Individual variations in IGF-1 levels are not explained by changes in glucose, insulin, or IGF-binding proteins. A-D Linear regression analysis between changes in IGF-1 (Δ IGF-1) and Δ IGFBP-1, Δ IGFBP-3, Δ glucose, and Δ insulin for the intervention (yellow) and control (blue) arms. Delta (Δ) represents the value at follow up minus the value at baseline. Each dot represents an individual (n = 19 per group). The shaded areas show the 95% confidence bands for the best-fit line. R2 values quantify the strength of the correlation, with R2 < 0.2 suggesting a lack of association
Discussion
This finding challenges the notion that protein intake alone can regulate circulating IGF-1 of IGFBPs levels, as suggested by previous epidemiological studies [7]. Fasting for 3 to 5 days or undergoing severe food restriction (e.g., reducing daily energy intake by over 50%, leading to severe protein restriction) can swiftly and consistently cause a significant decrease in circulating IGF-1 levels in humans [8, 9]. Hence, the absence of an impact on IGF-1 levels from our isocaloric protein restriction cannot be ascribed to a short study duration but rather to the interplay of calorie and protein restriction. Notably, individuals adhering to raw vegetarian diets, maintaining an average daily energy and protein intake of around 1989 kcal and 0.73 g of protein per kg of body weight, respectively, demonstrate lower serum levels of IGF-1 when compared to master athletes of similar BMI [10]. On the other hand, those following high-protein calorie-restricted diets (1772 kcal/day with a protein intake of 1.73 g/kg) did not exhibit lower serum IGF-1 levels, unless there was a concomitant substantial reduction in protein intake [5]. Therefore, a combination of CR and PR is needed to reduce serum IGF-1.
The development of interventions capable of safely and consistently reducing IGF-1 levels, especially in combination with lower insulin, testosterone and inflammation, is of paramount importance, particularly for prostate cancer patients with a high risk of recurrence post-surgery [1, 11]. Approximately 20% to 35% of individuals with prostate cancer who undergo surgery or radiation therapy for localized disease will experience biochemical recurrence [12]. Additional research is required to elucidate what dietary interventions can safely reduce serum IGF-1 levels, particularly in patients with cancers where IGF-1 plays a pivotal role in its development and progression.
Supplementary Information
Acknowledgements
Acknowledgments and role of funding sources. This work was supported by grants to L.F. from the Bakewell Foundation, the Longer Life Foundation (an RGA/Washington University Partnership), the National Center for Research Resources (UL1 RR024992), the Australian NHMRC Investigator Grant (APP1177797), and Australian Youth and Health Foundation. G.F. was supported by the Italian Ministry of Health through “Ricerca Corrente'' and “5x1000”. M.L.C. was supported by a Schmidt Science Fellowship. The funding sources were not involved in any form with the findings presented in the study and its submission for publication. The article was not commissioned. No author was precluded access to data.
Role of funding sources
The funding sources were not involved in any form with the findings presented in the study and its submission for publication. The article was not commissioned. No author was precluded access to data.
Authors’ contributions
Conception and design (L.F.). Trial and sample collection (L.F., B.B., E.C., F.S., N.V., V.T., A.B., R.F., G.A.). Data analysis (G.F., M.C., A.M., V.T.). Interpretation of results and manuscript writing (M.C., L.F.). Review and editing (T.P., G.R.). Funding acquisition (L.F., G.F., M.C). All authors reviewed and approved the final version of the manuscript.
Availability of data and materials
The data underlying this article are available in the article and in its online supplementary material. Request for additional information can be made to the corresponding author.
Declarations
Ethics approval and consent to participate
This study focuses on the secondary outcome (IGF-1 axis) of the trial “Does Protein Restriction Inhibit Prostrate Cancer Growth” (registry: https://www.clinicaltrials.gov/; registration number:NCT01692587; registration date: 2012–09-07). This was a randomized controlled trial performed at Washington University in St. Louis in the United States, aimed to evaluate the effects of isocaloric PR in otherwise healthy men diagnosed with localized prostate cancer. The study adhered to the Declaration of Helsinki 1975 (1983), and the protocol was approved by the institutional review board of Washington University Medical School, St. Louis, MO, United States (IRB #201011804). Written informed consent was obtained from all study volunteers, and oversight was provided by a data and safety monitoring board.
Consent for publication
All data presented in this publication are de-identified and do not contain individual information.
Competing interests
The authors report no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Basu R, Kopchick JJ. GH and IGF1 in cancer therapy resistance. Endocr Relat Cancer. 2023;30(9):e220414. [DOI] [PubMed]
- 2.Breen KJ, O’Neill A, Murphy L, Fan Y, Boyce S, Fitzgerald N, et al. Investigating the role of the IGF axis as a predictor of biochemical recurrence in prostate cancer patients post-surgery. Prostate. 2017;77(12):1288–300. 10.1002/pros.23389 [DOI] [PubMed] [Google Scholar]
- 3.Green CL, Lamming DW, Fontana L. Molecular mechanisms of dietary restriction promoting health and longevity. Nat Rev Mol Cell Biol. 2022;23(1):56–73. 10.1038/s41580-021-00411-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fontana L, Villareal DT, Das SK, Smith SR, Meydani SN, Pittas AG, et al. Effects of 2-year calorie restriction on circulating levels of IGF-1, IGF-binding proteins and cortisol in nonobese men and women: a randomized clinical trial. Aging Cell. 2016;15(1):22–7. 10.1111/acel.12400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fontana L, Weiss EP, Villareal DT, Klein S, Holloszy JO. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell. 2008;7(5):681–7. 10.1111/j.1474-9726.2008.00417.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fontana L, Cummings NE, Arriola Apelo SI, Neuman JC, Kasza I, Schmidt BA, et al. Decreased consumption of branched-chain amino acids improves metabolic health. Cell Rep. 2016;16(2):520–30. 10.1016/j.celrep.2016.05.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giovannucci E, Pollak M, Liu Y, Platz EA, Majeed N, Rimm EB, et al. Nutritional predictors of insulin-like growth factor I and their relationships to cancer in men. Cancer Epidemiol Biomarkers Prev. 2003;12(2):84–9. [PubMed] [Google Scholar]
- 8.Clifton KK, Ma CX, Fontana L, Peterson LL. Intermittent fasting in the prevention and treatment of cancer. CA: a cancer journal for clinicians. 2021;71(6):527–46. [DOI] [PubMed] [Google Scholar]
- 9.Isley WL, Underwood LE, Clemmons DR. Dietary components that regulate serum somatomedin-C concentrations in humans. J Clin Invest. 1983;71(2):175–82. 10.1172/JCI110757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fontana L, Klein S, Holloszy JO. Long-term low-protein, low-calorie diet and endurance exercise modulate metabolic factors associated with cancer risk. Am J Clin Nutr. 2006;84(6):1456–62. 10.1093/ajcn/84.6.1456 [DOI] [PubMed] [Google Scholar]
- 11.Liu G, Zhu M, Zhang M, Pan F. Emerging role of IGF-1 in prostate cancer: a promising biomarker and therapeutic target. Cancers (Basel). 2023;15(4):1287. [DOI] [PMC free article] [PubMed]
- 12.Rischke HC, Knippen S, Kirste S, Grosu AL. Treatment of recurrent prostate cancer following radical prostatectomy: the radiation-oncologists point of view. Q J Nucl Med Mol Imaging. 2012;56(5):409–20. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material. Request for additional information can be made to the corresponding author.