Abstract
As a consequence of being diploid viruses, members of the Retroviridae have a high recombination rate. To measure recombination between two identical sequences within the same RNA molecule per round of retroviral replication cycle, a murine leukemia virus based vector (JZ442 + 3′ Hyg) has been constructed. It carries a drug resistance gene, hyg, and a 290-bp repeat sequence of the 3′ hyg gene inserted into the 3′ untranslated region of the green fluorescent protein gene (gfp). Under fluorescence microscopy, Hygr cells containing the recombinant proviruses were clear, while a green color was observed in the drug-resistant cells carrying the parental proviruses. The rate of recombination was determined by the ratio of the number of clear colonies to the total number of Hygr colonies (green and clear colonies). The rate of recombination was found to be 62% by this method. The intermolecular recombination rate between an infectious virus bearing two copies of the 290-bp segment and a noninfectious chimeric RNA virus containing only a single copy of this sequence was also measured.
The presence of two genomic RNA molecules in retroviral virions results in a high rate of recombination (4). To study recombination, two different proviruses were introduced into one retroviral helper cell line. The recombinants were then judged by the expression of markers from both parental proviruses. Evidence shows that intermolecular recombinations occur frequently (8, 17). Previously, it was deduced that recombination may occur between two sequences within the same RNA molecule (intramolecular) as well as between sequences within two separate RNA molecules (intermolecular) (15). If intramolecular recombination occurs, it will result in a deletion within the progeny provirus. However, there is no direct evidence indicating that intramolecular recombination occurs. This is because, in the experiments described above, retroviral particles were able to package either two different RNA molecules or two identical RNA molecules. The deletion can be the result of an intramolecular event or the recombination of an upstream sequence from one RNA molecule with a downstream sequence from the other RNA molecule. Since current technology cannot separate viral particles containing two identical molecules (homogeneous) from those containing two different molecules (heterogeneous), intermolecular recombination cannot be excluded as the source of deletion recombinants.
Previously, retroviral vectors containing two identical sequences separated by a packaging signal within the same RNA molecule were constructed and used to infect cells to examine deletion rates (5, 9). The investigators found that the rate of deletion in progeny proviruses was 57 to 93% after one round of replication. This result does not exclude the possibility that the high rate of deletion resulted from the packaging signal being a hot spot for reverse transcriptase template switching events (9). In addition, because the viruses analyzed were from a pool of transfected cells (5), it could not be determined if the deletion occurred during reverse transcription or occurred during transfection with the proviral DNA.
To further study recombination between two identical sequences within the same RNA molecule, murine leukemia virus (MLV)-based vector (JZ442 + 3′ Hyg) has been constructed. This vector contains a whole hyg gene and an unselected color marker reporter gene, the green fluorescent protein gene (gfp). Additionally, it carries an extra 3′ hyg gene segment of 290 bp inserted into the 3′ untranslated region of the gfp gene. This construct allows us to demonstrate that a high rate of deletion does not relate to the packaging signal sequence. The intermolecular recombination rate between an infectious virus bearing two copies of the 290-bp segment and a chimeric RNA virus containing a single copy of this sequence was also measured. The rate of intermolecular recombination in the presence of two copies of identical sequences on the infectious RNA molecule did not increase much compared with the rate (62%) of recombination between the two identical sequences on the same RNA molecule.
MATERIALS AND METHODS
Nomenclature.
Plasmids are designated as, for example, pJZ442; viruses made from these plasmids are designated as, for example, JZ442. Some infectious Moloney murine leukemia virus (MLV) vectors contained a 290-bp sequence (3′ hyg). When the 290-bp sequence was inserted at the 5′ end of the neo gene, the number of nucleotides inserted is on the left of the “N” (N stands for neo) (for example, pL290N). When the 290-bp sequence was inserted at the 3′ end of the neo gene, the number of nucleotides inserted is on the right of the “N” (for example, pLN290).
Vector constructions.
All recombinant techniques were carried out by conventional procedures (14). All vector sequences are available upon request.
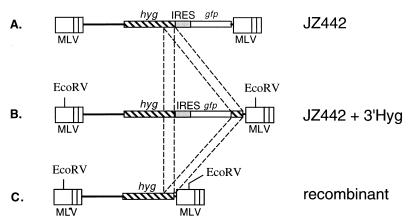
(i) Construction of pJZ442 and pJZ442 + 3′ Hyg (Fig. 1A and B).
FIG. 1.
Structures of retrovirus vectors used for determination of the recombination rate between two identical sequences within the same RNA molecule. (A) Structure of the retrovirus vector containing the hyg gene and the gfp gene. The hyg gene is expressed from the 5′ MLV LTR, and the gfp gene is expressed from an encephalomyocarditis virus IRES. (B) Structure of the retrovirus containing two identical sequences. JZ442 + 3′ Hyg is similar to JZ442, except that JZ442 + 3′ Hyg also contains 290 bp of the 3′ hyg gene sequence downstream of the gfp gene. (C) Structure of the recombinant provirus. After one round of replication, the downstream 3′ hyg gene sequence will recombine with the identical upstream hyg gene sequence and result in the deletion of the gfp gene. Recombinants, therefore, contain only the hyg gene. The broken lines between JZ442, JZ442 + 3′ Hyg, and the recombinant provirus indicate the identical 3′ hyg gene sequences.
The pJZ442 construct, from 5′ to 3′, was assembled as follows. The 5.4-kb NdeI-BamHI fragment (from positions 2990 to 1630) was isolated from pLN (12) and contains the neo gene and the two MLV long terminal repeats (LTRs). The 0.7-kb BamHI-SalI fragment (from positions 1631 to 2387) was isolated from pEGFP-1 (Clontech, Palo Alto, Calif.). The 0.6-kb SalI-NdeI fragment (from positions 2388 to 2989) was isolated from pCITE-1 (Novagen, Madison, Wis.) and contains the internal ribosome entry segment (IRES) sequence. In pJZ442 + 3′ Hyg, the SacII-HindIII fragment (290 bp long) of the 3′ hyg sequence was inserted at the NotI and ClaI sites of pJZ442, which are located downstream of the open reading frame of the gfp gene.
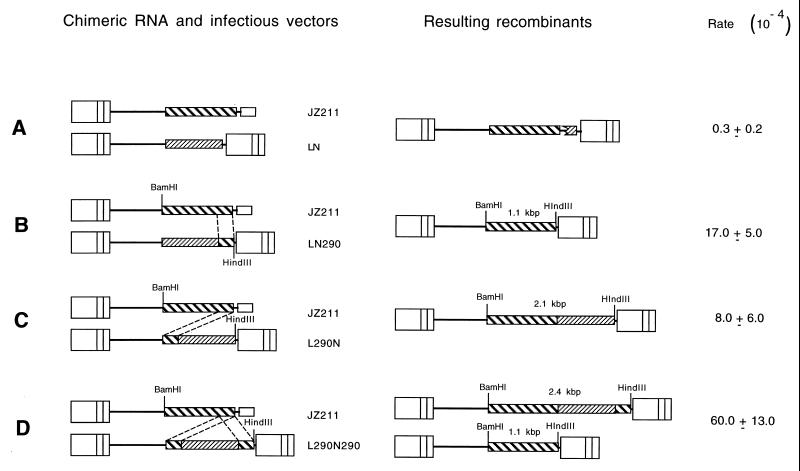
(ii) Construction of pJZ211, pLN290, pL290N, and pL290N290 (Fig. 2).
FIG. 2.
Chimeric RNA vector, infectious virus vectors, and resulting recombinants. JZ211 contains only the 5′ MLV LTR, while the infectious vectors LN, LN290, L290N, and L290N290 contain two MLV LTRs. The recombinant proviruses containing the hyg gene form only when recombination occurs between JZ211 and an infectious vector such that the hyg gene is flanked by two LTRs. Recombination between JZ211 and LN is nonhomologous (17). Most recombinations between JZ211 and LN290, L290N, and L290N290 occurred between the 290-bp identical sequences. The broken lines between the chimeric RNA vector and the infectious vectors indicate the identical 290-bp 3′ hyg sequences in the two vectors. The resulting recombinants correspond to individual pairs of chimeric RNA and infectious vectors. The lengths of the BamHI-HindIII fragments which hybridized to a hyg probe are shown for the recombinants. Two recombinants resulted from recombination between JZ211 and L290N290: one, utilizing the upstream hyg sequence, gives a 2.4-kb BamHI-HindIII fragment, while the other, utilizing the downstream hyg sequence, gives a 1.1-kb BamHI-HindIII fragment. Rates of recombination are shown on the right.
The pJZ211 and pLN290 constructs were described previously (17, 18). The 290-bp hyg sequence in pL290N and pL290N290 was cloned as the SacII-HindIII fragment from pJZ211.
Cells, transfection, and infection.
The processing of D17 cells (a dog osteosarcoma cell line; ATCC CRL-8468), PA317 helper cells (ATCC CRL-9078), and PG13 helper cells (ATCC CRL-10686), DNA transfections, virus harvesting, and virus infections were as previously described (17).
Introduction of JZ442 + 3′ Hyg (and JZ442) into helper cell line PG13.
Plasmid DNA of pJZ442 + 3′ Hyg (and pJZ442) was transfected into an MLV amphotropic helper cell line, PA317 (10). The supernatant media containing the viruses were collected and designated STEP 1 virus stock. The STEP 1 viruses were used to infect an MLV xenotropic helper cell line, PG13 (11). The viruses released from infected PG13 cells were unable to infect NIH 3T3 derivatives, including PG13 (11). This procedure ensured that the infection of D17 cells with viruses collected from PG13 cells represented only a single round of infection. Infected cells were selected for hygromycin resistance (Hygr). Visible colonies appeared about 10 days after selection. The cells of well-separated green colonies were isolated and designated STEP 2 cells.
Fluorescence microscopy.
A fluorescence inverted microscope (Zeiss Axiovert 25) with a mercury arc lamp (100 W) and a fluorescence filter set (CZ909) consisting of a 470- to 40-nm exciter, a 515-nm emitter, and a 500-nm beam splitter was used to detect green fluorescent protein in living cells.
RESULTS
Determination of the recombination rate within one retroviral RNA molecule.
A bicistronic MLV-based vector (pJZ442) that carries a drug resistance gene, hyg, and an unselected color reporter gene, gfp (3), along with an IRES sequence between the two genes, has been constructed (Fig. 1A). The IRES sequence of the encephalomyocarditis virus origin allows the ribosome to bind to the internal AUG that initiates the translation of the second gene independently of the upstream gene (1, 2). To measure the recombination rate between two identical sequences within the same RNA molecule, another vector (pJZ442 + 3′ Hyg) that also contains the hyg and gfp genes but also includes the insertion of a sequence homologous to 290 bp of the 3′ hyg gene into the 3′ untranslated portion of the gfp gene (downstream of the gfp gene or after the stop codon of the gfp gene) has been constructed (Fig. 1B).
pJZ442 + 3′ Hyg was used to transfect PG13 cells, and transfected cells were selected for Hygr. Hygr cells were analyzed under a fluorescence microscope. Green cells contained parental JZ442 + 3′ Hyg, and clear cells contained a gfp gene deletion (or mutation) in the transfected provirus. Approximately 48.8% ± 10.2% of the transfected Hygr PG13 cells were clear. Therefore, transfection alone caused a high frequency of deletion (or mutation) between the two identical sequences in the same plasmid DNA. To avoid a high frequency of deletion during transfection, JZ442 and JZ442 + 3′ Hyg were introduced by infection into the helper cell line PG13 described in Materials and Methods. The viruses released from each PG13 clone, which contained JZ442 or JZ442 + 3′ Hyg provirus, were used to infect D17 cells; the infected D17 cells were selected for Hygr. After about 12 days of selection, visible Hygr colonies appeared, and these were designated STEP 3 cells. Because D17 cells do not contain viral gag-pol and env gene products for retroviral replication, no progeny viruses were released from these cells (17). Therefore, each colony of Hygr cells represented a single viral infection.
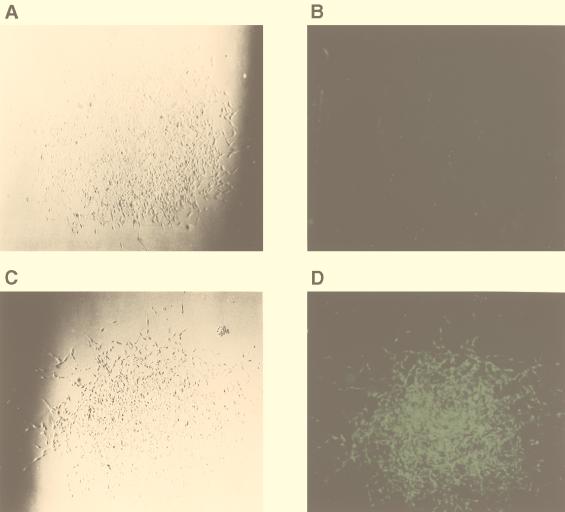
Individual Hygr colonies were examined under a fluorescence microscope. The clear colonies (Fig. 3A and B) represented cells containing the proviruses with the gfp deletion. The green colonies (Fig. 3C and D) and represented cells containing the parental proviruses. Results from counting green and clear colonies (Table 1) indicated that the rate of deletion between the two identical sequences in the same RNA molecule was very high (62% ± 9% per replication cycle). In comparison, only 1% of the colonies infected with JZ442 were clear. This result indicates that the rate of mutation of the gfp gene in the JZ442 vector during a single round of retroviral replication is about the same as previously reported (13). To determine the nature of the recombinants, genomic DNAs from clear and green STEP 3 cells were digested with EcoRV. EcoRV digested within the LTRs of the vectors; the parental provirus produced a 4.2-kb fragment, while the recombinant provirus with the deletion of the gfp gene produced a 2.5-kb fragment (Fig. 4). The recombinant formed a distinct 2.5-kb band, indicating that most deletions of the gfp gene resulted from recombination between the two identical sequences.
FIG. 3.
Microscopic analyses of D17 cells infected with viral vector JZ442 + 3′ Hyg containing the gfp gene. (A) Visible-light microscopy of a hygromycin-resistant colony containing recombinant JZ442 + 3′ Hyg provirus. (B) Fluorescence microscopy of a hygromycin-resistant colony (same colony as in panel A) containing recombinant JZ442 + 3′ Hyg provirus. (C) Visible-light microscopy of a hygromycin-resistant colony containing parental JZ442 + 3′ Hyg provirus. (D) Fluorescence microscopy of a hygromycin-resistant colony (same colony as in panel C) containing parental JZ442 + 3′ Hyg provirus.
TABLE 1.
Microscopic analysis of D17 cells infected with JZ442 and JZ442 + 3′ Hyga
| JZ442 clone | No. of colonies that were
|
JZ442 + 3′ Hyg clone | No. of colonies that were
|
|||||
|---|---|---|---|---|---|---|---|---|
| Green | Clear | Mixture | Green | Clear | Mixture | |||
| 1 | 124 | 1 | 1 | 1 | 27 | 55 | 1 | |
| 2 | 124 | 1 | 0 | 2 | 14 | 70 | 3 | |
| 3 | 105 | 1 | 0 | 3 | 33 | 53 | 5 | |
| 4 | 112 | 2 | 0 | 4 | 13 | 19 | 0 | |
| 5 | 72 | 0 | 1 | 5 | 19 | 25 | 0 | |
| 6 | 132 | 2 | 0 | 6 | 33 | 46 | 0 | |
| 7 | 28 | 32 | 0 | |||||
| Total | 669 | 7 | 2 | Total | 167 | 300 | 9 | |
Each clone was an individual clone of PG13 cells containing the JZ442 or JZ442 + 3′ Hyg provirus. The colonies were analyzed under a fluorescence microscope. The rates of gfp deletion were 1% for JZ442 clones and 62% ± 9% for JZ442 + 3′ Hyg clones.
FIG. 4.
Southern analysis of chromosomal DNA of Hygr cells. Cellular DNA isolated from STEP 3 cells was digested with EcoRV and hybridized with a hyg gene probe. Hygr cells had a deletion between the two identical sequences in one RNA molecule of vector JZ442 + 3′ Hyg. Pooled DNA was isolated from more than 500 Hygr STEP 3 colonies. The clear clone is an individual Hygr colony resulting from deletion of the gfp gene between the two 3′ hyg gene segments. The green clone is an individual Hygr colony. Molecular sizes are shown on the left.
Intermolecular recombination between a noninfectious RNA and an infectious RNA containing two identical sequences.
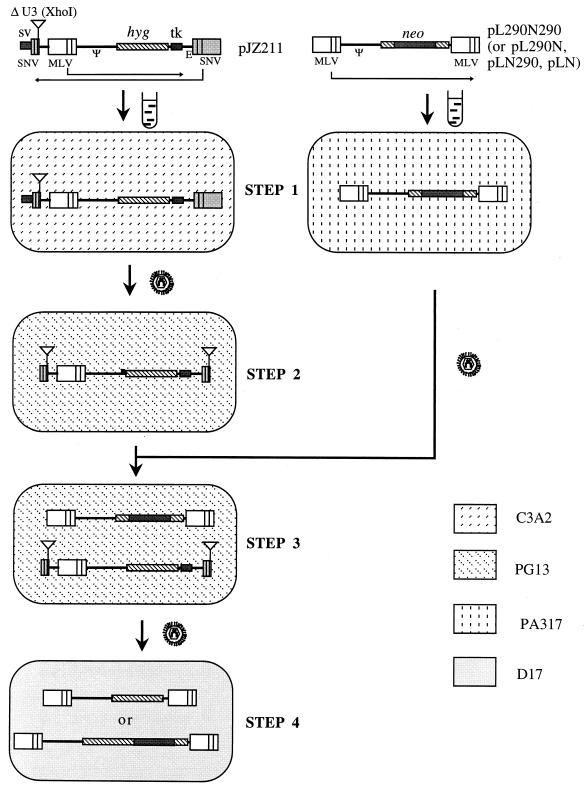
The retroviral vectors and protocol used to measure rates of recombination have been described previously (17). In order to study recombination between chimeric and infectious MLV RNAs, a chimeric RNA, JZ211 (Fig. 2), was prepared. JZ211, derived from spleen necrosis virus (SNV), contained a deletion in the U3 region of the 3′ SNV LTR as well as an XhoI restriction site linker in the deletion site (Fig. 5). This vector also contained a truncated MLV vector between the two SNV LTRs, in a transcriptional orientation opposite that of the SNV LTRs. In this truncated MLV vector, the hyg gene was expressed from the 5′ MLV LTR, and a herpes simplex virus thymidine kinase poly(A) addition signal replaced the completely deleted 3′ MLV LTR. The infectious MLV vectors contained a neo gene between the two MLV LTRs (17, 18) (Fig. 2 and 5). pJZ211 DNA was transfected into the SNV C3A2 helper cell line (containing the SNV gag-pol and env genes) (16) (Fig. 5). The cells were selected for Hygr, and resistant cells were pooled and designated STEP 1 cells (Fig. 5, STEP 1). Virus from STEP 1 cells was used to infect the MLV helper cell line PG13. Infected cells were selected for Hygr, and individual clones were isolated and designated STEP 2 cells. The structure of the proviruses formed from the SNV U3-negative vector in the PG13 cells was monitored by Southern (DNA) analysis. The XhoI linker in JZ211 was duplicated in the 5′ LTR during the formation of the STEP 2 provirus (Fig. 5, STEP 2) (7). The STEP 2 clones that contained the expected XhoI fragment, which hybridized to a hyg probe, were used for further analysis (Fig. 5, STEP 2). To test whether any virus capable of forming Hygr colonies was produced by STEP 2 cells, the supernatant medium (3 ml) from each STEP 2 cell clone was used to infect D17 cells, and the infected cells were selected for Hygr. No Hygr D17 colonies were detected. This was because the deletion of the U3 region (promoter and enhancer) in the SNV 5′ LTR prevented transcription from the SNV vector (6).
FIG. 5.
Outline of an experimental approach for the determination of the rate of recombination during a single cycle of retroviral replication between a chimeric RNA vector, JZ211, and infectious vectors. No plasmid backbone sequences are shown. The directions of transcription in SNV and MLV are shown by the long thin arrows. Transfections are indicated by test tube shapes. Infections are indicated by virion shapes. The different backgrounds represent the indicated cell lines. SV, late polyadenylation signal of simian virus 40; Ψ and E, encapsidation sequences of MLV and SNV, respectively; tk, thymidine kinase. The lines in the LTR separate the U3, R, and U5 regions.
The infectious MLV vectors LN, LN290, L290N, and L290N290 (Fig. 2) were transfected individually into the helper cell line PA317 (Fig. 5). Cells exhibiting the Neor phenotype were pooled, viruses from the PA317 cells were used to superinfect STEP 2 cells containing JZ211, and the infected cells were selected for Neor. Individual Neor clones were isolated and designated STEP 3 cells (Fig. 5, STEP 3). Each STEP 3 cell clone contained a single JZ211 integration and a single integration of LN, LN290, L290N, or L290N290. Viruses from each STEP 3 clone were used to infect D17 cells, and the infected cells were selected separately for Hygr and for Neor. The resulting cells were designated STEP 4 cells (Fig. 5, STEP 4). With this approach, Hygr colonies form only when recombination between JZ211 and any one of the three vectors occurs at the shared 290-bp homologous sequences, so that the hyg gene is flanked by two LTRs. If a nonhomologous recombination event occurs between JZ211 and LN, it results in a Hygr colony. The rate of recombination is much lower than the rates for vectors containing the 290-bp sequence when there are shared homologous sequences (Fig. 2A) (17, 18). The target cells do not contain viral gag-pol and env gene products for retrovirus replication; therefore, no progeny virus can be released from them (17). Consequently, these vector viruses had undergone only one cycle of replication. LN290 and L290N contained the same 290-bp 3′ hyg sequence, except that the 290-bp sequence of LN290 was inserted at the 3′ end of neo and served as the 3′ untranslated sequence, whereas the 290-bp sequence of L290N was inserted at the 5′ end of neo and served as the 5′ untranslated sequence (Fig. 2B and C). This 290-bp sequence does not contain an ATG motif; therefore, neo translation should not be affected by the 5′ insertion (Fig. 2A and B). The ratios of Hygr CFU to Neor CFU produced were 8 × 10−4 ± 6 × 10−4 and 17 × 10−4 ± 5 × 10−4, respectively, for LN290 and L290N. L290N290 contained two copies of sequences identical to that in JZ211. Specifically, the 290-bp 3′ hyg sequence was inserted both upstream and downstream of the neo gene within this vector. The rate of recombination between JZ211 and L290N290 was 60 × 10−4 ± 13 × 10−4, and the rate of recombination between JZ211 and LN was only 0.3 × 10−4 ± 0.2 × 10−4.
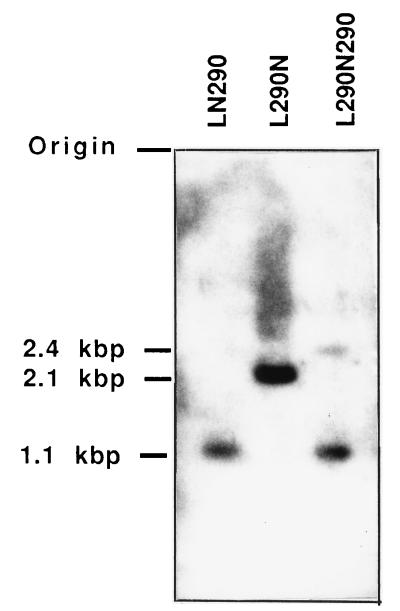
To determine whether recombination had occurred at the 5′ or at the 3′ end of the hyg sequence, the DNA of pooled STEP 4 cells was digested with BamHI and HindIII and hybridized with a hyg probe. The proviruses resulting from recombination between JZ211 and L290N290 utilizing upstream and downstream hyg sequences produced different sizes of BamHI-HindIII fragments that hybridized with a hyg probe (Fig. 2). The recombinants utilizing the upstream hyg sequence produced a 2.4-kb BamHI-HindIII hyg fragment, and those utilizing the downstream hyg sequence produced a 1.1-kb BamHI-HindIII hyg fragment. The pooled recombinants from JZ211 and L290N290 yielded a 1.1-kb fragment and a 2.4-kb fragment, indicating that both identical sequences were used to form the recombinants between the chimeric RNA and the infectious vector (Fig. 6).
FIG. 6.
Southern analysis of recombinants between JZ211 and L290N290. Chromosomal DNA of Hygr STEP 4 cells was used. Cellular DNAs isolated from STEP 4 cells were pooled from more than 100 Hygr colonies, digested with BamHI and HindIII, and hybridized with a hyg gene probe (Fig. 2). LN290, L290N, and L290N290 represent Hygr cells resulting from recombination between JZ211 and LN290, L290N, and L290N290, respectively. Molecular sizes are shown on the left.
DISCUSSION
Because they have two genomic RNA molecules in their virions, retroviruses undergo recombination at a high rate. Our data indicate that the high rate of recombination between two identical sequences within the same RNA molecule was not dependent on the packaging signal. The rate of intermolecular nonhomologous deletion in a single RNA molecule (10−5 bp per replication cycle) is about 1,000 times higher than that of intermolecular nonhomologous recombination (10−8 bp per replication cycle) (13).
Recombination between sequences within the same RNA molecule can be intermolecular or intramolecular. Recombination between a chimeric RNA vector and infectious vectors containing one or two copies of the identical sequences was examined. The results indicated that the presence of two copies of identical sequences on the infectious RNA increased the rate of recombination very little (6 × 10−3) compared with a 60% increase in the rate of recombination (6 × 10−1) between the same 290-bp identical sequences in the same RNA molecule. In addition, the actual titers of the infectious viruses, as measured by Neor, were even higher. This result would be due to intrastrand recombination within the two identical sequences on either side of the neo gene. From the data presented in this study, the deletion rate should be nearly 50%, which would account for the discrepancy observed. However, it is interesting to observe that most intermolecular recombination events either have involved the downstream stretch of identical sequence or have already involved an intramolecular recombination event between the two copies of the 290-bp stretch. This finding suggests the phenomenon of negative interference, which indicates that intramolecular recombination increases the chance of intermolecular recombination or vice versa. However, the individual rate of inter- versus intramolecular recombination between two identical sequences within the same RNA molecule remains unresolved.
ACKNOWLEDGMENTS
We thank William Bargmann, Chih-Li Hsu, Alan Kaplan, Alan Simmons, and Ting Li for helpful comments on the manuscript.
This research was supported by Public Health Service research grant CA70407 from the National Institutes of Health.
REFERENCES
- 1.Adam M A, Ramesh N, Miller A D, Osborne W R. Internal initiation of translation in retroviral vectors carrying picornavirus 5′ nontranslated regions. J Virol. 1991;65:4985–4990. doi: 10.1128/jvi.65.9.4985-4990.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boris-Lawrie K A, Temin H M. Recent advances in retrovirus vector technology. Curr Opin Genet Dev. 1993;3:102–109. doi: 10.1016/s0959-437x(05)80349-1. [DOI] [PubMed] [Google Scholar]
- 3.Chalfie M, Tu Y, Euskirchen G, Ward W W, Prasher D C. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- 4.Coffin J M, Hughes S H, Varmus H. Retroviruses. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1997. [PubMed] [Google Scholar]
- 5.Delviks K A, Hu W-S, Pathak V K. Ψ− vectors: murine leukemia virus-based self-inactivating and self-activating retroviral vectors. J Virol. 1997;71:6218–6224. doi: 10.1128/jvi.71.8.6218-6224.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dornburg R, Temin H M. Retroviral vector system for the study of cDNA gene formation. Mol Cell Biol. 1988;8:2328–2334. doi: 10.1128/mcb.8.6.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dougherty J P, Temin H M. Determination of the rate of base-pair substitution and insertion mutations in retrovirus replication. J Virol. 1988;62:2817–2822. doi: 10.1128/jvi.62.8.2817-2822.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu W S, Temin H M. Genetic consequences of packaging two RNA genomes in one retroviral particle: pseudodiploidy and high rate of genetic recombination. Proc Natl Acad Sci USA. 1990;87:1556–1560. doi: 10.1073/pnas.87.4.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Julias J G, Hash D, Pathak V K. E− vectors: development of novel self-inactivating and self-activating retroviral vectors for safer gene therapy. J Virol. 1995;69:6839–6846. doi: 10.1128/jvi.69.11.6839-6846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller A D, Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986;6:2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller A D, Garcia J V, von Suhr N, Lynch C M, Wilson C, Eiden M V. Construction and properties of retrovirus packaging cells based on gibbon ape leukemia virus. J Virol. 1991;65:2220–2224. doi: 10.1128/jvi.65.5.2220-2224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller A D, Rosman G J. Improved retroviral vectors for gene transfer and expression. BioTechniques. 1989;7:980–982. , 984–986, 989–990. [PMC free article] [PubMed] [Google Scholar]
- 13.Pulsinelli G A, Temin H M. Characterization of large deletions occurring during a single round of retrovirus vector replication: novel deletion mechanism involving errors in strand transfer. J Virol. 1991;65:4786–4797. doi: 10.1128/jvi.65.9.4786-4797.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 15.Skalka A M, Goff S. Reverse transcriptase. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1993. [PubMed] [Google Scholar]
- 16.Watanabe S, Temin H M. Construction of a helper cell line for avian reticuloendotheliosis virus cloning vectors. Mol Cell Biol. 1983;3:2241–2249. doi: 10.1128/mcb.3.12.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, Temin H M. Rate and mechanism of nonhomologous recombination during a single cycle of retroviral replication. Science. 1993;259:234–238. doi: 10.1126/science.8421784. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Temin H M. Retrovirus recombination depends on the length of sequence identity and is not error prone. J Virol. 1994;68:2409–2414. doi: 10.1128/jvi.68.4.2409-2414.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]