Abstract
Background:
Understanding blood alcohol concentrations (BAC) achieved after drinking is critical to predicting alcohol exposure to the brain and other organs and alcohol effects. However, predicting such end-organ exposures is challenging, as there is a wide variation in BAC achieved after drinking a similar amount of alcohol. This variation is partly due to differences in body composition and alcohol elimination rates (AER), but there is limited data on how obesity affects AER. Here we assess associations between obesity, fat-free mass (FFM), and AER in women and determine whether bariatric surgeries, which are linked to increased risk for alcohol misuse, affect these associations.
Methods:
We conducted an analysis of data from three studies that used similar intravenous alcohol clamping procedures to estimate AER in 143 women (21-64 years old) with a wide range of body mass index (BMI;18.5-48.4 kg/m2). Body composition was measured in a subgroup using dual-energy X-ray absorptiometry (n=42) or Bioimpedance (n=60), and 19 of them underwent bariatric surgery 2.1 ± 0.3 years before participation. We analyzed data using multiple linear regression analyses.
Results:
Obesity and older age were associated with a faster AER (BMI: rs= 0.70 and age: rs= 0.61, both P< 0.001). Compared to women with normal weight, AER was 52% faster (95% Confidence Interval: 42-61%) in women with obesity. However, BMI lost predictive value when adding fat-free mass (FFM) to the regression model. Age, FFM, and its interaction explained 72% of individual variance in AER (F(4, 97) = 64.3, P < 0.001). AER was faster in women with higher FFM, particularly for women in the top tertile of age After controlling for FFM and age, bariatric surgery was not associated with differences in AER (P= 0.74).
Conclusions:
Obesity is associated with a faster AER, but this association is mediated by an obesity-related increase in FFM, particularly in older women. Previous findings of a reduced alcohol clearance post-bariatric surgery compared to pre-surgery are likely explained by a reduction in FFM post-surgery.
Keywords: Alcohol Elimination Rate, BMI, Obesity, Fat-free mass, Bariatric surgery
Introduction
Alcohol is the most widely used psychoactive drug in the world and the leading cause of premature death among people aged 15-49 years, according to the Wealth Health Organization (Griswold et al., 2018). The short and long-term pharmacologic and toxic effects of alcohol depend substantially on blood alcohol concentrations (BAC) reached after drinking (Graham et al., 1998). Therefore, understanding the fate of alcohol while it moves throughout the body (i.e., alcohol's pharmacokinetics), which determines the BAC-time profile achieved after alcohol ingestion, is critical to predict exposure to the brain and other organs and, in turn, the pharmacologic and toxic effects of alcohol (i.e., alcohol’s pharmacodynamics).
However, predicting individual BAC after alcohol ingestion is challenging. The same dose of alcohol per kilogram of body weight consumed over the same time can result in different BAC across individuals (Ramchandani et al., 1999) and, to a lesser but still significant degree, within individuals under similar conditions (Norberg et al., 2003, Jones and Jönsson, 1994). Variations in the rate of absorption from the gut and in the amount of ingested alcohol that is metabolized before reaching the bloodstream (i.e., first-pass metabolism) are among the most important factors underlying large inter- and intra-individual variations in BAC (Saldich et al., 2021, Lee et al., 2006). Nevertheless, even when researchers administer alcohol intravenously on a dose/kg-related basis, bypassing the highly variable absorption processes and alcohol's first-pass metabolism, BAC achieved can vary by 1.5- to 2.0-fold (Hahn et al., 1994).
Two important contributing factors to individual differences in BAC achieved after intravenous administration of alcohol are liver volume and body composition (Kwo et al., 1998, Jones, 2019). Studies that administered alcohol intravenously have used a single bolus dose (e.g., Hahn et al., 1994) or an alcohol clamp technique (e.g., Kwo et al., 1998). The clamp technique involves infusing alcohol intravenously following pre-estimated personalized infusion-rate profiles computed using a physiologically-based pharmacokinetic (PBPK) model that takes into consideration the individual’s age, height, weight, and gender to achieve and maintain breath alcohol concentrations (BrAC) at a target level for a prolonged period (Ramchandani et al., 1999). Using this technique, it has been shown that the systemic alcohol elimination rate (AER) is positively associated with liver volume in both men and women (Kwo et al., 1998). The dependence of AER on liver volume is logical because most post-absorbed alcohol (95-98%) is removed from the body via oxidative metabolism in the hepatocyte, mainly by alcohol dehydrogenase (ADH) (Jones, 2019). Body composition, particularly the fat-to-lean tissue ratio, affects the volume of distribution of alcohol (Vd), as distribution into fat is almost negligible (Endres and Grüner, 1994). Therefore women, who generally have a higher percentage of body fat than men, reach higher peak BAC when both sexes receive the same intravenous dose of alcohol per kilogram of body weight (Hahn et al., 1994).
Given the critical role that body composition has on BACs achieved after alcohol ingestion, and even when administered intravenously, the best strategy to estimate BAC achieved after drinking in people with obesity is unclear. The Vd for alcohol is disproportionately low for people with obesity (Jones, 2010, Maudens et al., 2014). Furthermore, the two-fold increase in the risk of developing alcohol use disorders after undergoing some bariatric surgeries (Mellinger et al., 2021, King et al., 2012, Ostlund et al., 2013, King et al., 2017, Ibrahim et al., 2019) highlights the need to understand better the influence of obesity and body composition on alcohol pharmacokinetics. The two most commonly performed bariatric surgeries in the world, i.e., Roux-en-Y-gastric bypass and sleeve gastrectomy, can double peak BAC when drinking a similar alcohol dose than before surgery (Pepino et al., 2015, Acevedo et al., 2018). While these surgeries remarkably reduce body weight and increase alcohol bioavailability by accelerating alcohol absorption (Pepino et al., 2015; Acevedo et al., 2018, Klockhoff et al., 2002, Steffen et al., 2013) and minimize alcohol first-pass metabolism (Seyedsadjadi et al., 2022), their effect on AER is incompletely understood. At least two observations suggest alcohol clearance is slower after bariatric surgery. First, a study on patients who underwent gastric bypass surgery shows that patients took longer to reach zero BAC than non-operated normal weight controls after drinking the same amount of alcohol (Hagedorn et al., 2007). Second, we have previously shown that the total amount of alcohol eliminated from the body per hour is higher in women assessed before bariatric surgery than in women assessed post-bariatric surgery (Acevedo et al., 2018). However, the differences in alcohol eliminated per hour between pre-surgery and post-surgery groups became negligible when corrected for body weight (Acevedo et al., 2018). Of importance, in these two previous studies, alcohol was given orally, and therefore changes in alcohol elimination kinetics were confounded by changes in absorption kinetics.
The present study aimed to assess associations between AER and body composition in women with a wide range of body mass index (BMI). Using the alcohol clamp, which avoids the confounding effects of alcohol absorption processes, we measured AER in women whose BMI resulted in categorizations ranging from normal to severe obesity. Unlike other methods for estimating AER, the alcohol clamp is especially suited to individuals with obesity because, during the steady state “clamped” exposure, no assumptions about Vd or other kinetic parameters are required. When BrAC is held constant, the rate of alcohol infusion is equivalent to the rate of alcohol elimination from the body (Ramchandani and O'Connor, 2006). As a secondary aim, we also investigated whether bariatric surgeries were associated with a slower AER in women independent of changes in body weight or composition.
Methods
Study design and experimental procedures
Using a cross-sectional design approach, we conducted a secondary data analysis of three studies that used the Computer-Assisted Alcohol Infusion System (CAIS) to conduct an alcohol clamping procedure to estimate AER (see section The Computer-assisted Alcohol Infusion System (CAIS) and calculation of alcohol elimination rate (AER)) (Kwo et al., 1998). Studies were conducted at the University of Illinois at Urbana-Champaign or Carle Foundation Hospital (Urbana, IL; n=42) and Indiana University (Indianapolis, IN; n=101) and were approved by the respective Institutional Review Boards.
Because women comprise 80% of the patients undergoing bariatric surgery (Fuchs et al 2015), our studies at Urbana Champaign included only women. Given the significant sex-related differences in alcohol metabolism (Kwo et al., 1998; Hahn et al., 1994) and the fact that all participants in the bariatric study were women, this secondary data analysis excluded men. Potential participants were excluded if they were pregnant or breastfeeding, were taking any prescribed medication that could affect alcohol metabolism, had a history of alcohol or substance dependence, or a history of substance abuse disorder (based on DSM-IV criteria), severe alcohol-induced flushing reactions, had liver abnormalities or abnormal levels of liver enzymes, renal impairment, a history of heart disease, including congestive heart failure and coronary artery disease, or gastrointestinal disorders, or were currently smoking cigarettes or quit smoking less than six months before the study.
Participants
The combined study sample comprised 143 women between 21- 64 years of age (81.1% Caucasian, 12.6% African American, 6.3% Asian), with a BMI (calculated from measured height and weight) ranging from 18.5 to 48.4 kg/m2 (Table 1). Fat-free mass (FFM) was assessed in a subsample of these women (n= 102) by using Bioelectrical impedance analysis system (RJL Quantum X Body Composition Analyzer) (n=60) or by using a dual-energy X-ray absorptiometry scan (DXA) with a Hologic Horizon W (APEX Software version 5.6.0.5; Hologic) (n=42). Nineteen of these women underwent bariatric surgery on average 2.1 years before participating in the study (range: 0.3- 5.5 years). Fourteen underwent sleeve gastrectomy, two Roux-en-Y gastric bypass, and three laparoscopic adjustable gastric banding. Each woman gave informed written consent for the study in which she participated, including consent for secondary analyses.
Table 1.
Characteristics of study participants
| Age (year) |
Weight (kg) |
Height (cm) |
BMI (kg/m2) |
FFM (kg) |
Body Fat (%) |
||
|---|---|---|---|---|---|---|---|
| All women (n=143) | 29.4 (10.0) |
76.6 (20.2) |
165.9 (6.4) |
27.9 (7.3) |
- | - | |
| Subgroup with FFM data | All (n=102) | 31.1 (11.3) |
79.8 (21.0) |
166.3 (6.4) |
28.9 (7.7) |
47.5 (6.7) |
38.6 (8.9) |
| Normal weight (n=44) | 23.4 (2.6)a |
62.9 (6.3)a |
167.2 (6.4) |
22.5 (1.7)a |
43.3 (4.2)a |
31.1 (4.4)a |
|
| Overweight (n=22) | 28.2 (7.8)b |
76.9 (5.8)b |
167.2 (4.6) |
27.5 (1.4)b |
47.9 (4.9)b |
37.7 (4.4)b |
|
| Obesity (n=36) | 42.4 (10.6)c |
102.3 (17.7)c |
164.6 (7.1) |
37.7 (5.8)c |
52.3 (6.8)c |
48.3 (4.9)c |
|
| Metabolic surgery (n=19)* | 41.1 (9.3) |
89.1 (14.6) |
164.6 (7.6) |
33.0 (5.7) |
50.3 (5.4) |
42.8 (6.3) |
|
All values are means (standard deviations), FFM: Fat-free Mass, *Evaluated on average 2.1 years post-metabolic surgery (range: 0.3 - 5.5 years). Groups that do not share the same letters are significantly different from each other at P < 0.005.
The Computer-Assisted Alcohol Infusion System (CAIS) and calculation of alcohol elimination rate (AER)
Participants arrived at the clinical research facility at about 8:00 AM and were instructed to abstain from food and any alcoholic drinks for at least 9 hours before their appointment. Pregnancy status was checked by using a urine pregnancy test upon arrival. The CAIS was used to administer an IV infusion of 6% (V/V) ethanol in half-normal saline using an alcohol clamp paradigm with a PBPK modeling system that calculated the infusion rate needed to carefully control BrAC (breath alcohol concentration) (Ramchandani et al., 1999, Kwo et al., 1998, Ramchandani and O'Connor, 2006, Ramchandani et al., 2006, Zimmermann et al., 2013). The model was programmed to reach a target BrAC of 60 mg%. BrAC followed a linear ascent from 0 to the targeted BAC mg% precisely in 15 minutes, then held pseudo-constant (clamped) for at least 120 minutes. Steady-state infusion rates were achieved after 75 min of clamping. Serial BrAC readings were collected during the ascending limb and the clamped state using a breathalyzer machine (Dräger Alcotest 7410 Plus or 6510, Germany). Readings were entered into the PBPK model-based algorithm, to allow online adjustment of the infusion rate to maintain the targeted BrAC within 5 mg%. The AER was calculated by multiplying the average infusion rate during the steady-state by the infusate concentration (Kwo et al., 1998).
Statistical analysis
Prior to data analysis, the Shapiro-Wilk and Kolmogorov-Smirnov tests were used to evaluate the distributional properties of variables. Correlations between variables were analyzed using Pearson's or Spearman's correlation coefficients, as appropriate. Multiple linear regression analyses were conducted to analyze the association between AER as the dependent variable and BMI, FFM, age, and bariatric surgery as predictors. Regression models were built in three steps. In the first step, the model included age, BMI, and the interaction term of age and BMI. In this model, AER data was positively skewed and required a logarithmic transformation to approximate normal distribution. The second model included age, BMI, FFM, and the interaction term of age and FFM, and the third model included age, BMI, FFM, bariatric surgery, and the interaction term of age and FFM. FFM, BMI, and age were centered on their means prior to running the linear regression analyses to reduce co-linearity among predictor variables. Collinearity was tested with Variance of Inflation (VIF) in regression models, and VIF values less than four were considered non-collinear. Based on their BMI values, women were assigned to three groups: normal weight (BMI> 18.4 and < 25.0 kg/m2), overweight (BMI > 24.9 and < 30.0 kg/m2), or obesity (BMI > 29.9 kg/m2). One-way ANOVA was used to assess statistical differences in the means of continuous variables between BMI groups. Levene's Test of Equality of variances was applied to check the homogeneity of variances between BMI groups. When variances were not homogenous or distributions were not normal, a Welch's ANOVA or Kruskal Wallis H test was used to determine statistically significant differences among groups. All statistical data analyses were performed with SPSS version 27 for Windows (IBM Corp, Armonk, NY), and a criterion of P value ≤ 0.05 was considered for statistical significance.
Results
Obesity and Alcohol Elimination Rate
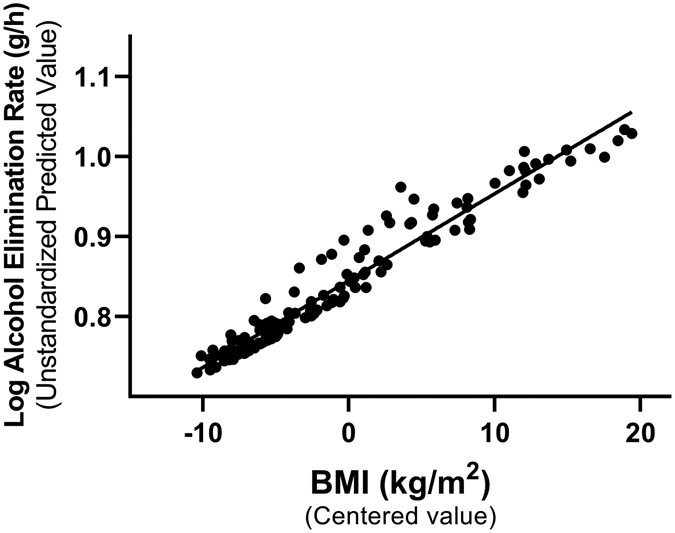
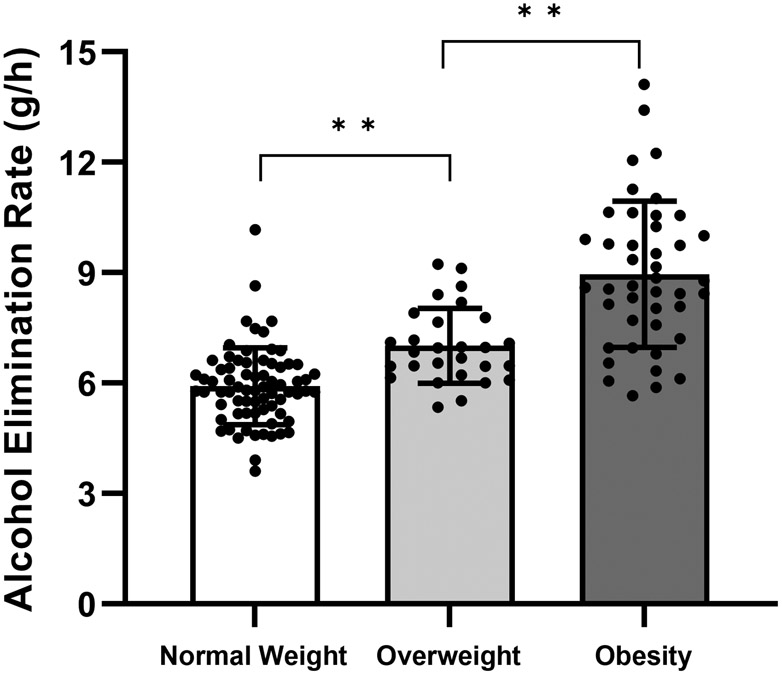
Obesity was associated with faster AER (rS (143) = 0.70, P < 0.001; Figure 1 A) and older age (rS (143) = 0.64, P < 0.001), and older age was associated with faster AER (rS (143) = 0.61, P < 0.001). Multiple regression analysis revealed no interaction between BMI and age (t(142) = −1.34, P = 0.18). Age and BMI were independent predictors and accounted for 54% of the variation in AER (F(3, 139) = 56.99, P < 0.001) (Table 2). Group comparison among categories of BMI showed that mean AER differed among normal weight, overweight, and obesity groups (FWelch (2, 64.5) = 47.34, P < 0.001). AER was 52% faster (95% confidence interval, 42%- 61%) in women in the group with obesity compared to the group with normal weight (Figure 1B).
Figure 1.
Obesity was associated with a faster alcohol elimination rate (AER) in women. (A) Age and body mass index (BMI) were independent predictors and accounted for 54% of the individual variation in AER. Data are shown as the unstandardized predicted values of AER from regression models that controlled for age versus mean-centered values of BMI (n = 143). (B) AER differed across groups with normal weight (BMI= 18.5 to 25.0 kg/m2, n = 72), overweight (BMI = 25.0 to 30.0 kg/m2, n = 28), and obesity (BMI > 30.0 kg/m2, n = 43). Data are presented as mean ± standard deviation (SD) and include individual data points within each BMI group. *value significantly different from the other group at P ≤ 0.001.
Table 2.
Multiple linear regression analyses for the association between alcohol elimination rate (AER) as the dependent variable and potential predictors (age (year), BMI (kg/m2), FFM (kg), and bariatric surgery) assessed with three models. VIF: Variance Inflation Factor.
| Models | n | Adj. R2 | Predictor | β coefficient |
Standard Error |
t | P | VIF |
|---|---|---|---|---|---|---|---|---|
| Model a * | 143 | 0.54 | age | 0.003 | 0.001 | 3.01 | 0.003 | 2.61 |
| BMI | 0.008 | 0.001 | 6.90 | 0.000 | 2.08 | |||
| age X BMI | 0.000 | 0.000 | −1.34 | 0.182 | 1.55 | |||
| Model b | 102 | 0.72 | age | 0.072 | 0.014 | 5.02 | 0.000 | 2.50 |
| BMI | 0.001 | 0.027 | 0.03 | 0.978 | 3.97 | |||
| FFM | 0.145 | 0.023 | 6.42 | 0.000 | 2.18 | |||
| age X FFM | 0.004 | 0.002 | 2.00 | 0.019 | 1.22 | |||
| Model c | 102 | 0.72 | age | 0.074 | 0.012 | 6.35 | 0.000 | 1.65 |
| FFM | 0.146 | 0.017 | 8.38 | 0.000 | 1.30 | |||
| age X FFM | 0.003 | 0.002 | 2.14 | 0.035 | 1.37 | |||
| Surgery | −0.104 | 0.308 | −0.34 | 0.737 | 1.37 |
FFM, BMI, and age were centered on their means prior to running the linear regression analyses to reduce co-linearity among predictor variables.
Log AER was used to normalize the residuals of model a
Fat-Free Mass, Bariatric surgery, and Alcohol Elimination Rate
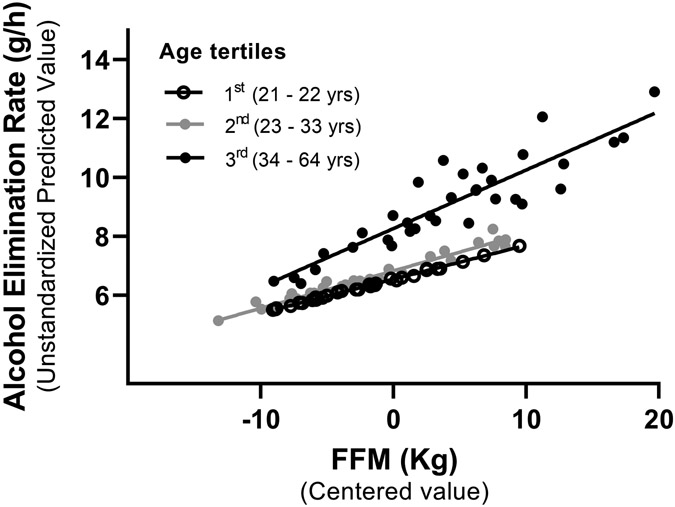
As expected, women with higher BMI had higher FFM than women with lower BMI (FWelch (2, 51.5) = 25.94, P < 0.001) (Table 1). Therefore, we determined the contribution of FFM to the association between AER and BMI by assessing the subgroup of participants with measured FFM. When FFM, BMI, and age, and the interaction between FFM and age were included in the regression model, BMI was no longer a predictor of AER (BMI main effect: t(101) = 0.03, P = 0.98). Age (t(101) = 5.02, P <0.001), FFM (t(101) = 6.42, P <0.001), and its interaction (t(101) = 2.39, P < 0.05) were significant predictors and accounted for 72% of the individual variations in AER (F(4, 97) = 64.26, P < 0.001). To illustrate the interaction between FFM and age, we stratified women by age tertiles (Figure 2) and by decade of life (Supplementary Figure 1). We found that higher fat-free mass (FFM) was associated with a faster AER, ,particularly in women in the oldest subgroups (Figure 2 and Supplementary Figure 1). Bariatric surgery was not associated with changes in AER in the regression model that included FFM and age (t(101) = −0.34, P = 0.74) (Table 2).
Figure 2.
Higher fat-free mass (FFM) was associated with faster alcohol elimination rates (AER), particularly in women in the oldest tertile group. Age, FFM, and the interaction between age and FFM accounted for 72% of the individual variation in AER. Data are shown as the unstandardized AER predicted values versus mean-centered values of FFM stratified by age tertiles (n = 102).
Discussion
A better understanding of factors that affect BAC achieved after alcohol ingestion is critical to estimate exposure of the brain and other organs to alcohol and, in turn, the toxic effects of alcohol. The degree of adiposity and liver volume (which correlates positively with obesity) (Grant et al., 2020), significantly impact the BAC experienced after alcohol administration (Kwo et al., 1998). In this study, we investigated the relationships between body composition and systemic AER using the alcohol clamp technique in women whose BMI ranged from normal weight to severe obesity. The clamping methodology eliminates variability arising from oral alcohol absorption and first-pass metabolism, and provides a direct measure of elimination from the amount of alcohol infused to maintain a steady-state exposure, which obviates any pharmacokinetic modeling assumptions (Ramchandani et al., 2006). We found that obesity and older age were associated with 52% faster AER in women. Nevertheless, BMI was not a significant predictor of AER when FFM and the interaction between FFM and age were incorporated into the regression model, suggesting that FFM is a better predictor for faster AER in women with obesity. Further, our data support the notion that metabolic surgery does not affect AER independently of surgically-related changes in body weight. For both, non-operated and operated women, FFM and age together accounted for 72% of women's inter-individual variability of systemic AER.
In absolute terms, people with obesity have larger FFM and fat mass than people of the same sex, age, and height, without obesity (Forbes and Welle, 1983). A recent study in 100 adult individuals with a wide range of BMI (from 16.9- 51.8 kg/m2) showed that FFM was a strong predictor of lean liver volume, the part of the liver that is functional for drug metabolism (Sinha et al., 2020). Therefore, it is likely that the strong dependence of AER on FFM and age in our findings is due to the robust relationship between FFM and lean liver size —a key determinant of AER (Kwo et al., 1998, Vatsalya et al., 2022). Findings from previous studies of a reduced AER following metabolic surgery-associated weight loss (Acevedo et al., 2018) are most likely explained by a reduction in FFM (and liver size) post-surgery.
The findings of this study may have implications with regard to estimating doses to achieve specific target BACs in alcohol challenge studies, particularly in populations with obesity. Most alcohol administration studies compute standardized doses based on body weight or anthropometrically-estimated total body water to adjust for sources of variability in alcohol Vd (Goist and Sutker, 1985, Jones, 2019). However, these approaches can significantly impact the target BACs achieved due to the critical impact of adiposity on the Vd (which the body weight or total body water is attempting to control for). FFM measured using DXA method, may serve as a more appropriate metric for the Vd of alcohol, and hence a better measure to standardize doses of administered alcohol in studies that include groups with obesity (Acevedo et al., 2020, Seyedsadjadi et al., 2022, Pepino et al., 2015, Acevedo et al., 2018). Taken together, results from this and previous studies (Acevedo et al., 2018, Acevedo et al., 2020) suggest that the scaling of alcohol dose by FFM is effective as it helps adjust for individual differences not only in alcohol Vd, influencing peak BAC, but also in AER.
Our finding of the positive association between age and AER is consistent with another study showing that older adults have higher AER than younger adults (Fiorentino and Moskowitz, 2013). However, a recent clamp study showed no significant group differences in AER between younger (21-25 years) and older (55-65 years) men and women and found that sex-related differences in AER were mostly attributed to differences in FFM and liver volume. The age-related association with AER might be due to several anatomical and physiological changes associated with the progression of aging that can affect alcohol metabolism (Mangoni and Jackson, 2004). For example, there is a progressive reduction in FFM and total body water, which reduces Vd (Watson et al., 1980) along with a decrease in liver volume and liver blood flow (Wynne et al., 1989) and changes in hormones that affect hepatic ADH activity with advancing age (Cederbaum, 2012) which may impact AER. However, not all anatomical and physiological processes age at the same rate or by the same magnitude. Therefore, AER may be higher at age ranges when Vd is decreased, but liver volume is still conserved and hepatic function remains constant. Although the above-proposed setup for Vd, liver volume, and hepatic function is hypothetical, our finding of a significant interaction between FFM and age in predicting AER with a steeper upward tilt of the curve for the group of women who were 34-64 years of age compared to those in the younger group might illustrate such a scenario. In addition, the effect of hormones on hepatic ADH activity is complex (Cederbaum, 2012, Mezey, 2000). For example, estrogens increase, but thyroid and testosterone reduce ADH activity (Mezey, 2000, Mardh et al., 1986). Therefore, it is possible that changes in these hormones with perimenopause and menopause, explain some of the observed associations between AER and age in this group of women.
The results of our study should be interpreted considering its strengths and limitations. The major strengths of our study are the use of the highly controlled intravenous alcohol clamp technique, which circumvented the confounding effects of gastric absorption and distribution kinetics on AER, and the large sample size that included a broad representation of all BMI categories. The major limitations of this study are that we only included women and a relatively small subgroup underwent bariatric surgery. The well-established sex-related differences in alcohol's pharmacokinetics stress the need for future AER studies to include men with a wide BMI range. In addition, because the cross-sectional study design does not allow for inferring causality, longitudinal studies are needed to further evaluate changes in AER after metabolic surgery Finally, some drawbacks of using DXA to assess FFM in individuals with obesity merit attention. Weight limits and table dimensions of densitometers could preclude the participation of some individuals or require the use of half-body scans (Shepherd et al., 2017). Despite being recognized as the preferred method for bone density and body composition (Shepherd et al., 2017), DXA systematically overestimates FFM in men and women (Schoeller et al., 2005; Jensen et al., 2019), and the error increases with increases in fat mass (Jensen et al., 2019). Nevertheless, the overestimates of FFM by DXA are within 5-10% (Schoeller et al., 2005; Jensen et al., 2019). To put this in perspective, for a scaling of 0.5 grams of alcohol by kg of FFM (the dose used in all our studies in the bariatric population), a 10% overestimation of FFM would result in an additional ~3 ml of alcohol in the beverage. This amount of extra alcohol can be considered trivial because typically this same amount of alcohol is added onto the surface of cups to serve as a smell and flavor mask in studies that include a placebo control beverage (Mennella et al., 2005; Acevedo et al., 2020).
In conclusion, our study findings show that obesity is associated with a faster alcohol elimination rate, but the association is strongly dependent on FFM, a good predictor of lean liver volume (Sinha et al., 2020). In women with BMI status ranging from normal weight to extreme obesity, FFM and age account for most of the variability in AER.
Supplementary Material
Acknowledgment
We acknowledge the invaluable contribution of Belen Acevedo, PhD and Raul Alfaro, PhD (University of Illinois at Urbana Champaign) by recruiting participants and conducting study visits for a significant portion of the data collected at the University of Illinois at Urbana Champaign. We also acknowledge Rafael Troconis, MD (University of Illinois at Urbana Champaign), and Danisa Nieto, MD (University of Illinois at Urbana Champaign) for the expert technical assistance with phlebotomy procedures, Carle Pharmacy for the preparation of 6% v/v infusate, and the supportive assistance with study coordination of Christine Canfield, BS, CCRP, and Adam Wright, BS, CCRP (Carle Foundation Hospital). The cooperation and support of the IU research pharmacy in the preparation of 6% alcohol v/v infusate, and of the Indiana Clinical and Translational Sciences Institute Clinical Research Center personnel in the preparation of participants for testing were essential for the IU component of this research project. The programming expertise of Victor Vitvitskiy was vital to this project and most sincerely appreciated. The authors would also like to thank all study participants for taking part in this study.
Invitations: Investigator interested in adapting the capabilities of the Computer-assisted Alcohol Infusion System to their own research is welcome and those interested should send an email to mplaweck@iupui.edu.
This study was supported in part by the National Institutes of Health (NIH) grants R01 AA 024103 (MYP), R01 AA027236 (MHP), P60 AA07611 (MHP, SOC, AEK), NIAAA DICBR (Z1A AA 000466) (VAR) and the USDA National Institute of Food and Agriculture Hatch Project number 698-921 (MYP).
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References:
- Acevedo MB, Eagon JC, Bartholow BD, Klein S, Bucholz KK & Pepino MY (2018) Sleeve gastrectomy surgery: when 2 alcoholic drinks are converted to 4. Surgery for Obesity and Related Diseases, 14, 277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo MB, Teran-garcia M, Bucholz KK, Eagon JC, Bartholow BD, Burd NA, et al. (2020) Alcohol sensitivity in women after undergoing bariatric surgery: a cross-sectional study. Surgery for Obesity and Related Diseases, 16, 536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederbaum AI (2012) Alcohol metabolism. Clinics in Liver Disease, 16, 667–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres HG & Grüner O (1994) Comparison of D2O and ethanol dilutions in total body water measurements in humans. Clinical Investigation, 72, 830–837. [DOI] [PubMed] [Google Scholar]
- Fiorentino DD & Moskowitz H (2013) Breath alcohol elimination rate as a function of age, gender, and drinking practice. Forensic Science International, 233, 278–282. [DOI] [PubMed] [Google Scholar]
- Forbes GB & Welle SL (1983) Lean body mass in obesity. International Journal of Obesity, 7, 99–107. [PubMed] [Google Scholar]
- Fuchs HF, Broderick RC, Harnsberger CR, Chang DC, Sandler BJ, Jacobsen GR, et al. (2015) Benefits of bariatric surgery do not reach obese men. Journal of laparoendoscopic & advanced surgical techniques. Part A, 25, 196–201. [DOI] [PubMed] [Google Scholar]
- Goist KC & Sutker PB (1985) Acute alcohol intoxication and body composition in women and men. Pharmacology Biochemistry and Behavior, 22, 811–814. [DOI] [PubMed] [Google Scholar]
- Graham K, Wilsnack R, Dawson D & Vogeltanz N (1998) Should alcohol consumption measures be adjusted for gender differences? Addiction, 93, 1137–1147. [DOI] [PubMed] [Google Scholar]
- Grant H, Zhang Y, Li L, Wang Y, Kawamoto S, Penisson S, et al. (2020) Larger organ size caused by obesity is a mechanism for higher cancer risk. bioRxiv, 2020.07.27.223529. [Google Scholar]
- Griswold MG, Fullman N, Hawley C, Arian N, Zimsen SRM, Tymeson HD, et al. (2018) Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet, 392, 1015–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn JC, Encarnacion B, Brat GA & Morton JM (2007) Does gastric bypass alter alcohol metabolism? Surgery for Obesity and Related Diseases, 3, 543–8; discussion 548. [DOI] [PubMed] [Google Scholar]
- Hahn RG, Norberg A, Gabrielsson J, Danielsson A & Jones AW (1994) Eating a meal increases the clearance of ethanol given by intravenous infusion. Alcohol and Alcoholism, 29, 673–677. [PubMed] [Google Scholar]
- Ibrahim N, Alameddine M, Brennan J, Sessine M, Holliday C & Ghaferi AA (2019) New onset alcohol use disorder following bariatric surgery. Surgical Endoscopy, 33, 2521–2530. [DOI] [PubMed] [Google Scholar]
- Jensen B, Braun W, Geisler C, Both M, Klückmann K Müller MJ, et al. (2019) Limitations of Fat-Free Mass for the Assessment of Muscle Mass in Obesity. Obesity Facts, 12, 307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AW (2010) Evidence-based survey of the elimination rates of ethanol from blood with applications in forensic casework. Forensic Science International, 200, 1–20. [DOI] [PubMed] [Google Scholar]
- Jones AW (2019) Alcohol, its absorption, distribution, metabolism, and excretion in the body and pharmacokinetic calculations. WIREs Forensic Science, 1. [Google Scholar]
- Jones AW & Jonsson KA (1994) Between-subject and within-subject variations in the pharmacokinetics of ethanol. British Journal of Clinical Pharmacology, 37, 427–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King WC, Chen JY, Courcoulas AP, Dakin GF, Engel SG, Flum DR, et al. (2017). Alcohol and other substance use after bariatric surgery: prospective evidence from a U.S. multicenter cohort study. Surgery for Obesity and Related Diseases, 13, 1392–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King WC, Chen JY, Mitchell JE, Kalarchian MA, Steffen KJ, Engel SG, et al. (2012) Prevalence of alcohol use disorders before and after bariatric surgery. Jama, 307, 2516–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klockhoff H, Naslund I & Jones AW (2002) Faster absorption of ethanol and higher peak concentration in women after gastric bypass surgery. British Journal of Clinical Pharmacology, 54, 587–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwo PY, Ramchandani VA, O'Connor S, Amann D, Carr LG, Sandrasegaran K, et al. (1998) Gender differences in alcohol metabolism: Relationship to liver volume and effect of adjusting for body mass. Gastroenterology, 115, 1552–1557. [DOI] [PubMed] [Google Scholar]
- Lee SL, Chau GY, Yao CT, Wu CW & Yin SJ (2006) Functional assessment of human alcohol dehydrogenase family in ethanol metabolism: significance of first-pass metabolism. Alcohol Clin Exp Res, 30, 1132–1142. [DOI] [PubMed] [Google Scholar]
- Mangoni AA & Jackson SH (2004) Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. British Journal of Clinical Pharmacology, 57, 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardh G, Dingley AL, Auld DS & Vallee BL (1986) Human Class II (pi) Alcohol Dehydrogenase Has a Redox-Specific Function in Norepinephrine Metabolism. Proceedings of the National Academy of Sciences of the United States of America, 83, 8908–8912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maudens KE, Patteet L, Van nuijs ALN, Van broekhoven C, Covaci A & Neels H (2014) The influence of the body mass index (BMI) on the volume of distribution of ethanol. Forensic Science International, 243, 74–78. [DOI] [PubMed] [Google Scholar]
- Mellinger JL, Shedden K, Winder GS, Fernandez AC, Lee BP, Waljee J., et al. (2021) Bariatric surgery and the risk of alcohol-related cirrhosis and alcohol misuse. Liver International, 41, 1012–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella JA, Pepino MY, Teff KL (2005) Acute alcohol consumption disrupts the hormonal milieu of lactating women. The Journal of clinical endocrinology and metabolism, 90, 1979–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezey E. (2000) Influence of sex hormones on alcohol metabolism. Alcoholism: Clinical and Experimental Research, 24, 421. [PubMed] [Google Scholar]
- Norberg A, Jones AW, Hahn RG & Gabrielsson JL (2003) Role of Variability in Explaining Ethanol Pharmacokinetics. Clinical Pharmacokinetics, 42, 1–31. [DOI] [PubMed] [Google Scholar]
- Ostlund MP, Backman O, Marsk R, Stockeld D, Lagergren J, Rasmussen F, et al. (2013) Increased admission for alcohol dependence after gastric bypass surgery compared with restrictive bariatric surgery. JAMA Surgery, 148, 374–377. [DOI] [PubMed] [Google Scholar]
- Pepino MY, Okunade AL, Eagon JC, Bartholow BD, Bucholz K & Klein S (2015) Effect of Roux-en-Y Gastric Bypass Surgery: Converting 2 Alcoholic Drinks to 4. JAMA Surgery, 150, 1096–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandani VA, Bolane J, Li TK & O'Connor S (1999) A Physiologically-Based Pharmacokinetic (PBPK) Model for Alcohol Facilitates Rapid BrAC Clamping. Alcoholism: Clinical and Experimental Research, 23, 617–623. [PubMed] [Google Scholar]
- Ramchandani VA & O'Connor S (2006) Studying alcohol elimination using the alcohol clamp method. Alcohol Research & Health, 29, 286–290. [PMC free article] [PubMed] [Google Scholar]
- Ramchandani VA, O'Connor S, Neumark Y, Zimmermann US, Morzorati SL & De Wit H (2006) The Alcohol Clamp: Applications, Challenges, and New Directions – An RSA 2004 Symposium Summary. Alcoholism: Clinical and Experimental Research, 30, 155–164. [DOI] [PubMed] [Google Scholar]
- Saldich EB, Wang C, Rosen IG, Bartroff J & Luczak SE (2021) Effects of stomach content on the breath alcohol concentration-transdermal alcohol concentration relationship. Drug and Alcohol Review, 40, 1131–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeller DA, Tylavsky FA, Baer DJ, Chumlea WC, Earthman CP, Fuerst T, et al. (2005) QDR 4500A dual-energy X-ray absorptiometer underestimates fat mass in comparison with criterion methods in adults. American Journal of Clinical Nutrition, 81: 1018–1025. [DOI] [PubMed] [Google Scholar]
- Seyedsadjadi N, Acevedo MB, Alfaro R, Ramchandani VA, Plawecki MH, Rowitz B et al. (2022) Site of Alcohol First-Pass Metabolism Among Women. JAMA Network Open, 5, e223711–e223711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JA, Ng BK, Sommer MJ, Heymsfield SB (2017) Body composition by DXA. Bone, 104, 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha J, Duffull SB, Green B & Al-Sallami HS (2020) Evaluating the Relationship Between Lean Liver Volume and Fat-Free Mass. Clinical Pharmacokinetics, 59, 475–483. [DOI] [PubMed] [Google Scholar]
- Steffen KJ, Engel SG, Pollert GA, Li C & Mitchell JE (2013) Blood alcohol concentrations rise rapidly and dramatically after Roux-en-Y gastric bypass. Surgery for Obesity and Related Diseases, 9, 470–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatsalya V, Byrd ND, Stangl BL, Momenan R & Ramchandani VA (2022) Influence of Age and Sex on Alcohol Pharmacokinetics and Subjective Pharmacodynamic Responses following Intravenous Alcohol Exposure in Humans. Alcohol, S0741-8329(22)00094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson PE, Watson ID & Batt RD (1980) Total body water volumes for adult males and females estimated from simple anthropometric measurements. The American Journal of Clinical Nutrition, 33, 27–39. [DOI] [PubMed] [Google Scholar]
- Wynne HA, Cope LH, Mutch E, Rawlins MD, Woodhouse KW & James OFW (1989) The effect of age upon liver volume and apparent liver blood flow in healthy man. Hepatology, 9, 297–301. [DOI] [PubMed] [Google Scholar]
- Zimmermann US, O’Connor S, Ramchandani VA (2013) Modeling alcohol self-administration in the human laboratory, in Behavioral Neurobiology of Alcohol Addiction (Sommer WH, Spanagel R). pp 315–353. Springer, Berlin. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.