Abstract
Pathology laboratories are undergoing digital transformations, adopting innovative technologies to enhance patient care. Digital pathology systems impact clinical, education, and research use cases where pathologists use digital technologies to perform tasks in lieu of using glass slides and a microscope. Pathology professional societies have established clinical validation guidelines, and the US Food and Drug Administration have also authorized digital pathology systems for primary diagnosis, including image analysis and machine learning systems. Whole slide images, or digital slides, can be viewed and navigated similar to glass slides on a microscope. These modern tools not only enable pathologists to practice their routine clinical activities, but can potentially enable digital computational discovery. Assimilation of whole slide images in pathology clinical workflow can further empower machine learning systems to support computer assisted diagnostics. The potential enrichment these systems can provide is unprecedented in the field of pathology. With appropriate integration, these clinical decision support systems will allow pathologists to increase the delivery of quality patient care. This review describes the digital pathology transformation process, applicable clinical use cases, incorporation of image analysis and machine learning systems in the clinical workflow, as well as future technologies that may further disrupt pathology modalities to deliver quality patient care.
Keywords: artificial intelligence, digital pathology, digital transformation, machine learning, patient care, personalized medicine
1 |. INTRODUCTION
Pathology is the foundation of modern healthcare, responsible for the accurate and timely diagnosis of patient specimens. Traditionally, pathologists have used glass slides and a bright-field microscope to examine and interpret tissue or fluid samples retrieved from patients. While this workflow has persisted for many years, it has certain limitations, such as the need for physical space to store and transport glass slides, the time-consuming process of manually aggregating, collating, and reviewing glass slides, as well as the limited access to expert pathologists in geographically remote or resource-restricted areas. To address these challenges, innovative technologies are becoming increasingly adopted by pathology laboratories to provide a novel patient care delivery model. While digital pathology systems impact clinical, education, and research use cases, this review will focus on the clinical applications in pathology that affect patient care.
Digital pathology systems convert glass slides into digital images that can be viewed, shared, and analyzed on a computer. The use of digital pathology systems allows pathologists to access and analyze patient specimens remotely, remotely collaborate with colleagues, and compare specimens for more accurate diagnoses. Appropriate infrastructure to support integration and implementation of digital pathology systems is critical to ensure these systems can be successfully used by pathologists in the clinical setting. Pathologists can then confidently use digital technologies instead of using glass slides and a microscope.
Pathology regulatory organizations (e.g., College of American Pathologists [CAP], Royal College of Pathologists) have established clinical validation guidelines and best practices,1,2 and the US Food and Drug Administration have also authorized digital pathology systems for primary diagnosis, including image analysis and machine learning systems.3–7 These approvals have provided the necessary regulatory framework for pathologists to adopt digital pathology tools with assurance when properly validated. Clinical use cases include primary diagnosis, consultation including intraoperative consultation (e.g., frozen section), molecular pathology, clinical conferences, interpretation of immunohistochemistry or special stains, among many others.8,9 Digital pathology systems have many benefits, including improved accuracy, increased efficiency, enhanced collaboration, and the potential for computational biomarker discovery. For instance, the use of digital tools can reduce the time and cost associated with the preparation and transport of physical slides.10,11 Additionally, digital pathology systems can enable pathologists to use image analysis and machine learning systems to assist in diagnosis, providing more accurate and consistent results. These benefits can ultimately lead to improved patient care.
Despite the numerous advantages of digital pathology systems, challenges still exist. One of the most significant challenges is the need for specialized equipment and expertise, which can be costly and challenging to integrate with existing clinical laboratory and information systems.12 Additionally, the integration of new digital tools into available analog clinical workflows requires careful planning and validation to ensure that patient care is not compromised.
This review describes the digital pathology transformation process, applicable clinical use cases, incorporation of image analysis and machine learning systems in the clinical workflow, as well as upcoming technologies that may further disrupt pathology modalities to deliver quality patient care. The integration of digital tools into pathology practice has the potential to evolve patient care through improved accuracy of diagnoses while bolstering efficient workflows.
2 |. DIGITAL TRANSFORMATION
2.1 |. Infrastructure
Digital pathology transformation involves the adoption of innovative technologies to enhance patient care, resulting in a significant shift from the traditional glass slides and microscope-based approach to specimen review and analysis. For clinical adoption, a suitable, multi-faceted digital pathology infrastructure must be in place. Each laboratory and organization will require similar, basic foundational components, including hardware, software, and network components to support the digital pathology system. However, the exact infrastructure blueprint will depend on the laboratory use cases, resources, and deployment strategy. In addition, appropriate component validation must be completed, followed by adequate training and support for the pathologists and laboratory staff to ensure proper use of the digital pathology systems.
The digital pathology ecosystem is composed of three main components: information systems, digital pathology systems, and system tools (Figure 1). These systems should ideally support interoperability in an integrated digital workflow between all of the other upstream and downstream components. The digital pathology system is the central component that enables a digital pathology workflow, allowing pixel data to flow from an acquisition device (e.g., whole slide scanner) to a viewer application (e.g., whole slide image viewer, image management system). System tools such as image analysis and computer-assisted diagnosis can further be integrated into the pathologist’s workflow to analyze whole slide images. The hardware required for digital pathology systems includes slide scanners, computers, display monitors, and input devices. The high-definition cameras in the whole slide scanners capture high-fidelity digital images of patient samples (e.g., histology sections of tissue), while the display monitors enable pathologists to view and analyze the digital slides (Figure 2). The reliability and quality of the hardware and derivative digital slides are essential for accurate diagnosis and the components must be validated to ensure consistent and reliable results. Due to the high resolution of whole slide images, their resultant file sizes are larger than most medical images. This necessitates a thoroughly designed information technology network infrastructure to support digital pathology and enable data storage and retrieval. High-speed internet connections are necessary for the transmission of digital images; digital storage solutions such as on-premise or cloud-based storage can be deployed for tiered short or long-term storage. Software plays a critical role in digital pathology systems as it enables pathologists to pan, zoom, focus (i.e., comparable to a bright-field microscope) and examine the digital slides. This includes whole slide image viewing by a pathologists’ eyes vis-à-vis image analysis or machine learning system. These software solutions allow pathologists to digitally annotate the whole slide images or provide a basis for machine learning systems to present visualizations to the pathologist for similar classification tasks (e.g., tumor detection, subtyping, mitosis quantification). These machine learning-based systems and image analysis software can be used as an adjunct to assist pathologist in rendering a diagnosis.
FIGURE 1.
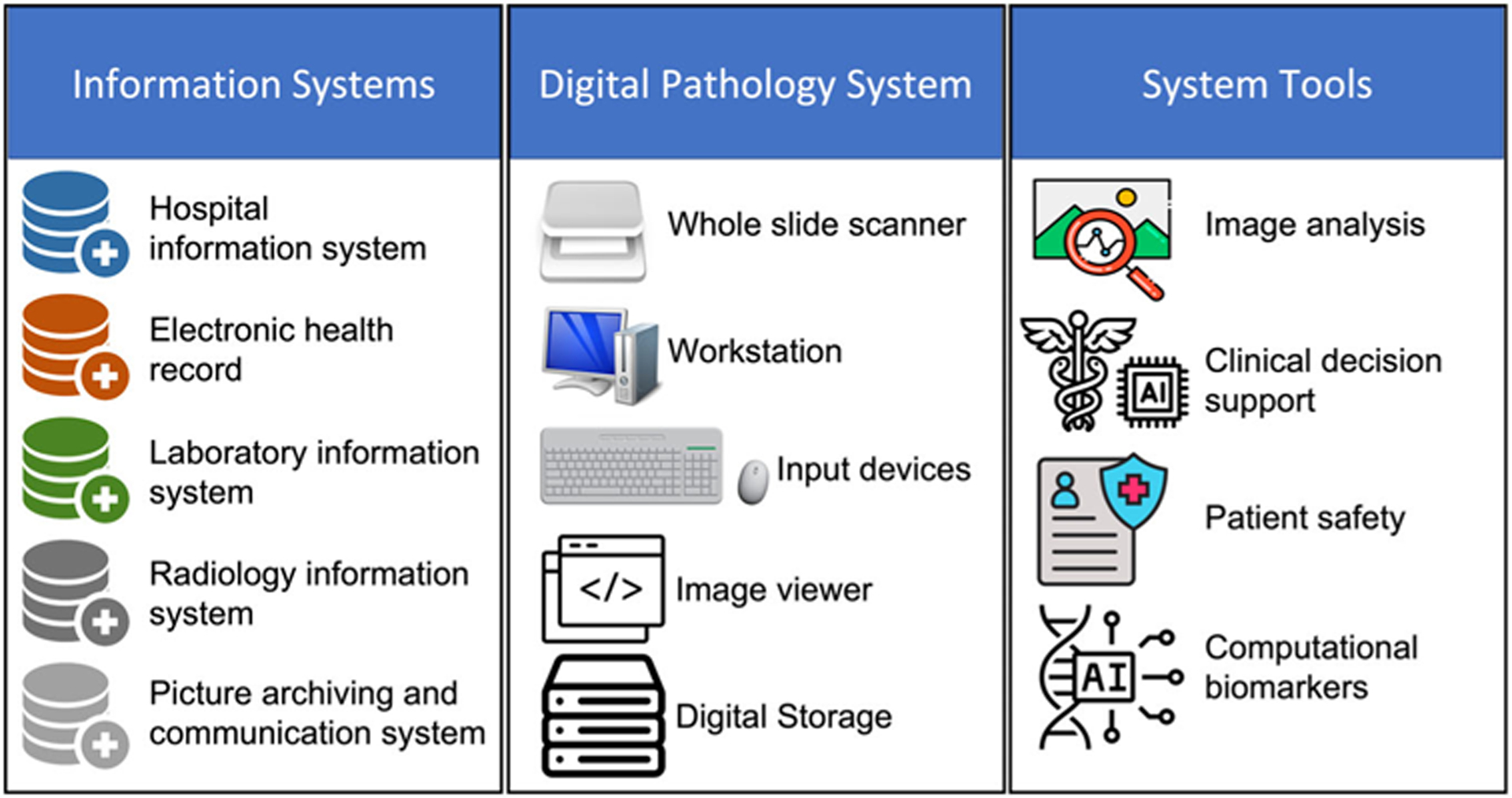
Digital pathology ecosystem.
FIGURE 2.
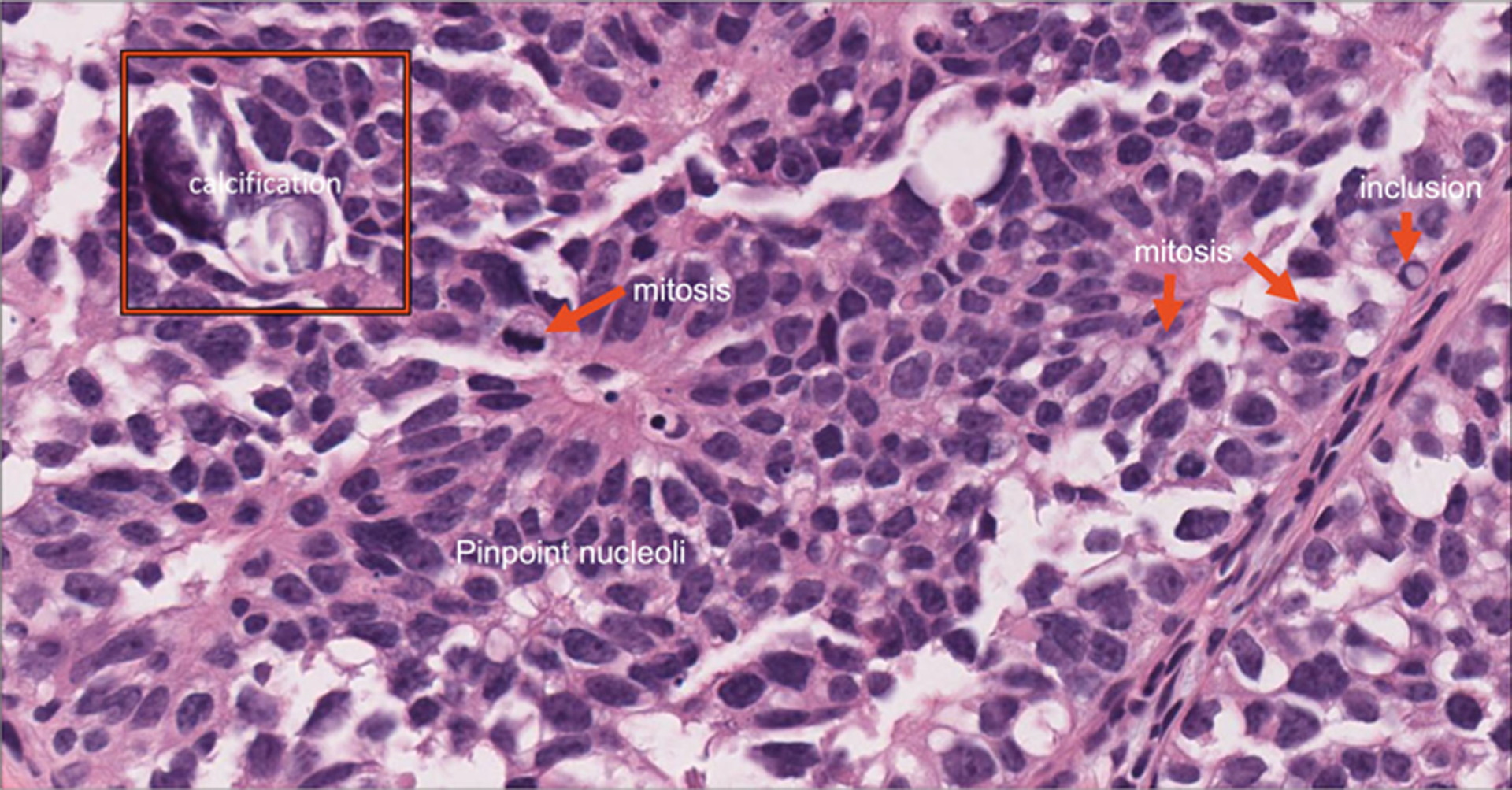
Representative high-resolution view of a tissue section showing identifiable histology features.
2.2 |. Integration and interoperability
Integration of digital pathology systems can be discussed from an operational and technical perspective. Operationally, whole slide scanning devices intended for clinical purposes may be best suited in the laboratory where the glass slides are being generated (e.g., sectioned, stained, coverslipped). This may not always be possible as most laboratory space was designed without planning for digital pathology hardware bench space. However, centralization of the histology laboratory workflow and digital distribution of cases may be a more efficient solution for distributed health networks. Ensuring a lean operation from a laboratory workflow perspective will mitigate increased patient report turnaround times through decreased courier services or additive scanning time of glass slides. Integrating digital pathology for clinical workflows ultimately makes the whole slide scanning operation into a critical process where whole slide scanning devices must be treated as clinical grade instruments. Sufficient hardware (e.g., whole slide scanners) is needed to meet the laboratory’s peak clinical glass slide volumes. Similarly, an appropriate number of trained staff are required to operate and troubleshoot the whole slide scanners as needed for the laboratory schedule and specimen volume. Review of laboratory pre-analytical instrument (e.g., slide coverslipper) compatibility is also needed to evaluate slide rack compatibility. Downstream operations such as clinical review of whole slide images by pathologists may require laboratory information system interfaces allowing pathologists ease of access to the patient’s pathology record and digital slides, including archived cases, if applicable. Additional integration of clinical decision support tools may require mapping of laboratory information system data to guarantee the appropriate image analysis or machine learning tools are being correctly computed on the right specimen or slide. For instance, ensuring tissue sampling from a prostate biopsy has a prostatic adenocarcinoma detection model deployed on the appropriate H&E and not a breast specimen. These operational underpinnings will provide substantiative groundwork for the clinical deployment of digital pathology.
From the technical perspective, there still exist interoperability limitations between certain digital pathology systems due to the proprietary whole slide image file formats and software developed by vendors. This results in viewer software being limited to a specified vendor formats, which curtails interoperability if their respective WSI file format is not supported by another vendor viewer software. As a result, when multiple whole slide scanning systems from different vendors are integrated in the clinical operation, a vendor-agnostic viewer may be appropriate so that all digital slides can be viewed on a single image viewer with consistent digital tools and user interface. Trying to advance interoperability, the Digital Imaging and Communications in Medicine (DICOM) standard includes a core file format and network transmission protocol. The DICOM network protocol enables imaging devices to exchange data and securely transfer images and patient information over a defined network protocol. While predominantly used in radiology, dermatology, and ophthalmology, DICOM Working Group 26 is specific to the field of Pathology and provides a framework to incorporate pathology specimen identification, WSI storage and retrieval, and annotations. The success of DICOM in enabling multiple imaging devices from different vendors to integrate and interface together based on a single standard has led to vendor initiation in support DICOM interoperability in digital pathology.13–15 A DICOM standard could allow for pathology-designed picture archiving and communication system digital workflow.
2.3 |. Implementation
Laboratories have several options regarding their digital pathology roadmap implementation. Many choose a phased approach to address challenges as they arise, while other organizations with dedicated resources or specified initiatives may opt for a comprehensive implementation for all intended use cases. As aforementioned, digital pathology systems typically consist of hardware, such as whole slide scanners and monitors, and software components, such as whole slide image viewers and decision support tools. The specific quantity and variety of each component will depend on the intended use cases and laboratory specimen volumes. Additionally, no single whole slide scanner can meet all of the needs of a high complexity pathology laboratory, including the imaging of cellular specimens (e.g., cytology, hematology), traditional and whole mount glass slide formats (e.g., 1 × 3 in., 2 × 3 in.), continuous loading capabilities, bright-field/fluorescence abilities, high-resolution scanning, and rapid scan speeds. Consequently, institutions with high complexity pathology testing may choose to acquire multiple digital pathology vendor systems, deploying the preferred technology for each intended use case. For the software component, performance of whole slide image viewers is needed for expeditious navigation, such as launching cases from the laboratory information system or image management system, loading and rendering the digital slides, and field of view navigation (e.g., rendering of images during pan and zoom functions).
Quality control in the pathology laboratory is standard practice and extends to any clinically validated test. Quality control of the deployed digital pathology system is critical to uphold laboratory standard practices and mitigate any pre-scan slide defects (e.g., air bubbles, fingerprints, ink marks, etc.).16 A standard operating procedure will additionally bolster the quality control practice to support high-fidelity glass slide digitization into whole slide images. Automated software can help expedite flagging of digital defects and minimize manual reviews of digital slides.
Validation is a critical requirement for any digital pathology system used for clinical purposes. The systems should be validated based on how they will be used clinically. The CAP has published updated guidelines1,2 for the validation of whole slide imaging systems, and many validation studies have been published following these guidelines.17,18 It is also important to revalidate the clinical utilization of digital pathology systems whenever a significant change is made to any of the prior validated components or the intended use. Validation guidelines for clinical use of machine learning systems are still emerging as the clinical use of these systems are relatively early. As these tools continue to become clinically used, published data will contribute to providing formal validation guidelines in the future.
Training and support are essential for the successful adoption of digital pathology tools in the clinical setting. Pathologists and laboratory staff must be trained on the use of digital pathology hardware and software to ensure proper use and interpretation of digital images. Additionally, ongoing support must be provided to ensure that any technical issues are promptly addressed, minimizing downtime and ensuring the quality of patient care.
2.4 |. Regulatory
Regulatory authorization supports the safety and efficacy of vendor provided systems. Regulatory geographic jurisdiction determines the requirements for authorization. Digital pathology systems can be authorized in the European Union under then In Vitro Diagnostic Devices Directive (IVDD 98/79/EC) and be granted CE-IVD (Conformité Européenne-In Vitro Diagnostic) marking.19 CE marking indicates that an in vitro diagnostic device may be legally commercialized in the European Union. The CE mark is not recognized in the United States of America. Products that are marketed in the United States must comply with relevant Food and Drug Administration federal or state regulations.20 As of April 2023, the FDA has authorized three slide scanning devices for primary diagnosis on formalin fixed, paraffin-embedded tissue, and two for hematology peripheral blood smears; seven viewers and/or image management systems; four display devices (e.g., monitors); and four classification decision support systems (Figure 3). However, regardless of regulatory authorization status, laboratories are still required to internally validate systems as per accreditation body guidelines. Validation should be performed to analyze the digital pathology system as it would be clinically implemented. Many laboratories have validated and clinically used digital pathology systems that did not have Food and Drug Administration authorization; however, institutions have varied risk-profiles and may opt to only validate regulatorily authorized systems.21–47
FIGURE 3.
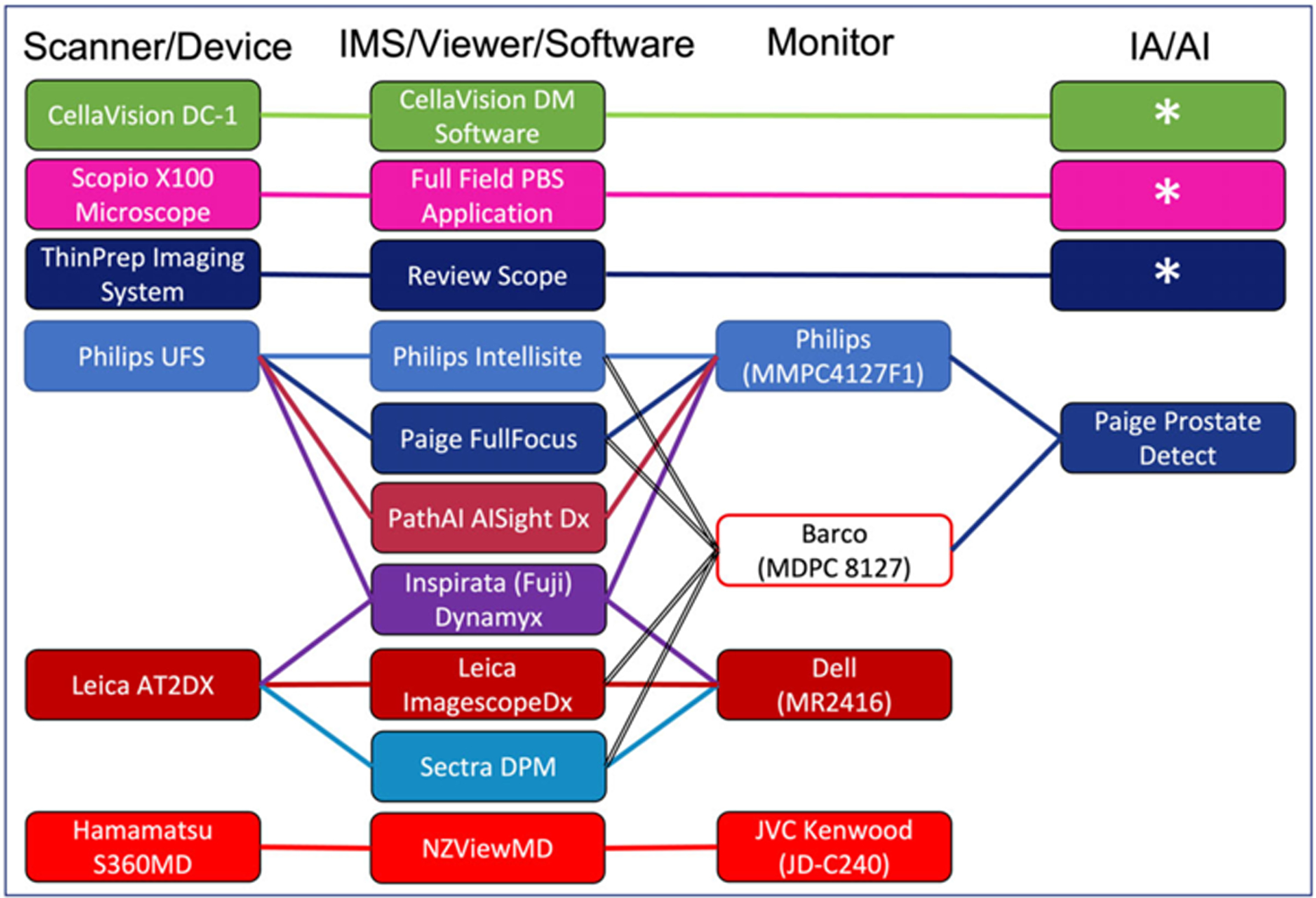
Regulatory authorized digital pathology systems. Represents the current Food and Drug Administration (FDA) authorized digital pathology systems as of April 12, 2023. Asterisks (*) represent FDA authorized devices that incorporate image analysis/machine learning as part of their integrated solution. AI, artificial intelligence; DPM, digital pathology module; IA, image analysis; IMS, image management system; PBS, peripheral blood smear
3 |. CLINICAL USE CASES
Clinical patient care delivery models in pathology have evolved slowly over hundreds of years. Microscopy and cellular pathology were instituted with the invention of the microscope around the 1600s by Zacharias Janssen, Robert Hooke, and Antonie van Leeuwenhoek. Around the 1800s, morphologic understanding of pathology was refined by Rudolf Virchow. Tissue processing, embedding, microtomy, staining, and coverslipping workflows advanced over this era as well. Immunohistochemistry evolved understanding of cellular expression in pathology toward to latter 1900s, and genomic testing becoming mainstream in the early 2000s. The evolution of pathology has continued to progress with digital and computational pathology. Aside from digital pathology systems improving workflow solutions such as creating paper-less workflows and automatically collating and aggregating patient slides to the laboratory information system, there are additional significant diagnostic implications. Pathologists may continue to use all tools of each evolutionary era as part of an armamentarium of systems available to diagnose and prognosticate patients to the highest standard of care. Digital pathology systems allow pathologists newfound tools to not only replicate but enrich their ability to perform clinical tasks.
3.1 |. Primary diagnosis
Digital pathology systems are becoming increasingly prevalent in the field of pathology, and there is growing interest in using them for primary diagnosis. Primary diagnosis refers to the process of diagnosing a patient specimen based on digitized images (e.g., whole slide image) as the primary means of diagnosis, without the need for reviewing the glass slides on a microscope. At baseline, these systems allow pathologists to review digital slides with sufficient resolution to render a histopathology diagnosis. Additionally, digital pathology may increase efficiency and speed up the diagnostic process, as pathologists can review cases remotely and collaborate with other experts in real time.10,11 Review of whole slide images on a display device may require time to adjust to, and initially may be less efficient.46,48–50 However with appropriate training, familiarity, and technology (i.e., high definition monitor, input devices, decision support tools), pathologists may become more accurate and/or efficient compared to conventional workflows.51–54 Remote digital pathology review will be described in more detail in Section 3.2. Primary diagnosis using digital tools can also aid pathologists to observe regions on the slide they have already reviewed using a slide coverage map. This can be especially useful in slides with multiple tissue levels or fragmented tissue and provide a way for pathologists to quickly reference the slide coverage visualization. Co-registration is another tool that is not feasible using traditional glass slides and a microscope. This allows pathologists to select a number of slides to be copresented in the same window that can synchronize tissue review across all selected slides.55 Digital pathology systems interfaced with the laboratory information system also provide ready-access to the digital images from the patient’s previous encounters. This may mitigate the time and cost of retrieving glass slides from the glass slide pathology archival storage. Anecdotally, this relative low-hanging fruit was transformational in proving the technology worthiness for pathologists.12 Digital patient timelines can facilitate review of key findings and compare current specimens to previous pathology (e.g., compare morphology of a metastatic tumor to a primary site of origin). In certain subspecialties such as cytology or microbiology, identification of disease or microorganisms is performed using coarse and fine-focusing of the plane of view on a microscope. Review of these findings may be enhanced through digitization of glass slides at multiple planes of focus (e.g., z-planes). This capability is supported by various scanner devices and can be employed as needed for specific use cases. Another feature in the digital toolset is the capability to label the image using digital annotations. Annotations are familiar to pathologists as ink markings on glass slides, however digital annotations enable several options for marking up the digital image such as point(s) or regions of interest that can have associated text, also with varying colors. Digital annotations are searchable, making retrieval of annotations easy. Digital pathology systems store annotations such that they can also have auditing and tracking functionality. Screen capture images with or without annotations can be embedded into diagnostic pathology reports to showcase findings or be saved for other nonclinical purposes. Similarly, gross specimen image capture has also been interfaced into laboratory information systems such that still images of gross specimens can be captured and annotated (Figure 4). These images can be referred to by pathologists for specimen sectioning and can increase the understanding of specimen orientation or sampling. Finally, primary diagnosis using clinical decision support tools are emerging and will be further discussed in Section 4.
FIGURE 4.
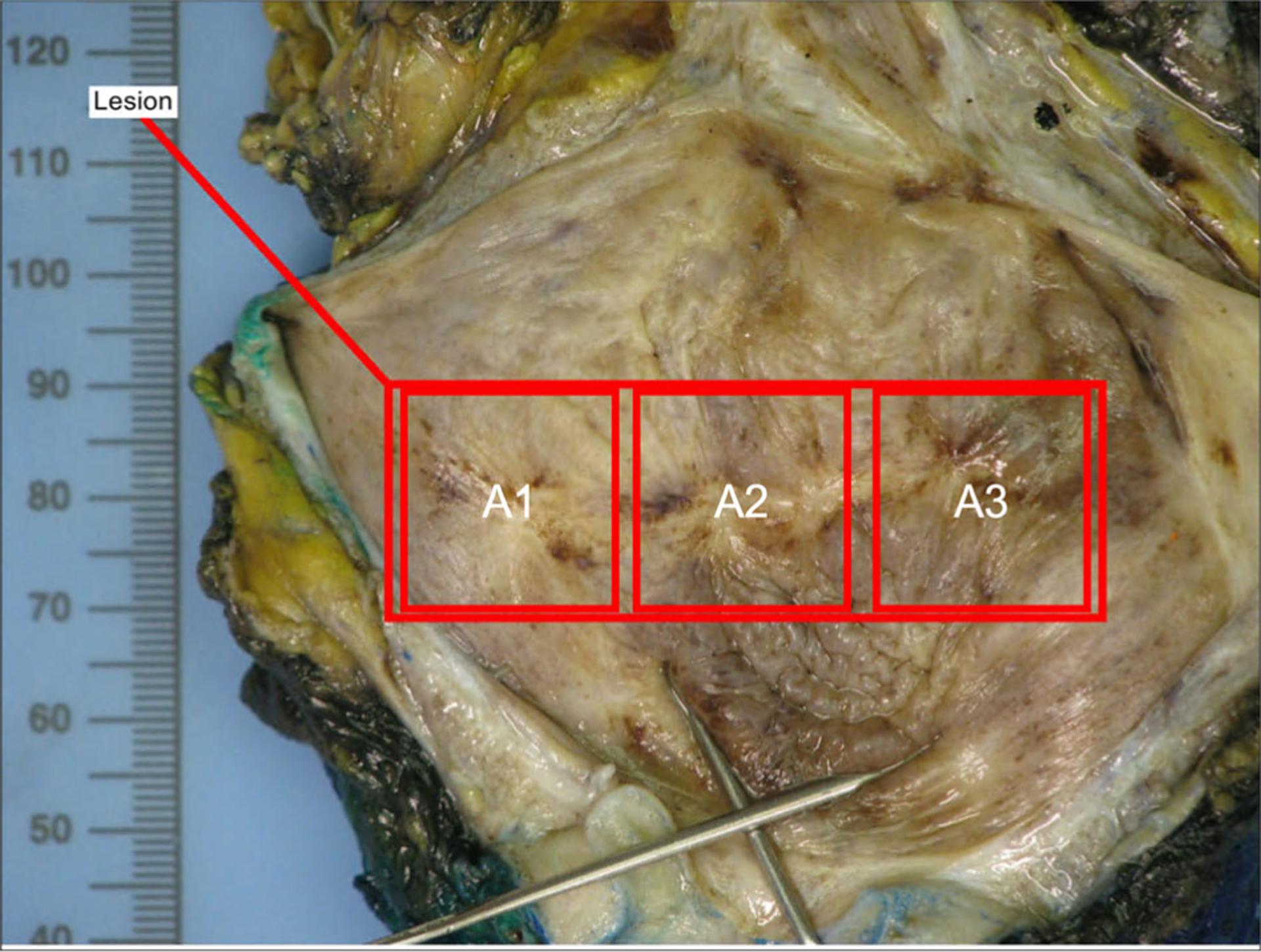
Representative gross specimen static image with block label annotations.
3.2 |. Consultation (telepathology)
Digital pathology systems can also be used for internal or remote consultation, allowing pathologists to share digital images and collaborate on patient cases. This collaborative workflow is familiar to pathologists who may share cases internally with colleagues for informal consultations, or for formal second opinion, sharing the patient slides with pathologists who are located at another institution. This may also be for tertiary care centers where pathology review for patients being managed at the local institution is required prior to any performed procedure. Use of telepathology for consultations can be especially useful for cases that necessitate subspecialty expertise or for laboratories that are in remote or underserved locations.56–62
Remote consultation can be done synchronously in real time, with both pathologists viewing the same image simultaneously and discussing the case over audio/video conference. This is more feasible for laboratories within similar geographic locations and time zones. Alternatively, asynchronous review can be performed where a submitting laboratory can request consultation on digital images, upload them to a secure server, and be provided asynchronous feedback or a report with the consulting institution’s findings. Digital pathology can also facilitate consultation with experts in other countries, allowing pathologists to access specialized knowledge and expertise that may not be available locally. This can be particularly important in areas where certain diseases are more prevalent or where access to specialized equipment or testing is limited.
3.3 |. Intraoperative consultation
Telepathology can also be used for a specific use case described in this section for intraoperative consultations (e.g., frozen sections). Use of telepathology for intraoperative consultation refers to the remote real-time assessment of patient specimens obtained during surgery, which are rapidly frozen and examined by a pathologist for a stat diagnosis. The pathologist then can communicate their findings with the surgeon during the operative procedure to help guide surgical decisions. Digital pathology systems can enhance the efficiency and accuracy of frozen section telepathology consultations.63,64 There are three methods in which digital pathology systems can be utilized for intraoperative consultation. First, a real-time video camera feed can be reviewed by a pathologist. This method requires a local expert operator to navigate to and show the appropriate field of view. Second, using a whole slide scanner, the frozen tissue specimen can be digitally scanned and shared with a remote pathologist for interpretation in real time. This practically allows for a single z-plane scanned digital slide that the pathologist can review in its entirety for an interpretation. Finally, specialized real-time “hybrid scanners” can be used, where the prepared slides can be loaded into, and the pathologist can remotely operate the device. These devices allow for similar navigation (e.g., panning, zooming, etc.), however, provide the added benefit of real time focusing of the tissue on the glass slide. This method has been primarily adopted for intraoperative consultations.65–69 All these digital pathology methods enable pathologists to render a prompt diagnosis, to aid the surgical team in making informed decisions for patient undergoing the procedure in real time.
An advantage of using telepathology during for frozen sections is the ability to involve a pathologist who may not be physically present in the frozen section area. This can help serve off-site surgical centers or may be useful when subspecialty pathology expertise is required when not available in the same physical location. Additionally, telepathology enables sharing intraoperative consultations with multiple pathologists simultaneously, facilitating collaboration and consultation as needed without having to courier glass slides between pathologists’ offices. This can lead to more accurate and timely diagnoses for patients, and ultimately better patient outcomes through appropriate surgical management.
3.4 |. Molecular pathology
Molecular testing technologies are also increasingly being utilized by pathology laboratories to provide a more comprehensive and accurate diagnosis to patients. With the increasing demand for personalized medicine and targeted therapies, the role of molecular testing in patient diagnosis and treatment has become standard of care in many scenarios. One operational use case where digital pathology systems support molecular workflows is the integration of histopathology images during region of interest selection for macro/microdissection. These technologies allow for digital annotation on the whole slide image, which can construct a coordinate map to then automate tissue capture on unstained level sections of the same tissue block. This can create more precise macrodissection and microdissection of tissue compared to conventional manual tissue scraping and minimize specimen contamination. From a clinical perspective, integrated reporting can facilitate accurate diagnostic interpretation of patient specimens. The ability to simultaneously visualize the morphology of patient specimens via whole slide images as well as molecular data in a single view can improve diagnostic accuracy and reduce turnaround time and errors in patient reports by avoiding review of each modality in insolation. Integration of both the digital images of patient tissue, along with associated molecular data (e.g., targeted gene expression profiles) makes this possible. Furthermore, clinical sampling and digitization can be leveraged for patient specimens being stored in biorepositories. Clinical studies can be designed to review the morphologic findings in patient specimens, further analyze the mechanism of disease, or discover potential targeted therapy. The use of digital pathology systems for large specimen biorepositories allows for ready-access digital retrieval and review of specimens, enabling large-scale studies that would not be possible with traditional analog methods. The use of digital pathology systems in molecular workflows has the potential to further evolve patient diagnosis and treatment by providing more accurate and personalized care to patients.
3.5 |. Multidisciplinary team meetings
Digital pathology systems have been increasingly used in multidisciplinary team (MDT) meetings to facilitate the sharing of pathology digital images and allow discussions among healthcare professionals from various medical domains (e.g., pathology, radiology, medicine, surgery, oncology, etc.). These conferences are used to congregate healthcare professionals from various medical disciplines and discuss patient cases to provide consensus on patient management decisions. Using digital pathology systems, pathologists can access patient digital images and other relevant data remotely. When using whole slide images, this can be impactful for non-pathology healthcare practitioners to review digital slides in a more comprehensive fashion in a team environment. Additionally, pathologists can annotate the digital slides prior to the MDT for a streamlined review and discussion of the key pathology findings. Furthermore, pathologist presentations in reviewing and communicating patient pathology results can be performed remotely using these digital technologies. This can improve the quality of patient management related discussions in reviewing the complete pathology record, including the entire patient timeline, as well any relevant pathology materials that were not originally prepared for a traditional static presentation.
These systems can enhance the education and training of pathologists and other healthcare providers. Since these digital assets are available, trainees or practicing physicians can observe and participate in MDT discussions remotely, allowing them to learn from and participate in the discussions. This can improve the quality of medical education and training, ultimately leading to better patient care. Overall, digital pathology systems can positively augment the efficiency and effectiveness of the MDT. As these digital technologies in pathology continue to mature and become adopted, it is likely that their use in MDTs will become even more prevalent in the future.
4 |. IMAGE ANALYSIS AND MACHINE LEARNING IN PATHOLOGY
4.1 |. Image analysis for biomarkers
Image analysis is the process of extracting meaningful information from digital images using mathematical and computational algorithms. It involves transforming raw image data into quantitative measurements, which can be used for several applications (i.e., diagnosis, research, and quality control). Image analysis is increasingly being used in pathology for immunohistochemistry quantification, especially for predictive and prognostic biomarkers. These digital pathology systems can capture high-resolution images of patient tissue prepared on glass slides, including all staining preparations such as special stains, immunohistochemistry, bright-field and dark-field in situ hybridization. Clinically the pathology laboratory uses these types of stained tissue samples to allow for accurate and precise assessment of biomarker spatial expression patterns. After digitization, the glass slides can now be converted to pixel imaging data where qualitative assessment can now be quantitative (e.g., percentage of cells staining positive for a particular marker or the intensity of staining). This objective data can be used to assist pathologist interpretation and further guide clinical decision-making. There are numerous image analysis software available for immunohistochemistry quantification from commercial vendors, academic medical centers, or open-access (e.g., QuPath). The FDA has already authorized several products using motorized microscopes and digital cameras or using whole slide images for various biomarkers including ER, PR, HER2, Ki-67, and p53.6 However this quantification is not limited to immunohistochemistry and includes DNA or RNA in situ hybridization, or even karyotypic analysis.70–72 These technologies have allowed for increased accuracy and standardization of the biomarker quantification.73 In recognition of these emerging digital pathology systems and their clinical use, guidelines specific to HER2 immunohistochemistry quantitative image analysis have been published by the CAP.74
4.2 |. Machine learning in pathology
Machine learning and augmented intelligence are increasingly being applied to various pathology applications to improve accuracy, productivity, and discovery of computational biomarkers. These technologies have shown promise in various aspects of pathology, including classification, segmentation, and quantifications tasks. These systems are to be purposed in assistive workflows to pathologists and may be used in clinical decision support, quantification of biomarkers, or predicting patient outcomes. There are myriads of applications currently in various development stages, and several that are commercially available. Augmented intelligence is being used in this context in place of the term artificial intelligence, as there are no specific use cases in pathology to date that are being used in isolation without the oversight of a pathologist.75 As a tool for diagnostic decision support, machine learning classification systems can be trained on high quality datasets of pathology images and associated metadata, and allow visualization of regions of interest for pathologist’s directed review. This can be used as an additional patient safety application where digital pathology systems can help improve the accuracy and constancy in diagnosis. Steiner et al. studied 240 prostate biopsies by 20 pathologists, unassisted and assisted with a machine learning model to classify prostate cancer Gleason grades.76 The article specifically addressed the potential for machine learning to aid in the standardization of pathology diagnoses, specifically in areas with high interobserver variability, such as grading of prostate biopsies. The study found that a combination of artificial intelligence and pathologist assessment led to higher interobserver agreement and accuracy in grading compared to pathologist assessment alone.77–81 The results showed an absolute increase in agreement for all 240 biopsies of 5.6%, which suggests this approach could lead to more standardized and accurate diagnoses in the future, including reduction of overgrading patients leading to overtreatment if not needed. The results are in keeping with showing machine learning models used in an assistive approach have high accuracy and increase interobserver agreement compared to the model outputs individually, or pathologists alone.
In addition to classification, machine learning systems in pathology can drive productivity in several ways, including assistive triaging of specimens for pathologist review,82–84 screening tasks and directed review,85–88 and democratization of “expert” trained knowledge.89 In a typical workday for a pathologist using the analog workflow, they are presented with a pile of trays, each holding glass slides and paperwork, manually collated by patient accession. They are generally not sorted in any particular order, and do not have any indication of pathology that may be present within the tissue awaiting review. Digital workflows allow for automated aggregation of slides for each patient, and can layer machine learning model predictions for specimens for specific findings (e.g., invasive carcinoma, metastasis, etc.). These predictions can be presented as alerts or visual indicators to the pathologist who can then selectively review the cases assigned to them based on priority, or order additional predictive/prognostic biomarker tests to be started more expeditiously in the laboratory.90 The classification task itself can prove valuable for providing the pathologist with a directed review. Furthermore, tedious screening tasks such as: identification of mitoses, mycobacteria, small foci of metastatic tumor; can be presented to the pathologist for directed review showing the highest probability of a suspicious finding for pathologist eventual interpretation. One publication showed pathologists had an average 47% decrease in time to detection of micrometastatic breast carcinoma, but also a 19% decrease review time for benign lymph nodes; suggesting that with pathologist trust and comfortability—screening tools can improve efficiencies for negative findings as well.91 Another area of productivity relates to the democratization of expert knowledge. These digital technologies can also mitigate shipping of patient specimens to reduce reporting turnaround times. Machine learning models trained on expert level data can be exported to rural or resource restricted geographic locations where specific expertise may be lacking.89
Perhaps one of the most hopeful areas surrounding machine learning models in pathology relates to the discovery of computational biomarkers to provide better diagnoses or prognostication for patients. Machine learning can aid in the precision medicine era encouraging discovery of novel biomarkers and targets by identifying patterns and associations between different multimodal data (e.g., histopathology, genomic, proteomic, clinical data). Machine learning models have shown high performance in predicting site of origin from cancers of unknown primary,92 or virtual prediction of cellular protein expression,93,94 and molecular aberrations from hematoxylin and eosin glass slides alone.95–103 With appropriate training data, multi-omics information can provide quantitative data for machine learning models to predict patient outcomes (e.g., survival, response to treatment).104–119 These systems have potential to change how pathology is practiced; however, machine learning models are not replacement for human expertise. With any new technology, there are challenges to overcome120,121; however, pathologists are still needed to apply oversight to these systems and will continue to play a vital role in specimen diagnosis as well as integration and validation of machine learning results into clinical practice.
5 |. FUTURE TECHNOLOGIES IN PATHOLOGY
5.1 |. Multispectral imaging
Multispectral imaging is a technique that captures imaging data from varied, specific wavelengths of light, allowing for the detection of spectral excitation characteristics of tissues. Multispectral imaging differs from the aforementioned whole slide scanners in the color spectrum capture (e.g., RGB vs. entire spectral wavelength). In pathology, multispectral imaging has the potential to enhance diagnostic accuracy by improving the visualization and characterization of cells, as well as their spatial relationships through the simultaneous imaging and quantitation of numerous antibodies/antigens on the same formalin fixed paraffin embedded tissue section. This can be an improvement compared to conventional immunohistochemical stains such that multispectral imaging can capture data from multiple wavelengths of light on the same tissue section, instead of having numerous immunohistochemical stained sections. Image viewers compatible to review multispectral images allow users to review permutations of cellular expression and can allow for more rapid and comprehensive analysis of the specimen. Multispectral imaging techniques can provide cellular level characterization of various protein expression permutations. Data from these images can be used for vast quantification of cells in the tissue section as a coordinate system for downstream analysis. These detailed analyses can provide more accurate information compared to traditional methods of imaging.57 Clinical utility of this technology has not been mainstream, and is used currently in the research space; however, the technology can further understanding of disease and cellular spatial orientation, having implications for treatment planning and patient outcomes.
5.2 |. Advanced slide-less pathology imaging
Advanced imaging techniques in pathology are emerging in the research domain, however if they become mainstream, could have significant clinical implications for the practice of pathology and patient diagnostics.122 Imaging techniques such as multiphoton imaging with or without clearing histology, fluorescence imitating bright-field imaging (FIBI), or light sheet microscopy emerging “slide-free” are examples of technologies that can be used to nondestructively directly image patient tissue specimens in minutes to hours, a relative short amount of time compared to current histology laboratory practices. Aside from disrupting the conventional glass slide production of the laboratory, this technology can be a valuable tool for clinical practice since it is nondestructive to the tissue and can be salvaged for molecular or other downstream testing. Multiphoton imaging is a nonlinear imaging technique that has been purposed as an ex vivo microscopy imaging solution. It uses ultrafast laser pulses to excite multiple photons in a specimen, leading to fluorescence emission. The laser pulses can penetrate tissue samples and capture high-resolution images in three dimensions, making it a valuable tool for studying tissue structure and function.123–125 In conjunction with tissue clearing techniques such as clearing histology with multiphoton microscopy, these technologies can create a more detailed and complete picture of tissue samples, and recreate familiar hematoxylin and eosin stained images using fluorescent dyes.126 FIBI is based on the use of patient specimens absorbing stains, then illuminated at 405 nanometer wavelength to capture the excitation autofluorescence which appears as familiar hematoxylin and eosin stained images.127 Another nondestructive technology called open-top light sheet microscopy uses low natural aperture illumination to capture a three-dimensional fluorescent image. This technique allows for the direct generation of images of tissue sections, enabling the visualization of complex structures and cellular interactions in their native context.128–130 All of these technologies have shown promise as potential disruptive technologies in pathology to analyze morphology, cellular organization, and composition of tissues with high spatial resolution. They can impact patient care through their nondestructive and relatively short turnaround time capture of high-resolution images of tissues and cells. These advanced imaging techniques can aid in the diagnosis of disease and have the potential to advance our understanding of pathology as medical discipline.
6 |. CONCLUSION
Pathology laboratories have access to and are undergoing digital transformations, adopting innovative technologies to enhance patient care. Digital transformation in pathology involves setting up an appropriate infrastructure to support these technologies, integration into clinical practice, implementation to ensure sufficient quality, and understanding the regulatory implications. Infrastructure involves the acquisition and storage of digital images, and integration refers to the incorporation of digital pathology systems with other clinical systems, such as laboratory information systems or electronic medical records. Implementation involves the use of digital pathology systems for routine clinical use cases. Pathology professional societies have established clinical validation guidelines, and the US Food and Drug Administration has approved numerous digital pathology systems across varied applications in pathology. Once properly validated, these digital pathology systems can be used for many clinical use cases such as primary diagnosis (e.g., surgical pathology, cytology, microbiology, hematology, etc.), consultation, intraoperative consultation, molecular pathology, and MDT meetings. For consultations, digital pathology systems enable pathologists to share digital slides with other pathologists for remote consultation. Intraoperative consultation has also been facilitated through using these technologies, allowing pathologists to remotely review patient specimens retrieved during surgical procedures. In molecular pathology, digital pathology systems can be used to facilitate analysis of tissue samples for genetic aberrations. MDT meetings have also been facilitated through digital pathology systems, allowing clinicians across medical domains to collaborate and discuss patient cases. As technologies continue to emerge, disruption of the pathology domain may be evidenced in advanced imaging devices that do not require glass slide preparation and can capture images from patient tissue directly with cellular resolution. This group of technologies can enable faster image acquisition and tissue review compared to current analog methods, delivering even higher quality patient care. Digital pathology systems have enabled pathologists to practice their routine clinical activities, using digital workflows instead of analog processes. These technologies are poised to stimulate digital computational discovery. Image analysis and machine learning systems have provided pathologists with decision support tools. Incorporating these emerging technologies into the digital pathology workflow has the potential to further enhance the accuracy, efficiency, and quality of patient care. As these technologies continue to evolve the field of pathology, pathologists should keep abreast of such systems and adopt them where added value is successfully proven to improve pathology practice and ultimately patient care.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1.Pantanowitz L, Sinard JH, Henricks WH, et al. Validating whole slide imaging for diagnostic purposes in pathology: guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med. 2013;137(12):1710–1722. doi: 10.5858/arpa.2013-0093-CP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans AJ, Brown RW, Bui MM, et al. Validating whole slide imaging systems for diagnostic purposes in pathology: guideline update from the College of American Pathologists in collaboration with the American Society for Clinical Pathology and the Association for Pathology Informatics. Arch Pathol Lab Med. 2022;146(4):440–450. doi: 10.5858/arpa.2020-0723-CP [DOI] [PubMed] [Google Scholar]
- 3.Mukhopadhyay S, Feldman MD, Abels E, et al. Whole slide imaging versus microscopy for primary diagnosis in surgical pathology. Am J Surg Pathol. 2018;42(1):39–52. doi: 10.1097/PAS.0000000000000948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borowsky AD, Glassy EF, Wallace WD, et al. Digital whole slide imaging compared with light microscopy for primary diagnosis in surgical pathology. Arch Pathol Lab Med. 2020;144(10):1245–1253. doi: 10.5858/arpa.2019-0569-OA [DOI] [PubMed] [Google Scholar]
- 5.US Food and Drug Administration. FDA Authorizes Software that can Help Identify Prostate Cancer. FDA News Release; 2021. Accessed November 9, 2021. https://www.fda.gov/news-events/press-announcements/fda-authorizes-software-can-help-identify-prostate-cancer [Google Scholar]
- 6.Cornish TC. Clinical application of image analysis in pathology. Adv Anat Pathol. 2020;27(4):227–235. doi: 10.1097/PAP.0000000000000263 [DOI] [PubMed] [Google Scholar]
- 7.510(k) Premarket Notification. X100 with full field peripheral blood smear (PBS) application. 2020. Accessed November 9, 2021. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K201301
- 8.Hanna MG, Parwani A, Sirintrapun SJ. Whole slide imaging: technology and applications. Adv Anat Pathol. 2020;27(4):251–259. doi: 10.1097/PAP.0000000000000273 [DOI] [PubMed] [Google Scholar]
- 9.Luo W, Hassell LA. Use cases for digital pathology. In: Kaplan KJ, Rao LKF, eds. Digital Pathology: Historical Perspectives, Current Concepts & Future Applications. Springer International Publishing; 2016: 5–15. doi: 10.1007/978-3-319-20379-9_2 [DOI] [Google Scholar]
- 10.Evans AJ, Vajpeyi R, Henry M, Chetty R. Establishment of a remote diagnostic histopathology service using whole slide imaging (digital pathology). J Clin Pathol. 2021;74(7):421–424. doi: 10.1136/jclinpath-2020-206762 [DOI] [PubMed] [Google Scholar]
- 11.Baidoshvili A, Bucur A, van Leeuwen J, van der Laak J, Kluin P, van Diest PJ. Evaluating the benefits of digital pathology implementation: time savings in laboratory logistics. Histopathology. 2018;73(5): 784–794. doi: 10.1111/his.13691 [DOI] [PubMed] [Google Scholar]
- 12.Hanna MG, Ardon O, Reuter VE, et al. Integrating digital pathology into clinical practice. Mod Pathol. 2022;35(2):152–164. doi: 10.1038/s41379-021-00929-0 [DOI] [PubMed] [Google Scholar]
- 13.Gorman C, Punzo D, Octaviano I, et al. Interoperable slide microscopy viewer and annotation tool for imaging data science and computational pathology. Nat Commun. 2023;14(1):1572. doi: 10.1038/s41467-023-37224-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrmann MD, Clunie DA, Fedorov A, et al. Implementing the DICOM standard for digital pathology. J Pathol Inform. 2018;9:37. doi: 10.4103/jpi.jpi_42_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalinski T, Zwönitzer R, Roßner M, Hofmann H, Roessner A, Guenther T. Digital Imaging and Communications in Medicine (DICOM) as standard in digital pathology. Histopathology. 2012; 61(1):132–134. doi: 10.1111/j.1365-2559.2012.04243.x [DOI] [PubMed] [Google Scholar]
- 16.Williams BJ, Knowles C, Treanor D. Maintaining quality diagnosis with digital pathology: a practical guide to ISO 15189 accreditation. J Clin Pathol. 2019;72(10):663–668. doi: 10.1136/jclinpath-2019-205944 [DOI] [PubMed] [Google Scholar]
- 17.Azam AS, Miligy IM, Kimani PKU, et al. Diagnostic concordance and discordance in digital pathology: a systematic review and meta-analysis. J Clin Pathol. 2021;74(7):448–455. doi: 10.1136/jclinpath-2020-206764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goacher E, Randell R, Williams B, Treanor D. The diagnostic concordance of whole slide imaging and light microscopy: a systematic review. Arch Pathol Lab Med. 2017;141(1):151–161. doi: 10.5858/arpa.2016-0025-RA [DOI] [PubMed] [Google Scholar]
- 19.García-Rojo M International clinical guidelines for the adoption of digital pathology: a review of technical aspects. Pathobiol J Immunopathol Mol Cell Biol. 2016;83(2–3):99–109. doi: 10.1159/000441192 [DOI] [PubMed] [Google Scholar]
- 20.Abels E, Pantanowitz L. Current state of the regulatory trajectory for whole slide imaging devices in the USA. J Pathol Inform. 2017;8:23. doi: 10.4103/jpi.jpi_11_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Araújo ALD, Amaral-Silva GK, Fonseca FP, et al. Validation of digital microscopy in the histopathological diagnoses of oral diseases. Virchows Arch Int J Pathol. 2018;473(3):321–327. doi: 10.1007/s00428-018-2382-5 [DOI] [PubMed] [Google Scholar]
- 22.Zelic R, Giunchi F, Lianas L, et al. Interchangeability of light and virtual microscopy for histopathological evaluation of prostate cancer. Sci Rep. 2021;11(1):3257. doi: 10.1038/s41598-021-82911-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diniz PB, Sena-Filho M, Graner KM, et al. Comparison of the whole slide imaging and conventional light microscopy in the grading of oral epithelial dysplasia: a multi-institutional study. Med Oral Patol Oral Cir Bucal. 2021;26(1):e8–e13. doi: 10.4317/medoral.23854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alassiri A, Almutrafi A, Alsufiani F, et al. Whole slide imaging compared with light microscopy for primary diagnosis in surgical neuropathology: a validation study. Ann Saudi Med. 2020;40(1):36–41. doi: 10.5144/0256-4947.2020.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al Habeeb A, Evans A, Ghazarian D. Virtual microscopy using whole-slide imaging as an enabler for teledermatopathology: a paired consultant validation study. J Pathol Inform. 2012;3:2. doi: 10.4103/2153-3539.93399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Araújo ALD, do Amaral-Silva GK, Pérez-de-Oliveira ME, et al. Fully digital pathology laboratory routine and remote reporting of oral and maxillofacial diagnosis during the COVID-19 pandemic: a validation study. Virchows Arch Int J Pathol. 2021;479:585–595. doi: 10.1007/s00428-021-03075-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bauer TW, Schoenfield L, Slaw RJ, Yerian L, Sun Z, Henricks WH. Validation of whole slide imaging for primary diagnosis in surgical pathology. Arch Pathol Lab Med. 2013;137(4):518–524. doi: 10.5858/arpa.2011-0678-OA [DOI] [PubMed] [Google Scholar]
- 28.Brunelli M, Beccari S, Colombari R, et al. iPathology cockpit diagnostic station: validation according to College of American Pathologists Pathology and Laboratory Quality Center recommendation at the Hospital Trust and University of Verona. Diagn Pathol. 2014;9(Suppl 1):S12. doi: 10.1186/1746-1596-9-S1-S12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buck TP, Dilorio R, Havrilla L, O’Neill DG. Validation of a whole slide imaging system for primary diagnosis in surgical pathology: a community hospital experience. J Pathol Inform. 2014;5(1):43. doi: 10.4103/2153-3539.145731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fonyad L, Krenács T, Nagy P, et al. Validation of diagnostic accuracy using digital slides in routine histopathology. Diagn Pathol. 2012;7: 35. doi: 10.1186/1746-1596-7-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jukic DM, Drogowski LM, Martina J, Parwani AV. Clinical examination and validation of primary diagnosis in anatomic pathology using whole slide digital images. Arch Pathol Lab Med. 2011;135(3):372–378. doi: 10.1043/2009-0678-OA.1 [DOI] [PubMed] [Google Scholar]
- 32.Mpunga T, Hedt-Gauthier BL, Tapela N, et al. Implementation and validation of telepathology triage at cancer referral center in rural Rwanda. J Glob Oncol. 2016;2(2):76–82. doi: 10.1200/JGO.2015.002162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campbell WS, Hinrichs SH, Lele SM, et al. Whole slide imaging diagnostic concordance with light microscopy for breast needle biopsies. Hum Pathol. 2014;45(8):1713–1721. doi: 10.1016/j.humpath.2014.04.007 [DOI] [PubMed] [Google Scholar]
- 34.Campbell WS, Lele SM, West WW, Lazenby AJ, Smith LM, Hinrichs SH. Concordance between whole-slide imaging and light microscopy for routine surgical pathology. Hum Pathol. 2012;43(10): 1739–1744. doi: 10.1016/j.humpath.2011.12.023 [DOI] [PubMed] [Google Scholar]
- 35.Cheng CL, Azhar R, Sng SHA, et al. Enabling digital pathology in the diagnostic setting: navigating through the implementation journey in an academic medical centre. J Clin Pathol. 2016;69(9):784–792. doi: 10.1136/jclinpath-2015-203600 [DOI] [PubMed] [Google Scholar]
- 36.Houghton JP, Ervine AJ, Kenny SL, et al. Concordance between digital pathology and light microscopy in general surgical pathology: a pilot study of 100 cases. J Clin Pathol. 2014;67(12):1052–1055. doi: 10.1136/jclinpath-2014-202491 [DOI] [PubMed] [Google Scholar]
- 37.Snead DRJ, Tsang YW, Meskiri A, et al. Validation of digital pathology imaging for primary histopathological diagnosis. Histopathology. 2016;68(7):1063–1072. doi: 10.1111/his.12879 [DOI] [PubMed] [Google Scholar]
- 38.Tabata K, Mori I, Sasaki T, et al. Whole-slide imaging at primary pathological diagnosis: validation of whole-slide imaging-based primary pathological diagnosis at twelve Japanese academic institutes. Pathol Int. 2017;67(11):547–554. doi: 10.1111/pin.12590 [DOI] [PubMed] [Google Scholar]
- 39.Al-Janabi S, Huisman A, Nap M, Clarijs R, van Diest PJ. Whole slide images as a platform for initial diagnostics in histopathology in a medium-sized routine laboratory. J Clin Pathol. 2012;65(12):1107–1111. doi: 10.1136/jclinpath-2012-200878 [DOI] [PubMed] [Google Scholar]
- 40.Al-Janabi S, Huisman A, Vink A, et al. Whole slide images for primary diagnostics in dermatopathology: a feasibility study. J Clin Pathol. 2012;65(2):152–158. doi: 10.1136/jclinpath-2011-200277 [DOI] [PubMed] [Google Scholar]
- 41.Al-Janabi S, Huisman A, Nikkels PGJ, ten Kate FJW, van Diest PJ. Whole slide images for primary diagnostics of paediatric pathology specimens: a feasibility study. J Clin Pathol. 2013;66(3):218–223. doi: 10.1136/jclinpath-2012-201104 [DOI] [PubMed] [Google Scholar]
- 42.Al-Janabi S, Huisman A, Jonges GN, ten Kate FJW, Goldschmeding R, van Diest PJ. Whole slide images for primary diagnostics of urinary system pathology: a feasibility study. J Renal Inj Prev. 2014;3(4):91–96. doi: 10.12861/jrip.2014.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arnold MA, Chenever E, Baker PB, et al. The College of American Pathologists guidelines for whole slide imaging validation are feasible for pediatric pathology: a pediatric pathology practice experience. Pediatr Dev Pathol. 2015;18(2):109–116. doi: 10.2350/14-07-1523-OA.1 [DOI] [PubMed] [Google Scholar]
- 44.Krishnamurthy S, Mathews K, McClure S, et al. Multi-institutional comparison of whole slide digital imaging and optical microscopy for interpretation of hematoxylin-eosin-stained breast tissue sections. Arch Pathol Lab Med. 2013;137(12):1733–1739. doi: 10.5858/arpa.2012-0437-OA [DOI] [PubMed] [Google Scholar]
- 45.Ordi J, Castillo P, Saco A, et al. Validation of whole slide imaging in the primary diagnosis of gynaecological pathology in a university hospital. J Clin Pathol. 2015;68(1):33–39. doi: 10.1136/jclinpath-2014-202524 [DOI] [PubMed] [Google Scholar]
- 46.Hanna MG, Reuter VE, Hameed MR, et al. Whole slide imaging equivalency and efficiency study: experience at a large academic center. Mod Pathol. 2019;32(7):916–928. doi: 10.1038/s41379-019-0205-0 [DOI] [PubMed] [Google Scholar]
- 47.Hanna MG, Reuter VE, Ardon O, et al. Validation of a digital pathology system including remote review during the COVID-19 pandemic. Mod Pathol. 2020;33(11):2115–2127. doi: 10.1038/s41379-020-0601-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Randell R, Ruddle RA, Thomas RG, Mello-Thoms C, Treanor D. Diagnosis of major cancer resection specimens with virtual slides: impact of a novel digital pathology workstation. Hum Pathol. 2014;45(10): 2101–2106. doi: 10.1016/j.humpath.2014.06.017 [DOI] [PubMed] [Google Scholar]
- 49.Thrall MJ, Wimmer JL, Schwartz MR. Validation of multiple whole slide imaging scanners based on the guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med. 2015;139(5):656–664. doi: 10.5858/arpa.2014-0073-OA [DOI] [PubMed] [Google Scholar]
- 50.Mills AM, Gradecki SE, Horton BJ, et al. Diagnostic efficiency in digital pathology: a comparison of optical versus digital assessment in 510 surgical pathology cases. Am J Surg Pathol. 2018;42(1):53–59. doi: 10.1097/PAS.0000000000000930 [DOI] [PubMed] [Google Scholar]
- 51.Abel JT, Ouillette P, Williams CL, et al. Display characteristics and their impact on digital pathology: a current review of pathologists’ future “microscope”. J Pathol Inform. 2020;11(1):23. doi: 10.4103/jpi.jpi_38_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clarke EL, Munnings C, Williams B, Brettle D, Treanor D. Display evaluation for primary diagnosis using digital pathology. J Med Imaging. 2020;7(2):027501. doi: 10.1117/1.JMI.7.2.027501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim D, Pantanowitz L, Schüffler P, et al. (Re) defining the high-power field for digital pathology. J Pathol Inform. 2020;11:33. doi: 10.4103/jpi.jpi_48_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vodovnik A Diagnostic time in digital pathology: a comparative study on 400 cases. J Pathol Inform. 2016;7(1):4. doi: 10.4103/2153-3539.175377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hanna MG, Pantanowitz L. Feasibility of using the Omnyx digital pathology system for cytology practice. J Am Soc Cytopathol. 2019; 8(4):182–189. doi: 10.1016/j.jasc.2019.01.003 [DOI] [PubMed] [Google Scholar]
- 56.Zhao C, Wu T, Ding X, et al. International telepathology consultation: three years of experience between the University of Pittsburgh Medical Center and KingMed diagnostics in China. J Pathol Inform. 2015;6:63. doi: 10.4103/2153-3539.170650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pantanowitz L, Wiley CA, Demetris A, et al. Experience with multimodality telepathology at the University of Pittsburgh Medical Center. J Pathol Inform. 2012;3:45. doi: 10.4103/2153-3539.104907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen J, Jiao Y, Lu C, Zhou J, Zhang Z, Zhou C. A nationwide telepathology consultation and quality control program in China: implementation and result analysis. Diagn Pathol. 2014;9(Suppl 1): S2. doi: 10.1186/1746-1596-9-S1-S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chong T, Palma-Diaz MF, Fisher C, et al. The California telepathology service: UCLA’s experience in deploying a regional digital pathology subspecialty consultation network. J Pathol Inform. 2019; 10:31. doi: 10.4103/jpi.jpi_22_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dunn BE, Almagro UA, Choi H, et al. Dynamic-robotic telepathology: department of veterans affairs feasibility study. Hum Pathol. 1997; 28(1):8–12. doi: 10.1016/s0046-8177(97)90271-9 [DOI] [PubMed] [Google Scholar]
- 61.Montgomery ND, Tomoka T, Krysiak R, et al. Practical successes in telepathology experiences in Africa. Clin Lab Med. 2018;38(1): 141–150. doi: 10.1016/j.cll.2017.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Têtu B, Perron É, Louahlia S, Paré G, Trudel MC, Meyer J. The eastern Québec telepathology network: a three-year experience of clinical diagnostic services. Diagn Pathol. 2014;9(Suppl 1):S1. doi: 10.1186/1746-1596-9-S1-S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Menter T, Nicolet S, Baumhoer D, Tolnay M, Tzankov A. Intraoperative frozen section consultation by remote whole-slide imaging analysis-validation and comparison to robotic remote microscopy. J Clin Pathol. 2020;73(6):350–352. doi: 10.1136/jclinpath-2019-206261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thrall MJ, Rivera AL, Takei H, Powell SZ. Validation of a novel robotic telepathology platform for neuropathology intraoperative touch preparations. J Pathol Inform. 2014;5(1):21. doi: 10.4103/2153-3539.137642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dietz RL, Hartman DJ, Zheng L, Wiley C, Pantanowitz L. Review of the use of telepathology for intraoperative consultation. Expert Rev Med Devices. 2018;15(12):883–890. doi: 10.1080/17434440.2018.1549987 [DOI] [PubMed] [Google Scholar]
- 66.Baskota SU, Wiley C, Pantanowitz L. The next generation robotic microscopy for intraoperative teleneuropathology consultation. J Pathol Inform. 2020;11:13. doi: 10.4103/jpi.jpi_2_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Laurent-Bellue A, Poullier E, Pomerol JF, et al. Four-year experience of digital slide telepathology for intraoperative frozen section consultations in a two-site French academic Department of Pathology. Am J Clin Pathol. 2020;154(3):414–423. doi: 10.1093/ajcp/aqaa055 [DOI] [PubMed] [Google Scholar]
- 68.Ribback S, Flessa S, Gromoll-Bergmann K, Evert M, Dombrowski F. Virtual slide telepathology with scanner systems for intraoperative frozen-section consultation. Pathol Res Pract. 2014;210(6):377–382. doi: 10.1016/j.prp.2014.02.007 [DOI] [PubMed] [Google Scholar]
- 69.Słodkowska J, Pankowski J, Siemiatkowska K, Chyczewski L. Use of the virtual slide and the dynamic real-time telepathology systems for a consultation and the frozen section intra-operative diagnosis in thoracic/pulmonary pathology. Folia Histochem Cytobiol. 2009;47(4): 679–684. doi: 10.2478/v10042-010-0009-z [DOI] [PubMed] [Google Scholar]
- 70.Jamalzadeh S, Häkkinen A, Andersson N, et al. QuantISH: RNA in situ hybridization image analysis framework for quantifying cell type-specific target RNA expression and variability. Lab Investig J Tech Methods Pathol. 2022;102(7):753–761. doi: 10.1038/s41374-022-00743-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McNamara G, Difilippantonio M, Ried T, Bieber FR. Microscopy and image analysis. Curr Protoc Hum Genet. 2017;94(1):4.4.1–4.4.89. doi: 10.1002/cphg.42 [DOI] [PubMed] [Google Scholar]
- 72.Baumgartner A, Ferlatte Hartshorne C, Polyzos AA, Weier HUG, Weier JF, O’Brien B. Full karyotype interphase cell analysis. J Histochem Cytochem. 2018;66(8):595–606. doi: 10.1369/0022155418771613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stålhammar G, Fuentes Martinez N, Lippert M, et al. Digital image analysis outperforms manual biomarker assessment in breast cancer. Mod Pathol. 2016;29(4):318–329. doi: 10.1038/modpathol.2016.34 [DOI] [PubMed] [Google Scholar]
- 74.Bui MM, Riben MW, Allison KH, et al. Quantitative image analysis of human epidermal growth factor receptor 2 immunohistochemistry for breast cancer: guideline from the College of American Pathologists. Arch Pathol Lab Med. 2019;143(10):1180–1195. doi: 10.5858/arpa.2018-0378-CP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.American Medical Association. Augmented intelligence in health care. 2019. Accessed November 9, 2021. https://www.ama-assn.org/system/files/2019-01/augmented-intelligence-policy-report.pdf
- 76.Steiner DF, Nagpal K, Sayres R, et al. Evaluation of the use of combined artificial intelligence and pathologist assessment to review and grade prostate biopsies. JAMA Netw Open. 2020;3(11):e2023267. doi: 10.1001/jamanetworkopen.2020.23267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Burchardt M, Engers R, Müller M, et al. Interobserver reproducibility of Gleason grading: evaluation using prostate cancer tissue microarrays. J Cancer Res Clin Oncol. 2008;134(10):1071–1078. doi: 10.1007/s00432-008-0388-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Veloso SG, Lima MF, Salles PG, Berenstein CK, Scalon JD, Bambirra EA. Interobserver agreement of Gleason score and modified Gleason score in needle biopsy and in surgical specimen of prostate cancer. Int Braz J Urol. 2007;33(5):639–646. doi: 10.1590/s1677-55382007000500005 [DOI] [PubMed] [Google Scholar]
- 79.Egevad L, Ahmad AS, Algaba F, et al. Standardization of Gleason grading among 337 European pathologists. Histopathology. 2013; 62(2):247–256. doi: 10.1111/his.12008 [DOI] [PubMed] [Google Scholar]
- 80.Ozdamar SO, Sarikaya S, Yildiz L, Atilla MK, Kandemir B, Yildiz S. Intraobserver and interobserver reproducibility of WHO and Gleason histologic grading systems in prostatic adenocarcinomas. Int Urol Nephrol. 1996;28(1):73–77. doi: 10.1007/BF02550141 [DOI] [PubMed] [Google Scholar]
- 81.Abdollahi A, Meysamie A, Sheikhbahaei S, et al. Inter/intra-observer reproducibility of Gleason scoring in prostate adenocarcinoma in Iranian pathologists. Urol J. 2012;9(2):486–490. [PubMed] [Google Scholar]
- 82.Kers J, Bülow RD, Klinkhammer BM, et al. Deep learning-based classification of kidney transplant pathology: a retrospective, multicentre, proof-of-concept study. Lancet Digit Health. 2022;4(1):e18–e26. doi: 10.1016/S2589-7500(21)00211-9 [DOI] [PubMed] [Google Scholar]
- 83.Dov D, Assaad S, Syedibrahim A, et al. A hybrid human-machine learning approach for screening prostate biopsies can improve clinical efficiency without compromising diagnostic accuracy. Arch Pathol Lab Med. 2022;146(6):727–734. doi: 10.5858/arpa.2020-0850-OA [DOI] [PubMed] [Google Scholar]
- 84.Xander LU, Wagner J, Hahn H. Improved computer-assisted reading of identification and shortened MIC data for reporting on urine specimens at a Berlin university hospital. Zentralbl Bakteriol Mikrobiol Hyg. 1988;268(3):295–305. doi: 10.1016/s0176-6724(88)80014-2 [DOI] [PubMed] [Google Scholar]
- 85.Pantanowitz L, Hartman D, Qi Y, et al. Accuracy and efficiency of an artificial intelligence tool when counting breast mitoses. Diagn Pathol. 2020;15(1):80. doi: 10.1186/s13000-020-00995-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tadrous PJ. Computer-assisted screening of Ziehl-Neelsen-stained tissue for mycobacteria. Algorithm design and preliminary studies on 2,000 images. Am J Clin Pathol. 2010;133(6):849–858. doi: 10.1309/AJCPMR3BLVBH8THV [DOI] [PubMed] [Google Scholar]
- 87.Obstfeld AE. Hematology and machine learning. J Appl Lab Med. 2023;8(1):129–144. doi: 10.1093/jalm/jfac108 [DOI] [PubMed] [Google Scholar]
- 88.Eloy C, Marques A, Pinto J, et al. Artificial intelligence-assisted cancer diagnosis improves the efficiency of pathologists in prostatic biopsies. Virchows Arch Int J Pathol. 2023;482(3):595–604. doi: 10.1007/s00428-023-03518-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Campanella G, Hanna MG, Geneslaw L, et al. Clinical-grade computational pathology using weakly supervised deep learning on whole slide images. Nat Med. 2019;25(8):1301–1309. doi: 10.1038/s41591-019-0508-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pantanowitz L, Quiroga-Garza GM, Bien L, et al. An artificial intelligence algorithm for prostate cancer diagnosis in whole slide images of core needle biopsies: a blinded clinical validation and deployment study. Lancet Digit Health. 2020;2(8):e407–e416. doi: 10.1016/S2589-7500(20)30159-X [DOI] [PubMed] [Google Scholar]
- 91.Steiner DF, MacDonald R, Liu Y, et al. Impact of deep learning assistance on the histopathologic review of lymph nodes for metastatic breast cancer. Am J Surg Pathol. 2018;42(12):1636–1646. doi: 10.1097/PAS.0000000000001151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lu MY, Chen TY, Williamson DFK, et al. AI-based pathology predicts origins for cancers of unknown primary. Nature. 2021;594(7861): 106–110.doi: 10.1038/s41586-021-03512-4 [DOI] [PubMed] [Google Scholar]
- 93.Jackson CR, Sriharan A, Vaickus LJ. A machine learning algorithm for simulating immunohistochemistry: development of SOX10 virtual IHC and evaluation on primarily melanocytic neoplasms. Mod Pathol. 2020;33(9):1638–1648. doi: 10.1038/s41379-020-0526-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.de Haan K, Zhang Y, Zuckerman JE, et al. Deep learning-based transformation of H&E stained tissues into special stains. Nat Commun. 2021;12(1):4884. doi: 10.1038/s41467-021-25221-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mayer C, Ofek E, Fridrich DE, et al. Direct identification of ALK and ROS1 fusions in non-small cell lung cancer from hematoxylin and eosin-stained slides using deep learning algorithms. Mod Pathol. 2022;35(12):1882–1887. doi: 10.1038/s41379-022-01141-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Milewski D, Jung H, Brown GT, et al. Predicting molecular subtype and survival of rhabdomyosarcoma patients using deep learning of H&E images: a report from the children’s oncology group. Clin Cancer Res. 2023;29(2):364–378. doi: 10.1158/1078-0432.CCR-22-1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen M, Zhang B, Topatana W, et al. Classification and mutation prediction based on histopathology H&E images in liver cancer using deep learning. npj Precis Oncol. 2020;4:14. doi: 10.1038/s41698-020-0120-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tomita N, Tafe LJ, Suriawinata AA, et al. Predicting oncogene mutations of lung cancer using deep learning and histopathologic features on whole-slide images. Transl Oncol. 2022;24:101494. doi: 10.1016/j.tranon.2022.101494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Coudray N, Ocampo PS, Sakellaropoulos T, et al. Classification and mutation prediction from non-small cell lung cancer histopathology images using deep learning. Nat Med. 2018;24(10):1559–1567. doi: 10.1038/s41591-018-0177-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Choi YS, Bae S, Chang JH, et al. Fully automated hybrid approach to predict the IDH mutation status of gliomas via deep learning and radiomics. Neuro-Oncol. 2021;23(2):304–313. doi: 10.1093/neuonc/noaa177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cao R, Yang F, Ma SC, et al. Development and interpretation of a pathomics-based model for the prediction of microsatellite instability in colorectal cancer. Theranostics. 2020;10(24):11080–11091. doi: 10.7150/thno.49864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kather JN, Heij LR, Grabsch HI, et al. Pan-cancer image-based detection of clinically actionable genetic alterations. Nat Cancer. 2020;1(8):789–799. doi: 10.1038/s43018-020-0087-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jang HJ, Lee A, Kang J, Song IH, Lee SH. Prediction of clinically actionable genetic alterations from colorectal cancer histopathology images using deep learning. World J Gastroenterol. 2020;26(40): 6207–6223. doi: 10.3748/wjg.v26.i40.6207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Madabhushi A, Agner S, Basavanhally A, Doyle S, Lee G. Computer-aided prognosis: predicting patient and disease outcome via quantitative fusion of multi-scale, multi-modal data. Comput Med Imaging Graph. 2011;35(7–8):506–514. doi: 10.1016/j.compmedimag.2011.01.008 [DOI] [PubMed] [Google Scholar]
- 105.Sammut SJ, Crispin-Ortuzar M, Chin SF, et al. Multi-omic machine learning predictor of breast cancer therapy response. Nature. 2022; 601(7894):623–629. doi: 10.1038/s41586-021-04278-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chaudhary K, Poirion OB, Lu L, Garmire LX. Deep learning-based multi-omics integration robustly predicts survival in liver cancer. Clin Cancer Res. 2018;24(6):1248–1259. doi: 10.1158/1078-0432.CCR-17-0853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhong Y, She Y, Deng J, et al. Deep learning for prediction of N2 metastasis and survival for clinical stage I non-small cell lung cancer. Radiology. 2022;302(1):200–211. doi: 10.1148/radiol.2021210902 [DOI] [PubMed] [Google Scholar]
- 108.Vanguri RS, Luo J, Aukerman AT, et al. Multimodal integration of radiology, pathology and genomics for prediction of response to PD-(L)1 blockade in patients with non-small cell lung cancer. Nat Cancer. 2022;3(10):1151–1164. doi: 10.1038/s43018-022-00416-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sui Q, Chen Z, Hu Z, et al. Cisplatin resistance-related multi-omics differences and the establishment of machine learning models. J Transl Med. 2022;20(1):171. doi: 10.1186/s12967-022-03372-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nazha A, Komrokji R, Meggendorfer M, et al. Personalized prediction model to risk stratify patients with myelodysplastic syndromes. J Clin Oncol. 2021;39(33):3737–3746. doi: 10.1200/JCO.20.02810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen RJ, Lu MY, Williamson DFK, et al. Pan-cancer integrative histology-genomic analysis via multimodal deep learning. Cancer Cell. 2022;40(8):865–878.e6. doi: 10.1016/j.ccell.2022.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bera K, Velcheti V, Madabhushi A. Novel quantitative imaging for predicting response to therapy: techniques and clinical applications. Am Soc Clin Oncol Educ Book. 2018;38(38):1008–1018. doi: 10.1200/EDBK_199747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Corredor G, Toro P, Koyuncu C, et al. An imaging biomarker of tumor-infiltrating lymphocytes to risk-stratify patients with HPV-associated oropharyngeal cancer. J Natl Cancer Inst. 2022;114(4): 609–617. doi: 10.1093/jnci/djab215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Whitney J, Corredor G, Janowczyk A, et al. Quantitative nuclear histomorphometry predicts oncotype DX risk categories for early stage ER+ breast cancer. BMC Cancer. 2018;18:610.doi: 10.1186/s12885-018-4448-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xie J, Pu X, He J, et al. Survival prediction on intrahepatic cholangiocarcinoma with histomorphological analysis on the whole slide images. Comput Biol Med. 2022;146:105520. doi: 10.1016/j.compbiomed.2022.105520 [DOI] [PubMed] [Google Scholar]
- 116.Lu C, Romo-Bucheli D, Wang X, et al. Nuclear shape and orientation features from H&E images predict survival in early-stage estrogen receptor-positive breast cancers. Lab Investig J Tech Methods Pathol. 2018;98(11):1438–1448. doi: 10.1038/s41374-018-0095-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li H, Bera K, Toro P, et al. Collagen fiber orientation disorder from H&E images is prognostic for early stage breast cancer: clinical trial validation. npj Breast Cancer. 2021;7(1):1–10.doi: 10.1038/s41523-021-00310-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li H, Whitney J, Bera K, et al. Quantitative nuclear histomorphometric features are predictive of oncotype DX risk categories in ductal carcinoma in situ: preliminary findings. Breast Cancer Res. 2019;21(1):114. doi: 10.1186/s13058-019-1200-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lexe G, Monaco J, Doyle S, et al. Towards improved cancer diagnosis and prognosis using analysis of gene expression data and computer aided imaging. Exp Biol Med. 2009;234(8):860–879. doi: 10.3181/0902-MR-89 [DOI] [PubMed] [Google Scholar]
- 120.Reis-Filho JS, Kather JN. Overcoming the challenges to implementation of artificial intelligence in pathology. J Natl Cancer Inst. 2023; 115:djad048. doi: 10.1093/jnci/djad048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nakagawa K, Moukheiber L, Celi LA, et al. AI in pathology: what could possibly go wrong? Semin Diagn Pathol. 2023;40(2):100–108. doi: 10.1053/j.semdp.2023.02.006 [DOI] [PubMed] [Google Scholar]
- 122.Liu JTC, Glaser AK, Bera K, et al. Harnessing non-destructive 3D pathology. Nat Biomed Eng. 2021;5(3):203–218. doi: 10.1038/s41551-020-00681-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Torres R, Velazquez H, Chang JJ, et al. Three-dimensional morphology by multiphoton microscopy with clearing in a model of cisplatin-induced CKD. J Am Soc Nephrol JASN. 2016;27(4):1102–1112. doi: 10.1681/ASN.2015010079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jain D, Torres R, Celli R, Koelmel J, Charkoftaki G, Vasiliou V. Evolution of the liver biopsy and its future. Transl Gastroenterol Hepatol. 2021;6:20. doi: 10.21037/tgh.2020.04.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Olson E, Levene MJ, Torres R. Multiphoton microscopy with clearing for three dimensional histology of kidney biopsies. Biomed Opt Express. 2016;7(8):3089–3096. doi: 10.1364/BOE.7.003089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Torres R, Vesuna S, Levene MJ. High-resolution, 2- and 3-dimensional imaging of uncut, unembedded tissue biopsy samples. Arch Pathol Lab Med. 2014;138(3):395–402. doi: 10.5858/arpa.2013-0094-OA [DOI] [PubMed] [Google Scholar]
- 127.Borowsky AD, Levenson RM, Gown AM, et al. A pilot validation study comparing FIBI, a slide-free imaging method, with standard FFPE H&E tissue section histology for primary surgical pathology diagnosis. medRxiv. 2022. doi: 10.1101/2022.03.10.22272226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Glaser AK, Reder NP, Chen Y, et al. Multi-immersion open-top light-sheet microscope for high-throughput imaging of cleared tissues. Nat Commun. 2019;10(1):2781. doi: 10.1038/s41467-019-10534-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen Y, Xie W, Glaser AK, et al. Rapid pathology of lumpectomy margins with open-top light-sheet (OTLS) microscopy. Biomed Opt Express. 2019;10(3):1257–1272. doi: 10.1364/BOE.10.001257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xie W, Glaser AK, Vakar-Lopez F, et al. Diagnosing 12 prostate needle cores within an hour of biopsy via open-top light-sheet microscopy. J Biomed Opt. 2020;25(12):126502. doi: 10.1117/1.JBO.25.12.126502 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.


