Abstract
Multipurpose prevention technology intravaginal rings (MPT IVRs) may offer a promising solution for addressing women’s multiple sexual and reproductive health needs. We describe MPT IVR acceptability perspectives and examine user experiences of 25 cisgender women aged 18–34 years enrolled in a phase IIa randomized, partially blinded, placebo-controlled evaluation of tenofovir-based IVRs with and without contraceptive co-formulation. All took part in an individual, audio-recorded, semi-structured qualitative interview. A thematic analysis of transcribed interviews was completed in MaxQDA. Participants shared little to no knowledge of or experience with IVRs prior to joining the study. Four MPT IVR themes were identified: physical well-being, method reliability, personal management, and societal endorsement. Commonly cited of concern, but less described as being experienced, were physical discomforts (e.g., painful insertion/removal; inability to carry out daily activities/chores; foreign body sensation; expulsion; sexual interference, or debilitating side effects). Uncertainty regarding efficacy influenced perspectives about intended prevention benefits. Personal choices in managing reproduction and sexual behaviors had to be congruent with sociocultural values and norms for acceptance beyond the individual user level. Participants viewed broader community acceptance as likely to be mixed given community opposition to the use of modern family planning methods. They also shared concerns that IVR use could lead to infertility, especially among nulliparous women, or that it would encourage premarital sex or high-risk sexual behaviors among adolescent and young women. While a MPT IVR may not be suitable for all women, first-hand testimonials could help influence collective receptivity. Additional community acceptability research is needed.
Clinical Trial Registration
The study is registered at http://ClinicalTrials.gov under the identifier NCT03762382.
Keywords: HIV prevention, Multipurpose intravaginal rings, Acceptability, User experience, Women, Kenya
1. Introduction
For sexually active women of reproductive age, reducing risks for human immunodeficiency virus (HIV) infection, other sexually transmitted infections (STIs), and unintended pregnancy remain public health priorities, especially in high prevalence settings. While condoms are effective in preventing pregnancy, HIV, and some STIs, partner agreement with correct and consistent use are required. Multipurpose technologies (MPTs) offer a promising solution to simultaneously address women’s sexual and reproductive health prevention needs [1]. Given that women have different preferences and circumstances, MPT products include a variety of drug configurations (some with hormones), delivery methods, designs, and indications or purposes [2]. Among 24 MPT products currently in development or testing, 16 are used vaginally (e.g., vaginal gels, films), with intravaginal rings (IVRs) accounting for a little over half of the vaginal MPTs [2]. The availability of a single, multi-indication, user-friendly, and discreet prevention product may also help reduce stigma associated with use of existing HIV and STI or pregnancy prevention options, increase product acceptability and adherence, and provide consumer cost-savings [3]. Market research suggests women in sub-Saharan Africa would prefer MPTs offering simultaneous protection against HIV and unintended pregnancy, rather than separate methods [4].
Daily oral pre-exposure prophylaxis with tenofovir disoproxil fumarate/emtricitabine (daily oral PrEP) can effectively prevent HIV among women at-risk of infection [5]; however, behavioral, social, and structural factors may have a role in limiting awareness and uptake of daily oral PrEP among women globally [6,7]. Recently, a dapivirine IVR [8] and long-acting injectable cabotegravir [9] were found to be safe and effective for HIV prevention in cisgender women aged 18 years or older. Like daily oral PrEP, these two non-MPT products do not offer a more holistic sexual and reproductive health approach for women.
Studies of acceptability of HIV prevention technologies are plentiful [10]. A systematic review of MPT IVR acceptability among women in 25 low- and middle-income countries, including 17 sub-Saharan African countries, suggests high acceptability but platform preferences may not be generalizable within or across age groups and settings [11]. Given the potential complexities of MPT IVR acceptance and uptake, available lessons learned from daily oral PrEP [12,13] and contraception [14,15] should be considered. A systematic review and meta-analysis of IVRs for HIV or pregnancy prevention found IVRs acceptable among most women who used it but received low hypothetical acceptability among non-IVR users [16]. Qualitative research (acceptability and user experience studies) may enhance our understandings of intended users’ needs, preferences, circumstances and concerns, and to gain insights on contextual factors that may influence decision-making and action-taking. We describe acceptability perspectives and personal experiences of Kenyan women enrolled in a phase IIa clinical trial.
2. Material and methods
2.1. Study design
A phenomenological, lived experience theoretical framework was used for our qualitative research design [17,18]. This approach examines individuals’ narratives of personal feelings and experiences related to a specific event, space, or object (things) with the aim of being able to understand and describe its “essence” (i.e., the essential structure of meaning) [17,18]. Research participants are asked to reflect on a specific experience they have already lived through. Our focus was on the lived experience of using an investigational IVR that might prevent both HIV and pregnancy.
2.2. Sample
Our qualitative sample was derived from women taking part in a phase IIa randomized, partially blinded, placebo-controlled IVR clinical trial (henceforward referred to as the Kisumu Combined Ring Study (KCRS)). All KCRS participants were eligible to take part in a one-time, individual, audio-recorded qualitative interview. The duration of study IVR use depended on each participant’s date of enrollment relative to the August 2019 expiry date for all study IVRs. Hence, qualitative interviews corresponded with participant-level completion of study IVR removals following one, two, or three menstrual cycles, respectively.
2.2.1. Brief Kisumu combined ring study overview
KCRS evaluated the safety, pharmacokinetics, pharmacodynamics, markers of tolerability, and acceptability of two types of 90-day continuous delivery tenofovir (TFV)-based IVRs for HIV prevention with and without pregnancy prevention at one clinical research site in Kisumu, Kenya. It enrolled healthy women aged 18–34 years who scored ≤4 on the validated Vaginal and Oral Interventions to Control the Epidemic (VOICE) HIV risk assessment tool [19]. KCRS clinical trial analyses and results are being published elsewhere.
Briefly, trial participants were randomized (2:2:1) to use one of the three IVRs: a TFV-alone IVR; a TFV/levonorgestrel (LNG) IVR, or a placebo IVR. Insertion and removal of IVRs was performed by a study clinician. The study clinic staff and participants were blinded to the randomization assignments; however, the trial was partially blinded because the TFV/LNG IVR appeared different from the TFV-only IVR. Although the TFV/LNG and placebo IVRs appeared the same, study clinicians may have been able to detect differences in the IVRs during insertion and removal. All participants visually inspected and briefly practiced self-insertion and removal of a placebo study IVR before randomization following standard biosafety and operation procedures. At all study visits, participants received non-spermicidal male condoms (as needed), HIV and pregnancy risk reduction counseling, and discussion of any potential side effects of using the IVRs. Participants were provided with 24-h telephone phone number to report all medical occurrences whether or not they were product related. Additionally, general health and genital examinations were completed at each study visit and participants were asked to document in a guidebook and to report all side-effects experienced. Clinical evaluation and management of side effects were handled separately from the qualitative interview. Participants randomized to use the TFV/LNG IVR might have also experienced changes in menstrual bleeding due to possible LNG-induced anovulatory cycles or breakthrough bleeding. Blinding was maintained until 1) the last enrolled participant had completed follow-up, 2) all endpoint data were entered into the study database, and 3) these data were verified as ready for final analysis. Planned IVR removal varied depending on each participant’s date of enrollment and ranged from one to three menstrual cycles.
2.3. Data collection
The Scientific and Ethical Review Unit of the Kenya Medical Research Institute and an institutional review board at the University of Washington reviewed and approved the study protocol, written informed consent, and the interview guide. Qualitative interviews were conducted using a semi-structured interview guide to gather trial participants’ perspectives on the acceptability of a MPT IVR and to describe their IVR lived experiences. Data were collected from April 2019 to August 2019. Participants received 1000 Kenya Shillings (approximately $10 USD) for taking part in the qualitative interview.
Two interviewers with language proficiency in English, Kiswahili, and Dholuo used a semi-structured interview guide in the language preference of each participant. Interviewers were part of a community mobilization team and were not involved in the clinical care of any participants. The English interview guide developed by the research team underwent Swahili and Dholuo translation and interviews of non-English speaking participants were translated into English by Kisumu team members. Table 1 provides questions specific to this analysis (i.e., the textual data set). Written consent in the participant’s preferred language (English, Kiswahili, or Dholuo) to take part in a qualitative interview was obtained as part of trial enrollment. Before conducting the qualitative interview, a verbal re-consenting process was completed.
Table 1.
Interview guide acceptability and experiential questions, Kisumu Combined Ring Study, 2019.
| Domain of Inquiry: Dual Pregnancy and HIV Prevention Acceptability |
| In your community, how acceptable is it to have a family planning method that also prevents HIV? |
| How would other women that you know react to a family planning method that also prevents HIV? |
| What about men, what would their reaction to be? |
| What are ways that non-acceptance can be overcome? |
| What information would help create dialogue in the community about methods that prevent both pregnancy and HIV? |
| Domain of Inquiry: Study Intravaginal Ring Experiences |
| What did you know about the vaginal rings before you became involved in this study? |
| Tell me about your experiences using the ring. |
| Tell me about any instances when either the ring fell out or felt like it was about to fall out. |
| How did your partner react to you using the ring? |
| Describe any times that the ring was removed by you or at the request of your partner. (omit if participant has not had sex during the ring use phase) |
| What were your experiences with the ring during sexual intercourse? (omit if no sex during the ring use phase) |
| Tell me about any hygiene concerns that you had with keeping the ring in for 90 days. |
| How satisfied with the ring were you? |
To describe the qualitative sample, we used demographic and behavioral data collected at clinical trial screening via electronic case report forms and IVR data were collected post enrollment. Variables are described below.
2.4. Data analysis
All audio recordings underwent transcription in preparation for thematic analysis. The local study team completed an English translation and transcription for each non-English audio recording. Each transcript was reviewed for accuracy against its audio recording by the original interviewer. Codebook development closely followed the approach described by MacQueen, et al. [20] A systematic, iterative, and inductive thematic analysis method was applied. Specifically, the process outlined by Braun and Clarke [21] was used to perform the thematic analysis. Coding and analysis of each English-based transcript was done in MaxQDA (VERBI Software, GmbH, Berlin, Germany, 2018). Thomas and Harden’s [22] coding consistency approach was used to verify coding. Each transcript underwent independent coding by three analysts (EML, SD, and JB), which was followed by group discussions of overlaps and divergences, to resolve coding differences, and to update the MaxQDA analysis project. Coding constructs, analytical scheme, and thematic findings were reviewed independently by three in-country authors (KO, BN, and VM) overseeing the data collection.
Given that key themes were multi-faceted, representational elements of participants’ views and lived experiences, they were not firmly quantifiable, fixed, or ordered. Descriptive indicators (e.g., common, shared, occasional, rare, unique) were used to present thematic findings. To reduce the possibility of inadvertent participant identification, quotes do not include participant demographic or behavioral details. To avoid too many quotes from a single participant, we limited each interview to a maximum of two quote extractions. We followed Roller and Lavrakas’ approach for selecting quotes that best help to illustrate our key data interpretations [23]. In selecting divergent views, quotes selection was often limited to a few participants or a singular one. It should also be noted that quotes were not extracted from all interviews. Quotes selected to illustrate a particular finding may include information on other thematic constructs.
Descriptive statistics summarizing demographic information collected at study screening (age, highest level of education), participant behaviors at screening (sexually active in the past 3 months, family planning use in past 6 months), and post-IVR insertion data (randomization arm, days of IVR use, IVR discontinuation before end of study IVR removal) were calculated using SAS 9.4 (SAS Institute Inc., Cary, NC, USA). Family planning methods response options included none, hormonal or non-hormonal intrauterine devices, implants, injectables, male or female condoms, and natural methods, such as withdrawal or rhythm method.
3. Results
3.1. Sample characteristics
Twenty-five out of the 27 trial enrollees consented to take part in a qualitative interview. Ten had been randomized to the TFV-only IVR arm, 10 to the TFV/LNG arm, and 5 to the placebo arm. Reasons for declination were not collected from the two enrollees who did not complete a qualitative interview.
While 36.0% of the participants had fully completed planned IVR use before taking part in a qualitative interview, none had undergone intervention arm unblinding. As shown in Table 2, the mean participant age was 21.9 years (range 18–34 years) and 68.0% reported having completed secondary education or higher. At screening, more than half (64.0%) reported having been sexually active in the past 3 months. Overall, 88.0% of the qualitative interview sample reported that they had not used any family planning method over the past 6 months. No HIV seroconversions took place. The median days of IVR use was 70 (range 16 to 92 days). Five of the qualitative interview participants (24.0%) discontinued IVR use earlier than planned. One participant reported experiencing vulvovaginitis and repeated IVR dislodgment.
Table 2.
Demographic and behavioral characteristics of qualitative interview participants (n = 25) by treatment group, Kisumu Combined Ring Study, 2019.
| n (%) | ||||
|---|---|---|---|---|
| TFV/LNG IVR (n = 10) | TFV-only IVR (n = 10) | Placebo IVR (n = 5) | Total (N = 25) | |
| Screening visit characteristic | ||||
| Age (mean (SD)) | 21.9 (3.0) | 26.6 (5.5) | 23.8 (4.8) | 24.2 (4.8) |
| Highest level of education completed | ||||
| None | 0 (0.0) | 0 (0.0) | 1 (20.0) | 1 (4.0) |
| Primary school | 0 (0.0) | 1 (10.0) | 1 (20.0) | 2 (8.0) |
| Some secondary school | 1 (10.0) | 4 (40.0) | 0 (0.0) | 5 (20.0) |
| Secondary school | 8 (80.0) | 3 (30.0) | 2 (40.0) | 13 (52.0) |
| College/university | 1 (10.0) | 2 (20.0) | 1 (20.0) | 4 (16.0) |
| Current relationship status | ||||
| Single/never married | 8 (80.0) | 6 (60.0) | 4 (80.0) | 18 (72.0) |
| Married/living as married | 2 (20.0)a | 3 (30.0) | 1 (20.0) | 6 (24.0) |
| Divorced/separated | 0 (0.0) | 1 (10.0) | 0 (0.0) | 1 (4.0) |
| Widowed | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Sexually active in the past 3 months | ||||
| Yes | 7 (70.0) | 6 (60.0) | 3 (60.0) | 16 (64.0) |
| No | 2 (20.0) | 4 (40.0) | 2 (40.0) | 8 (32.0) |
| No Response | 1 (10.0) | 0 (0.0) | 0 (0.0) | 1 (4.0) |
| Main or steady sexual partner in the past 3 months | ||||
| Yes | 8 (80.0) | 7 (70.0) | 5 (100.0) | 20 (80.0) |
| No | 2 (20.0) | 3 (30.0) | 0 (0.0) | 5 (20.0) |
| Currently lives with a sexual partner | ||||
| Yes | 2 (20.0) | 2 (20.0) | 0 (0.0) | 4 (16.0) |
| No | 8 (80.0) | 8 (80.0) | 5 (100.0) | 21 (84.0) |
| Ever been pregnant | ||||
| Yes | 4 (40.0) | 6 (60.0) | 4 (80.0) | 14 (56.0) |
| No | 6 (60.0) | 4 (40.0) | 1 (20.0) | 11 (44.0) |
| Contraceptive use in the past 6 months | ||||
| None | 8 (80.0) | 9 (90.0) | 5 (100.0) | 22 (88.0) |
| Oral Contraception | 1 (10.0) | 1 (10.0) | 0 (0.0) | 2 (8.0) |
| Male Condom | 2 (20.0) | 0 (0.0) | 0 (0.0) | 2 (8.0) |
| Days of IVR use (mean (SD)) | 54.7 (29.9) | 76.3 (27.0) | 73.0 (20.6) | 67.0 (28.0) |
| IVR discontinuation before end of studyb | ||||
| Yes | 3 (30.0) | 2 (20.0) | 0 (0.0) | 5 (20.0) |
| No | 7 (70.0) | 8 (80.0) | 5 (100.0) | 20 (80.0) |
TFV = tenofovir; LNG = levonorgestrel; IVR = intravaginal ring.
One participant reported that she was in a polygamous marriage.
Multiple clinical indications for early IVR removal may have been present for a participant. Reasons included menorrhagia related to IVR use, symptomatic BV, recurrent dislodgement of the IVR for one participant, vulvovaginitis, and pregnancy (clinical outcomes data published elsewhere).
3.2. Key themes
Figs. 1 and 2 display the thematic classifications applied to recurrent types of information shared by participants. Our analysis identified four themes with one or more intersections (or co-occurrences) related to MPT IVR acceptability: 1) physical well-being; 2) method reliability: 3) personal management, and 4) societal endorsement. For societal endorsement, we further found that that if a MPT IVR was incongruent with either sociocultural reproductive management practices or sexual norms, broader community acceptance was unlikely.
Fig. 1.
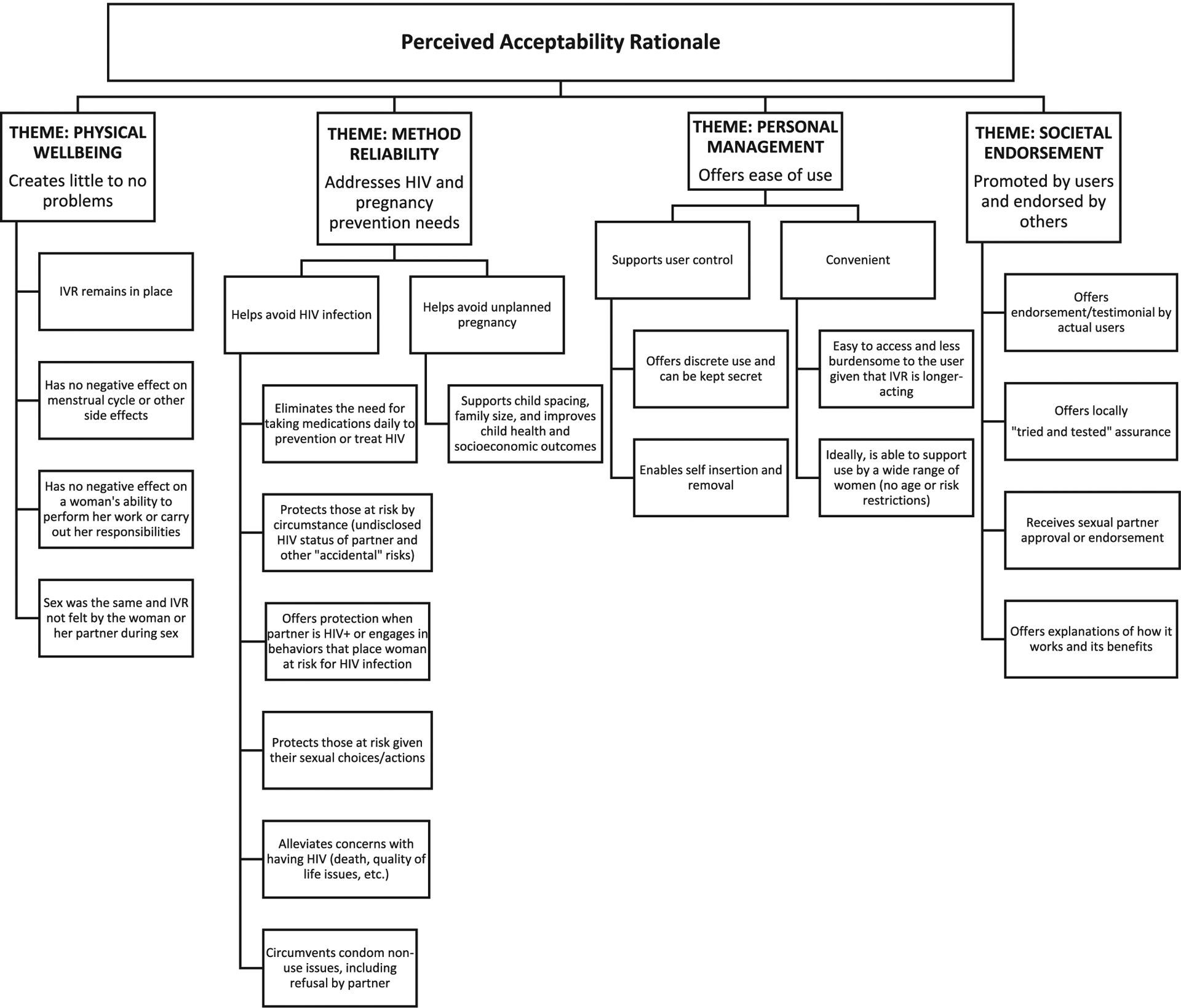
Favorable receptivity toward a multipurpose prevention technology intravaginal ring. IVR = intra vaginal ring.
Fig. 2.
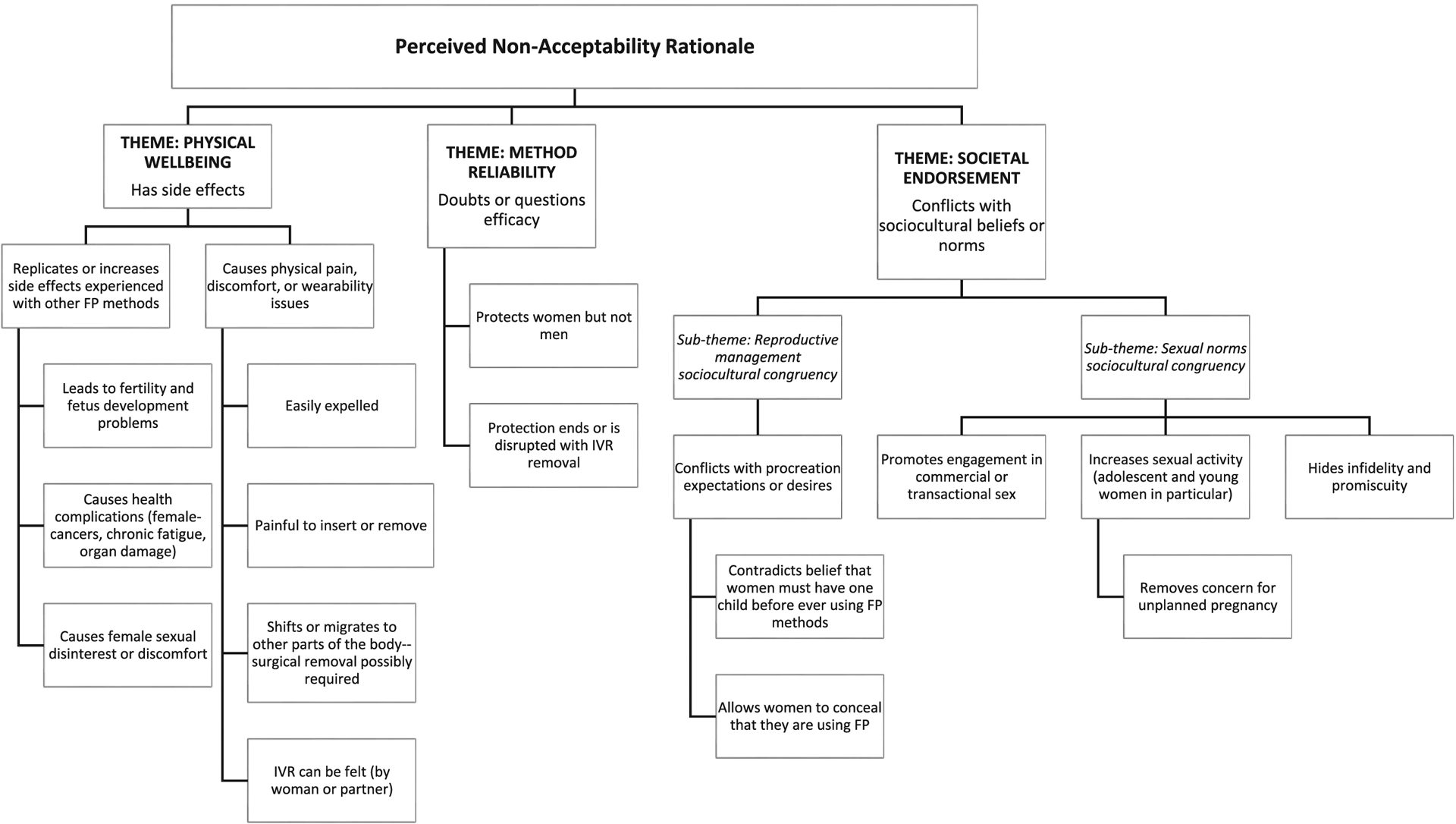
Non-favorable receptivity toward a multipurpose prevention technology intravaginal ring. FP = family planning.
3.2.1. Physical well-being
Participants commonly expressed that they had little to no prior knowledge of IVRs before study recruitment activities took place in their communities. Participant recall of their initial reactions to IVR use focused on physical well-being fears that emphasized the likelihood of painful vaginal insertion and removal of the IVR; non-tolerability with prolonged use; physical discomfort (unnatural or foreign body sensation); the potential for the IVR to fall out or move within the body and possibly require surgical removal; disruption in women’s daily activities or inability to perform chores; non-acceptance by the participant’s significant others; the IVR being felt during sex, and concerns regarding infertility or other side effects that were comparable to or worse than those experienced with modern family planning methods.
“The first time I heard about the ring I was not willing to use it. I was afraid that maybe it had side effects and maybe it could cause infertility for someone who has not delivered…. As for me I was scared since I thought even my parents would not allow me to use it and my partner too. So, the first time I heard about it, I told them give me time and that I was still going to think about it and ask my partner. If they were comfortable with it, then I would contact you.”
(60-day user randomized to TFV-only IVR).
Some participants expressed that overall safety of a MPT IVR was not a concern given that they had been assured by study staff that the government would not “allow something harmful to come to Kenya”. However, for a small minority of participants this translated to mean that the study IVRs had no side effects. With minor exceptions, participants described their IVR use experiences as positive (e.g., “very much okay”, “very satisfied”, “very happy”, “I loved it”). Feelings toward the IVR following actual use were depicted in terms of contentment (pleased with product) or discontentment (displeased with product). Product contentment was predominately attributed to having little to no side effects, physical discomfort, hindrance of daily activities, interference with sexual activities, or IVR expulsion. Some participants emphasized the benefit of not feeling the IVR and the ability to forget that it was in place.
“Sometimes I would not even be thinking about having the ring, but they [friends] would ask me how I was feeling on that day the way girls love to joke. I would ask them what they were talking about, and they would tell me to stop pretending. At that point they would remind me that I was wearing the ring and that was when I would remember that I had the ring and tell them I was not feeling anything. They would then accuse me of lying to them.”
(30-day user randomized to the TFV/LNG IVR).
Overall, participants indicated that study IVR side effects were temporary or manageable. While increased vaginal discharge was described by participants from each IVR group, mention by those randomized to the TFV/LNG IVR was more common. Participants indicated that increased vaginal wetness made daily use of pantyliners (provided by the study) necessary. They stated that the potential for increased vaginal wetness had been explained to them early on, so they felt prepared for it to occur. Despite considering the study IVR better than other contraceptive methods, one participant expressed product discontentment:
“The one [side effect] that I experienced that I was not happy about is that I was so much wet every now and then.”
(30-day user randomized to the TFV/LNG IVR).
Concerns commonly noted by participants before IVR insertion included menstrual cycle suppression or increased bleeding. After initiating IVR use, abnormal vaginal bleeding (e.g., between cycle spotting, heavy menstrual flow) was occasionally described by participants. Heavy or prolonged menstrual cycles were rare. Lighter, and shorter menstrual cycles were reported by some participants. Participants did not express if lighter bleeding and shorter menstrual days were preferred. Those with a prior hormonal contraceptive history, commonly freely elicited assessments that the IVR made their periods “normal”.
“My menses are shorter unlike before it was 5 [days] then it reduced to 3 [days] and this is less. I mean the flow is not heavy like before I would get heavy periods. When you are on your period, I could wear a pad early in the morning then by 6:30 have to change. Right now, if I wear a pad at 7:00 in the morning, I can wear it up to 1:30 pm and then you change and get a fresh one at night.”
(90-day user randomized to TFV/LNG IVR).
Among the occasional participants who reported experiencing side effects associated with study IVRs, some indicated that the side effects diminished over time, while others encountered the same side effect or set of side effects throughout the study IVR use period. In some cases, side effects perceived to be related to IVR use may have been due to an undiagnosed medical condition (e.g., anemia as suggested by the quote below).
“Okay the ring is good, but it has a lot of side effects for me. But I think now it is good because when we use it, it doesn’t hinder any of your activities. Okay, as in, you have something like craving for something—some foods, but it usually comes sometimes like you just feel like eating this, next week you feel like eating...chips/soil [dirt]. Yes, I mean odowa [a type of soft stone], cookies, the queen cakes and yoghurt. Okay you feel cold, and then you have an appetite for food, and then sometimes when you stand you feel like…I don’t know, something is pulling down something in your vagina. You feel like something is about to be expelled from your vagina. Then you feel like your breasts are enlarged and some pain on this sides, and sometimes you get a headache then it disappears. And you sleep a lot.”
(90-day user randomized to TFV/LNG IVR).
Along with the participant quoted above, a few participants described sensations of the IVR repositioning, slipping down, or slipping out of place.
“One day, I felt as if it was hanging but it did not come out. I just washed my hands and I pushed it inside…. I pushed it in, and it did not come down again.”
(30-day user randomized to TFV-only IVR).
“The ring made me feel uncomfortable. The ring was inserted, and I was told to come back after one week. In the evening, I started feeling the ring and it turned out to be a routine that I kept positioning the ring every morning by pushing it upward. So, I do not know if this happened to everyone (laughs)….and that is why I think the ring was removed early.”
(User randomized to TFV/LNG IVR who underwent IVR discontinuation within ~30 days).
Participants otherwise indicated that the IVR remained in place despite their initial concerns that menses, physical activities, sex, or body elimination processes would expel it. Being able to feel the IVR created some physical discomfort to a few participants.
“What bothered me after wearing the ring is just that I felt it…. I had abdominal pain. It was like it was moving. It was taking its position. You just feel like something is moving. I contacted them [study staff], and they told me that it was usual, and I would be just comfortable.”
(60-day user randomized to TFV-only IVR).
Despite frequent statements by participants by that side effects would greatly influence IVR acceptance, descriptions regarding side effect reporting and management experiences were limited. While the majority of participants who experienced side effects stated that they “did as instructed” and sought out assistance from the study clinicians in managing their side effects, there was one participant who acknowledged inconsistent reporting.
“Okay, there are some that I usually share with them, but they just saw that it is not an issue, and that they never heard from people, and I just keep quiet.”
(90-day user, randomized to TFV/LNG IVR).
Participant comments related to the IVR’s physical properties were rare. A few participants mentioned that they recalled having size concerns (i.e., “too big”) prior to using the study IVR. After experiencing IVR use, only one participant commented that the community may not accept an MPT IVR unless the size is made smaller.
3.2.2. Method reliability
While participants acknowledged the unproven efficacy of study IVRs and spoke of being randomized to one of three rings, a few participants were unclear on the prevention aim of all the IVRs beyond there being one to prevent HIV. While unique, the design (“wide open”) of the IVR raised pre-use questions for a few participants as to how the IVR worked or could be effective. Other participants either questioned how the drug would get released into their bodies from the IVR or if the IVR would be “a protective” delivery mode [i.e., would actually work in providing protection from pregnancy or HIV].
In discussing acceptance, participants commonly framed their responses around a MPT IVR “helping women”, “saving lives” and “being useful” and recommended that the IVR’s benefits be prominently highlighted. Participants indicated that questions or uncertainties regarding efficacy would nonetheless be present; however, such concerns could best be addressed by allowing users to share their experiences with the community at large or inviting women the opportunity to try it out for themselves.
Some participants stressed the importance of not restricting use of a MPT IVR to particular groups. These participants highlighted the appropriateness of making an efficacious MPT IVR accessible to adolescent and young women. Conversely, other participants firmly maintained that a MPT IVR should not be used by women without a history of at least one childbirth or by those who are not yet married.
“… I can just talk about how others were telling me. Like they were telling me that, ‘you know you are young, you are not supposed to wear that ring. That ring is for women with 5 children and above and you know you just have one. So, you are not supposed to wear it. So, you want to be a specimen and it will maybe cause you not to have children and yet you do not have children.’ So, I was worried.”
(User randomized to TFV-only IVR who underwent IVR removal within~30 days).
Participants explained that the availability of an IVR for preventing HIV infection would help alleviate societal and personal HIV burdens, especially given that there is no available cure for HIV. Concerns for the welfare of children who had one or both parents die from HIV were common. The idea of a MPT IVR providing an effective method for avoiding unplanned pregnancy, especially among adolescent girls and young women, was generally viewed favorably (e.g., supports child spacing, improves health and socioeconomic outcomes for women and their children). This was also considered potentially controversial given personal and hearsay accounts of side effects due to family planning methods and viewpoints that family planning methods could cause infertility or negative birth outcomes. Participants also indicated that a MPT IVR would help get around needing to use a condom or convincing a sexual partner that condoms were necessary. The need to combine condom use with a MPT IVR to prevent STIs was rarely mentioned.
Participants often spoke of an accidental HIV exposure (e.g., needle stick, coming into contact with an injured person’s blood, unknown HIV status of a sexual partner) or pregnancy resulting from non-consensual sex. Hence, a MPT IVR was viewed as being highly beneficial in the context of physically forced or unwanted [tricked, manipulated, or coerced] sex, having an HIV-positive partner, or having a sexual partner who engaged in sexual behaviors that placed a woman at increased risk for HIV infection. Although not a property of MPT IVRs, a few women believed that a MPT IVR would also afford men protection.
“…you know there are different kinds of men. There are people who are rude, they will reject it, but you are the one who is supposed to tell the person how it is going to help him, not only the woman but also him, because even for him, she cannot tell what he does when he is out there. And if she has it [the IVR], if the man goes out there to be with other partners, he will still not get infected.”
(90-day user randomized to TFV-only IVR).
3.2.3. Personal management
A commonly perceived benefit of a MPT IVR centered on avoiding daily medication necessary to either treat or prevent HIV. Along with a MPT IVR being longer acting than oral contraceptives, it was viewed as less burdensome (no need to remember to take daily) given that the user could potentially insert and remove the IVR, especially if pregnancy was desired or side effects were problematic. A minority of participants mentioned that a drawback of the user removing the MPT IVR for any reason was the immediate absence of protection. One participant shared that curiosity led her to remove the study IVR temporarily to observe changes in the device. Of note, while all study IVRs remained the same size when inside and outside of the vagina, this participant held that environmental-related expansion and contraction differences were observed.
“[I] wanted to see if the drugs were getting absorbed or they were just constant…the drugs were still there though they had moved a bit. And again, the more it stays inside is the more it continues to swell and when you remove it. It resembles a tortoise that has just come out from water and shrinks. If the ring takes two hours outside the vagina it will reduce, that is how I know it.”
(90-day user randomized to the TFV-only IVR).
Participants indicated that having to expend less time and effort (e. g., travel time, time away from work, frequency of clinic visits) to access a MPT IVR through existing medical channels could further product appeal and acceptance. Participants stated that the fewer challenges a woman faced in using a MPT IVR, the better user compliance and adherence could potentially be for them. A MPT IVR was also described by some participants as having the added benefits of either minimizing or helping to overcome stigma (i.e., “won’t look down upon you”). They explained that unlike daily oral PrEP or contraceptives, such as oral pills or an implant, others in the community would not likely be aware that a woman was using a MPT IVR.
Although all participants reiterated the dual HIV and pregnancy prevention in discussing a potential MPT IVR, they rarely explicitly stated (as illustrated by the quote below) that having two products in one offered convenience to the user, as opposed to having to use various products for each indication.
“We can tell them the advantages of this method. It is one method, but it prevents two things. Instead of using this method, and again use the other one, I mean if it is a tablet, it is one tablet, but it prevents two things and it will be easy to use. If they accept this thing is something that after insertion it stays there until its expiry date, it is not something that is not inserted every day and you will not feel it, you will just be the same.”
(60-day user randomized to TFV/LNG IVR).
3.2.4. Societal endorsement
After initiating IVR use, participants commonly held that an IVR that simultaneously protected against HIV and pregnancy would be favorably received by others in the community. However, participants also acknowledged a stronger likelihood that receptivity within the community would be mixed and that it was mainly attributed to the contraceptive co-formulation of a MPT IVR. Participants shared that despite community recognition of the negative socioeconomic, health, interpersonal, and structural consequences of unplanned pregnancy, some sexual partners, family members, influential leaders, and decision-makers would be reticent to accept a MPT IVR. Participants noted that the hesitancy could be due to existing unfavorable attitudes toward modern family planning methods, including condoms. Often, family planning methods were described as being inconvenient, requiring compliance (on-time, every time) which was often difficult, and leading to potential side effects, such as heavy prolonged bleeding, the inability to conceive, and cervical cancer.
User testimonial and endorsement by local health professionals were perceived to be essential in attaining community support. Opposition by family, friends, village elders, religious leaders, and others could discourage IVR knowledge and use. Men were described as less willing to accept a MPT IVR. In addition to the health and female sexuality concerns, any potential for diminished sexual interest or desires on a woman’s part or the IVR being felt during sex was perceived to limit acceptance by men. Participants stressed the importance of educating the community about MPT IVR risks and benefits given the significant role that rumors, and misinformation could have on acceptance and permission by partners and family members to use it. Having a MPT IVR that was tried and tested within the community was perceived to further foster support for the IVR.
From a sociocultural perspective, a few participants shared that the IVR should only be used by women who had already given birth to one or more children, given the potential for the IVR to prevent them from having children in the future. Community members opposed to a MPT IVR were described as likely to focus on family planning concerns, to believe that an IVR would increase premarital and extramarital sexual activity, sexual promiscuity, and participation in transactional sex by women.
“I know they cannot accept because there are side effects, there are people who believe that family planning causes cancer, so some people do not agree easily if they are not taught, and they understand. Again, you also find that some people believe that if you use family planning when you are still a girl/young woman, you cannot give birth; it is not easy for you to get a child later.”
(90-day user randomized to TFV-only IVR).
“But now if that method is made available it will make people not care they just… when they put that method on then they say that even if I do this I will not get this HIV and I will not become pregnant, they will be irresponsible….I mean it can lead young girls into doing bad things like being prostitutes because they know they will not become pregnant.”
(90-day user randomized to TFV/LNG IVR).
Pros and cons of being able to discreetly use an IVR without a partner’s knowledge were discussed by most participants. Non-acceptance by partners was often attributed to concerns regarding a woman’s fidelity.
“Now it is better for a wife to be open and tell her husband that ‘the reason I am using this thing is because I want to protect our status and also to preserve our life plus our children’”.
(90-day user randomized to Placebo IVR).
Some participants informed their sexual partners about the IVR in advance given that study staff had indicated that male partners might be able to feel the IVR during sexual intercourse. Others opted not to disclose study IVR use to anyone, including their partners. Descriptions of partners being able to feel a study IVR were uncommon.
4. Discussion
This is the first lived experience qualitative study of women in sub-Saharan Africa evaluating an IVR with intended to prevent HIV and pregnancy. Our findings suggest that MPT IVR acceptance and user experience may be influenced under four broad themes: physical well-being, method reliability, personal management, and societal endorsement. Acceptance beyond the individual-user level may necessitate that personal choices in managing reproduction and sexual behaviors had to be congruent with sociocultural values and norms. HIV microbicide research has similarly shown that community [24,25] and partner acceptance [26] are critical in targeting prevention technologies for women.
Our findings indicate participants’ recollections of their initial reactions about the study IVRs (i.e., their pre-use expectations) did not align with their actual lived experience of using the study IVRs. In a previous crossover study of four vaginal dosage forms, delivery by IVR increased the most in popularity between pre-insertion and after use interviews [27]. Consistent with single-purpose IVR studies [11,28,29], we found that women were pleased with the IVR and experienced minimal side effects, physical discomfort, sexual interference, and expulsions. While not reported as problematic, increased vaginal wetness was present across study groups but more common among the TFV/LNG IVR group. Similar findings were reported for at least two US-based studies of HIV prevention-only IVRs [30,31]. Longitudinal examination of changes in abundances of individual bacterial taxa for the KCRS trial showed no evidence that either the TFV/LNG or TFV IVR significantly affected a woman’s genital bacterial or diversity of Candida sp. [32] While increased vaginal discharge may not require treatment [33], it could negatively affect MPT IVR acceptance, uptake, and continued use.
Consistent with other research, our participants expressed concerns about partner acceptance and negative partner reaction to feeling the IVR during sex [11,34,35]. The potential for unfavorable MPT receptivity given beliefs that contraception should not be used by nulliparous women in light of perceived potential fertility side effects has also been shown in other research [36,37]. Misperceptions that contraceptive drugs will lead to an inability for a woman to have children may also have broader negative social and psychological consequences [38]. Apprehensions about the potential for female sexual promiscuity and behavioral disinhibition have been addressed in oral PrEP studies with no indication that risk compensation occurred [39]. Further insights on the connection between the sociocultural context and MPT preferences and expectations would be useful. Limited research is available on the influence that perceived community norms [34] and spousal acceptance [35] may have on individual choices and decision making. Recent research on covert contraceptive use suggests that implants and injectables are preferred among married women in Kenya [40]. Systematic review of motivating factors for covert contraceptive use are largely accounted for by women’s misconceptions or side–effects, along with male partner disapproval, and fertility cultural beliefs/social expectations [41]. A qualitative study in three sub-Saharan African countries proposes that covert contraceptive use as an act of female disobedience within a patriarchal social system [42]. Tanner et al. [43] emphasize social context, in particular the idea that women’s bodies are shared space; this idea is important in positioning women’s microbicide product preferences. Conversely, a number of reproductive [44], sexual, and HIV treatment studies have shown that the sociocultural context supports a “my body, my choice” body ownership. Participants in our study frequently spoke of the influence that sexual partners, family, and others in the community made in their choices, including decisions on disclosing or not disclosing IVR use. While our study possibly hints at a key role partners, family, and community may have in MPT IVR acceptance, closer examination is warranted.
5. Limitations
Interviews with participants before IVR insertion may have been useful given the possibility that both recall issues and an epistemological shift due to actual IVR use may have made it challenging for participants to fully return to their initial pre-IVR use thoughts and concerns. Interviews with participants’ sexual partners were not conducted. Given the importance of participants’ perceptions of partner acceptance, future trials may benefit from collecting data from partners to better understand relationship factors supporting or hindering actual IVR use. The time point for conducting the qualitative interview differed for each participant, based on the IVR insertion date and IVR expiry date compelling removal. We, however, did not discern any thematic differences based on duration of IVR use. IVR use in our study relied heavily on clinician assistance and may have consequentially influenced positive perspectives toward and experiences with the study IVRs. Sparse details regarding side effect reporting and management may have been influenced by routine clinical trial inquiry regarding such events. Participants may have been reluctant to talk about side effects that had not been previously introduced or those that differed from their own family planning experiences or expectations. Social desirability as well as interviewer rapport may have also affected experiences shared.
6. Summary
In this study of women with experience using an IVR, acceptability was good and product contentment was common. Women speculated that a MPT IVR may not be suitable for all women, and that there may be non-acceptance by partners, family, and the community. While first-hand testimonials may help foster MPT IVR awareness, closer examination of broader community acceptance as well as the role of partners and family in decisions around MPT IVR use may be needed to develop appropriate education, messaging, and pathways for message delivery.
Acknowledgements
We extend our appreciation to the women who took part in the study, the broader Kisumu community, Jecinter A. Oruko, Isdorah Akoth Odero, and Eucabeth Awuonda for their roles in in the conduct of these interviews. A special thanks to Drs. Kenneth Ngure, Jeffrey Wiener, Elizabeth Bukusi, and Taraz Samandari for their technical guidance and support.
Funding statement
This work was supported by the United States Centers for Disease Control and Prevention (CDC) and the United States Agency for International Development (USAID, under the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR)) through cooperative agreement U01PS005183, 2017-2021 (registered at http://ClinicalTrials.gov under the identifier NCT03762382), and was made possible by the support of the American people through these governmental agencies and programs. Funding from USAID’s Cooperative Agreements with CONRAD/Eastern Virginia Medical School (AID-OAA-A-14-00010 and AID-OAA-A-14-00011) was used to support the manufacturing and clinical qualification of the intravaginal rings and CONRAD’s personnel time and effort.
Abbreviations:
- CDC
United States Centers for Disease Control and Prevention
- HIV
Human immunodeficiency virus
- IVR
Intravaginal ring
- KCRS
Kisumu Combined Ring Study
- LNG
Levonorgestrel
- MPTs
Multipurpose prevention technologies
- PrEP
Pre-exposure prophylaxis for HIV infection
- STIs
Sexually transmitted infections
- TFV
Tenofovir
- US
United States
- USAID
United States Agency for International Development
- USD
United States dollars
- VOICE
Vaginal and Oral Interventions to Control the Epidemic
Footnotes
Disclaimer statement
The contents in this article are the sole responsibility of the authors and do not necessarily reflect the official position of the institutions, CDC, USAID, PEPFAR, and/or the United States Government.
Financial conflicting interests statement
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Non-financial competing interests statement
The authors declare that they have no non-financial competing interests.
Data availability statement
The data that support the findings of this study are available from the CDC but restrictions apply to the availability of these data, which were used under license for the current study, so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of CDC.
References
- [1].Brady M, Manning J, Lessons from reproductive health to inform multipurpose prevention technologies: don’t reinvent the wheel, Antivir. Res 100 (Suppl) (2013) S25–S31, 10.1016/j.antiviral.2013.09.019. [DOI] [PubMed] [Google Scholar]
- [2].Coalition Advancing Multipurpose Innovations (CAMI), MPT Product Development Database, Public Health Institute, Sacramento, CA, 2022. http://mpts101.org/mpt-database/mpts-gels. Accessed December 4, 2020. [Google Scholar]
- [3].Abdool Karim SS, Abdool Karim Q, Kharsany AB, et al. , Tenofovir gel for the prevention of herpes simplex virus type 2 infection, N. Engl. J. Med 373 (6) (2015) 530–539, 10.1056/NEJMoa1410649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].El-Sahn M, Lucas J, Aikenhead M, et al. , Understanding the potential for multipurpose prevention of pregnancy and HIV: Results from surveys assessing four hypothetical concept profiles of multipurpose prevention technologies (MPTs) in Uganda, Nigeria and South Africa: AVAC: Global Advocacy for HIV Prevention. http://www.theimpt.org/documents/UnderstandingPotentialMPT-HIVpregnancy.pdf, 2016. [Google Scholar]
- [5].Thomson KA, Baeten JM, Mugo NR, et al. , Tenofovir-based oral PrEP prevents HIV infection among women, Curr. Opin. HIV AIDS 11 (1) (2016) 18, 10.1097/COH.0000000000000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bradley E, Forsberg K, Betts JE, et al. , Factors affecting pre-exposure prophylaxis implementation for women in the United States: a systematic review, J. Women’s Health 28 (9) (2019) 1272–1285, 10.1089/jwh.2018.7353. [DOI] [PubMed] [Google Scholar]
- [7].Warren EA, Paterson P, Schulz WS, et al. , Risk perception and the influence on uptake and use of biomedical prevention interventions for HIV in sub-Saharan Africa: a systematic literature review, PLoS One 13 (6) (2018), 10.1371/journal.pone.0198680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nel A, Kapiga S, Bekker LG, Devlin B, Borremans M, Rosenberg Z for the IPM 027/The ring study research center teams. Safety and efficacy of dapivirine vaginal ring for HIV-1 prevention in African women (Abstract #110LB), CROI, Boston, MA. [abstract], 2016, 2020 February 22–25, https://www.croiconference.org/abstract/safety-and-efficacy-dapivirine-vaginal-ring-hiv-1-prevention-african-women. Accessed July 26 2021. [Google Scholar]
- [9].World Health Organization (WHO), Trial results reveal that long-acting injectable cabotegravir as PrEP is highly effective in preventing HIV acquisition in women, World Health Organization Newsletter, Geneva, 2022. https://www.who.int/news/item/09-11-2020-trial-results-reveal-that-long-acting-injectable-cabotegravir-as-prep-is-highly-effective-in-preventing-hiv-acquisition-in-women. Accessed November 10 2020. [Google Scholar]
- [10].Beckham SW, Crossnohere NL, Gross M, Bridges JF, Eliciting preferences for HIV prevention technologies: a systematic review, Patient 14 (2) (2021. Mar) 151–174, 10.1007/s40271-020-00486-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Griffin JB, Ridgeway K, Montgomery E, et al. , Vaginal ring acceptability and related preferences among women in low- and middle-income countries: a systematic review and narrative synthesis, PLoS One 14 (11) (2019), e0224898, 10.1371/journal.pone.0224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Celum C, Baeten J, PrEP for HIV prevention: evidence, global scale-up, and emerging options, Cell Host Microbe 27 (4) (2020) 502–506, 10.1016/j.chom.2020.03.020. [DOI] [PubMed] [Google Scholar]
- [13].Eakle R, Venter F, Rees H, Pre-exposure prophylaxis (PrEP) in an era of stalled HIV prevention: can it change the game? Retrovirology 15 (1) (2018) 29, 10.1186/s12977-018-0408-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Delany-Moretlwe S, Mullick S, Eakle R, et al. , Planning for HIV preexposure prophylaxis introduction: lessons learned from contraception, Curr. Opin. HIV AIDS 11 (1) (2016) 87–93, 10.1097/coh.0000000000000221. [DOI] [PubMed] [Google Scholar]
- [15].Seidman D, Weber S, Carlson K, et al. , Family planning providers’ role in offering PrEP to women, Contraception 97 (6) (2018) 467–470, 10.1016/j.contraception.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mayo AJ, Browne EN, Montgomery ET, Torjesen K, Palanee-Phillips T, Jeenarain N, Seyama L, Woeber K, Harkoo I, Reddy K, Tembo T, Acceptability of the dapivirine vaginal ring for HIV-1 prevention and association with adherence in a phase III trial, AIDS Behav. 25 (8) (2021) 2430–2440, 10.1007/s10461-021-03205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Moustakas C, Phenomenological research methods, Sage, Thousand Oaks, CA, 1994. [Google Scholar]
- [18].van Manen M, Researching lived experience: Human science for an action sensitive pedagogy, SUNY Press, New York, NY, 1990. [Google Scholar]
- [19].Balkus JE, Brown ER, Palanee-Phillips T, et al. , Performance of a validated risk score to predict HIV-1 acquisition among African women participating in a trial of the dapivirine vaginal ring, JAIDS 77 (1) (2018), e8, 10.1097/QAI.0000000000001556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].MacQueen KM, McLellan E, Kay K, Milstein B, Codebook development for team-based qualitative analysis, Cam. J 10 (2) (1998) 31–36. [Google Scholar]
- [21].Braun V, Clarke V, Using thematic analysis in psychology, Qual. Res. Psychol 3 (2) (2006) 77–101, 10.1191/1478088706qp063oa. [DOI] [Google Scholar]
- [22].Thomas J, Harden A, Methods for the thematic synthesis of qualitative research in systematic reviews, BMC Med. Res. Methodol 8 (1) (2008) 45, 10.1186/1471-2288-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Roller MR, Lavrakas PJ, Applied Qualitative Research Design: A Total Quality Framework Approach, Guilford Press, New York, 2015. [Google Scholar]
- [24].Domanska CA, Teitelman AM, Factors that affect acceptance of HIV microbicides among women, Collegian 19 (2012) 23–32, 10.1016/j.colegn.2011.09.006. [DOI] [PubMed] [Google Scholar]
- [25].Nuttall J, Microbicides in the prevention of HIV infection, Drugs 70 (2010) 1231–1243, 10.2165/10898650-000000000-00000. [DOI] [PubMed] [Google Scholar]
- [26].Lanham M, Wilcher R, Montgomery ET, et al. , (2014), engaging male partners in women’s microbicide use: evidence from clinical trials and implications for future research and microbicide introduction, J. Int. AIDS Soc 17 (2014) 19159, 10.7448/IAS.17.3.19159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Montgomery ET, Beksinska M, Mgodi N, et al. , End-user preference for and choice of four vaginally delivered HIV prevention methods among young women in South Africa and Zimbabwe: the Quatro clinical crossover study, J. Int. AIDS Soc 22 (5) (2019. May), 10.1002/jia2.25283 e25283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Montgomery ET, van der Straten A, Chitukuta M, et al. , Acceptability and use of a dapivirine vaginal ring in a phase III trial, AIDS 31 (8) (2017) 1159–1167, 10.1097/qad.0000000000001452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].van der Straten A, Browne EN, Shapley-Quinn MK, et al. , First impressions matter: how initial worries influence adherence to the dapivirine vaginal ring, JAIDS 81 (3) (2019) 304–310, 10.1097/QAI.0000000000002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Watnick D, Keller MJ, Stein K, et al. , Acceptability of a tenofovir disoproxil fumarate vaginal ring for HIV prevention among women in new York City, AIDS Behav. 22 (2) (2018) 421–436, 10.1007/s10461-017-1962-8. [DOI] [PubMed] [Google Scholar]
- [31].van der Straten A, Panther L, Laborde N, et al. , Adherence and acceptability of a multidrug vaginal ving for HIV prevention in a phase I study in the United States, AIDS Behav. 20 (11) (2016) 2644–2653, 10.1007/s10461-016-1299-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dabee S, Mugo N, Mudhune V, et al. , Genital microbiota of women using a 90 day tenofovir or tenofovir and levonorgestrel intravaginal ring in a placebo controlled randomized safety trial in Kenya, Sci. Rep 12 (2022) 12040, 10.1038/s41598-022-13475-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lete I, Cuesta MC, Marin JM, et al. , Vaginal health in contraceptive vaginal ring users–a review, Eur J Contracept Reprod Health Care 18 (4) (2013) 234–241, 10.3109/13625187.2013.801954. [DOI] [PubMed] [Google Scholar]
- [34].Dynes M, Stephenson R, Rubardt M, et al. , The influence of perceptions of community norms on current contraceptive use among men and women in Ethiopia and Kenya, Health Place 18 (4) (2012) 766–773, 10.1016/j.healthplace.2012.04.006. [DOI] [PubMed] [Google Scholar]
- [35].Lasee A, Becker S, Husband-wife communication about family planning and contraceptive use in Kenya, Int. Fam. Plan. Perspect 23 (1997) 15–33, 10.2307/2950781. [DOI] [Google Scholar]
- [36].Kungu W, Khasakhala A, Agwanda A, Use of long-acting reversible contraception among adolescents and young women in Kenya, PLoS One 15 (11) (2020), e0241506, 10.1371/journal.pone.0241506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Black K, Lotke P, Buhling KJ, Zite NB, Intrauterine contraception for nulliparous women: translating research into action (INTRA) group. A review of barriers and myths preventing the more widespread use of intrauterine contraception in nulliparous women, Eur J Contracept Reprod Health Care 17 (5) (2012) 340–350, 10.3109/13625187.2012.700744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ochako R, Mbondo M, Aloo S, et al. , Barriers to modern contraceptive methods uptake among young women in Kenya: a qualitative study, BMC Public Health 15 (1) (2015) 118, 10.1186/s12889-015-1483-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Fonner V, Dalglish S, Kennedy C, Oral tenofovir-based HIV pre-exposure prophylaxis (PrEP) for all populations: a systematic review and meta-analysis of effectiveness, safety, behavioural, and reproductive health outcomes, AIDS 30 (12) (2016) 1973–1983, 10.1097/QAD.0000000000001145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Akoth C, Oguta JO, Gatimu SM, Prevalence and factors associated with covert contraceptive use in Kenya: a cross-sectional study, BMC Public Health 21 (1) (2021) 1–8, 10.1186/s12889-021-11375-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Blackstone SR, Nwaozuru U, Iwelunmor J, Factors influencing contraceptive use in sub-Saharan Africa: a systematic review, Int. Q. Commun. Health Educ 37 (2) (2017) 79–91, 10.1177/0272684x16685254. [DOI] [PubMed] [Google Scholar]
- [42].Kibira Simon P.S., Karp Celia, Wood Shannon N., Desta Selamawit, Galadanci Hadiza, Makumbi Fredrick E., Omoluabi Elizabeth, et al. , Covert use of contraception in three sub-Saharan African countries: a qualitative exploration of motivations and challenges, BMC Public Health 20 (2020) 1–10, 10.1186/s12889-020-08977-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Tanner AE, Zimet G, Fortenberry JD, et al. , Young women’s use of a vaginal microbicide surrogate: the role of individual and contextual factors in acceptability and sexual pleasure, J. Sex Res 46 (1) (2009) 15–23, 10.1080/00224490802398407. [DOI] [PubMed] [Google Scholar]
- [44].Sundstrom B, Szabo C, Dempsey A, “My body. My choice”: a qualitative study of the influence of trust and locus of control on postpartum contraceptive choice, J. Health Commun 23 (2) (2018) 162–169, 10.1080/10810730.2017.1421728. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the CDC but restrictions apply to the availability of these data, which were used under license for the current study, so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of CDC.


