Abstract
The outcome of viral infections is dependent on the amount of tissue destruction caused either by direct lysis of infected cells and/or by immunopathology resulting from the immune response to the virus. We investigated whether induction of tolerance to only one viral protein could reduce immunopathology caused by nonlytic lymphocytic choriomeningitis virus (LCMV) in perforin-deficient hosts. Earlier studies had shown that LCMV infection results in aplastic anemia and death in most of these mice and that this is associated with bone marrow infiltration by antiviral cytotoxic T lymphocytes (CTL) that secrete inflammatory cytokines. We report here that perforin-deficient mice exhibit severe immunopathology in multiple organs that is characterized by infiltration of anti-LCMV CTL that secrete large amounts of gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α). Importantly, this immunopathology is significantly reduced and long-term survival of LCMV infection is increased in perforin-deficient mice expressing LCMV nucleoprotein (NP) in the thymus (and therefore deleting most of their LCMV-NP CTL) compared to the situation in thymus nonexpressors. This is due to the selective reduction of NP-specific CTL responses and their inflammatory-cytokine (IFN-γ and TNF-α) secretion and to a lack of pathogenetically relevant compensatory responses to other viral proteins. Thus, “selective reduction” of the antiviral immune response to only one viral protein can significantly reduce inflammatory immunopathology and might be a therapeutic possibility for certain nonlytic infections.
Immunopathology caused by the immune response against a virus can be instrumental in determining the outcome of an infection (6, 12, 29). Consequently, nonlytic or latent viruses can persist in the absence of a strong immune response without causing immunopathology; these include cytomegalovirus (1), herpes simplex virus (16, 19, 20), and at-birth-transmitted lymphocytic choriomeningitis virus (LCMV) (6). In contrast, nonlytic infections can cause death due to immunopathology if they induce immune responses that localize to more sensitive areas of the body such as the brain, which is affected in intracranial LCMV infection (15). Consequently, a strong immune response is beneficial in clearing infections with lytic viruses so as to limit tissue destruction or prevent infection-associated immunosuppression as seen in measles (8), but for many viral infections the precise in vivo balance between direct lysis of infected cells and immune system-mediated damage is not known. Predictions are hampered by the fact that in vitro cytopathic effects cannot be directly translated into in vivo pathology, because the types and quantity of specific cells infected in vivo may vary considerably depending on the properties of the virus (21). Thus, the best treatment strategy is difficult to define, and the principal goal in antiviral therapy has been the use of antiviral drugs in situations where protective immunity (reviewed in reference 27 for LCMV) prior to first exposure cannot be induced. However, many infections, for example human immunodeficiency virus, can persist even in the presence of an initially strong immune response (5) or in the presence of antiviral therapy (28). For precisely these situations it might be beneficial to dampen antiviral immunity, especially since direct antiviral agents would be able to control the potentially higher viral titers. Some recent studies have demonstrated that depleting cytotoxic CD8 T lymphocytes (CTL) is beneficial in reducing immunopathology (4). However, depletion of whole T-lymphocyte subsets in vivo can result in generalized severe immunosuppression. Therefore, the goal of our present study was to investigate whether tolerance to only one viral protein could reduce immunopathology in an infection model with a noncytopathic virus. Earlier studies by us (24) and others had shown that lowering the response to one viral protein increased compensatory responses to other viral proteins. We sought to determine whether such compensatory responses would negate any beneficial effect that selective tolerance might have in chronic immunopathology.
The model system we chose was LCMV infection of perforin-deficient mice that express the viral nucleoprotein (NP) as a transgene in the thymus. We chose central (thymic) over peripheral tolerance, since deletion is permanent and not potentially transient (peripheral immunization [2]) and therefore offers a “cleaner” experimental system to test the general feasibility of our hypothesis. In normal H-2b mice, LCMV is recognized by CTL directed to three major epitopes located in the glycoprotein (GP-1 and GP-2) and nucleoprotein (NP) (27). Transgenic mice expressing the LCMV-NP in their thymus were previously described by us (24, 25) and delete the majority of their high-affinity LCMV-NP-specific CTL via negative selection. Some CTL can still emerge to the periphery, probably due to the affinity dependence of the thymic selection process (3). The number of NP CTL in the periphery is much lower in H-2b mice than in H-2d thymic expressor mice, most probably because the affinity recognition of the NPb peptide is higher than that of the NPd peptide and therefore negative selection may occur more efficiently (24). To achieve optimal lowering of NP CTL, we therefore chose the H-2b background for the present study. The LCMV NP thymic expressor line we used expresses NP in the thymus and pancreas but not in other organs (RIP-NP) (25). Diabetes and islet infiltration usually observed in RIP-NP H-2b C57BL/6J mice following LCMV infection (25) does not occur in any offspring when these mice are crossed with perforin-deficient SV129J mice, because the SV129 (H-2b) background due to major histocompatibility complex (MHC) nonlinked genes (25a) conveys resistance to islet destruction and diabetes.
Perforin-deficient SV129 H-2b mice have been generated by several laboratories (13, 26). These mice become chronically infected with LCMV because, among other factors, lytic activity of CTL is required to eliminate LCMV from infected cells (14). Ongoing immune system activation leads to bone marrow infiltration of LCMV CD8+ lymphocytes, which results in profound aplastic anemia and death of the majority of animals within 1 to 2 months (4). Thus, LCMV-infected perforin-deficient mice are a good model for persistent infection with ongoing immunopathology.
In the present investigation, we found that perforin-deficient mice crossed to LCMV-NP thymic expressors profit from dampening their virus specific immune response to only one viral protein when chronically infected with LCMV. Cytokine secretion in response to LCMV-NP and, consequently, immunopathology was significantly reduced, and more mice survived. Importantly, no pathogenically relevant, compensatory increase of immune responses to other viral proteins was observed. Viral titers were not altered. However, the long-term survivors exhibited lower LCMV-specific cytokine production, resulting in a stable but not progressive degree of immunopathology. These findings show that in chronic viral infections, dampening of the immune response can be beneficial and might have potential importance for improving the outcome of some infections, if a strategy for inducing selective peripheral immunotolerance can be devised.
MATERIALS AND METHODS
Transgenic mice.
RIP-NP H-2b (backcross F9 to C57BL/6J) transgenic mice expressing LCMV NP in their thymus and pancreas but not in other organs were generated in our laboratory and previously characterized (25). Perforin-deficient H-2b SV129 mice were obtained from Craigh Walsh (26) and were crossed with the RIP-NP transgenic mice. F1 animals were crossed back to the perforin-deficient mice for one generation, and the resulting F2 backcrosses were used for all experiments. Four experimental groups were obtained: perforin-competent (heterozygous) or -deficient mice that did or did not express LCMV-NP in their thymus. The nomenclature we are using throughout this paper is as follows: perforin heterozygous, NP negative = p+/− NP−; perforin heterozygous, NP positive = p+/− NP+; perforin negative, NP negative = p−/− NP−; perforin negative, NP positive = p−/− NP+. In some studies, regular C57BL/6J (H-2b perforin-competent) mice were used as an additional control. These are labeled B6 p+/+ or p+/+ (b).
RNA analysis and RNase protection assays.
RNA was extracted from cells and organs by the guanidinium isothiocyanate method (7). Total RNA (10 μg) was analyzed by RNase protection assay exactly as described previously (10). The mIL-1α(B), mIL-1β(A), mIL-2(A), mIL-3(B), mIL-4(B), mIL-5(C), mIL-6(B), mIFNγ(B), mTNFα(A), mTNFβ(A), and mL32(A) subclones in the pGEM-4 transcription vector were described in a previous report (11). The mCD4(IC), mCD3γ(IC), mCD8α(DM), and F480 subclones in the pGEM-4 vector were also previously described (10). The content of T-cell and macrophage marker and cytokine RNA in various organs was quantitated by phosphorImager analysis with Optiquant image analysis software (Packard, Meriden, Conn.).
Analysis of glucose levels in blood.
Blood samples were obtained from the retro-orbital plexus of mice. The levels of glucose were determined by using the ACCUCHECK II instrument (25).
CTL and antibody assays.
CTL activity was measured in a 5- to 6-h in vitro 51Cr release assay as described previously (23–25). To determine CTL recognition and lysis, syngeneic or allogeneic target cells were infected with LCMV strain ARMSTRONG at a multiplicity of infection (MOI) of 1, recombinant vaccinia viruses expressing the full-length LCMV ARM GP (MOI = 3) or full-length NP (MOI = 3), or uninfected target cells coated with LCMV peptides GP from amino acids (aa) 33 to 41 or 276 to 286 or NP from aa 396 to 404, all H-2b (Db) restricted, or NP aa 118 to 127 that is H-2d (Ld) restricted. We used 20 to 0.02 μg of peptide per 104 target cells for in vitro CTL affinity assessment, unless otherwise indicated. Assays with splenic lymphocytes used effector-to-target ratios of 50:1, 25:1, and 12.5:1, while those with CTL clones or secondary CTL lines used ratios of 5:1, 2.5:1, and 1:1.
Histology and immunocytochemistry.
Tissues taken for histologic analysis were placed in Bouin’s fixative and then stained with hematoxylin and eosin. Immunochemical studies were carried out on 6- to 10-μm cryomicrotome sections as described in our previous publications (23). This allowed immunostaining of organs for expression of MHC class I and II, CD4, CD8, B220, F4/80, and NLDC. These antibodies are available from Pharmingen (San Diego, Calif.).
Viruses.
Virus stocks consisted of LCMV ARM (clone 53b) and vaccinia virus-LCMV GP and NP recombinants that expressed LCMV GP aa 1 to 398 and LCMV NP aa 1 to 558. The viruses were plaque purified three times on Vero cells, and stocks were prepared by a single passage on BHK-21 cells. Stocks of recombinant vaccinia viruses were prepared by infection of 143 TK− cells in medium containing bromodeoxyuridine.
Proliferation assays and secondary in vitro stimulations for harvesting of tissue culture supernatants for cytokine enzyme-linked immunosorbent assays (ELISAs).
Spleen cells were harvested at various times after LCMV infection of transgenic mice. For some experiments, splenocytes were sorted by fluorescence-activated cell sorting (FACS) (see below). Antigen-presenting cells (APCs) in the assay mixture consisted of 2 × 105 irradiated syngeneic spleen cells per well or APCs from the native spleen population (for assays not quantitating proliferation but cytokine production in the supernatant of proliferating cultures) infected with LCMV or coated with LCMV NP or GP (see “CTL and antibody assays” above). The medium was RPMI containing 7% fetal calf serum and glutamine. For assessment of LCMV antigen-specific gamma interferon (IFN-γ) production, cells were cultivated for 5 h in the presence of syngeneic APCs (peritoneal exudate macrophages), infected with LCMV, and irradiated.
FACS analysis: phenotyping lymphocytes and intracellular cytokine analysis.
Cultured lymphocytes were phenotyped by FACS analysis with monoclonal antibodies to murine CD4 and CD8 and various cytokines, as directed by the manufacturer (Pharmingen). Intracellular cytokine analysis was done as described previously (18) and with antibodies provided by Pharmingen.
Assessment of cytokine production by ELISA.
Cytokines (interleukin 4 [IL-4], IL-6, IL-10, IL-2, tumor necrosis factor alpha, and IFN-γ) produced by splenocytes were detected by ELISA (Pharmingen). Briefly, 96-well Millititer HA plates (Millipore, Bedford, Mass.) were coated with the capture antibodies for IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ diluted to 2 μg/ml. After overnight incubation at 4°C, the plates were washed four times with phosphate-buffered saline (PBS) containing 0.05% Tween 20 and preincubated for 1 h at room temperature with PBS containing 10% fetal calf serum (FCS). Tissue culture supernatants and standards were added at various dilutions in PBS containing 10% FCS and 0.05% Tween 20, and plates were incubated for 2 to 4 h at room temperature. Thereafter, the plates were washed four times with PBS–0.05% Tween, and the respective detection antibodies for the cytokines were added at 1 μg/ml in PBS–0.05% Tween–10% FCS. The plates were incubated at room temperature for 1 h and washed four times in PBS-Tween before streptavidin-peroxidase conjugate (Boehringer Mannheim, Indianapolis, Ind.) was added at a 1:1,000 dilution. After a 30-min incubation at room temperature, the color substrate solution 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS) was added and left on the plates for 10 to 30 min. The plates were then counted in a ELISA reader at 490 nm.
sALT analysis.
The extent of hepatocellular injury during LCMV infection was monitored by measuring serum alanine aminotransferase (sALT) activity at multiple time points after infection. sALT activity was measured in a Paramax chemical analyzer (Baxter Diagnostics Inc., McGaw Park, Ill.) exactly as previously described (10).
CTL precursor measurements.
For precursor frequency analysis, spleen cells were harvested on days 7, 28, 60, 90, and 120 after primary LCMV infection. These cells were diluted serially and cultivated in 96-well flat-bottom plates in the presence of T-cell growth factor and syngeneic irradiated LCMV-infected (103 PFU/ml) spleen cells or syngeneic feeder cells coated with 10−5 M LCMV NP or GP H-2b peptides (105/well). After 5 to 9 days, each well was assayed for CTL lysis (described above) on target cells that were uninfected or infected with LCMV, peptide coated, or infected with vaccinia viruses and expressing GP or NP. The fraction of negative cultures (lysis of <3 standard errors above background) was determined for each dilution and correlated on a semilogarithmic scale with the number of splenocytes per well. CTL precursors (pCTL) were determined by the formula pCTL(f) = [4.6 − ln (percentage of negative wells)]/number of splenocytes per well.
Blood analysis.
Blood samples were obtained from the retro-orbital plexus of mice anesthetized with Metophane, and erythrocyte (RBC) counts were determined with a hemocytometer after 1:5 dilution of blood samples.
RESULTS
Thymic expression of the LCMV nucleoprotein reduces numbers of and cytokine secretion by NP-specific CD8+ CTL in perforin-deficient and -competent mice, and no compensatory responses to other viral proteins are noted.
Perforin-competent p+/+ (H-2b) mice and four experimental groups of F2 backcrosses between perforin-deficient SV129 (H-2b) and H-2b-transgenic mice expressing LCMV NP in their thymus (NP expressors) were infected with 105 PFU of LCMV intraperitoneally, and the numbers of pCTL were determined as described in Materials and Methods. The numbers of LCMV- NP- and GP-specific pCTL found in these five groups are displayed in Table 1. Numbers of NP pCTL were reduced by more than 10-fold in NP thymic expressor mice. In contrast numbers of GP pCTL were not affected by thymic expression of LCMV NP, and no significant compensatory increase was noted. Thus, NP CTL were deleted in the thymus of NP expressors. As expected, no lytic pCTL were detectable in p−/− mice.
TABLE 1.
Transgenic H-2b mice expressing LCMV NP in their thymus generate smaller numbers of LCMV NP CTL than do their nontransgenic littermatesa
| Type of mouse | LCMV pCTL countsb on cells coated with:
|
|
|---|---|---|
| GP-1 pept | NP-pept | |
| p+/+ (H-2b) | 1/180, 1/55, 1/320, 1/150 | 1/300, 1/125, 1/88, 1/450 |
| p+/− NP− | 1/230, 1/98, 1/255, 1/225 | 1/250, 1/78, 1/126, 1/380 |
| p+/− NP+ | 1/420, 1/133, 1/550, 1/480 | 1/4,550, 1/3,670, 1/5,670, 1/8,500 |
| p−/− NP− | NDc | ND |
| p−/− NP+ | ND | ND |
Groups of four RIP-NP thymic expressors and nontransgenic littermates were infected with 105 PFU of LCMV intraperitoneally. At 7 days postinfection, single-cell suspensions were prepared from spleens and tested for numbers of pCTL by limiting-dilution analysis (see Materials and Methods). The cutoff for positive wells for each responder cell concentration was killing of >3 standard errors over background 51Cr release. CTL activity was assessed by a 51Cr release assay in vitro, using syngeneic MC57 (H-2b) and MHC mismatched BalbCL/7 (H-2d) fibroblasts as targets. LCMV NP peptide (FQPQNGQFI) or GP-1 peptide was added to target cells in order to assess CTL specificity. The standard error for controls was <10%, and nonspecific 51Cr release was <5%. All control samples were run in triplicates with standard error of <5%; specific control killing of B6 spleens (day 7 postinfection) was 65% on NP peptide (NP-pept)-coated MC57 cells and 41% on GP-1 (GP-1 pept)-coated MC57 cells.
Results are given individually for the four mice in each group.
ND, not detected.
Therefore, because LCMV NP-specific CD8+ CTL activity in perforin-deficient mice cannot be assessed directly through testing lytic activity, another test system was used. IFN-γ was quantitated in tissue culture supernatants from splenocytes obtained on day 7 after LCMV infection and stimulated in the presence or absence of MHC class I-restricted LCMV H-2b NP or GP-1 peptides or LCMV-infected antigen presenting cells, respectively. The data clearly demonstrate that NP- but not GP-1-specific CD8+-mediated IFN-γ production is reduced in cultures from NP thymic-expressor perforin-competent mice compared to nonexpressors (Table 2). Most importantly, no significant compensatory increase of GP-specific IFN-γ production or IFN-γ secretion in response to whole virus was noted. As a consequence, IFN-γ production in NP expressors was reduced in response to whole virus, which is pathogenetically important. In addition, it became evident that lymphocytes in perforin-deficient hosts were secreting much more IFN-γ on day 7 after LCMV infection than were those in perforin-competent mice, suggesting that their immune response is much more strongly activated by the viral infection. Similarly, relatively large amounts of IFN-γ were present in cultures from perforin-deficient mice without antigen (LCMV peptide)-specific stimulation, probably because the virus grows to higher titers in protein-deficient mice (Table 3). Importantly, it became evident that splenocytes from perforin-deficient NP expressors made less IFN-γ than did those from thymic nonexpressors. Thus, the strong activation of lymphocytes found in perforin-deficient mice after LCMV infection and IFN-γ secretion by antigen-specific CD8 lymphocytes was significantly reduced by expression of the viral NP in the thymus, and no significant compensatory responses were seen. The observed differences were confirmed by direct ex vivo intracellular cytokine analysis after a 5-h stimulation in the presence of LCMV antigen (see Materials and Methods) (88.0% ± 10% IFN-γ-positive CD8 lymphocytes in p−/− NP− mice; 21% ± 5% in p−/− NP+ mice; 35% ± 8% in p+/− NP− mice; and 15% ± 3% in p+/− NP+ mice.
TABLE 2.
IFN-γ production in response to LCMV NP is reduced in thymic NP expressors without a compensatory increase in response to other viral proteinsa
| Type of effector spleno-cyte and time (days) after infection | IFN-γ production (ng/100 μl of day 6 supernatant) in presence of:
|
|||
|---|---|---|---|---|
| No peptide | LCMV | GP-1 peptide | NP peptide | |
| p+/+ (b) day 7 | NDb | 1.0 ± 0.24 | 0.3 ± 0.1 | 0.5 ± 0.13 |
| p+/− NP− day 7 | ND | 0.8 ± 0.1 | 0.25 ± 0.08 | 0.43 ± 0.1 |
| p+/− NP+ day 7 | ND | 0.25 ± 0.12 | 0.24 ± 0.1 | 0.1 ± 0.1 |
| p−/− NP− day 7 | 1.4 ± 0.23 | 2.5 ± 0.53 | 1.3 ± 0.5 | 2.7 ± 1.1 |
| p−/− NP+ day 7 | 0.4 ± 0.13 | 0.9 ± 0.12 | 1.1 ± 0.3 | 0.2 ± 0.07 |
| p+/+ (b) day 180 | ND | 3.3 ± 1.1 | 2.3 ± 0.2 | 2.5 ± 0.1 |
| p+/− NP− day 180 | ND | 3.1 ± 0.8 | 2.0 ± 0.1 | 2.9 ± 0.1 |
| p+/− NP+ day 180 | ND | 2.3 ± 0.3 | 1.65 ± 0.05 | 1.0 ± 0.04 |
| p−/− NP− day 180 | ND | 1.5 ± 0.24 | 0.6 ± 0.05 | 1.4 ± 0.1 |
| p−/− NP+ day 180 | ND | 0.6 ± 0.12 | 0.7 ± 0.1 | 0.3 ± 0.15 |
| p+/− NP+ or NP− uninf. | ND | 0.1 ± 0.02 | ND | ND |
| p−/− NP+ or NP− uninf. | ND | 0.12 ± 0.0 | ND | ND |
All groups of three or four mice were infected with 105 PFU of LCMV intraperitoneally. Splenocytes were harvested at the times indicated and cultured in 7% RPMI in the presence or absence of LCMV peptides (10−5 M final concentration). After 6 days, tissue culture supernatants were harvested and tested for IFN-γ concentrations in ELISAs (see Materials and Methods). Note the defect in IFN-γ production in response to LCMV-NP in p−/− NP+ mice. Interestingly, on day 35 (data not shown) and later (day 180 shown), the surviving perforin-deficient mice made less IFN-γ.
ND, not detected (<0.05 ng/100 μl).
TABLE 3.
Viral titers in organs from LCMV-infected perforin-deficient and -competent NP transgenic or nontransgenic mice that survived long-terma
| Type of mouse and time postinfection | Viral titer (PFU/g of tissue)
|
|||
|---|---|---|---|---|
| Liver | Kidney | Pancreas | Spleen | |
| Day 7 | ||||
| p+/− NP− | 2 × 102 ± 70 | ND | ND | ND |
| p+/− NP+ | NDb | ND | ND | ND |
| p−/− NP− | 0.8 × 105 ± 3 × 104 | 2 × 103 ± 1 × 103 | 1 × 103 ± 400 | 7 × 104 ± 4 × 104 |
| p−/− NP+ | 5 × 104 ± 2 × 104 | 5 × 102 ± 200 | 2 × 102 ± 150 | 1.5 × 105 ± 6 × 104 |
| Day 60 | ||||
| p+/− NP− | ND | ND | ND | ND |
| p+/− NP+ | ND | ND | ND | ND |
| p−/− NP− | 1 × 105 ± 4 × 104 | 1.4 × 104 ± 4 × 103 | 2 × 104 ± 1 × 104 | 3 × 104 ± 2 × 104 |
| p−/− NP+ | 1 × 105 ± 7 × 104 | 1 × 104 ± 5 × 103 | 1.6 × 104 ± 1 × 104 | 3 × 104 ± 1.1 × 104 |
Three or four mice in each group were infected with 105 PFU of LCMV intraperitoneally. Viral titers in the spleen, liver, thymus, kidney, pancreas and brain were determined by a plaque assay at 7 or 60 days postinfection. No significant differences were found between thymic expressors and nonexpressors. Brains and thymuses of all perforin-deficient mice also contained virus as assessed by direct immunohistochemistry (data not shown).
ND, nondetected.
The higher degree of organ infiltration and secretion of cytokines by lymphocytes at early times after LCMV infection in perforin-deficient mice correlates with increased viral titers.
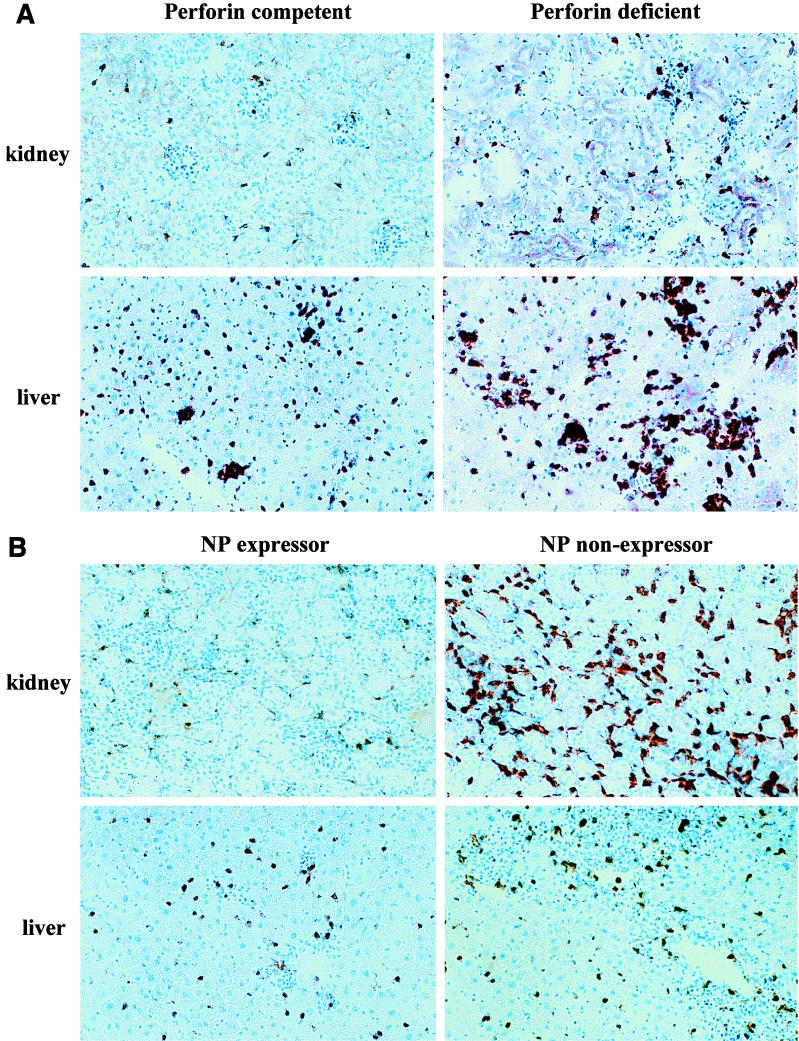
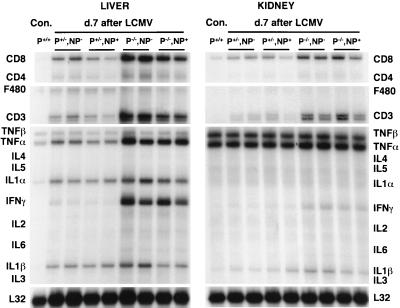
RNase protection analysis for cytokines and T-cell markers and plaque assays were performed on livers, spleens, and kidneys from perforin-deficient or -competent mice 7 days after LCMV infection. We found that viral titers were consistently at least 2 to 3 log units higher in perforin-deficient mice (Table 3). This correlated with increased lymphocytic infiltration by CD8 cells and cytokine secretion in many major organs. Histologically, liver and kidney infiltration by CD8 lymphocytes was the most pronounced (Fig. 1A). RNase protection analysis (Fig. 2 and Table 4) clearly shows that CD8 infiltration on day 7 after LCMV infection was increased up to fivefold in the liver in perforin-deficient mice. In parallel, IFN-γ production was 20-fold higher and TNF-α production was 2- to 5-fold higher. Interestingly, no apparent differences in liver and kidney immunopathology were noted at this early time (day 7 postinfection) between p−/− thymic expressors and nonexpressors, despite already clear differences in lymphocyte IFN-γ production (Table 2) and pronounced anemia in some p−/− mice that died early, at 1 month postinfection. Collectively, these results indicate that the inability of perforin-deficient mice to control LCMV replication is paralleled by increased activation of CD8 lymphocytes and cytokine secretion, resulting in multiorgan inflammation.
FIG. 1.
Immunopathology present in the pancreas and kidneys of perforin-deficient mice following LCMV infection is reduced by thymic expression of LCMV NP. Groups of three mice were infected with 105 PFU of LCMV intraperitoneally, and organs were harvested and stained immunohistochemically (see Materials and Methods) for CD4, CD8, NLDC-145 (dendritic cell marker), F 4/80 (macrophage marker), and B220 (B-lymphocytes) on day 7 or 60 postinfection. The liver, kidney, and spleen showed the most profound immunopathology. (A) Comparison of CD8+ lymphocytes in livers and kidneys in perforin-competent (p+/+) and perforin-deficient (p−/−) mice 7 days after LCMV infection. (B) Liver and kidney sections stained for CD8 from perforin-deficient mice (p−/−) on day 60 postinfection comparing thymic NP expressors and nonexpressors.
FIG. 2.
RNase protection analysis for T-cell and macrophage markers and cytokine RNA in organs of perforin-deficient and -competent thymic expressor or nonexpressor mice 7 days after LCMV infection. Groups of two to three mice were infected with LCMV at 105 PFU intraperitoneally, and organs were harvested and RNA was extracted for RNase protection analysis on day 7 postinfection (see Materials and Methods). The results were compared to those observed in organs derived from age-matched, nontransgenic uninfected controls (Con.). Results for two representative animals per group are shown. Note that organs from perforin-deficient mice show a higher degree of immune system activation, since CD8+ cells are found more abundantly and larger amounts of IFN-γ and TNF-α are produced than in perforin-competent littermates (Table 4). Virus was found in all areas of the spleen of perforin-deficient mice (data not shown). No apparent differences were noted in thymic expressors and nonexpressors at this early stage postinfection (Table 4).
TABLE 4.
CD8, TNF-α, and IFN-γ mRNA content in organs of perforin-deficient and -competent NP expressors and nonexpressorsa
| Type of mouse and time post-infection | Relative mRNA contentb
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Liver
|
Kidney
|
Spleen
|
|||||||
| CD8 | TNF-α | IFN-γ | CD8 | TNF-α | IFN-γ | CD8 | TNF-α | IFN-γ | |
| Day 7 | |||||||||
| p+ NP− | 8.2 | 1.4 | 4.3 | 2.4 | 0.9 | 1.3 | 1.7 | 0.9 | 4.5 |
| p+ NP+ | 6.2 | 1.4 | 3.6 | 2.2 | 0.9 | 3.7 | 1.4 | 0.9 | 3.8 |
| p− NP− | 42.3 | 3.9 | 99.9 | 4.8 | 1.2 | 19.5 | 4.8 | 2.0 | 59.3 |
| p− NP+ | 32.2 | 3.9 | 133 | 5.0 | 0.8 | 15.5 | 3.2 | 2.4 | 55.1 |
| Day 60 | |||||||||
| p− NP− | 13.1 | 1.7 | 25 | 11.6 | 1.2 | 82.2 | 6.4 | 1.9 | 10 |
| p− NP+ | 2.6 | 1.3 | 1.5 | 3.2 | 0.86 | 4.9 | 0.72 | 1.0 | 2.9 |
Total RNA extracted from the indicated organs of two representative mice per group was analyzed by the RNase protection assay for CD8, TNF-α, and IFN-γ RNA contents (Fig. 2 and 4) and quantitated by phosphorImager analysis.
CD8, TNF-α, and IFN-γ RNA values were normalized to the values of the housekeeping gene L32, divided by the respective RNA values detected in uninfected controls, and expressed as mean values, thus representing fold increase relative to L32 controls. Boldfaced values reflect important differences between perforin-deficient thymic expressors and nonexpressors.
Thymic expression of LCMV-NP reduces immunopathology in perforin-deficient mice after LCMV infection and increases their long-term survival.
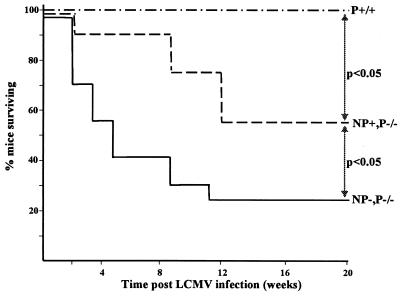
Next, we documented the long-term survival of perforin-deficient thymic expressors and nonexpressors. Most (75%) but not all perforin-deficient animals died after LCMV infection (Fig. 3). In contrast, death occurred later and the overall rate was decreased (to 45%) when LCMV-NP was expressed in the thymus of perforin-deficient mice. Thus, survival is increased in perforin-deficient mice that have a dampened immune response to one viral protein. Immunopathology was evaluated histologically (Fig. 1B) and showed profound organ destruction in perforin-deficient thymic nonexpressors on day 60 postinfection, which was reflected as early as day 9 postinfection by elevated sALT levels (129 to 195 U/liter in perforin-deficient mice versus 87 to 99 U/liter in normal mice). In contrast, perforin-deficient thymic NP expressors exhibited less immunopathology than did their NP-nonexpressing perforin-deficient littermates. Inflammatory disease of the liver (Fig. 1B), kidneys (Fig. 1B), and spleen (data not shown) 40 to 60 days after LCMV infection was drastically reduced. The difference was less pronounced in the pancreas (data not shown). Fewer CD8 cells and, to a lesser extent fewer CD4 cells (data not shown) and B lymphocytes (data not shown), and less macrophage infiltration was present in the livers and kidneys of thymic-expressor NP mice. This was confirmed by RNase production analysis for T-cell and cytokine markers, as shown in Table 4 and Fig. 4 for CD8 cells. Perforin-deficient thymic expressors showed a 5-fold-lower degree of CD8 infiltration and a 15-fold-lower level of IFN-γ secretion in kidneys and livers (and to a lesser degree in spleens) compared to perforin-deficient thymic nonexpressors (Table 4 and Fig. 4). All groups of perforin-competent mice showed neither organ infiltration nor cytokine upregulation at this point (Fig. 1). Thus, immunopathology is dampened in perforin NP+ mice, which correlates well with the selective depletion of NP CTL and no generation of pathologically significant compensatory responses to other viral proteins.
FIG. 3.
Survival of LCMV infection is increased in perforin-deficient mice expressing LCMV NP in their thymuses. Groups of 20 mice were infected with 105 PFU of LCMV intraperitoneally and maintained under specific-pathogen-free conditions in viral containment for the duration of the study. Statistically significant differences (survival log-rank test used for calculation of P value; P < 0.05 was considered significant) were found between the groups p−/− NP−, p−/− NP+, and p+/+ (H-2b). p+/− NP+ or p+/− NP− mice survived infection similar to the p+/+ controls shown.
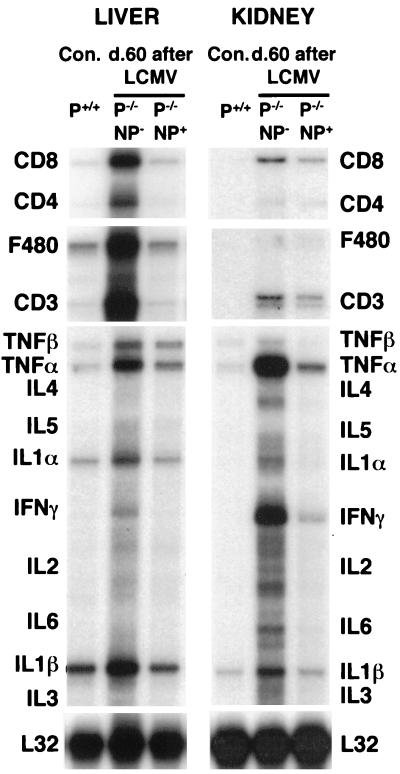
FIG. 4.
RNase protection analysis for T-cell and macrophage markers and cytokine RNA in organs of perforin-deficient thymic expressors or nonexpressors. Groups of two or three mice were infected with LCMV at 105 PFU intraperitoneally, and organs were harvested and RNA was extracted for RNase protection analysis on day 40 to 60 postinfection (see Materials and Methods). Results were compared to those observed in organs derived from age-matched, nontransgenic uninfected controls (Con.). Results from one representative animal per group are shown. Note that organs from perforin-deficient mice show signs of chronic immune system activation; i.e., CD8 cells are found abundantly, and large amounts of IFN-γ and TNF-α are produced (Table 4). Virus was found in all areas of the spleen of perforin-deficient mice (data not shown). Perforin-deficient thymic NP expressors showed much less IFN-γ production and CD8 infiltration than did nonexpressing littermates.
Spleens were analyzed in more detail (data not shown). Disruption of splenic architecture was evident 40 days postinfection in all groups of perforin-deficient mice, although it was less pronounced in RIP-NP-positive littermates (data not shown). In these analyses, complete disruption of red and white pulp demarcation reflects the chronic ongoing immune system activation in perforin-deficient mice whereas some reorganization of white pulp centers is indicative of a greatly reduced inflammatory response in perforin-deficient NP expressors. In contrast, spleen architecture appeared normal in perforin-competent or heterozygous littermates that had cleared the virus. In addition, we evaluated the occurrence and severity of anemia in comparing perforin-deficient thymic expressors to nonexpressors and in comparing survivors to moribund animals. Interestingly, pronounced anemia was found only in those perforin-deficient animals that died within 1 month of infection (RBC count = 2.2 × 109/ml ± 10%); the anemia was much less severe in mice dying later (RBC count = 7 × 109/ml ± 12%). Survivors had essentially normal RBC counts (8 × 109/ml ± 5% compared to 8.8 × 109/ml ± 3% in uninfected controls). Therefore, it appears likely that the cause of death, especially in perforin-deficient animals surviving longer than 1 month, is multifactorial and due to other factors in addition to the anemia. This was the rationale to test for immunopathology in multiple organs, as well as the RBC counts. Thus, multiorgan immunopathology occurs in perforin-deficient mice after LCMV infection and is dampened by tolerizing the NP-specific T-cell response.
Perforin-deficient long-term survivors expressing LCMV NP in their thymus do not clear LCMV and have equivalent viral titers in organs to those of perforin-deficient thymic nonexpressors.
To determine whether lower LCMV NP responses in NP transgenic perforin-deficient hosts would affect viral titers and/or clearance, the LCMV titers in several organs were determined 60 days postinfection (Table 3). No apparent differences emerged, and the viral titers were still similarly high up to day 180 postinfection (data not shown). Thus, perforin-deficient mice are unable to clear LCMV and become persistently infected independent of thymic expression of LCMV NP.
Perforin-deficient long-term survivors show decreased cytokine production by LCMV-specific CD8 lymphocytes at later times postinfection and exhibit stable levels of immunopathology.
Lymphocytes in perforin-deficient mice produce strikingly large amounts of cytokines, especially IFN-γ, on day 7 following LCMV infection (Tables 2 and 4). A large part of this activation is apparent in in vitro assays without antigen specific stimulation (Tables 2 and 4). This reflects an in vivo viral presence that is probably also transferred to in vitro cultures (Table 3). Not only is the IFN-γ level increased (Tables 2 and 4; Fig. 4), but also the number of TNF-α-producing cells is increased. Organ infiltration and cytokine production are much less pronounced at day 60 postinfection in the livers, kidneys, and spleens of perforin-deficient NP expressors than of thymic nonexpressors (Fig. 1B and 4; Table 4). Interestingly, they are even lower at later times postinfection in perforin-deficient long-term survivors (day 180, Table 2), and a loss of CD8 cells parallels this decrease in activation in all groups of perforin-deficient mice (7 to 12% CD8+ lymphocytes by FACS in perforin-deficient long-term survivors compared to 32 to 42% CD8+ lymphocytes in normal mice 180 days after LCMV infection or in uninfected perforin-deficient mice). The underlying mechanism is either exhaustion of CD8+ lymphocytes by activation-induced cell death (9, 17) or induction of a form of anergy due to the high levels of viral antigen present (9). The degree of immunopathology in the long-term survivors (day 180) was less pronounced than that shown in Fig. 1. Long-term survivors are therefore characterized by viral persistence but dampened antiviral cytokine (IFN-γ and TNF-α) responses (Table 2), which probably contributes to their ability to cope with the infection for longer.
DISCUSSION
The outcome of viral infections is governed by the direct lysis of cells infected with the virus and by the amount of immunopathology caused by the immune response initiated by the virus (29). We selected perforin-deficient mice that are persistently infected with nonlytic LCMV as a model for a chronic viral infection with immunopathology that leads to death in most of the animals. We found that thymic expression of one viral protein, NP, leads to selective tolerization of NP-specific CD8+ lymphocytes and consequently to a significant decrease in IFN-γ production following infection with LCMV. Importantly, no pathogenically significant compensatory increase in responses to other viral proteins was noted. Therefore, the amount of chronic inflammatory disease in the liver, kidney, and spleen, disruption of splenic architecture, and rate and incidence of deaths were reduced. Interestingly, these perforin-deficient, NP-expressing long-term survivors were characterized by a selective loss of LCMV-specific IFN-γ production, which is probably one of the reasons that these mice are able to tolerate this chronic infection long-term.
Our present and previous findings and those of others with the LCMV model illustrate this correlation between immunopathology and outcome of a viral infection as described in the following paragraphs. First, mice acutely infected intraperitoneally with LCMV clear the virus by relying mostly on a strong CTL response. In this case, only transient immunopathology and transient infiltration of multiple organs resulted, since the virus was eliminated rather rapidly (days 7 to 10 [Fig. 2]) (6). However, if the affinity of the CTL is lower (for example, in mice expressing a viral protein in the thymus) and, at the same time, the antiviral effect of IFN-γ is genetically eliminated, chronic infection results (23). The majority of such mice die due to immunopathology reflected in multiorgan inflammation and loss of APCs killed by CTL. Similarly, perforin-deficient mice develop persistent LCMV infection with very strong ongoing immune system activation, because CTL cannot lyse target cells but are constantly activated by “professional” APCs such as dendritic cells and macrophages that are LCMV infected. This leads to secretion of high levels of inflammatory cytokines such as IFN-γ (Tables 2 and 4) or TNF-α and to multiorgan inflammatory disease (Fig. 1). In this article, we show that genetically engineered thymic expression of antigen can be used to down-regulate one arm of the T-cell response in these perforin-deficient mice and thus to modulate inflammation and disease. Other previous observations support the notion that dampening of the antiviral immune response is beneficial in persistent infections. For example, mice infected with LCMV at birth are mostly tolerant on the T-cell level and therefore survive for >1 year although they have high viral titers in all organs. This finding also illustrates the low cytopathicity of LCMV in vivo. Furthermore, perforin-deficient mice that are depleted of CD8 lymphocytes do not succumb to aplastic anemia following LCMV infection (4). Our present study extends these concept to a therapeutic approach that selectively attempts to tolerize the immune system to one viral protein, thus leaving its other functions intact in order to not immunocompromise the host.
Based on our present data, the following chain of events takes place in perforin-deficient thymic expressors and nonexpressors after infection with LCMV. First, since neither NK cells nor CTL generated in response to LCMV have the ability to kill infected cells through the perforin pathway, virus is not cleared. This occurs despite the production of substantial amounts of IFN-γ and an intact FAS pathway. Viral presence in many lymphoid and nonlymphoid organs as well as in “professional” (costimulation-competent) APCs continues to drive antiviral lymphocytes that secrete IFN-γ and TNF-α (Tables 2 and 4; Fig. 2 and 4) but do not kill target cells (Table 1). This explains why much higher levels of these cytokines are produced in perforin-deficient mice than in perforin-competent littermates. The high IFN-γ production found in perforin-deficient mice without addition of viral antigens in vitro (Table 1) is therefore probably due to the fact that virus is present in all spleen samples, resulting in a higher degree of lymphocyte activation compared to that in perforin-competent littermates that have already started to clear virus at that point (Table 3). Clearly, thymic expression of NP specifically reduces the activation of NP-specific CTL (Tables 1, 2, and 4) in both perforin-competent and -deficient hosts. Overall antiviral immunity is still sufficient to mediate viral clearance by GP-specific CTL in perforin-competent mice. In contrast, as expected, virus is not cleared in perforin-deficient mice regardless of thymic LCMV NP expression (Fig. 3). Most importantly, no pathologically relevant compensatory increase in responses to other viral proteins is observed, reflected in equally reduced cytokine responses in p−/− NP+ mice in the presence of LCMV. However, the reduction of the immune response to this one viral antigen (NP but not GP [Tables 1 and 2]) is sufficient to suppress multiorgan inflammation (Fig. 1, 2, and 4) and increase survival (Fig. 3). In the perforin-deficient mice that survive LCMV infection long-term (50% of perforin-deficient thymic NP expressors and 30% of perforin-deficient thymic NP nonexpressors), CD8 lymphocytes secrete lower levels of LCMV-specific cytokines and their overall level is depleted over time (Table 2). This is the result of either activation-induced cell death occurring at a high rate in LCMV-specific lymphocytes (and maybe bystander-activated CD8 lymphocytes [22]), leading to their exhaustion (9, 17), or induction of anergy due to high systemic levels of LCMV antigen.
An important question is the precise cause of death in perforin-deficient mice. From our data, it appears to be multifactorial. Early deaths within 1 month (accounting for less than 50% of the overall deaths [Fig. 3]) are clearly associated with anemia. Deaths occurring later, however, are not accompanied by pronounced anemia but, rather, by stronger immunopathology in the liver, spleen, and kidneys (Fig. 1B), which is not present at early times after infection (Fig. 1A). As shown in Tables 2 and 4, low levels of inflammatory cytokines (IFN-γ and TNF-α) in the spleen appear to better correlate with survival, probably reflecting overall decreased immune system activation.
In summary, we conclude from our study that it might be worthwhile to take a second look at the degree of chronic immune system activation in persistent viral infections with comparatively low in vivo cytopathicity. In some situations, selective suppression of the antiviral response might be beneficial, if it can be achieved in vivo, especially since compensatory responses appear not to be pathogenically relevant. When combined with effective antiviral drug treatment to avoid excessively high titers and increased direct cytopathic effects, this might achieve a symbiotic relationship between virus and host in some situations.
ACKNOWLEDGMENTS
This research was supported by the National Institutes of Health and Juvenile Diabetes Foundation. Matthias G. von Herrath is supported by National Institutes of Health grants DK51091, AI44451, and AG04342 Project V and Juvenile Diabetes Foundation Career Development Award JDFI 296120. Luca G. Guidotti is supported by National Institutes of Health grant AI40696.
We thank Diana Frye for help with the manuscript and J. Lindsay Whitton and Mari Manchester for helpful discussions.
Footnotes
Publication 11705-NP from the Division of Virology, Department of Neuropharmacology, The Scripps Research Institute.
REFERENCES
- 1.Ahn K, Angulo A, Ghazal P, Peterson P A, Yang Y, Fruh K R. Human cytomegalovirus inhibits antigen presentation by a sequential multistep process. Proc Natl Acad Sci USA. 1996;93:10990–10995. doi: 10.1073/pnas.93.20.10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aichele P, Brduscha-Riem K, Oehen S, Odermatt B, Zinkernagel R M, Hengartner H, Pircher H. Peptide antigen treatment of naive and virus-immune mice: antigen-specific tolerance versus immunopathology. Immunity. 1997;6:519–529. doi: 10.1016/s1074-7613(00)80340-4. [DOI] [PubMed] [Google Scholar]
- 3.Ashton-Richardt P A, Bandeira A, Delaney J, Van Kaer L, Pircher H P, Zinkernagel R M, Tonegawa S. Evidence for a differential avidity model of T-cell selection in the thymus. Cell. 1994;76:651–663. doi: 10.1016/0092-8674(94)90505-3. [DOI] [PubMed] [Google Scholar]
- 4.Binder D, van den Broek M F, Kagi D, Bluethmann H, Fehr J, Hengartner H, Zinkernagel R M. Aplastic anemia rescued by exhaustion of cytokine-secreting CD8+ T cells in persistent infection with lymphocytic choriomeningitis virus. J Exp Med. 1998;187:1903–1920. doi: 10.1084/jem.187.11.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borrow P, Lewicki H, Hahn B H, Shaw G M, Oldstone M B A. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchmeier M, Welsh R, Dutko F, Oldstone M B A. The virology and immunobiology of LCMV infection. Adv Immunol. 1980;30:275–331. doi: 10.1016/s0065-2776(08)60197-2. [DOI] [PubMed] [Google Scholar]
- 7.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guadinium thiocyanate-phenol chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 8.Cutts F T, Markowitz L E. Successes and failures in measles control. J Infect Dis. 1994;170:S32–S41. doi: 10.1093/infdis/170.supplement_1.s32. [DOI] [PubMed] [Google Scholar]
- 9.Gallimore A, Glithero A, Godkin A, Tissot A C, Pluckthun A, Elliott T, Hengartner H, Zinkernagel R. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J Exp Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guidotti L G, Ishikawa T, Hobbs M V, Matzke B, Schreiber R, Chisari F V. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity. 1996;4:25–36. doi: 10.1016/s1074-7613(00)80295-2. [DOI] [PubMed] [Google Scholar]
- 11.Hobbs M V, Weigle W O, Noonan D J, Torbett B E, McEvilly R J, Koch R J, Cardenas G J, Ernst D N. Patterns of cytokine gene expression by CD4+ T cells from young and old mice. J Immunol. 1993;150:3602–3614. [PubMed] [Google Scholar]
- 12.Hoffsten P E, Oldstone M B A, Dixon F J. Immunopathology of adoptive immunization in mice chronically infected with lymphocytic choriomeningitis virus. Clin Immunol Immunopathol. 1977;7:44–52. doi: 10.1016/0090-1229(77)90028-9. [DOI] [PubMed] [Google Scholar]
- 13.Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen K J, Podack E R, Zinkernagel R M, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells greatly impaired in perforin-deficient mice. Nature. 1994;369:1–7. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 14.Kagi D, Ledermann B, Burki K, Zinkernagel R M, Hengartner H. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Annu Rev Immunol. 1996;14:207–232. doi: 10.1146/annurev.immunol.14.1.207. [DOI] [PubMed] [Google Scholar]
- 15.Klavinskis L, Whitton J L, Joly E, Oldstone M B A. Vaccination and protection from a lethal viral infection: identification, incorporation and use of a CTL glycoprotein epitope. Virology. 1990;178:393–400. doi: 10.1016/0042-6822(90)90336-p. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell B M, Leung A, Stevens J G. Murine cytomegalovirus DNA in peripheral blood of latently infected mice is detectable only in monocytes and polymorphonuclear leukocytes. Virology. 1996;223:198–207. doi: 10.1006/viro.1996.0468. [DOI] [PubMed] [Google Scholar]
- 17.Moskophidis D, Lechner F, Pircher H, Zinkernagel R M. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 18.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J D, Zajac A J, Miller J D, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 19.Rice G P A, Schrier R D, Southern P J, Nelson J, Casali P, Oldstone M B A. Cytomegalovirus and measles virus: in vitro models for virus-mediated immunosuppression. In: Gilmore N, Wainberg M A, editors. Viral mechanisms of immunosuppression: progress in leukocyte biology. I. Alan R. New York, N.Y: Liss, Inc.; 1985. pp. 15–29. [Google Scholar]
- 20.Shimeld C, Whiteland J L, Nicholls S M, Grinfeld E, Easty D L, Gao H, Hill T J. Immune cell infiltration and persistence in the mouse trigeminal ganglion after infection of the cornea with herpes simplex virus type 1. J Neuroimmunol. 1995;61:7–16. doi: 10.1016/0165-5728(95)00068-d. [DOI] [PubMed] [Google Scholar]
- 21.Stanberry L R. The concept of immune-based therapies in chronic viral infections. J Acquired Immune Defic Syndr. 1994;7:S1–S5. [PubMed] [Google Scholar]
- 22.Tough D F, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 23.von Herrath M G, Coon B, Oldstone M B A. Low-affinity cytotoxic T-lymphocytes requires IFN-gamma to clear an acute viral infection. Virology. 1997;229:349–359. doi: 10.1006/viro.1997.8442. [DOI] [PubMed] [Google Scholar]
- 24.von Herrath M G, Dockter J, Nerenberg M, Gairin J E, Oldstone M B A. Thymic selection and adaptability of cytotoxic T lymphocyte responses in transgenic mice expressing a viral protein in the thymus. J Exp Med. 1994;180:1901–1910. doi: 10.1084/jem.180.5.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Herrath M G, Dockter J, Oldstone M B A. How virus induces a rapid or slow onset insulin-dependent diabetes mellitus in a transgenic model. Immunity. 1994;1:231–242. doi: 10.1016/1074-7613(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 25a.von Herrath, M. G. Unpublished observations.
- 26.Walsh C M, Matloubian M, Liu C C, Ueda R, Kurahag M T, Young J D, Ahmed R, Clark W R. Immune function in mice lacking the perfogene. Proc Natl Acad Sci USA. 1994;91:10854–10858. doi: 10.1073/pnas.91.23.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitton J L. Lymphocytic choriomeningitis virus CTL. Semin Virol. 1990;1:257–262. [Google Scholar]
- 28.Wolf D G, Yaniv I, Honigman A, Kassis I, Schonfeld T, Ashkenazi S. Early emergence of ganciclovir resistant CMV strains in children with primary combined immunodeficiency. J Infect Dis. 1998;178:535–538. doi: 10.1086/517468. [DOI] [PubMed] [Google Scholar]
- 29.Zinkernagel R M. Are HIV-specific CTL responses salutary or pathogenic? Curr Opin Immunol. 1995;7:462–470. doi: 10.1016/0952-7915(95)80089-1. [DOI] [PubMed] [Google Scholar]