SUMMARY
The mitochondrial proteome is comprised of approximately 1,100 proteins1, all but 12 of which are encoded by the nuclear genome in C. elegans. The expression of nuclear-encoded mitochondrial proteins varies widely across cell lineages and metabolic states2–4, but the factors that specify these programs are not known. Here we identify mutations in two nuclear-localized mRNA processing proteins, CMTR1/CMTR-1 and SRRT/ARS2/SRRT-1, which we show act via the same mechanism to rescue the mitochondrial complex I mutant NDUFS2/gas-1(fc21). CMTR-1 is an FtsJ-family RNA methyltransferase that in mammals 2’-O-methylates the first nucleotide 3’ to the mRNA CAP to promote RNA stability and translation5–8. The mutations isolated in cmtr-1 are dominant and lie exclusively in the regulatory G-patch domain. SRRT-1 is an RNA binding partner of the nuclear cap-binding complex and determines mRNA transcript fate9. We show that cmtr-1 and srrt-1 mutations activate embryonic expression of NDUFS2/nduf-2.2, a paralog of NDUFS2/gas-1 normally expressed only in dopaminergic neurons, and that nduf-2.2 is necessary for the complex I rescue by the cmtr-1 G-patch mutant. Additionally, we find that loss of the cmtr-1 G-patch domain cause ectopic localization of CMTR-1 protein to processing bodies (P-bodies), phase-separated organelles involved in mRNA storage and decay10. P-body localization of the G-patch mutant CMTR-1 contributes to the rescue of the hyperoxia sensitivity of the NDUFS2/gas-1 mutant. This study suggests that mRNA methylation at P-bodies may control nduf-2.2 gene expression, with broader implications for how the mitochondrial proteome is translationally remodeled in the face of tissue-specific metabolic requirements and stress.
Graphical Abstract

In Brief
Meisel et al. reveal that a C. elegans mitochondrial complex I mutant is rescued by mutations in the G-patch domain of the mRNA methyltransferase CMTR-1, which cause ectopic localization of the CMTR-1 protein to Processing bodies and widespread expression of the NDUFS2 paralog nduf-2.2 that is normally restricted to dopaminergic neurons.
RESULTS AND DISCUSSION
CMTR-1 G-patch mutations rescue the mitochondrial NDUFS2/gas-1(fc21) mutant
In an effort to identify genetic regulators of the mitochondrial electron transport chain (ETC) across eukaryotes, we performed a forward genetic selection in C. elegans for mutations that rescue survival of the complex I mutant NDUFS2/gas-1(fc21). NDUFS2 is a core subunit of complex I of the ETC that contributes to forming the quinone binding site11–13. The fc21 allele encodes a hypomorphic R290K missense mutation that reduces complex I function to cause low broodsize and a moderate slowing of growth at 21% oxygen14,15. However at 50% oxygen – a condition that does not affect the growth of wild type animals – the gas-1(fc21) mutant arrests development at the L2 stage16. We randomly mutagenized gas-1(fc21) animals at the permissive 21% oxygen concentration, and then transferred F2 animals containing thousands of newly-induced mutations to the non-permissive 50% oxygen. Nearly all F2 animals arrested development at 50% oxygen, but rare animals that reached adulthood due to suppressor mutations that improved ETC function were selected, allowed to reproduce, retested for suppression of gas-1(fc21) hyperoxia sensitivity, and subjected to whole genome sequencing.
From this genetic selection and genome sequencing, we identified 13 independent alleles of the gene CMTR1/cmtr-1 which encodes a nuclear FtsJ-family RNA methyltransferase, the human orthologue of which has been shown biochemically to 2’-O-methylate the first transcribed nucleotide of mRNAs (Figure 1A). The 13 independent alleles we identified include 8 missense mutations (M97I, M97T, G106E, G106R, G111D, G111S, G126E, and G126R), all disrupting essential sequence features of the G-patch domain17 of CMTR-1 (Figure 1B & Table S1). Most of these mutations were isolated as heterozygotes in the strains that survived the selection, suggesting that these mutations confer a dominant phenotype. This G-patch domain, including the specific methionine and glycine residues substituted in the cmtr-1 C. elegans mutants, is conserved in mammalian CMTR1 (Figure 1B) and mediates an interaction with the RNA helicase DHX15/DDX-1518,19, but reports conflict about whether this interaction promotes or inhibits CMTR-1 methyltransferase activity. We confirmed that backcrossed screen isolates carrying mutations in the G-patch domain of CMTR-1 suppress the growth arrest of gas-1(fc21) at 50% and 100% oxygen (Figures 1C & S1A). cmtr-1 G-patch mutations also completely suppressed induction of the hsp-6::gfp reporter for mitochondrial stress by gas-1(fc21) at 21% and 100% oxygen (Figures 1D & S1B), suggesting G-patch mutation results in healthier mitochondria in all oxygen tensions. Interestingly, 100% oxygen induces hsp-6::gfp expression in wild-type animals, and this response was also blunted in cmtr-1 G-patch mutants (Figures 1D & S1B), suggesting that these G-patch mutations may be protective from oxygen-induced mitochondrial stress even in wild type.
Figure 1. CMTR-1 G-patch mutations restore health of NDUFS2/gas-1(fc21) mutants.

A. CMTR1/CMTR-1 is a SAM-dependent RNA methyltransferase that has been shown in mammalian cells to 2’-O-methylate the first transcribed nucleotide of nuclear-encoded mRNAs. B. Mutations in cmtr-1 that allow the NDUFS2/gas-1(fc21) mutant to survive hyperoxia are confined to conserved features of the G-patch domain17 (Pfam Signature PF01585). C. Growth of animals after 5 days exposure to 50% oxygen (left) or 5 days exposure to 100% oxygen followed by 3 days recovery at 21% oxygen (right). D. Mean intestinal fluorescence of the mitochondrial stress reporter hsp-6::gfp in L4 stage animals incubated at 21% or 100% oxygen for 1 day at 20°C. Exposure time = 100 ms, magnification = 69x, quantified from images in Figure S1B. E-F. Growth of animals following 4 days exposure to 50% oxygen. G. Images of animal growth and reproduction follow 4 days exposure to 50% oxygen. H. Total progeny produced from individual animals incubated at 21% oxygen. I. Growth of animals following 5 days exposure to 50% oxygen. J. Growth of animals following 2 days exposure to 21% oxygen. K. Growth of animals exposed to 3 days of 50% oxygen followed by recovery for 3 days at 21% oxygen. For all panels statistical significance was calculated using one-way ANOVA followed by Tukey’s Multiple Comparison Test. Error bars represent standard deviation. n.s. = not significant, * = p value <0.05, ** = p value <0.01, *** = p value <0.001. See also Figure S1 and Table S1.
CMTR-1 G-patch mutants were classically dominant with respect to gas-1(fc21) suppression, consistent with the heterozygous mutants that emerged from our selection (Figures S1C–D). Additionally, over-expression of transgenic cmtr-1(G111D) or cmtr-1-(G126R) in a wild-type cmtr-1 background rescued gas-1(fc21) growth at 50% oxygen (Figure 1E), confirming a dominant genetic mode-of-action, and arguing against haploinsufficiency. In contrast, over expression of wild-type cmtr-1 had no effect on gas-1(fc21) growth in high oxygen (Figure 1E), suggesting a gain-of-function activity of the mutant rather than a higher level of wild-type gene activity. Over-expression of cmtr-1 harboring an in-frame deletion of the entire G-patch domain was also an excellent gas-1(fc21) suppressor at 50% oxygen (Figure 1F), pointing towards the G-patch domain being a negative regulator of CMTR-1 rescue activity. Indeed, single-copy insertion of cmtr-1 harboring an in-frame G-patch deletion (with wild-type cmtr-1 present at the endogenous locus) completely suppressed the growth arrest of gas-1(fc21) in hyperoxia and restored gas-1(fc21) brood size at 21% oxygen to wild-type levels (Figures 1G–H). Over-expression of G-patch mutant cmtr-1(G111D) with additional point mutations K244A and D379A that disrupt the catalytic site for RNA 2’-O-methylation8,20 did not rescue gas-1(fc21) in hyperoxia (Figure 1I), demonstrating that RNA methylation activity is required for G-patch mutant CMTR-1 rescue activity. Taken together, these results suggest that loss of the CMTR-1 G-patch domain leads to gain-of-function RNA methylation activity that rescues the gas-1(fc21) complex I mutant.
gas-1(fc21) mutants display a slight growth defect at 21% oxygen, which was curiously not rescued by cmtr-1 mutants isolated from our screen (Figures 1J & S1E). However we observed that cmtr-1 mutants have a recessive growth defect at 21% oxygen themselves, which may be responsible for the observed lack of rescue (Figure 1J). Indeed, over-expression of transgenic cmtr-1(G126R) with wild-type cmtr-1 at the endogenous locus did rescue the slow growth of gas-1(fc21) at 21% oxygen (Figure 1J). Consistent with homozygous cmtr-1 mutants having a recessive growth liability, heterozygous cmtr-1 mutants rescued gas-1(fc21) growth rate in 50% oxygen better than homozygous mutants (Figure S1C), while homozygous cmtr-1 mutations were a stronger suppressor of hsp-6::gfp induction (Figures 1D, S1B, and S1D). Additionally, over-expression of a cmtr-1(G126R) transgene rescued growth at 50% oxygen better than mutating cmtr-1(G126R) at the endogenous locus (Figure S1F). Taken together, these results demonstrate that cmtr-1 G-patch loss rescues all aspects of the gas-1(fc21) mutant phenotype, and that endogenous cmtr-1 G-patch loss slows wild-type growth.
We tested if cmtr-1 G-patch mutations could rescue other complex I mutants, but transgenic over-expression of cmtr-1(G126R) did not improve growth of NDUFS4/lpd-5(mg746) null animals at 21% or 1% oxygen (Figure S1G) or NDUFS7/nduf-7(et19) hypomorph mutants at 21% or 50% oxygen (Figure 1K & S1H), and in fact tended to make these complex I mutants grow more slowly. Additionally, the nduf-7(et19); cmtr-1(G111D) double mutant displayed synthetic sickness, growing extremely slowly at 21% oxygen and producing an embryonic lethality phenotype, which neither the nduf-7(et19) nor cmtr-1 single mutants displayed (Figure S1I). These results point to a rescuing activity of G-patch mutant CMTR-1 specific to the gas-1(fc21) mutant that may be detrimental in the context of other complex I lesions.
G-patch mutant CMTR-1 is ectopically localized to Processing bodies
To understand how loss of the CMTR-1 G-patch domain activates CMTR-1 and confers gas-1(fc21) rescue activity, we tagged integrated single-copy cmtr-1 transgenes with GFP to assess changes in expression or localization. We first confirmed that C-terminally tagged CMTR-1::GFP was functional, by showing that cmtr-1(ΔG-patch)::gfp rescued gas-1(fc21) growth arrest at 50% oxygen (Figure 1G). We used confocal microscopy to examine the expression pattern of wild-type CMTR-1, and as expected for an mRNA cap1 methyltransferase found it to be localized to all nuclei in the animal (Figure 2A). CMTR-1 carrying the G-patch deletion mutation that activates CMTR-1 activity also localized to nuclei, but, surprisingly, also localized to cytosolic foci in many cells of the animal (Figure 2A). These results were consistent when transgenes were driven by a strong ribosomal promoter (Prpl-28) or the endogenous cmtr-1 promoter, arguing against the localization pattern being an artifact of over-expression (Figures 2A & S2A). Additionally, the gas-1(fc21) mutation has no effect on the localization of wild-type or G-patch mutant CMTR-1 (Figure S2B).
Figure 2. G-patch mutant CMTR-1::GFP is ectopically localized to P-bodies, which is necessary for its rescue activity.

A. Confocal microscopy of cmtr-1(wt)::gfp and cmtr-1(ΔG-patch)::gfp driven by the ribosomal promoter Prpl-28. Brightfield image is inset, white box corresponds to enlarged image on right. B. Confocal microscopy (left) and quantification (right) of cmtr-1(ΔG-patch)::gfp co-localization with the P-body marker dcap-1::DsRed. White box in brightfield image corresponds to fluorescent images on right. C. Bioinformatic analysis reveals three nuclear localization sequences in CMTR-1 and an N-terminal region predicted to form an intrinsically disordered domain. Deletion of amino acids 11–42 removes the first NLS and a portion of the IDD. D. Confocal microscopy of animals carrying extra-chromosomal arrays encoding G-patch mutant cmtr-1(G126R)::gfp or cmtr-1(Δ11–42 G126R)::gfp. E-F. Growth of animals following 5 days (E) or 2 days (F) exposure to 50% oxygen. Statistical significance was calculated using one-way ANOVA followed by Tukey’s Multiple Comparison Test. Error bars represent standard deviation. n.s. = not significant, *** = p value <0.001. See also Figure S2.
To determine the identity of the cytosolic foci, we introduced fluorescently-tagged proteins with known localization patterns into the cmtr-1(ΔG-patch)::GFP strain. dcap-1::DsRed is a marker of processing bodies (P-bodies)21 and co-localized with 90% of CMTR-1(ΔG-patch) foci (Figure 2B). Conversely, 80% of P-body foci contained cmtr-1(ΔG-patch)::GFP (Figure 2B), confirming these phase-separated condensates as sites of CMTR-1 ectopic localization if bearing a G-patch domain mutation. P-bodies are membraneless, ribonucleoprotein-based organelles involved in mRNA storage, silencing, and decay10.
We sought to determine whether nuclear or P-body localized CMTR-1(ΔG-patch) mediates gas-1(fc21) rescue activity, and so attempted to construct a cmtr-1 G-patch mutant lacking the nuclear localization sequence. Surprisingly our cmtr-1(Δ11–42 G126R)::gfp strain maintained its nuclear localization pattern and instead showed dramatically decreased P-body localization (Figures 2C–D). Further bioinformatic analysis revealed three regions of CMTR-1 that may function as bipartite nuclear localization sequences (NLS), two of which were retained in our cmtr-1(Δ11–42 G126R) construct (Figures 2C & S2C), explaining its nuclear localization. Additionally, we found the N-terminal region deleted in the cmtr-1(Δ11–42 G126R) construct is predicted to lie in an intrinsically disordered domain (IDD) that may contribute to phase separation and P-body localization (Figures 2C & S2C). Notably, the predicted IDD and downstream NLS are both present in human CMTR1 (Figure S2C), suggesting P-body localization of mammalian CMTR1 may also occur if its G-patch activity is altered in particular physiological conditions.
We tested whether this ΔIDD cmtr-1(Δ11–42 G126R) derivative could rescue the gas-1(fc21) mutant growth arrest in hyperoxia and found that CMTR-1 lacking P-body expression had significantly reduced rescue activity (Figure 2E). As a control, extra-chromosomal arrays encoding cmtr-1(G126R)::gfp were localized to P-bodies and nuclei and rescued gas-1(fc21) growth arrest (Figures 2D–E), ruling out an effect of array mosaicism. Additionally, both sets of transgenes had no effect on growth rate in hyperoxia in a wild-type genetic background (Figure 2F). These data show that P-body localization of G-patch mutant CMTR-1 is partially necessary for its gas-1 mutant complex I rescue activity. We do not exclude the possibility that G-patch mutant CMTR-1 localized to the dilute phase of the cytosol also has gas-1 rescue activity.
G-patch mutant CMTR-1 rescues NDUFS2/gas-1(fc21) by activating expression of its paralog NDUF-2.2
Because P-bodies are sites of mRNA storage and translational control, we hypothesized that CMTR-1 may rescue gas-1(fc21) by activating translation of a particular transcript or set of transcripts. A strong candidate was NDUFS2/nduf-2.2, a paralog of NDUFS2/gas-1 which is 96% identical after the mitochondrial targeting sequence15. For unknown reasons, gene duplication of the complex I NDUFS2 subunit has occurred many times in evolution and is present in diverse nematode species and helminths22. The C. elegans gas-1(fc21) R290K mutant sensitivity to volatile anesthetics is rescued by expression of nduf-2.2 from the gas-1 promoter but not the native nduf-2.2 promoter, demonstrating that they are functionally equivalent complex I subunits, but that nduf-2.2 may not be widely expressed15. In contrast to the lethal phenotype of a gas-1 null mutant, nduf-2.2 null mutant animals are viable with no growth defect, or anesthetic or oxygen sensitivity23, but do have axon regeneration defects24,25. We made a transgenic reporter for nduf-2.2 using 3kb of upstream promoter and the endogenous 67 bp nduf-2.2 5’UTR (which could be methylated by CMTR-1 and/or subject to trans-splicing) fused to gfp (Figure 3A). In wild-type animals, we observed expression of nduf-2.2 in dopaminergic neurons, as identified by co-localization with the dopamine transporter gene dat-1::mCherry (Figures 3B & S3A). When this nduf-2.2 reporter gene with the nduf-2.2 promoter and 5’UTR was introduced into the cmtr-1 G-patch mutant, we observed radically expanded pattern of nduf-2.2 expression in many cells of embryos and adult tissues (Figures 3C–E and S3B). No change in nduf-2.2 expression was observed in the gas-1(fc21) background (Figure 3E), consistent with that mutant’s complex I deficiency. This data shows that the cmtr-1 G-patch mutation activates nduf-2.2 transcription or translation in additional cells and is consistent with the hypothesis that misexpression of the nduf-2.2 paralog in the cmtr-1 G-patch mutants is the molecular basis of the gas-1(fc21) mutant suppression.
Figure 3. G-patch mutant CMTR-1 rescues NDUFS2/gas-1(fc21) by activating expression of the paralog NDUF-2.2.

A. GFP reporter for nduf-2.2 expression was constructed using 3 kb of upstream promoter sequence and 67 bp of the endogenous 5’UTR which is the target for cap1 2’-O-methylation by CMTR-1. B. nduf-2.2::gfp reporter in wild-type animals is weakly expressed in a subset of head neurons indicated by white arrows. Exposure time = 2 seconds, magnification = 100x. C. nduf-2.2::gfp reporter (green arrow) in wild-type or cmtr-1(G126R) adult animals. myo-2::mCherry co-injection marker (red arrow) is visible. Exposure time = 2 seconds, magnification = 40x. D. nduf-2.2::gfp reporter in wild-type and cmtr-1(G126R) embryos. White arrows indicate embryos expressing no GFP. Exposure time = 2 seconds, magnification = 90x. E. Quantification of nduf-2.2::gfp fluorescence in embryos from wild type and cmtr-1(G126R) (panel D) and gas-1(fc21) (not pictured). Plotted are maximum fluorescence values with background subtracted. F. Total progeny produced from individual animals incubated at 21% oxygen. G. Images of nematode growth at 21% oxygen after 1 generation. Statistical significance was calculated using t-test (E) or one-way ANOVA followed by Tukey’s Multiple Comparison Test (F). Error bars represent standard deviation. n.s. = not significant, * = p value <0.05, ** = p value <0.01, *** = p value <0.001. See also Figure S3.
To prove this hypothesis, we tested whether nduf-2.2 was required for the rescue of gas-1(fc21) by a cmtr-1 G-patch mutant. Although nduf-2.2(ok437) null mutants are viable and do not grow slowly, gas-1(fc21) is synthetic maternal effect lethal with nduf-2.2 loss23. We constructed gas-1(fc21); nduf-2.2(ok437) double mutants with the use of a balancer chromosome for gas-1 and confirmed that the double mutants which segregated were sterile (Figures 3F–G). This synthetic sterility was rescued by nuo-3(G60D), an intra-complex suppressor mutation in complex I we have reported to boost complex I forward activity in the gas-1(fc21) mutant16 (Figures 3F–G). cmtr-1 is a stronger gas-1(fc21) suppressor than nuo-3(G60D) with respect to many phenotypes including hsp-6::gfp attenuation, growth rate rescue, and hyperoxia resistance, but unlike nuo-3(G60D), the G-patch mutant cmtr-1 was not able to rescue the synthetic lethality of gas-1(fc21) and nduf-2.2(ok437) (Figures 3F–G). This data proves that cmtr-1 rescue of gas-1(fc21) is dependent on the paralog NDUFS2/nduf-2.2.
The NDUFS2 duplication is present in all Caenorhabditis species as well as a diverse subset of nematode species (e.g. Ascaris suum, Brugia malayi, Onchocerca volvulus) separated by hundreds of millions of years of nematode evolution22, and specific amino acid differences between GAS-1 and NDUF-2.2 are highly conserved in these nematode species (Figures S3C–D). This data, along with the endogenous expression in dopaminergic neurons and neuronal phenotypes of nduf-2–2, raises the question of what the functional differences are between gas-1 and nduf-2.2, and whether they might be relevant for the rescue by cmtr-1 G-patch mutants. It has been hypothesized that paralogs nduf-2.2 and sdha-2 may produce alternate ETC complexes I and II (respectively) that use rhodoquinone (RQ) to couple forward activity of complex I to reverse activity of complex II in states of hypoxia22. RQ synthesis is dependent on tryptophan degradation and modification by the kynurenine pathway; a kynu-1 mutation that disables a step in rhodoquinone synthesis disrupts the production of RQ26. We introduced kynu-1(tm4924) into the gas-1(fc21) mutant and found that G-patch mutant cmtr-1 was still able to rescue the growth arrest in hyperoxia (Figure S3E), demonstrating that rescue does not require RQ and therefore it is unlikely complex II runs in reverse in these mutants. Instead, we hypothesize that forward flow through the ETC is restored by cmtr-1 mutation and nduf-2.2 expression in the gas-1(fc21) mutants.
Mutation of the RNA binding protein Serrate rescues NDUFS2/gas-1(fc21)
To further understand the mechanism by which CMTR-1 localization at P-bodies may activate NDUFS2/nduf-2.2 expression, we returned to our gas-1(fc21) suppressor screen and searched for additional lesions in mRNA processing genes in the sequenced isolates that did not contain cmtr-1 mutations. We identified three independent alleles of Serrate/SRRT/ARS2/srrt-1 that encode G310E or R497H missense mutations and confer a recessive gas-1(fc21) rescue phenotype (Figure 4A & Table S1). Serrate is a nuclear RNA binding protein that interacts with single stranded RNA and the cap binding complex in plants and animals to target transcribed RNAs to distinct cellular fates27. We used CRISPR-Cas9 to introduce the srrt-1(G310E) mutation into a clean genetic background and confirmed that srrt-1(G310E) rescued gas-1(fc21) growth rate at 21% and 50% oxygen (Figures 4B–C). Over-expression of wild-type srrt-1 rescued the srrt-1(G310E) mutant, resulting in slower growth in a gas-1(fc21) mutant background at 50% oxygen (Figure 4D), confirming the reduction-of-function nature of the srrt-1(G310E) allele. Over-expression of srrt-1(wt) had no effect on growth at 50% oxygen in a wild-type background (Figure S4A). Both SRRT/srrt-1 mutations isolated in our screen lie in highly conserved residues present in all metazoa (Figure 4E). By introducing a negative charge the G310E mutation may disrupt the RNA recognition motif (RRM) domain, a basic patch of positively charged Arg and Lys residues that interacts with single stranded RNA (Figure 4F)27.
Figure 4. Mutation of the RNA binding protein Serrate activates nduf-2.2 and rescues gas-1(fc21).
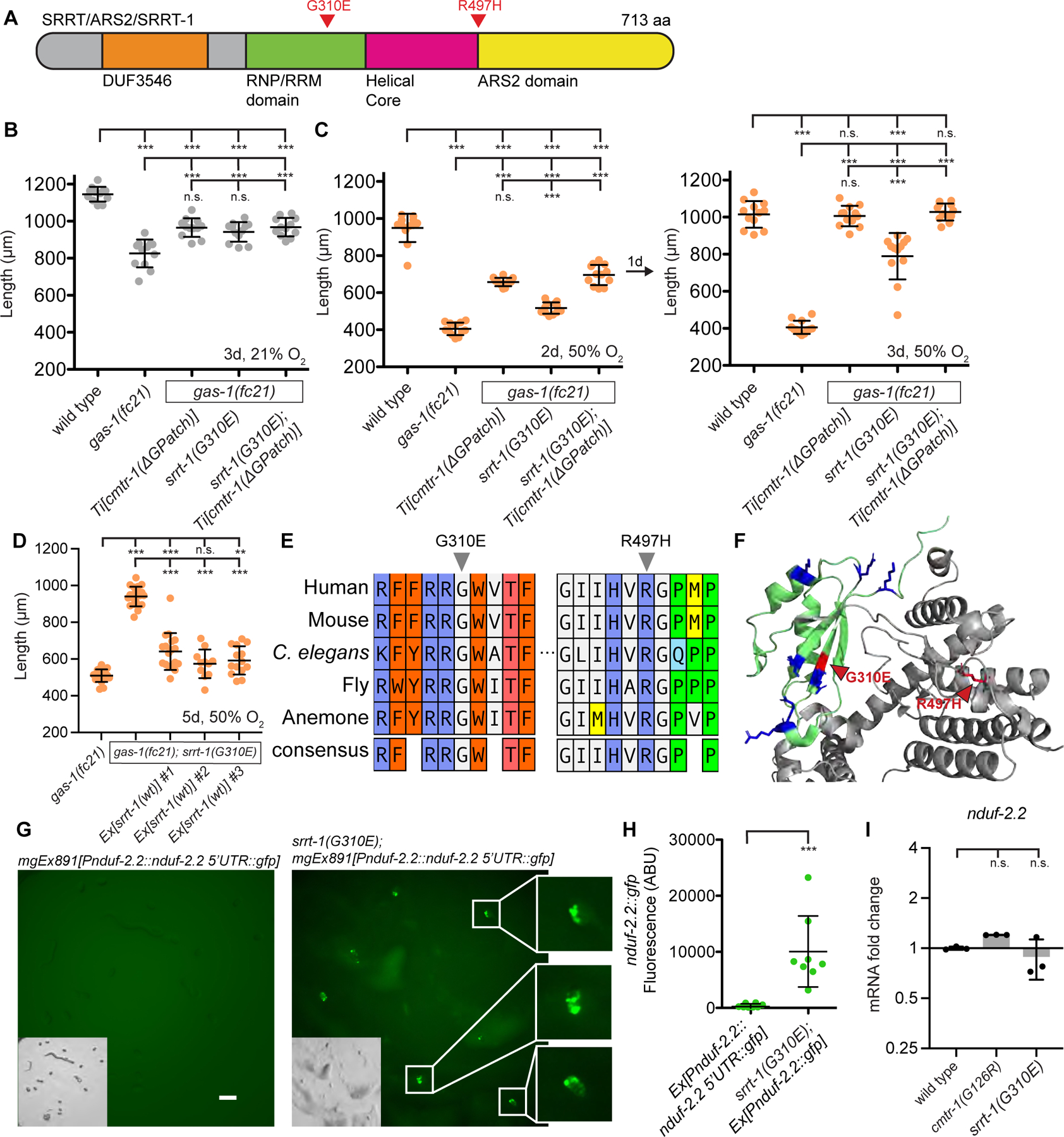
A. Domain structure of RNA binding protein Serrate. B. Growth of animals following 3 days exposure to 21% oxygen. C. Growth of animals following 2 days (left) or 3 days (right) exposure to 50% oxygen. D. Growth of animals following 5 days exposure to 50% oxygen. E. Multiple sequence alignment of SRRT/srrt-1 homologs from animals made with ClustalW. Labelled residues correspond to suppressor mutations isolated in C. elegans srrt-1. F. Crystal structure of Human SRRT/ARS2 (PDB: 6F7J27). Highlighted in green is the RNA-binding RRM domain. Blue residues correspond to positively charged amino acids; red residues correspond to mutations isolated in this study. G. nduf-2.2::gfp reporter in wild-type and srrt-1(G310E) embryos. White arrows indicate embryos expressing no GFP. Exposure time = 1 second, magnification = 90x. H. Quantification of GFP fluorescence in embryos from panel G. Plotted are maximum fluorescence values with background subtracted. I. Quantitative real-time PCR of nduf-2.2 mRNA normalized to the housekeeping gene rps-23. For all panels statistical significance was calculated using one-way ANOVA followed by Tukey’s Multiple Comparison Test. Error bars represent standard deviation. n.s. = not significant, * = p value <0.05, ** = p value <0.01, *** = p value <0.001. See also Figure S4.
To determine if srrt-1 and cmtr-1 act via a shared mechanism to rescue gas-1(fc21) mutants, we constructed the srrt(G310E); cmtr-1(G-patch); gas-1(fc21) triple mutant and observed that cmtr-1 and srrt-1 were not additive for gas-1(fc21) rescue at 21% or 50% oxygen (Figures 4B–C), consistent with both genes acting in the same genetic pathway. In contrast, nuo-3(G60D) and cmtr-1(G111D) were additive for gas-1(fc21) suppression (Figure S4B), indicating that improvement of the cmtr-1 rescue of gas-1(fc21) is possible. Importantly, like the cmtr-1(G-patch) mutant, the srrt-1(G310E) mutation induced expression of the nduf-2.2::gfp reporter in embryos (Figures 4G–H), confirming cmtr-1 and srrt-1 both activate nduf-2.2 expression. We performed qPCR using primers specific for nduf-2.2 (Figure S4C) and found that neither cmtr-1(G126R) nor srrt-1(G310E) mutation caused a significant increase in nduf-2.2 mRNA (Figure 4I), despite a dramatic increase in embryonic nduf-2.2::gfp expression. Notably, single-cell transcriptomics from wild-type embryos detected nduf-2.2 mRNA in 94/409 cells28, consistent with a post-transcriptional regulatory mechanism. Taken together these results strongly suggest that mutations in cmtr-1 and srrt-1 rescue gas-1(fc21) by increasing the translation of nduf-2.2 mRNA.
We asked if the srrt-1 suppressor mutation acts directly through CMTR-1 by affecting its subcellular localization. However srrt-1(G310E) did not alter the wild-type cmtr-1::gfp localization pattern which remained exclusively nuclear (Figure S4D), showing that srrt-1(G310E) does not rescue gas-1(fc21) by causing ectopic localization of CMTR-1 to P-bodies. Additionally, srrt-1(G310E) did not alter the mutant cmtr-1(ΔG-patch)::gfp localization to nuclei and P-body foci, demonstrating that the srrt-1 mutation does not act by disrupting P-body condensates (Figure S4E). Instead, we hypothesize that srrt-1 mutation alters the localization of a specific mRNA transcript such as nduf-2.2, resulting in a fate equivalent to hyperactive RNA methylation by G-patch mutant CMTR-1. For example, SRRT-1 protein may normally traffic nduf-2.2 mRNA to P-bodies, thus repressing its translation.
Conclusions
Post-transcriptional chemical modification of RNA molecules is widespread, molecularly diverse, and confers dramatic impacts on gene expression29,30. CMTR-1 and cap1 methylation of mRNAs have not been implicated in playing a regulatory role in gene expression but rather are thought to be a feature of the 5’ mRNA cap on essentially all eukaryotic mRNAs31. In this study we show that loss of the highly conserved regulatory G-patch domain of CMTR-1 causes ectopic localization of the CMTR-1 protein to P-bodies and activation of the duplicated NDUFS2/gas-1 paralog NDUFS2/nduf-2.2 in many cells of the embryo, which in turn rescues NDUFS2/gas-1(fc21) complex I mutants from the toxicity of high oxygen. These results implicate a highly specific target mRNA for methylation by the CMTR-1 G-patch mutant and are generally consistent with human cell culture experiments that showed transgenic expression of G-patch mutant CMTR-1 caused no transcriptomic changes but rather increased translation of certain mRNAs18.
cmtr-1 is one of five C. elegans members of the ftsJ RNA methyltransferase gene family that is conserved across the Tree of Life. Our genetic analysis generated gain-of function mutations that disrupt the G-patch domain of CMTR-1, one of 14 G-patch domain proteins in C. elegans, many of which bind RNA. CMTR-1 is the only ftsJ homolog among the five with a G-patch domain, which is a universal feature of animal CMTR1 orthologues. The other four C. elegans ftsJ RNA methyltransferase genes are: cmtr-2, an animal-specific ftsJ domain protein that methylates the second transcribed nucleotide of mRNAs32, and three other ftsJ genes (F45G2.9, H06I04.3, and R74.7) that have clear bacterial and archaeal orthologues and methylate their targets at RNA stem-loop structures33,34. Salient to our discovery of CMTR-1 regulation of mitochondrial electron transport gene expression, F45G2.9 is the orthologue of the mitochondrial-localized mammalian MRM2 that methylates the mitochondrial 16S ribosomal RNA. CMTR-1 may also have more RNA substrates in addition to its biochemical assignment as a cap1 methyltransferease, as deletion of cmtr-2 in C. elegans did not affect levels of cap2 methylation35. Our study suggests the cap1 methylation and/or other RNA methylations mediated by CMTR-1 could be a rate-limiting step in the translation of particular target mRNAs rather than a constitutive feature of all mRNAs.
While the CMTR-1 G-patch mutant suppression of NDUFS2/gas-1(fc21) oxygen-sensitivity by expression of an NDUFS2 paralog is a simple single target gene mechanism, the implications for translational regulation of gene expression at P-bodies are profound. P-bodies were originally thought to be sites of RNA decay based on the presence of exonucleases and decapping enzymes, but subsequent evidence has shown that mRNAs in P-bodies are merely silenced and can reenter the cytosol and be translated10. Internal (non-cap) m6A mRNA methylation by YTHDF2 can target mRNAs to P-bodies36, but what modifications control the fate of P-body-localized mRNA remain unknown. We propose that CMTR1/CMTR-1-mediated RNA methylation may be an important regulatory mark that determines whether mRNAs at P-bodies initiate translation. In this study we focus on expression of NDUFS2/nduf-2.2, a subunit of complex I of the mitochondrial ETC. Future studies will characterize the full set of genes activated by mutant CMTR-1 and SRRT-1, and whether they belong to a shared biological process. We note that P-bodies are physically associated with mitochondria37 and that during glucose starvation mitochondrial transcripts are upregulated and stored in P-bodies but not decayed38.
Mitochondrial proteomes vary across developmental and metabolic states3,4, and the cytosolic and mitochondrial translational machinery are coupled in the face of increased demand for respiratory metabolism39, implicating a post-transcriptional regulatory mechanism. Indeed, nuclear mRNA splicing factors (e.g. U1 snRNP) were identified in a genome-wide CRISPR screen for gene disruptions that promote the shift to oxidative phosphorylation40. Our screen identified CMTR-1 and SRRT-1 as two more nuclear mRNA factors that may affect translation of nuclear-encoded mitochondrial proteins, and tantalizingly SRRT interacts with the U1 snRNP, promoting its loading onto most transcripts9. Thus our recessive mutations in SRRT may phenocopy the disruption of U1 snRNP activity. Whether mutations in SRRT also affect cap1 methylation or localization of certain mRNAs to P-bodies is an area of future study.
The NDUFS2/nduf-2.2 paralog requires either a cmtr-1 activating mutation or srrt-1 loss-of-function mutation to be widely expressed, but we observed normal physiological expression of nduf-2.2 in a subset of neurons that includes the dopaminergic neurons. This is consistent with neuronal phenotypes of the nduf-2.2 mutant, which displays axon regeneration defects in the PLM mechanosensory neurons that express a D1-like dopamine receptor. Dopaminergic neurons experience a high burden of oxidative stress41 and lowered complex I activity may underlie the death of dopaminergic neurons in Parkinson’s disease42–44. The amino acid differences between GAS-1 and NDUF-2.2 are highly conserved across the taxonomy of nematodes and platyhelminths. We hypothesize that the duplicated NDUF-2.2 subunit may produce a specialized complex I adapted to the metabolic state of dopaminergic neurons. The G-patch of C. elegans CMTR-1 is required to negatively regulate expression of nduf-2.2 in cells other than dopaminergic neurons. Future work will investigate whether the activities of CMTR-1 or SRRT-1 are altered in specific cells or under particular conditions (for example, oxygen tension or high NADH levels) and may play a physiological role in the expression of nduf-2-2, potentially delineating mechanisms underlying the variation in the mitochondrial proteome across cell lineages or metabolic states.
STAR Methods
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Gary Ruvkun (ruvkun@molbio.mgh.harvard.edu).
Materials availability
C. elegans strains and plasmids generated in this study are available upon request from the lead contact.
Data and code availability
All data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODELS AND STUDY PARTICIPANT DETAILS
Strain maintenance and generation
C. elegans were propagated on NGM plates seeded with E. coli strain OP5045. A complete list of strains used in this study can be found in the Key Resources Table. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). To generate mutants with CRISPR/Cas9, 30 pmol S. pyogenes Cas9 (IDT) was injected into C. elegans gonads along with 90 pmol tracrRNA (IDT), 95 pmol crRNA (IDT), ssODN repair template (when applicable), and 40 ng/μl PRF4::rol-6(su1006) plasmid was used as a marker of successful injections46. To generate transgenic animals carrying extra-chromosomal arrays, a mix consisting of 50 ng/μl plasmid DNA of interest and 50 ng/μl plasmid DNA containing ofm-1::gfp was injected into C. elegans P0 gonads. F1 progeny displaying the co-injection marker were singled to new plates and screened for lines in which the array was inherited by the F2 generation; at least three independent lines were generated for each construct. To generate animals carrying single-copy integrated transgenes, a plasmid mix consisting of 50 ng/μl Mos1 transposase, 2 ng/μl myo-2::mCherry, 2 ng/μl myo-3::mCherry, and 12 ng/μl miniMos transgene was injected into unc-119(ed3) mutant C. elegans47. Injected P0s were immediately placed at 25°C to avoid transgene silencing, and integrated non-unc non-red transgenic animals were confirmed by backcrossing and Mendelian segregation.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Experimental models: Organisms/strains | ||
| C. elegans strain CW152: gas-1(fc21) X +22.9 | CGC | CW152 |
| C. elegans strain GR3408: nduf-7(et19) I | Ruvkun lab16, derived from QC134 (CGC) | GR3408 |
| C. elegans strain GR3414: hsp-6::gfp; gas-1(fc21) | Ruvkun Lab16 | GR3414 |
| C. elegans strain GR3489: cmtr-1(mg780[G126R]); gas-1(fc21) | This study | GR3489 |
| C. elegans strain GR3490: cmtr-1(mg779[G111D]); gas-1(fc21) | This study | GR3490 |
| C. elegans strain GR3491: cmtr-1(mg779[G111D]); hsp-6::gfp; gas-1(fc21) | This study | GR3491 |
| C. elegans strain GR3492: cmtr-1(mg779[G111D]); hsp-6::gfp | This study | GR3492 |
| C. elegans strain GR3493: cmtr-1(mg780[G126R]); hsp-6::gfp | This study | GR3493 |
| C. elegans strain GR3494: cmtr-1(mg780[G126R]); hsp-6::gfp; gas-1(fc21) | This study | GR3494 |
| C. elegans strain GR3495: gas-1(fc21); mgEx878[Prpl-28::cmtr-1 cDNA::GFP + myo-2::mCherry] | This study | GR3495 |
| C. elegans strain GR3496: gas-1(fc21); mgEx879[Prpl-28::cmtr-1 cDNA::GFP + myo-2::mCherry] | This study | GR3496 |
| C. elegans strain GR3497: gas-1(fc21); mgEx880[Prpl-28::cmtr-1(G111D) cDNA::GFP + myo-2::mCherry] | This study | GR3497 |
| C. elegans strain GR3498: gas-1(fc21); mgEx881[Prpl-28::cmtr-1(G111D) cDNA::GFP + myo-2::mCherry] | This study | GR3498 |
| C. elegans strain GR3499: gas-1(fc21); mgEx882[Prpl-28::cmtr-1(G126R) cDNA::GFP + myo-2::mCherry] | This study | GR3499 |
| C. elegans strain GR3500: gas-1(fc21); mgEx883[Prpl-28::cmtr-1(G126R) cDNA::GFP + myo-2::mCherry] | This study | GR3500 |
| C. elegans strain GR3501: gas-1(fc21); mgEx884[Prpl-28::cmtr-1(DeltaG-Patch) cDNA::GFP + myo-2::mCherry] | This study | GR3501 |
| C. elegans strain GR3502: gas-1(fc21); mgEx885[Prpl-28::cmtr-1(DeltaG-Patch) cDNA::GFP + myo-2::mCherry] | This study | GR3502 |
| C. elegans strain GR3503: gas-1(fc21); mgEx886[Prpl-28::cmtr-1(DeltaG-Patch) cDNA::GFP + myo-2::mCherry] | This study | GR3503 |
| C. elegans strain GR3504: gas-1(fc21); mgTi63[Prpl-28::cmtr-1(DeltaG-Patch) cDNA::GFP] | This study | GR3504 |
| C. elegans strain GR3505: gas-1(fc21); mgEx887[Prpl-28::cmtr-1(G111D K244A D379A) cDNA::GFP + myo-2::mCherry] | This study | GR3505 |
| C. elegans strain GR3506: gas-1(fc21); mgEx888[Prpl-28::cmtr-1(G111D K244A D379A) cDNA::GFP + myo-2::mCherry] | This study | GR3506 |
| C. elegans strain GR3507: gas-1(fc21); mgEx889[Prpl-28::cmtr-1(G111D K244A D379A) cDNA::GFP + myo-2::mCherry] | This study | GR3507 |
| C. elegans strain GR3508: cmtr-1(mg780[G126R]) II | This study | GR3508 |
| C. elegans strain GR3509: nduf-7(et19); mgEx882[Prpl-28::cmtr-1(G126R) cDNA::GFP + myo-2::mCherry] | This study | GR3509 |
| C. elegans strain SJ4100: zcIs13[hsp-6::GFP] V | CGC | SJ4100 |
| C. elegans strain GR3513: unc-119(ed3) III; mgTi64[Prpl-28::cmtr-1 cDNA::GFP] | This study | GR3513 |
| C. elegans strain GR3514: unc-119(ed3) III; mgTi63[Prpl-28::cmtr-1(DeltaG-Patch) cDNA::GFP] | This study | GR3514 |
| C. elegans strain GR3515: unc-119(ed3) III; mgTi65[Pcmtr-1::cmtr-1(no G-patch) cDNA::GFP + unc-119(+)]; bpIs37[dcap-1p::dcap-1::DsRed + rol-6(su1006)] | This study | GR3515 |
| C. elegans strain GR3516: gas-1(fc21); mgEx890[Prpl-28::cmtr-1(Delta11–42 G126R) cDNA::GFP + myo-2::mCherry] | This study | GR3516 |
| C. elegans strain GR3519: mgIs86[Pnduf-2.2(3 kb)::gfp + myo-2::mCherry] 8xBC | This study | GR3519 |
| C. elegans strain GR3520: mgEx891[Pnduf-2.2(3 kb)::gfp + myo-2::mCherry] | This study | GR3520 |
| C. elegans strain GR3521: cmtr-1(mg780[G126R]); mgEx891[Pnduf-2.2(3 kb)::gfp + myo-2::mCherry] | This study | GR3521 |
| C. elegans strain VC393: nduf-2.2(ok437) III | CGC | VC393 |
| C. elegans strain GR3522: nduf-2.2(ok437); gas-1(fc21)/tmC24[F23D12.4(tmIs1233) unc-9(tm9718)] | This study | GR3522 |
| C. elegans strain GR3523: nduf-2.2(ok437); nuo-3(mg748[G60D]); gas-1(fc21)/tmC24[F23D12.4(tmIs1233) unc-9(tm9718)] | This study | GR3523 |
| C. elegans strain GR3524: nduf-2.2(ok437) mgTi67[Prpl-28::cmtr-1(G111D) cDNA::GFP]; gas-1(fc21)/tmC24[F23D12.4(tmIs1233) unc-9(tm9718)] | This study | GR3524 |
| C. elegans strain GR3528: srrt-1(mg781[G310E]); gas-1(fc21) | This study | GR3528 |
| C. elegans strain GR3529: srrt-1(mg781[G310E]); mgTi63[Prpl-28::cmtr-1(DeltaG-Patch) cDNA::GFP]; gas-1(fc21) | This study | GR3529 |
| C. elegans strain GR3446: gas-1(fc21); mgEx869[Prpl-28::nuo-3(G60D) cDNA + ofm-1::gfp] | Ruvkun Lab16 | GR3446 |
| C. elegans strain GR3530: gas-1(fc21); mgTi68[Prpl-28::cmtr-1(G111D) cDNA::GFP] | This study | GR3530 |
| C. elegans strain GR3531: gas-1(fc21); mgTi68[Prpl-28::cmtr-1(G111D) cDNA::GFP]; mgEx869[Prpl-28::nuo-3(G60D) cDNA + ofm-1::gfp] | This study | GR3531 |
| C. elegans strain GR3532: srrt-1(mg781[G310E]); mgEx891[Pnduf-2.2(3 kb)::gfp + myo-2::mCherry] | This study | GR3532 |
| C. elegans strain GR3406: lpd-5(mg746[354bp DEL])/hT2 I | Ruvkun Lab16 | GR3406 |
| C. elegans strain GR3510: lpd-5(mg746[354bp DEL])/hT2; mgEx882[Prpl-28::cmtr-1(G126R) cDNA::GFP + myo-2::mCherry] | This study | GR3510 |
| C. elegans strain GR3511: cmtr-1(mg779[G111D]) II | This study | GR3511 |
| C. elegans strain GR3512: nduf-7(et19); cmtr-1(mg779[G111D]) | This study | GR3512 |
| C. elegans strain GR3517: unc-119(ed3) III; mgTi66[Pcmtr-1::cmtr-1 cDNA::GFP + unc-119(+)] | This study | GR3517 |
| C. elegans strain GR3518: unc-119(ed3) III; mgTi65[Pcmtr-1::cmtr-1(no G-patch) cDNA::GFP + unc-119(+)] | This study | GR3518 |
| C. elegans strain GR3525: otIs181[Pdat-1::mCherry; Pttx-3::mCherry] III; mgIs86[Pnduf-2.2(3 kb)::gfp + myo-2::mCherry] | This study | GR3525 |
| C. elegans strain GR3526: gas-1(fc21); kynu-1(tm4924) | This study | GR3526 |
| C. elegans strain GR3527: gas-1(fc21); kynu-1(tm4924); mgEx882[Prpl-28::cmtr-1(G126R) cDNA::GFP + myo-2::mCherry] | This study | GR3527 |
| C. elegans strain GR3533: srrt-1(mg781[G310E]); unc-119(ed3); mgTi66[Pcmtr-1::cmtr-1 cDNA::GFP + unc-119(+)] | This study | GR3533 |
| C. elegans strain GR3534: srrt-1(mg781[G310E]); unc-119(ed3); mgTi65[Pcmtr-1::cmtr-1(no G-patch) cDNA::GFP + unc-119(+)] | This study | GR3534 |
| C. elegans strain N2: wild type | CGC | N2 |
| C. elegans strain GR3584: him-5(e1490) oxTi405 V; gas-1(fc21) X | This study | GR3584 |
| C. elegans strain GR3585: mgEx882[Prpl-28::cmtr-1(G126R) cDNA::GFP + myo-2::mCherry] | This study | GR3585 |
| C. elegans strain GR3586: mgEx883[Prpl-28::cmtr-1(G126R) cDNA::GFP + myo-2::mCherry] | This study | GR3586 |
| C. elegans strain GR3587: mgEx890[Prpl-28::cmtr-1(Delta11–42 G126R) cDNA::GFP + myo-2::mCherry] | This study | GR3587 |
| C. elegans strain GR3588: mgEx899[Prpl-28::cmtr-1(Delta11–42 G126R) cDNA::GFP + myo-2::mCherry] | This study | GR3588 |
| C. elegans strain GR3589: mgEx900[Prpl-28::cmtr-1(Delta11–42 G126R) cDNA::GFP + myo-2::mCherry] | This study | GR3589 |
| C. elegans strain GR3590: gas-1(fc21); mgEx901[Prpl-28::cmtr-1(Delta11–42 G126R) cDNA::GFP + myo-2::mCherry] | This study | GR3590 |
| C. elegans strain GR3591: gas-1(fc21); mgEx902[Prpl-28::cmtr-1(Delta11–42 G126R) cDNA::GFP + myo-2::mCherry] | This study | GR3591 |
| C. elegans strain GR3592: gas-1(fc21); mgTi65[Pcmtr-1::cmtr-1(DeltaG-patch) cDNA::GFP + unc-119(+)] | This study | GR3592 |
| C. elegans strain GR3593: gas-1(fc21); mgTi66[Pcmtr-1::cmtr-1 cDNA::GFP + unc-119(+)] | This study | GR3593 |
| C. elegans strain GR3594: gas-1(fc21); mgEx891[Pnduf-2.2(3 kb)::gfp + myo-2::mCherry] | This study | GR3594 |
| C. elegans strain GR3595: srrt-1(mg781[G310E]) I | This study | GR3595 |
| C. elegans strain GR3596: srrt-1(mg781[G310E]); gas-1(fc21); mgEx903[Prpl-28::srrt-1 cDNA 50 ng/μl] | This study | GR3596 |
| C. elegans strain GR3597: srrt-1(mg781[G310E]); gas-1(fc21); mgEx904[Prpl-28::srrt-1 cDNA 50 ng/μl] | This study | GR3597 |
| C. elegans strain GR3598: srrt-1(mg781[G310E]); gas-1(fc21); mgEx905[Prpl-28::srrt-1 cDNA 50 ng/μl] | This study | GR3598 |
| C. elegans strain GR3599: mgEx906[Prpl-28::srrt-1 cDNA 50 ng/μl] | This study | GR3599 |
| C. elegans strain GR3600: mgEx907[Prpl-28::srrt-1 cDNA 50 ng/μl] | This study | GR3600 |
| C. elegans strain GR3601: mgEx908[Prpl-28::srrt-1 cDNA 50 ng/μl] | This study | GR3601 |
| C. elegans strain GR3602: mgEx909[Prpl-28::srrt-1 cDNA 50 ng/μl] | This study | GR3602 |
| Oligonucleotides | ||
| rps-23 qPCR Forward: aaggctcacattggaactcg | This study | N/A |
| rps-23 qPCR Reverse: aggctgcttagcttcgacac | This study | N/A |
| nduf-2.2 qPCR Forward: TGAAGTTTCCCGCTCGTATC | This study | N/A |
| nduf-2.2 qPCR Reverse: TTCCACTTCCACGAACCATC | This study | N/A |
METHOD DETAILS
Genetic screens and sequence analysis
To screen for genetic suppressors of gas-1(fc21) in hyperoxia thousands of L4 animals were exposed to 47 mM EMS (ethyl methanesulfonate) (Sigma M0880) for four hours while rocking. Animals were then washed twice with M9 buffer and allowed to recover on standard NGM plates. F1 animals were bleach prepped as described above to generate a synchronized L1 stage population of mutagenized F2 animals, which were then dropped onto standard NGM plates at 50% oxygen. Plates were checked daily and F2 individuals capable of growing to adulthood were transferred onto new plates. Fertile isolates were retested using F3 or F4 progeny to confirm their phenotype and then genomic DNA for whole genome sequencing was isolated using Gentra Puregene Tissue Kit (Qiagen 158667).
To identify candidate suppressor mutations in screen isolates we sheared genomic DNA using a Covaris S2 sonicator and prepared libraries using the NEBNext DNA library prep kit for Illumina. Libraries with unique barcodes were quantified using the Qubit dsDNA HS Assay Kit (Life Technologies Q32851) and pooled in sets of 24 and sequenced using Illumina HiSeq48. Raw FASTQ files were analyzed on the Galaxy platform (usegalaxy.org) with the following workflow: TrimGalore! to trim reads, Map with BWA to align reads to the C. elegans reference genome, MiModD to call variants, and SnpEff to identify mutations that may affect protein function. Lists of protein-altering mutations from each suppressor strain were then compared to identify genes with multiple mutant alleles. These candidate genes were then verified using targeted CRISPR/Cas9-based editing.
For protein sequence comparisons, homologs were identified using BlastP and, in the case of NDUFS2, from https://caenorhabditis.org/49 and alignments made with ClustalW. For annotation of C. elegans CMTR-1 and human CMTR1 sequence features, predicted nuclear localization signals were identified using cNLS mapper50 and intrinsically disordered domains were identified using both DEPICTER51 and PrDOS52.
Growth and fluorescent reporter assays
To measure C. elegans growth and development crowded plates of gravid animals were washed into tubes in M9 buffer [3 g KH2PO4, 6 g Na2HPO4, 5 g NaCl, 1 ml 1 M MgSO4, H2O to 1 liter] and incubated with 20% bleach and 10% 5M KOH for 5 minutes while vortexing. The resulting embryos were washed 3x in M9 buffer and allowed to hatch overnight while rocking in M9. The following day arrested L1 animals were dropped onto E. coli OP50 plates and incubated at 20°C. For assays in hyperoxia (50% or 100% oxygen) plates were sealed in a modular chamber (Stemcell Technologies #27310) and flushed for 3 minutes with either a 50:50 mixture of oxygen and nitrogen, or with pure oxygen gas. For assays in hypoxia (1% oxygen), animals were incubated in a Hypoxic in vitro cabinet (Coy Laboratory Products, Inc) at room temperature. To measure animal length, images were acquired using a ZEISS Axio Zoom V16 microscope with ZEN PRO software and the midline of individual animals was quantified in FIJI software. Brood size measurements were made by transferring adult C. elegans to new plates daily and counting all progeny generated over the course of egg-laying adulthood.
To measure hsp-6::gfp and nduf-2.2::gfp fluorescence, animals were mounted on agar pads, immobilized in sodium azide, and imaged at 70x magnification using a ZEISS Axio Zoom V16 microscope with ZEN PRO software. Fluorescent images were quantified by calculating the mean fluorescence along the midline of the intestine (for hsp-6::gfp) or by calculating the maximum fluorescence in embryos (for nduf-2.2::gfp) using FIJI software. To visualize cmtr-1::gfp localization patterns, images were acquired with a Nikon A1R confocal microscope, using a 60X/1.49 NA oil objective using 488nm excitation at 290nm/pixel. Worms were immobilized on 10% agarose pads with 0.3 μl of 0.1 μm diameter polystyrene microspheres (Polysciences 00876-15, 2.5% w/v suspension). To quantify colocalization of cmtr-1(ΔG-patch)::gfp and dcap-1::DsRed, green or red foci from three independent animals were blindly selected and the presence or absence of foci in the other channel was scored.
Quantitative PCR
To measure nduf-2.2 mRNA levels by qPCR, total RNA was isolated from mixed stage animals using TRIzol Reagent (ThermoFisher 15596026). RNA was DNase treated using DNA-free Kit (Invitrogen AM1906) and cDNA was synthesized by the ProtoScript II First Strand cDNA Synthesis Kit (NEB E6560). Quantitative real-time PCR was performed using iQ SYBR Green Supermix (Biorad) on a BIORAD CFX Real-Time System. Three biological replicates were analyzed for each genotype, and three technical replicates per sample were performed in each qPCR run. Negative controls included (1) cDNA samples synthesized without reverse transcriptase and (2) cDNA synthesized from nduf-2.2 deletion mutants, confirming that the nduf-2.2 primers were specific. Delta Cq values were normalized to the housekeeping gene rps-23. All primer sequences are available in the Key Resources Table.
QUANTIFICATION AND STATISTICAL ANALYSYS
All statistical analyses were performed using GraphPad Prism software. Statistical tests are detailed in the Figure Legends. Typically, statistical significance was calculated using one-way ANOVA followed by correction for multiple hypothesis testing. Error bars represent standard deviation and ‘n’ refers to the number of animals tested in a single experiment. n.s. = not significant, * = p value <0.05, ** = p value <0.01, *** = p value <0.001.
Supplementary Material
Highlights.
cmtr-1 G-patch mutations activate broad expression of a neuronal complex I subunit
NDUFS2/nduf-2.2 is normally restricted to the dopaminergic neurons in C. elegans
G-patch loss causes ectopic localization of CMTR-1 protein to Processing bodies
Mutations in the mRNA binding protein Serrate phenocopy CMTR-1 G-patch mutations
ACKNOWLEDGEMENTS
We thank Peter Breen and Stephen Nurrish for technical assistance. We thank Philip Morgan, Margaret Sedensky, Luisa Cochella, Geraldine Seydoux, and all members of the Ruvkun and Mootha labs for helpful discussions. This work was supported in part by the National Institutes of Health (K99GM140217 to J.D.M., R01NS124679 to V.K.M., R01AG016636 to G.R.). J.D.M. was supported by The Jane Coffin Childs Memorial Fund for Medical Research. V.K.M. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
V.K.M. is listed as an inventor on patents filed by MGH on therapeutic uses of hypoxia.
V.K.M. is a paid advisor to 5am Ventures.
REFERENCES
- 1.Rath S, Sharma R, Gupta R, Ast T, Chan C, Durham TJ, Goodman RP, Grabarek Z, Haas ME, Hung WHW, et al. (2020). MitoCarta3.0: an updated mitochondrial proteome now with sub-organelle localization and pathway annotations. Nucleic Acids Res 49, D1541–D1547. 10.1093/nar/gkaa1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong S-E, Walford GA, Sugiana C, Boneh A, Chen WK, et al. (2008). A Mitochondrial Protein Compendium Elucidates Complex I Disease Biology. Cell 134, 112–123. 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grimsrud PA, Carson JJ, Hebert AS, Hubler SL, Niemi NM, Bailey DJ, Jochem A, Stapleton DS, Keller MP, Westphall MS, et al. (2012). A Quantitative Map of the Liver Mitochondrial Phosphoproteome Reveals Posttranslational Control of Ketogenesis. Cell Metab 16, 672–683. 10.1016/j.cmet.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuhn KM, DeRisi JL, Brown PO, and Sarnow P (2001). Global and Specific Translational Regulation in the Genomic Response of Saccharomyces cerevisiae to a Rapid Transfer from a Fermentable to a Nonfermentable Carbon Source. Mol Cell Biol 21, 916–927. 10.1128/mcb.21.3.916-927.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Picard-Jean F, Brand C, Tremblay-Létourneau M, Allaire A, Beaudoin MC, Boudreault S, Duval C, Rainville-Sirois J, Robert F, Pelletier J, et al. (2018). 2’-O-methylation of the mRNA cap protects RNAs from decapping and degradation by DXO. PLoS ONE 13, e0193804. 10.1371/journal.pone.0193804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muthukrishnan S, Morgan M, Banerjee AK, and Shatkin AJ (1976). Influence of 5’-terminal m7G and 2’--O-methylated residues on messenger ribonucleic acid binding to ribosomes. Biochemistry 15, 5761–5768. 10.1021/bi00671a012. [DOI] [PubMed] [Google Scholar]
- 7.Haline-Vaz T, Silva TCL, and Zanchin NIT (2008). The human interferon-regulated ISG95 protein interacts with RNA polymerase II and shows methyltransferase activity. Biochem. Biophys. Res. Commun 372, 719–724. 10.1016/j.bbrc.2008.05.137. [DOI] [PubMed] [Google Scholar]
- 8.Bélanger F, Stepinski J, Darzynkiewicz E, and Pelletier J (2010). Characterization of hMTr1, a human Cap1 2’-O-ribose methyltransferase. Journal of Biological Chemistry 285, 33037–33044. 10.1074/jbc.m110.155283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lykke-Andersen S, Rouvière JO, and Jensen TH (2021). ARS2/SRRT: at the nexus of RNA polymerase II transcription, transcript maturation and quality control. Biochem Soc T 49, 1325–1336. 10.1042/bst20201008. [DOI] [PubMed] [Google Scholar]
- 10.Ivanov P, Kedersha N, and Anderson P (2018). Stress Granules and Processing Bodies in Translational Control. Csh Perspect Biol 11, a032813. 10.1101/cshperspect.a032813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu J, Vinothkumar KR, and Hirst J (2016). Structure of mammalian respiratory complex I. Nature 536, 354–358. 10.1038/nature19095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiedorczuk K, Letts JA, Degliesposti G, Kaszuba K, Skehel M, and Sazanov LA (2016). Atomic structure of the entire mammalian mitochondrial complex I. Nature 538, 406–410. 10.1038/nature19794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zickermann V, Wirth C, Nasiri H, Siegmund K, Schwalbe H, Hunte C, and Brandt U (2015). Mechanistic insight from the crystal structure of mitochondrial complex I. Science 347, 44–49. 10.1126/science.1259859. [DOI] [PubMed] [Google Scholar]
- 14.Kayser EB, Morgan PG, and Sedensky MM (1999). GAS-1: a mitochondrial protein controls sensitivity to volatile anesthetics in the nematode Caenorhabditis elegans. Anesthesiology 90, 545–554. 10.1097/00000542-199902000-00031. [DOI] [PubMed] [Google Scholar]
- 15.Kayser EB, Morgan PG, Hoppel CL, and Sedensky MM (2001). Mitochondrial expression and function of GAS-1 in Caenorhabditis elegans. J. Biol. Chem 276, 20551–20558. 10.1074/jbc.m011066200. [DOI] [PubMed] [Google Scholar]
- 16.Meisel JD, Miranda M, Skinner OS, Wiesenthal PP, Wellner SM, Jourdain AA, Ruvkun G, and Mootha VK (2024). Hypoxia and intra-complex genetic suppressors rescue complex I mutants by a shared mechanism. Cell. 10.1016/j.cell.2023.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aravind L, and Koonin EV (1999). G-patch: a new conserved domain in eukaryotic RNA-processing proteins and type D retroviral polyproteins. Trends Biochem Sci 24, 342–344. 10.1016/s0968-0004(99)01437-1. [DOI] [PubMed] [Google Scholar]
- 18.Inesta-Vaquera F, Chaugule VK, Galloway A, Chandler L, Rojas-Fernandez A, Weidlich S, Peggie M, and Cowling VH (2018). DHX15 regulates CMTR1-dependent gene expression and cell proliferation. Life Sci Alliance 1, e201800092. 10.26508/lsa.201800092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toczydlowska-Socha D, Zielinska MM, Kurkowska M, Astha, Almeida CF, Stefaniak F, Purta E, and Bujnicki JM (2018). Human RNA cap1 methyltransferase CMTr1 cooperates with RNA helicase DHX15 to modify RNAs with highly structured 5’ termini. Philosophical Transactions of the Royal Society B: Biological Sciences 373, 20180161. 10.1098/rstb.2018.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feder M, Pas J, Wyrwicz LS, and Bujnicki JM (2003). Molecular phylogenetics of the RrmJ/fibrillarin superfamily of ribose 2′-O-methyltransferases. Gene 302, 129–138. 10.1016/s0378-1119(02)01097-1. [DOI] [PubMed] [Google Scholar]
- 21.Sun Y, Yang P, Zhang Y, Bao X, Li J, Hou W, Yao X, Han J, and Zhang H (2011). A genome-wide RNAi screen identifies genes regulating the formation of P bodies in C. elegans and their functions in NMD and RNAi. Protein Cell 2, 918–939. 10.1007/s13238-011-1119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otero L, Martínez-Rosales C, Barrera E, Pantano S, and Salinas G (2019). Complex I and II Subunit Gene Duplications Provide Increased Fitness to Worms. Frontiers Genetics 10, 1043. 10.3389/fgene.2019.01043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kayser E-B, Sedensky MM, and Morgan PG (2004). The effects of complex I function and oxidative damage on lifespan and anesthetic sensitivity in Caenorhabditis elegans. Mechanisms of Ageing and Development 125, 455–464. 10.1016/j.mad.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Knowlton WM, Hubert T, Wu Z, Chisholm AD, and Jin Y (2017). A Select Subset of Electron Transport Chain Genes Associated with Optic Atrophy Link Mitochondria to Axon Regeneration in Caenorhabditis elegans. Front Neurosci-switz 11, 263. 10.3389/fnins.2017.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen L, Wang Z, Ghosh-Roy A, Hubert T, Yan D, O’Rourke S, Bowerman B, Wu Z, Jin Y, and Chisholm AD (2011). Axon Regeneration Pathways Identified by Systematic Genetic Screening in C. elegans. Neuron 71, 1043–1057. 10.1016/j.neuron.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borrello SD, Lautens M, Dolan K, Tan JH, Davie T, Schertzberg MR, Spensley MA, Caudy AA, and Fraser AG (2019). Rhodoquinone biosynthesis in C. elegans requires precursors generated by the kynurenine pathway. Elife 8, e48165. 10.7554/elife.48165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulze WM, Stein F, Rettel M, Nanao M, and Cusack S (2018). Structural analysis of human ARS2 as a platform for co-transcriptional RNA sorting. Nat Commun 9, 1701. 10.1038/s41467-018-04142-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Packer JS, Zhu Q, Huynh C, Sivaramakrishnan P, Preston E, Dueck H, Stefanik D, Tan K, Trapnell C, Kim J, et al. (2019). A lineage-resolved molecular atlas of C. elegans embryogenesis at single-cell resolution. Science 365. 10.1126/science.aax1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roundtree IA, Evans ME, Pan T, and He C (2017). Dynamic RNA Modifications in Gene Expression Regulation. Cell 169, 1187–1200. 10.1016/j.cell.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frye M, Harada BT, Behm M, and He C (2018). RNA modifications modulate gene expression during development. Science 361, 1346–1349. 10.1126/science.aau1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akichika S, Hirano S, Shichino Y, Suzuki T, Nishimasu H, Ishitani R, Sugita A, Hirose Y, Iwasaki S, Nureki O, et al. (2019). Cap-specific terminal N6-methylation of RNA by an RNA polymerase II-associated methyltransferase. Science 363, eaav0080. 10.1126/science.aav0080. [DOI] [PubMed] [Google Scholar]
- 32.Werner M, Purta E, Kaminska KH, Cymerman IA, Campbell DA, Mittra B, Zamudio JR, Sturm NR, Jaworski J, and Bujnicki JM (2011). 2′-O-ribose methylation of cap2 in human: function and evolution in a horizontally mobile family. Nucleic Acids Res 39, 4756–4768. 10.1093/nar/gkr038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caldas T, Binet E, Bouloc P, Costa A, Desgres J, and Richarme G (2000). The FtsJ/RrmJ heat shock protein of Escherichia coli is a 23 S ribosomal RNA methyltransferase. J Biological Chem 275, 16414–16419. 10.1074/jbc.m001854200. [DOI] [PubMed] [Google Scholar]
- 34.Pintard L, Lecointe F, Bujnicki JM, Bonnerot C, Grosjean H, and Lapeyre B (2002). Trm7p catalyses the formation of two 2′-O-methylriboses in yeast tRNA anticodon loop. Embo J 21, 1811–1820. 10.1093/emboj/21.7.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dix T, Haussmann I, Brivio S, Nallasivan M, Hadzhiev Y, Muller F, Pettitt J, Müller B, and Soller M (2022). CMTr mediated 2`-O-ribose methylation status of cap adjacent-nucleotides across animals. RNA 28, rna.079317.122. 10.1261/rna.079317.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, Fu Y, Parisien M, Dai Q, Jia G, et al. (2014). N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 505, 117–120. 10.1038/nature12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang L, Mollet S, Souquere S, Roy FL, Ernoult-Lange M, Pierron G, Dautry F, and Weil D (2011). Mitochondria Associate with P-bodies and Modulate MicroRNA-mediated RNA Interference*. J Biol Chem 286, 24219–24230. 10.1074/jbc.m111.240259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang C, Schmich F, Srivatsa S, Weidner J, Beerenwinkel N, and Spang A (2018). Context-dependent deposition and regulation of mRNAs in P-bodies. Elife 7, e29815. 10.7554/elife.29815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Couvillion MT, Soto IC, Shipkovenska G, and Churchman LS (2016). Synchronized mitochondrial and cytosolic translation programs. Nature 533, 499–503. 10.1038/nature18015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jourdain AA, Begg BE, Mick E, Shah H, Calvo SE, Skinner OS, Sharma R, Blue SM, Yeo GW, Burge CB, et al. (2021). Loss of LUC7L2 and U1 snRNP subunits shifts energy metabolism from glycolysis to OXPHOS. Mol Cell 81, 1905–1919.e12. 10.1016/j.molcel.2021.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berman SB, and Hastings TG (1999). Dopamine Oxidation Alters Mitochondrial Respiration and Induces Permeability Transition in Brain Mitochondria. J Neurochem 73, 1127–1137. 10.1046/j.1471-4159.1999.0731127.x. [DOI] [PubMed] [Google Scholar]
- 42.Schapira AHV, Cooper JM, Dexter D, Jenner P, Clark JB, and Marsden CD (1989). MITOCHONDRIAL COMPLEX I DEFICIENCY IN PARKINSON’S DISEASE. Lancet 333, 1269. 10.1016/s0140-6736(89)92366-0. [DOI] [PubMed] [Google Scholar]
- 43.Nicklas WilliamdotJ., Vyas I, and Heikkila RE (1985). Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sci 36, 2503–2508. 10.1016/0024-3205(85)90146-8. [DOI] [PubMed] [Google Scholar]
- 44.González-Rodríguez P, Zampese E, Stout KA, Guzman JN, Ilijic E, Yang B, Tkatch T, Stavarache MA, Wokosin DL, Gao L, et al. (2021). Disruption of mitochondrial complex I induces progressive parkinsonism. Nature 599, 650–656. 10.1038/s41586-021-04059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brenner S (1974). The genetics of Caenorhabditis elegans. Genetics 77, 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghanta KS, and Mello CC (2020). Melting dsDNA Donor Molecules Greatly Improves Precision Genome Editing in Caenorhabditis elegans. Genetics 216, 643–650. 10.1534/genetics.120.303564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frøkjær-Jensen C, Davis MW, Sarov M, Taylor J, Flibotte S, LaBella M, Pozniakovsky A, Moerman DG, and Jorgensen EM (2014). Random and targeted transgene insertion in Caenorhabditis elegans using a modified Mos1 transposon. Nat. Methods 11, 529–534. 10.1038/nmeth.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lehrbach NJ, Ji F, and Sadreyev R (2017). Next-Generation Sequencing for Identification of EMS-Induced Mutations in Caenorhabditis elegans. Curr Protoc Mol Biology 117, 7.29.1–7.29.12. 10.1002/cpmb.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stevens L, Rooke S, Falzon LC, Machuka EM, Momanyi K, Murungi MK, Njoroge SM, Odinga CO, Ogendo A, Ogola J, et al. (2020). The Genome of Caenorhabditis bovis. Curr Biol 30, 1023–1031.e4. 10.1016/j.cub.2020.01.074. [DOI] [PubMed] [Google Scholar]
- 50.Kosugi S, Hasebe M, Tomita M, and Yanagawa H (2009). Systematic identification of cell cycle-dependent yeast nucleocytoplasmic shuttling proteins by prediction of composite motifs. Proc National Acad Sci 106, 10171–10176. 10.1073/pnas.0900604106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barik A, Katuwawala A, Hanson J, Paliwal K, Zhou Y, and Kurgan L (2020). DEPICTER: Intrinsic Disorder and Disorder Function Prediction Server. J Mol Biol 432, 3379–3387. 10.1016/j.jmb.2019.12.030. [DOI] [PubMed] [Google Scholar]
- 52.Ishida T, and Kinoshita K (2007). PrDOS: prediction of disordered protein regions from amino acid sequence. Nucleic Acids Res 35, W460–W464. 10.1093/nar/gkm363. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.


