ABSTRACT
Highly pathogenic viruses from family Phenuiviridae, which are mainly transmitted by arthropods, have intermittently sparked epidemics worldwide. In particular, tick-borne bandaviruses, such as severe fever with thrombocytopenia syndrome virus (SFTSV), continue to spread in mountainous areas, resulting in an average mortality rate as high as 10.5%, highlighting the urgency and importance of vaccine development. Here, an mRNA vaccine developed based on the full-length SFTSV glycoprotein, containing both the receptor-binding domain and the fusion domain, was shown to confer complete protection against SFTSV at a very low dose by triggering a type 1 helper T cell-biased cellular immune response in rodents. Moreover, the vaccine candidate elicited long-term immunity and protection against SFTSV for at least 5 months. Notably, it provided complete cross-protection against other bandaviruses, such as the Heartland virus and Guertu virus, in lethal challenge models. Further research revealed that the conserved epitopes among bandaviruses within the full-length SFTSV glycoprotein may facilitate broad-spectrum protection mediated by the cellular immune response. Collectively, these findings demonstrate that the full-length SFTSV glycoprotein mRNA vaccine is a promising vaccine candidate for SFTSV and other bandaviruses, and provide guidance for the development of broad-spectrum vaccines from conserved antigens and epitopes.
IMPORTANCE
Tick-borne bandaviruses, such as SFTSV and Heartland virus, sporadically trigger outbreaks in addition to influenza viruses and coronaviruses, yet there are no specific vaccines or therapeutics against them. mRNA vaccine technology has advantages in terms of enabling in situ expression and triggering cellular immunity, thus offering new solutions for vaccine development against intractable viruses, such as bandaviruses. In this study, we developed a novel vaccine candidate for SFTSV by employing mRNA vaccination technology and using a full-length glycoprotein as an antigen target. This candidate vaccine confers complete and durable protection against SFTSV at a notably low dose while also providing cross-protection against Heartland virus and Guertu virus. This study highlights the prospective value of full-length SFTSV-glycoprotein-based mRNA vaccines and suggests a potential strategy for broad-spectrum bandavirus vaccines.
KEYWORDS: severe fever with thrombocytopenia syndrome virus, glycoprotein, cellular immunology, broad-spectrum protection, conserved epitope
INTRODUCTION
Ticks, mosquitoes, and sandflies are ubiquitous vectors that facilitate the global spread of viruses and occasionally transmit arboviruses from domesticated animals and poultry to humans (1–3). These arboviruses, including flaviviruses, togaviruses, and bunyaviruses, cause various serious infectious diseases and even death (4, 5). Among them, the tick-borne severe fever with thrombocytopenia syndrome virus (SFTSV) and Heartland virus (HRTV), belonging to the genus Bandavirus, family Phenuiviridae, and order Bunyavirales, cause similar severe clinical manifestations, including fever, thrombocytopenia, leukopenia, multisystem organ failure, and even death (6, 7). Specifically, SFTSV has an average case-fatality rate of 10.5%, and HRTV has a mortality rate ranging from 13% to 30% (8–11). In addition, an increasing number of tick-borne bandaviruses, such as Guertu virus (GTV), which was discovered in Xinjiang, China, have been found to be potentially pathogenic to humans (12). Viruses other than bandaviruses in the family Phenuiviridae, including the tick-borne Khasan virus (KHAV) (13) and Razdan virus (RAZV) (14), the mosquito-transmitted Rift Valley fever virus (RVFV) (15), and the sandfly-transmitted sandfly fever Sicilian virus (SFSV) (16), also cause sporadic deaths worldwide. Together, viruses constitute a global public health threat, in addition to respiratory viruses, such as influenza viruses and coronaviruses, highlighting the urgent need to develop specific prevention and treatment strategies (4).
Classical bandaviruses are enveloped and segmented, negative-stranded RNA viruses. Their genomes consist of small (S), medium (M), and large (L) segments encoding the nucleoprotein (NP), non-structural protein (NS), envelope glycoprotein (GP), and large polymerase protein (LP), respectively (17). Using SFTSV as an example to illustrate the life cycle of bandaviruses, the M segment expresses a glycoprotein precursor within the endoplasmic reticulum, which is then cleaved into the Gn and Gc subunits that subsequently form heterodimers and are immediately incorporated into virions as pentons and hexons within the Golgi apparatus (18). Next, mature virions are transported to the plasma membrane via vesicles, culminating in the release and completion of the viral replication cycle (19). When SFTSV enters a host cell, Gn initiates an infection by interacting with dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin and C-C motif chemokine receptor 2 to implement binding (20–22), which is accompanied by membrane fusion induced by Gc within acidic endosomes (23). Given the essential roles of Gn and Gc in the viral life cycle, GPs containing both Gn and Gc are regarded as attractive targets for therapeutic antibodies and vaccines (24–27).
Because of the complex conformation of the SFTSV GP, which is difficult to reproduce in vitro, previously reported vaccines based on recombinant Gn or Gc proteins have not provided satisfactory protective effects (28). However, mRNA technology overcomes these intricacies by allowing the direct expression of antigens in situ, eliminating much of the uncertainty associated with traditional vaccine preparation (29). The efficacy and safety of mRNA vaccines have been substantiated through extensive real-world evidence, as exemplified during the coronavirus disease 2019 pandemic (30, 31), thereby affirming their potential for application against a broader range of pathogens. In this study, an mRNA vaccine was constructed based on the full-length SFTSV GP, and its immunogenicity and efficacy were investigated. The vaccine provided complete protection against SFTSV and broad-spectrum protection against other bandaviruses. The cellular immune response, which may be partially engaged by the conserved epitopes, was deemed to be the main mechanism. This study principally focused on the efficacy of a full-length GP mRNA vaccine candidate and its associated mechanisms to propose a viable approach for developing broad-spectrum vaccines.
RESULTS
The GP mRNA vaccine elicited humoral and type 1 helper T cell-biased cellular immune responses in mice
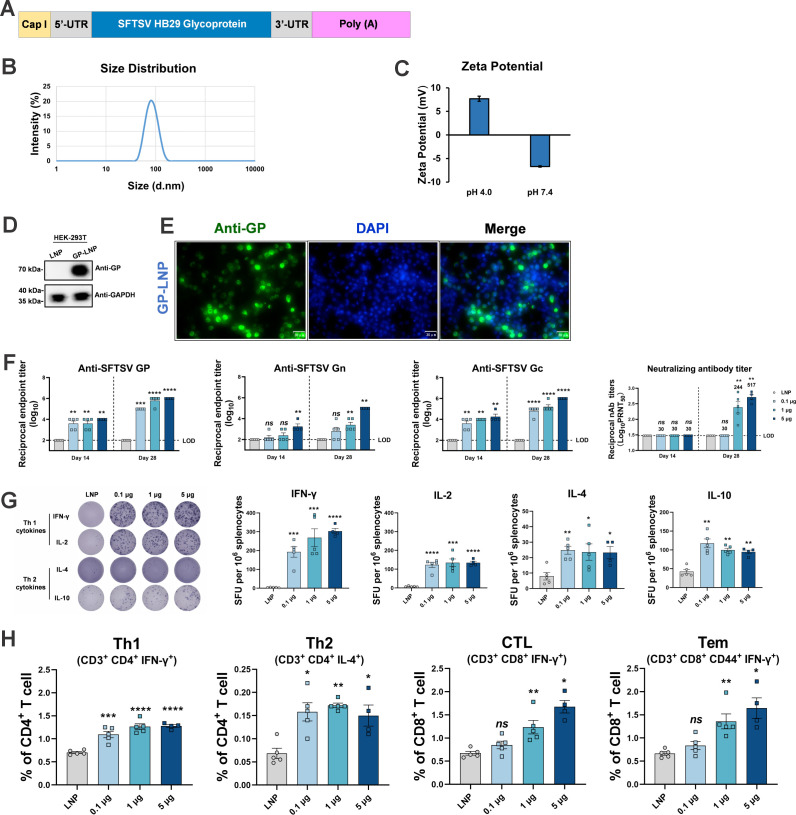
For vaccine preparation, a template containing codon-optimized full-length SFTSV GP was de novo synthesized and then in vitro transcribed into mRNA, with uridines substituted by N1-methyl-pseudouridines (Fig. 1A). Microchip capillary electrophoresis showed that the GP mRNA had a length of 3,490 nt, which is consistent with the theoretical size of the transcript, and a purity of 98.3% (Fig. S1). Subsequently, the mRNA was encapsulated within lipid nanoparticles (LNPs), which were formulated with a specific ratio of ionizable cationic lipids, phosphatidylcholine, cholesterol, and polyethylene glycol lipids, to produce GP-LNPs. Dynamic light scattering showed that the GP-LNPs had a particle size of approximately 80 nm (Fig. 1B), and the zeta potentials at pH 4.0 and 7.4 were 7.69 and −6.70, respectively (Fig. 1C), suggesting its appropriate uniformity and stability. Successful expression in mammalian cells was confirmed in HEK-293T cells by Western blotting and immunofluorescence (Fig. 1D and E; Fig. S2).
Fig 1.
Immunogenicity evaluation of the GP mRNA vaccine in BALB/c mice. (A) Schematic diagram of the full-length SFTSV GP mRNA vaccine. (B and C) Size distribution and zeta potential of the mRNA-LNPs. (D and E) The expression of SFTSV GP mRNA in HEK-293T cells was detected by Western blotting with glyceraldehyde-phosphate dehydrogenase used as the control (also shown in Fig. S2), and visualized in the cytoplasm using immunofluorescence with nuclei stained with 4′,6-diamidino-2-phenylindole. (F–H) Female BALB/c mice were allocated into the following four groups with five per group: empty LNPs, 0.1, 1.0, or 5.0 μg of GP-LNPs. Vaccinations followed a standard prime-boost regimen with a 3-week interval. The levels of binding antibodies against SFTSV GP, Gn, and Gc were determined by enzyme-linked immunosorbent assay with a starting dilution of 1:100 (LOD). Neutralizing antibodies were ascertained using a plaque reduction neutralization test, with geometric mean titers indicated above the corresponding bars (LOD of 1:30) (F). The cellular immune response was detected by either ELISpot or flow cytometry. Representative images (left panel) and spots of IFN-γ, IL-2, IL-4, and IL-10 (right panel) are shown (G), and the proportions of Th1 cells, Th2 cells, CTLs, and Tem cells were analyzed via ICS assay (H). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. CTL, cytotoxic T cell; DAPI, 4′,6-diamidino-2-phenylindole; ELISpot, enzyme-linked immunospot assay; ICS, intracellular cytokine staining assay; GP, glycoprotein; IFN-γ, interferon gamma; IL, interleukin; LNP, lipid nanoparticle; LOD, limit of detection; ns, not significant; SFTSV, severe fever with thrombocytopenia syndrome virus; Tem, effector memory T; Th1, type 1 helper T; Th2, type 2 helper T; UTR, untranslated region.
To investigate the immunogenicity of the GP mRNA vaccine, immunocompetent BALB/c mice were intramuscularly (i.m.) vaccinated with various doses of GP-LNPs ranging from 0.1 to 5.0 µg. After receiving a two-shot vaccination regimen with a 3-week interval and biweekly sampling, the mice were sacrificed to analyze both the humoral and cellular immune responses 10 days after the booster vaccination. As expected, the GP-LNPs elicited high specific binding titers against SFTSV GP, Gn, and Gc, which peaked at ~1:106 after booster vaccination with 5 µg of GP-LNPs, in a dose-dependent manner. Additionally, the neutralizing antibody titers exhibited the same trend as the binding antibody titers, reaching a reciprocal geometric mean titer (GMT) of 1:517 after booster vaccination with 5 µg of GP-LNPs, despite the absence of significant neutralizing antibodies after priming vaccination (Fig. 1F).
The cellular immune response assessed by enzyme-linked immunospot assay (ELISpot) showed robust type 1 helper T (Th1) cytokines [interferon gamma (IFN-γ) and interleukin (IL)-2] production by the SFTSV GP peptide pool stimulation even at a vaccination dose as low as 0.1 µg, compared with the relatively weak Th2-type cytokine (IL-4 and IL-10) production (Fig. 1G). Consistent with these results, the proportions of Th1 cells (CD3+, CD4+, and IFN-γ+), cytotoxic T cells (CD3+, CD8+, and IFN-γ+), and effector memory T cells (CD3+, CD8+, CD44+, and IFN-γ+) detected by flow cytometry were dose-dependently elevated with the vaccination doses after stimulation with the SFTSV GP peptide pool, while those of Th2 cells (CD3+, CD4+, and IL-4+) remained at a low level despite having statistical significance (Fig. 1H; Fig. S3). Collectively, these results demonstrated that both humoral and Th1-biased cellular immune responses can be elicited by the SFTSV GP mRNA vaccine, enabling a subsequent efficacy evaluation.
The GP mRNA vaccine conferred complete protection against lethal SFTSV challenge at a low dose
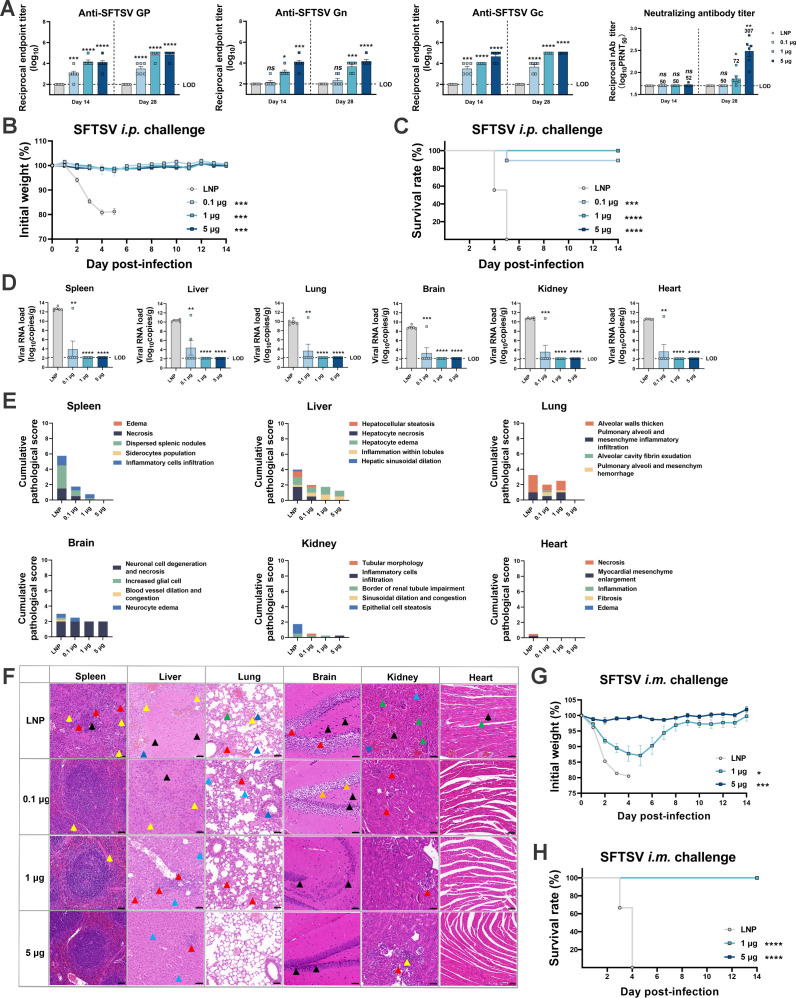
The efficacy of the GP mRNA vaccine was evaluated in a type I interferon receptor-deficient (IFNαR−/−) C57BL/6 J mouse (A129) model, with the same vaccination regimen used in the immunogenicity investigation. After the vaccination, A129 mice were intraperitoneally (i.p.) challenged with 100, 50% tissue culture infectious dose (TCID50) of SFTSV, followed by monitoring the body weight and survival rate for 2 weeks. As shown in Fig. 2A, the antibody responses, including binding antibodies against GP, Gn, and Gc, and neutralizing antibodies against SFTSV, in A129 mice were consistent with those in BALB/c mice, and a dose-dependent effect was seen. It should be noted that significant neutralization occurred at a dose of 5 µg, while a dose less than 1 µg elicited negligible neutralizing antibodies.
Fig 2.
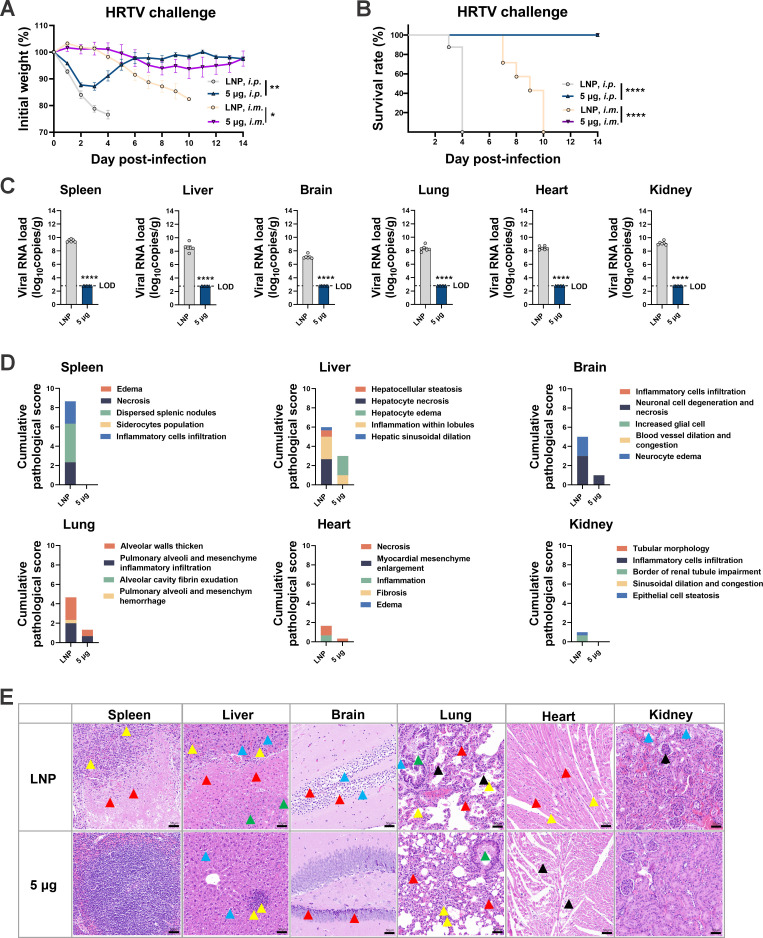
Dose-dependent efficacy of the GP mRNA vaccine in A129 mice. A129 mice received vaccinations with empty LNPs, 0.1, 1.0, or 5.0 µg of GP-LNPs with a regular two-shot regimen, followed by i.p. challenge with 100 TCID50 of SFTSV 10 days after the booster vaccination. (A) Binding and neutralizing antibodies were detected using ELISAs for GP, Gn, or Gc and PRNT, respectively. The LODs for the ELISA and PRNT were 1:100 and 1:50, respectively. (B and C) Body weight and survival were monitored over 14 days post-challenge. (D) The viral loads in the spleen, liver, lung, brain, kidney, and heart were detected by reverse transcription-quantitative polymerase chain reaction based on the standard curve method with a pair of primers targeting the NP gene. The LOD for qPCR was 138 copies per reaction, and converted copies are presented. (E) Cumulative pathological scores for different tissues from each mouse were calculated based on pathological indicators; the details of the scoring criteria are provided in the supplemental materials. (F) Representative images from each group were analyzed by hematoxylin and eosin staining. The colored arrows in the images marking obvious pathological features and their detailed meanings are listed as follows: (i) lungs: red indicates alveolar epithelial cells proliferated, alveolar atrophy, and alveolar walls thickened; green denotes congested and dilated capillaries within the alveolar wall; blue indicates protein mucus present in the bronchus lumen; yellow denotes inflammatory cell infiltration; (ii) liver: red indicates hepatocellular necrosis, nuclear fragmentation, and karyopyknosis; green denotes hepatocyte steatosis; black indicates hepatic sinusoidal dilatation; blue denotes hepatocyte edema; yellow indicates inflammatory cell infiltration; (iii) kidneys: red denotes glomerular atrophy and decreased number of mesangial cells; green indicates glomeruli were lobulated or had interstitial hemorrhages; black denotes degeneration of renal tubular epithelial cells; blue denotes protein mucus present in the kidney tubules; yellow denotes inflammatory cell infiltration; (iv) spleen: red denotes necrosis, nuclear fragmentation, and karyopyknosis of lymphocytes; black indicates scattered splenic nodules and blurred boundary between the red pulp; yellow denotes inflammatory cell infiltration; (v) brain: red indicates neuronal cell degeneration, karyopyknosis, and basophilia were enhanced; blue denotes neuronal cell edema; yellow denotes increased number of glial cells; green indicates congested and dilated blood vessels; (vi) heart: black denotes myocardial fibrosis and rupture; blue: indicates disordered arrangement of myocardial fibers and connective tissue hyperplasia; yellow denotes inflammatory cell infiltration. (G and H) A129 mice received vaccinations with empty LNPs, 1 or 5 µg of GP-LNPs with a regular two-shot regimen, followed by i.m. challenge with 1 × 105 TCID50 of SFTSV 10 days after the booster vaccination. Body weight and survival were monitored for 14 days. ELISA, enzyme-linked immunosorbent assay; i.m., intramuscular; i.p., intraperitoneal; PRNT, plaque reduction neutralization test; qPCR, quantitative PCR; TCID50, 50% tissue culture infectious dose.
After the challenge, vehicle-treated mice experienced sustained body weight loss and died within 5 days post-infection (dpi). In contrast, mice vaccinated with 1- and 5-µg GP-LNPs maintained their body weights and no deaths occurred within 14 days, whereas one mouse vaccinated with 0.1 µg of GP-LNPs died at day 5 (one of nine) (Fig. 2B and C). At the experimental endpoint, the spleens, livers, lungs, brains, kidneys, and hearts were dissected to examine viral loads and pathological lesions. Compared to those in the vehicle-treated group (viral loads as high as 1010 copies/g), the viral loads in these tissues of surviving mice were effectively reduced by the GP mRNA vaccine in a dose-dependent manner (Fig. 2D). Consistent with the viral loads, pathological injuries to these tissues were eliminated or alleviated by the GP mRNA vaccine (Fig. 2E and F).
To evaluate the efficacy of the GP mRNA vaccine under a more realistic infectious route, another set of SFTSV challenge experiments was conducted by i.m. inoculating A129 mice with 1 × 105 TCID50 of SFTSV. Unlike the i.p. challenge, vehicle-treated mice experienced more rapid weight loss and died within 4 dpi. Although the body weight showed more dramatic fluctuations in the 1-µg than the 5-µg group, all of the mice survived (Fig. 2G and H). These data indicated the explicit protection provided by the GP mRNA vaccine against SFTSV at a low dose under different infection conditions.
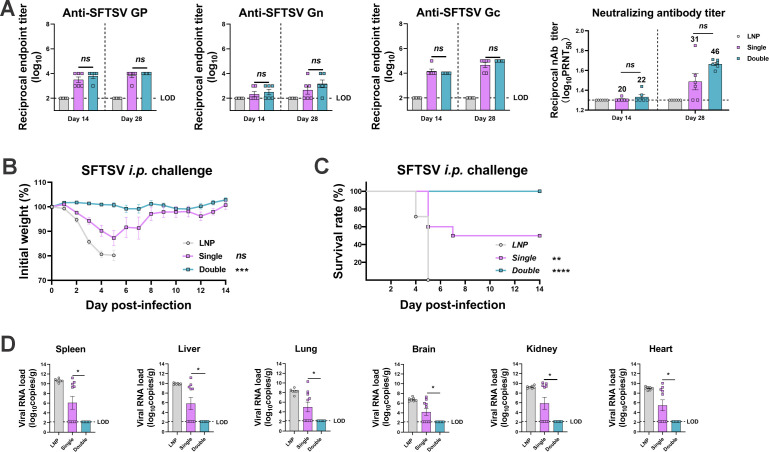
A two-shot regimen was required for complete protection by a low dose of the GP mRNA vaccine
Next, we fixed the dose at 1 µg and changed the vaccination frequency to preliminarily investigate the vaccination procedure. From the antibody results, it can be concluded that the GP-LNPs triggered limited production of binding or neutralization antibodies, regardless of whether a single- or double-dose regimen was applied (Fig. 3A). According to the challenge data, only 50% of the mice (5 of 10) in the single-dose group survived, whereas 100% of the mice in the double-dose group survived (Fig. 3B and C). As shown in Fig. 3D, the double-dose regimen significantly reduced the viral loads in tissues such as the spleen, liver, lung, brain, kidney, and heart, whereas the single-dose regimen alone did not, which is consistent with the body weight and survival rate data. These results further confirm that a booster vaccination is necessary for the GP mRNA vaccine to give rodents complete protection, particularly when administered at a relatively low dose, such as 1 µg. Moreover, the data suggested that the protective effect can manifest without a strong humoral immune response, which emphasizes the role of the cellular immune response in the protection offered by this GP mRNA vaccine.
Fig 3.
Efficacy of the GP mRNA vaccine in single-dose and double-dose regimens. A129 mice received vaccinations with empty LNPs or 1 µg of GP-LNPs in a single- or double-dose regimen, followed by i.p. challenge with 100 TCID50 of SFTSV. (A) Binding antibodies against SFTSV GP, Gn, and Gc were detected by ELISA (LOD, 1:100). Neutralizing antibodies were detected by PRNT, with the GMTs indicated above the corresponding bars (LOD, 1:20). (B and C) Curves of body weight and survival, which were monitored for 14 days post-challenge. (D) Viral loads in the spleen, liver, lung, brain, kidney, and heart were detected by reverse transcription-quantitative PCR (LOD, 138 copies). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
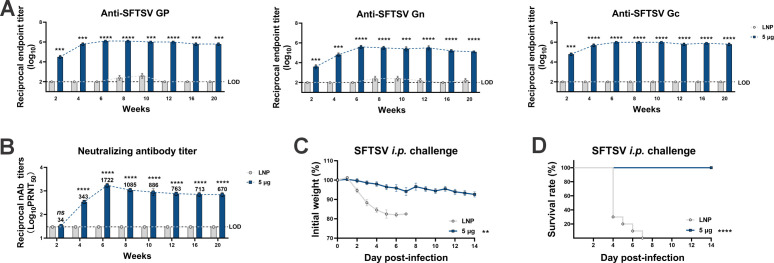
The SFTSV GP mRNA vaccine conferred long-term protection against lethal challenge
Encouraging outcomes associated with the GP mRNA vaccine prompted us to investigate the effective protection period. Although 1 µg was confirmed to be the optimal dose, 5 µg was used in this study to trigger a more robust humoral response to be convenient for immune indicator detection. Following the standard two-dose regimen, the mice were subjected to biweekly sampling for 20 weeks (approximately 5 months) and were then challenged in the 21st week. The results showed that the peak titers of GP-, Gn-, and Gc-specific binding antibodies all occurred in week 6 and subsequently stabilized at approximately 1:106 or experienced only a marginal decline over the 20-week span. Similarly, the neutralizing antibody titers peaked in week 6 and then decreased slightly but remained high until week 20 (Fig. 4A and B). Upon challenge, it was determined that complete protection was achieved, as reflected by the maintenance of body weight and 100% survival rate over 14 days (Fig. 4C and D). These results demonstrated the long-term immune response and protection offered by the GP mRNA vaccine, further indicating its efficacy.
Fig 4.
Long-term immune response and protection provided by the GP mRNA vaccine. A129 mice were vaccinated with empty LNPs or 5 µg of GP-LNPs following a two-shot regimen and were then were challenged with 100 TCID50 of SFTSV (i.p.) 21 weeks later. (A) The titers of binding antibodies specific to SFTSV GP, Gn, or Gc lasting for 20 weeks were detected by ELISA (LOD, 1:100). (B) The neutralizing antibody titers within 20 weeks were detected by PRNT (LOD, 1:30). (C and D) The body weight and survival rate were monitored for 14 days post-challenge. **P < 0.01, ***P < 0.001, ****P < 0.0001.
The GP mRNA vaccine provided cross-protection against other viruses from the genus Bandavirus, including Heartland virus and Guertu virus
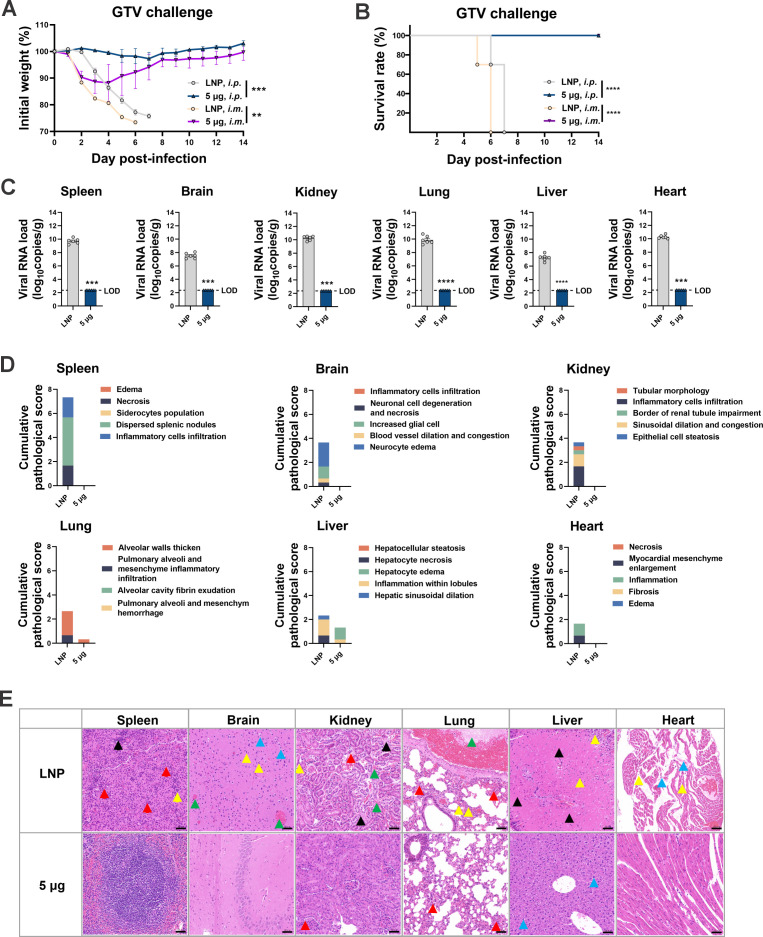
Based on the abovementioned results, the protective scope of the full-length SFTSV GP mRNA vaccine was investigated among bandaviruses, such as HRTV and GTV, which are evolutionarily close to SFTSV. Following a two-shot regimen with 5 µg of GP-LNPs, A129 mice and AG129 mice (IFNα/β/γR−/−) (32) were challenged with HRTV (A129, 1 × 107 TCID50 i.p.; AG129, 1 × 105 TCID50 i.m.) or GTV (A129, 100 TCID50 i.p.; A129, 1 × 105 TCID50 i.m.). Unexpectedly, all GP-LNP-vaccinated mice survived from both HRTV and GTV challenge, regardless of the mouse background or challenge route (the HRTV data are shown in Fig. 5, and the GTV data are shown in Fig. 6). As exhibited in Fig. 5, mice in the vehicle group that received the i.m. challenge died later than those that received the i.p. challenge, yet the GP mRNA vaccine saved the mice from both challenge routes while maintaining their body weights. The viral loads and pathological data from the i.p. challenge group showed sterilizing viral clearance and obvious pathological elimination or alleviation in the spleens, livers, brains, lungs, hearts, and kidneys. As shown in Fig. 6, after receiving i.m. or i.p. challenge, all the mice in the vehicle group died at approximately 6 dpi, while the GP mRNA vaccine saved the mice from both challenge routes. Similarly, the viral loads and pathological lesions were thoroughly eliminated or significantly alleviated.
Fig 5.
Cross-protection offered by the GP mRNA vaccine against Heartland virus. A129 or AG129 mice were vaccinated with empty LNPs or 5 µg of GP-LNPs in a two-shot regimen and were subsequently challenged with HRTV (A129, i.p., 1 × 107 TCID50; AG129, i.m., 1 × 105 TCID50). (A and B) Body weight and survival were monitored for 14 days post-challenge. (C) viral loads in the tissues after i.p. challenge were quantified using reverse transcription-quantitative PCR, as described above (LOD, 629 copies). (D and E) Cumulative pathological scores and representative images from each tissue are presented. A detailed description of the pathological analysis is listed in the supplemental materials, and annotations for the colored arrows can be referred to in the Fig. 2 legend. *P < 0.05, **P < 0.01, ****P < 0.0001. HRTV, Heartland virus.
Fig 6.
Cross-protection of the GP mRNA vaccine against Guertu virus. A129 mice were vaccinated with empty LNPs or 5 µg of GP-LNPs using a two-shot regimen and were subsequently challenged with GTV (i.p., 100 TCID50; i.m., 1 × 105 TCID50). (A and B) Body weight and survival were monitored for 14 days post-challenge. (C) Viral loads in tissues after i.p. challenge were quantified using reverse transcription-quantitative PCR, as described above (LOD, 232 copies). (D and E) Cumulative pathological scores and representative images from each tissue are presented. A detailed description of the pathological analysis is listed in the supplemental materials, and annotations for the colored arrows can be referred to in the Fig. 2 legend. **P < 0.01, ***P < 0.001, ****P < 0.0001. GTV, Guertu virus.
Despite cross-protection, no cross-binding or neutralizing antibodies were detected against HRTV or GTV (Fig. S4), suggesting that cross-cellular immunity might have occurred behind the protection. Collectively, these findings demonstrate the broader application scope of the full-length SFTSV GP mRNA vaccine and emphasize the crucial role of cellular immunity for cross-protection.
Conserved epitopes within SFTSV GP among Phenuiviridae pathogens may contribute to cross-protection
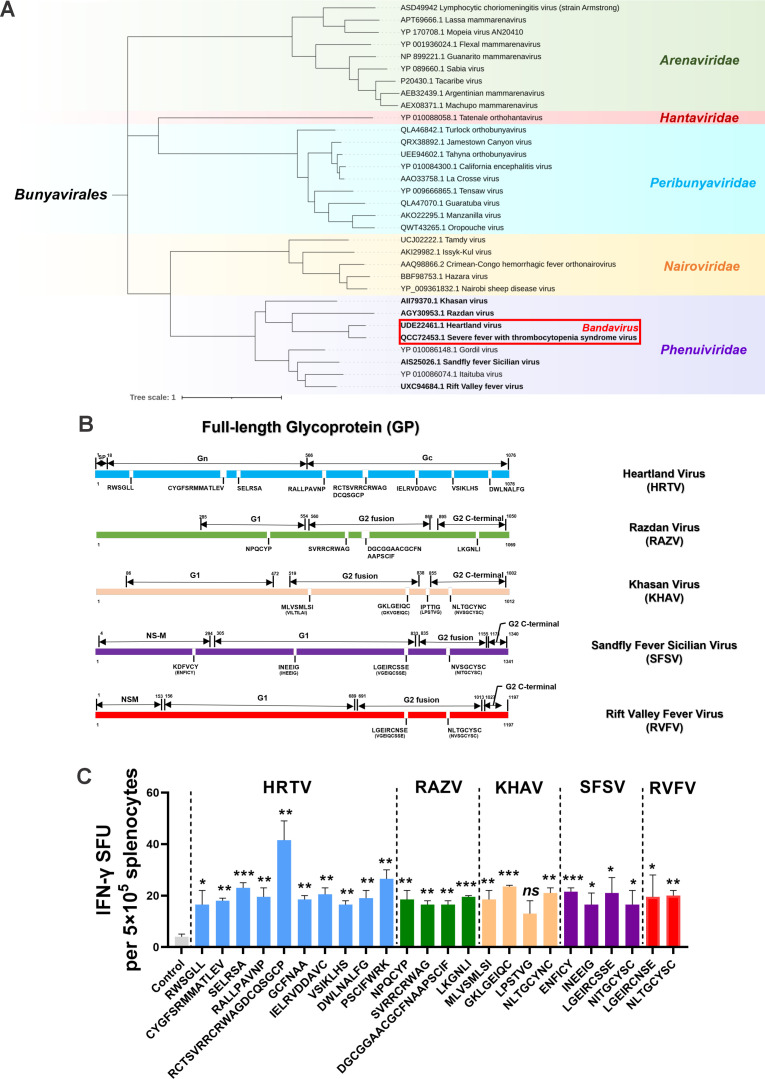
Inspired by the above results, GPs of Phenuiviridae pathogens, including HRTV, RAZV, KHAV, SFSV, and RVFV, were subjected to sequence alignment and T-cell epitope analysis, and a set of potentially conserved epitopes was founded in the SFTSV GP, particularly in the Gc domain. Among the five pathogens, HRTV had the most conserved epitopes with SFTSV, which was consistent with their evolutionary distance (Fig. 7A and B). Using an ELISpot assay, we used 24 peptides independently for in vitro stimulation of splenocytes from mice vaccinated with the SFTSV GP mRNA vaccine. As shown in Fig. 7C, all but one of the peptides induced significantly IFN-γ release. This experiment identified a set of potential protective T-cell epitopes within the SFTSV GP while also revealing the probable underlying mechanism of the observed cross-protection, suggesting the possibility of developing a broad-spectrum vaccine against Phenuiviridae pathogens based on conserved epitopes and antigens containing these epitopes.
Fig 7.
Identification of the potential protective epitopes from SFTSV GP. (A) Phylogenetic tree of the pathogens in the order Bunyavirales and pathogens from the genus Bandavirus, family Phenuiviridae, is highlighted. (B) Mapping of conserved and similar peptides from the SFTSV GP protein sequences among pathogenic Phenuiviridae pathogens. (C) IFN-γ secretion upon restimulation with each peptide (50 µg/mL) was detected by ELISpot, followed by statistical analyses comparing with the control. *P < 0.05, **P < 0.01, ***P < 0.001.
DISCUSSION
Pathogens carried by vectors, such as mosquitoes, ticks, sandflies, and midges, pose a great threat to human health when they spill over into humans, particularly during outdoor activities. Members of the Phenuiviridae family are globally prevalent, with a heightened incidence in underdeveloped regions, yet few vaccines or therapeutics are available. Given the similarities in terms of infection mechanism, transmission route, and virological and genetic properties, there is both a need and the potential for the development of broad-spectrum vaccines to combat these pathogens.
In this study, we present a candidate mRNA vaccine that employs the complete sequence of the SFTSV GP, leaving the secretory signal peptides unaltered and retaining the transmembrane and intracellular domains, thus ensuring its in situ expression and processing in a manner as natural as possible. This approach resulted in good efficacy not only against SFTSV but also against related bandaviruses, such as HRTV and GTV, exhibiting serendipitous effects despite seemly being unsophisticated. The glycoproteins of bandaviruses and Phenuiviridae viruses typically consist of a loose complex of subunits with irregular distribution on the viral membrane surface, which complicates their reproduction in an authentic conformation, posing a considerable challenge to recombinant protein vaccine development (19). Previous attempts to generate vaccines or therapeutic antibodies using recombinant Gn proteins have not yielded satisfactory results (33–35). Hence, preserving the active conformation while simultaneously inducing a robust cellular immune response is critical for a promising bandavirus vaccine, a view that is widely accepted among vaccine researchers. Thus, the use of mRNA technology to express the full-length glycoprotein in situ is necessary and feasible to some extent.
Consistent with our findings, Kim et al. reported that an mRNA vaccine basing the Gn head domain of SFTSV that elicits a robust humoral immune response and confers complete protection against SFTSV infection (27). Kwak et al. described the effectiveness of a DNA vaccine encoding Gn and Gc using a eukaryotic expression plasmid that successfully shielded ferrets from lethal challenge (36). These findings, along with the results of our study, corroborate the efficacy of nucleic acid vaccines derived from the SFTSV GP. Notably, our research illuminates the broad-spectrum potential of a full-length GP mRNA vaccine against bandaviruses and identifies protective epitopes on SFTSV GP. Moreover, our findings suggested that complete protection could be achieved by full-length GP-induced humoral and Th1-biased cellular immune responses or, in certain cases, solely by cellular responses. This outcome somewhat differs from those of previous studies, which reported that only a humoral response elicited by Gn/Gc or only a cellular response prompted by a combination of NP, NS, and LP can offer complete protection. The discrepancies between these studies may be attributed to differences in vaccine reactivity among species and individuals.
However, Kwak’s study and ours have jointly proposed an approach to develop an SFTSV vaccine inducing cellular immunity, which can be strongly triggered by nucleic acid vaccines, whether or not the antigens are from the receptor-binding domain. This concept can be employed in the development of broad-spectrum vaccines if conserved antigens are not limited to the receptor-binding domain or envelope glycoproteins. This idea was previously validated in a SARS-CoV-2 study (37). Zhong et al. reported the efficacy of a multigenic SARS-CoV-2 vaccine, which contained both spike and nucleoprotein genes, that significantly reduced viral loads and inflammatory cytokine production even in the absence of neutralizing antibodies (38). In addition, various approaches for developing broad-spectrum vaccines, such as using combinations of multiple antigens (39), employing sequential immunizations with different antigens (40), and incorporating combinations of conserved antigens or epitopes (41), have been proposed. Despite those, this study underscores the importance of utilizing antigens with as many conserved T-cell epitopes as possible.
Overall, although our study demonstrates the effectiveness of full-length SFTSV GP mRNA at providing broad-spectrum protection and reveals the potential role of conserved T-cell epitopes, cautions should be taken using large proteins as antigens, since the present technical bottleneck in preparing oversized mRNAs and the possible presence of adverse epitopes that may influence the final outcomes. In addition, the use of animal models for efficacy evaluations in preclinical studies may lead to deviations in protection, especially for bandaviruses like SFTSV, which lack both immunocompetent and low-cost rodent models (42, 43). Therefore, it seems necessary to evaluate the efficacy of this candidate vaccine in additional animal models before proceeding to clinical trials and clinical application.
MATERIALS AND METHODS
Proteins, antibodies, cell lines, and viruses
The ectodomains of SFTSV Gn (20–452 aa) and Gc (563–1,035 aa), GP (a heterodimer of ecto-Gn and Gc), and NP were recombinantly expressed in Expi293F cells, and subsequently purified via affinity chromatography. Polyclonal antibodies against SFTSV NP were prepared by immunization of New Zealand white rabbits.
Vero-ATCC and HEK-293T cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS, Gibco) and maintained at 37°C with 5% CO2.
Authentic SFTSV (HBMC16 strain), HRTV (MO-4 strain), and GTV (DXM strain) specimens were preserved and obtained from the National Virus Resource Center, Wuhan Institute of Virology, Chinese Academy of Sciences, and propagated with Vero-ATCC cells. Animal experiments involving SFTSV and HRTV were conducted in an ABSL-3 laboratory.
Peptides and peptide pools
The SFTSV GP sequences were uploaded to the Immune Epitope Database (https://www.iedb.org/) to predict major histocompatibility complex class I and II binding epitopes, after which the top 10 epitopes from either Gn or Gc were chosen for synthesis (GenScript, Nanjing, China). The peptides were dissolved in dimethyl sulfoxide and phosphate-buffered saline (PBS) and then mixed to form the SFTSV GP peptide pool.
The conserved or similar regions of HRTV, RAZV, KHAV, SFSV, and RVFV within SFTSV GP were identified as follows. First, the amino acid sequences of the HRTV, RAZV, KHAV, SFSV, and RVFV GPs were aligned with the SFTSV GP sequence using a local alignment method (Smith-Waterman) and conserved, or similar peptides containing six or more amino acids were selected for further analysis. Next, these conserved or similar peptides were evaluated for their T-cell immunogenicity using VaxiJen v.2.0 (44) and IFNepitope (https://webs.iiitd.edu.in/raghava/ifnepitope/application.php). Ultimately, 10 peptides were selected from HRTV; 4 peptides were selected from RAZV, KHAV, or SFSV; and 2 peptides were selected from RVFV.
Generation and characterization of the GP-mRNA-LNPs
A full-length GP mRNA vaccine was developed based on SFTSV strain HB29 (GenBank: YP_006504094.1). The mRNAs and LNPs were produced using the Liverna Therapeutics platform (China patent: ZL201911042634.2). Briefly, mRNA was synthesized using an optimized T7 RNA polymerase-mediated transcription reaction in vitro with the complete replacement of uridine with N1-methyl-pseudouridine. The reaction was performed using a DNA template bearing an open reading frame bordered by 5′ and 3′ untranslated regions, and concluded with the incorporation of a poly A tail. The length and purity of the in vitro-transcribed mRNAs were further validated through microchip capillary electrophoresis (5200 Fragment Analyzer system; Agilent, Santa Clara, USA), and the mRNAs were then encapsulated in LNPs according to a refined procedure, wherein an ethanolic lipid mixture of ionizable cationic lipids, phosphatidylcholine, cholesterol, and polyethylene glycol lipids was rapidly mixed with an aqueous solution containing the mRNA products. Subsequent analytical assessments involved measuring the particle size, polydispersity index, encapsulation efficiency, pH, endotoxin contamination, and bioburden.
Animal immunization and challenge
BALB/c mice (aged 6–8 weeks, n = 5) and A129 or AG129 mice (aged 6–10 weeks, n = 6–10) received intramuscular vaccinations according to a prime-boost regimen at 3-week intervals. Serum samples were collected biweekly until sacrifice or challenge. Ten days after the booster vaccination, the mice were i.p. inoculated with 100 TCID50 of SFTSV (A129), 1 × 107 TCID50 of HRTV (A129), or 100 TCID50 of GTV (A129), or i.m. inoculated with 1 × 105 TCID50 of SFTSV (A129), HRTV (AG129), and GTV (A129). Body weights and survival were recorded daily from day 0 to 14. At the experimental endpoint, the spleens, livers, lungs, brains, kidneys, and hearts were dissected for viral detection and histopathological examination.
Enzyme-linked immunosorbent assay
Antibody titers specific to SFTSV Gn, Gc, or GP were determined using classical enzyme-linked immunosorbent assays. Briefly, the Gn, Gc, and GP proteins were diluted to 2 µg/mL in coating buffer (0.1-M sodium carbonate, 0.1-M sodium bicarbonate, pH 9.6), and then dispensed into each well of a 96-well polystyrene high-binding flat-bottom plate (Greiner, Frickenhausen, Germany) at 100 µL/well, followed by incubation at 4°C overnight. The next day, the plates were washed three times with PBS with 0.05% Tween 20 (PBST) and then incubated with blocking buffer (PBST, 5% skim milk) for 1 hour. Serially diluted sera (beginning with a 1:100 dilution) samples were added to the wells and incubated at room temperature for 1 hour. After further washing, the wells were incubated with horseradish peroxidase-conjugated goat anti-mouse IgG (H + L) (ABclonal, Wuhan, China) at a dilution of 1:4,000 for 1 hour. After incubation, a tetramethylbenzidine solution (Proteintech, Rosemont, IL, USA) was added to each well, and the reaction was stopped with 2 M H2SO4 after 10 minutes. Finally, the absorbance was measured at 450 nm using a Synergy H1 microplate reader (BioTek, Winooski, VT, USA). Absorbance values greater than twice that of the control (PBS) were considered positive.
ELISpot assays
Splenocytes from vaccinated mice were processed through a 70-µm mesh filter, followed by suspension in RBC lysis buffer (Cell Signaling Technology, Danvers, MA, USA) for 10 minutes at room temperature, shielded from light. Subsequently, 1 × 106 cells were cultured with the SFTSV GP pool (2 µg/mL each peptide) in ELISpot plates (MabTech, Stockholm, Sweden) at 37°C for 36 hours, employing phorbol 12-myristate 13-acetate/ionomycin and RPMI 1640 medium as positive and negative controls, respectively. After incubation, spots indicative of IFN-γ, IL-2, IL-4, and IL-10 secretion were detected as per the ELISpot Plus assay protocol (MabTech) and enumerated using an ImmunoSpot S6 plate reader (Cellular Technology Limited, Shaker Heights, OH, USA).
To identify conserved peptides, splenocytes from vaccinated mice were cultured in ELISpot plates at a density of 5 × 105 cells per well. The cells were then stimulated with each peptide (50 µg/mL) derived from HRTV, RAZV, KHAV, SFSV, and RVFV individually for 36 hours at 37°C. The subsequent procedures were performed according to the methodology described above.
Intracellular cytokine staining assay and flow cytometry
Splenocytes were stimulated with the SFTSV GP peptide pool (2 µg/mL each peptide) supplemented with 5-µg/mL brefeldin A (Absin, Shanghai, China), followed by a 10-hour incubation prior to cell labeling. First, live and dead cells were distinguished using the Zombie Aqua Fixable Viability Kit (BioLegend, San Diego, CA, USA). Then, the Fc receptors were blocked with purified rat anti-mouse CD16/CD32 antibodies (clone 2.4G2; BD Pharmingen, San Diego, CA, USA). Next, primary antibodies, including anti-CD3e-APC-Cy7 (clone 145–2C11), anti-CD4-PE (clone RM4-5), anti-CD8a-PE-Cy7 (clone 53–6.7), and anti-CD44-APC (clone IM7), all from BD Pharmingen, were added to the cells and incubated at 4°C for 30 minutes. Following cell surface labeling, the splenocytes were fixed and permeabilized using a fixation/permeabilization kit (BD Pharmingen) before the addition of anti-IFN-γ-BV786 (clone XMG1.2) and anti-IL-4-BV711 (clone 11B11) antibodies. The data were collected using a BD LSRFortessa instrument (BD Biosciences, Franklin Lakes, NJ, USA), and subsequent analysis was conducted using FlowJo v.10 (BD Biosciences).
Plaque reduction neutralization test (PRNT)
Vero-ATCC cells were seeded into 48-well plates at a density of 2 × 105 cells/well and cultured overnight. The next day, serially diluted sera (beginning with a 1:15 dilution) in DMEM containing 2% FBS were incubated with 200 plaque-forming units of SFTSV, HRTV, or GTV at 37°C for 1 hour. The serum-virus mixture was then added to duplicate wells and incubated with the cells for an additional hour. After incubation, the mixture was discarded, and the cell monolayer was washed with PBS and overlaid with 1.25% methylcellulose and DMEM containing 2% FBS. Three days later, the overlay was removed and the cells were fixed in 4% formaldehyde and incubated with 0.2% Triton X-100 in 5% skim milk for 30 minutes. Immunoplaques were detected using laboratory-prepared anti-SFTSV NP rabbit polyclonal antibodies (1:1,000), horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (1:1,000) (ABclonal), and an Enhanced HRP-DAB Chromogenic Kit (TIANGEN, Beijing, China). The spots were recorded using an ImmunoSpot S6 reader (Cellular Technology Limited), and PRNT50 and GMT values were calculated using GraphPad Prism v.9 (GraphPad, San Diego, CA, USA).
Quantification of viral copies in tissues
Total RNA was extracted from spleens, livers, lungs, brains, kidneys, and hearts using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Subsequently, 3 µL of RNA from a 30-µL elution was reverse transcribed using the HiScript III All-In-One RT SuperMix Perfect for quantitative PCR (qPCR) kit (Vazyme, Nanjing, China) in a reaction volume of 20 µL. Then, 2 µL of cDNA was amplified by qPCR utilizing the Taq Pro Universal SYBR qPCR Master Mix kit (Vazyme), with primers specific for the NP genes of SFTSV, HRTV, or GTV. Amplification was conducted using a QuantStudio v.6 Pro thermocycler (ABI, Natick, MA, USA), and viral copies were quantified using a standard curve generated from plasmids encoding NP.
Histopathology
Tissues dissected from animals were immediately fixed in 4% paraformaldehyde. The fixed tissues were subsequently dehydrated with an ethanol gradient and embedded in paraffin. Sections (4 µm thick) were cut from the embedded tissues, dried at 60°C, then dewaxed and stained with hematoxylin and eosin. The stained sections were dehydrated, sealed, and imaged using a Nikon Digital Sight DS-FI2 microscopic imaging system (Nikon, Tokyo, Japan). Pathological scoring was performed by a professional animal pathology analysis agency (Wuhan BaiQianDu Biotechnology Co., Ltd., Wuhan, China) using objective scoring criteria (see supplemental materials).
Data analysis
Data were processed using GraphPad Prism v.9.0 software, and the results are displayed as the mean ± standard error of the mean. Statistical analyses comprised both unpaired Student’s t-tests and Mann-Whitney U tests, with significance denoted as not significant (ns) for P > 0.05, * for P < 0.05, ** for P < 0.01, *** for P < 0.001, and **** for P < 0.0001.
ACKNOWLEDGMENTS
This research was financially supported by the Knowledge Innovation Program of Wuhan Basic Research (grant no. 2022020801010147) and the Youth Innovation Promotion Association CAS (grant no. 2021333) awarded to X.P.
We are particularly grateful to the Center for Instrumental Analysis and Metrology and the Center for Experimental Animals at the Institutional Center for Shared Technologies and Facilities of the Wuhan Institute of Virology for their technical support. We also thank Professor Deng Fei and Professor Deng Zengqin at the Wuhan Institute of Virology, CAS, for providing the relevant experimental materials.
J.L. characterized immunogenicity, evaluated efficacy, and identified epitopes. J.L. and Y.P. designed and prepared the mRNA vaccines with crucial help from S.C. Y.W. and X.H. assisted with the animal experiments. G.X., X.C., and X.Z. assisted with data collection and analysis. Y.P. and G.X. coordinated the project. J.L. and X.P. designed the experiments and drafted the manuscript. X.P. contributed to the interpretation of the results and revised and approved the manuscript.
Contributor Information
Yucai Peng, Email: pengyucai@live-rna.com.
Gengfu Xiao, Email: xiaogf@wh.iov.cn.
Xiaoyan Pan, Email: panxy@wh.iov.cn.
Jae U. Jung, Lerner Research Institute, Cleveland Clinic, Cleveland, Ohio, USA
ETHICS APPROVAL
All animal experiments were conducted in strict compliance with the Regulations for the Administration of Affairs on Experimental Animals in China. The protocols were approved by the Laboratory Animal Care and Use Committee of the Wuhan Institute of Virology, Chinese Academy of Sciences (Wuhan, China), with the assigned ethics number WIVA25202204. Female BALB/c mice were procured from GemPharmatech Co., Ltd. (Nanjing, China), whereas A129 and AG129 mice were maintained under specific pathogen-free conditions. The challenge experiments were performed in an animal biosafety level 3 laboratory.
DATA AVAILABILITY
All data associated with this study are included in the main text and supplemental material. Inquiries regarding materials, data, and the elaboration of methods should be addressed to the primary contact, Xiaoyan Pan (panxy@wh.iov.cn).
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/jvi.00769-24.
Figures S1 to S4; detailed pathological scoring criteria for each kind of tissue.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Shi J, Hu Z, Deng F, Shen S. 2018. Tick-borne viruses. Virol Sin 33:21–43. doi: 10.1007/s12250-018-0019-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang Y-J, Higgs S, Vanlandingham DL. 2019. Emergence and re-emergence of mosquito-borne arboviruses. Curr Opin Virol 34:104–109. doi: 10.1016/j.coviro.2019.01.001 [DOI] [PubMed] [Google Scholar]
- 3. Ayhan N, Charrel RN. 2017. Of phlebotomines (sandflies) and viruses: a comprehensive perspective on a complex situation. Curr Opin Insect Sci 22:117–124. doi: 10.1016/j.cois.2017.05.019 [DOI] [PubMed] [Google Scholar]
- 4. Zhou H, Xu L, Shi W. 2023. The human-infection potential of emerging tick-borne viruses is a global public health concern. Nat Rev Microbiol 21:215–217. doi: 10.1038/s41579-022-00845-3 [DOI] [PubMed] [Google Scholar]
- 5. Mansfield KL, Jizhou L, Phipps LP, Johnson N. 2017. Emerging tick-borne viruses in the twenty-first century. Front Cell Infect Microbiol 7:298. doi: 10.3389/fcimb.2017.00298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gai Z-T, Zhang Y, Liang M-F, Jin C, Zhang S, Zhu C-B, Li C, Li X-Y, Zhang Q-F, Bian P-F, Zhang L-H, Wang B, Zhou N, Liu J-X, Song X-G, Xu A, Bi Z-Q, Chen S-J, Li D-X. 2012. Clinical progress and risk factors for death in severe fever with thrombocytopenia syndrome patients. J Infect Dis 206:1095–1102. doi: 10.1093/infdis/jis472 [DOI] [PubMed] [Google Scholar]
- 7. Yu X-J, Liang M-F, Zhang S-Y, Liu Y, Li J-D, Sun Y-L, Zhang L, Zhang Q-F, Popov VL, Li C, et al. 2011. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med 364:1523–1532. doi: 10.1056/NEJMoa1010095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li J, Li S, Yang L, Cao P, Lu J. 2021. Severe fever with thrombocytopenia syndrome virus: a highly lethal bunyavirus. Crit Rev Microbiol 47:112–125. doi: 10.1080/1040841X.2020.1847037 [DOI] [PubMed] [Google Scholar]
- 9. Bopp NE, Kaiser JA, Strother AE, Barrett ADT, Beasley DWC, Benassi V, Milligan GN, Preziosi M-P, Reece LM. 2020. Baseline mapping of severe fever with thrombocytopenia syndrome virology, epidemiology and vaccine research and development. NPJ Vaccines 5:111. doi: 10.1038/s41541-020-00257-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brault AC, Savage HM, Duggal NK, Eisen RJ, Staples JE. 2018. Heartland virus epidemiology vector association, and disease potential. Viruses 10:498. doi: 10.3390/v10090498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Staples JE, Pastula DM, Panella AJ, Rabe IB, Kosoy OI, Walker WL, Velez JO, Lambert AJ, Fischer M. 2020. Investigation of heartland virus disease throughout the United States, 2013–2017. Open Forum Infect Dis 7:faa125. doi: 10.1093/ofid/ofaa125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shen S, Duan X, Wang B, Zhu L, Zhang Y, Zhang J, Wang J, Luo T, Kou C, Liu D, Lv C, Zhang L, Chang C, Su Z, Tang S, Qiao J, Moming A, Wang C, Abudurexiti A, Wang H, Hu Z, Zhang Y, Sun S, Deng F. 2018. A novel tick-borne phlebovirus, closely related to severe fever with thrombocytopenia syndrome virus and Heartland virus, is a potential pathogen. Emerg Microbes Infect 7:95. doi: 10.1038/s41426-018-0093-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lvov DK, Leonova GN, Gromashevsky VL, Skvortsova TM, Shestakov VI, Belikova NP, Berezina LK, Gofman YP, Klimenko SM, Safonov AV, Sazonov AA, Zakaryan VA. 1978. Khasan virus, a new ungrouped bunyavirus isolated from Haemaphysalis longicornis ticks in the Primorie region. Acta Virol 22:249–252. [PubMed] [Google Scholar]
- 14. Lvov DK, Gromashevsky VL, Zakaryan VA, Skvortsova TM, Berezina LK, Gofman YP, Klimenko SM, Chubkova AL. 1978. Razdan virus, a new ungrouped bunyavirus isolated from Dermacentor marginatus ticks in Armenia. Acta Virol 22:506–508. [PubMed] [Google Scholar]
- 15. Nair N, Osterhaus A, Rimmelzwaan GF, Prajeeth CK. 2023. Rift valley fever virus—infection, pathogenesis and host immune responses. Pathogens 12:1174. doi: 10.3390/pathogens12091174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alkan C, Bichaud L, de Lamballerie X, Alten B, Gould EA, Charrel RN. 2013. Sandfly-borne phleboviruses of Eurasia and Africa: epidemiology, genetic diversity, geographic range, control measures. Antiviral Res 100:54–74. doi: 10.1016/j.antiviral.2013.07.005 [DOI] [PubMed] [Google Scholar]
- 17. Boshra H. 2022. An overview of the infectious cycle of bunyaviruses. Viruses 14:2139. doi: 10.3390/v14102139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hofmann H, Li X, Zhang X, Liu W, Kühl A, Kaup F, Soldan SS, González-Scarano F, Weber F, He Y, Pöhlmann S. 2013. Severe fever with thrombocytopenia virus glycoproteins are targeted by neutralizing antibodies and can use DC-SIGN as a receptor for pH-dependent entry into human and animal cell lines. J Virol 87:4384–4394. doi: 10.1128/JVI.02628-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Du S, Peng R, Xu W, Qu X, Wang Y, Wang J, Li L, Tian M, Guan Y, Wang J, Wang G, Li H, Deng L, Shi X, Ma Y, Liu F, Sun M, Wei Z, Jin N, Liu W, Qi J, Liu Q, Liao M, Li C. 2023. Cryo-EM structure of severe fever with thrombocytopenia syndrome virus. Nat Commun 14:6333. doi: 10.1038/s41467-023-41804-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tani H, Shimojima M, Fukushi S, Yoshikawa T, Fukuma A, Taniguchi S, Morikawa S, Saijo M. 2016. Characterization of glycoprotein-mediated entry of severe fever with thrombocytopenia syndrome virus. J Virol 90:5292–5301. doi: 10.1128/JVI.00110-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lozach P-Y, Kühbacher A, Meier R, Mancini R, Bitto D, Bouloy M, Helenius A. 2011. DC-SIGN as a receptor for phleboviruses. Cell Host Microbe 10:75–88. doi: 10.1016/j.chom.2011.06.007 [DOI] [PubMed] [Google Scholar]
- 22. Zhang L, Peng X, Wang Q, Li J, Lv S, Han S, Zhang L, Ding H, Wang C-Y, Xiao G, Du X, Peng K, Li H, Liu W. 2023. CCR2 is a host entry receptor for severe fever with thrombocytopenia syndrome virus. Sci Adv 9:eadg6856. doi: 10.1126/sciadv.adg6856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu J, Xu M, Tang B, Hu L, Deng F, Wang H, Pang D, Hu Z, Wang M, Zhou Y. 2019. Single‐particle tracking reveals the sequential entry process of the bunyavirus severe fever with thrombocytopenia syndrome virus. Small 15. doi: 10.1002/smll.201803788 [DOI] [PubMed] [Google Scholar]
- 24. Wu Y, Zhu Y, Gao F, Jiao Y, Oladejo BO, Chai Y, Bi Y, Lu S, Dong M, Zhang C, Huang G, Wong G, Li N, Zhang Y, Li Y, Feng W-H, Shi Y, Liang M, Zhang R, Qi J, Gao GF. 2017. Structures of phlebovirus glycoprotein Gn and identification of a neutralizing antibody epitope. Proc Natl Acad Sci U S A 114:E7564–E7573. doi: 10.1073/pnas.1705176114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang B, Huang B, Li X, Guo Y, Qi G, Ding Y, Gao H, Zhang J, Wu X, Fang L. 2022. Development of functional anti‐Gn nanobodies specific for SFTSV based on next‐generation sequencing and proteomics. Protein Sci 31:e4461. doi: 10.1002/pro.4461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim D, Kim E, Kim S, Chung Y, Lai C-J, Cha I, Cho S-D, Choi Y, Dai X, Kim S, Kang S, Kwak M-J, Liu Z, Choi Y, Park S-H, Choi YK, Jung JU. 2023. Self-assembling Gn head ferritin nanoparticle vaccine provides full protection from lethal challenge of in aged ferrets. mBio 14:e0186823. doi: 10.1128/mbio.01868-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim D, Lai C-J, Cha I, Kang S, Yang W-S, Choi Y, Jung JU. 2023. SFTSV Gn‐Head mRNA vaccine confers efficient protection against lethal viral challenge. J Med Virol 95:e29203. doi: 10.1002/jmv.29203 [DOI] [PubMed] [Google Scholar]
- 28. Kang J-G, Jeon K, Choi H, Kim Y, Kim H-I, Ro H-J, Seo YB, Shin J, Chung J, Jeon YK, Kim YS, Lee KH, Cho N-H. 2020. Vaccination with single plasmid DNA encoding IL-12 and antigens of severe fever with thrombocytopenia syndrome virus elicits complete protection in IFNAR knockout mice. PLoS Negl Trop Dis 14:e0007813. doi: 10.1371/journal.pntd.0007813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu S, Yang K, Li R, Zhang L. 2020. mRNA vaccine era—mechanisms, drug platform and clinical prospection. Int J Mol Sci 21:6582. doi: 10.3390/ijms21186582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Qin S, Tang X, Chen Y, Chen K, Fan N, Xiao W, Zheng Q, Li G, Teng Y, Wu M, Song X. 2022. mRNA-based therapeutics: powerful and versatile tools to combat diseases. Signal Transduct Target Ther 7:166. doi: 10.1038/s41392-022-01007-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. 2020. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 383:2603–2615. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bosco-Lauth AM, Calvert AE, Root JJ, Gidlewski T, Bird BH, Bowen RA, Muehlenbachs A, Zaki SR, Brault AC. 2016. Vertebrate host susceptibility to heartland virus. Emerg Infect Dis 22:2070–2077. doi: 10.3201/eid2212.160472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim KH, Kim J, Ko M, Chun JY, Kim H, Kim S, Min J-Y, Park WB, Oh M-D, Chung J. 2019. An anti-Gn glycoprotein antibody from a convalescent patient potently inhibits the infection of severe fever with thrombocytopenia syndrome virus. PLoS Pathog 15:e1007375. doi: 10.1371/journal.ppat.1007375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu X, Li Y, Huang B, Ma X, Zhu L, Zheng N, Xu S, Nawaz W, Xu C, Wu Z. 2020. A single-domain antibody inhibits SFTSV and mitigates virus-induced pathogenesis in vivo. JCI Insight 5:e136855. doi: 10.1172/jci.insight.136855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim S, Jeon K, Choi H, Jeong D-E, Kang J-G, Cho N-H. 2024. Comparative analysis of the efficacy of vaccines using structural protein subunits of the severe fever with thrombocytopenia syndrome virus. Front Microbiol 15:1348276. doi: 10.3389/fmicb.2024.1348276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kwak J-E, Kim Y-I, Park S-J, Yu M-A, Kwon H-I, Eo S, Kim T-S, Seok J, Choi W-S, Jeong JH, Lee H, Cho Y, Kwon JA, Jeong M, Maslow JN, Kim Y-E, Jeon H, Kim KK, Shin E-C, Song M-S, Jung JU, Choi YK, Park S-H. 2019. Development of a SFTSV DNA vaccine that confers complete protection against lethal infection in ferrets. Nat Commun 10:3836. doi: 10.1038/s41467-019-11815-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tan CW, Valkenburg SA, Poon LLM, Wang L-F. 2023. Broad-spectrum pan-genus and pan-family virus vaccines. Cell Host Microbe 31:902–916. doi: 10.1016/j.chom.2023.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhong C, Xia H, Adam A, Wang B, Hajnik RL, Liang Y, Rafael GH, Zou J, Wang X, Sun J, Soong L, Barrett ADT, Weaver SC, Shi P-Y, Wang T, Hu H. 2021. Mucosal vaccination induces protection against SARS-CoV-2 in the absence of detectable neutralizing antibodies. NPJ Vaccines 6:139. doi: 10.1038/s41541-021-00405-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhai L, Tumban E. 2016. Gardasil-9: a global survey of projected efficacy. Antiviral Res 130:101–109. doi: 10.1016/j.antiviral.2016.03.016 [DOI] [PubMed] [Google Scholar]
- 40. Song S, Zhou B, Cheng L, Liu W, Fan Q, Ge X, Peng H, Fu Y-X, Ju B, Zhang Z. 2022. Sequential immunization with SARS-CoV-2 RBD vaccine induces potent and broad neutralization against variants in mice. Virol J 19:2. doi: 10.1186/s12985-021-01737-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lo C-Y, Misplon JA, Li X, Price GE, Ye Z, Epstein SL. 2021. Universal influenza vaccine based on conserved antigens provides long-term durability of immune responses and durable broad protection against diverse challenge virus strains in mice. Vaccine 39:4628–4640. doi: 10.1016/j.vaccine.2021.06.072 [DOI] [PubMed] [Google Scholar]
- 42. Sun J, Min Y-Q, Li Y, Sun X, Deng F, Wang H, Ning Y-J. 2021. Animal model of severe fever with thrombocytopenia syndrome virus infection. Front Microbiol 12:797189. doi: 10.3389/fmicb.2021.797189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yoshikawa T. 2021. Vaccine development for severe fever with thrombocytopenia syndrome. Viruses 13:627. doi: 10.3390/v13040627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Doytchinova IA, Flower DR. 2007. VaxiJen: a server for prediction of protective antigens, tumour antigens and subunit vaccines. BMC Bioinformatics 8:4. doi: 10.1186/1471-2105-8-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1 to S4; detailed pathological scoring criteria for each kind of tissue.
Data Availability Statement
All data associated with this study are included in the main text and supplemental material. Inquiries regarding materials, data, and the elaboration of methods should be addressed to the primary contact, Xiaoyan Pan (panxy@wh.iov.cn).