Abstract
Objective
Existing behavioral weight management interventions produce clinically meaningful weight loss. The onset of the COVID‐19 pandemic led to the quick transition of such interventions from in‐person to virtual platforms. This provided a unique opportunity to compare engagement and outcomes for an in‐person versus virtually delivered weight management intervention.
Methods
A non‐randomized comparison of engagement and weight outcomes was performed between two cohorts who participated in a weight management intervention in person (N = 97) versus three who participated virtually via videoconference (N = 134). Various metrics of engagement were examined, including group class and individual phone call attendance and duration, and retention for weight assessments. Behavioral targets of daily caloric intake and step‐counts and the clinical weight outcome were explored.
Results
Cohorts (mean [standard deviation] age 47.3 (11.5), 67.1% women: 86.8% White) that participated virtually attended more group sessions (p < 0.001) and had maintenance telephone calls that were of a longer duration (p < 0.001). No other engagement or weight outcomes significantly differed by delivery modality.
Conclusions
Virtual weight management programs are promising and may generate similar outcomes to those delivered in‐person. Future research should seek to understand how best to promote and sustain engagement in virtual interventions.
Keywords: COVID‐19, engagement, videoconference, virtual intervention, weight loss intervention, weight management
COVID‐19 led many weight management interventions to transition to virtual modes of delivery. In the present study, cohorts who received the intervention virtually had boosts in some engagement (attendance) outcomes but not all outcomes (retention of weight data). Virtually delivered weight management programs show promise for producing outcomes similar to those delivered in‐person.
1. INTRODUCTION
Efficacious weight management programs involving didactic information and behavioral skills training help people lose weight and, to some extent, maintain weight loss over time. 1 , 2 Although these programs yield clinically significant (i.e., 5%) weight loss on average, there exists considerable variability in response, 3 which plausibly stems from a lack of consistent participant engagement among other possible factors. Engagement has been defined in many ways 4 and is associated with weight outcomes in weight management trials. 5 , 6 Engagement is defined here as carrying out an intervention task or activity. Identifying strategies to promote sustained engagement in weight loss interventions to achieve and maintain clinically significant weight loss remains an ongoing challenge. 3
Historically, such interventions were conducted in person, 1 , 2 , 3 requiring participants to travel to and attend weekly or biweekly sessions over several months. The sudden onset of COVID‐19 restrictions forced the Diabetes Prevention Program, Weight Watchers program, and research teams to quickly transition from in‐person to remote delivery platforms such as phone calls, mobile health (mHealth) applications, and videoconferencing. 7 , 8 , 9 , 10 , 11 The problem of engagement for many behavioral weight‐loss interventions was plausibly exacerbated by this recent transition from in‐person to virtual delivery.
1.1. The promise of remotely delivered interventions
The term “remote” often refers to any intervention not delivered in‐person, including those delivered by telephone and virtually by videoconference. Remotely delivered interventions are promising for increasing the reach to individuals who struggle to access intensive in‐person programs or find such programs burdensome or stressful. 12 They may also increase the engagement of underrepresented populations who identify with racial and ethnic minority groups, reside in rural areas, or are socioeconomically disadvantaged. 13 , 14
Few direct comparisons of in‐person versus remotely delivered weight management interventions exist. Weight‐loss research before the COVID‐19 pandemic indicated that engagement and weight loss outcomes are as good for telephone‐based as for in‐person interventions, particularly for adults residing in rural areas. 6 , 15 Although videoconference‐based group intervention programs have demonstrated feasibility and the potential to improve health‐related treatment outcomes, 16 they may be associated with decreased feelings of therapeutic alliance 17 and inadequately mimic in‐person weight‐loss programs. Although videoconferencing allows for real‐time interactions, participants often turn off their cameras and can mute or walk away from the session entirely. Further, despite emerging evidence supporting enhanced engagement in virtual settings, 18 declines still occur over time. 4
The few evaluations of videoconference‐based interventions that emerged during the COVID‐19 pandemic suggested generally promising but also mixed results for engagement. 19 Hu and colleagues 20 found in a 6‐month mobile phone‐based behavioral weight‐loss trial that participants attended (i.e., logged into) videoconference‐based counseling sessions 85.7% of the time. However, many turned off their cameras during the session, and adherence to app‐based self‐monitoring of dietary intake steadily declined over time, suggesting poor intervention engagement over time. Leahey et al. compared the adherence of pre‐pandemic in‐person sessions to post‐pandemic videoconference sessions and found that treatment attendance was greater in the remote cohort, particularly among Hispanic populations. 10 However, other research showed reduced meeting attendance in weight‐loss group meetings delivered over videoconference during the COVID‐19 lockdown compared with in‐person meetings pre‐pandemic. 21
Beyond engagement, research suggests that delivering weight‐loss programs in‐person or remotely may have no significant effect on weight loss or retention rates. 20 Yucel and Yucel, 22 however, found that both phone‐and video‐counseling were more effective at promoting weight loss than traditional in‐person group counseling sessions. Taken together, these findings suggest that further evidence is needed to understand the effectiveness of remotely delivered interventions in fostering sustained engagement and weight‐loss maintenance.
1.2. Present investigation
This investigation sought to determine how engagement and weight outcomes changed in an intervention that transitioned from in‐person to virtual delivery at the onset of the COVID‐19 pandemic. To do so, a non‐randomized comparison of engagement between cohorts of participants who received a weight management intervention in‐person or by videoconference was performed. Exploratory analyses were conducted on data from a randomized trial comparing a participant‐only to a partner‐assisted weight management intervention over 24 months. 3 During this time, participants experienced several life‐changing events, including stay‐at‐home orders, indoor mask mandates, vaccine availability, return to work guidelines, and the proliferation of videoconferencing for personal, work, and educational activities. These unanticipated changes provided a rare opportunity for the research team to compare the engagement of individuals from the time when the weight loss portion of the intervention was delivered in‐person to when it was delivered remotely over videoconference.
This research explores various indicators of engagement, including attendance at, and duration of, group classes and individual phone calls, and retention for weight assessments. The behavioral targets of daily caloric intake and step‐counts as well as the primary outcome of weight over time are explored. Due to the mixed evidence concerning whether virtually delivered interventions promote or disrupt intervention engagement and weight outcomes, there were no a priori hypotheses.
2. PARTICIPANTS
Community‐dwelling index participants currently residing with a romantic partner in the greater Madison, WI metropolitan area were recruited into one of five cohorts ranging from 38 to 50 dyads each. Participants were enrolled in the study for 24 months (Figure S1). Study sample size calculations, recruitment strategies, and exclusion and inclusion criteria for participants and their partners have been described. 3 Briefly, index participants had either a body mass index (BMI) of 27 to 29.9 plus at least one obesity‐related comorbidity or a BMI of at least 30 kg/m2. Additionally, they had to live and have regular contact with a partner, speak English, be aged 18–74, and not have medical conditions that would contraindicate weight loss or affect weight. See Table 1 and Results for sample characteristics.
TABLE 1.
Sample characteristics.
| Overall | In‐person (Cohorts 1–2) | Virtual (Cohorts 3–5) | |
|---|---|---|---|
| N | 231 | 97 | 134 |
| Date of first group session | 12 March 2019 | 10 March 2020 | |
| Timing relative to COVID‐19 onset | Pre COVID‐19 | Post COVID‐19 | |
| Age (M(SD)) | 47.3 (11.5) | 48.4 (11.6) | 46.5 (11.5) |
| Race (%) a | |||
| • White | 198 (86.8%) | 84 (88.4%) | 114 (85.7%) |
| • Black or African American | 8 (3.5%) | 2 (2.1%) | 6 (4.5%) |
| • Asian | 12 (5.3%) | 7 (7.4%) | 5 (3.8%) |
| • American Indian or Alaskan native | 3 (1.3%) | 1 (1.1%) | 2 (1.5%) |
| • Multiracial | 7 (3.1%) | 1 (1.1%) | 6 (4.5%) |
| Hispanic/Latinx (%) | 10 (4.3%) | 3 (3.1%) | 7 (5.2%) |
| Gender Identity (%) | |||
| • Women | 155 (67.1%) | 61 (62.9%) | 94 (70.1%) |
| • Men | 74 (32.0%) | 34 (35.1%) | 40 (29.9%) |
| • Genderqueer | 1 (0.45%) | 1 (1.0%) | 0 (0.0%) |
| • Multi‐gender | 1 (0.45%) | 1 (1.0%) | 0 (0.0%) |
| Employed full‐time (%) a | 176 (76.5%) | 77 (79.4%) | 99 (74.4%) |
| BMI kg/m2 (M(SD)) | 37.1 (6.4) | 37.8 (7.3) | 36.7 (5.8) |
| Weight kg (M(SD)) | 106.6 (19.4) | 108.4 (20.7) | 105.2 (18.4) |
| Caloric intake (kcal) | 2143 (733.4) | 2088 (781) | 2182 (698) |
| Step count | 8114 (3513) | 7998 (3397) | 8209 (3616) |
221 participants reported their race, and 230 participants reported their employment status.
3. MATERIALS AND METHODS
3.1. Design
Partner2Lose 3 was a parallel, two‐arm randomized controlled trial wherein participants were randomized with equal probability to either a participant‐only or partner‐assisted intervention. Across 24 months, index participants in both study arms were first enrolled in 6 months of weight‐loss programming, then 12 months of weight‐loss maintenance intervention, and finally 6 months of no‐intervention contact. The romantic partners of participants assigned to the partner‐assisted arm participated in half of the group classes and all phone calls, where the couples received additional instruction on and practice with communication skills. Partners of participants assigned to the participant‐only arm did not take part in the intervention. Outcome assessments were collected every 6 months, with weight‐loss at 24 months being the primary outcome.
3.1.1. Key study details
The 6‐month weight loss phase involved 13 group class sessions that met every other week. These classes were co‐led by a registered dietician (RD) and an exercise physiologist, with 60–90 min focusing on nutrition education and behavioral strategies such as goal setting and mindfulness, and 15 min focusing on exercise education and demonstration. 3 The subsequent 12‐month maintenance period involved: (i) 3 monthly group meetings and 3 monthly individual phone calls alternating every two weeks in months 7–9, followed by (ii) 3 monthly individual phone calls in months 10–12, and lastly (iii) 3 individual phone calls delivered every 2 months between months 13–18. All intervention content was consistent throughout the trial, regardless of delivery modality. In the first group session of each cohort, the principal investigator gave an overview of the study and emphasized the need to return for outcome assessments even if participants missed some or all of the intervention. The explanation was accompanied by an infographic demonstrating the potential bias associated with missing data, a strategy shown to be effective for increasing participant knowledge and trust. 23 One month prior to each outcome assessment, the study team mailed a letter to participants asking them to schedule their outcome assessment visit and included the same infographic to remind participants about the importance of outcome assessments.
3.1.2. Pre and post COVID‐19 protocol modifications
Important procedures and key changes made to the protocol in response to the 2020 COVID‐19 stay‐at‐home orders are outlined here. Many details were kept consistent with the pre COVID‐19 intervention plan, 3 with modifications focused on enabling effective virtual delivery of the intervention. The most significant change from the original Partner2Lose protocol was that all cohorts transitioned to remote intervention procedures at various points in the study (Figure S1). Cohort 1 completed all 16 group‐based classes in‐person at a community location. 3 Cohort 2 completed the 13 weight loss classes in person and then had three maintenance group sessions by videoconference. These two cohorts were combined into an “in‐person intervention delivery” group. Cohort 3 had one class in person and then transitioned to videoconference, and Cohorts 4 and 5 completed the entire group‐based intervention remotely. These three cohorts were combined into a “virtual intervention delivery” group (Table 1). The decision to combine Cohorts 1 and 2 into an “in‐person intervention delivery” group and Cohorts 3, 4, and 5 into a “virtual intervention delivery” group was made based on the primary delivery modality of the 13 weight‐loss‐focused group classes. Both Cohorts 1 and 2 attended all weight loss group classes in person, and Cohorts 3, 4, and 5 attended all weight loss group classes virtually except for the first class in Cohort 3. Participants in Cohorts 1, 2, and 3 received exercise bands and Fitbit activity trackers at in‐person baseline visits; Cohorts 4 and 5 received them by mail. All cohorts were mailed digital bathroom scales after the pandemic began because in‐person outcome assessments were prohibited for several months during 2020.
Exercises for all cohorts were meant to be completed at home without equipment, except for a few that required exercise bands provided by the study team. The pre COVID‐19 in‐person cohorts (1–2) participated in 15 min of exercise education followed by exercise physiologist‐led demonstrations. For the post COVID‐19 virtual cohorts (3–5), the study team recorded videos of the exercise physiologist demonstrating exercises at the standard level, with a separate window depicting a study team member demonstrating modified, stepped‐down versions of the exercises that could be performed while seated in a chair. These videos were closed captioned and included content markers to promote greater accessibility. They were shown during the virtual classes.
3.1.3. Measures
Data on participant engagement, captured in Research Electronic Data Capture (REDCap) surveys, 24 , 25 included attendance in 13 weight‐loss group meetings during the 6‐month weight‐loss phase, attendance in three monthly group sessions and nine telephone calls during the 12‐month maintenance phase, and completion of primary outcome assessments every 6 months across the 24‐month study period.
The behavioral targets of dietary intake and physical activity were captured every 6 months. Self‐reported dietary intake was collected using the ASA‐24. 26 This measure is analyzed using proprietary algorithms to estimate daily macronutrient and micronutrient intake. Participants were prompted by e‐mail to complete one survey on a weekday and one on a weekend day within a 7‐day window at each 6‐month assessment period. A video created by the team demonstrated how to enter data into the software. Daily steps were recorded with a Fitbit activity tracker. Participants were asked to wear them daily for 7 days during each 6‐month assessment period. 3
The original protocol required participants to attend an in‐person assessment where they stepped on a study scale and completed surveys on a tablet. During the stay‐at‐home order in 2020, all cohorts were asked to complete outcome assessments at home. Each member of the couple was sent a link to a REDCap survey and instructed to manually type in their weight and then upload a photo of their weight displayed on the study‐provided scale. A member of the study team compared the uploaded photo to the weight typed into the REDCap survey. For one outcome assessment period in late 2020, after the stay‐at‐home order was lifted, participants were asked to complete an outdoor drive‐by visit where they stepped on the study scale and to weigh at home using the previously described method on the same day (Figure S2). In September 2021, the study resumed in‐person assessments and offered remote assessments for participants who were unwilling to attend in‐person. Participants were paid $40 for outcome assessments at 6, 12, and 18 months. Cohorts 1–4 were paid $60 for month 24, and this incentive was increased to $70 for Cohort 5 to boost retention. All procedures described here were approved by the institutional review board (protocol #2018‐1400).
3.1.4. Data preparation and analysis
Results of the main study outcomes and details on adverse events (in partner and participant‐only arms: 3% and 4% were serious, 3% and 1% expected, 0% and 0% probably or definitely related, respectively) will be described in a separate paper. The purpose of the current examination was to compare participant engagement in the Partner2Lose intervention before and after the onset of the COVID‐19 pandemic when participants transitioned from in‐person to virtually delivered intervention.
As there were no differences in primary and secondary outcomes between the participant‐only and partner‐assisted intervention arms, data were collapsed across arms for the present exploratory analyses comparing in‐person to virtual groups. Exploratory descriptive and inferential data analyses were performed on measures of engagement, behavioral targets, and weight. Data were summarized via mean (standard deviation [SD]), median (interquartile range [IQR]), or N (%) where appropriate. Demographic and patient characteristics were compared using t‐tests or chi‐square tests. Wilcoxon rank sum tests were performed on the attendance data, and repeated measures analyses were conducted to assess changes in the behavioral targets and weight outcome over time. Covariates were not included because demographic variables were relatively balanced (Table 1). Due to the exploratory nature of the analyses, an unadjusted 5% significance level was used for all tests. Analyses were performed using R for statistical computing version 4.3.
4. RESULTS
4.1. Sample characteristics
Details on participant enrollment, allocation, and retention are depicted in Figure S3. Across cohorts, there were 231 index participants with mean (SD) age 47.3 (11.5), who self‐identified as 67.1% women, 32.0% men, 0.9% other gender, 86.8% White, 3.5% Black or African American, 5.3% Asian, 1.3% American Indian or Alaskan Native, 3.1% Multiracial, and 4.3% Hispanic/Latinx. Additional information on participant characteristics and baseline equivalency of in‐person and virtual cohorts is outlined in Table 1.
4.2. Engagement measures
Group session attendance was significantly lower for in‐person cohorts (Median [IQR] = 11.0 [5.0–14.0] visits) compared to virtual cohorts (13.0 [10.0–15] visits; W = 4603.5, p < 0.001, d = 0.25, 95% confidence interval (CI) [−3.0, −1.0]) (Figure 1). However, the duration of in‐person classes (M = 73.4, SD = 11.6 min) was similar to virtual classes (M = 65.9, SD = 14.2 min), t = 1.12, p = 0.28, d = 0.37, 95% CI [−4.8, 15.3]. The mean number of maintenance calls attended was nearly identical when comparing the in‐person (Median [IQR]: 9.0 [6.0–9.0] visits) and virtual cohorts (8.0 [6.0–9.0] visits; W = 4114.5, p = 0.32, d = 0.08, 95% CI [0, 0]) (Figure S4). However, the call duration was longer for cohorts who attended the group sessions virtually (M = 29.8, SD = 11.3 min) than in‐person (M = 22.3, SD = 9.1 min), t = −8.35, d = 0.82, 95% CI [–9.6, −5.9].
FIGURE 1.
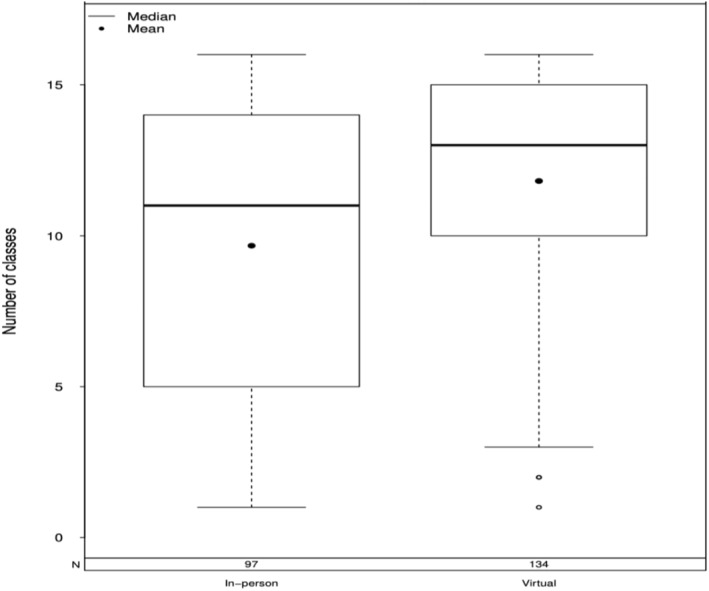
Average number of group classes attended (out of 16) for in‐person versus virtual cohorts. Box and whisker plots indicate the median (solid line), mean (dot), and interquartile range within the dimensions of the boxes.
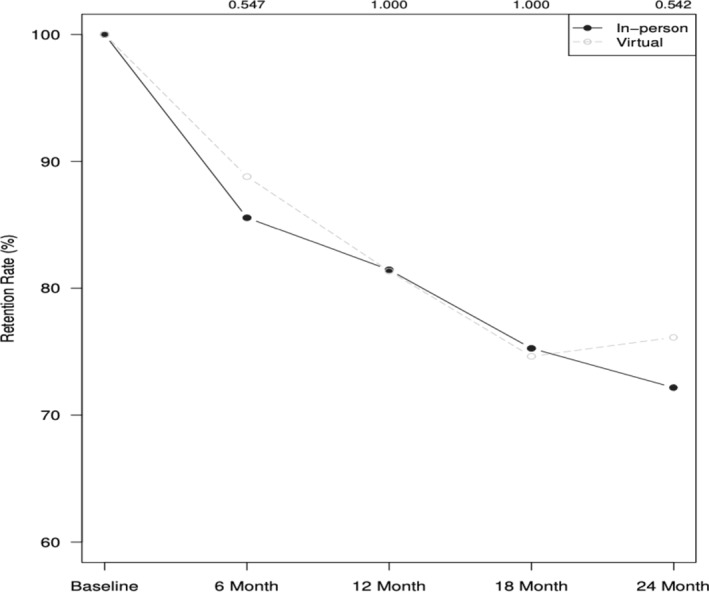
A steep decline was observed in retention for primary outcome assessments in both the in‐person and virtual groups. Provision of weight data dropped from 100% at baseline to 72.2% in the in‐person cohorts and 76.1% in the virtual cohorts at 24 months, with no differences at 6 months (x 2 = 0.28, p = 0.60, w = 0.04), 12 months (x 2 = 0.00, p = 0.99, w = 0.00), 18 months (x 2 = 0.00, p = 0.99, w = 0.00), or 24 months (x 2 = 0.28, p = 0.60, w = 0.04) (Figure 2).
FIGURE 2.
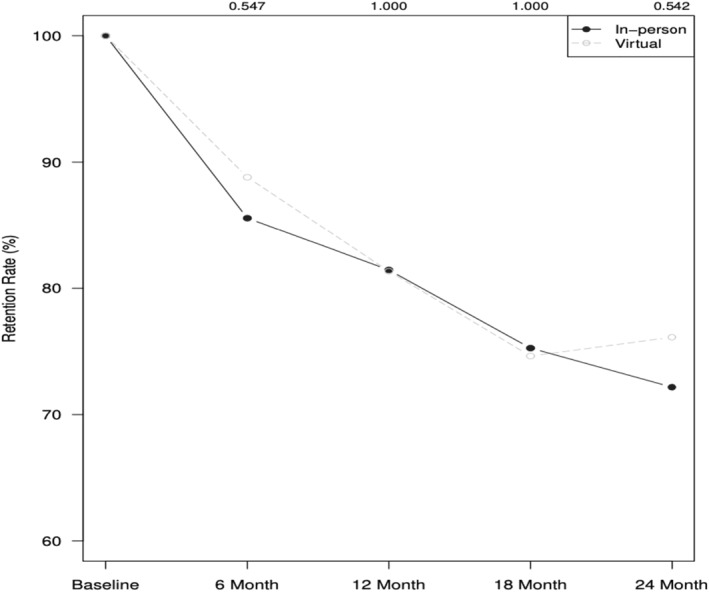
Retention of weight data over time for in‐person and virtual cohorts.
4.3. Behavioral targets
At 6‐month intervals across the 24‐month intervention, there were nearly identical patterns for in‐person and virtual cohorts (Figure 3) in change for average daily caloric intake (kcal) at 6 months (t = 0.16, p = 0.87, d = 0.01, 95% CI[−176,208]), 12 months (t = 0.10, p = 0.93, d = 0.01, 95% CI[−187,205]), 18 months [t = 0.14, p = 0.89, d = 0.01, 95% CI [−186,214]), and 24 months (t = −0.17, p = 0.86, d = −0.013, 95% CI [−225, 188]). The same was true for average daily step counts at 6 months (t = −0.69, p = 0.49, d = 0.06, 95% CI [−1095,525]), 12 months (t = −1.58, p = 0.11, d = 0.13, 95% CI [−1530,165]), 18 months (t = −1.82, p = 0.07, d = 0.15, 95% CI [−1672, 66]), and 24 months (t = −1.16, p = 0.25, d = 0.10, 95% CI [−1446, 373]).
FIGURE 3.
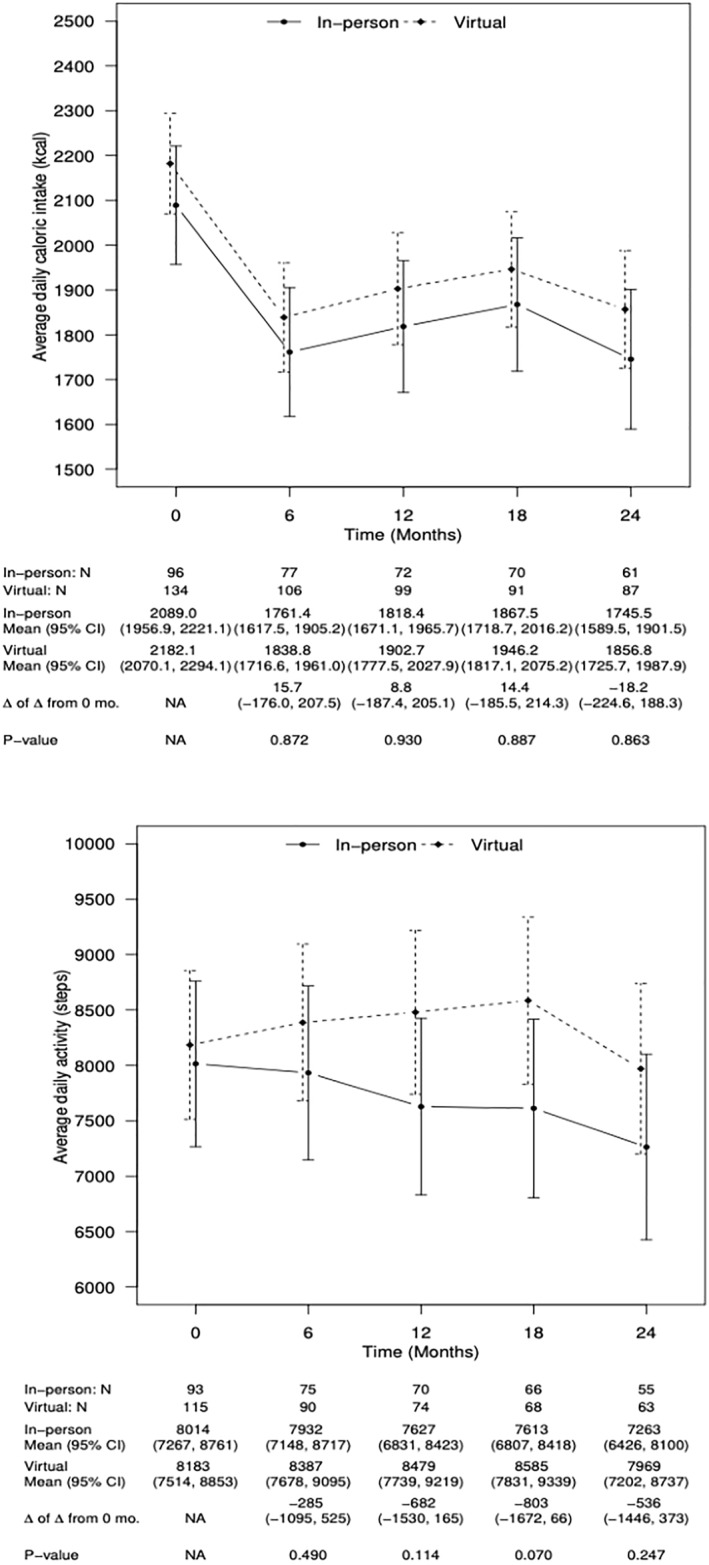
Estimated daily caloric intake and daily steps for in‐person versus virtual cohorts.
4.4. Weight
The pattern of change in participant weight was similar for the in‐person and virtual cohorts (Figure 4) at 6 months (t = 0.81, p = 0.42, d = 0.06, 95% CI [−1.0, 2.5]), 12 months (t = −0.15, p = 0.88, d = 0.01, 95% CI [−2.0, 1.7]), 18 months (t = −0.75, p = 0.45, d = 0.06, 95% CI [−2.6, 1.1]), and 24 months (t = −0.42, p = 0.68, d = 0.03, 95% CI [−2.3, 1.5]), suggesting no major difference by intervention delivery modality.
FIGURE 4.
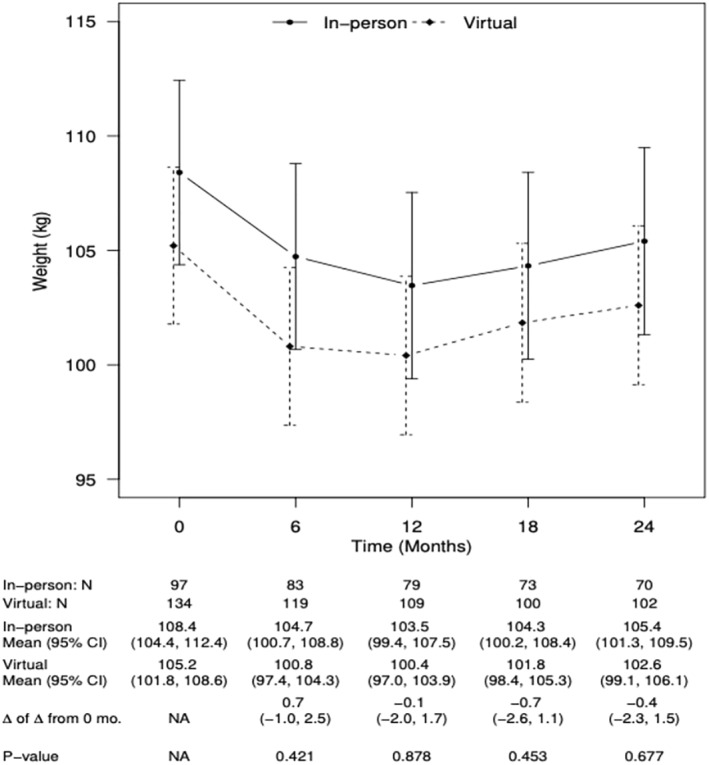
Observed weight across time for in‐person versus virtual cohorts.
5. DISCUSSION
Exploratory results of the Partner2Lose trial suggest similar patterns of engagement with the intervention and related behavioral targets, as well as weight, for in‐person and virtually attended group sessions. The exceptions were significantly greater attendance at group sessions and longer maintenance call duration among cohorts that participated virtually due to COVID‐19. These increases did not translate to improved health behaviors or weight loss.
This boost in attendance in the videoconference relative to the in‐person sessions is consistent with the patterns of attendance reported by Hu and colleagues 20 and Leahey and colleagues. 10 There are several plausible explanations for why attendance may have increased in the virtual cohorts relative to the in‐person cohorts. Participants in the virtual cohorts attended group sessions following the onset of COVID‐19, and the stay‐at‐home orders and shifts in daily routines likely led participants to experience feelings of social isolation, 27 negative emotions like boredom and sadness, 28 to recognize weight gain, 29 and to shift daily activities toward more sedentary behaviors. 30 These factors may have increased the appeal of attending virtual sessions during the height of the pandemic. The change in daily routines (including a transition for some to remote work) may also have reduced transportation barriers as participants no longer needed to travel (including in inclement weather) to attend sessions in‐person. The use of videoconference technology also provided a platform on which participants could safely interact with others.
Although patterns of engagement in phone call attendance and retention of weight data, key behavioral targets, and the longer‐term clinical weight outcome were similar for in‐person and virtual cohorts, engagement still declined in all cohorts over time. Declines in attendance and adherence to behavioral targets are common in weight loss studies. Financial incentives have been shown to increase adherence 31 , 32 ; however, financial incentives for session attendance or for meeting behavioral targets were not provided in this study. Participants were incentivized for providing weight outcome data. When retention for the primary end point was below the pre‐specified target of 80% for the first four cohorts, the team increased the incentive for Cohort 5. However, even with additional financial incentives and education about the importance of providing outcomes, the loss to follow‐up could not be overcome because Cohort 5 had a smaller sample size. This suggests a need for involving community advisory boards and facilitating ongoing community engagement to better identify retention strategies beyond financial incentives for outcome assessments. 32 Future research should also replicate and extend these findings to better evaluate their generalizability to more diverse populations and intervention settings.
Finally, digital engagement is a multifaceted construct that involves the simultaneous investment of affective, cognitive, and physical energies (e.g., emotions, attention, information processing, actions) directed toward a specific task or activity. 4 This can be distinguished from adherence, defined as participants following specific instructions to complete a task (e.g., logging into a virtual session). 4 Adherence behaviors may be necessary, but not sufficient, to sustain engagement over time. Existing research often conflates these concepts and defines both engagement and adherence based on low‐investment behaviors such as clicking on a link, attending a videoconference session, or opening a smartphone application, without requiring additional emotional or attentional investment. Indeed, the maintenance of engagement over time likely involves investment beyond mere adherence.
The present examination has some limitations. As participants' emotional and cognitive investment was not measured, it is not possible to precisely distinguish these concepts in the engagement measure that was collected, namely attendance. Further, behavioral proxies of engagement that reflect investment were not recorded, such as whether participants turned their videos on, unmuted to speak during sessions, and/or sent messages through chat windows. Measuring these behaviors would have provided more in‐depth information regarding participant engagement during the sessions and represents a direction for future research. As this was a non‐randomized comparison, causal arguments cannot be made. Moreover, there is a potential for confounding by unmeasured factors, and the unique circumstances participants faced during COVID‐19 may reduce generalizability of the findings. Finally, although most weight loss sessions were virtual in Cohort 3, the first session was in‐person; thus, this cohort did not experience the entire weight loss component of the intervention virtually.
These limitations are countered by several strengths. Data were systematically collected on attendance, and participants were provided with several options to provide outcomes to accommodate individual differences in comfort. Intervention fidelity was also assessed throughout the trial duration through review by doctoral trained investigators of 75% of group classes and 10% of telephone calls using fidelity checklists.
This research provides a foundational step in examining participant engagement with in‐person versus virtually delivered weight maintenance interventions and brings to light the importance of studying engagement in different ways to understand its impact more precisely. Although virtual weight management programs are promising and may generate similar outcomes to those delivered in‐person, more work is necessary to understand how best to promote engagement in these interventions. Given that engagement is associated with better outcomes in multiple studies, 5 , 6 future research should determine how to conceptualize and measure varying levels of engagement with the goals of (i) effectively promoting and sustaining engagement across time and intervention components and (ii) having a clinically meaningful impact on weight outcomes. Future research should also identify strategies that promote and sustain engagement and determine which aspects of engagement are predictive of enhanced clinical outcomes. Such research will improve both the effectiveness and accessibility of weight maintenance programs.
AUTHOR CONTRIBUTIONS
All authors were involved in the writing of the paper, with Stephanie M. Carpenter, Armaan Shetty, and Corrine I. Voils writing the original draft, and Laura S. Porter, Kristen E. Gray, Ryan J. Shaw, Megan A. Lewis, Heather M. Johnson, Samantha Pabich, William S. Yancy Jr., Katya Garza, Felix Elwert, Lu Mao, and Scott J. Hetzel reviewing and providing critical edits. Corrine I. Voils, Laura S. Porter, Kristen E. Gray, Ryan J. Shaw, Megan A. Lewis, Heather M. Johnson, Samantha Pabich, and William S. Yancy Jr conceived and designed the experiments. Armaan Shetty, Katya Garza, and Samantha Pabich performed the experiments. Scott J. Hetzel, Felix Elwert, Lu Mao, Corrine I. Voils, and Stephanie M. Carpenter were involved in analyzing and interpreting the data, with Scott J. Hetzel conducting formal analyses. Felix Elwert, Lu Mao, and Corrine I. Voils contributed materials and analysis tools.
CONFLICT OF INTEREST STATEMENT
Samantha Pabich is a Consultant for Eli Lilly and Company, and a Consultant for Dynamed. Ryan Shaw is a Consultant for Cerner Enviza. No other authors declare any interests.
Supporting information
Supporting Information S1
ACKNOWLEDGMENTS
The authors thank Kate Cronin, MPH, and Beth Jeanes, MS for project coordination; Rachel Adler, DrPH, RD for contributions to the intervention; Logan Moore, Amanda Haban, and Alissa Turnquist from the Wisconsin Research Education Network for data collection; Shubhangi Sneha and Cynthia Villatoro for logistics; Sean McMullin for database programming; Whitney Sharp, MPH, RD and Andrea von Helms, MPH, RD for intervention delivery; Allison Hung, MPH, for data cleaning; Michaela Kiernan, PhD, for consulting on retention strategies; Alice Yuroff, PhD for data collection; and Kara Gavin, PhD, MPH for support with logistics and intervention fidelity. We also extend our gratitude to Katrina Phelps, PhD and the University of Wisconsin Community Advisors on Research Design and Strategies for providing suggestions about our recruitment letter and to members of the Institute for Clinical and Translational Research Data Monitoring Committee for safety oversight. This study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases [R01 DK111491‐01]. The funder did not have a role in study design, execution, statistical analysis, manuscript preparation or interpretation, or the decision to submit this paper for publication. Dr. Voils was supported by VA Research Career Scientist award [RCS 14–443] and Dr. Gray by Career Development Award [CDA 16–154] from the Health Services Research and Development service of the Department of Veterans Affairs (VA).
Carpenter SM, Shetty A, Hetzel SJ, et al. A non‐randomized comparison of engagement and outcomes for in‐person versus virtual delivery of the Partner2Lose weight management trial. Obes Sci Pract. 2024;e778. 10.1002/osp4.778
The views represented in this article represent those of the authors and not those of the institution or of the VA or the United States Government.
REFERENCES
- 1. Foster GD, Wyatt HR, Hill JO, et al. Weight and metabolic outcomes after 2 years on a low‐carbohydrate versus low‐fat diet: a randomized trial. Ann Intern Med. 2010;153(3):147‐157. 10.7326/0003-4819-153-3-201008030-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johns DJ, Hartmann‐Boyce J, Jebb SA, Aveyard P, Behavioural Weight Management Review Group . Diet or exercise interventions vs combined behavioral weight management programs: a systematic review and meta‐analysis of direct comparisons. J Acad Nutr Diet. 2014;114(10):1557‐1568. 10.1016/j.jand.2014.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Voils CI, Shaw R, Adler R, et al. Protocol for Partner2Lose: a randomized controlled trial to evaluate partner involvement on long‐term weight loss. Contemp Clin Trials. 2020;96:106092. 10.1016/j.cct.2020.106092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nahum‐Shani I, Shaw SD, Carpenter SM, Murphy SA, Yoon C. Engagement in digital interventions. Am Psychol. 2022;77(7):836‐852. 10.1037/amp0000983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Voils CI, Adler R, Strawbridge E, et al. Early‐phase study of a telephone‐based intervention to reduce weight regain among bariatric surgery patients. Health Psychol. 2020;39(5):391‐402. 10.1037/hea0000835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Perri MG, Shankar MN, Daniels MJ, et al. Effect of telehealth extended care for maintenance of weight loss in rural US communities: a randomized clinical trial. JAMA Netw Open. 2020;3(6):e206764. 10.1001/jamanetworkopen.2020.6764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pagoto S, Xu R, Bullard T, et al. An evaluation of a personalized multicomponent commercial digital weight management program: single‐arm behavioral trial. JMIR. 2023;25:e44955. 10.2196/44955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barron E, Bradley D, Safazadeh S, et al. Effectiveness of digital and remote provision of the healthier you: NHS Diabetes prevention programme during the COVID‐19 pandemic. Diabet Med. 2023;40(5):e15028. 10.1111/dme.15028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wilson HK, Averill B, Cook G, Campbell CL. Implementation of the national Diabetes prevention program in FCS extension during the COVID‐19 pandemic: participant experiences, lessons learned. J Fam Consum Sci. 2022;114(3):11‐19. 10.14307/jfcs114.3.11 [DOI] [Google Scholar]
- 10. Leahey TM, Pham J, Denmat Z, et al. Feasibility of online behavioral clinical trials: the future of weight management randomized clinical trials? Obes Sci Pract. 2022;8(6):811‐815. 10.1002/osp4.615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lohnberg JA, Salcido L, Frayne S, et al. Rapid conversion to virtual obesity care in COVID‐19: impact on patient care, interdisciplinary collaboration, and training. Obes Sci Pract. 2021;8(1):131‐136. PMID: 34540265; PMCID: PMC8441727. 10.1002/osp4.550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cliffe M, Di Battista E, Bishop S. Can you see me? Participant experience of accessing a weight management programme via group videoconference to overcome barriers to engagement. Health Expect. 2021;24(1):66‐76. 10.1111/hex.13148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hswen Y, Viswanath K. Beyond the hype: mobile technologies and opportunities to address health disparities. J Mobile Tech Med. 2015;4(1):39‐40. 10.7309/jmtm.4.1.9 [DOI] [Google Scholar]
- 14. Anderson‐Lewis C, Darville G, Mercado RE, Howell S, Di Maggio S. mHealth technology use and implications in historically underserved and minority populations in the United States: systematic literature review. JMIR mHealth uHealth. 2018;6(6):e128. 10.2196/mhealth.8383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Befort CA, VanWormer JJ, Desouza C, et al. Effect of behavioral therapy with in‐clinic or telephone group visits vs in‐clinic individual visits on weight loss among patients with obesity in rural clinical practice: a randomized clinical trial. JAMA. 2021;325:363‐372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clark DO, Keith N, Weiner M, Xu H. Outcomes of an RCT of videoconference vs. in‐person or in‐clinic nutrition and exercise in midlife adults with obesity. Obes Sci Pract. 2019;5(2):111‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tate DF, Valle CG, Crane MM, et al. Randomized trial comparing group size of periodic in‐person sessions in a remotely delivered weight loss intervention. Int J Behav Nutr Phys Activ. 2017;14(144):1‐11. 10.1186/s12966-017-0599-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carpenter SM, Menictas M, Nahum‐Shani I, Wetter DW, Murphy SA. Developments in mobile health just‐in‐time adaptive interventions for addiction science. Curr Addict Rep. 2020;7(3):280‐290. 10.1007/s40429-020-00322-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gentry MT, Lapid MI, Clark MM, Rummans TA. Evidence for telehealth group‐based treatment: a systematic review. J Telemed Telecare. 2019;25(6):327‐342. 10.1177/1357633x18775855 [DOI] [PubMed] [Google Scholar]
- 20. Hu L, Illiano P, Pompeii ML, et al. Challenges of conducting a remote behavioral weight loss study: lessons learned and a practical guide. Contemp Clin Trials. 2021;108:106522. PMID: 34352387; PMCID: PMC8491412. 10.1016/j.cct.2021.106522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fischer M, Weimann T, Oberänder N, Schupitza L, Hösel J, Weimann A. Remote treatment successfully delivers a usual care weight loss and lifestyle intervention in adults with morbid obesity. Ann Nutr Metab. 2022;78(6):328‐335. 10.1159/000526475 [DOI] [PubMed] [Google Scholar]
- 22. Yücel ÜÖ, Yücel M. The effect of phone and video counselling given to participants living with obesity on weight loss and quality of life during the COVID‐19 pandemic: a randomised controlled trial. J Hum Nutr Diet. 2023;36(4):1417‐1424. 10.1111/jhn.13126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kiernan M, Oppezzo MA, Resnicow K, Alexander GL. Effects of a methodological infographic on research participants’ knowledge, transparency, and trust. Health Psychol. 2018;37(8):782‐786. 10.1037/hea0000631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inf. 2009;42(2):377‐381. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inf. 2019;95:103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Subar AF, Thompson FE, Potischman N, et al. Formative research of a quick list for an automated self‐administered 24‐hour dietary recall. J Am Diet Assoc. 2007;107(6):1002‐1007. 10.1016/j.jada.2007.03.007 [DOI] [PubMed] [Google Scholar]
- 27. Brodeur A, Clark AE, Fleche S, Powdthavee N. COVID‐19, lockdowns and well‐being: evidence from google trends. J Publ Econ. 2021;193:104346. 10.1016/j.jpubeco.2020.104346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Droit‐Volet S, Gil S, Martinelli N, et al. Time and Covid‐19 stress in the lockdown situation: time free, «Dying» of boredom and sadness. PLoS One. 2020;15(8):e0236465. 10.1371/journal.pone.0236465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zeigler Z. COVID‐19 self‐quarantine and weight gain risk factors in adults. Curr Obes Rep. 2021;10(3):423‐433. 10.1007/s13679-021-00449-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stockwell S, Trott M, Tully M, et al. Changes in physical activity and sedentary behaviours from before to during the COVID‐19 pandemic lockdown: a systematic review. BMJ Open Sport Exerc. 2021;7(1):e000960. 10.1136/bmjsem-2020-000960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Voils CI, Pendergast J, Hale SL, et al. A randomized feasibility pilot trial of a financial incentives intervention for dietary self‐monitoring and weight loss in adults with obesity. Transl Behav Med. 2021;11(4):954‐969. 10.1093/tbm/ibaa102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leahey TM, Subak LL, Fava J, et al. Benefits of adding small financial incentives or optional group meetings to a web‐based statewide obesity initiative. Obesity. 2015;23(1):70‐76. 10.1002/oby.20937 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1


