Abstract
Background
Accurate identification of pain generators in the context of low back and spine-related pain is crucial for effective treatment. This review aims to evaluate the potential usefulness of single photon emission computed tomography with computed tomography (SPECT/CT) as an imaging modality in guiding clinical decision-making.
Methods
A broad scoping literature review was conducted to identify relevant studies evaluating the use of SPECT/CT in patients with spine-related pain. Studies were reviewed for their methodology and results.
Results
SPECT/CT appears to have advantages over traditional modalities, such as magnetic resonance imaging and CT, in certain clinical scenarios. It may offer additional information to clinicians and improve the specificity of diagnosis. However, further studies are needed to fully assess its diagnostic accuracy and clinical utility.
Conclusions
SPECT/CT is a promising imaging modality in the evaluation of low back pain, particularly in cases where magnetic resonance imaging and CT are inconclusive or equivocal. However, the current level of evidence is limited, and additional research is needed to determine its overall clinical relevance.
Clinical Relevance
SPECT/CT may have a significant impact on clinical decision-making, particularly in cases in which traditional imaging modalities fail to provide a clear diagnosis. Its ability to improve specificity could lead to more targeted and effective treatment for patients with spinal pathology.
Level of Evidence
4.
Keywords: SPECT/CT, diagnostic imaging, axial back pain, lumbar degenerative disease, pain generators
Introduction
Identifying the cause of pain is often a diagnostic dilemma plaguing the treatment of patients with degenerative pathology in the spine. Discs, facet joints, nerves, muscles, ligaments, and spinal instability may all be sources of neck and back pain. While magnetic resonance imaging (MRI) is a highly sensitive diagnostic modality that provides excellent resolution of bone, soft tissue, and neurological structures, it has been shown that MRI findings in the aging spine are often asymptomatic and thus poorly specific.1
Successful outcomes after lumbar spine surgery are commonly dependent on accurate identification of the patient’s dominant pain generator. Attempts to avoid incomplete surgical treatment of axial pain may lead surgeons to pursue multilevel fusion procedures. This approach can lead to unfavorable outcomes as complication rates and unintended long-term consequences increase with the magnitude of surgery.2 Accurately identifying sources of axial pain may also help guide targeted nonsurgical treatment. Given that the majority of care delivered for axial pain is nonoperative, improvements in diagnostic methods have the potential to improve outcomes and reduce costs on a large scale.
The shortcomings of MRI in localizing sources of axial pain in patients with spine-related pain and multilevel degenerative pathology have led to a search for more clinically useful imaging modalities. Bone single photon emission computed tomography with computed tomography (SPECT/CT) has emerged as a potentially useful tool in this patient population. This review summarizes the scientific basis for bone SPECT/CT imaging of the spine and provides a broad overview of the clinical scenarios in which this modality may be helpful.
Methods
This study was conducted as a scoping literature review, focusing on the use of SPECT/CT in spine surgery. We utilized major databases available through Google Scholar to gather relevant literature. The search was restricted to the English language only, allowing for a broad and inclusive review of the topic.
The search strategy was designed to capture all articles that discussed the use of SPECT/CT in the diagnosis, management, and treatment of various spinal conditions. The search terms included combinations of “SPECT/CT,” “spine surgery,” “diagnosis,” “management,” “treatment,” and “outcomes.” After the initial search, all article titles and abstracts were screened for relevance. The full texts of potentially relevant articles were then reviewed in detail. Any studies that did not provide sufficient information on the use of SPECT/CT in spine surgery were excluded.
The data extracted from each study included the study design, patient population, spinal condition being treated, use of SPECT/CT, and the reported outcomes. These data were then synthesized and analyzed to provide a comprehensive overview of the current state of knowledge on the use of SPECT/CT in spine surgery.
Basic Science of SPECT/CT Imaging
Nuclear SPECT/CT imaging is an extension of conventional gamma camera planar imaging but is acquired 3-dimensionally and merged with a CT image.3 Some of the commonly used radiotracers in SPECT/CT include Technetium 99m-methyl diphosphonate (99mTc-MDP), Gallium-67 (67Ga), and Indium 111-tagged white blood cell (111In-WBC), and radiotracer choice is dependent on tissue/study of interest.4 99mTc-MDP binds to calcium and is commonly used in bone scintigraphy and can be detected by gamma camera planar or SPECT/CT. Like contrast, 99mTc-MDP biodistribution depends on the time interval between administration and imaging. Immediate imaging after administration would highlight vascular structures, while imaging after a few minutes will demonstrate more uptake in soft tissues. With late-phase imaging, up to several hours after 99mTc-MDP administration, uptake is expected to be predominantly within skeletal structures and the genitourinary system. Biochemically, this is due to 99mTc-MDP diffusion to extracellular space then binding to hydroxyapatite crystals and calcium salts. In bones, binding is due to radiotracer chemisorption to the hydroxyapatite matrix, particularly at the osteoid and osteocyte lacunae (or mineralization front of the bone); radiotracer uptake is minimal or absent near osteoclast sites. Radiotracer that is not bound must be metabolized or excreted. MDP is a disphosphonate compound belonging to a class of bisphosphonates not significantly metabolized but renally excreted. Within 2 to 3 hours, 99mTc-MDP that is not bound to the skeleton is eliminated. As elimination is renally dependent, glomerular function is an important consideration with SPECT/CT, and patients should be well hydrated to reduce adverse effects and improve image quality.5
SPECT/CT combines images from both SPECT and CT. Gamma cameras are the basis for SPECT imaging. Gamma cameras detect gamma rays emitted from radionuclides that have been ingested or injected into a patient through crystals that detect gamma photons and accumulate counts within the camera. Gamma camera performance is typically evaluated on image sharpness, efficiency of radiation detection, ability to measure energy of radiation, and the counting rate without dead time losses.6 The result is a 2-dimensional image similar to radiographs. In contrast, tomographic gamma cameras used in SPECT imaging yield slices through the body similar to a cross-section CT or MRI.7
Aligning SPECT and CT images is a complex task that involves a process known as coregistration. Specialized software algorithms are used for this integration of anatomical and functional images, similar to how positron emission tomography (PET) scans are aligned with CT scans.5 For each patient, an attenuation map showing the distribution of attenuation coefficients is created, which interactive reconstruction algorithms use to correct the emission data. The process converts Hounsfield units into attenuation coefficients at the SPECT radionuclide’s energy, using either segmentation, scaling, or a hybrid technique. This combination of SPECT and CT allows for consistently coregistered anatomical images in a single study, providing convenience for the patient. Reports suggest coregistration accuracy can be as specific as 3 mm or better, allowing for 3-dimensional visualization of anatomical regions with increased radiotracer uptake.8
Clinical Utility of SPECT/CT In The Degenerative, Surgically Naïve Spine
Facetogenic Pain
Axial back and neck pain represents a complex spectrum of clinical entities influenced by diverse risk factors and etiologies across geographical and socioeconomic spectrums.9,10 It represents a significant economic burden, amounting to an estimated $134.5 billion of health care expenditure in the United States in 2016.11 Despite debates over the correlation between degenerative changes in advanced imaging and pain/disability, intervertebral discs and facet joints are recognized as potential sources of axial pain.
Facet joints, characterized as true synovial joints with cartilaginous articular surfaces, are crucial for spinal motion, load transmission, and stability.12 They can generate an inflammatory response and activate nociceptive nerve endings in response to cartilage compression, leading to facetogenic pain.13,14 This pain affects 15% to 41% of chronic low back pain sufferers, with osteoarthritis being the most common cause.12,15
Facet joints lead to facetogenic pain, a type of axial mechanical pain, typically provoked by specific body positions such as extension, and can overlap with other spinal pathologies. Localization of pain generators through clinical evaluation or CT/MR imaging is challenging.16–21 Although the most reliable diagnostic test in the setting of a painful facet joint involves an intra-articular diagnostic injection or medial branch block, this test is invasive and carries some risk.22–24 This has led to the exploration of bone SPECT/CT (henceforth referred to as just “SPECT/CT”) for better characterization of the source of facetogenic pain.
The potential of SPECT/CT first emerged in 2007 when patients reported an average improvement of 4.4 + 1.6 visual analog scale (VAS) points following SPECT/CT-guided lumbar facet joint injections (Figure 1).25 Subsequent research in 2013 by Matar et al supported these findings, demonstrating that SPECT/CT identified potential pain sources in 92% of cervical spine scans and 86% of lumbar spine scans and guided injection therapies in 60% of cases.26 Such findings underscore the potential utility of SPECT/CT in the diagnosis and treatment of facetogenic pain.
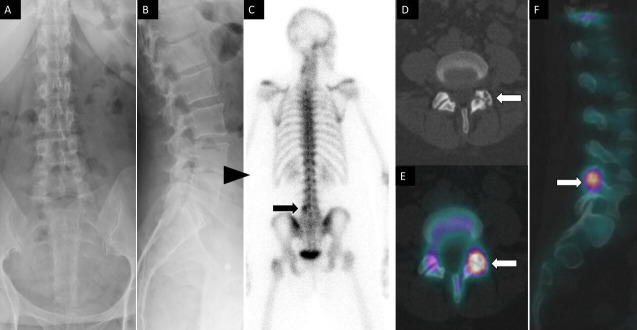
Figure 1.
A 52-year-old woman had lumbar back pain. Anteroposterior (A) and lateral (B) spine radiographs show mild multilevel degenerative disc narrowing most notable at L4 to L5 (arrowhead). Posterior planar 99m-methyl diphosphonate bone scan image (C) shows focal increased osteoblastic activity within the left lateral aspect of L5 vertebra. Axial noncontrast computed tomography (CT) (D) and fused axial (E) and coronal (F) single photon emission CT with CT images demonstrate increased osteoblastic activity within the left L4 to L5 facet joint with associated subchondral cysts and joint space narrowing compatible with degenerative facet arthropathy.
SPECT/CT’s sensitivity in detecting pain generators was corroborated in a study by Ravindra et al, where all 7 patients exhibited facet joint hypertrophy with degeneration corresponding to SPECT/CT hotspots after undergoing posterior cervical fusion and decompression. Postoperative VAS scores improved by 4 points on average, and the Neck Disability Index improved by 20%. These findings suggest that SPECT/CT can enhance the detection of symptomatic facet joint pathology and improve operative outcomes.27 In contrast, in a case series of 99 patients by Russo et al, 40% showed discrepancies between scintigraphic uptake and facet joint degeneration on CT images, suggesting that conventional imaging might not always identify pain generators. Using Pathria’s grading system, 69% of grade 3, 16.8% of grade 2, and 5.5% of grades 0–1 patients were positive on SPECT/CT, indicating its potential in guiding treatment planning.3
In addition, Lehman et al found in their retrospective series that activity on SPECT/CT did not always correlate with clinical findings.28 In 74 patients who underwent hybrid imaging, they found that 70% had discrepant imaging findings and treatment selections for at least 1 facet joint, while 46% had a side (right vs left) discrepancy. In another study examining the correlation between scintigraphic uptake and conventional imaging, they found that only 52 of 716 (7.3%) facet joints were rated positive on SPECT/CT as compared with 149 of 720 (20.7%) on fat-suppressed MRI.29 Without a gold standard or clinical information, it is not possible to make any conclusions regarding the clinical relevance of this study.
In summary, when evaluating for presumptive facetogenic pain, clinical findings should be carefully correlated with SPECT/CT findings. Furthermore, once a particular facet joint(s) has been identified as the suspected pain generator, at minimum, further interrogation with a diagnostic injection would be prudent.
Discogenic Pain
Intervertebral discs provide shock absorption of axial compressive forces as well as flexibility throughout the vertebral column. Each disc consists of a sturdy outer annulus fibrosus, a gelatinous inner nucleus pulposus, and hyaline cartilage end plates.30 These discs are innervated by sinuvertebral nerves and gray rami communicantes, enabling pain signal transmission from the disc.31,32
Discogenic pain is estimated to account for approximately 40% of low back pain cases.33,34 It arises as a consequence of intervertebral disc pathology in the absence of nerve root compression or segmental instability.35 In the degenerated disc, the presence of radial fissures upregulates inflammatory modulators and growth factors that increase the density of nociceptive nerve fibers in the area of the tear.36
Discogenic pain is typically characterized by a deep, dull ache that is provoked by axial loading and improved with recumbence. Some patients may be able to identify an acute onset of pain following a bending, twisting, or lifting event. However, history and physical examination alone are often unreliable in diagnosing or ruling out discogenic pain.19 Radiographs are commonly performed as part of the initial workup for back pain, though their utility in identifying pathology related to the disc may be limited. MRIs have been the most reliable tool for examining the disc, but the correlation between symptom severity and the extent of degenerative changes on imaging is unclear.37–40 Provocative discography, which involves fluoroscopic evaluation of the intervertebral disc with the placement of needles, is controversial but can help identify primary pain generators, especially in the setting of multiple positive discs on MRI. However, this invasive procedure poses risks, such as potentially exacerbating an existing disc pathology or causing degeneration in an otherwise normal disc. Current literature suggests that discography may further increase disc degeneration over time41 and yield a high number of false positives.42
Given these diagnostic challenges, attention has been turned toward the assessment of discogenic pain with SPECT/CT. In a prospective series, Harisankar et al found that the presence of increased uptake in the anterior body on SPECT/CT correlated with degenerative disc changes on MRI and CT in 7 patients with low back pain.43 Another case series by Kato et al described the utility of SPECT/CT in identifying pain generators in 3 patients with degenerative disc disease.41 This led to the decision to carry out selective, short-segment spinal fusions for all 3 patients.
Adding to this, a prospective study by Russo et al revealed a strong association between Modic changes observed on MRI and heightened activity on SPECT/CT images. In a cohort of 99 subjects with low back pain, they found that 71% of MRI findings resulted in scintigraphically active endplates and disc spaces on SPECT/CT.3 Interestingly, 96% of end plates with type 1 Modic changes exhibited high osteoblastic activity on SPECT/CT. These findings led them to conclude that SPECT/CT hybrid imaging could offer valuable supplementary insights for tailoring treatment options.
In a comparative study by Van de Kelft et al, SPECT/CT was used to identify hot spots in patients with and without chronic low back pain.44 Out of 94 patients with chronic low back pain, 45 (47.87%) were found to have increased vertebral end plate uptake vs 19 out of 103 (18.45%) in the control group. However, approximately one-third of patients with chronic low back pain did not exhibit increased uptake on SPECT/CT. The authors hypothesized that in symptomatic individuals, disc degeneration may cause pain prior to the onset of bony changes, resulting in a normal SPECT/CT image despite a potential pain generator. However, in asymptomatic individuals, chronic changes to the bone may occur without inflammation, resulting in increased uptake on SPECT/CT despite the absence of pain. Therefore, while SPECT/CT offers promise for diagnosing patients with chronic back pain, additional research is needed to clarify its role in evaluating discogenic pain.
In summary, while the understanding of discogenic pain and the diagnostic specificity of SPECT/CT continues to evolve, both are pivotal in comprehending the pathophysiological processes of pain generation. The role of the end plate in the diagnosis of discogenic pain is not fully understood. The presence of Modic changes underpins some diagnostic algorithms, implying its significance in pain causation. The exact mechanism of discography remains unclear as well, with both annular strain and end plate pressure potentially contributing to positive results. Although SPECT/CT seems more adept at detecting end plate abnormalities, treatment options for both disc and end plate pathologies appear similar. Presently, in patients demonstrating potential discogenic pain, a combination of discography, SPECT/CT, and MRI may enhance diagnostic specificity despite the inherent risks of discography (Figure 2).7,42 SPECT/CT may potentially aid in establishing indications for basivertebral nerve ablation, as this seems to target the end plate of the disc in the setting of discogenic or vertebrogenic pain, but more research is needed.45,46
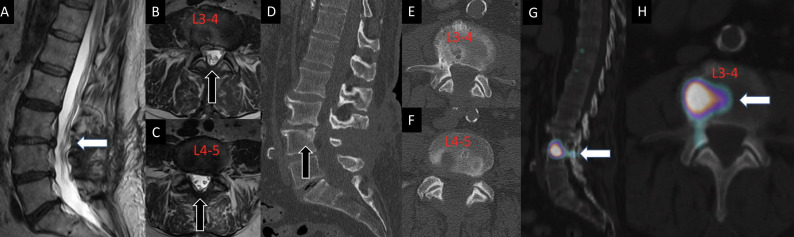
Figure 2.
A 70-year-old man had previously undergone L3 to L5 decompression for lumbar spinal stenosis with leg dominant radicular pain. After the decompression, the patient presented with a new complaint of axial flexion-dominant mid-lumbar pain in the absence of leg symptoms. Advanced imaging demonstrated no significant residual/recurrent stenosis on sagittal and axial T2-weighted MRI sequences (1, 2, and 3), and appropriate decompression on sagittal and axial computed tomography images without evidence of pars interarticularis fracture or subtotal facetectomy (4, 5, and 6). Single photon emission computed tomography with computed tomography findings demonstrated increased radiotracer uptake in the L3 to L4 disc (7 and 8). The L3 to L4 disc was determined to be the dominant pain generator, resulting in a discogenic pain pattern. The patient was then referred for further physical therapy and physical medicine and rehabilitation.
SPECT/CT In The Diagnosis and Management of Spinal Fractures
SPECT/CT plays a crucial role in diagnosing and managing various types of spinal fractures, including sacral insufficiency fractures (SIFs), osteoporotic vertebral compression fractures (OVCFs), and pars interarticularis fractures in children and young adults.
Sacral Insufficiency Fractures
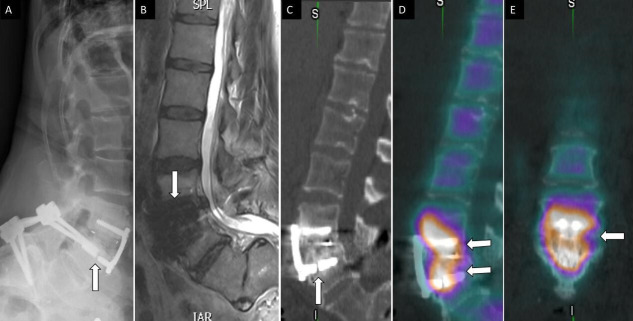
SIFs are a treatable and often underreported cause of axial low back pain.47 SIFs can occur in the setting of rheumatoid arthritis, osteoporosis, prolonged corticosteroid use, and after pelvic irradiation.47–49 Patients with multilevel lumbar fusion rostral to the sacrum are also at risk, given the high-stress concentration at the sacrum.50–52 Rarer etiologic risk factors for SIFs include osteomalacia, Paget’s Disease, osteodystrophy, and hyperparathyroidism.53 The gold standard for diagnosing SIFs is MRI.54–56 However, SPECT/CT can visualize these fractures as a Honda sign and is particularly useful in health care settings where MRI accessibility is limited or contraindicated52,57 (Figure 3).
Figure 3.
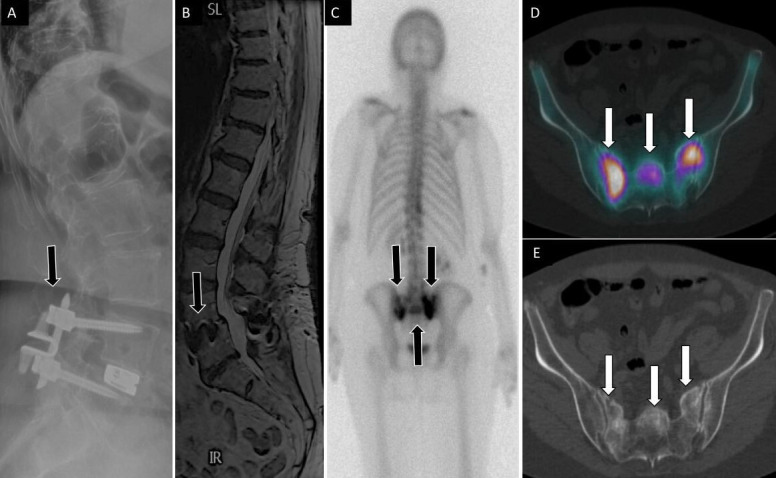
71-year-old woman with low back, buttock, and bilateral leg pain. Lateral (A) spine radiograph shows L4 to L5 instrumentation with prior interspinous spacer and unilateral pedicle screws with interbody device (arrow) without evidence of complications. Lumbar spine T2 sagittal magnetic resonance image (B) shows no central canal stenosis, also seen on axial (not displayed) and metal artifact at L4 to L5 (arrow) related to instrumentation. Posterior planar 99mTc-MDP bone scan (C) and axial fused single photon emission computed tomography with computed tomography (D) images show H-shaped radiotracer uptake within the sacrum, with associated sclerotic changes on computed tomography (E), compatible with sacral insufficiency fracture.
Osteoporotic Vertebral Compression Fracture
OVCFs are a common pathology primarily affecting older patients and can lead to pain, disability, and kyphotic deformity. While these injuries are visible on plain films and CT images, MRI remains the gold standard imaging modality for distinguishing acute and subacute fractures from chronic injuries.58 However, numerous studies have demonstrated that SPECT/CT is of comparable efficacy for diagnosing acute and subacute OVCFs59 (Figure 4). Furthermore, there is some evidence to suggest that in the setting of OVCF, SPECT/CT can be useful in predicting the patient’s response to vertebral cement augmentation. Sola et al performed SPECT/CT on 33 OVCF patients intended for cement augmentation and noted clinical improvement in 91% of patients with positive SPECT/CT and identification of additional pain generators in 5 patients.60
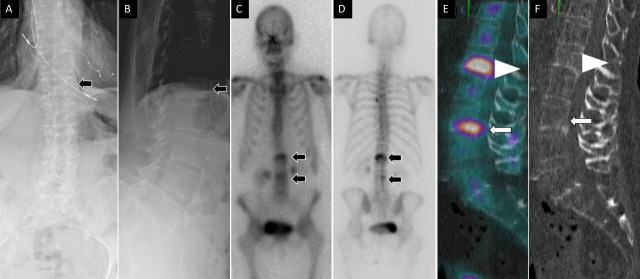
Figure 4.
A 76-year-old woman presented with acute-onset low back pain. Anteroposterior (A) and lateral (B) spine radiographs show mild height loss at the level of the L1 vertebral body (arrow). Anterior (C) and posterior (D) planar 99mTc-MDP bone scan images show focal increased osteoblastic activity at L1 and L3 vertebral levels (arrows). Sagittal fused single photon emission computed tomography with computed tomography (E) demonstrates increased activity at the L1 and L3 vertebral bodies. Noncontrast computed tomography images (F) demonstrate corresponding height loss (arrowhead) at the L1 vertebral body and inferior endplate irregularity and linear sclerotic changes (arrow) at the L3 vertebral body, compatible with healing vertebral compression fractures.
Pars Interarticularis Fracture in Children and Young Adults
Spondylolysis or fracture of the pars interarticularis is a common source of axial low back pain in adolescents and young adults participating in activities such as diving, gymnastics, and weight lifting, which involve repetitive hyperextension of the lumbar spine. Spondylolysis in this setting most commonly involves the L5 pars interarticularis. CT is the gold standard imaging study to detect a pars defect. Planar SPECT can be utilized to diagnose this clinical entity, and this modality has been shown to be more accurate than plain radiographs alone.61,62 However, there may be advantages of SPECT/CT (Figure 5) over planar SPECT, specifically with regard to delay in treatment. In a retrospective comparison of a cohort of young athletes with pars interarticularis fractures who underwent SPECT and a similar cohort who underwent SPECT/CT, a median (interquartile range) delay of treatment of 7 (8) days was observed in the SPECT cohort compared with no delay with SPECT/CT.63 SPECT/CT is only diagnostic when there is increased metabolic activity at the pars. In an established nonunion, it would become nondiagnostic. For individuals with asymptomatic spondylolysis, the use of SPECT/CT as a diagnostic tool remains unexplored.
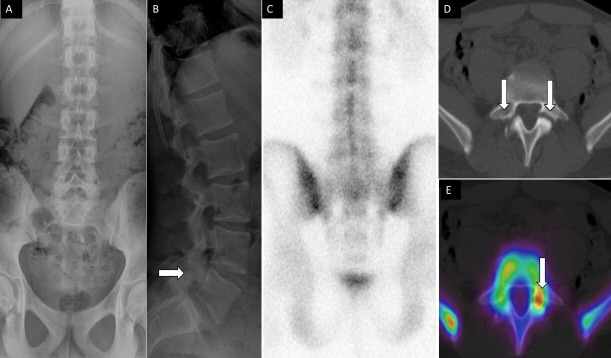
Figure 5.
A 14-year-old male football player presenting with low back pain. Anteroposterior (A) and lateral (B) spine radiographs show subtle lucency on the lateral view within the L5 pars interarticularis (arrow). Posterior planar 99mTc-MDP bone scan image (C) was unremarkable. Axial noncontrast computed tomography (CT (D) and fused single photon emission CT with CT (E) images demonstrate bilateral pars defects, with asymmetric increased activity within the left pars defect, respectively.
SPECT/CT In The Previously Operated Spine
Diagnosis of Pseudarthrosis and Instrumentation Loosening
SPECT/CT’s utility extends to diagnosing pseudarthrosis and instrumentation loosening after spinal fusion, although these data are less robust.64 Rager et al described the results of SPECT/CT and CT alone in 10 consecutive patients with recurrence of back pain and suspicion of pseudarthrosis by radiograph. SPECT/CT performed identically to CT in the detection of screw loosening and was more sensitive than CT for facet joint degeneration (Figures 6 and 7). It was also noted that SPECT/CT was negative in 3 of 5 patients who had nonunion through/around the cages on CT alone.65
Figure 6.
A 56-year-old woman presented with low back pain after prior L4 to S1 posterior instrumented fusion and subsequent standalone L3 to L4 anterior lumbar interbody fusion with anterior plate fixation. Lateral lumbar spine radiograph (A) shows fractured screw related to anterior instrumentation at the level of L4 (arrow). Sagittal T2 magnetic resonance imaging (B) shows no evidence of acute central canal stenosis and metal artifact (arrow) related to instrumentation. Sagittal noncontrast computed tomography (C) demonstrates fractured screw into the L4 vertebral body and sclerotic changes within the bone. Fused sagittal and coronal images demonstrate increased osteoblastic activity and radiotracer uptake within the L3 to L4 disc space concerning for hardware loosening and pseudoarthrosis.
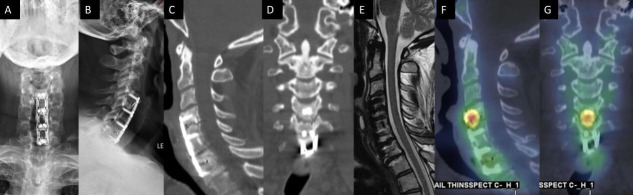
Figure 7.
A 54-year-old man presented with persistent axial mechanical neck pain after previously undergoing C4-7 anterior cervical discectomy and fusion 2 years earlier. Anteroposterior (A) and lateral (B) plain films demonstrated all implants from the index surgery were appropriately positioned. Sagittal (C) and coronal computed tomography (CT) reconstructions did not demonstrate any bridging bone across the C4-5 disc space. No residual/recurrent central stenosis was seen on mid-sagittal T2-weighted magnetic resonance imaging (E). Sagittal (F) and coronal (G) single photon emission CT with CT images demonstrated increased radiotracer uptake at the C4-5 level, which suggested symptomatic pseudarthrosis. The patient subsequently underwent posterior cervical instrumented fusion with good result.
Further investigation of SPECT/CT’s role was highlighted in another series that focused on 8 patients with surgically confirmed pseudarthroses. SPECT/CT successfully identified pseudarthroses in 7 of these cases.66 One of the largest studies evaluating the utility of SPECT/CT, conducted by Heimburger et al, involved 54 patients with axial pain after lumbar fusion. They showed an 81% sensitivity and 83% specificity for pseudarthrosis following posterolateral fusion, as well as 100% sensitivity coupled with 60% specificity in detecting interbody pseudarthrosis.67
While SPECT/CT can add useful diagnostic information in cases where there is uncertainty about fusion status, this modality should not be used in isolation to determine the need for reoperation. When clinical evaluation and other imaging and laboratory studies result in uncertain determination of symptomatic pseudarthrosis, SPECT/CT can be considered as an additional diagnostic source of information.
SPECT/CT In Predicting Response to Spine Fusion
SPECT/CT also appears to have utility in predicting the response of axial neck and back pain after spinal fusion procedures, though current supporting evidence is limited. Ravindra et al utilized SPECT/CT in a series of 7 patients to assist in the diagnosis of upper cervical facet arthropathy. These 7 patients all had focal unilateral uptake within the facet joints at either C1-2 or C2-3 and went on to have a selective single-level posterior fusion surgery. Despite varying responses to image-guided injection into these joints, all but 1 patient had significant reduction in their neck pain following surgery.28 Similarly, Brusko et al reported in their results that 82% of 23 patients undergoing cervical or lumbar fusion for axial symptoms, targeted based on SPECT/CT, saw substantial pain reduction at 1-year follow-up.68
The findings of these studies were corroborated by a large study of SPECT/CT in surgically treated patients. Tender et al studied 189 patients with positive SPECT/CT. Of these, 86 patients had scans that were focally positive at 2 or fewer areas and were offered surgery. Of these, 48 patients underwent 1- or 2-level cervical or lumbar fusion procedures for axial pain-related complaints. The authors reported a significant reduction in self-reported VAS scores of axial pain from 9.0 ± 1.4 to 4.3 ± 2.3 (P = 0.03).69 However, the lack of a control group complicates evaluation of surgical success based solely on history, examination, and MRI appearance vs preoperative SPECT/CT. Figure 6 showcases the superiority of SPECT/CT in delineating hardware loosening and pseudoarthrosis after anterior cervical discectomy and fusion as compared with conventional imaging.
These preliminary studies indicate SPECT/CT’s potential in predicting axial pain relief after fusion surgery. If replicated in larger studies, SPECT/CT would have tremendous potential to address a long-standing dilemma surrounding the indications for spine fusion.
Malignancy
Metastatic disease of the spine and associated pathological fractures is common and is associated with significant morbidity.70,71 SPECT/CT has been extensively researched for assessing bone metastases, demonstrating higher specificity (84%–98.6%) in detecting equivocal bone lesions in cancer patients compared with SPECT alone and planar bone scintigraphy (Figure 8).72–78
Figure 8.
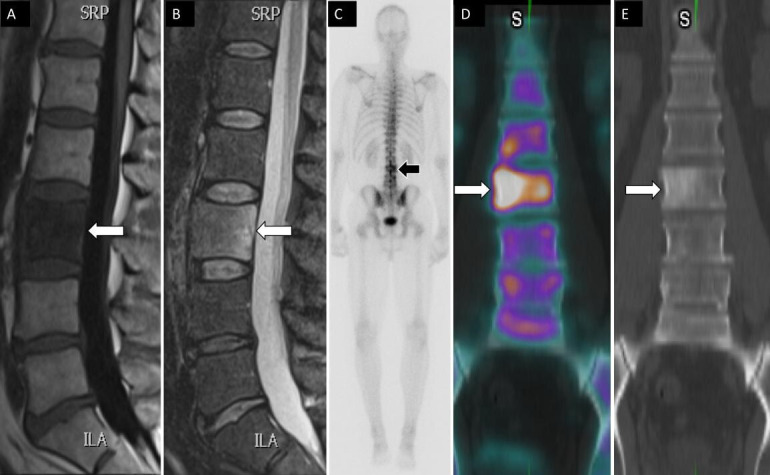
A 49-year-old man had a history of tonsillar squamous cell carcinoma and low back pain. Sagittal T1 (A) and STIR (B) magnetic resonance images show low T1 and high T2 signal changes within the L3 vertebral body (arrows). Posterior planar 99mTc-MDP bone scan image (C) shows increased osteoblastic activity at L3 vertebral body. Coronal fused single photon emission computed tomography with computed tomography image (D) demonstrates an osteoblastic lesion within the right L3 vertebral body with associated sclerotic changes on computed tomography (E) compatible with metastatic bone lesion.
Focusing specifically on spinal metastases, 2 SPECT/CT studies by Iqbal et al and Zhang et al revealed how SPECT/CT considerably reduces indeterminate findings and enhances sensitivity.79,80 Iqbal et al identified 50 oncology patients and 30 nononcology patients with solitary vertebral lesions by planar bone scintigraphy and performed SPECT/CT on the same day to further evaluate. Lesions were classified as benign, malignant, or indeterminate by 2 nuclear medicine physicians. The majority (63.8%) of lesions were indeterminate by planar bone scintigraphy but only 13.8% were indeterminate after SPECT/CT. Additionally, SPECT/CT improved sensitivity from 6.1% with planar bone scintigraphy to 78.8% and was able to accurately differentiate degenerative disc disease, facet arthropathies, and disc infection from focal metastases.79 Zhang et al performed a similar study by using SPECT/CT on 90 solitary spinal hypermetabolic lesions that were equivocal on planar bone scintigraphy. After clinical follow-up and histopathology, 28% of the lesions were malignant and 72% were benign.
SPECT/CT reduced equivocal cases to 5.6% compared with 40% using SPECT alone. This resulted in significantly better diagnostic accuracy of 91.1% in SPECT/CT compared with 58.9% in SPECT.80 Although MRI is the gold standard for diagnosing metastatic disease, SPECT/CT outperforms planar bone scintigraphy and generally compares favorably to MRI. A direct comparison by Jambor et al found SPECT/CT to be as specific but less sensitive than MRI.81 Thus, for patients with multiple spinal metastases or several potentially painful yet morphologically alike lesions, SPECT/CT may allow for more precise treatment. It may also be a useful diagnostic tool for spinal metastatic disease when MRI is inaccessible or contraindicated.
Pyogenic Infections of The Spine
Spinal infections, including osteodiscitis and epidural abscesses, can also lead to significant back pain, particularly affecting the elderly and those with weakened immune systems.82 While once rare, the incidence of spinal infections is now increasing.82,83 Osteomyelitis-discitis often presents as nonspecific back pain, making them challenging to distinguish from Modic changes on MRI and often resulting in late diagnosis. Jean et al reported an average diagnostic delay of 45.5 days after symptoms in a prospective study of 88 patients with vertebral osteomyelitis.84 This delay can heighten the risk of complications, neurological deficits, and mortality.85 SPECT/CT may have a role in increasing the diagnostic yield of imaging in cases of spinal infection and reducing delays in treatment.
Several single photon-emitting agents have been studied in the diagnosis of vertebral osteomyelitis, all with similar results.67 Gallium-MDP SPECT/CT is the most studied modality.86–89 In a retrospective review of 34 spondylodiscitis patients who underwent 67Ga-MDP SPECT/CT, Tamm et al found both the sensitivity and specificity to be impressively high—94% and 100%, respectively, leading them to conclude that SPECT/CT holds up to MRI in diagnosing spondylodiscitis.64 Echoing this result, the Dominguez et al study, in which 9 spondylodiscitis patients underwent 67Ga-MDP SPECT/CT, found a 100% sensitivity and an enhanced capacity to detect adjacent soft tissue infections compared with planar imaging.87 Fuster et al presented a different angle in their prospective analysis of 34 patients with spondylodiscitis. They found that 67Ga-MDP SPECT/CT showed 79% sensitivity and 81% specificity. They also explored PET/CT as an alternate modality and found it to be superior to SPECT/CT by all comparisons, recommending 67Ga-MDP SPECT/CT as a potentially handy tool in cases where planar bone scan and 67Ga-MDP pattern point toward spondylodiscitis.88
111In-Biotin, a radioactive isotope of vitamin B7, has also been used to identify spondylodiscitis. Lazzeri et al prospectively studied 110 patients with either hematogenous spread (n = 71) or postoperative infection (n = 39) and found that planar imaging and SPECT alone had a sensitivity and specificity of 93% and 90%, respectively.90 Lazzeri et al conducted a follow-up investigation 2 years later that compared SPECT/CT with SPECT alone and found identical sensitivities and specificities. They did note, however, that SPECT/CT was able to better localize the infection compared with SPECT alone, and the ability to delineate soft tissue vs bone infection had an impact on patient management.91 111In-Biotin remains a suitable option to detect infection as it is minimally absorbed by bone marrow and emits less radiation than 67Ga; however, it is not widely available in most of the world.92 Moreover, despite the potential for SPECT/CT in diagnosing pyogenic infections of the spine, MRI remains the gold standard diagnostic imaging modality.
Sacroiliac Joint Dysfunction
Sacroiliac joint (SIJ) dysfunction is an increasingly studied cause of low back pain but remains difficult to accurately diagnose.93 While patient history and examination findings may suggest the diagnosis in some cases, patient complaints related to SIJ dysfunction have significant overlap with pain from other sources. Commonly used imaging studies are generally not effective at differentiating SIJ dysfunction from other causes of low back pain. CT, MRI, and ultrasonography may help evaluate other pain generators; however, these have not been demonstrated to have significant diagnostic value with respect to pathology or dysfunction of the SIJ, and the gold standard for diagnosis, to date, is the response to a diagnostic injection.94–97 Pain resulting from the pathology of the SIJ is, therefore, typically a clinical diagnosis.98–103 The ability of SPECT/CT to accurately distinguish between the various sources of axial pain makes it an appealing supplementary study in the evaluation of SIJ dysfunction.104,105
Cusi et al identified 100 patients with a diagnosis of SIJ incompetence secondary to peripartum dysfunction (48%) or trauma (52%) and 80 control patients with other sources of pathology resulting in axial symptoms or nonspecific low back pain. In the setting of SIJ dysfunction, SPECT/CT resulted in a sensitivity and specificity of 95% and 99%, respectively. Furthermore, positive predictive value (PPV), negative predictive value (NPV), and Kappa values were 99%, 94%, and 0.85, and they concluded that SPECT/CT reliably demonstrates metabolic alterations at the SIJ in patients with SIJ dysfunction. It was also postulated that MRI is not helpful because of the chronicity and absence of edema, and therefore the lack of a proton signal, in these patients. They postulated that the high accuracy of SPECT/CT was a result of the pathophysiology of SIJ dysfunction; repetitive ligament microtrauma leading to calcium deposition and uptake of the nuclear tracer.104 Tofuku et al105 performed SPECT/CT on 32 patients with recalcitrant SIJ dysfunction and analyzed tracer uptake values as a prognostic indicator. They found that higher amounts of tracer accumulation had a positive correlation with severity of symptoms and requirement of advanced treatments.
While current research suggests that SPECT/CT may be a helpful supplementary tool for diagnosing and evaluating the prognosis of SIJ dysfunction, due to limitations in the data, there is currently no formal recommendation in support of SPECT/CT.
Discussion
Our literature review suggests that SPECT-CT may have a particularly useful role in cases where traditional imaging modalities, such as MRI and CT, fail to provide a clear diagnosis. Its ability to improve specificity could lead to more targeted and effective treatment in specific clinical scenarios. For example, it may be particularly useful in diagnosing symptomatic SIJ pathology by reliably demonstrating metabolic alterations at the SIJ in patients with SIJ dysfunction, which may not be as apparent on MRI due to chronicity and absence of edema. Furthermore, SPECT-CT may be beneficial in cases of diagnostic uncertainty in patients with primarily axial symptoms and/or a history of prior spine surgery when CT and MRI are equivocal. However, further research is needed to more definitively establish the clinical situations in which SPECT-CT is most beneficial.
While SPECT-CT can provide valuable additional information in certain cases, it is important to consider its potential shortcomings. It is not 100% sensitive or specific, so results should be interpreted within the context of all relevant clinical information. Another key consideration is cost-effectiveness. Although SPECT-CT offers more diagnostic information, it is more expensive than traditional imaging modalities. Therefore, its use should be judiciously considered, balancing the potential benefits against the costs. There are also specific contraindications for SPECT-CT. For instance, patients with multiple spinal metastases or multiple potentially painful but morphologically similar lesions may not be suitable candidates for this imaging modality. Similarly, in patients with spinal infections, SPECT-CT may not be the best choice because it can be challenging to differentiate these infections from Modic changes on MRI.
Additionally, the risks associated with SPECT-CT should not be overlooked. The procedure does involve exposure to radiation, which can be a concern for some patients. However, it is worth noting that the level of radiation exposure is generally low and is considered safe for most patients. Thus, while SPECT-CT can be a powerful tool in the diagnosis and management of spinal conditions, its use should be carefully evaluated on a case-by-case basis, taking into account all these factors.
Limitations
The studies included in our review had several shortcomings. Many of them were limited by small sample sizes, which may have affected the reliability of the results. Additionally, the studies varied in their methodologies, making it difficult to compare results across studies. Some studies also lacked long-term follow-up data, which is crucial for assessing the lasting impact of SPECT-CT on patient outcomes.
Conclusion
This literature review has highlighted the potential utility of SPECT-CT in specific clinical scenarios where traditional imaging modalities, such as MRI and CT, may fail to provide a clear diagnosis. SPECT-CT’s ability to improve diagnostic specificity could help spine specialists provide more targeted and effective treatments, particularly in diagnosing SIFs and in cases of diagnostic uncertainty. However, it is crucial to consider the potential limitations of SPECT-CT, including its sensitivity and specificity, cost-effectiveness, contraindications, and associated risks such as radiation exposure. Furthermore, the studies included in our review had several shortcomings, including small sample sizes, varied methodologies, and a lack of long-term follow-up data. Therefore, while SPECT-CT can be a powerful tool in the diagnosis and management of spinal conditions, its use should be carefully evaluated on a case-by-case basis. Further research is needed to more definitively establish the clinical situations in which SPECT-CT is most beneficial.
References
- 1. Boden SD, McCowin PR, Davis DO, Dina TS, Mark AS, Wiesel S. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72(8):1178–1184. [PubMed] [Google Scholar]
- 2. Zheng F, Cammisa FP, Sandhu HS, Girardi FP, Khan SN. Factors predicting hospital stay, operative time, blood loss, and transfusion in patients undergoing revision posterior lumbar spine decompression, fusion, and segmental instrumentation. Spine (Phila Pa 1976). 2002;27(8):818–824. 10.1097/00007632-200204150-00008 [DOI] [PubMed] [Google Scholar]
- 3. Russo VM, Dhawan RT, Dharmarajah N, Baudracco I, Lazzarino AI, Casey AT. Hybrid bone single photon emission computed tomography imaging in evaluation of chronic low back pain: correlation with modic changes and degenerative disc disease. World Neurosurg. 2017;104:816–823. 10.1016/j.wneu.2017.03.107 [DOI] [PubMed] [Google Scholar]
- 4. Wehling M. Principles of Translational science in medicine: from bench to bedside. Principles of Translational Science in Medicine: From Bench to Bedside. Elsevier; 2015. [Google Scholar]
- 5. Wale DJ, Wong KK, Savas H, Kandathil A, Piert M, Brown RKJ. Extraosseous findings on bone scintigraphy using fusion SPECT/CT and correlative imaging. AJR Am J Roentgenol. 2015;205(1):160–172. 10.2214/AJR.14.13914 [DOI] [PubMed] [Google Scholar]
- 6. Cherry SR, Sorenson J, Phelps ME, Methé BM. Physics in nuclear medicine. Med Phys. 2004;31(8):2370–2371. 10.1118/1.1776595 [DOI] [Google Scholar]
- 7. Cuellar JM, Stauff MP, Herzog RJ, Carrino JA, Baker GA, Carragee EJ. Does provocative discography cause clinically important injury to the lumbar Intervertebral disc? A 10-year matched cohort study. Spine J. 2016;16(3):273–280. 10.1016/j.spinee.2015.06.051 [DOI] [PubMed] [Google Scholar]
- 8. Bybel B, Brunken RC, DiFilippo FP, Neumann DR, Wu G, Cerqueira MD. SPECT/CT imaging: clinical utility of an emerging technology. Radiographics. 2008;28(4):1097–1113. 10.1148/rg.284075203 [DOI] [PubMed] [Google Scholar]
- 9. Hoy D, Bain C, Williams G, et al. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012;64(6):2028–2037. 10.1002/art.34347 [DOI] [PubMed] [Google Scholar]
- 10. Patrick N, Emanski E, Knaub MA. Acute and chronic low back pain. Med Clin North Am. 2014;98(4):777–789. 10.1016/j.mcna.2014.03.005 [DOI] [PubMed] [Google Scholar]
- 11. Dieleman JL, Cao J, Chapin A, et al. US health care spending by payer and health condition, 1996-2016. JAMA. 2020;323(9):863–884. 10.1001/jama.2020.0734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maas ET, Juch JNS, Ostelo RWJG, et al. Systematic review of patient history and physical examination to diagnose chronic low back pain originating from the facet joints. Eur J Pain. 2017;21(3):403–414. 10.1002/ejp.963 [DOI] [PubMed] [Google Scholar]
- 13. Zhang S, Zhao E, Winkelstein BA. A nociceptive role for integrin signaling in pain after mechanical injury to the spinal facet capsular ligament. Ann Biomed Eng. 2017;45(12):2813–2825. 10.1007/s10439-017-1917-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lu Y, Chen C, Kallakuri S, Patwardhan A, Cavanaugh JM. Development of an in vivo method to investigate biomechanical and neurophysiological properties of spine facet joint capsules. Eur Spine J. 2005;14(6):565–572. 10.1007/s00586-004-0835-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anaya JEC, Coelho SRN, Taneja AK, Cardoso FN, Skaf AY, Aihara AY. Differential diagnosis of facet joint disorders. Radiographics. 2021;41(2):543–558. 10.1148/rg.2021200079 [DOI] [PubMed] [Google Scholar]
- 16. McCall IW, Park WM, O’Brien JP. Induced pain referral from posterior lumbar elements in normal subjects. Spine (Phila Pa 1976). 1979;4(5):441–446. 10.1097/00007632-197909000-00009 [DOI] [PubMed] [Google Scholar]
- 17. Dreyer SJ, Dreyfuss PH. Low back pain and the zygapophysial (facet) joints. Arch Phys Med Rehabil. 1996;77(3):290–300. 10.1016/s0003-9993(96)90115-x [DOI] [PubMed] [Google Scholar]
- 18. Schwarzer AC, Wang SC, O’Driscoll D, Harrington T, Bogduk N, Laurent R. The ability of computed tomography to identify a painful zygapophysial joint in patients with chronic low back pain. Spine (Phila Pa 1976). 1995;20(8):907–912. 10.1097/00007632-199504150-00005 [DOI] [PubMed] [Google Scholar]
- 19. Schwarzer AC, Aprill CN, Derby R, Fortin J, Kine G, Bogduk N. The prevalence and clinical features of internal disc disruption in patients with chronic low back pain. Spine (Phila Pa 1976). 1995;20(17):1878–1883. 10.1097/00007632-199509000-00007 [DOI] [PubMed] [Google Scholar]
- 20. Weishaupt D, Zanetti M, Hodler J, Boos N. MR imaging of the lumbar spine: prevalence of Intervertebral disk extrusion and sequestration, nerve root compression, end plate abnormalities, and osteoarthritis of the facet joints in asymptomatic volunteers. Radiology. 1998;209(3):661–666. 10.1148/radiology.209.3.9844656 [DOI] [PubMed] [Google Scholar]
- 21. Stojanovic MP, Sethee J, Mohiuddin M, et al. MRI analysis of the lumbar spine: can it predict response to diagnostic and therapeutic facet procedures Clin J Pain. 2010;26(2):110–115. 10.1097/AJP.0b013e3181b8cd4d [DOI] [PubMed] [Google Scholar]
- 22. Boswell MV, Manchikanti L, Kaye AD, et al. A best-evidence systematic appraisal of the diagnostic accuracy and utility of facet (zygapophysial) joint injections in chronic spinal pain. Pain Physician. 2015;18(4):E497–E533. [PubMed] [Google Scholar]
- 23. Marks RC, Houston T, Thulbourne T. Facet joint injection and facet nerve block: a randomised comparison in 86 patients with chronic low back pain. Pain. 1992;49(3):325–328. 10.1016/0304-3959(92)90239-8 [DOI] [PubMed] [Google Scholar]
- 24. Bogduk N. International spinal injection society guidelines for the performance of spinal injection procedures. Part 1: zygapophysial joint blocks. Clin J Pain. 1997;13(4):285–302. 10.1097/00002508-199712000-00003 [DOI] [PubMed] [Google Scholar]
- 25. McDonald M, Cooper R, Wang MY. Use of computed tomography-single-photon emission computed tomography fusion for diagnosing painful facet arthropathy. FOC. 2007;22(1):1–4. 10.3171/foc.2007.22.1.2 [DOI] [PubMed] [Google Scholar]
- 26. Matar HE, Navalkissoor S, Berovic M, et al. Is hybrid imaging (SPECT/CT) a useful adjunct in the management of suspected facet joints arthropathy Int Orthop. 2013;37(5):865–870. 10.1007/s00264-013-1811-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ravindra VM, Mazur MD, Bisson EF, Barton C, Shah LM, Dailey AT. The usefulness of single-photon emission computed tomography in defining painful upper cervical facet arthropathy. World Neurosurg. 2016;96:390–395. 10.1016/j.wneu.2016.09.012 [DOI] [PubMed] [Google Scholar]
- 28. Lehman VT, Murphy RC, Kaufmann TJ, et al. Frequency of discordance between facet joint activity on technetium Tc99m methylene diphosphonate SPECT/CT and selection for percutaneous treatment at a large multispecialty institution. AJNR Am J Neuroradiol. 2014;35(3):609–614. 10.3174/ajnr.A3731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lehman VT, Murphy RC, Schenck LA, et al. Comparison of facet joint activity on 99Mtc-MDP SPECT/CT with facet joint signal change on MRI with fat suppression. Diagn Interv Radiol. 2016;22(3):277–283. 10.5152/dir.2015.15203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Raj PP. Intervertebral disc: anatomy-physiology-pathophysiology-treatment. Pain Pract. 2008;8(1):18–44. 10.1111/j.1533-2500.2007.00171.x [DOI] [PubMed] [Google Scholar]
- 31. Raoul S, Faure A, Robert R, et al. Role of the sinu-vertebral nerve in low back pain and anatomical basis of therapeutic implications. Surg Radiol Anat. 2003;24(6):366–371. 10.1007/s00276-002-0084-8 [DOI] [PubMed] [Google Scholar]
- 32. Zhao Q, Cheng L, Yan H, et al. The anatomical study and clinical significance of the sinuvertebral nerves at the lumbar levels. Spine (Phila Pa 1976). 2020;45(2):E61–E66. 10.1097/BRS.0000000000003190 [DOI] [PubMed] [Google Scholar]
- 33. DePalma MJ, Ketchum JM, Saullo T. What is the source of chronic low back pain and does age play a role. Pain Med. 2011;12(2):224–233. 10.1111/j.1526-4637.2010.01045.x [DOI] [PubMed] [Google Scholar]
- 34. Schwarzer AC, Aprill CN, Derby R, Fortin J, Kine G, Bogduk N. Clinical features of patients with pain stemming from the lumbar zygapophysial joints. Is the lumbar facet syndrome a clinical entity? Spine (Phila Pa 1976). 1994;19(10):1132–1137. 10.1097/00007632-199405001-00006 [DOI] [PubMed] [Google Scholar]
- 35. Fujii K, Yamazaki M, Kang JD, et al. Discogenic back pain: literature review of definition, diagnosis, and treatment. JBMR Plus. 2019;3(5):e10180. 10.1002/jbm4.10180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. García-Cosamalón J, del Valle ME, Calavia MG, et al. Intervertebral disc, sensory nerves and neurotrophins: who is who in discogenic pain J Anat. 2010;217(1):1–15. 10.1111/j.1469-7580.2010.01227.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976). 2001;26(17):1873–1878. 10.1097/00007632-200109010-00011 [DOI] [PubMed] [Google Scholar]
- 38. Takatalo J, Karppinen J, Niinimäki J, et al. Does lumbar disc degeneration on magnetic resonance imaging associate with low back symptom severity in young Finnish adults. Spine (Phila Pa 1976). 2011;36(25):2180–2189. 10.1097/BRS.0b013e3182077122 [DOI] [PubMed] [Google Scholar]
- 39. Jensen MC, Brant-Zawadzki MN, Obuchowski N, Modic MT, Malkasian D, Ross JS. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331(2):69–73. 10.1056/NEJM199407143310201 [DOI] [PubMed] [Google Scholar]
- 40. Kjaer P, Leboeuf-Yde C, Korsholm L, Sorensen JS, Bendix T. Magnetic resonance imaging and low back pain in adults: a diagnostic imaging study of 40-year-old men and women. Spine (Phila Pa 1976). 2005;30(10):1173–1180. 10.1097/01.brs.0000162396.97739.76 [DOI] [PubMed] [Google Scholar]
- 41. Kato S, Demura S, Matsubara H, et al. Utility of bone SPECT/CT to identify the primary cause of pain in elderly patients with degenerative lumbar spine disease. J Orthop Surg Res. 2019;14(1):185. 10.1186/s13018-019-1236-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carragee EJ, Don AS, Hurwitz EL, Cuellar JM, Carrino JA, Herzog R. 2009 ISSLS prize winner: does discography cause accelerated progression of degeneration changes in the lumbar disc: a ten-year matched cohort study. Spine (Phila Pa 1976). 2009;34(21):2338–2345. 10.1097/BRS.0b013e3181ab5432 [DOI] [PubMed] [Google Scholar]
- 43. Harisankar CNB, Mittal BR, Bhattacharya A, Singh P, Sen R. Utility of single photon emission computed tomography/computed tomography imaging in evaluation of chronic low back pain. Indian J Nucl Med. 2012;27(3):156–163. 10.4103/0972-3919.112720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Van de Kelft E, Verleye G, Van de Kelft A-S, Melis K, Van Goethem J. Validation of topographic hybrid single-photon emission computerized tomography with computerized tomography scan in patients with and without nonspecific chronic low back pain. A prospective comparative study. Spine J. 2017;17(10):1457–1463. 10.1016/j.spinee.2017.05.007 [DOI] [PubMed] [Google Scholar]
- 45. Khalil JG, Smuck M, Koreckij T, et al. A prospective, randomized, multicenter study of intraosseous basivertebral nerve ablation for the treatment of chronic low back pain. Spine J. 2019;19(10):1620–1632. 10.1016/j.spinee.2019.05.598 [DOI] [PubMed] [Google Scholar]
- 46. Huang J, Delijani K, Jones J, et al. Basivertebral nerve ablation. Semin Intervent Radiol. 2022;39(2):162–166. 10.1055/s-0042-1745794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Peh WC, Khong PL, Yin Y, et al. Imaging of pelvic insufficiency fractures. Radiographics. 1996;16(2):335–348. 10.1148/radiographics.16.2.8966291 [DOI] [PubMed] [Google Scholar]
- 48. Lapina O, Tiškevičius S. Sacral insufficiency fracture after pelvic radiotherapy: a diagnostic challenge for a radiologist. Medicina (Kaunas). 2014;50(4):249–254. 10.1016/j.medici.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 49. Sakaguchi M, Maebayashi T, Aizawa T, Ishibashi N. Risk factors for sacral insufficiency fractures in cervical cancer after whole pelvic radiation therapy. Anticancer Res. 2019;39(1):361–367. 10.21873/anticanres.13120 [DOI] [PubMed] [Google Scholar]
- 50. Holderread BM, Shin CP, Syed IY, Avramis I, Rizkalla JM. Sacral insufficiency fracture after lumbosacral decompression and fusion. Proc (Bayl Univ Med Cent). 2022;35(4):451–454. 10.1080/08998280.2022.2058832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ha K-Y, Kim Y-H, Park H-Y, et al. Sacral insufficiency fracture after instrumented lumbosacral fusion: focusing pelvic deformation - a retrospective case series. J Clin Neurosci. 2021;83:31–36. 10.1016/j.jocn.2020.11.034 [DOI] [PubMed] [Google Scholar]
- 52. Seo J-Y, Ha K-Y, Kim Y-H, et al. Analysis of fracture patterns and characteristics in sacral insufficiency fracture: do sacral fractures occur in patients who had previous lumbosacral fusion insufficiency fractures or stress fractures? Asian Spine J. 2021;15(6):769–777. 10.31616/asj.2020.0466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yoo J-I, Ha Y-C, Ryu H-J, et al. Teriparatide treatment in elderly patients with sacral insufficiency fracture. J Clin Endocrinol Metab. 2017;102(2):560–565. 10.1210/jc.2016-3582 [DOI] [PubMed] [Google Scholar]
- 54. Kinoshita H, Miyakoshi N, Kobayashi T, Abe T, Kikuchi K, Shimada Y. Comparison of patients with diagnosed and suspected sacral insufficiency fractures. J Orthop Sci. 2019;24(4):702–707. 10.1016/j.jos.2018.12.004 [DOI] [PubMed] [Google Scholar]
- 55. Bencardino JT, Stone TJ, Roberts CC. Expert panel on musculoskeletal imaging: et al. ACR appropriateness criteria® stress (fatigue/insufficiency) fracture, including Sacrum, excluding other vertebrae. J Am Coll Radiol. 2017;14(5):S293–S306. 10.1016/j.jacr.2017.02.035 [DOI] [PubMed] [Google Scholar]
- 56. Kim YY, Chung BM, Kim WT. Lumbar spine MRI versus non-lumbar imaging modalities in the diagnosis of sacral insufficiency fracture: a retrospective observational study. BMC Musculoskelet Disord. 2018;19(1):257. 10.1186/s12891-018-2189-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Al-faham Z, Rydberg JN, Oliver Wong C-Y. Use of SPECT/CT with 99Mtc-MDP bone scintigraphy to diagnose sacral insufficiency fracture. J Nucl Med Technol. 2014;42(3):240–241. 10.2967/jnmt.114.137547 [DOI] [PubMed] [Google Scholar]
- 58. McConnell CT, Wippold FJ, Ray CE, et al. ACR appropriateness criteria management of vertebral compression fractures. J Am Coll Radiol. 2014;11(8):757–763. 10.1016/j.jacr.2014.04.011 [DOI] [PubMed] [Google Scholar]
- 59. Li Y-B, Zheng X, Wang R, et al. SPECT-CT versus MRI in localizing active lesions in patients with osteoporotic vertebral compression fractures. Nucl Med Commun. 2018;39(7):610–617. 10.1097/MNM.0000000000000857 [DOI] [PubMed] [Google Scholar]
- 60. Solá M, Pérez R, Cuadras P, et al. Value of bone SPECT-CT to predict chronic pain relief after percutaneous vertebroplasty in vertebral fractures. Spine J. 2011;11(12):1102–1107. 10.1016/j.spinee.2011.11.003 [DOI] [PubMed] [Google Scholar]
- 61. Spencer HT, Sokol LO, Glotzbecker MP, et al. Detection of pars injury by SPECT in patients younger than age 10 with low back pain. J Pediatr Orthop. 2013;33(4):383–388. 10.1097/BPO.0b013e318285c3be [DOI] [PubMed] [Google Scholar]
- 62. Takemitsu M, El Rassi G, Woratanarat P, Shah SA. Low back pain in pediatric athletes with unilateral tracer uptake at the pars interarticularis on single photon emission computed tomography. Spine (Phila Pa 1976). 2006;31(8):909–914. 10.1097/01.brs.0000209308.19642.96 [DOI] [PubMed] [Google Scholar]
- 63. Gaddikeri S, Matesan M, Alvarez J, Hippe DS, Vesselle HJ. MDP-SPECT versus hybrid MDP-SPECT/CT in the evaluation of suspected pars interarticularis fracture in young athletes. J Neuroimaging. 2018;28(6):635–639. 10.1111/jon.12533 [DOI] [PubMed] [Google Scholar]
- 64. Al-Riyami K, Gnanasegaran G, Van den Wyngaert T, Bomanji J. Bone SPECT/CT in the postoperative spine: a focus on spinal fusion. Eur J Nucl Med Mol Imaging. 2017;44(12):2094–2104. 10.1007/s00259-017-3765-6 [DOI] [PubMed] [Google Scholar]
- 65. Rager O, Schaller K, Payer M, Tchernin D, Ratib O, Tessitore E. SPECT/CT in differentiation of pseudarthrosis from other causes of back pain in lumbar spinal fusion: report on 10 consecutive cases. Clin Nucl Med. 2012;37(4):339–343. 10.1097/RLU.0b013e318239248b [DOI] [PubMed] [Google Scholar]
- 66. Damgaard M, Nimb L, Madsen JL. The role of bone SPECT/CT in the evaluation of lumbar spinal fusion with metallic fixation devices. Clin Nucl Med. 2010;35(4):234–236. 10.1097/RLU.0b013e3181d18cdd [DOI] [PubMed] [Google Scholar]
- 67. Heimburger C, Hubele F, Charles YP, Steib J-P, Namer I-J, Rust E. Évaluation de critères D’Interprétation de la tomoscintigraphie D’Émission monophotonique au 99Mtc-HMDP pour le diagnostic des complications tardives des arthrodèses rachidiennes. Médecine Nucléaire. 2015;39(2):105–121. 10.1016/j.mednuc.2015.02.008 [DOI] [Google Scholar]
- 68. Brusko GD, Perez-Roman RJ, Tapamo H, Burks SS, Serafini AN, Wang MY. Preoperative SPECT imaging as a tool for surgical planning in patients with axial neck and back pain. Neurosurg Focus. 2019;47(6):2019.9.FOCUS19648. 10.3171/2019.9.FOCUS19648 [DOI] [PubMed] [Google Scholar]
- 69. Tender GC, Davidson C, Shields J, et al. Primary pain generator identification by CT-SPECT in patients with degenerative spinal disease. Neurosurg Focus. 2019;47(6):2019.9.FOCUS19608. 10.3171/2019.9.FOCUS19608 [DOI] [PubMed] [Google Scholar]
- 70. Ghosh P. The role of SPECT/CT in skeletal malignancies. Semin Musculoskelet Radiol. 2014;18(2):175–193. 10.1055/s-0034-1371019 [DOI] [PubMed] [Google Scholar]
- 71. Sciubba DM, Petteys RJ, Dekutoski MB, et al. Diagnosis and management of metastatic spine disease. J Neurosurg Spine. 2010;13(1):94–108. 10.3171/2010.3.SPINE09202 [DOI] [PubMed] [Google Scholar]
- 72. Horger M, Eschmann SM, Pfannenberg C, et al. Evaluation of combined transmission and emission tomography for classification of skeletal lesions. AJR Am J Roentgenol. 2004;183(3):655–661. 10.2214/ajr.183.3.1830655 [DOI] [PubMed] [Google Scholar]
- 73. Helyar V, Mohan HK, Barwick T, et al. The added value of multislice SPECT/CT in patients with equivocal bony metastasis from carcinoma of the prostate. Eur J Nucl Med Mol Imaging. 2010;37(4):706–713. 10.1007/s00259-009-1334-3 [DOI] [PubMed] [Google Scholar]
- 74. Zhao Z, Li L, Li F, Zhao L. Single photon emission computed tomography/spiral computed tomography fusion imaging for the diagnosis of bone metastasis in patients with known cancer. Skeletal Radiol. 2010;39(2):147–153. 10.1007/s00256-009-0764-0 [DOI] [PubMed] [Google Scholar]
- 75. Palmedo H, Marx C, Ebert A, et al. Whole-body SPECT/CT for bone scintigraphy: diagnostic value and effect on patient management in oncological patients. Eur J Nucl Med Mol Imaging. 2014;41(1):59–67. 10.1007/s00259-013-2532-6 [DOI] [PubMed] [Google Scholar]
- 76. Sharma P, Singh H, Kumar R, et al. Bone scintigraphy in breast cancer: added value of hybrid SPECT-CT and its impact on patient management. Nucl Med Commun. 2012;33(2):139–147. 10.1097/MNM.0b013e32834e3b14 [DOI] [PubMed] [Google Scholar]
- 77. Sharma P, Kumar R, Singh H, et al. Indeterminate lesions on planar bone scintigraphy in lung cancer patients: SPECT, CT or SPECT-CT Skeletal Radiol. 2012;41(7):843–850. 10.1007/s00256-011-1304-2 [DOI] [PubMed] [Google Scholar]
- 78. Fleury V, Ferrer L, Colombié M, et al. Advantages of systematic trunk SPECT/CT to planar bone scan (PBS) in more than 300 patients with breast or prostate cancer. Oncotarget. 2018;9(60):31744–31752. 10.18632/oncotarget.25860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Iqbal B, Currie GM, Wheat JM, Raza H, Kiat H. The incremental value of SPECT/CT in characterizing solitary spine lesions. J Nucl Med Technol. 2011;39(3):201–207. 10.2967/jnmt.111.088351 [DOI] [PubMed] [Google Scholar]
- 80. Zhang Y, Shi H, Cheng D, et al. Added value of SPECT/spiral CT versus SPECT in diagnosing solitary spinal lesions in patients with extraskeletal malignancies. Nucl Med Commun. 2013;34(5):451–458. 10.1097/MNM.0b013e32835fa552 [DOI] [PubMed] [Google Scholar]
- 81. Jambor I, Kuisma A, Ramadan S, et al. Prospective evaluation of planar bone scintigraphy, SPECT, SPECT/CT, 18F-Naf PET/CT and whole body 1.5t MRI, including DWI, for the detection of bone metastases in high risk breast and prostate cancer patients: SKELETA clinical trial. Acta Oncol. 2016;55(1):59–67. 10.3109/0284186X.2015.1027411 [DOI] [PubMed] [Google Scholar]
- 82. Lam KS, Webb JK. Discitis. Hosp Med. 2004;65(5):280–286. 10.12968/hosp.2004.65.5.13703 [DOI] [PubMed] [Google Scholar]
- 83. Sato K, Yamada K, Yokosuka K, et al. Pyogenic spondylitis: clinical features, diagnosis and treatment. Kurume Med J. 2019;65(3):83–89. 10.2739/kurumemedj.MS653001 [DOI] [PubMed] [Google Scholar]
- 84. Jean M, Irisson J-O, Gras G, et al. Diagnostic delay of pyogenic vertebral osteomyelitis and its associated factors. Scand J Rheumatol. 2017;46(1):64–68. 10.3109/03009742.2016.1158314 [DOI] [PubMed] [Google Scholar]
- 85. Gupta A, Kowalski TJ, Osmon DR, et al. Long-term outcome of pyogenic vertebral osteomyelitis: a cohort study of 260 patients. Open Forum Infect Dis. 2014;1(3):ofu107. 10.1093/ofid/ofu107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tamm AS, Abele JT. Bone and gallium single-photon emission computed tomography-computed tomography is equivalent to magnetic resonance imaging in the diagnosis of infectious spondylodiscitis: a retrospective study. Can Assoc Radiol J. 2017;68(1):41–46. 10.1016/j.carj.2016.02.003 [DOI] [PubMed] [Google Scholar]
- 87. Domínguez ML, Lorente R, Rayo JI, et al. SPECT-CT with 67Ga-citrate in the management of spondylodiscitis. Revista Española de Medicina Nuclear e Imagen Molecular (English Edition). 2012;31(1):34–39. 10.1016/j.remngl.2011.04.003 [DOI] [PubMed] [Google Scholar]
- 88. Fuster D, Solà O, Soriano A, et al. A prospective study comparing whole-body FDG PET/CT to combined planar bone scan with 67Ga SPECT/CT in the diagnosis of spondylodiskitis. Clin Nucl Med. 2012;37(9):827–832. 10.1097/RLU.0b013e318262ae6c [DOI] [PubMed] [Google Scholar]
- 89. Liévano P, De la Cueva L, Navarro P, Arroyo E, Añaños M, Abós MD. 67Ga SPECT/low-dose CT. A case report of spondylodiscitis and schmorl’s node. Rev Esp Med Nucl. 2009;28(6):288–290. 10.1016/j.remn.2009.07.003 [DOI] [PubMed] [Google Scholar]
- 90. Lazzeri E, Erba P, Perri M, et al. Scintigraphic imaging of vertebral osteomyelitis with 111In-biotin. Spine (Phila Pa 1976). 2008;33(7):E198–E204. 10.1097/BRS.0b013e31816960c9 [DOI] [PubMed] [Google Scholar]
- 91. Lazzeri E, Erba P, Perri M, Doria R, Tascini C, Mariani G. Clinical impact of SPECT/CT with In-111 biotin on the management of patients with suspected spine infection. Clin Nucl Med. 2010;35(1):12–17. 10.1097/RLU.0b013e3181c36173 [DOI] [PubMed] [Google Scholar]
- 92. Palestro CJ, Glaudemans AWJM, Dierckx RAJO. Multiagent imaging of inflammation and infection with radionuclides. Clin Transl Imaging. 2013;1(6):385–396. 10.1007/s40336-013-0041-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Dreyfuss P, Michaelsen M, Pauza K, McLarty J, Bogduk N. The value of medical history and physical examination in diagnosing sacroiliac joint pain. Spine (Phila Pa 1976). 1996;21(22):2594–2602. 10.1097/00007632-199611150-00009 [DOI] [PubMed] [Google Scholar]
- 94. Weksler N, Velan GJ, Semionov M, et al. The role of Sacroiliac joint dysfunction in the genesis of low back pain: the obvious is not always right. Arch Orthop Trauma Surg. 2007;127(10):885–888. 10.1007/s00402-007-0420-x [DOI] [PubMed] [Google Scholar]
- 95. Bäcklund J, Clewett Dahl E, Skorpil M. Is CT indicated in diagnosing sacroiliac joint degeneration? Clin Radiol. 2017;72(8):693. 10.1016/j.crad.2017.03.006 [DOI] [PubMed] [Google Scholar]
- 96. Gupta AD. Sacroiliac joint pathologies in low back pain. BMR. 2009;22(2):91–97. 10.3233/BMR-2009-0221 [DOI] [PubMed] [Google Scholar]
- 97. Sáenz-Navarro I, Möller I, Iagnocco A, Naredo E, Barcelona Sonoanatomy Group . Ultrasound assessment of the sacroiliac joint. J Clin Ultrasound. 2011;39(2):93–94. 10.1002/jcu.20773 [DOI] [PubMed] [Google Scholar]
- 98. Cook C, Massa L, Harm-Ernandes I, et al. Interrater reliability and diagnostic accuracy of pelvic girdle pain classification. J Manipulative Physiol Ther. 2007;30(4):252–258. 10.1016/j.jmpt.2007.03.008 [DOI] [PubMed] [Google Scholar]
- 99. Kokmeyer DJ, Van der Wurff P, Aufdemkampe G, Fickenscher TCM. The reliability of multitest regimens with sacroiliac pain provocation tests. J Manipulative Physiol Ther. 2002;25(1):42–48. 10.1067/mmt.2002.120418 [DOI] [PubMed] [Google Scholar]
- 100. Laslett M, Aprill CN, McDonald B, Young SB. Diagnosis of sacroiliac joint pain: validity of individual provocation tests and composites of tests. Man Ther. 2005;10(3):207–218. 10.1016/j.math.2005.01.003 [DOI] [PubMed] [Google Scholar]
- 101. Robinson HS, Brox JI, Robinson R, Bjelland E, Solem S, Telje T. The reliability of selected motion- and pain provocation tests for the sacroiliac joint. Manual Therapy. 2007;12(1):72–79. 10.1016/j.math.2005.09.004 [DOI] [PubMed] [Google Scholar]
- 102. Telli H, Telli S, Topal M. The validity and reliability of provocation tests in the diagnosis of sacroiliac joint dysfunction. Pain Physician. 2018;21(4):E367–E376. [PubMed] [Google Scholar]
- 103. Vleeming A, Albert HB, Ostgaard HC, Sturesson B, Stuge B. European guidelines for the diagnosis and treatment of pelvic girdle pain. Eur Spine J. 2008;17(6):794–819. 10.1007/s00586-008-0602-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Cusi M, Saunders J, Van der Wall H, Fogelman I. Metabolic disturbances identified by SPECT-CT in patients with a clinical diagnosis of sacroiliac joint incompetence. Eur Spine J. 2013;22(7):1674–1682. 10.1007/s00586-013-2725-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Tofuku K, Koga H, Komiya S. The diagnostic value of single-photon emission computed tomography/computed tomography for severe sacroiliac joint dysfunction. Eur Spine J. 2015;24:859–863. 10.1007/s00586-014-3375-y [DOI] [PubMed] [Google Scholar]