Abstract
The ability of antibodies to interfere with anterograde transmission of herpes simplex virus (HSV) from neuronal axons to the epidermis was investigated in an in vitro model consisting of human fetal dorsal root ganglia innervating autologous skin explants in a dual-chamber tissue culture system. The number and size of viral cytopathic plaques in epidermal cells after axonal transmission from HSV type 1 (HSV-1)-infected dorsal root ganglionic neurons were significantly reduced by addition to the outer chamber of neutralizing polyclonal human sera to HSV-1, of a human recombinant monoclonal group Ib antibody to glycoprotein D (gD), and of rabbit sera to HSV-1 gB and gD but not by rabbit anti-gE or anti-gG. A similar pattern of inhibition of direct infection of epidermal cells by these antibodies was observed. High concentrations of the monoclonal anti-gD reduced transmission by 90%. Rabbit anti-gB was not taken up into neurons, and human anti-gD did not influence spread of HSV in the dorsal root ganglia or axonal transport of HSV antigens when applied to individual dissociated neurons. These results suggest that anti-gD and -gB antibodies interfere with axonal spread of HSV-1, possibly by neutralizing HSV during transmission across an intercellular gap between axonal termini and epidermal cells, and thus contribute to control of HSV spread and shedding. Therefore, selected human monoclonal antibodies to protective epitopes might even be effective in preventing epidermis-to-neuron transmission during primary HSV infection, especially neonatal infection.
The herpes simplex viruses (HSV) establish lifelong latent infections in the sensory neurons of the host dorsal root ganglia (DRG), where they undergo periodic reactivations (42). Such recurrences can be spontaneous or can be associated with different external stimuli such as physical or emotional stress, fever, exposure to UV light, tissue and/or nerve damage, or immunosuppression. The viral and host factors that lead to the establishment and the maintenance of HSV latency and the eventual recurrences are still poorly understood. Following reactivation from latency, HSV is transported axonally back to the originally infected dermatomes or to adjacent ones, resulting in recurrent clinical lesions or asymptomatic viral shedding (5, 10, 27, 37, 44). T lymphocytes, macrophages and their products (such as cytokines and chemokines), and perhaps natural killer (NK) cells have been shown to restrict viral replication in the skin and genital mucosa (1, 30–32, 40). However, the exact role for antibodies in controlling HSV infection is unclear, especially in humans, where correlation with antibody levels may also reflect T-cell responses. In animal models there is clear evidence for a protective effect of antibody against HSV infection and spread within the nervous system, acting by neutralization directed against glycoprotein B (gB) and gD or by antibody-dependent cytotoxicity (ADCC) against gB, gD, and gC (9, 22, 24, 29, 33). It has been suggested that ADCC in the presence of competent effector cells (NK cells) is more effective against higher challenge doses of virus than neutralizing antibodies.
In humans a role for antibody has been suggested by studies of vertical transmission resulting in neonatal herpes, where passive transmission of neutralizing antibody or antibody titers associated with ADCC have been reported to correlate with protection against disease (23, 25, 26, 47). However, some groups have not been able to find such an association (45). Furthermore, although the risk of neonatal herpes following a primary infection is more than 10-fold greater than that following recurrent infection, this may be related to the higher titers and longer duration of viral shedding in the genital tract associated with primary infection (2, 36). Studies of children with agammaglobulinaemia have also not provided a clear indication of susceptibility to primary HSV infection (25).
Clinical recurrences of herpes simplex are often associated with levels of neutralizing antibody higher than those in asymptomatic seropositive controls, and antibody titers do not change significantly after dermal recurrences (10, 49). Furthermore, chronic indolent and spreading herpetic ulcers in immunocompromised patients with AIDS, leukemia, or transplantation usually have T-cell defects but not diminished specific antibody levels (17, 41). Nevertheless, whether recurrences with humans are controlled solely by cellular immunity or whether the humoral arm of the immune response plays a modulating role remains the subject of much debate.
Previously we have developed an in vitro model consisting of human fetal DRG neurons and autologous epidermal cells (ECs) (DRG-EC model) in two separate chambers to study anterograde axonal transport of HSV type 1 (HSV-1) (35). HSV-1 infection of the human DRG neurons results in separate axonal transport of glycoproteins and nucleocapsids (35), which are likely to assemble into mature virions before crossing the intercellular gap between axonal termini and ECs (6, 35). With this system, glycoprotein and nucleocapsid antigens are detectable by immunohistochemistry and confocal microscopy at 20 h in ECs, and subsequent development of HSV-1 cytopathic plaques can be observed over the next 48 h (35). Here we utilized the DRG-EC model to study the effect of neutralizing antibodies on transmission of HSV from human axons to the epidermis in comparison with direct infection of ECs.
MATERIAL AND METHODS
Human fetal tissue.
Human fetal tissue age 16 to 18 weeks was obtained from therapeutic terminations with informed consent and Western Sydney Area Health Service Ethics Committee approval.
Preparation of the human fetal DRG-EC model.
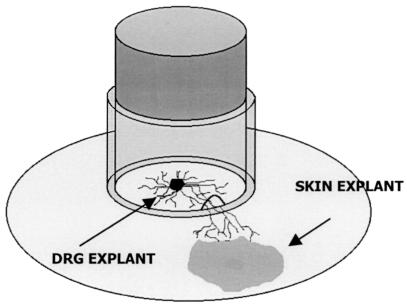
The in vitro model consists of a growth chamber which comprises a stainless steel cylinder attached with silicone grease to the substratum (Thermanox plastic coverslip; Nalge Nunc International, Naperville, Ill.) in each well of a six-well tissue culture plate, dividing each into an inner chamber and an outer chamber (35). Two transverse grooves on the opposite inferior surfaces of the stainless steel ring were filled with agarose (2% [wt/vol] in phosphate-buffered saline [PBS]) to prevent outward diffusion of HSV-1. Two fetal skin explants cleaned of dermal tissue were placed on the coverslip outside the ring, and autologous DRG were placed opposite the skin explants inside the ring (Fig. 1). Growth medium contained Dulbecco modified Eagle medium base with Earle’s salts (Gibco, Rockville, Md.) supplemented with (per liter) 200 mM l-glutamine (Gibco), 5.12 g of d-glucose, 50 ml of Monomed A (CSL, Sydney, Australia), 10 μg of epidermal growth factor (Sigma, St. Louis, Mo.), 60 μg of nerve growth factor (Boehringer, Mannheim, Germany), and 9% fetal calf serum (FCS) (CSL). Axons grew out from the ganglia, penetrated the agarose without causing leaks, and interacted with ECs within 8 to 10 days. The integrity of the seal was tested by sampling for infectious HSV at 0, 2, and 6 h after infection of the DRG neurons in the inner chamber. Fewer than 20% of outer chamber samples were positive, and they were excluded from further studies.
FIG. 1.
Diagram of the fetal human DRG-EC model.
Preparation and culture of dissociated DRG neurons.
Isolated DRG were dissociated into a monocell suspension by using 0.25% trypsin (CSL)–0.05% collagenase (Worthington Biomedical Co., Lakewood, N.J.) in Hanks balanced salt solution (HBSS) for 30 min at 37°C and then washed by centrifugation (800 × g for 7 min) three times at 4°C. The cells were then plated onto Matrigel (Collaborative Biomedical Products, Bedford, Mass.) (diluted 1:10 with HBSS)-coated 14-mm-diameter glass coverslips placed in the wells of a 24-well plate (Nunc International) and cultured (2 × 105 to 3 × 105 cells per well) in growth medium supplemented with 4% FCS (CSL) instead of 9% FCS.
HSV infection of DRG neurons in the DRG-EC model.
The second passages of the HSV-1 clinical isolate WM-1 or the HSV-2 clinical isolate WM-4 (only for anti-gG2 experiments) were used to infect the DRG neurons of the model at 1,000 and 5,000 tissue culture infective doses (TCID50)/ganglion (approximately 0.001 and 0.005 TCID50/ganglionic neuron as determined after estimates of the total number of neurons in DRG by staining with hematoxylin and eosin and for Nissl granules and somatophysin).
HSV infection of neurons in dissociated DRG cultures.
The neurons in the dissociated cell cultures were infected at a multiplicity of infection (MOI) of 5 TCID50/cell for 1 h to ensure that a high proportion (>80%) were infected. The inoculum was then removed, and the cells were washed once carefully with HBSS and incubated with the optimal neutralizing dilution of antibody or growth medium. They were later processed for confocal microscopy.
Neutralizing antibodies.
Polyclonal human sera (with high neutralizing titers for HSV-1 or HSV-2) were obtained from individuals with frequent recurrences of HSV-1 or HSV-2 and filtered. Monospecific rabbit polyclonal anti-gB1, anti-gC1, and anti-gD1 sera were kindly donated by G. Cohen and R. Eisenberg (University of Pennsylvania) (20, 21), polyclonal rabbit anti-gG2 serum was kindly donated by R. Courtney (Pennsylvania State University) (43), and a polyclonal rabbit antibody to gE expressed in baculovirus was kindly donated by H. Friedman (University of Pennsylvania) (11). The human recombinant monoclonal anti-HSV-1 antibody (HSV8) used in this study was previously described (3, 38, 46). This type-common antibody recognizes the highly conserved and protective antigenic site Ib (8, 12). It efficiently neutralizes both laboratory strains and low-passage clinical isolates of both HSV serotypes, inhibits cell fusion by a syncytium-inducing HSV-1 strain, and inhibits cell-to-cell spread of HSV-1 and -2 in inhibition-of-plaque development assays (3, 8). It also proved to be protective against viral challenge in nude mice (38, 39).
All of the sera and antibodies used in this study were treated at 56°C for 20 min to inactivate complement, were nontoxic for ECs, and did not neutralize an unrelated virus (cocksackie virus B1). HSV-1- and HSV-2-negative human sera and an unrelated monoclonal antibody (mouse anti-Leu 3a+3b antibody; Becton Dickinson, Franklin Lakes, N.J.) were used as controls. The neutralization titer (50% plaque reduction) for all antibodies was initially determined in HEp-2 cell cultures infected with HSV-1 at an MOI of 5 TCID50/cell grown in 12-well plates. The titers were 1:5,000 for polyclonal sera to both HSV-1 and HSV-2, 1:2,500 for both anti-gB1 and anti-gD1, and 1:5,000 (200 ng/ml) for the human monoclonal anti-gD. Antibodies were used at the optimal dilutions as well as dilutions fivefold lower and twofold higher (1:500 and 1:10,000, respectively, for polyclonal sera to HSV-1, anti-gB1, and anti-gD1 and 1:25,000 [40 ng/ml] and 1:2,500 [400 ng/ml], respectively, for human anti-gD antibody) to cover an appropriate range of concentrations given that neutralization potency can differ according to cell type (28). Human anti-gD antibody was later used at much higher concentrations (1:1,000 to 1:25 or 1, 2, 4, and 40 μg/ml).
Use of neutralizing antibodies in the DRG-EC model.
The HSV inoculum was applied to the DRG in the inner chamber of the model and aspirated after a 1-h incubation, and then DRG were washed once carefully with HBSS. The antibodies (or growth medium) were incubated with ECs for 2 h in the outer chamber of the model at 24 and 12 h before and 0, 12, 18, 26, and 32 h after infection of the DRG neurons in the inner chamber at the optimal neutralizing dilution. Anti-gC1, anti-gG2, and anti-gE1 antibodies did not show neutralizing activity and were used at fivefold dilutions surrounding the optimum dilution for immunofluorescence staining (1:50 to 1:5,000). HSV-infected ECs cultivated alone in 12-well plates or in the outer chamber of the DRG-EC model were fixed with electron microscopy (EM)-grade methanol 48 h after infection and stained with monoclonal anti-gC1 (Goodwin Institute for Cancer Research, Plantation, Fla.) (dilution, 1:100), anti-gD1 (Cymbus Bioscience, Hants, United Kingdom) (dilution, 1:100), or anti-gC2 rabbit polyclonal antibody (SmithKline Beecham, Rixensart, Belgium) (dilution, 1:200) for 45 min. After washing with HBSS, biotinylated sheep anti-mouse antibody (Biosource International, Camarillo, Calif.) (dilution, 1:200) or biotinylated goat anti-rabbit antibody (Biosource International) (dilution, 1:200) was used as a secondary antibody (with staining for 45 min at room temperature [RT]), followed by washing with HBSS and treatment with streptavidin-horseradish peroxidase conjugate (Biosource International) (dilution, 1:4000; staining for 45 min at RT) (34).
Detection of neutralizing antibodies within neurons in the dissociated DRG cultures.
To determine whether neutralizing anti-HSV antibody can be taken up by neurons, especially axons, and transported to cellular compartments where virus accumulates to subsequently inhibit viral assembly or egress, the neurons in dissociated cell cultures were infected or mock infected with HSV-1 for 1 h (at 5 TCID50/cell), washed carefully twice with HBSS, and then incubated with neutralizing rabbit anti-gB antibody or growth medium for 2 h. The cells were then incubated with growth medium for a further 5, 10, or 15 h, fixed for immunofluorescence staining for rabbit antibody, and examined by confocal microscopy. For the detection of rabbit anti-gB antibody uptake by dissociated DRG neurons, cells were fixed in 2.5% formaldehyde (ProSci Tech, Thuringowa, Queensland, Australia) in Sorensons buffer (pH 7.4) for 30 min, permeabilized with 0.1% Triton X-100 (Sigma) in PBS for 20 min, and stained with fluorescein isothiocyanate-conjugated goat anti-rabbit antibody (Sigma) (1:40 dilution) for 45 min at RT. The cultures were rinsed three times with HBSS, mounted in mounting fluid (Syva Microtrak, Tamarillo, Calif.), and examined with a Bio-Rad MRC 600 confocal microscope.
Statistical evaluation.
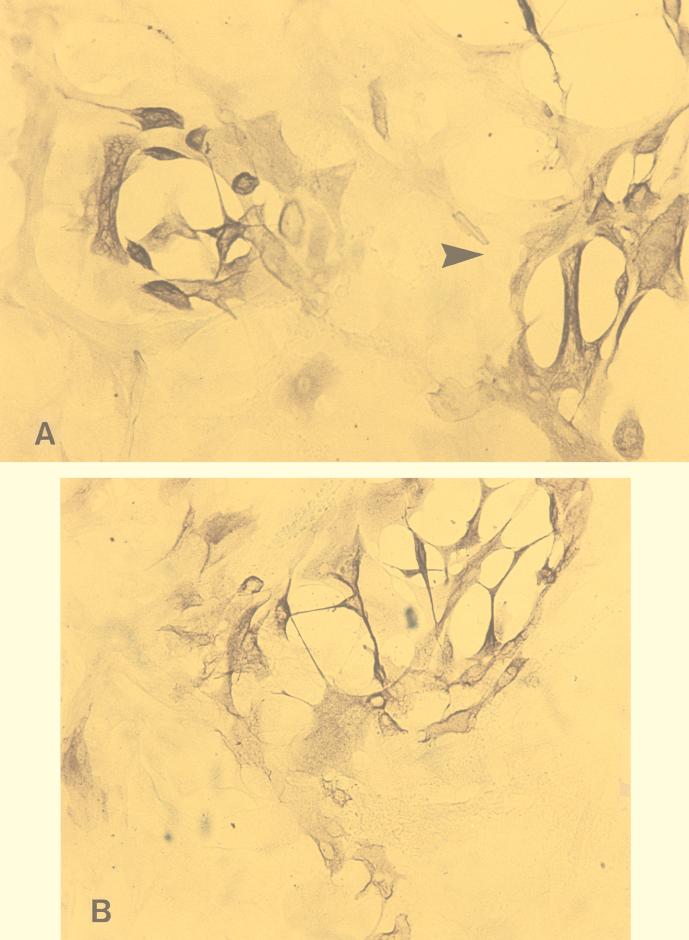
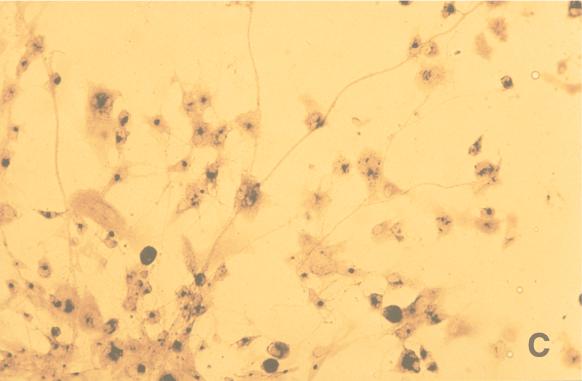
The differences in numbers and sizes of the HSV-1 plaques with and without different treatments in ECs were compared. The diameters of plaques were measured with an ocular micrometer, and the plaques were classified into three groups: small (<1 mm), medium (1 to 2 mm), and large (>2 mm) (Fig. 2). The results were calculated as the means from experiments using 12 different sets of fetal tissue, each performed in triplicate. Differences between the numbers and sizes of plaques after various treatments were assessed for statistical significance by the Student t test and expressed as mean percent reductions in number or size of HSV plaques.
FIG. 2.
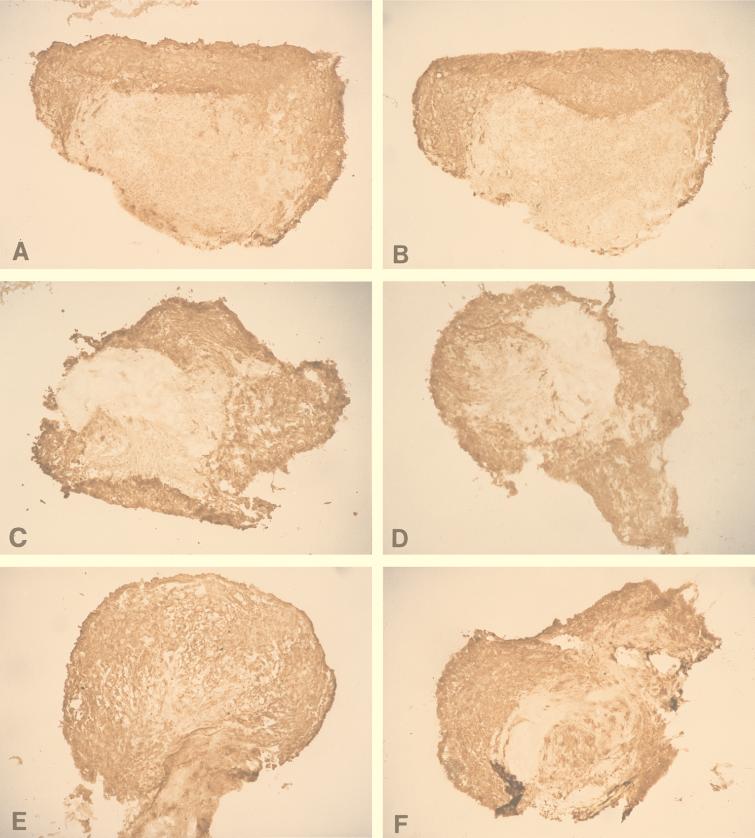
(A and B) Small and large (arrowhead) (A) and medium (B) cytopathic plaques produced by HSV-1 infection in ECs after axonal transmission in the DRG-EC model. Magnification ×320. (C) HSV-1-infected ECs in the outer chamber. Magnification, ×100. Staining was for HSV-1 gC antigen by the immunoperoxidase technique.
In the experiments examining the effect of neutralizing antibody on HSV spread through the DRG, the proportion of the DRG neurons which were HSV infected (i.e., gC antigen positive) was determined as follows: 10 whole perpendicular sections (1 per 20 to 40 sections, depending on DRG diameter) covering both the central and peripheral portions of each DRG were examined by light microscopy after immunoperoxidase staining, and the proportion of infected neurons from each of the sections was estimated (19). Two DRG were examined for each experimental group (treated with control medium or human anti-gD neutralizing antibody) at each time point in three different experiments. The proportions of HSV-infected DRG neurons were calculated as the means and standard errors (SEs) of readings from each section for each experimental group.
In the HSV-infected dissociated neuronal cell cultures treated with control or neutralizing human anti-gD antibody, at least 20 neurons were examined per culture. The proportion of infected neurons with full expression of HSV antigens in the cytoplasm and especially in the axon was calculated from five replicate cultures in five separate experiments, and results for antibody-treated and control cultures were compared. Mean control and experimental values and their SEs were compared, and significance was calculated by use of the Student t test adjusted for unequal variances.
RESULTS
Effect of neutralizing antibodies on HSV-1 cytopathic plaques in ECs after axonal transmission (DRG-EC model) or after direct infection.
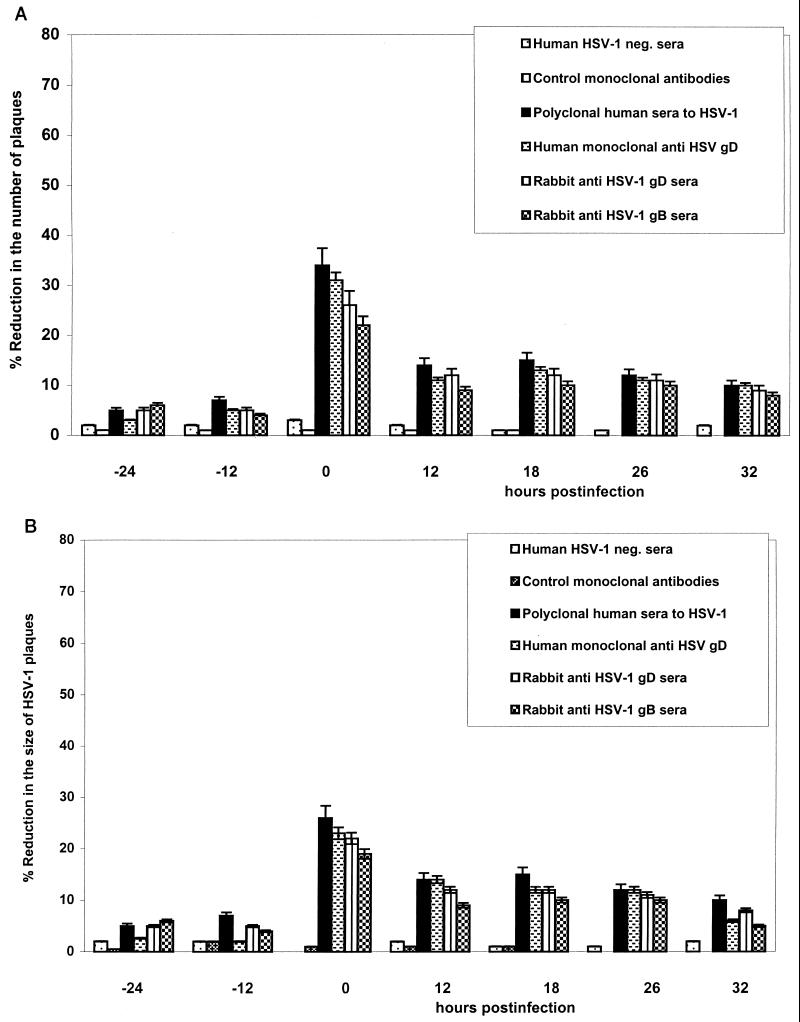
As expected, the extent of infection of ECs was dependent on the size of the HSV inoculum. After direct infection of ECs, 0.005 TCID50/EC produced significantly more plaques than 0.001 TCID50/EC (P < 0.001; data not shown). An MOI of 5,000 TCID50/DRG in the inner chamber produced more plaques than 1,000 TCID50/DRG in the DRG-EC model. The addition of the neutralizing human polyclonal sera against HSV-1, the human monoclonal anti-gD antibody, or the neutralizing polyclonal monospecific rabbit sera against HSV-1 gB or gD to the outer chamber of the DRG-EC model or EC cultures alone significantly reduced both the number and the size of HSV-1 cytopathic plaques in ECs. Addition of anti-gC1, anti-gG2, or anti-gE did not affect the number or size of HSV-1 plaques in ECs in either setting.
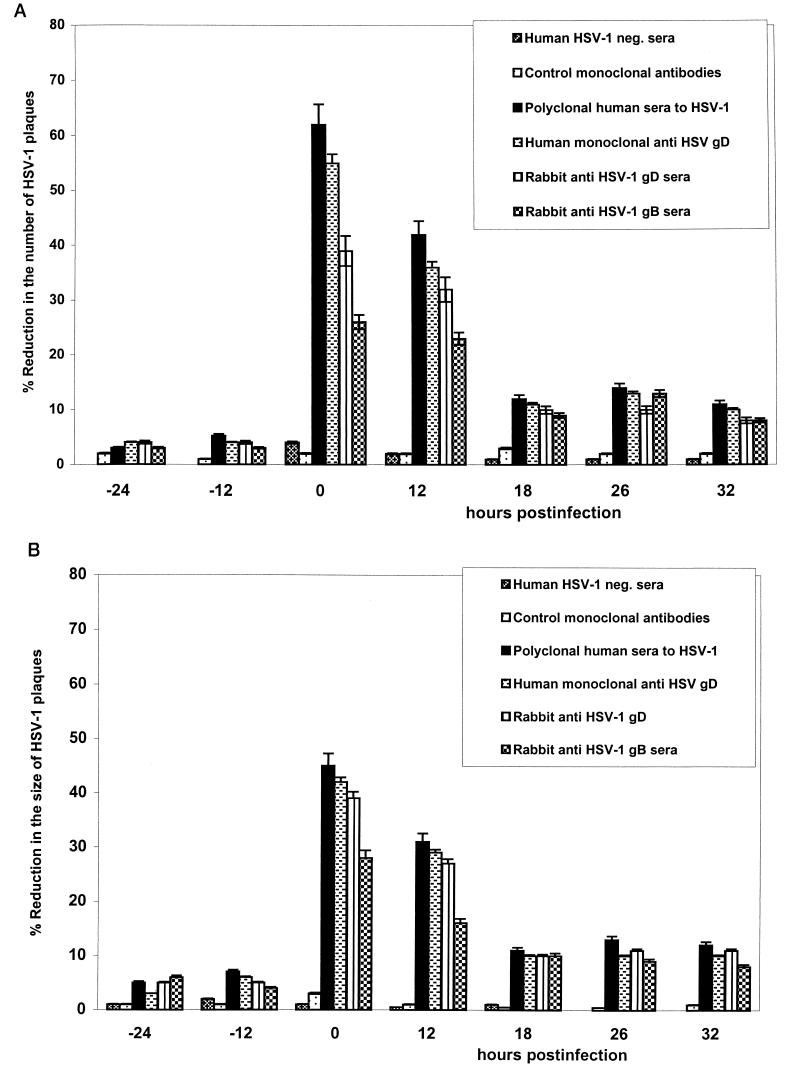
Human HSV-1-neutralizing antibody and rabbit polyclonal anti-gB1 and anti-gD1 sera, at the optimal 50% neutralizing concentrations for HSV-infected HEp-2 cells, markedly reduced the number (by 25 to 55%) and the size (by >20%) of EC plaques in the DRG-EC model at 0 and 12 h postinfection (hpi) (Fig. 2 and 3). Polyclonal human HSV-1-neutralizing sera and the human monoclonal anti-gD were slightly more potent in inhibiting axonal spread of HSV-1 to ECs than predicted by their neutralizing titer in HEp-2 cells, whereas anti-gD1 or anti-gB1 rabbit polyclonal sera were less potent at each time point (Fig. 3). The reduction in plaque number and size by human polyclonal HSV-1-neutralizing sera, rabbit anti-gD1 and -gB1 sera, and human anti-gD antibody was significantly less (P < 0.05) at 18, 26, and 32 hpi (for six of six tested) (Fig. 3). When ECs were infected directly with HSV-1, polyclonal human neutralizing sera and the human monoclonal anti-gD were again most effective relative to their neutralizing titers on HEp-2 cells, more so than polyclonal human anti-gD1 and anti-gB1. However, a marked neutralizing effect (>20% reduction in number and size of HSV plaques) was observed only with coincubation of HSV and antibody, with much less inhibition at 12, 18, 26, or 32 hpi (Fig. 4).
FIG. 3.
Effect of neutralizing and control antibodies on the number (A) and size (B) of cytopathic plaques induced by HSV-1 in ECs after axonal transmission in the DRG-EC model. The HSV inoculum was aspirated after 1 h of incubation, and the DRG in the inner chamber of the model were carefully washed once with HBSS. The antibodies (or growth medium) were incubated for 2 h with ECs in the outer chamber of the model at 24 and 12 h before and 0, 12, 18, 26, and 32 h after infection of the DRG neurons in the inner chamber at the optimal neutralizing dilution. MOI for HSV inoculum, 0.005 TCID50/neuron. neg., negative. Error bars show SEs.
FIG. 4.
Effect of neutralizing and control antibodies on the number (A) and size (B) of cytopathic plaques induced by HSV-1 in ECs after direct infection of the EC monolayers. Monolayers of autologous ECs were obtained by treating the epidermal explants grown to 90% confluence with 0.25% trypsin-EDTA solution (CSL) in HBSS for 2 min at 37°C, washed by centrifugation (800 × g for 7 min), resuspended in growth medium containing 9% FCS, and seeded as a single cells in 12-well plates (Nunc). Twenty-four hours later the autologous ECs were infected at 0.001 and 0.005 TCID50/EC (to approximate the low MOI for EC infection in the DRG-EC model) and treated with antibodies at the same time points as in the DRG-EC model. The cells were washed twice with HBSS after incubation with antibody or HSV-1 infection, fixed with EM-grade methanol, and then stained by the immunoperoxidase method. MOI for HSV inoculum, 0.005 TCID50/EC. neg., negative. Error bars show SEs.
Effect of high concentrations of neutralizing antibodies on HSV-1 cytopathic plaques in epidermal cells after axonal transmission (DRG-EC model).
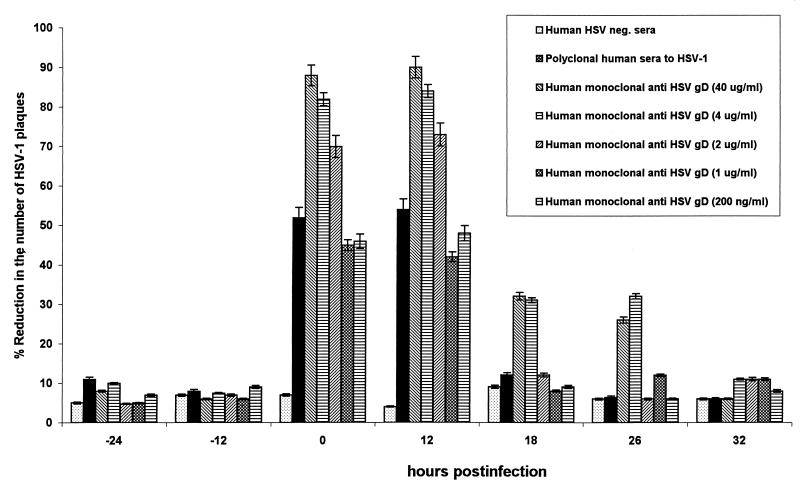
To determine whether it was possible to completely block HSV-1 infection of ECs after axonal transmission, concentrations of human anti-gD antibody much higher than the 50% HSV-HEp 2 neutralizing concentrations were added to the terminal axons and ECs in the outer chamber. Incubation with 40, 4, 2, and 1 μg and 200 ng of antibody per ml decreased the number of HSV plaques in ECs by approximately 90, 80 to 85, 70, and 40 to 50%, respectively (Fig. 5).
FIG. 5.
Effect of high concentrations of neutralizing human anti-gD monoclonal antibody on the number of cytopathic plaques induced by HSV-1 in ECs in the DRG-EC model. One, 2, 4, or 40 μg of antibody per ml was added to the terminal axons and ECs in the outer chamber at 24 and 12 h before and 0, 12, 24, and 36 h after infection, and the antibody was maintained at this concentration for up to 48 h after infection. After fixation with EM-grade methanol for 10 min, the cultures were stained for gC antigen by immunoperoxidase staining as described in Materials and Methods. MOI for HSV inoculum 0.005 TCID50/neuron. neg., negative. Error bars show SEs.
Do the neutralizing antibodies diffuse to the inner chamber and inhibit the viral transport within the ganglion in the DRG-EC cell model?
In experiments determining whether neutralizing antibody could diffuse into the inner chamber and inhibit HSV spread through the DRG, the DRG in the inner chamber were first infected (or mock infected) for 1 h, and then the supernatant fluid was carefully aspirated and replaced with growth medium. The ECs in the outer chamber were incubated with a 1:2,500 dilution (400 ng/ml) of human anti-gD monoclonal antibody (or growth medium) for a longer period of 12 h. The DRG were sectioned and stained for gC antigen with immunoperoxidase (Fig. 6).
FIG. 6.
Sections through DRG (isolated from the thoracic region) from HSV-infected DRG-EC cultures at 36 (A and B), 48 (C and D), and 72 (E and F) hpi stained for HSV gC antigen. Human anti-gD (A, C, and E) or control medium (B, D, and F) was added to the outer chamber immediately after infection and left for 12 h. At 36, 48, and 72 hpi, snap-frozen (for 30 s in liquid nitrogen) HSV-infected and mock-infected DRG were mounted on a freezing cryotome (Shandon E-600) at −20°C and sectioned (perpendicularly to the coverslip) into 5-μm-thick sections. The sections were air dried on glass slides, stained with murine anti-gC1 antibody (1:100) (Goodwin Institute for Cancer Research) for 45 min, washed with HBSS and double-distilled water, and stained with biotinylated sheep anti-mouse antibody (Biosource International) (dilution, 1:200) for 45 min at RT. After two washes, sections were treated with streptavidin-horseradish peroxidase conjugate (Biosource International) at a 1:4,000 dilution. The proportion of all DRG neurons which were gC antigen positive was quantified in frozen sections of the whole mounted DRG (as described in Materials and Methods). Actual size of frozen DRG, 1.2 to 2.2 mm.
As shown in Table 1 and Fig. 6, the proportion of DRG positively stained for viral antigen was similar at each time point in viral controls and in the antibody-treated DRG-EC model. There were no significant differences (P > 0.1 by the Student t test).
TABLE 1.
Spread of HSV-1 through human fetal DRG in the presence or absence of neutralizing human anti-gD added to the outer chambera
| Anti-gD | % gC antigen-positive DRG neurons (mean ± SE) at hpi:
|
||||
|---|---|---|---|---|---|
| 0 | 24 | 36 | 48 | 72 | |
| Absent (control) | 0 | 22 ± 7 | 35 ± 9 | 60 ± 8 | 77 ± 8 |
| Present | 0 | 19 ± 8 | 40 ± 9 | 65 ± 5 | 80 ± 8 |
Calculated from readings from each section for each experimental group. Results from a representative experiment are shown.
Is neutralizing antibody to HSV internalized by neurons in dissociated DRG cultures?
Three cell types were present in the human dissociated DRG cultures: neurons, Schwann cells, and fibroblasts. Neurons comprised approximately 90 to 93%, Schwann cells comprised 1 to 2%, and fibroblasts comprised 5 to 10% of the total DRG cell population after 4 days in culture. All cell types were easily distinguishable by their characteristic cellular morphology. After passage through the Percoll gradient, the proportions of Schwann cells and fibroblasts were diminished (<5% nonneuronal cell types).
To determine whether neutralizing anti-HSV antibody can be internalized by neurons and inhibit viral assembly or egress, HSV-infected neurons in dissociated cell cultures were incubated with neutralizing rabbit anti-gB antibody (at 400 ng/ml) or growth medium for 2 h after two washes with HBSS. The cells were then incubated with growth medium for a further 5, 10, or 15 h and examined by confocal microscopy for immunofluorescence staining for rabbit antibody. The antibody-treated infected and uninfected cells showed no evidence of uptake of intracellular rabbit antibody at any time (data not shown).
Effect of neutralizing antibodies on viral replication in dissociated DRG neuronal cell cultures.
After infection of dissociated DRG cultures, HSV antigen could be detected in all cell types by immunofluorescence and confocal microscopy. However, in dissociated DRG cultures, neurons are easily distinguishable from other cell types on the basis of their distinctive morphology.
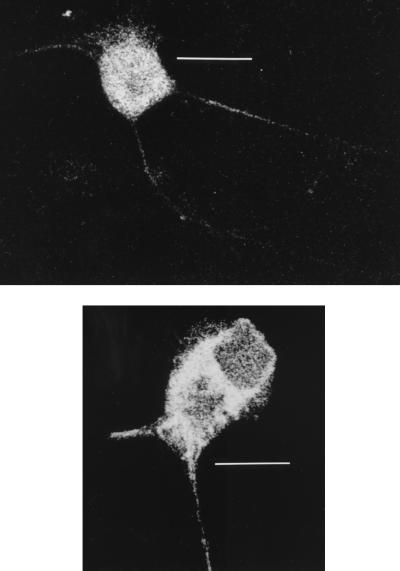
To determine whether antibody could directly affect replication in neurons, human anti-gD antibody at 400 ng/ml or growth medium was added to the cells in cultures, left for 2 h, and then replaced with growth medium alone for another 10 or 15 h. Viral controls and antibody-treated cells showed the same proportion of anti-gC-stained neurons (80 to 90%) and the same kinetic patterns and intensities of gC antigen distribution by immunofluorescence and confocal microscopy. In all stained neurons, gC antigen was distributed in both the cytoplasm and axon hillock at 10 h and was distributed in the cytoplasm, axon hillock, and especially the axon at 15 h. Thus, there were no marked differences in viral replication as shown by kinetics of gC antigen distribution and no delay in anterograde axonal transport in any of the cultures examined (Fig. 7).
FIG. 7.
Confocal micrographs of HSV-infected neurons stained for gC antigen at 15 hpi and after addition of human anti-gD monoclonal antibody (top) or control medium (bottom). The HSV inoculum (5 TCID50/cell) was aspirated after 1 h of incubation, and the cells were carefully washed once with HBSS. The HSV-infected or mock-infected dissociated neuronal cultures, incubated with a 1:2,500 dilution (400 ng/ml) of human anti-gD antibody, were fixed in 2.5% formaldehyde (ProSci Tech) in Sorensons buffer (pH 7.4) for 30 min and permeabilized with 0.1% Triton X-100 (Sigma) in PBS for 20 min. Nonspecific staining was blocked by incubation with 5% mouse serum in HBSS for 15 min. The cells on coverslips were then incubated with fluorescein isothiocyanate-conjugated anti-gC1 antibody (Syva Microtrak) (1:100 dilution), rinsed three times with HBSS, and mounted in mounting fluid (Syva Microtrak). Stained neurons were examined with a Bio-Rad MRC 600 confocal microscope. Note the similar distributions of gC antigen in the axon and cytoplasm in both micrographs. Bars, 40 μm (top) and 20 μm (bottom).
DISCUSSION
In this study both monoclonal and polyclonal antibodies to gB1 and gD1 inhibited axonal spread of HSV-1 from neurons to epithelial cells in an in vitro DRG-EC model. The reduction in number and size of HSV-1 plaques suggested inhibition of axon-EC HSV transmission and probably also some secondary viral spread in ECs. Human hyperimmune sera, human recombinant group Ib antibody to gD, and rabbit monospecific antibodies to gD and gB showed significant inhibition. The kinetics of marked inhibition at 0 and 12 h after HSV infection in the DRG-EC model compared with a similar effect at only 0 h in ECs, followed by lesser but still significant neutralization in both culture systems up to 32 h, are consistent with inhibition of transmission from axon termini to ECs.
The alternate explanations are that these neutralizing antibodies to essential glycoproteins may diffuse back into the inner chamber and inhibit spread of virus through the DRG or, alternatively, may be taken up by the neurons or axons and inhibit intracellular viral replication, especially assembly and egress. However, a series of experiments found no evidence for these alternatives. In the intact DRG-EC model, incubation of anti-gD human monoclonal antibody in the outer chamber at concentrations inhibiting axon-EC transmission did not inhibit spread of HSV through the DRG. In animal studies, spinal cord motoneurons, hypothalamic neurons, and cerebellar Purkinje cells have been shown to take up immunoglobulins (4, 14). In postmortem human studies, intracellular immunoglobulins were also demonstrated in some of these neurons (13, 15, 16). However, after incubation of HSV-infected and uninfected neurons with very high concentrations of rabbit anti-gB antibody, no antibody could be demonstrated at 5, 10, and 15 h after infection by using immunofluorescence and confocal microscopy.
Furthermore, infected neurons bathed in high concentrations of human anti-gD antibody showed kinetics and intensity of HSV gC antigen appearance in the cytoplasm and of anterograde transport to the axon terminus that were similar to those of controls. The times selected for fixation and staining of infected neurons for gC were guided by more-detailed kinetic studies of gC, gD, and gB distribution in the same neurons (32a). Therefore, unlike the effect of immune nonneutralizing bivalent anti-E2 antibody on Sindbis virus replication in mice in vivo and in DRG neurons in vitro, neutralizing (anti-gD) antibody does not shut down HSV replication in neurons (18). The effect of the anti-Sindbis virus antibodies is partly due to inhibition of viral budding. This might occur with HSV at the axon termini.
It could be argued that the initial experiments demonstrating only 25 to 55% inhibition of axonal transmission to ECs leave open the possibility that the majority of HSV is transmitted via mechanisms which are not susceptible to exogenous neutralization, such as viral transmission across fused axon-EC membranes. Although we have previously reported that there is an intercellular gap between fine (200-nm-diameter) axon termini and ECs (34) and we have observed viral nucleocapsid in ECs subjacent to this intercellular gap (6, 19a), this does not exclude the possibility of some intercellular membrane fusion, allowing direct transmission of some HSV. However, the demonstration of 90% inhibition of axon-EC transmission with very high concentrations of neutralizing human anti-gD (in the absence of HSV infection of neurons) suggests that the vast majority of transmitted virus passes across an intercellular gap. Ultrastructural studies have shown that axons are usually buried deep within the convulated membranes of ECs, suggesting that there may be slow antibody diffusion to the intercellular gap around the axon terminus (34).
The inhibitory effects of human polyclonal HSV-1-neutralizing sera, human monoclonal anti-gD antibody, and rabbit monospecific anti-gD1 and anti-gB1 on cell-free and axonally transmitted HSV infection of ECs but a lack of effect of anti-gC1, anti-gG2, and anti-gE were qualitatively similar. Collectively, the above data suggest that HSV in the intercellular gap, after assembly in axon termini, has a glycoprotein constitution similar to that of cell-free HSV generated by infection of nonneural cells.
As we have previously discussed, the DRG-EC model used is likely to be representative of the axonal spread of HSV to the skin in vivo in view of the similarity of cultured ECs to the cell types surrounding the arborizing plexus of nonmyelinated free sensory nerve ending within the stratum granulosum (7). The present results suggest that neutralizing antibodies should be included in the immune factors that can determine the degree of cytopathology in ECs after axonal transmission of HSV. Despite the lack of an inverse correlation of neutralizing antibody titer with the occurrence, severity, or frequency of clinical recurrent herpes simplex, we hypothesize that there may be a threshold effect such that the basal neutralizing antibody titers may limit the extent of recurrent clinical lesions and may also reduce the titers of virus shed symptomatically or asymptomatically (10, 49).
The present observations also suggest a potential preventive role for administration of human monoclonal antibodies in controlling the spread of herpes simplex infections where there may be absent or deficient endogenous neutralizing antibodies (e.g., in B-cell immunodeficiencies). Such antibodies are unlikely to be effective in recurrent herpes simplex, where there are high titers of neutralizing antibody. However, the inhibition of transmission of HSV across the intercellular gap between the axon terminus and EC demonstrated here is also likely to be relevant to transmission in the reverse direction, which probably occurs in primary infection prior to the development of endogenous neutralizing antibodies. For example, this may have potential in the prevention of neonatal herpes.
Few human monoclonal antibodies have been produced to date because of the limited efficacy of conventional technologies in their production. The human monoclonal antibody used here is part of a panel of antibodies to HSV established by recombinant techniques from combinatorial Fab libraries expressed on the surface of M13 bacteriophage (3, 39, 46). The potential shown in this study needs to be extended by testing combinations of appropriate anti-HSV human monoclonal antibodies such as anti-gD and anti-gB for inhibition of the retrograde transmission of HSV from epidermal explants to sensory axons in a model of primary infection currently under development (46, 48).
ACKNOWLEDGMENTS
The National Health and Medical Research Council of Australia supported this work through grant no. 970738 to A. L. Cunningham. P. P. Sanna is the recipient of Public Health Service grant AI37582 and an NARSAD Young Investigator Award.
Rabbit neutralizing antibodies against gB1, gC1, and gD1 were kindly donated by G. Cohen and R. Eisenberg (University of Pennsylvania), anti-gC2 antibody was kindly donated by R. Courtney (Pennsylvania State University College of Medicine), and anti-gE was kindly donated by H. Friedman (University of Pennsylvania). We thank Bill Sinai and Jane Milliken (Histopathology Unit, Westmead Hospital) for their help with sectioning and immunoperoxidase staining of DRG.
REFERENCES
- 1.Biron C A, Byron K S, Sullivan J L. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320:1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 2.Brown Z A, Benedetti J, Ashley R, Burchett S, Selke S, Berry S, Vontver L A, Corey L. Neonatal herpes simplex virus infection in relation to asymptomatic maternal infection at the time of labor. N Engl J Med. 1991;324:1247–1252. doi: 10.1056/NEJM199105023241804. [DOI] [PubMed] [Google Scholar]
- 3.Burioni R, Williamson R A, Sanna P P, Bloom F E, Burton D R. Recombinant human Fab to glycoprotein D neutralizes infectivity and prevents cell-to-cell transmission of herpes simplex viruses 1 and 2 in vitro. Proc Natl Acad Sci USA. 1994;91:355–359. doi: 10.1073/pnas.91.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burlet A J, Menzaghi F, Tilders F J, Oers J W, Nicholas J P, Burlet C R. Uptake of monoclonal antibody to corticotropin-releasing factor (CRF) into rat hypothalamic neurons. Brain Res. 1990;28:283–293. doi: 10.1016/0006-8993(90)91039-j. [DOI] [PubMed] [Google Scholar]
- 5.Corey L, Adams H G, Brown Z A, Holmes K K. Genital herpes simplex virus infection: clinical manifestations, course and complications. Ann Intern Med. 1983;98:958–972. doi: 10.7326/0003-4819-98-6-958. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham A L, Penfold M E T, Mikloska Z, Armati P J, Holland D. Strategies for control of asymptomatic herpes and consequent transmission: insights from in vitro pathogenetic studies, abstr. S20. In Abstracts of the 20th International Herpesvirus Workshop, Groningen, The Netherlands. 1995. [Google Scholar]
- 7.Dalsgaard C J, Rydh M, Haegerstrand A. Cutaneous innervation in man visualized with protein gene product 9.5 (PGP 9.5) antibodies. Histochemistry. 1989;92:385–390. doi: 10.1007/BF00492495. [DOI] [PubMed] [Google Scholar]
- 8.De Logu A, Williamson R A, Rozenshteyn R, Simpson C D, Ramiro-Ibanez F, Burton D R, Sanna P P. Characterization of a recombinant antibody to herpes simplex virus with high therapeutic potential. J Clin Microbiol. 1998;36:3198–3204. doi: 10.1128/jcm.36.11.3198-3204.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dix R D, Pereira L, Baringer J R. Use of monoclonal antibody directed against herpes simplex virus glycoproteins to protect mice against acute virus-induced neurological disease. Infect Immun. 1981;34:192–199. doi: 10.1128/iai.34.1.192-199.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Douglas R G, Couch R B. A prospective study of chronic herpes simplex virus infection and recurrent herpes labialis in humans. J Immunol. 1970;104:289–295. [PubMed] [Google Scholar]
- 11.Dubin G, Basu S, Mallory D L, Basu M, Tal-Singer R, Friedman H M. Characterization of domains of herpes simplex virus type 1 glycoprotein E involved in Fc binding activity for immunoglobulin G aggregates. J Virol. 1994;68:2478–2485. doi: 10.1128/jvi.68.4.2478-2485.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenberg R J, Long D, Ponce de Leon M, Matthews J T, Spear P G, Gibson M G, Lasky L A, Berman P, Golub E, Cohen G H. Localization of the epitopes of herpes simplex virus type 1 glycoprotein D. J Virol. 1985;53:634–644. doi: 10.1128/jvi.53.2.634-644.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fishman P S, Drachman D B. Internalization of IgG in motoneurons of patients with ALS: selective or nonselective? Neurology. 1995;45:1551–1554. doi: 10.1212/wnl.45.8.1551. [DOI] [PubMed] [Google Scholar]
- 14.Fishman P S, Farrand D A, Kristt D A. Internalization of plasma proteins by cerebellar Purkinje cells. J Neurol Sci. 1990;100:43–49. doi: 10.1016/0022-510x(90)90011-b. [DOI] [PubMed] [Google Scholar]
- 15.Fishman P S, Farrand D A, Kristt D A. Penetration and internalization of plasma proteins in the human spinal cord. J Neurol Sci. 1991;104:166–175. doi: 10.1016/0022-510x(91)90306-r. [DOI] [PubMed] [Google Scholar]
- 16.Frantatoni S A, Dubrovsky A L, Uchitel O D. Uptake of immunoglobulin G from amyotrophic lateral sclerosis patients by motor nerve terminals in mice. J Neurol Sci. 1996;137:97–102. doi: 10.1016/0022-510x(95)00345-3. [DOI] [PubMed] [Google Scholar]
- 17.Greenberg M S, Friedman H, Cohen G H, Oh S H, Laster L, Starr S. A comparative study of herpes simplex infections in renal transplant and leukemic patients. J Infect Dis. 1987;156:280–287. doi: 10.1093/infdis/156.2.280. [DOI] [PubMed] [Google Scholar]
- 18.Griffin D, Levine B, Tyor W, Ubol S, Despres P. The role of antibody in recovery from alphavirus encephalitis. Immunol Rev. 1997;159:155–161. doi: 10.1111/j.1600-065x.1997.tb01013.x. [DOI] [PubMed] [Google Scholar]
- 19.Hinojosa R, Seligsohn R, Lerner S A. Ganglion cell counts in the cochlea of patients with normal audiograms. Acta Otolaryngol. 1985;99:8–13. doi: 10.3109/00016488509119139. [DOI] [PubMed] [Google Scholar]
- 19a.Holland, D. J., et al. Anterograde transport of herpes simplex viral proteins in axons of peripheral human fetal neurons. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 20.Hung C L, Srinivasan S, Friedman H M, Eisenberg R J, Cohen G H. Structural basis of C3b binding by glycoprotein C of herpes simplex virus. J Virol. 1992;66:4013–4027. doi: 10.1128/jvi.66.7.4013-4027.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isola V J, Eisenberg R J, Siebert G R, Heilman C J, Wilcox W C, Cohen G H. Fine mapping of antigenic site II of herpes simplex virus glycoprotein D. J Virol. 1989;63:2325–2334. doi: 10.1128/jvi.63.5.2325-2334.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohl S, Cahall D L, Walters D L, Schaffner V E. Murine antibody-dependent cellular cytotoxicity to herpes simplex virus-infected target cells. J Immunol. 1979;123:25–30. [PubMed] [Google Scholar]
- 23.Kohl S, West M S, Prober C G, Loo L S, Sullender W, Arvin A M. Neonatal antibody-dependent cellular cytotoxic antibody levels are associated with the clinical presentation of neonatal herpes simplex virus infection. J Infect Dis. 1989;160:770–776. doi: 10.1093/infdis/160.5.770. [DOI] [PubMed] [Google Scholar]
- 24.Kohl S, Strynadka N C J, Hodges R S, Pereira L. Analysis of the role of antibody-dependent cellular cytotoxicity antibody activity in murine neonatal herpes simplex virus infection with antibodies to synthetic peptides of glycoprotein D and monoclonal antibodies to glycoprotein B. J Clin Invest. 1990;86:273–278. doi: 10.1172/JCI114695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohl S. Role of antibody-dependent cellular cytotoxicity in neonatal infection with herpes simplex virus. Rev Infect Dis. 1991;13:S950–S952. doi: 10.1093/clind/13.supplement_11.s950. [DOI] [PubMed] [Google Scholar]
- 26.Kohl S. The role of antibody in herpes simplex virus infection in humans. Curr Top Microbiol Immunol. 1992;179:75–88. doi: 10.1007/978-3-642-77247-4_5. [DOI] [PubMed] [Google Scholar]
- 27.Koutsky L A, Stevens C E, Holmes K K. Underdiagnosis of genital herpes by current clinical and viral isolation procedures. N Engl J Med. 1992;326:1533–1539. doi: 10.1056/NEJM199206043262305. [DOI] [PubMed] [Google Scholar]
- 28.Mannini-Palenzona A, Moschella A, Costanzo F, Manservigi R, Monini P. Cell-type dependent sensitivity of herpes simplex virus 1 mutants to plaque development inhibition by an anti-gD monoclonal antibody. New Microbiol. 1995;18:351–358. [PubMed] [Google Scholar]
- 29.Mester J C, Glorioso J C, Rouse B T. Protection against zosteriform spread of herpes simplex virus by monoclonal antibodies. J Infect Dis. 1991;163:263–269. doi: 10.1093/infdis/163.2.263. [DOI] [PubMed] [Google Scholar]
- 30.Mikloska Z, Cunningham A L. Glycoproteins gB, gC, gD are targets for CD4 cytotoxic T lymphocytes in IFN-gamma pretreated human epidermal keratinocytes. J Gen Virol. 1998;79:353–361. doi: 10.1099/0022-1317-79-2-353. [DOI] [PubMed] [Google Scholar]
- 31.Mikloska Z, Danis V A, Adams S, Lloyd A R, Adrian D L, Cunningham A L. In vivo production of cytokines and (C-C) chemokines in human recurrent herpes simplex lesions. Do virus infected keratinocytes contribute to their production? J Infect Dis. 1998;177:827–838. doi: 10.1086/515236. [DOI] [PubMed] [Google Scholar]
- 32.Mikloska Z, Kesson A M, Penfold M E T, Cunningham A L. Herpes simplex protein targets for CD4 and CD8 lymphocyte cytotoxicity in cultured epidermal keratinocytes treated with interferon-gamma. J Infect Dis. 1996;173:7–17. doi: 10.1093/infdis/173.1.7. [DOI] [PubMed] [Google Scholar]
- 32a.Miranda-Saksena, M., et al. Unpublished data.
- 33.Openshaw H, Asher L V S, Wohlenberg C, Sekizawa T, Notkins A L. Acute and latent infection of sensory ganglia with herpes simplex virus: immune control and virus reactivation. J Gen Virol. 1979;44:205–215. doi: 10.1099/0022-1317-44-1-205. [DOI] [PubMed] [Google Scholar]
- 34.Penfold M E T, Armati P J, Mikloska Z, Cunningham A L. The interaction of human fetal neurons and epidermal cells in vitro. Cell Dev Biol Anim. 1996;32:420–426. doi: 10.1007/BF02723004. [DOI] [PubMed] [Google Scholar]
- 35.Penfold M E T, Armati P, Cunningham A L. Axonal transport of herpes simplex virions to epidermal cells: evidence for a specialized mode of virus transport and assembly. Proc Natl Acad Sci USA. 1994;91:6529–6533. doi: 10.1073/pnas.91.14.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prober C G, Sullender W M, Yasukawa L L, Au D S, Yeager A S, Arvin A M. Low risk of herpes simplex virus infections in neonates exposed to the virus at the time of vaginal delivery to mothers with recurrent genital herpes simplex virus infections. N Engl J Med. 1987;316:240–244. doi: 10.1056/NEJM198701293160503. [DOI] [PubMed] [Google Scholar]
- 37.Roizman B, Sears A E. Herpes simplex viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1995. pp. 2231–2295. [Google Scholar]
- 38.Sanna P P, De Logu A, Williamson R A, Hom Y L, Straus S E, Bloom F E, Burton D R. Protection of nude mice by passive immunization with a type-common human recombinant monoclonal antibody against HSV. Virology. 1996;215:101–116. doi: 10.1006/viro.1996.0011. [DOI] [PubMed] [Google Scholar]
- 39.Sanna P P, Williamson R A, De Logu A, Bloom F E, Burton D R. Directed selection of recombinant human monoclonal antibodies to herpes simplex glycoproteins from phage display libraries. Proc Natl Acad Sci USA. 1995;92:6439–6443. doi: 10.1073/pnas.92.14.6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmid D S, Rouse B T. The role of T cell immunity in control of herpes simplex virus. Curr Top Microbiol Immunol. 1992;179:57–74. doi: 10.1007/978-3-642-77247-4_4. [DOI] [PubMed] [Google Scholar]
- 41.Siegal F P, Lopez C, Hammer G S, Brown A E, Kornfeld S J, Gold J, Hassett J, Hirschman S Z, Cunningham-Rundles C, Adelberg B R, et al. Severe acquired immunodeficiency in male homosexuals, manifested by chronic perianal ulcerative herpes simplex lesions. N Engl J Med. 1981;305:1439–1444. doi: 10.1056/NEJM198112103052403. [DOI] [PubMed] [Google Scholar]
- 42.Stevens J G, Cook M L. Latent herpes simplex virus in spinal ganglia of mice. Science. 1971;173:843–845. doi: 10.1126/science.173.3999.843. [DOI] [PubMed] [Google Scholar]
- 43.Su H K, Fetherston J D, Smith M E, Courtney R J. Orientation of the cleavage site of the herpes simplex glycoprotein G-2. J Virol. 1993;67:2954–2959. doi: 10.1128/jvi.67.5.2954-2959.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wald A, Zeh J, Selke S, Ashley R L, Corey L. Virologic characterization of subclinical and symptomatic genital herpes infection. N Engl J Med. 1995;333:770–775. doi: 10.1056/NEJM199509213331205. [DOI] [PubMed] [Google Scholar]
- 45.Whitely R J, Yaeger A, Kartus B, Bryson Y, Connor J D, Alford C A, Nahmias A, Soong S-J. Neonatal herpes simplex virus infection: follow-up evaluation of Vidarabine therapy. Pediatrics. 1983;72:778–785. [PubMed] [Google Scholar]
- 46.Williamson R A, Burioni R, Sanna P P, Partridge L J, Barbas C D, Burton D R. Human monoclonal antibodies against a plethora of viral pathogens from single combinatorial libraries. Proc Natl Acad Sci USA. 1993;90:4141–4145. doi: 10.1073/pnas.90.9.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeager A S, Arvin A M, Urbani L J, Kemp J A. Relationship of antibody to outcome in neonatal herpes simplex virus infections. Infect Immun. 1980;29:532–538. doi: 10.1128/iai.29.2.532-538.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeitlin L, Whaley K J, Sanna P P, Moench T R, Bastidas R, DeLogu A, Williamson R A, Burton D R, Cone R A. Topically applied human recombinant monoclonal IgG1 antibody and its Fab and F(ab′)2 fragments protect mice from vaginal transmission of HSV-2. Virology. 1996;225:213–215. doi: 10.1006/viro.1996.0589. [DOI] [PubMed] [Google Scholar]
- 49.Zweerink H J, Stanton L W. Immune response to herpes simplex virus infections: virus specific antibodies in sera from patients with recurrent facial infections. Infect Immun. 1981;31:624–630. doi: 10.1128/iai.31.2.624-630.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]