Abstract
Background
Robot-guided lumbar spine surgery has evolved rapidly with evidence to support its utility and feasibility compared with conventional freehand and fluoroscopy-based techniques. The objective of this study was to assess trends among the top 25 most-cited articles pertaining to robotic-guided lumbar spine surgery.
Methods
An “advanced document search” using Boolean search operator terms was performed on 16 November 2022 through the Web of Science and SCOPUS citation databases to determine the top 25 most-referenced articles on robotic lumbar spine surgery. The articles were compiled into a directory and hierarchically organized based on the total number of citations.
Results
Cumulatively, the “Top 25” list for robot-assisted navigation in lumbar spine surgery received 2240 citations, averaging 97.39 citations annually. The number of citations ranged from 221 to 40 for the 25 most-cited articles. The most-cited study, by Kantelhardt et al, received 221 citations, averaging 18 citations per year.
Conclusions
As utilization of robot-guided modalities in lumbar spine surgery increases, this review highlights the most impactful studies to support its efficacy and implementation. Practical considerations such as cost-effectiveness, however, need to be better defined through further longitudinal studies that evaluate patient-reported outcomes and cost-utility.
Clinical Relevance
Through an overview of the top 25 most-cited articles, the present review highlights the rising prominence and technical efficacy of robotic-guided systems within lumbar spine surgery, with consideration to pragmatic limitations and need for additional data to facilitate cost-effective applications.
Level of Evidence
5
Keywords: robotic spine surgery, lumbar, pedicle screw, navigation, clinical outcomes
Introduction
With continuous strides toward refining minimally invasive surgery (MIS) techniques and optimizing clinical outcomes, the cross integration of technological developments has given rise to advancements within the field of spine surgery. The advent of robotic surgical systems is one such area of growth aimed at improving surgical precision and has gained considerable traction since its foremost applications within joint arthroplasty procedures.1,2 Within spine surgery, robotic-assisted technology has been shown to confer benefits to both surgeons and patients by minimizing fatigue, visual errors, and perioperative complications.3,4
In view of its initial success, robotics guidance has expanded surgical capabilities in the field of lumbar spine surgery. Studies have shown that robotics-assisted posterior instrumentation in lumbar spine fusion is both safe and highly effective and moreover allows spine surgeons to preserve surgical accuracy and fine motor control through extended procedures.5 In assessments of pedicle screw placement accuracy, reports across the literature have demonstrated significant reductions in screw malpositioning, postoperative complications, and subsequent revision risk with robotic-assisted procedures compared with conventional freehand techniques and fluoroscopy-guided navigation.4,6 Incorporation of robotic surgical platforms further enables additionally detailed preoperative planning for tailored selection of implants fitted to patients’ individualized anatomy.7 With evidence to support its utility, applications of robotic systems have conjointly expanded with that of newly developing techniques within the field, including single-position lumbar interbody fusions.8,9 As evidence unfolds to substantiate contemporary iterations of robotic surgical platforms, the use of robotic-guided modalities within spine surgery will likely continue to evolve. As such, understanding and awareness of the most impactful studies to support the application, feasibility, and efficacy of robotics within spine surgery is important to contend with its expanding role; to accomplish these aims, this review examines the top 25 most-cited articles on robotic lumbar surgery.
Materials and Methods
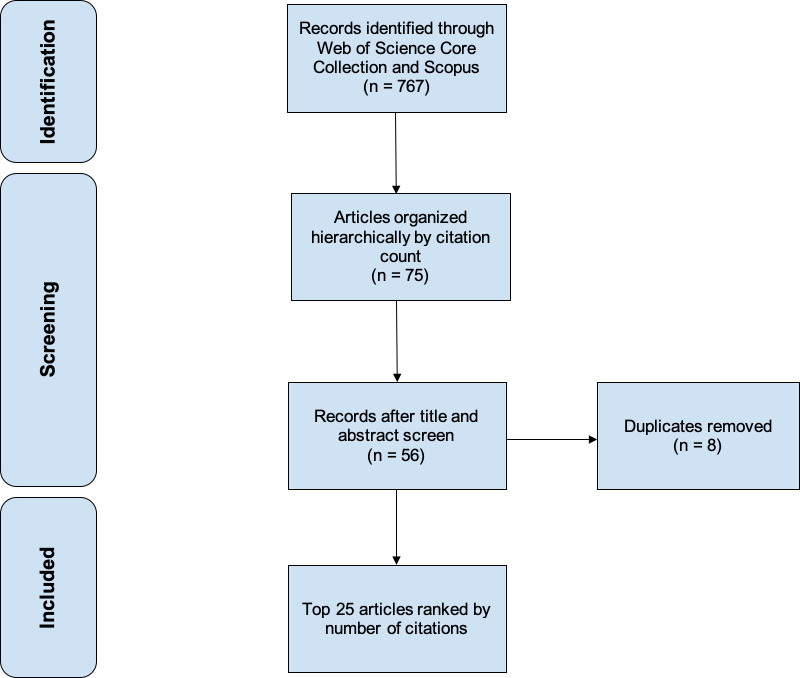
Articles of interest were queried through a systematic search performed on November 2022 using Boolean operator terms within SCOPUS and Clarivate Analytics’ Web of Science database, which comprises the Web of Science Core Collection, MEDLINE (Pubmed), and BIOSIS databases. These databases were selected based on their ability to hierarchically organize peer-reviewed articles by number of citations, which is suited for the purposes of this study. Only articles written in English were included in our study. The 25 most-cited articles were compiled into a ranked list after sorting results in descending order based on the total number of citations (Figure 1). The following details were extracted from the 25 articles: year of publication, journal title, total citation count, the average number of citations annually, and study summary. Duplicate entries between the Web of Science and SCOPUS databases were excluded. Article content was assessed to determine a summative “Top 25” list on robotic-assisted lumbar spine surgery.
Figure 1.
Flowchart diagram outlining the systematic review process used to identify the top 25 most-cited articles.
Results
An initial search of the Web of Science database for robotic-assisted lumbar spine surgery yielded 445 articles, amassing a total of 5927 citations. Similarly, the SCOPUS database search generated 322 articles with a combined 3951 citations. The leading 25 articles were ranked based on total citation count (Table 1).4–6,10–31 The resulting list was further screened to ensure adherence to the inclusion criteria and pertinence to the intended topic search.
Table 1.
Top 25 most-cited articles on robotic-assisted lumbar spine surgery ranked hierarchically by cumulative number of citations.
| Author and Publication Year | Journal | Summary Description | Times Cited (Total) | Citations Per Year | Robot System |
| 1. Kantelhardt et al (2011)10 | European Spine Journal | Retrospective cohort analysis of 112 consecutive patients demonstrating shorter intraoperative fluoroscopy time and increased screw accuracy within open robotic-assisted vs conventional open pedicle screw placement. | 221 | 20 | SpineAssist |
| 2. Devito et al (2010)11 | Spine | Retrospective observational case series of 3271 pedicle screw and guide-wire insertions with SpineAssist guidance across 14 hospitals from June 2005 to June 2009, wherein clinical acceptance and accuracy were assessed and compared with freehand techniques as reported by the literature. | 215 | 18 | SpineAssist |
| 3. Hyun et al (2017)12 | Spine | Randomized clinical trial of 60 patients showing significantly reduced fluoroscopy exposure and length of stay in patients undergoing single- and two-level robotic-guided lumbar fusion relative to conventional fluoroscopic guidance. | 148 | 30 | Renaissance |
| 4. Schatlo et al (2014)13 | Journal of Neurosurgery: Spine | Retrospective cohort analysis of 95 consecutive patients with degenerative lumbar pathologies showing comparable surgical time, length of stay, and screw placement accuracy in robotic-assisted vs fluoroscopy. | 130 | 16 | SpineAssist |
| 5. Kim et al (2017)14 | International Journal of Medical Robotics and Computer-Assisted Surgery | Randomized controlled trial of 78 patients with lumbar spinal stenosis showing superior outcomes with respect to facet joint violation and convergence orientation with robotic-assisted PLIF vs conventional freehand techniques. | 122 | 24 | Renaissance |
| 6. Lieberman et al (2006)15 | Neurosurgery | Observational cadaveric case study substantiating screw placement accuracy with SpineAssist when comparing actual screw and planned screw trajectories on postprocedure computed tomography. | 120 | 8 | SpineAssist |
| 7. Pechlivanis et al (2009)16 | Spine | Prospective observational case series of 31 patients validating accuracy of pedicle screw placement in patients undergoing PLIF with percutaneous posterior pedicle screw insertion using passive guidance provided by a bone-mounted miniature robotic device (SpineAssist). | 118 | 9 | SpineAssist |
| 8. Lonjon et al (2016)4 | European Spine Journal | Prospective 1:1 matched-cohort analysis of 20 consecutive patients reporting higher screw placement precision using robotic-assistance (ROSA) over freehand techniques. | 111 | 19 | ROSA |
| 9. van Dijk et al (2015)17 | Spine | Retrospective case series of 112 consecutive patients undergoing PLIFs with SpineAssist demonstrating accurate screw placement, wherein intraoperative screw placement was consistent with preoperative plan. | 92 | 13 | SpineAssist |
| 10. Lieberman et al (2012)18 | Journal of Spinal Disorders and Techniques | Prospective cohort analysis of 12 cadavers across 17 surgeons showing decreased radiation exposure, fluoroscopy time per screw, procedure time, screw placement deviation, and pedicle wall breaches while maintaining increased accuracy of percutaneous pedicle screw placement with the use of the SpineAssist system compared with freehand techniques. | 91 | 9 | SpineAssist |
| 11. Keric et al (2017)19 | Neurosurgical Focus | Retrospective case series of 413 patients who underwent spinal screw implantation with Renaissance showing high reliability and accuracy in screw placement with lower peri- and early postoperative complications relative to other percutaneous screw placement techniques across the literature. | 81 | 16 | Renaissance |
| 12. Barzilay et al (2006)20 | International Journal of Medical Robotics and Computer-Assisted Surgery | Prospective case series of 15 patients who underwent robot-assisted lumbar spine fusion with the SpineAssist system which identified technical and clinical variables contributing to difficult cases. | 77 | 5 | SpineAssist |
| 13. Lefranc et al (2016)21 | Expert Review of Medical Devices | Technical review outlining surgical technique, indications for use, future directions, and advantages associated with use of the new ROSA robot in performing accurate pedicle screw placement and minimally invasive percutaneous surgical procedures. | 73 | 12 | ROSA |
| 14. Khan et al (2019)5 | Operative Neurosurgery | Retrospective case series of 20 patients who underwent robotically assisted pedicle screw insertion performed by a single surgeon with preliminary results showing 98.7% accuracy in 75 pedicle screw placements, reinforcing feasibility of robotic guidance in lumbar spine surgery. | 71 | 24 | Mazor X |
| 15. Kim et al (2015)22 | Spine | Prospective randomized controlled study that demonstrated similar quality of performance and accuracy as measured by a cumulative summation test in pedicle screw fixation in 20 patients who underwent robot-assisted MIS PLIF and 20 patients who underwent conventional open PLIF using freehand technique. | 67 | 10 | Renaissance |
| 16. Schatlo et al (2015)23 | Acta Neurochirurgica | Retrospective chart review of 258 patients requiring thoracic and/or lumbar spine surgery with posterior instrumentation showed that robot-assisted screw placement is safe with a 3.8% screw malposition rate. | 67 | 10 | TiRobot |
| 17. Gao et al (2018)24 | European Spine Journal | Systematic review and meta-analysis of 6 studies incorporating 158 patients (688 pedicle screws) in the robot-assisted group and 148 patients (672 pedicle screws) in the conventional freehand group demonstrated that both groups exhibited similar accuracy rate of pedicle screw implantation, but the robot-assisted technique was associated with longer operative time. | 65 | 16 | N/A (5 Mazor, 1 Tianji) |
| 18. Le et al (2018)6 | World Neurosurgery | Retrospective matched-cohort study of 58 patients undergoing pedicle screw insertion through the cortical bone for lumbar fixation that demonstrated perfect trajectory for 87.2% of robotic-assisted screw insertion and 66.9% of conventional freehand screw instrumentation. | 59 | 15 | Renaissance |
| 19. Urakov et al (2017)25 | Neurosurgical Focus | Retrospective review of prospectively collected data from 33 patients who underwent robot-assisted thoracolumbar pedicle instrumentation that showed no correlation regarding speed and accuracy of instrumentation between surgeon’s years of operative experience and commitment to spine surgery as their future speciality. | 49 | 10 | Renaissance |
| 20. Li et al (2020)26 | Spine | Meta-analysis of 9 randomized controlled trials with 696 patients demonstrated that robot-assisted pedicle screw placement reduced radiation dose and decreased intraoperative radiation exposure time while showing greater accuracy compared with pedicle screw instrumentation by conventional freehand technique. | 47 | 24 | TiRobot |
| 21. Schröder et al (2017)27 | Neurosurgical Focus | Retrospective cohort study of 72 patients who had undergone an MIS PLIF or MIS TLIF and completed a follow-up ≥12 months demonstrated that robot-guided screw trajectories are more accurate compared with trajectories established by freehand techniques which reduce rate of revision surgery for screw malposition and improve visual analog scale and Oswestry Disability Index scores. | 46 | 9 | Renaissance |
| 22. Fan et al (2017)28 | Medical Science Monitor | Prospective cohort study of 890 pedicle screws placed in 190 patients for treatment of degenerative lumbar disease demonstrated that robot-assisted technique unsuccessfully showed significant differences for accuracy of pedicle screw insertion compared with freehand technique but greatly reduced blood loss, fluoroscopy time per screw, and postoperative stay. | 45 | 9 | SpineAssist |
| 23. Kuo et al (2016)29 | PLoS ONE | Retrospective review of 64 patients who either underwent or did not undergo TLIF demonstrated that the Renaissance robotic system can accurately place pedicle screws, and secondary registration enhances accuracy by providing intraoperative evaluation of screw positioning. | 45 | 8 | Renaissance |
| 24. Tian et al (2020)30 | Neurospine | Case series of 62 thoracolumbar pedicle screws implanted in 12 patients using 5G telerobotic remote telecommunication showed the potential of utilizing telemedical service in the future. | 40 | 20 | TiRobot |
| 25. Wolf et al (2001)31 | Spine | Observational case series of morphometric data using computed tomography of the lumbar spine of 55 patients who provided additional information on vertebrae geometry and its relation to entry points for screw insertion for spinal procedures. | 40 | 2 | N/A |
Abbreviations: MIS, minimally invasive surgery; PLIF, posterior lumbar interbody fusion; TLIF, transforaminal lumbar interbody fusion.
Overall, the “Top 25” most-cited articles totaled 2240 citations, averaging 97.39 citations annually. The most-cited article by Kantelhardt et al received 221 citations in total, averaging 18 citations annually since publication in 2011.10 Devito et al authored the second most-cited article in 2010 with 215 citations overall and an annual average of 17 citations.11 Relative to the preceding studies, the third most-cited article was published more recently, in 2017, by Hyun et al and has since garnered 148 citations with an average of 30 per year.12 The 25 most-cited article by Wolf et al accrued 40 citations overall at a yearly rate of 2 citations since 2001.31
Article publication year ranged from 2001 to 2020. Among the top 25, the highest number of articles published on this topic was in 2017 (n = 6; Figure 2). Regarding publication trends, Spine published the highest number of articles, producing 7 of the 25 most-cited articles on robotic-assistance in lumbar spine surgery (Figure 3). Neurosurgical Focus and The European Spine Journal each published 3 articles, making these 2 journals tied for the second-highest number of publications on this subject.
Figure 2.
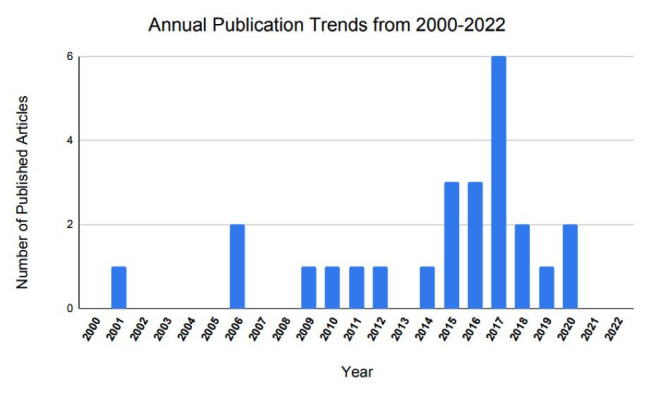
Annual publication trends in articles on robotic lumbar spine surgery from 2000 to 2022.
Figure 3.
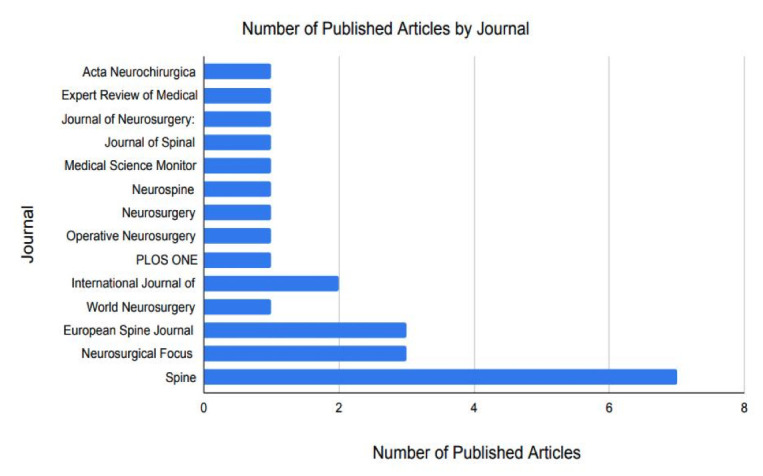
Number of published articles by journal on robotic lumbar spine surgery from 2000 to 2022.
A comprehensive review revealed that our “Top 25” cohort consisted of 18 case series, 7 cohort studies, 2 systematic reviews/meta-analyses, and 1 technical review. The Newcastle-Ottawa scale, a validated scoring metric, was used to assess the quality of case series included within our ranked list. This system considers several metrics including comparability, selection, and ascertainment of exposure/outcome of interest6,32,33 (Table 2). Articles are scored using a 9-point scale, with 0 corresponding to lower quality and 9 denoting the highest quality. Among our ranked list, scores ranged from 5 to 8, with the average score being 6.6. To evaluate cohort studies, the Joanna Briggs Institute critical appraisal tool was selected as it is the only validated instrument to assess cohort studies on the basis of trustworthiness, relevance, and results.34 This scoring system allocates scores from 0 to 10, with the lowest quality studies obtaining 0 and the highest quality studies achieving 10. Our cohort received scores ranging from 3 to 10, with the average score being 6.5 (Table 3).
Table 2.
Newcastle-Ottawa assessment scale of cohort studies within the top 25 most-cited articles ordered by citation ranking.
| Study | Representative of Cohorts | Selection of Nonexposed Cohort | Ascertainment of Exposure | Outcome of Interest Absent at Start of Study | Cohort Comparability | Outcome Assessment | Sufficient Follow-up | Adequacy of Follow-up | Total Score |
| 1. Kantelhardt et al (2011)10 | * | * | * | * | * | * | * | * | 8 |
| 3. Hyun et al (2017)12 | * | * | * | * | ** | * | * | * | 9 |
| 4. Schatlo et al (2014)13 | * | * | * | * | ** | * | * | * | 9 |
| 5. Kim et al (2017)14 | * | * | * | * | ** | * | * | * | 9 |
| 8. Lonjon et al (2016)4 | * | * | * | * | * * | * | * | * | 9 |
| 10. Lieberman et al (2012)18 | - | - | * | - | - | * | * | * | 4 |
| 15. Kim et al (2015)22 | * | * | * | * | - | * | * | * | 7 |
| 18. Le et al (2018)6 | - | - | * | * | * | * | * | * | 6 |
| 19. Urakov et al (2017)25 | - | - | - | - | - | - | - | - | 0 |
| 21. Schröder et al (2017)27 | - | - | - | - | - | - | - | - | 0 |
| 22. Fan et al (2017)28 | * | * | * | * | - | * | * | * | 7 |
Table 3.
Joanna Briggs Institute (JBI) critical appraisal tool assessments of case series within the top 25 most-cited articles ordered by citation ranking.
| JBI Assessment Categories | 2. Devito et al (2010) | 6. Lieberman et al (2006) | 7. Pechlivanis et al (2009) | 9. van Dijk et al (2015) | 11. Keric et al (2017) | 12. Barzilay et al (2006) | 14. Khan et al (2019) | 16. Schatlo et al (2015) | 23. Kuo et al (2016) | 24. Tian et al (2020) | 25. Wolf et al (2001) |
| Clear inclusion criteria | No | No | Yes | Yes | Yes | No | Yes | Yes | Yes | No | No |
| Reliable data collection | No | Yes | No | Yes | Yes | No | Yes | Yes | Yes | No | No |
| Valid methodology for assessing outcomes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | No | No |
| Consecutive series | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No |
| Inclusion of all possible participants/cases | No | No | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes |
| Relevant demographics reported | Yes | No | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes |
| Baseline clinical characteristics reported | No | No | No | No | Yes | Yes | No | No | Yes | No | No |
| Clear description of outcomes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Study site/setting described | Yes | No | No | No | Yes | Yes | No | No | Yes | Yes | No |
| Appropriate statistical analysis | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Total Score (/10) | 6 | 4 | 7 | 7 | 10 | 6 | 8 | 7 | 10 | 4 | 3 |
Discussion
The current study found that the top 25 most-cited articles on robotic-assisted lumbar spine surgery were largely descriptive case series followed by cohort studies. The most highly cited article was published in European Spine Journal by Kantelhardt et al in 2011, totaling 221 citations. The authors performed a single-institution retrospective cohort analysis using SpineAssist (Mazor Surgical Technologies, Caesarea, Israel) to compare intra- and perioperative metrics including pedicle screw placement accuracy, fluoroscopy duration, and postoperative opioid use between robotic-assisted approaches, both open and percutaneous, to conventional open procedures. The study included 112 consecutive cases with pedicle screw fixation from 2006 to 2009, with all approaches being performed across 6 surgeons.10 Kantelhardt et al describe improved screw accuracy and significantly decreased x-ray duration (34 vs 77 seconds), postoperative opioid use (46% vs 89%), and incidence of intraoperative adverse events (4.7% vs 9.1%) with robotics-guidance compared with conventional techniques.10 No significant differences were found with respect to operative duration, even when accounting for time attributed to configuring the robotics system. The heterogeneity of indications included within the study, along with its retrospective design, remains an important limitation to consider. In spite of this, these findings provide evidence to support implementation of robotic-assisted lumbar spine surgery.
The second most highly cited article, published in 2010 by Devito et al, was a multicenter retrospective case series in which the authors described the clinical acceptance and implant placement accuracy across 14 institutions using SpineAssist. Overall, the study involved 3721 pedicle screw and guide-wire insertions over 840 cases from 2005 to 2009, spanning the subsequent years after which SpineAssist—the first robotic-based platform in spine surgery—received US Food and Drug Administration approval in 2004.35 Clinical acceptance was established for 98% (n = 3204) of implants using intraoperative fluoroscopy. Accuracy was assessed using postoperative computed tomography (CT) across 646 placed pedicle screws, with 89.3% of screws placed entirely within the pedicle, and 9% of breached screws being within 2 mm.11 Stratifying by region, the authors found that 79% (30/38) thoracic and 90% (547/608) lumbar screw placements were contained entirely within the pedicle compared with 56% thoracic and 87.3% lumbar screws for freehand techniques.36 All comparisons, however, were made relative to the existing literature and, therefore, presented a limitation within this study. Nonetheless, the article was significantly impactful in establishing the clinical efficacy of robotic-assisted spine surgery—particularly in consideration of SpineAssist being the foremost model in clinical practice. The study has since garnered 215 citations, with an annual average of 19.5 citations since publication in 2010.
The third most-cited article was published in 2017 by Hyun et al. Herein, the authors designed a prospective randomized clinical trial to assess radiation exposure and patient-reported outcomes (PROs) between fluoroscopy-guided open procedures and robotic-assisted MIS approaches using the Renaissance system (Mazor Robotics Ltd) for patients undergoing lumbar fusion.12 Their study was conducted with consideration of the high levels of radiation exposure commensurate with the learning curves for MIS techniques. Their study revealed significantly reduced radiation exposure and hospital length of stay with robotic-guided MIS relative to fluoroscopic open procedures. Among PROs, visual analog scale back and leg pain scores were improved for robotic-assisted cases, while Oswestry Disability Index scores remained similar across both groups.12 Relative to the 2 preceding studies, the total number of citations, 148, for this article was noticeably reduced; it is important to note, however, that this article was published significantly later in 2017 compared with Devito et al in 2010 and Kantelhardt et al in 2011, respectively. Further analysis reveals that Hyun et al carried the highest average annual citation rate at 30 per year, which was the highest by this metric among the top 25 articles on robotic-assisted lumbar spine surgery.
Interestingly, 2017 was the most common publication year within this list—comprising 6 articles overall—followed by 2015 and 2016, with 3 articles for each.12,14,19,25,27,28 Publications from 2015 to 2017 represented 48% (12/25) of the articles within the top 25 most-cited list. Of the articles published in 2017, 5/6 (83%) reviewed the Renaissance robotic-guidance system, with the remaining study by Fan et al28 overviewing SpineAssist, the foremost iteration, which was designed by Mazor Robotics.
Despite other models such as ROSA Spine by Zimmer Biomet having received FDA clearance in 2016, Renaissance was approved in 2011 and therefore allowed for longitudinal studies spanning multiple years. Moreover, FDA clearance of SpineAssist in 2004 marked the introduction of robotics use in spine surgery; as such, subsequent models produced by Mazor Robotics had already gained considerable traction as the sole proprietor during this time.37 Relative to Renaissance, publications revolving around SpineAssist saw greater remportal distribution, which was likely due to limited accessibility as the first commercially available robotic system apt for spine surgery applications.38 Following acquisition of Mazor Robotics by Medtronic in 2018, newer models such as the Mazor X and Mazor X Stealth Edition have been implemented with improved preoperative planning and intraoperative navigation systems for the latter.39 These developments have augmented the minimally invasive nature of these systems and obviate the use of K wires and percutaneous pins as required by previous models.40 Nonetheless, the advent of robotic-assisted spine surgery has since fostered an array of models, including ROSA ONE Spine (Zimmer Biomet, Warsaw, IN), ExcelsiusGPS (Globus Medical Inc., Audubon, PA), and TiRobot (TINAVI Medical Technologies, Beijing, China).41 With technological advancements driving further growth in robotic capabilities, future developments should anticipate the integration of other evolving facets within spine surgery.
As the utilization of robotic systems in spine surgery expands, high-quality prospective studies evaluating clinical and patient-centered outcomes are crucial to substantiate pragmatic and cost-effective applications.42 While the evidence presented within the top 25 most-cited articles pointedly advocate for robotics use in spine surgery, the comparative analyses are entirely premised on freehand techniques and/or conventional fluoroscopic guidance. Intraoperative CT navigation offers another avenue by which pedicle screw instrumentation accuracy has improved and at a significantly lower cost than contemporary robotic systems.42 Comparative studies have primarily focused on implant-related metrics, wherein robotic systems enabled optimal implant dimensions with comparable screw accuracy, while reducing fluoroscopy and screw placement time over CT navigation alone.43,44 While CT navigation further provides granular visualization and real-time visualization, latest-generation robotic platforms integrate real-time CT navigation to optimize work-flow efficiency and instrumentation accuracy.40 Nonetheless, further biomechanical and clinical studies are required to assess potential long-term impact on clinical outcomes as current evidence indicates minimal benefits in PROs.45,46 Park et al conducted an randomized controlled trial and found no differences in ODI and VAS leg and back score improvement between patients who underwent posterior lumbar interbody fusion using robot-assisted pedicle screw fixation or conventional freehand techniques.47 As reimbursement shifts toward value-based care, understanding the impact of robotic platforms on PROs becomes increasingly important—especially in consideration of forthcoming technological developments.
Overall, economic advantages offered with robotics platforms are largely proposed upon improved implant accuracy and subsequent reduction in revision risk. Menger et al conducted a retrospective analysis of 557 thoracolumbar instrumentation cases and found that robotic assistance significantly reduced postoperative infection, length of stay, operative time, and revision risk, resulting in an estimated $608,546 in savings over a year.48 Ezeokoli et al reported higher variable direct costs for robotic-assisted spine procedures primarily owed to operating room time and supply -related expenses.49 Psasias et al likewise discovered higher rates of complications and costs for robotic-guided procedures compared with open and MIS techniques.50 Both studies, however, acknowledged potential confounding effects of surgeons’ learning curves on cost-effectiveness measures, which iwas supported by Hu and Lieberman, who demonstrated significant improvements with increasing experience.51 As such, an accurate cost-utility analysis therefore calls for longitudinal, prospective studies that are able to capture the entire scope of practical considerations with implementation of robotics systems in spine surgery.
Limitations
The present study has several limitations. First, self-citation is a potential confounding factor that may disproportionately inflate the citation count of articles with lesser impact. Additionally, our database search was restricted to articles written in English, which might have resulted in the exclusion of pertinent articles that may have contributed significantly to the subject matter. Furthermore, the heterogeneity of study designs precluded the application of a uniform, standardized assessment scale throughout our ranked list. Finally, our analysis merely represents a snapshot of the literature at the time our literature search was conducted. As new research emerges and our comprehension of robotic-assistance in lumbar spine surgery expands, relative citation counts may fluctuate, leading to alternative conclusions.
Conclusions
This study provides our analysis of the current literature on robotic-assistance within spine surgery. Utilizing the 25 most-cited articles relating to this topic, publication trends regarding the efficacy and expanded capabilities of various robotic models are discussed. The predominant representation of select models among the same developers, however, underlines the need for further high-quality studies focusing on alternative models, which have since been implemented within clinical practice. Given that robotics platforms are being increasingly adopted, this review is intended to serve as a resource that outlines some of the most impactful evidence for its utility in spine surgery as further technological advancements continue to unfold.
References
- 1. Bann S, Khan M, Hernandez J, et al. Robotics in surgery. J Am Coll Surg. 2003;196(5):784–795. 10.1016/S1072-7515(02)01750-7 [DOI] [PubMed] [Google Scholar]
- 2. Phillips FM, Lieberman IH, Polly DW, Wang MY. Minimally Invasive Spine Surgery: Surgical Techniques and Disease Management. New York: Springer Nature; 2019. 10.1007/978-3-030-19007-1 [DOI] [Google Scholar]
- 3. Ponnusamy K, Chewning S, Mohr C. Robotic approaches to the posterior spine. Spine (Phila Pa 1976). 2009;34(19):2104–2109. 10.1097/BRS.0b013e3181b20212 [DOI] [PubMed] [Google Scholar]
- 4. Lonjon N, Chan-Seng E, Costalat V, Bonnafoux B, Vassal M, Boetto J. Robot-assisted spine surgery: feasibility study through a prospective case-matched analysis. Eur Spine J. 2016;25(3):947–955. 10.1007/s00586-015-3758-8 [DOI] [PubMed] [Google Scholar]
- 5. Khan A, Meyers JE, Siasios I, Pollina J. Next-generation robotic spine surgery: first report on feasibility, safety, and learning curve. Oper Neurosurg (Hagerstown). 2019;17(1):61–69. 10.1093/ons/opy280 [DOI] [PubMed] [Google Scholar]
- 6. Le X, Tian W, Shi Z, et al. Robot-assisted versus fluoroscopy-assisted cortical bone trajectory screw instrumentation in lumbar spinal surgery: a matched-cohort comparison. World Neurosurg. 2018;120:e745–e751. 10.1016/j.wneu.2018.08.157 [DOI] [PubMed] [Google Scholar]
- 7. Good CR, Orosz L, Schroerlucke SR, et al. Complications and revision rates in minimally invasive robotic-guided versus fluoroscopic-guided spinal fusions: the MIS refresh prospective comparative study. Spine (Phila Pa 1976). 2021;46(23):1661–1668. 10.1097/BRS.0000000000004048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. North RY, Strong MJ, Yee TJ, Kashlan ON, Oppenlander ME, Park P. Navigation and robotic-assisted single-position prone lateral lumbar interbody fusion: technique, feasibility, safety, and case series. World Neurosurg. 2021;152:221–230. 10.1016/j.wneu.2021.05.097 [DOI] [PubMed] [Google Scholar]
- 9. Sinkov V, Lockey SD, Cunningham BW. Single position lateral lumbar interbody fusion with posterior instrumentation utilizing computer navigation and robotic assistance: retrospective case review and surgical technique considerations. Global Spine J. 2022;12(2_suppl):75S–81S. 10.1177/21925682221083909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kantelhardt SR, Martinez R, Baerwinkel S, Burger R, Giese A, Rohde V. Perioperative course and accuracy of screw positioning in conventional, open robotic-guided and percutaneous robotic-guided, pedicle screw placement. Eur Spine J. 2011;20(6):860–868. 10.1007/s00586-011-1729-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Devito DP, Kaplan L, Dietl R, et al. Clinical acceptance and accuracy assessment of spinal implants guided with spineassist surgical robot: retrospective study. Spine (Phila Pa 1976). 2010;35(24):2109–2115. 10.1097/BRS.0b013e3181d323ab [DOI] [PubMed] [Google Scholar]
- 12. Hyun S-J, Kim K-J, Jahng T-A, Kim H-J. Minimally invasive robotic versus open fluoroscopic-guided spinal instrumented fusions: a randomized controlled trial. Spine (Phila Pa 1976). 2017;42(6):353–358. 10.1097/BRS.0000000000001778 [DOI] [PubMed] [Google Scholar]
- 13. Schatlo B, Molliqaj G, Cuvinciuc V, Kotowski M, Schaller K, Tessitore E. Safety and accuracy of robot-assisted versus fluoroscopy-guided pedicle screw insertion for degenerative diseases of the lumbar spine: a matched cohort comparison. J Neurosurg Spine. 2014;20(6):636–643. 10.3171/2014.3.SPINE13714 [DOI] [PubMed] [Google Scholar]
- 14. Kim H-J, Jung W-I, Chang B-S, Lee C-K, Kang K-T, Yeom JS. A prospective, randomized, controlled trial of robot-assisted vs freehand pedicle screw fixation in spine surgery. Int J Med Robot. 2017;13(3). 10.1002/rcs.1779 [DOI] [PubMed] [Google Scholar]
- 15. Lieberman IH, Togawa D, Kayanja MM, et al. Bone-mounted miniature robotic guidance for pedicle screw and translaminar facet screw placement: part I-technical development and a test case result. Neurosurgery. 2006;59(3):641–650. 10.1227/01.NEU.0000229055.00829.5B [DOI] [PubMed] [Google Scholar]
- 16. Pechlivanis I, Kiriyanthan G, Engelhardt M, et al. Percutaneous placement of pedicle screws in the lumbar spine using a bone mounted miniature robotic system: first experiences and accuracy of screw placement. Spine (Phila Pa 1976). 2009;34(4):392–398. 10.1097/BRS.0b013e318191ed32 [DOI] [PubMed] [Google Scholar]
- 17. van Dijk JD, van den Ende RPJ, Stramigioli S, Köchling M, Höss N. Clinical pedicle screw accuracy and deviation from planning in robot-guided spine surgery: robot-guided Pedicle screw accuracy. Spine (Phila Pa 1976). 2015;40(17):E986–E991. 10.1097/BRS.0000000000000960 [DOI] [PubMed] [Google Scholar]
- 18. Lieberman IH, Hardenbrook MA, Wang JC, Guyer RD. Assessment of pedicle screw placement accuracy, procedure time, and radiation exposure using a miniature robotic guidance system. J Spinal Disord Tech. 2012;25(5):241–248. 10.1097/BSD.0b013e318218a5ef [DOI] [PubMed] [Google Scholar]
- 19. Keric N, Doenitz C, Haj A, et al. Evaluation of robot-guided minimally invasive implantation of 2067 pedicle screws. Neurosurg Focus. 2017;42(5):E11. 10.3171/2017.2.FOCUS16552 [DOI] [PubMed] [Google Scholar]
- 20. Barzilay Y, Liebergall M, Fridlander A, Knoller N. Miniature robotic guidance for spine surgery-introduction of a novel system and analysis of challenges encountered during the clinical development phase at two spine centres. Int J Med Robot. 2006;2(2):146–153. 10.1002/rcs.90 [DOI] [PubMed] [Google Scholar]
- 21. Lefranc M, Peltier J. Evaluation of the ROSATM spine robot for minimally invasive surgical procedures. Expert Rev Med Devices. 2016;13(10):899–906. 10.1080/17434440.2016.1236680 [DOI] [PubMed] [Google Scholar]
- 22. Kim H-J, Lee SH, Chang B-S, et al. Monitoring the quality of robot-assisted pedicle screw fixation in the lumbar spine by using a cumulative summation test. Spine (Phila Pa 1976). 2015;40(2):87–94. 10.1097/BRS.0000000000000680 [DOI] [PubMed] [Google Scholar]
- 23. Schatlo B, Martinez R, Alaid A, et al. Unskilled unawareness and the learning curve in robotic spine surgery. Acta Neurochir (Wien). 2015;157(10):1819–1823. 10.1007/s00701-015-2535-0 [DOI] [PubMed] [Google Scholar]
- 24. Gao S, Lv Z, Fang H. Robot-assisted and conventional freehand pedicle screw placement: a systematic review and meta-analysis of randomized controlled trials. Eur Spine J. 2018;27(4):921–930. 10.1007/s00586-017-5333-y [DOI] [PubMed] [Google Scholar]
- 25. Urakov TM, Chang KH-K, Burks SS, Wang MY. Initial academic experience and learning curve with robotic spine instrumentation. Neurosurg Focus. 2017;42(5):E4. 10.3171/2017.2.FOCUS175 [DOI] [PubMed] [Google Scholar]
- 26. Li H-M, Zhang R-J, Shen C-L. Accuracy of pedicle screw placement and clinical outcomes of robot-assisted technique versus conventional freehand technique in spine surgery from nine randomized controlled trials: a meta-analysis. Spine (Phila Pa 1976). 2020;45(2):E111–E119. 10.1097/BRS.0000000000003193 [DOI] [PubMed] [Google Scholar]
- 27. Schröder ML, Staartjes VE. Revisions for screw malposition and clinical outcomes after robot-guided lumbar fusion for spondylolisthesis. Neurosurg Focus. 2017;42(5):E12. 10.3171/2017.3.FOCUS16534 [DOI] [PubMed] [Google Scholar]
- 28. Fan Y, Du J, Zhang J, et al. Comparison of accuracy of pedicle screw insertion among 4 guided technologies in spine surgery. Med Sci Monit. 2017;23:5960–5968. 10.12659/msm.905713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kuo K-L, Su Y-F, Wu C-H, et al. Assessing the intraoperative accuracy of pedicle screw placement by using a bone-mounted miniature robot system through secondary registration. PLoS One. 2016;11(4):e0153235. 10.1371/journal.pone.0153235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tian W, Fan M, Zeng C, Liu Y, He D, Zhang Q. Telerobotic spinal surgery based on 5G network: the first 12 cases. Neurospine. 2020;17(1):114–120. 10.14245/ns.1938454.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wolf A, Shoham M, Michael S, Moshe R. Morphometric study of the human lumbar spine for operation-workspace specifications. Spine (Phila Pa 1976). 2001;26(22):2472–2477. 10.1097/00007632-200111150-00015 [DOI] [PubMed] [Google Scholar]
- 32. Rotenstein LS, Torre M, Ramos MA, et al. Prevalence of burnout among physicians: a systematic review. JAMA. 2018;320(11):1131–1150. 10.1001/jama.2018.12777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mata DA, Ramos MA, Bansal N, et al. Prevalence of depression and depressive symptoms among resident physicians: a systematic review and meta-analysis. JAMA. 2015;314(22):2373–2383. 10.1001/jama.2015.15845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Munn Z, Barker TH, Moola S, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. 2020;18(10):2127–2133. 10.11124/JBISRIR-D-19-00099 [DOI] [PubMed] [Google Scholar]
- 35. Theodore N, Ahmed AK. The history of robotics in spine surgery. Spine. 2018;43(7S):7S. 10.1097/BRS.0000000000002553 [DOI] [Google Scholar]
- 36. Kosmopoulos V, Schizas C. Pedicle screw placement accuracy: a meta-analysis. Spine (Phila Pa 1976). 2007;32(3):E111–20. 10.1097/01.brs.0000254048.79024.8b [DOI] [PubMed] [Google Scholar]
- 37. Kaushal M, Kurpad S, Choi H. Robotic-assisted systems for spinal surgery. Neurosurgical Procedures - Innovative Approaches. IntechOpen; 2020. 10.5772/intechopen.78468 [DOI] [Google Scholar]
- 38. Alluri RK, Avrumova F, Sivaganesan A, Vaishnav AS, Lebl DR, Qureshi SA. Overview of robotic technology in spine surgery. HSS J. 2021;17(3):308–316. 10.1177/15563316211026647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee NJ, Leung E, Buchanan IA, et al. A multicenter study of the 5-year trends in robot-assisted spine surgery outcomes and complications. J Spine Surg. 2022;8(1):9–20. 10.21037/jss-21-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. O’Connor TE, O’Hehir MM, Khan A, et al. Mazor X stealth robotic technology: a technical note. World Neurosurg. 2021;145:435–442. 10.1016/j.wneu.2020.10.010 [DOI] [PubMed] [Google Scholar]
- 41. Perfetti DC, Kisinde S, Rogers-LaVanne MP, Satin AM, Lieberman IH. Robotic spine surgery: past, present, and future. Spine (Phila Pa 1976). 2022;47(13):909–921. 10.1097/BRS.0000000000004357 [DOI] [PubMed] [Google Scholar]
- 42. Malham GM, Wells-Quinn T. What should my hospital buy next? Guidelines for the acquisition and application of imaging, navigation, and robotics for spine surgery. J Spine Surg. 2019;5(1):155–165. 10.21037/jss.2019.02.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Khan A, Meyers JE, Yavorek S, et al. Comparing next-generation robotic technology with 3-dimensional computed tomography navigation technology for the insertion of posterior Pedicle screws. World Neurosurg. 2019;123:e474–e481. 10.1016/j.wneu.2018.11.190 [DOI] [PubMed] [Google Scholar]
- 44. Shafi KA, Pompeu YA, Vaishnav AS, et al. Does robot-assisted navigation influence pedicle screw selection and accuracy in minimally invasive spine surgery? Neurosurg Focus. 2022;52(1):2021.10.FOCUS21526. 10.3171/2021.10.FOCUS21526 [DOI] [PubMed] [Google Scholar]
- 45. Kia C, Esmende S. Robotic-assisted spine surgery. Tech Orthop. 2020. [Google Scholar]
- 46. Chang M, Wang L, Yuan S, Tian Y, Zhao Y, Liu X. Percutaneous endoscopic robot-assisted transforaminal lumbar interbody fusion (PE RA-TLIF) for lumbar spondylolisthesis: a technical note and two years clinical results. Pain Physician. 2022;25(1):E73–E86. [PubMed] [Google Scholar]
- 47. Park SM, Kim HJ, Lee SY, Chang BS, Lee CK, Yeom JS. Radiographic and clinical outcomes of robot-assisted posterior pedicle screw fixation: two-year results from a randomized controlled trial. Yonsei Med J. 2018;59(3):438–444. 10.3349/ymj.2018.59.3.438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Menger RP, Savardekar AR, Farokhi F, Sin A. A cost-effectiveness analysis of the integration of robotic spine technology in spine surgery. Neurospine. 2018;15(3):216–224. 10.14245/ns.1836082.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ezeokoli EU, Pfennig M, John J, Gupta R, Khalil JG, Park DK. Index surgery cost of fluoroscopic freehand versus robotic-assisted pedicle screw placement in lumbar instrumentation: an age, sex, and approach-matched cohort comparison. J Am Acad Orthop Surg Glob Res Rev. 2022;6(12):12:e22.00137. 10.5435/JAAOSGlobal-D-22-00137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Passias PG, Brown AE, Alas H, et al. A cost benefit analysis of increasing surgical technology in lumbar spine fusion. Spine J. 2021;21(2):193–201. 10.1016/j.spinee.2020.10.012 [DOI] [PubMed] [Google Scholar]
- 51. Hu X, Lieberman IH. What is the learning curve for robotic-assisted pedicle screw placement in spine surgery? Clin Orthop Relat Res. 2014;472(6):1839–1844. 10.1007/s11999-013-3291-1 [DOI] [PMC free article] [PubMed] [Google Scholar]