Summary
Background
Although stereotactic body radiotherapy (SBRT) was progressively adopted in clinical practice in Belgium, a reimbursement request in 2011 was not granted because of remaining clinical and economic uncertainty. A coverage with evidence development (CED) program on SBRT started in 2013, with the aim to assess clinical and technical patterns-of-care in Belgium and monitor survival per indication, in view of supporting inclusion in the reimbursement system.
Methods
The Belgian National Institute for Health and Disability Insurance (NIHDI) initiated this prospective observational registry. Participating departments, using SBRT in clinical practice, signed the ‘NIHDI convention’. Eligible patients had a primary tumour (PT) or oligometastatic disease (OMD). Patient, tumour, and treatment characteristics were collected through an online module of the Belgian Cancer Registry, prerequisite for financing. Five-year overall survival (5YOS) and 30- and 90-days mortality were primary outcomes, derived from vital status information.
Findings
Between 10/2013 and 12/2019, 20 of the 24 accredited radiotherapy departments participated, 6 were academic. Registered cases per department ranged from 21 to 867. Of 5675 registrations analysed, the majority had good performance status and limited number of lesions. Enrolment of PTs remained stable over time, OMDs almost doubled. Peripheral lung lesions dominated in PTs as in OMDs. Other metastases were (para)spinal, ‘non-standard’ and hepatic. Thirty- and 90-days mortalities remained below 0.5% [95% CI 0.3%–0.8%] respectively 2.1% [95% CI 1.6%–2.7%]. 5YOS varied by indication, primary prostate patients performing best (85%, 95% CI [76%, 96%]), those with liver metastases worst (19%, 95% CI [15%, 24%]). Better OS was observed in academic departments, department size did not significantly impact survival. OMD survival was better in 2018–19.
Interpretation
CED can be used to define patterns-of-care and real-life outcome of innovative radiotherapy. As the observed survival for different indications was in line with outcome in emerging literature, SBRT was included in the Belgian reimbursement system as of January 2020.
Funding
NIHDI financed participating departments per registered case.
Keywords: Radiotherapy, Stereotactic body radiotherapy, Innovation, Implementation, Real-world data, Coverage with evidence development
Research in context.
Evidence before this study
Stereotactic body radiotherapy (SBRT) was broadly introduced in the clinic as of 2010, be it with considerable uncertainty regarding its clinical benefit: there were only few large-scale prospective, and no randomized controlled trials. In 2011, a demand to integrate SBRT in the Belgian reimbursement system was not granted, lacking clinical, technical and cost data pertaining to the Belgian context.
Recognising the low regulatory barriers for introducing new medical devices and techniques and awaiting further clinical validation of SBRT, the Belgian Health Care Knowledge Centre (KCE) and the National Institute for Health and Disability Insurance (NIHDI) proposed to introduce promising new radiotherapy techniques such as SBRT using coverage with evidence development (CED), based on robust cost calculations and documenting patterns-of-care and outcome in a prospective registry.
A cost-calculation study performed in 10 radiation oncology departments defined the financing of SBRT in the CED program. Clinical indications were identified in collaboration between the College for Physicians of Radiation Oncology Centres and different governmental stakeholders, amongst which KCE and NIHDI, based on a literature search for peer reviewed papers published up to 31/12/2010 in PubMed, using radiotherapy OR radiation therapy AND stereotactic OR SBRT OR SRT OR Cyberknife as terms. In addition, other national evaluation programs for SBRT (in France by the ‘Haute Autorité de Santé’ and in the UK by NICE) were reviewed, as were ongoing clinical trials and technical information on radiotherapy devices. The report of the NHS National Radiotherapy Implementation Group was found to be the most comprehensive overview on SBRT available and was used to guide the development of the CED program. More detailed information can be found in KCE Report 198.
Added value of this study
The SBRT CED project included all Belgian radiotherapy departments performing SBRT between 10/2013 and 12/2019. It captured real-world data on patient, tumour and treatment characteristics of both primary tumours and metastases, at a national level. Long-term survival data were obtained by linking patient cases with their vital status over time. With close to 6000 cases, this is–to our knowledge–the largest prospective data collection on SBRT at a national level.
Implications of all the available evidence
More prospective clinical evidence on SBRT is slowly emerging, with randomized phase II-III studies showing benefit. Also, a Commissioning through Evaluation scheme, using a similar prospective registry-based approach, supported the reimbursement of SBRT for metachronous oligometastatic disease in the UK. Our project adds to the current knowledge of the real-world use and outcome of SBRT.
In terms of methodology, a European project evaluated the available experience of CED programs for medical technologies and provided recommendations on how to use CED to aid decision-making and reimbursement of new medical devices. Our project demonstrated that CED can indeed be deployed at a national level to evaluate innovative (radiotherapy) devices and techniques, and that it can be used to support reimbursement and policymaking. Besides overall survival, future initiatives should focus on how to collect more granular outcome data and resource use and costs, at population level.
Introduction
Radiotherapy is one of the main pillars of cancer care, along with surgery and systemic treatments.1 Whereas reimbursement supporting the introduction of new cancer drugs in clinical practice typically ensues from evidence obtained in prospective clinical trials, this is less straightforward in non-systemic cancer treatments. Specifically in the field of radiation oncology, the breadth of innovations is considerable, ranging from new positioning devices over novel treatment indications, innovative techniques, and combinations with novel oncology drugs, up to the adoption of advanced treatment machines. Technologies and techniques often evolve over time and come with a strong operator-dependency, leading to learning curves and introducing uncertainty about the optimal timing to appraise the innovation. In addition to the associated costs for personnel training and quality assurance, the implementation of new radiotherapy interventions often requires sizable upfront investments in equipment and software, while the improved outcome, in terms of decreased side effects and better local control, survival or quality of life, may only become apparent years after their introduction.2
These characteristics render economic evaluation and health technology assessment challenging, and may delay access of patients to potentially beneficial interventions due to the absence of cost and effectiveness data, typically required by policymakers to grant reimbursement. This calls for a different and more blended approach to evidence generation, going beyond the mere reliance on randomised controlled trials (RCTs), also including assessments in real-life clinical practice. Which however implies the availability of and access to the intervention.3 A potential solution to this catch 22 is so-called ‘coverage with evidence development’ (CED), a form of performance-based risk-sharing arrangement. In this approach, reimbursement of a new intervention is temporary and conditional on further data being collected to reduce uncertainties about its clinical or cost-effectiveness.4,5 CED for medical devices has been evaluated in the EU funded COMED project, demonstrating that its use across Europe remains limited and highly variable.6, 7, 8
Stereotactic radiotherapy is an advanced external beam radiotherapy technique, using focused radiation beams to accurately target a well-defined tumour location and delivering large doses in only a few fractions. Originally developed for cerebral lesions in analogy with stereotactic brain surgery, it has rapidly been adopted to treat extra-cranial indications, such as liver metastases and early-stage, peripherally located non-small-cell lung cancer (ES-NSCLC). Interestingly, in the latter, real-world data provided evidence of clinical benefit for patients, unfit for or refusing surgery, well before randomised controlled trials were completed.9,10 More recently, stereotactic body radiotherapy (SBRT) has gained momentum in the radical treatment of oligometastatic disease (OMD).11 Here too, the clinical benefit remains difficult to prove in a randomised setting, due to the large diversity in presentation: different primary tumours with distinct prognosis, different numbers, sizes and locations of the metastatic lesions, different types and timing of the OMD, resulting in different combinations with systemic therapy.12,13
In 2011, the Belgian radiation oncology community submitted a request to the National Institute for Health and Disability Insurance (NIHDI) to include SBRT into the radiotherapy reimbursement system. This demand was not granted because of the remaining uncertainty regarding the clinical benefit, in the absence of randomised evidence. Moreover, there were no data about the effectiveness and the cost of SBRT in Belgium, hence there was no view on the possible cost-effectiveness or budgetary impact of introducing this innovative radiation therapy in the Belgian healthcare system. What followed was a multistakeholder dialogue, in which the Belgian Health Care Knowledge Centre (KCE) suggested to set up a CED program, as a close collaboration between the NIHDI, the Belgian Cancer Registry (BCR) and the radiation oncology professionals, under the auspices of the College for Physicians of Radiation Oncology Centres (“the College”). The aim was to gain insight in the practice patterns of SBRT in Belgium, and to evaluate the survival of the patients selected for this treatment. Prior to its initiation, the level of the provisional financing was defined through a time-driven activity-based costing (TD-ABC) project, computing the real-life cost of radiotherapy, amongst which SBRT, in Belgium.14,15 Subsequently, the inclusion criteria and the data capture, monitoring, and evaluation procedures of this CED project, starting in 2013 and aimed to run over a period of 4 years, were defined.
We report the practical development of the project with the patient, tumour, and treatment characteristics of the SBRT cases included throughout the entire period of the CED program, which was extended twice, ending December 31st of 2019. We also show the overall survival observed, by clinical indication and determinants of the participating radiation oncology departments. We finally examine this CED project in the light of the actual knowledge on CED for medical devices.
Methods
Study design and data sources
The indications for SBRT to be included in the prospective registration were defined in accordance with a clinical evidence review, performed by the NHS National Radiotherapy Implementation Group.16 SBRT was allowed for primary tumours (PTs) as well as for metastatic lesions. For the latter, the patient had to comply with a then accepted definition of OMD, that is, having a maximum of 3 active metastatic lesions, including brain metastases. No recommendations were made regarding the timing in the disease history or the status of the primary lesion. WHO/ECOG performance status ≤2 was requested for some indications, while no restrictions were made for others, in line with the NHS report.
Indications for which at the time ample literature evidence existed were referred to as ‘standard indications’ (i.e., peripherally located ES-NSCLC and liver, lung and paraspinal metastases), while all others, where clinical evidence was still deemed insufficient, were only accepted in the project under the condition that the patient was also enrolled in a clinical trial and referred to as ‘non-standard indications’. Detailed description of the in- and exclusion criteria, per indication and based on the NHS report, can be found in the table in the web Appendix, pg. 1. Cases not complying the project conditions were excluded from the analysis.
All patient, tumour, and treatment characteristics to be collected to qualify for provisional reimbursement were defined in agreement between the NIHDI, BCR and radiation oncology experts. Data capture was performed in an online registration module, governed by the BCR (web Appendix, pg. 4). After data reception, the BCR performed quality control and enrolling departments were contacted in case of missing or erroneous information.
For participation in the CED program, individual radiotherapy departments had to sign a contract (so-called ‘convention’) with the NIHDI. Only SBRT was included in the project, as intracerebral stereotactic irradiation (also of brain metastases) was already covered in the reimbursement system. Each radiotherapy plan had to be registered separately. Per patient and per year (defined as 12 running months, starting from the date of the first treatment fraction), a maximum of 3 radiotherapy treatment plans were accepted for registration and provisional reimbursement.
Outcomes
The study aimed to evaluate SBRT patterns-of-care in Belgium over time, with focus on patient, tumour, and treatment characteristics. Survival, defined by 5-year overall survival (5YOS) and 30- and 90-day mortality, was the primary outcome. Exploratory analysis of the impact of department type and size was undertaken.
Planned interim analyses were performed by the BCR and communicated to the NIHDI and the College in January 2016, 2017, and 2018. All included patient, tumour, and treatment characteristics, while 30- and 90-day mortality was first reported in 2018. The final report, including 5YOS per indication, was issued in October 2018 to support the renewed demand for including SBRT in the reimbursement system. Until this became effective, data collection was extended until December 31st, 2019.
Statistical analysis
For the entire data set presented, statistical analysis was performed with the statistical software package R version 4.2.2. A p-value <0.05 was considered statistically significant. Different statistical tests were used depending on the type of variables: for continuous variables: Mann–Whitney U; for categorical variables: Pearson's Chi-Squared; for counting variables: Poisson test. Sub-analyses were performed based on department type (academic vs non-academic) and size (0–50 registrations, 51–200 registrations, 201–500 registrations and more than 500 registrations) and evolution over time (2013–2015, 2016–2017, 2018–2019).
Kaplan–Meier curves were created to visualize survival probabilities from start of SBRT unto the date of analysis, April 1st, 2023. Vital status information was obtained from the Belgian Crossroads Bank for Social Security and linked using the National Social Security Identification Number. Patients that were still alive at analysis were censored at this date. Patients lost to follow-up were censored at the date of last information on vital status. Differences between the survival curves of the different indications were evaluated with the log-rank test taking pairwise comparisons into account with Bonferroni correction (using the pairwise_survdiff function in the R package survminer). Pairwise differences in survival between the levels of department type, department size, and incidence period corrected for indication were based on Cox regression models, using Tukey correction for multiple testing (averaged over indication using the R package emmeans).
Ethical considerations
This study was performed within the legal framework of the BCR. Based on the Coordinated Law of May 10th, 2015 (art. 138), BCR has a legal task to collect data on cancer and subject them to quality control before processing, analysing, and reporting them. In this regard, informed consent and additional approval by the ethics committee was not needed.
Role of the funding source
The NIHDI financed participating departments per treatment plan (3948€, 2013 value), based on the real-life costs calculated in the TD-ABC program,14,15 and charged per activities performed, conform the present radiotherapy reimbursement system. In the NIHDI convention, an additional fee (100€ res. 1000€, depending upon the indication) was foreseen per registration, to support data collection. The NIHDI participated in the design of the project, was informed about its progress, but did not intervene in the data collection, analysis, or interpretation. The NIHDI agreed with the final report and publication.
Results
The total project ran between 1/9/2013 until 31/12/2019. Analyses were performed as planned, but the data collection and provisional reimbursement was extended until formal inclusion of SBRT in the Belgian radiotherapy reimbursement, which became effective on January 1st, 2020.
In total, 20 (83%) of the 24 accredited radiotherapy departments participated in the convention, representing all departments offering SBRT in that period. Depending on the actual year, between 3 and 18 departments registered cases. Six (30%) departments were academic, the others were non-academic. Six (30%) departments included a total of 0–50 cases, 5 (25%) 51–200, 5 (25%) 201–500 and 4 (20%) departments had more than 500 registrations over the entire period.
A total of 6296 SBRT cases were registered, of which 621 did not correspond with the project conditions and were therefore excluded from the analysis (figure in web Appendix, pg. 12). The remaining 5675 registrations were retained for analysis, almost equally divided between PTs (N = 2885: 50.8%) and metastases (N = 2790; 49.2%). The vast majority of the PTs were peripherally-located ES-NSCLC (N = 2801; 97.1%), followed by prostate cancer (N = 49; 1.7%) and (para)spinal tumours (N = 16; 0.6%), while the other primaries (centrally located ES-NSCLC, kidney, pancreatic, head and neck or hepatic cancer) only accounted for 2–6 cases, representing 0.1–0.2% of all PTs. For the metastases, again lung lesions dominated (N = 1484, 53.2%), followed by (para)spinal metastases (N = 537; 19.2%), non-standard OMD (N = 442; 15.8) and hepatic metastases (N = 327, 11.7%).
Table 1 provides an overview of the main patient and tumour characteristics, for PTs vs metastases, divided over three consecutive time periods (2013–15; 2016–17 and 2018–19). While the number of included PT cases remained rather stable over time, there was a doubling in uptake of SBRT for OMD between the first and the last time period. About two thirds of the cases were in men; patients in the metastatic group were younger than those with PTs, yet with increasing age over time. Over 80% of the cases had an excellent patient performance status, with more missing values in OMD. Although as expected the average number of lesions treated per patient was higher in metastases than in PTs, it remained limited to about 1.25, with no increase over time.
Table 1.
Patient, tumour and treatment characteristics, primary tumours vs metastatic lesions, each for different time periods.
| Primary tumours |
Metastatic lesions |
|||||||
|---|---|---|---|---|---|---|---|---|
| Total | 2013–2015 | 2016–2017 | 2018–2019 | Total | 2013–2015 | 2016–2017 | 2018–2019 | |
| Registrations | 2885 | 921 | 838 | 1126 | 2790 | 660 | 806 | 1324 |
| Patient characteristics | ||||||||
| Age (years): median (IQR) | 72 (66–79) | 73 (66–79) | 72 (66–79) | 72 (66–79) | 68 (61–75)a | 66 (59–74) | 68 (62–75)b | 69 (62–75)b |
| Sex: male/female (%) | 66/34 | 69/31 | 67/33 | 63/37 | 63/37a | 60/40 | 63/37 | 63/37 |
| WHO performance: 0/1/unknown (%) | 45/45/1 | 45/46/0 | 48/40/1 | 44/47/1 | 43/38/16a | 48/37/12 | 45/35/16 | 39/41/17 |
| Tumour characteristics | ||||||||
| Number of lesions treated per patient: average (ranged) | 1.08 (1–3) | 1.04 (1–2) | 1.06 (1–2) | 1.07 (1–3) | 1.26 (1–5)a | 1.20 (1–3) | 1.20 (1–3) | 1.23 (1–4) |
| Lesion size (mm): median (IQR) | 19 (13–26) | 20 (15–28) | 18 (13–25)b | 18 (13–25.5)b | 19 (12–29) | 17 (11–25) | 20 (13–30)b | 19 (12–30)b |
| Treatment characteristics | ||||||||
| Total dose (Gy): median (IQR) | 54 (48–60) | 55 (48–60) | 55 (48–60) | 54 (48–60) | 48 (30–54)a | 50 (40–55) | 48 (30–50)b | 35 (30–50)b,c |
| Number of fractions: median (IQR) | 4 (4–5) | 4 (4–5) | 4 (3–5) | 4 (3–5) | 4 (3–5)a | 5 (3–8) | 4 (3–5)b | 3 (3–5)b,c |
| Treatment duration (days): median (IQR) | 10 (7–13) | 10 (8–13) | 10 (8–13) | 9 (7–12)b,c | 8 (6–12)a | 10 (7–15) | 9 (6–12.75)b | 7 (5–10)b,c |
| Treatment preparation | ||||||||
| Imaging modalities: CT-scan/PET-CT/MRI (%)e | 62/34/1 | 58/39/1 | 61/34/2 | 66/31/1 | 56/25/16a | 60/27/11 | 56/24/17 | 54/25/17 |
| Personalized immobilization (% yes) | 79 | 84 | 85 | 69b,c | 63a | 72 | 73 | 52b,c |
| Identification of tumor motion (% yes) | 98 | 97 | 97 | 99b,c | 69a | 84 | 74b | 57b,c |
| Image fusion for target delineation (% yes) | 68 | 64 | 66 | 73b,c | 74a | 70 | 76b | 76b |
| Treatment delivery | ||||||||
| Technique: 3D-CRT/IMRT/IMRT rot (%)e | 17/8/54 | 27/12/40 | 16/9/51 | 9/5/68 | 14/4/66a | 28/7/42 | 19/3/58 | 4/3/84 |
| IGRT: CBCT/stereoscopic X-rays/combination (%)e | 70/1/5 | 67/2/6 | 65/1/8 | 76/0/1b,c | 73/1/6 | 58/2/8 | 62/0/11b | 87/0/2b,c |
| Markers (% yes) | 8 | 9 | 9 | 7 | 12a | 23 | 14b | 6b,c |
| Tumor motion compensation (% yes) | 60 | 54 | 63b | 62b | 48a | 62 | 55b | 37b,c |
Patients were allocated to the period based on the start date of the radiotherapy course.
Abbreviations: IQR: interquartile range; WHO: World Health Organisation; Gy: Gray; CT: computed tomography; PET: positron emission tomography; MRI: magnetic resonance imaging; 3D-CRT: 3-dimensional conformal radiotherapy; IMRT: intensity modulated radiotherapy; IMRT rot: rotational IMRT; IGRT: image-guided radiotherapy; CBCT: cone beam computed tomography.
Significantly different from total primary tumor.
Significantly different from 2013 to 2015.
Significantly different from 2016 to 2017.
Range of number of registrations per patient.
3 most common categories.
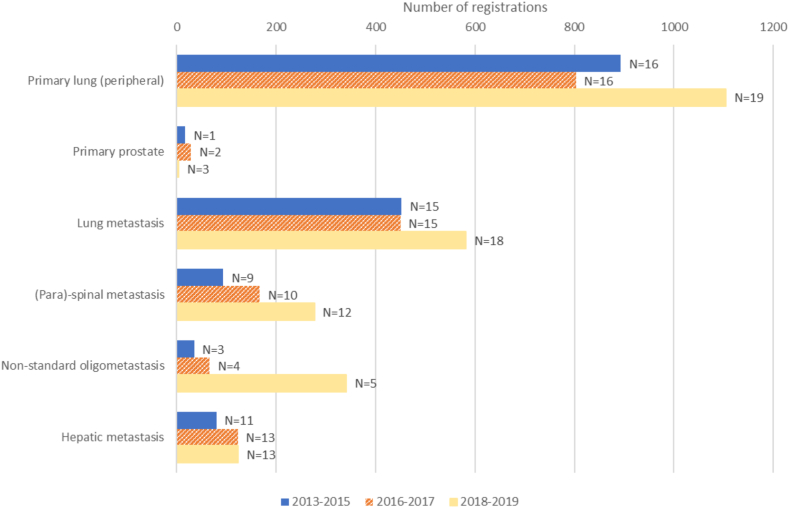
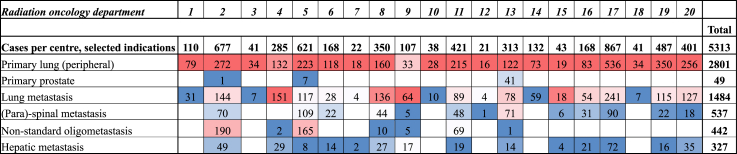
Fig. 1 shows the evolution of the 6 most frequent indications per time period, in terms of number of cases registered and number of departments participating. Of interest is that the number of lung lesions treated, PTs as well as metastases, increases in parallel to the number of departments treating these cases. In contrast, the number of other indications, especially paraspinal and non-standard OMDs, also increase considerably over time, but remain clustered in a few departments. This is also observed in Fig. 2: SBRT for prostate PT and non-standard OMD is only performed in a minority of departments, whereas all treat lung lesions. The departments only treating lung lesions are among those with the lowest number of SBRT cases registered.
Fig. 1.
Evolution of the numbers of registrations and centres (N) registering cases, for the most frequent indications, by predefined time period.
Fig. 2.
Registered cases per radiation oncology department, overall and per type of indication. Note: The figure shows the numbers of SBRT cases, overall and for selected indications, in all participating radiation oncology departments in Belgium, for the entire registration period 2013–2019. The departments are numbered by their start of participation in the CED project. The colour scale gives an indication about the % of a specific indication for each radiation oncology department. Red indicates a high %, blue indicates a low%.
Table 1 provides an overview of treatment characteristics, in terms of dose-fractionation and major technical aspects scored, differentiating between PTs and metastases, in the various time periods. On average, metastases were treated at lower total doses and number of fractions than PTs, further decreasing over time. In treatment preparation, personalised immobilisation and identification of tumour motion are more frequently applied in PTs than in OMDs, whereas the inverse holds for the use of MRI and image fusion. Rotational IMRT is the dominant treatment delivery technique, especially in metastases and in the most recent time period. Conebeam CT-scan is the most frequently used image-guidance modality, markers are hardly utilised. In line with the observations on motion management in treatment preparation, tumour motion compensation is less often used for metastases than for PTs.
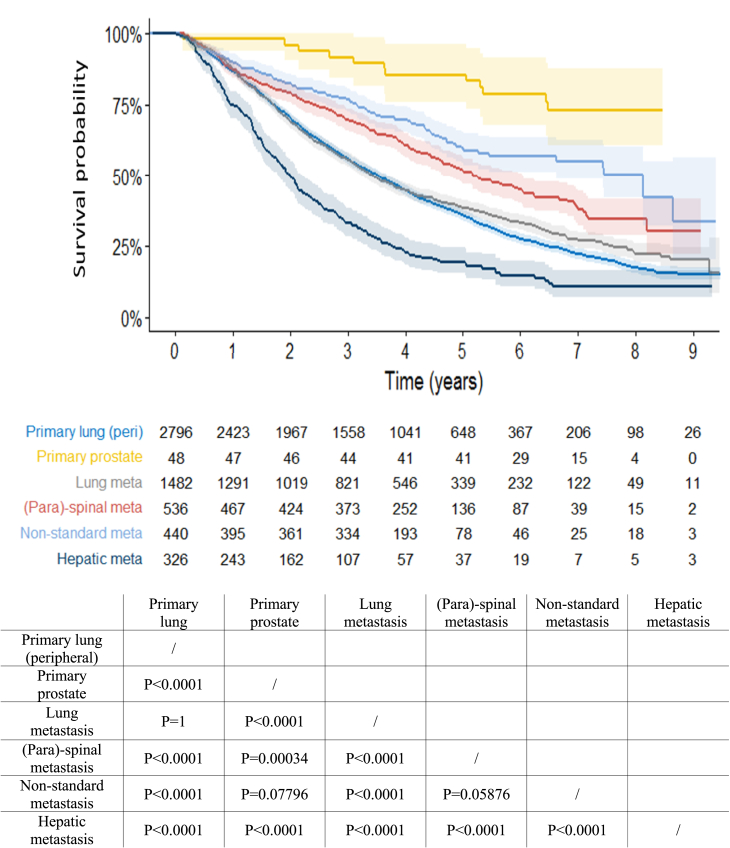
Thirty- and 90-days mortality were 0.5% [95% CI 0.3%–0.8%] res. 2.1% [95% CI 1.6%–2.7%] for all peripheral ES-NSCLC cases and 0.5% [95% CI 0.3%–0.9%] res. 1.9% [95% CI 1.4%–2.4%] for all metastatic patients. Fig. 3 and supplementary table (in web Appendix, pg. 13) show the OS of the 6 most frequent clinical indications. Thirty-nine patients were excluded from this analysis because no information about their vital status was available at date of final analysis. All indications show highly statistically significant differences in OS, except for peripheral ES-NSCLC and lung metastases, of which survival curves almost completely overlap up to 5 years (5YOS: 36% (95% CI [34%, 38%]) and 39% (95% CI [36%, 41%]); p = 1), and for non-standard metastases (5YOS: 60% (95% CI [54%, 65%]), where only a trend is observed compared to (para)spinal metastases (5YOS: 52% (95% CI [47%, 56%]); p = 0.059) and to primary prostate (5YOS: 85% (95% CI [76%, 96%]); p = 0.078). Primary prostate patients treated with SBRT overall perform best, those with liver metastases worst (5YOS: 19% (95% CI [15%, 24%])).
Fig. 3.
Overall survival of the 6 most frequent clinical indications. Note: the p-values are for pairwise comparison with Bonferroni correction.
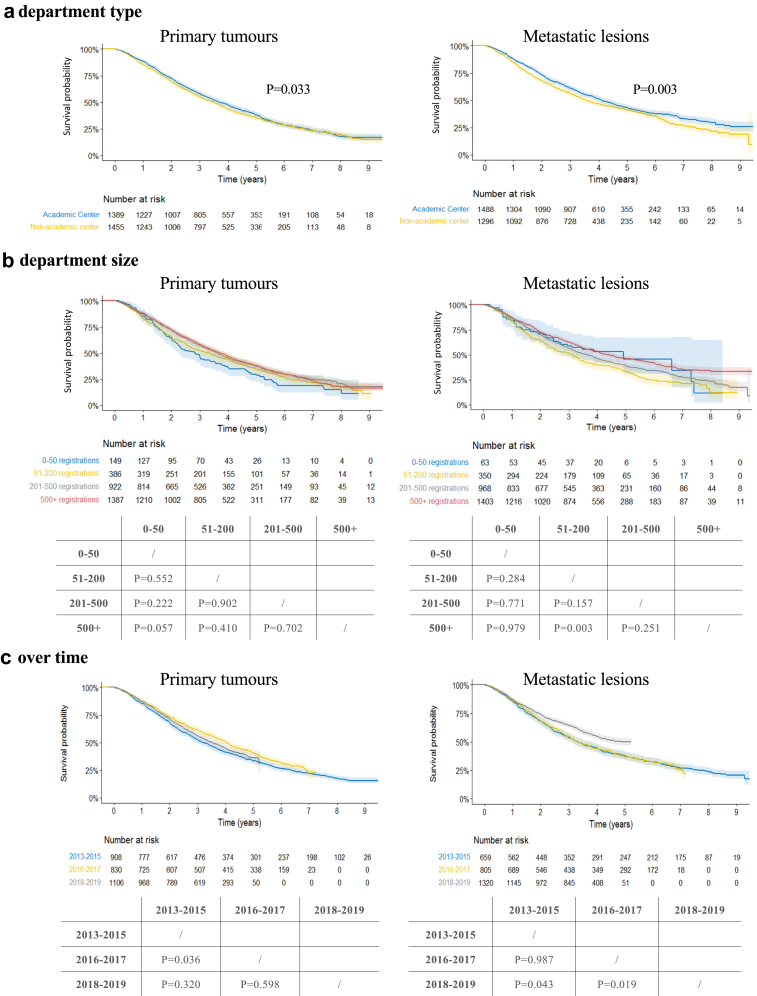
Fig. 4a–c present the OS curves of patients with PTs and metastases, analysed per type of radiation oncology department, number of cases registered throughout the project and time period (all corrected for type of indication). For PTs, there is a significant difference in OS between academic and non-academic departments (5YOS: 38% (95% CI [35%, 41%]) res. 35% (95% CI [33%, 38%]); p = 0.033). In the second time period, patients’ survival was significantly better than in the first period (5YOS: 41% (95% CI [38%, 44%]) res. 33% (95% CI [30%, 37%]); p = 0.036). For metastatic lesions, OS is also statistically significantly better in academic compared to non-academic departments (5YOS: 43% (95% CI [41%, 46%]) res. 41% (95% CI [38%, 44%]); p = 0.003). There is a trend towards better OS with increasing numbers of metastatic cases treated (5YOS of 33% (95% CI [28%, 39%]), 39% (95% CI [36%, 43%]) res. 46% (95% CI [43%, 49%]) for departments treating 51–200, 201–500 and >500 cases res.), yet only reaching statistical significance between departments treating 51–200 cases and >500 cases. Finally, a statistically significant improvement in OS is observed for OMD patients treated in 2018–19, compared to the earlier time periods (5YOS: 50% (95% CI [46%, 53%]) res. 37% (95% CI [34%, 40%]); p = 0.019 and 38% (95% CI [34%, 42%]); p = 0.043).
Fig. 4.
a–c: Overall survival for primary tumours and metastatic lesions, per department type (a), department size (b) and over time (c). Note: the p-values are for pairwise comparison, corrected for indication, based on Cox regression models.
Discussion
In 2013, the Belgian NIHDI launched a provisional reimbursement project to offer patients early access to SBRT, while prospectively gaining insight into clinical and technical practice patterns in Belgium and collecting survival data of patients selected for treatment with this innovative radiotherapy technique.
The project demonstrated a rapid uptake of SBRT across all academic and most non-academic Belgian radiation oncology departments. Especially SBRT for metastases increased considerably over time, in line with the rapidly accumulating literature evidence.17 Lung lesions—primary as well as metastases—were the most frequently treated sites, followed by paraspinal metastases. All were considered standard indications. In contrast, non-standard indications, that had to be included in a clinical trial, remained clustered in a few centres, be it with considerable variation in uptake: SBRT for primary prostate cancer remained stable, while the number of non-standard OMDs (very often lymph nodes and non-paraspinal bone metastases), increased significantly over time. The latter can be the consequence of centres participating in OMD trials initiated in Belgium, such as the STORM and CHEERS trials.18,19 As typically observed in other real-life SBRT cohorts, the performance status was good and the average number of lesions treated per patient low, even in case of OMD, indicating a careful patient selection.20, 21, 22, 23, 24 Dose-fractionation and technical characteristics were variable, in line with the variability in indications and treated sites, and reflecting evolving practice. For example, that the total dose delivered in OMDs decreased over time may be reflecting the higher uptake of SBRT in non-standard OMDs, often in prostate cancer patients, while the uptake of rotational IMRT increased as its technical implementation in departments evolved.
Most importantly, 30- and 90-days mortalities were reassuring, and the OS compared favourably with the then available evidence in the literature. As a matter of fact, for peripheral ES-NSCLC, a 2YOS of 70% was observed, compared to slightly above sixty percent in the retrospective analysis on 582 stage 1 NSCLC patients treated in 13 centres from the German Society for Radiation Oncology20 and 68% in the randomised phase II trial from the Scandinavian SBRT study group, where 49 patients were treated with SBRT in 9 centres.25 The only randomised phase III trial evaluating this indication, published in 2019, reported a 2YOS of 77% in 66 patients treated with SBRT, in 14 departments across Australia and New-Zealand.10 The 2YOS for all OMD cases in our cohort was 72% (95% CI [69%, 72%]), compared to 79% in the prospective UK registry on 1422 patients with predominantly metachronous extracranial OMD, treated in 17 institutions.23 Favourable survival data were the major argument supporting the negotiations towards formal inclusion of SBRT in the reimbursement system. In addition, there was a careful patient selection, with about half of the cases being ES-NSCLC, a broadly accepted evidence-based indication, and the other half OMD patients, with less strong evidence-base but with good overall performance status and limited number of active lesions. Lastly, some general trends in technical aspects, such as the use of motion management and IGRT, were in line with what could be expected based on the actual standards. Both patient and technical aspects were reassuring for the policymakers.
This is the largest CED program performed for radiotherapy so far, collecting both clinical and technical data, with prolonged follow-up data, demonstrating that such an approach is feasible at a national level. One of the strengths and dominant success factor of this project was the close collaboration between the NIHDI, the participating radiation oncology departments and BCR. The latter provided the technical application for data capture and storage, assured rigorous data cleaning and quality, performed the analyses, and provided linkage of the registered cases with the Belgian Crossroads Bank for Social Security, to obtain long-term survival information of the included patients. This role of the BCR fits perfectly in the ambition of European cancer registries to develop their portfolio beyond the mere collection of cancer incidence data, and become a central player in the regional or national data collection of population-based data, making these actionable to support health policy and decision-making.26,27
The broad participation of radiation oncology departments across Belgium was another asset in obtaining a nationwide oversight of SBRT practice. In contrast to other real-life cohorts where academic centres often dominate,20,23 the Belgian CED collected data from all departments providing SBRT in the timeframe of the program. Under the auspices of the College, the radiation oncology community was regularly updated on the evolution of the program, and the data collected.
The resulting very large nation-wide dataset allowed additional analyses to be performed, evaluating changes over time, not only in terms of practice, but also in terms of outcome. Survival indeed seemed to improve with time in the metastatic subset of patients. Moreover, the possible impact of department type and size (in terms of SBRT cases registered) on patients' survival could be assessed. Based on the analyses, accounting for the population mixes treated in the departments, academic departments seem to obtain better OS, especially so for OMD patients. Conversely, for department size, there is only a trend towards worse OS of PT's in the smallest centres, and for improved OS in OMDs as the number of cases treated increases. PTs are dominated by the large population of peripheral ES-NSCLC, which is a quite standardised indication, often the first to be implemented when introducing SBRT, hence with learning curves that have already been overcome. This concurs with the excellent results observed in the dosimetry audit program for lung SBRT in Belgium.28 The inverse holds for SBRT for metastatic lesions, a more recent and heterogeneous indication, where the population mixes treated vary considerably by department (Fig. 2). Still, even considering this, academic centres seem to perform better. Further analyses will be necessary to evaluate if time- and department-based variations still hold true for metastases of different primaries or in different organ sites, and whether patient characteristics or treatment-related factors are confounding these results. But the data are at least generating the hypothesis that more expertise may translate into better outcome. Of course, outcome in oncology patients is multifactorial, hence expertise may not only relate to the SBRT delivered, but also pertain to patient selection, for instance, or the other treatments all or not delivered in conjunction with SBRT.
The use of CED in radiotherapy, as in other technical disciplines, remains scarce. The other large CED program in radiotherapy, aiming to obtain reimbursement for SBRT in oligoprogressive extracranial OMD, was performed in England, the UK. The 1422 cases were included in 17 predominantly academic radiation oncology departments. While no technical aspects were reported, clinical data capture was performed over time and more detailed, including besides OS—obtained through linkage with mortality data from the UK Office for National Statistics—also data on local control, toxicity and QoL, more alike the methodology of a clinical trial.23 In general, CED programs are categorised under performance-based risk-sharing arrangements, in which the performance of a product, device or intervention is tracked in a defined patient population over a specified period of time, to gain insight into their real-world clinical and economic performance.4 The EU Horizon 2020 COMED project aimed to optimise the knowledge of and research on the evaluation and diffusion of medical devices.6 In this context, it analysed the challenges and current status of CEDs for medical devices in Europe, based on systematic literature review and interviews with stakeholders. The work culminated into a set of proposals for a good conduct of CEDs in medical devices, optimally aligning the actual practice in Europe with the economic theory behind CED schemes.7,8,29 Across seven evaluated European countries, it was found that only one of the recommendations (i.e., that the outcomes measured should be final and relevant, attributable to the device) was mainly followed. The other recommendations were only partially followed, or not at all. While SBRT is a technique that can be delivered with different types of radiotherapy devices, the structure presented by Drummond et al. provides a useful framework to evaluate this Belgian CED program (Table 2). Although the desirability to launch this project was not based on any formal assessment—except for a real-world cost calculation defining the financing level—its design and monitoring process was thoroughly discussed amongst stakeholders, resulting in clearly defined responsibilities, and planned interim assessments and reporting. While it started well before the publication of the COMED recommendations, the Belgian CED project implicitly adhered to many of the described principles and led to the inclusion of SBRT in the Belgian radiotherapy reimbursement system.
Table 2.
Evaluation of the Belgian coverage with evidence development program for stereotactic body radiotherapy in Belgium, based on recommendations of the European COMED project.
| Recommendations (Drummond8) | Current practice in Europe (Drummond) | CED SBRT in Belgium (this project) | Comments CED SBRT in Belgium (this project) |
|---|---|---|---|
| Assessing the desirability of CED schemes | |||
| Determine the need for a scheme based on an HTA including an economic evaluation. | Partially followed | Not followed | HTA was not performed. |
| Use VOI and ROA approaches to inform on the desirability, prioritization, and design of schemes. | Not followed | Not followed | VOI and ROA were not performed. |
| Compare the costs and consequences of CED schemes with other, alternative policy options. | Not followed | Not followed | The CED program was the only proposal made, no comparison with possible other options. |
| Only use CED when uncertainty can be reduced through further data collection. | Not followed | Mainly followed | Data on outcome, cost, cost-effectiveness and budget impact were lacking in Belgium; the aim of the CED was to generate part of this evidence. |
| Design of a scheme | |||
| The type of CED (e.g., OIR and OWR), and the study design (e.g., experimental vs observational) should be informed by explicit assessments on appropriateness, costs, and consequences of each option. | Not followed | Not followed | No explicit assessment took place. |
| The outcomes measured in a CED should be final, relevant outcomes attributable to the device. | Mainly followed | Mainly followed | OS and early mortality were deemed the most important outcome measures to be evaluated for the use of SBRT. |
| The length of the scheme should be primarily driven by the evidence requirements. | Partially followed | Mainly followed | A time frame of 5 years was set forward, to allow monitoring the uptake over time and generate OS data. Start was slower than expected, and the program was extended for 2 years to bridge the gap towards implementation of reimbursement. |
| Monitoring mechanisms, as well as stopping rules, should exist to ensure that schemes are proceeding as planned. | Partially followed | Partially followed | There was a strict monitoring scheme by the NIHDI and the BCR, but no stopping rules. |
| The criteria to inform policy actions at the outset of the scheme should be pre-specified at the beginning of the scheme. | Not followed | Partially followed | The criteria were that the observed outcome and practice should conform to the available evidence, but without formal targets defined. |
| Implementing CED schemes | |||
| Clearly identify the key responsibilities of various parties in providing funding, developing the study protocol, collecting and analysing data. Make the details of the scheme (e.g., uncertainties to be resolved, study design) publicly available. | Partially followed | Mainly followed | There was a clear definition of the responsibilities of the different stakeholders, with an upfront defined information scheme. |
| Anticipate possible adjustments of CED schemes, to deal with similar products entering the market, or product modifications | Not followed | Not followed | This was not anticipated, but in the context of the intervention concerned improbable. |
| Evaluating schemes | |||
| Assess whether the scheme achieved its aims. | Not followed/Not determined | Mainly followed | Interim and final evaluations were made. |
| Make appropriate decisions on reimbursement, coverage or price of the device based on the results of the scheme | Partially followed/Not determined | Mainly followed | Reimbursement for SBRT was included into the reimbursement system as of January 2020. |
Abbreviations: CED: coverage with evidence development; SBRT: stereotactic body radiotherapy; HTA: health technology assessment; VOI: value of information; ROA: real-options analysis; OIR: only in research; OWR: only with research; OS: overall survival; NIHDI: National Institute of Health and Disability Insurance; BCR: Belgian Cancer Registry.
Of course, prospective registry-based studies as the one presented should not be seen as an alternative, but rather as an addition to randomised controlled trials, in which comparative evidence is generated in a setting of clinical equipoise. Hence, emerging evidence from clinical trials is expected to further tailor the conclusions drawn upon this CED program.
The CED project by itself also had some limitations. Although inclusion criteria were defined, they were deliberately kept pragmatic, based on the NHS review available at the time of project development. Patient- and disease-related data elements were as much as possible aligned to ongoing data collection of the cancer registry as to allow all radiotherapy departments, also those without clinical trial expertise, to easily submit data with the available staff. An attempt was made to distinguish SBRT used for synchronous vs oligoprogressive OMD. However, the definition of OMD was still ill-defined at the time of initiation of the CED, rendering the attempt obsolete. Finally, only data on lesions treated within the convention were captured: 65 res. 17 patients had 1 res. 2 additional active lesions not treated with SBRT within the convention, which may have been treated radically with surgery, ablation, radiosurgery or more protracted radiotherapy schedules.
Postulating that survival is the most final clinical outcome for patient and society, overruling surrogate endpoints such as local control, only survival data were considered by linking the patients’ National Social Security Identification Number to their vital status in the Crossroads Bank for Social Security.6 This again was a pragmatic approach allowing data collection to be restricted to the moment of registration. This however also translated into the lack of information on subsequent treatments, toxicity or QoL. In addition, OS was not corrected for the patient characteristics. It is thus not possible to ensure that observed differences in outcome may not in part be caused by differences in patient variables such as WHO or age.
When designing the project, it was anticipated that treatment characteristics and technical factors may be very important for clinical outcome. These elements were thus captured at a rather granular level. Yet, the analyses showed large variations in practice and equipment, often building upon the historical investments made by the departments, but evolving over time, as new equipment was purchased, and indications evolved. It was therefore difficult to draw firm conclusions, beyond the observation of general trends, like the almost universal use of a form of motion identification strategy for lung lesions. The quality of the SBRT, and of the different steps in the treatment process, were not monitored within the frame of the CED project, as comprehensive clinical and dosimetric audits were performed through the College on a regular basis.28,30
Conclusion
This Belgian SBRT project demonstrated that CED for radiotherapy interventions is feasible at a national level. The overall data collected are reassuring, highlighting a careful patient selection for SBRT in the real-world setting, OS comparable to published evidence and variable technical aspects, reflecting historical investment for and implementation of SBRT. More granular analyses of specific indications are ongoing, while data from other prospective, international cohorts and randomised clinical trials, are accumulating—and needed—to further refine our knowledge on which patients favour most from SBRT.18,31, 32, 33, 34
The well-defined structure and collaboration amongst different stakeholders were key elements for success. This Belgian SBRT project also showed that linking case-specific data, collected by healthcare professionals at registration, with population-based information on vital status, can be used to inform policy. Not only did the long-term OS information support the decision to introduce SBRT in the reimbursement system, but it also shed light on department-specific characteristics possibly impacting patient outcome.
Although a cost calculation was part of the preparatory phase of this project, and an exploratory budget impact analysis was performed,14,15,35 future CED programs should not only focus on outcome -, but also invest in the prospective collection of resource use and cost data to fully appreciate the value of the new intervention compared to the available standard.
Meanwhile, this Belgian project illustrated that the theoretical merits of a CED program can be translated into practice, thus paving the way for a broader use of this methodology to support access to and reimbursement of emerging radiotherapy innovations, that cannot easily be addressed in randomised clinical trials.3,8 The same holds for other medical technologies and devices, especially when the dissemination process is prone to learning effects, both confounding clinical outcomes and impacting costs.
Contributors
YL, HE, NJ, LM, VR, KS, DV, CW and NVD contributed to the study concept and design. SJ, NVD, ML and YL analysed, critically reviewed and interpreted the data; YL drafted the manuscript. All authors (YL, SJ, ML, HE, XG, NJ, LM, VR, SR, KS, DV, CW, RW, NVD) had insight into the data and critically reviewed and approved the manuscript. Corresponding author YL had the final responsibility for the decision to submit for publication.
Data sharing statement
The study data are not publicly available because of data confidentiality and ethical restrictions. However, data can be made available from the corresponding author upon reasonable request. More concretely, pseudonymized data can be provided within the secured environment of the Belgian Cancer Registry after been guaranteed that all applicable GDPR regulations are considered.
Declaration of interests
All authors have completed the ICMJE disclosure form and declare: YL is recipient of the HERO-VBHC chair, with payments made to her institution and has unpaid leadership roles in ESTRO (Scientific Committee member and ESTRO-HERO co-chair), the Belgian College of Oncology (Board member) and in the EORTC-ESTRO E2-RADIATE project (PI); DV has research collaboration with RaySearch Laboratories and received speakers fees from BeSTRO and JASTRO and per diem payment as teacher in the ESTRO SBRT course, and reports advisory roles for the Belgian Supreme Health Council and the Medical Jury of the Belgian Federal Agency for Nuclear Control, treasurer of ESTRO and board member of ‘Stand Up Against Cancer’; ML receives a grant of the Foundation Against Cancer for proton radiotherapy in pregnancy (ProPOSE). None of the aforementioned payments or roles are in relation to the submitted work. RW receives fees as president of the College for Physicians of Radiation Oncology Centres, which are paid to her institution; SR is working for the NIHDI, HE was working for the NIHDI during the course of the CED program; SJ and NVD both work for the BCR; but none declare financial or personal interests that could have influenced the submitted work. XG, NJ, LM, VR, KS and CW reported no financial or other relationships that might impact the submitted work.
Acknowledgements
We thank Alexandre Audibert, Gregory Coucke and Geert Silversmit (Belgian Cancer Registry) for the statistical support and Prof. Mark Lawler (Queen's University Belfast) for his feedback on the project report.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2024.100992.
Appendix A. Supplementary data
References
- 1.Atun R., Jaffray D.A., Barton M.B., et al. Expanding global access to radiotherapy. Lancet Oncol. 2015;16:1153–1186. doi: 10.1016/S1470-2045(15)00222-3. [DOI] [PubMed] [Google Scholar]
- 2.Lievens Y., Audisio R., Banks I., et al. Towards an evidence-informed value scale for surgical and radiation oncology: a multi-stakeholder perspective. Lancet Oncol. 2019;20:e112–e123. doi: 10.1016/S1470-2045(18)30917-3. [DOI] [PubMed] [Google Scholar]
- 3.Borras J.M., Corral J., Aggarwal A., et al. Innovation, value and reimbursement in radiation and complex surgical oncology: time to rethink. Radiother Oncol. 2022;169:114–123. doi: 10.1016/j.radonc.2021.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Garrison L.P., Jr., Towse A., Briggs A., et al. Performance-based risk-sharing arrangements—good practices for design, implementation, and evaluation: report of the ISPOR good practices for performance-based risk-sharing arrangements task force. Value Health. 2013;16:703–719. doi: 10.1016/j.jval.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Gerkens S., Neyt M., San Miguel L., Vinck I., Thiry N., Cleemput I. Health Services Research (HSR) Brussels: Belgian Health Care Knowledge Centre (KCE); 2017. How to improve the Belgian process for Managed Entry Agreements? An analysis of the Belgian and international experience. KCE Reports 288. D/2017/10.273/41. [Google Scholar]
- 6.Torbica A., Tarricone R., Schreyögg J., Drummond M. Pushing the boundaries of evaluation, diffusion, and use of medical devices in Europe: insights from the COMED project. Health Econ. 2022;31(S1):1–9. doi: 10.1002/hec.4600. [DOI] [PubMed] [Google Scholar]
- 7.Federici C., Reckers-Droog V., Ciani O., et al. Coverage with evidence development schemes for medical devices in Europe: characteristics and challenges. Eur J Health Econ. 2021;22:1253–1273. doi: 10.1007/s10198-021-01334-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drummond M., Federici C., Reckers-Droog V., et al. Coverage with evidence development for medical devices in Europe: can practice meet theory? Health Econ. 2022;31(S1):179–194. doi: 10.1002/hec.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palma D., Visser O., Lagerwaard F.J., Belderbos J., Slotman B.J., Senan S. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small cell lung cancer: a population-based time-trend analysis. J Clin Oncol. 2010;28:5153–5159. doi: 10.1200/JCO.2010.30.0731. [DOI] [PubMed] [Google Scholar]
- 10.Ball D., Tao Mai G., Vinod S., et al. Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): a phase 3, open-label, randomised controlled trial. Lancet Oncol. 2019;20(4):494–503. doi: 10.1016/S1470-2045(18)30896-9. [DOI] [PubMed] [Google Scholar]
- 11.Lewis S.L., Porceddu S., Nakamura N., et al. Definitive stereotactic body radiotherapy (SBRT) for extracranial oligometastases: an international survey of >1000 radiation oncologists. Am J Clin Oncol. 2017;40:418–422. doi: 10.1097/COC.0000000000000169. [DOI] [PubMed] [Google Scholar]
- 12.Guckenberger M., Lievens Y., Bouma A.B., et al. Characterisation and classification of oligometastatic disease: a European society for radiotherapy and oncology and European organisation for research and treatment of cancer consensus recommendation. Lancet Oncol. 2020;21:e18–e28. doi: 10.1016/S1470-2045(19)30718-1. [DOI] [PubMed] [Google Scholar]
- 13.Palma D.A., Olson R., Harrow S., et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393(10185):2051–2058. doi: 10.1016/S0140-6736(18)32487-5. [DOI] [PubMed] [Google Scholar]
- 14.Hulstaert F., Mertens A.-S., Obyn C., et al. Health technology assessment (HTA) Brussels: Belgian health care knowledge centre (KCE) 2013. Innovative radiotherapy techniques: a multicentre time-driven activity-based costing study. KCE Reports 198C. D/2013/10.273/9. [Google Scholar]
- 15.Lievens Y., Obyn C., Mertens A.-S., Van Halewyck D., Hulstaert F. Stereotactic body radiotherapy for lung cancer: how much does it really cost? J Thorac Oncol. 2015;10(3):454–461. doi: 10.1097/JTO.0000000000000421. [DOI] [PubMed] [Google Scholar]
- 16.National Radiotherapy Implementation Group Report. Stereotactic Body Radiotherapy . NHS England; 2010. Clinical review of the evidence for SBRT. Version 1. [Google Scholar]
- 17.Lievens Y., Guckenberger M., Gomez D., et al. Defining oligometastatic disease from a radiation oncology perspective: an ESTRO-ASTRO consensus document. Radiother Oncol. 2020;148:157–166. doi: 10.1016/j.radonc.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Ost P., Shankar S., Brabrand S., et al. PEACE V-salvage treatment of OligoRecurrent nodal prostate cancer metastases (STORM): acute toxicity of a randomized phase 2 trial. Eur Urol Oncol. 2023;9(23):S2588–S9311. doi: 10.1016/j.euo.2023.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Spaas M., Sundahl N., Kruse V., et al. Checkpoint inhibitors in combination with stereotactic body radiotherapy in patients with advanced solid tumors: the CHEERS phase 2 randomized clinical trial. JAMA Oncol. 2023;9(9):1205–1213. doi: 10.1001/jamaoncol.2023.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guckenberger M., Allgäuer M., Appold S., et al. Safety and efficacy of stereotactic body radiotherapy for stage 1 non-small-cell lung cancer in routine clinical practice: a patterns-of-care and outcome analysis. J Thorac Oncol. 2013;8(8):1050–1058. doi: 10.1097/JTO.0b013e318293dc45. [DOI] [PubMed] [Google Scholar]
- 21.Davis J.N., Medbery I.I.I.C., Sharma C., Danish A., Mahadevan A. The RSSearch™ Registry: patterns of care and outcomes research on patients treated with stereotactic radiosurgery and stereotactic body radiotherapy. Radiat Oncol J. 2013;8:275. doi: 10.1186/1748-717X-8-275. http://www.ro-journal.com/content/8/1/275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendez Romero A., Schillemans W., van Os R., et al. The Dutch-Belgian registry of stereotactic body radiation therapy for liver metastases: clinical outcomes of 515 patients and 668 metastases. Int J Radiat Oncol Biol Phys. 2021;109(5):1377–1386. doi: 10.1016/j.ijrobp.2020.11.045. [DOI] [PubMed] [Google Scholar]
- 23.Chalkidou A., Macmillan T., Grzeda M.T., et al. Stereotactic ablative body radiotherapy in patients with oligometastatic cancers: a prospective, registry-based, single-arm, observational, evaluation study. Lancet Oncol. 2021;22:98–106. doi: 10.1016/S1470-2045(20)30537-4. [DOI] [PubMed] [Google Scholar]
- 24.Iyengar P., All S., Berry M.F., et al. Treatment of oligometastatic non-small cell lung cancer: an ASTRO/ESTRO clinical practice guideline. Pract Radiat Oncol. 2023;13:393–412. doi: 10.1016/j.prro.2023.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Nyman J., Hallqvist A., Lund J.A., et al. Space – a randomized study of SBRT vs. conventional fractionated radiotherapy in medically inoperable stage I NSCLC. Radiother Oncol. 2016;121:1–8. doi: 10.1016/j.radonc.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 26.ENCR Recommendations Treatment data recording – phase I. Version 28-06-2023. https://encr.eu/sites/default/files/Recommendations/Draft%20ENCR%20Recommendation%20treatment%20data%20recoding_20230628-for%20consultation.pdf
- 27.Giusti F., Martos C., Trama A., et al. Cancer treatment data available in European cancer registries: where are we and where are we going? Front Oncol. 2023;13 doi: 10.3389/fonc.2023.1109978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yalvac B., Reulens N., Reniers B. Early results of a remote dosimetry audit program for lung stereotactic body radiation therapy. Phys Imaging Radiat Oncol. 2024;29 doi: 10.1016/j.phro.2024.100544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reckers-Droog V., Federici C., Brouwer W., Drummond M. Challenges with coverage with evidence development schemes for medical devices: a systematic review. Health Policy Technol. 2020;9:146–156. doi: 10.1016/j.hlpt.2020.02.006. [DOI] [Google Scholar]
- 30.Vaandering A., Roels S., Yalvaç B., et al. Favouring quality improvement initiatives: the experience of the Belgian college of radiation oncology. Precis Cancer Med. 2023;6:4. doi.org/10.21037/pcm-22-15. [Google Scholar]
- 31.E2-RADIatE EORTC-ESTRO RADiotherapy InfrAstrucTure for Europe (E2-RADIatE). NCT03818503, OligoCare cohort. https://project.eortc.org/e2-radiate/cohorts/
- 32.2023. Stereotactic body radiotherapy for the treatment of OPD (HALT). NCT03256981. [Google Scholar]
- 33.2023. Stereotactic radiotherapy for oligo-progressive metastatic cancer (the STOP trial). NCT02756793. [Google Scholar]
- 34.Tsai C.J., Yang J.T., Shaverdian N., et al. Standard-of-care systemic therapy with or without stereotactic body radiotherapy in patients with oligoprogressive breast cancer or non-small-cell lung cancer (Consolidative Use of Radiotherapy to Block (CURB) oligoprogression): an open-label, randomized, controlled phase 2 study. Lancet. 2024;403(10422):171–182. doi: 10.1016/S0140-6736(23)01857-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nevens D., Kindts I., Defourny N., et al. The financial impact of SBRT for oligometastatic disease: a population-level analysis in Belgium. Radiother Oncol. 2020;145:215–222. doi: 10.1016/j.radonc.2020.01.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.