Abstract
Background:
When immunofluorescence on the frozen section is insufficient or unavailable, salvage immunofluorescence techniques can be used on formalin-fixed, paraffin-embedded tissue. The goal of the current investigation was to evaluate the diagnostic value of paraffin immunofluorescence following proteinase K digestion on skin biopsy samples in comparison to fresh frozen immunofluorescence.
Materials and Methods:
It was standardized and compared to the immunofluorescence on fresh frozen tissue (IF-Frozen) for paraffin immunofluorescence by proteinase K digestion of formalin-fixed paraffin-embedded skin biopsies (IF-FFPE). The study included 50 native skin biopsy cases, and fluorescein isothiocyanate-labeled IgA, IgG, IgM, and C3 intensity levels were evaluated in each case.
Results:
A total of 50 cases of native skin biopsy were included in the study, and their intensities for IgA, IgG, IgM, and C3 antibodies were compared. The average staining intensities in each disease group for the antibodies had equal intensity or had a minor difference (1+)/significant difference (2+). Paraffin immunofluorescence, proteinase K digestion had the best correlation, that is, had either equal or minor difference (1+) with fresh frozen immunofluorescence. The difference of 2+ intensity of antibodies between IF-FFPE and IF-Frozen was noted mainly in C3 antibody on paraneoplastic pemphigus. IF-FFPE showed a sensitivity of 100%, 97.6%, 100%, and 81.6% for IgA, IgG, IgM, and C3, respectively, whereas the specificity was 100% for IgA, IgG, IgM, and C3.
Limitations:
Small sample size and and the employment of one method of antigen retrieval in IF-FFPE.
Conclusion:
Immunofluorescence techniques done on formalin-fixed paraffin-embedded tissue can serve as salvage techniques in cases where immunofluorescence on the frozen section may not be adequate or may not be available.
Keywords: Antigen retrieval, direct immunofluorescence, formalin-fixed paraffin-embedded sections, proteinase, skin disease
Introduction
An easy, dependable, and reproducible approach in immunopathology is immunofluorescence (IF). It detects immunological deposits that are present in circulation and are implicated in the pathophysiology of many illnesses, particularly those that impact the skin and renal system.[1] Direct immunofluorescence on fresh frozen tissue has long been the gold standard.[1] Immunofluorescence on fresh frozen tissue does, however, have several drawbacks in clinical settings like thick frozen sections or the antigens’ potential for appearing diffusely distributed, which makes analysis challenging.[2] Additionally, insufficient frozen tissue may lead to a lower degree of diagnostic precision. Finally, re-evaluation cannot be done on the frozen portion after it has been done. In such cases, a salvage method using an alternate procedure on tissue that has been formalin-fixed and paraffin-embedded may be used.[2]
There are several antigen retrieval techniques used to get over the issue of antigen masking in formalin-fixed, paraffin-embedded tissues employing proteinase K, trypsin, and heat-induced antigen retrieval.[2,3,4]
Studies have shown that for the majority of pathogenic antibodies, immunofluorescence techniques on formalin-fixed, paraffin-embedded tissue yield equivalent results to those obtained on frozen sections.[2,5] However, most of the studies were on renal biopsies with only a few studies done on skin biopsies.[2,3,4]
The present study was undertaken to compare the diagnostic value of fresh frozen immunofluorescence versus paraffin immunofluorescence after proteinase K digestion on skin biopsies.
Materials and Methods
All formalin-fixed, paraffin-embedded blocks for routine hematoxylin and eosin (H and E)-stained slides for which corresponding direct immunofluorescence on a frozen section was available were gathered.
Proteinase K (Sigma-Aldrich, USA, Cat. P2308) was added to the slides made from the same blocks from which H and E-stained slides were created for the histopathological interpretation of skin diseases in order to carry out immunofluorescence on formalin-fixed, paraffin-embedded tissue (IF-FFPE).
Proteinase K technique
Slides coated with poly-L-lysine and cut into 3 μm sections were used for the proteinase K method of antigen retrieval. After deparaffinization, the sample was maintained in Tris-EDTA buffer with a pH of 9.0. Proteinase K was used to remove the antigens’ masks, and the slides were incubated in a humidified wet chamber at 37 centigrade for 30 minutes. IgA, IgG, IgM, and C3 fluorescence-conjugated polyclonal antibodies from Dako, Carpinteria, CA, USA, were added after incubation. Following phosphate-buffered saline (PBS) rinsing, slides were mounted with aqueous phosphate buffer glycerol. Direct immunofluorescence on frozen sections was used to compare the outcomes.
Frozen section technique
In the frozen section method (IF-Frozen), poly-L-lysine-coated slides with 3–4 μm sections cut in a cryostat were taken. The sections were first dried and washed in PBS three times at a pH of 7.4. Fluorescence-conjugated antibodies were added and incubated at 37°C.
Again, the slides were washed in PBS three times. The slides were then mounted with aqueous phosphate buffer glycerol.
Evaluation of immunofluorescence
The grading was performed, and the results were interpreted as follows: no staining (0), mild staining (1+), moderate staining (2+), moderate-to-high staining (3+), and high staining (4+). In terms of average intensity and intensity difference between IF-FFPE and IF-Frozen, the intensity of FITC-labeled IgA, IgG, IgM, and C3 antibodies was compared. The sensitivity and specificity of the IF-FFPE were compared to those of the IF-Frozen technique’s gold standard approach. One dermatopathologist, who was blinded to the results of the IF-Frozen, analyzed all of the IF-FFPE.
Results
The study comprised 50 cases of native skin biopsies. There were 17 cases of cutaneous lupus, six cases of pemphigus vulgaris, three cases of pemphigus foliaceous, two cases of paraneoplastic pemphigus, six cases of bullous pemphigoid, two cases of Hailey-Hailey disease, nine cases of leucocytoclastic vasculitis, and one case each of linear IgA bullous disease, erythema multiforme, porphyria cutanea tarda, psoriasis, and bullous drug reaction [Figure 1a–d]. In these 50 cases, IF-FFPE and IF-Frozen were compared for IgA, IgG, IgM, and C3 FITC-labeled antibody intensity [Table 1].
Figure 1.
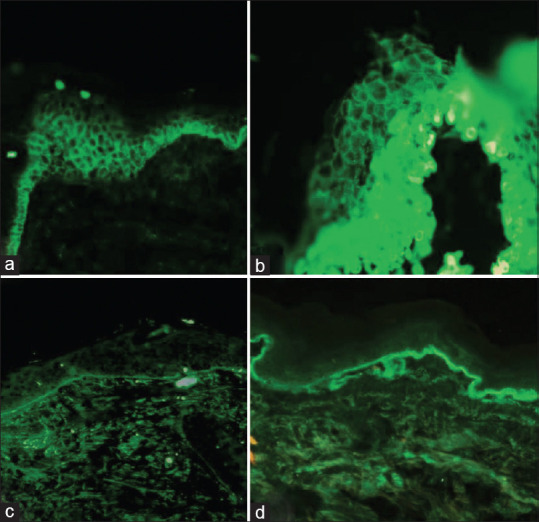
Immunofluorescence in a case of pemphigus vulgaris. (a) Intercellular space pattern showing 4+ IgG in frozen section (FITC-tagged antibody using IgG, 20×) versus (b) intercellular spaces pattern showing 4+ IgG in paraffin section (FITC-tagged antibody using IgG, 40×); Immunofluorescence in a case of cutaneous lupus erythematosus. (c) Basement membrane zone showing granular 3+ C3 in frozen section (FITC-tagged antibody using C3, 4×) versus (d) basement membrane zone showing granular 3+ C3 in paraffin section (FITC-tagged antibody using C3, 4×)
Table 1.
Average intensity with intensity difference by different paraffin immunofluorescence methods as compared with fresh frozen immunofluorescence
| Diagnosis | No. of cases | IgA | IgA | IgG | IgG | IgM | IgM | C3 | C3 |
|---|---|---|---|---|---|---|---|---|---|
| 50 | IF-F | IF-FFPE | IF-F | IF-FFPE | IF-F | IF-FFPE | IF-F | IF-FFPE | |
| Cutaneous lupus | 17 | 0.12 | 0.12 (equal) | 3 | 2.12 (1+) | 0.76 | 0.53 (1+) | 1.41 | 0.88 (1+) |
| Pemphigus vulgaris including vegetans | 6 | 0 | 0 | 3.67 | 2.67 (1+) | 0 | 0 | 2.67 | 2.17 (1+) |
| Pemphigus foliaceous | 3 | 0 | 0 | 2.33 | 1.67 (1+) | 0 | 0 | 1.33 | 0.67 (1+) |
| Paraneoplastic pemphigus | 2 | 0 | 0 | 4 | 3 (1+) | 0 | 0 | 1 | 0 |
| Bullous pemphigoid | 6 | 0 | 0 | 3.33 | 2.67 (1+) | 0 | 0 | 1.33 | 0.67 (1+/2) |
| Hailey-Hailey disease | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Linear IgA bullous disease | 1 | 4 | 3 (1+) | 0 | 0 | 0 | 0 | 1 | 1 (equal) |
| Leucocytoclastic vasculitis | 9 | 0 | 0 | 2.78 | 2.11 (1+) | 2.22 | 1.89 (1+) | 1.33 | 1 (1+) |
| Erythema multiforme | 1 | 0 | 0 | 3 | 2 (1+) | 0 | 0 | 3 | 2 (1+) |
| Porphyria cutanea tarda | 1 | 0 | 0 | 3 | 2 (1+) | 0 | 0 | 0 | 0 |
| Psoriasis | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bullous drug reaction | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Most of the cases showed a difference of 1+ intensity. A difference of 1+ intensity was observed in 50% of paraneoplastic pemphigus, 66% of bullous pemphigoid, 67% of leucocytoclastic vasculitis, 82% of cutaneous lupus, and 100% of pemphigus vulgaris, pemphigus foliaceous, linear IgA disease, erythema multiforme, and porphyria cutanea tarda. A difference of 2+ intensity was observed in 50% (n = 1, C3) of paraneoplastic pemphigus, followed by 17% (n = 1, C3) bullous pemphigoid and 12% (n = 2; IgG and C3) of cutaneous lupus [Table 2].
Table 2.
Immunofluorescence on fresh frozen tissue versus paraffin-embedded skin biopsies
| Diagnosis | Equal Intensity of Immunoglobulin/Complement between IF-F & IF-FFPE | Difference of 1+in Intensity of Immunoglobulin/Complement between IF-F & IF-FFPE | Difference of 2+ in Intensity of Immunoglobulin/Complement between IF-F & IF-FFPE |
|---|---|---|---|
| Cutaneous lupus (n=17) | 01 (06%) | 14 (82%) | 02 (12%) |
| Pemphigus vulgaris (n=6) | - | 06 (100%) | - |
| Pemphigus foliaceous (n=3) | - | 03 (100%) | - |
| Paraneoplastic pemphigus (n=2) | - | 01 (50%) | 01 (50%) |
| Bullous pemphigoid (n=6) | 01 (17%) | 04 (66%) | 01 (17%) |
| Hailey-Hailey disease (n=2) | 02 (100%) | - | - |
| Linear IgA bullous disease (n=1) | - | 01 (100%) | - |
| Leucocytoclastic vasculitis (n=9) | 03 (33%) | 06 (67%) | - |
| Erythema multiforme (n=1) | - | 01 (100%) | - |
| Porphyria cutanea tarda (n=1) | - | 01 (100%) | - |
| Psoriasis (n=1) | 01 (100%) | - | - |
| Bullous drug reaction (n=1) | 01 (100%) | - | - |
| n=50 | n=09 (18%) | n=37 (74%) | n=04 (08%) |
The specificity for IF-FFPE was 100% for IgA, IgG, IgM, and C3. The sensitivity was 100% for IgA and IgM. The sensitivity for IF FFPE was for IgG (97.6%), followed by C3 (81.6%) [Table 3].
Table 3.
Sensitivity and specificity for IgA, IgG, IgM and C3 by IF –FFPE method of 50 skin cases
| IF- Frozen/IF -FFPE | a (+/+) | b (-/+) | c (+/-) | d (-/-) | No. of Case | Sensitivity a/(a+c) | Specificity d/(d+b) |
|---|---|---|---|---|---|---|---|
| Ig A | 3 | 0 | 0 | 47 | 50 | 100% | 100% |
| IgG | 42 | 0 | 1 | 7 | 50 | 97.6% | 100% |
| IgM | 12 | 0 | 0 | 38 | 50 | 100% | 100% |
| C3 | 31 | 0 | 7 | 12 | 50 | 81.6% | 100% |
Discussion
Formalin may significantly impair intensity of immunofluorescence for select diseases depending on the time of exposure.[5] One of the masking mechanisms of formalin on immunofluorescence is protein cross-linking, which prohibits FITC-conjugated antibodies from interacting with the antigens during immunofluorescence on formalin-fixed, paraffin-embedded tissue.[6,7] Therefore, unmasking of the antigens is necessary for formalin-fixed, paraffin-embedded tissues using a variety of methods, such as enzyme digestion and heat treatment.[7,8] Proteinase K, trypsin, and heat-induced enzyme retrieval have been used for antigen retrieval in paraffin blocks of skin biopsies at various temperatures, concentrations, and times.[2,3,4]
Valencia-Guerrero et al.[2] used proteinase K for antigen retrieval in formalin-fixed, paraffin-embedded skin biopsies. The present study also utilized proteinase K for antigen retrieval in the skin biopsies done for various skin pathologies. Studies have shown that though the IF patterns in both IF-Frozen and IF-FFPE were identical, IF intensity of IF-FFPE was frequently lower than that of the frozen tissue.[2,9] So the present study focused on the comparison of the intensity of the antibodies in IF-FFPE versus IF-Frozen for skin biopsies. In the present study, a difference of 1+ intensity was observed in 50% of paraneoplastic pemphigus, 66% of bullous pemphigoid, 67% of leucocytoclastic vasculitis, 82% of cutaneous lupus, and 100% of pemphigus vulgaris, pemphigus foliaceous, linear IgA disease, erythema multiforme, and porphyria cutanea tarda. These findings are in concordance with Valencia-Guerrero et al.[2] and Mera et al.,[3] who reiterated the finding that a reduction in staining intensity is observed in FFPE skin biopsies when compared to IF-frozen tissue.
The present study showed that a majority of the cases showed a difference of 1+ intensity in IF-FFPE as compared to IF-Frozen. However, a significant difference of 2+ intensity was observed in 50% of paraneoplastic pemphigus, followed by 17% bullous pemphigoid and 12% of cutaneous lupus. Mera et al.[3] also analyzed the intensity of the antibodies IgG, IgM, IgA, C3, and fibrin in FFPE blocks of cases of pemphigus vulgaris, pemphigoid, and lupus erythematosus and compared the degree of intensity difference. They found a difference of 2+ intensity at least in one of the antibodies in 83% of pemphigus vulgaris, 33% of pemphigoid, and 100% cases of lupus erythematosus.[3] These higher percentages of cases showing a difference 2+ intensity could be attributed to a different methodology of antigen retrieval, that is, trypsin used by Mera et al.[3]
In this study, a difference of 2+ intensity was noted in antibodies of C3 in one case each of cutaneous lupus erythematosus, bullous pemphigoid, and paraneoplastic pemphigus. An intensity difference of 2+ for IgG was noted in one case of cutaneous lupus erythematosus. These findings are in concordance with the studies by Mera et al.[3] and Zhao et al.,[4] which showed C3 and IgG are the most difficult to demonstrate in formalin-fixed, paraffin-embedded material. In contrast, C3 was the most sensitive antibody and IgA was the most difficult antibody to demonstrate in the study by Valencia-Guerrero et al.[2] Thus, dermatopathologists should be aware of the pitfalls while interpreting C3 and IgG in IF-FFPE.
The sensitivity of IF-FFPE in pemphigus was 95.45%, and the specificity was 100% as per Zhao et al.[4] The sensitivity varied significantly among the different diseases ranging from 0% to79% as per Valencia-Guerrero et al.[2] The present study also attempted to analyze the sensitivity and specificity of IF-FFPE as compared to IF-Frozen of the antibodies. The sensitivity was 100% for IgA and IgM, whereas the sensitivity was 97.6% for IgG and 81.6% for C3. However, disadvantages of IF-FFPE in skin biopsies include higher background staining due to autofluorescence.
Limitations
The limitations of the study were a relatively smaller sample size with a lesser number of cases in each sub-group and employment of one method of antigen retrieval in IF-FFPE.
Conclusions
In situations when IF-Frozen may not be sufficient or available, IF-FFPE can be a beneficial strategy. When compared to IF-Frozen skin biopsies, the proteinase K digestion method for IF-FFPE skin tissue shows good agreement in terms of intensity. The authors propose that IF-FFPE tissue should not replace conventional IF-Frozen sections when evaluating skin biopsies and should be viewed as a salvage approach to improve the sensitivity and specificity of detecting immunoglobulins and complements in different skin pathologies.
Consent and ethics
The study was approved by university research ethics committee vide number VI/I (8)/UREC/EA/272/2015-6765 dated 19th August 2020.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Balakrishnan S, Kalappuravil NB. Immunofluorescence in dermatology: A brief review. J Skin Sex Transm Dis. 2023;5:66–74. [Google Scholar]
- 2.Valencia-Guerrero A, Deng A, Dresser K, Bouliane G, Cornejo KM. The value of direct immunofluorescence on proteinase-digested formalin-fixed paraffin embedded skin biopsies. Am J Dermatopathol. 2018;40:111–7. doi: 10.1097/DAD.0000000000000934. [DOI] [PubMed] [Google Scholar]
- 3.Mera SL, Young EW, Bradfield JW. Direct immunofluorescence of skin using formalin-fixed paraffin-embedded sections. J Clin Pathol. 1980;33:365–9. doi: 10.1136/jcp.33.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao W, Zhu H, Zhao X, Wu X, Sun F, Pan M, et al. Direct immunofluorescence of IgG on formalin-fixed paraffin-embedded tissue by heat-induced antigen retrieval as a sensitive method for the diagnosis of pemphigus. Clin Cosmet Investig Dermatol. 2023;16:1233–41. doi: 10.2147/CCID.S408613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arbesman J, Grover R, Helm TN, Beutner EH. Can direct immunofluorescence testing still be accurate if performed on biopsy specimens after brief inadvertent immersion in formalin? J Am Acad Dermatol. 2011;65:106–11. doi: 10.1016/j.jaad.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 6.Das N, Lakadong RO, Dey B, Raphael V. A comparative study of immunofluorescence on formalin-fixed, paraffin-embedded versus fresh frozen kidney biopsy. Cureus. 2023;15:e40978. doi: 10.7759/cureus.40978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nada R, Kumar A, Kumar VG, Gupta KL, Joshi K. Unmasking of complements using proteinase-K in formalin fixed paraffin embedded renal biopsies. Indian J Nephrol. 2016;26:182–7. doi: 10.4103/0971-4065.159558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brar A, Sharma A, Nauhria S, Nauhria S, Bhattacharjee A, Peela J, et al. Utility of direct immunofluorescence in cutaneous autoimmune bullous disorders. Cureus. 2021;13:e14562. doi: 10.7759/cureus.14562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Firth NA, Rich AM, Radden BG, Reade PC. Direct immunofluorescence of oral mucosal biopsies: A comparison of fresh-frozen tissue and formalin-fixed, paraffin-embedded tissue. J Oral Pathol Med. 1992;21:358–63. doi: 10.1111/j.1600-0714.1992.tb01365.x. [DOI] [PubMed] [Google Scholar]


