Abstract
Background and Aims:
Alcohol use disorder has been reported in patients undergoing bariatric procedures, but the pattern of alcohol consumption has not been evaluated. We investigated the prevalence, risk factors, and impact of binge drinking (BD) at the time of surgery and during follow-up.
Methods:
A prospective, longitudinal study of subjects undergoing bariatric surgery was included in the LABS-2 registry between 2006 and 2009. Participants with AUDIT questionnaire at the time of surgery and a minimum of 12 months follow-up were included. BD was defined as consuming ≥5 drinks on at least 1 occasion in the previous month. Liver biopsies were obtained during bariatric procedures in not all cases. Survival analysis was performed with the adjusted Cox regression model and competing risk.
Results:
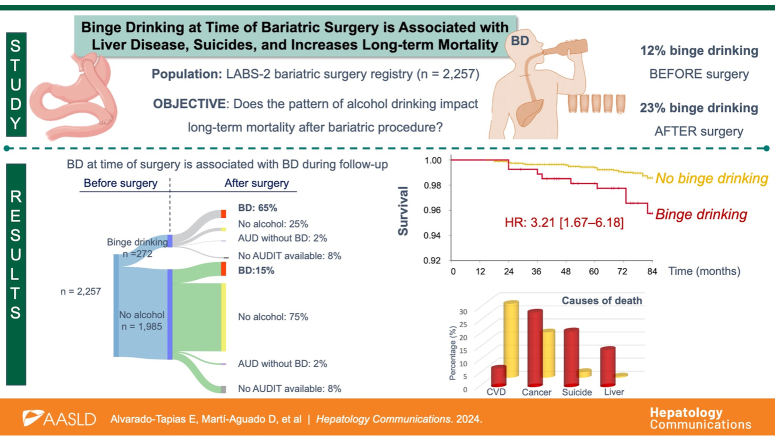
A total of 2257 subjects were included, with a median follow-up of 79 months. The prevalence of BD at time of surgery was 12%, and it raised up to 23% during follow-up. Patients with BD predominantly had a binge eating disorder (OR=1.35 [95% CI: 1.04–1.76]), regularly consumed fast food [OR=1.4 (95% CI: 1.07–1.85)] and used other drugs (OR=2.65 [95% CI: 1.74–4.04]). Within liver biopsies evaluation, BD showed higher hepatic iron deposits (OR=3.00 [95% CI: 1.25–7.21]). BD at the time of surgery was associated with a higher risk of BD during follow-up (OR=10.49 [95% CI: 7.86–14.00]) and long-term mortality (HR: 3.21 [95% CI: 1.67–6.18]). Specific causes of death in these patients with BD were liver disease (p=0.020), suicide (p=0.015), neoplasms (p=0.034), and respiratory (p=0.025).
Conclusions:
The prevalence of BD in patients undergoing bariatric surgery is high and increases the risk of postoperative liver disease, suicides, and long-term mortality.
INTRODUCTION
Bariatric surgery is the most effective long-term treatment for severe obesity and has shown effectiveness in reducing obesity-related comorbidities and increasing overall survival.1 Nevertheless, several studies have described an increased risk of alcohol use disorder (AUD) after surgery.2,3,4 The Longitudinal Assessment of Bariatric Surgery-2 (LABS-2) study, a prospective registry of US adults who underwent bariatric procedures, showed that the prevalence of AUD can double seven years after Roux-en-Y gastric bypass.2,4 These patients with AUD have a higher risk of developing alcohol-associated liver disease and alcohol-mental disorders.5
Several mechanisms have been suggested to explain the association between bariatric surgery and AUD.6,7 The presence of presurgical binge eating disorders, which are highly prevalent in these patients, can share behavioral similarities with substance use disorders. These conditions can be classified as binge behavioral disorders with a similar neuropsychological profile characterized by heavy episodic intakes (food, alcohol, or drugs)8 that can progress to an unhealthy stage.9 Alcohol Use Disorders Identification Test (AUDIT) is a widely validated questionnaire for measuring alcohol-associated problems. Specific AUDIT subscores for alcohol dependence or alcohol-associated harm define AUD as an impaired ability to control alcohol consumption with already established adverse social, occupational, or health consequences.10 Although AUD, symptoms of alcohol dependence, and alcohol-associated harm do not seem to increase mortality after bariatric surgery, the pattern of alcohol consumption has not been evaluated in this setting.11 Binge drinking (BD) is a pattern of alcohol consumption defined as consuming 4 or more drinks for females or 5 or more drinks for males on the same occasion on at least 1 day in the past 30 days.12,13 The rising prevalence of BD and its association with liver-related disease and death independently of average alcohol intake has raised awareness of the harmful repercussions of this overlooked drinking pattern.14
The primary objective of this study was to evaluate the prevalence of BD at the time of bariatric surgery and during follow-up, identify the risk factors associated with this pattern of alcohol consumption, and assess its long-term impact in a well-characterized cohort of the LABS-2 database. The secondary objective was to evaluate liver histological features that differ between patients with and without BD.
METHODS
Study design and participants
This longitudinal observational study was performed using the LABS-2 registry, a prospective cohort of US adults undergoing bariatric surgery at 10 hospitals between 2006 and 2009 (ClinicalTrials.gov: NCT00465829). Participants attended presurgery, 1-month, 6-month, and annual postoperative follow-up assessments for up to 7 years or until 2015, whichever came first. All adults with AUDIT information at the time of bariatric surgery were included. The exclusion criteria were a follow-up of <1 year. The LABS-2 database includes demographical, clinical, analytical, surgical, and follow-up information. The database was supplied by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Central Repository.15 The study was performed in accordance with the Declaration of Helsinki and good clinical practice guidelines. The Institutional Review Boards at each center approved the protocol, and all participants provided informed consent, including a liver biopsy review by the study pathologist. The local Institutional Review Board (University of Pittsburgh) approved the study protocol in May 2019 (STUDY19010143).
Data collection
Preoperative baseline clinical data were collected within 30 days of surgery at in-person visits by study-trained personnel using standardized protocols.2 Data were collected from patient self-reported information (age, sex, race, educational level, marital and employment status, cigarette smoking, alcohol consumption, and other drugs use), anthropometric measurements (mean arterial pressure, waist circumference, and body mass index) and review of medical records. Educational level was categorized as low (up to high school diploma) and high (some college up to graduate).16 Comorbidities (metabolic syndrome, arterial hypertension, diabetes, dyslipidemia, sleep apnea, cardiovascular disease [CVD], and chronic kidney disease) were defined using medical records, physical examination, participant interviews, and laboratory values.
The follow-up visits took place in person at the clinical centers. The underlying cause of death was obtained from death certificates and categorized according to the World Health Organization (WHO) Global Burden of Disease Study (Supplemental Table S1, http://links.lww.com/HC9/A997).17
Assessment of alcohol consumption and eating behaviors
Participants reported alcohol use, including frequency/quantity of consumption in the previous year. The AUDIT was used to assess alcohol consumption, including BD, through specific subscores.13 As established by the Substance Abuse and Mental Health Services Administration, BD was defined as 4 or more drinks for women or 5 or more drinks for men on the same occasion on at least 1 day in the past 30 days. Considering this definition, patients with BD were selected if an answer ≥3 was obtained for the third question of the AUDIT.12,13,18 The few patients (n=67) who reported daily alcohol consumption defined as AUDIT≥8, but did not referred heavy episodic drinking, were not included in BD category. During follow-up, the AUDIT questionnaire was assessed yearly, allowing the categorization of 4 groups after surgery: (1) no alcohol, (2) BD, (3) AUD without BD, (4) no AUDIT information available.
The self-administered LABS-2 Behavior Form includes a section on eating behaviors and disorders. This study assessed eating behavior, the frequency of eating each type of meal/week (breakfast, lunch, and dinner), the frequency of total snacks/meals per day, the frequency of eating meals at fast food and other restaurants, and the frequency of eating when not hungry or when uncomfortably full.
Eating disorders were defined as previously described using the Diagnostic and Statistical Manual of Mental Disorders-IV.19 Binge eating disorder was diagnosed by endorsing several items to determine the five criteria defined in the Diagnostic and Statistical Manual of Mental Disorders-IV. Participants reporting evening hyperphagia or nocturnal eating were considered to have night eating syndrome.
Histological evaluation
During surgery, liver biopsies were obtained from some participants according to the local standard of care.20 Biopsies were routinely stained with hematoxylin-eosin and Masson trichrome. The study pathologist was blinded to clinical and biological data. Biopsy adequacy was determined by measuring the length of the tissue sample. Features of steatotic liver disease were scored using the NASH Clinical Research Network scoring system.21 The NAFLD Activity Score was used as a composite measure of liver injury, adding the severity scores of steatosis, lobular inflammation, and ballooning. Inflammation was further evaluated using the Ishak scoring system, and iron deposits (Perls staining) were assessed according to Deugnier criteria.22,23 In addition to scoring, each biopsy was categorized into histological groups: steatohepatitis (defined as NAFLD Activity Score ≥4) and high-risk fibrotic steatohepatitis (defined as fibrosis stage≥2 and NAFLD Activity Score ≥4).24
Statistical analysis
Continuous variables are expressed as mean (SD) or median (IQR) whenever appropriate. Differences in continuous variables were tested by the ANOVA, Student t test, or Wilcoxon signed rank test for independent samples. Categorical variables were presented as frequency and percentage. Differences in the categorical variables were assessed by the χ2 test or by Fisher exact test. To evaluate differences between studies, tests on the equality of proportions were performed. Time was calculated from the day of surgery to the date of censoring: death or study closure, whichever came first. Time-to-event analysis was conducted using the Kaplan-Meier method. Survival curves were compared using the log-rank test to identify variables at the time of surgery that were associated with mortality. A multivariable Cox proportional hazard regression analysis was performed to determine the independent contribution of each factor to time to death, adjusted by age, sex, body mass index, education level, surgical procedure, smoking, AUD, substance use, and fibrosis score 4 index.25 A second multivariable Cox time-dependent model was performed with those variables available after surgery during follow-up (BD status, change in body mass index, and diabetes mellitus status). Those variables showing a p-value<0.10 in the univariate analysis were included in the multivariable models. A backward stepwise method was then used to identify independent predictors of mortality. The C-statistic assessed the discriminative ability of the models. The internal validity to assess the robustness of the multivariable model was tested for 500 bootstrap resamples using the “rms” package.26 The calibration of models was evaluated by the corresponding “slope” using the same package. The Fine and Gray competing risks regression model was used to determine the effect of the explanatory variable (BD) in the risk of each specific cause of death. Finally, a sensitivity analysis was performed using logistic regression analysis and was illustrated with a tornado plot, considering the pattern of alcohol consumption (BD on the y-axis) and the range of the outcome (mortality on the x-axis). The reference group was absence of BD at baseline and follow-up. A two-sided p-value<0.05 was considered statistically significant. Missing data were imputed using the “mice” package in R whenever necessary (m=5). The statistical analyses were performed using R (v.4.1.3) and the “Tornado” package; SPSS (v.25.0); Graph Pad Prism (v.9.1.1); and sankeymatic.com, statistical packages.
RESULTS
Baseline characteristics
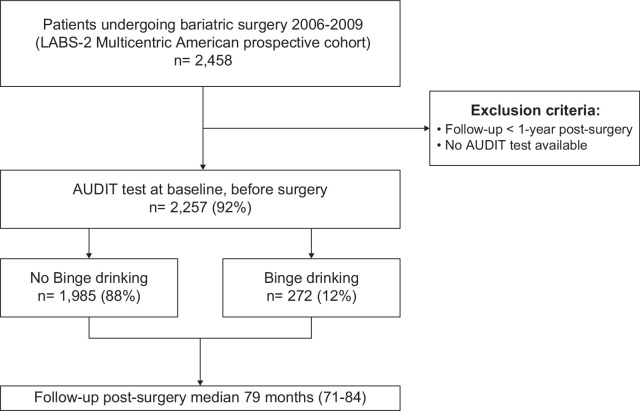
Participants’ flowchart is shown in Figure 1. A total of 2257 participants with AUDIT available at the time of bariatric surgery were included. Among them, 12% (n=272) had BD. Of the 88% (n=1,985) controls without BD, 3% (n=67) reported chronic daily alcohol consumption (AUDIT≥8). Baseline characteristics are summarized in Table 1.
FIGURE 1.
Flowchart showing patient selection from the LABS-2 database. Classification into binge drinking or no binge drinking was performed considering the baseline AUDIT test. Abbreviations: AUDIT, Alcohol Disorder Identification Test; LABS-2 Study, Longitudinal Assessment of Bariatric Surgery-2.
TABLE 1.
Baseline clinical characteristics and laboratory parameters in both cohorts with and without binge drinking
| Characteristics | Binge drinking (n=272) | No binge drinking (n=1985) | p |
|---|---|---|---|
| Age (y) | 40±10 | 46±11 | <0.001 |
| Sex, n (%) | — | — | 0.020 |
| Female | 199 (73) | 1572 (79) | — |
| Male | 73 (27) | 413 (21) | — |
| Race, n (%) | — | — | 0.050 |
| Hispanic | 20 (7) | 90 (5) | — |
| Not Hispanic | 252 (93) | 1893 (96) | — |
| Surgery performed, n (%) | — | — | 0.979 |
| RYGB | 193 (71) | 1410 (71) | — |
| LAGB and others | 79 (29) | 575 (29) | — |
| Education level, n (%) | — | — | 0.016 |
| Low | 47 (17) | 470 (24) | — |
| High | 225 (83) | 1503 (76) | — |
| Marital status, n (%) | — | — | 0.001 |
| Single | 65 (24) | 301 (15) | — |
| Married or living as married | 166 (61) | 1266 (64) | — |
| Divorced or separated | 39 (14) | 359 (18) | — |
| Widowed | 2 (1) | 48 (3) | — |
| Employment status, n (%) | — | — | <0.001 |
| Employed | 218 (81) | 1298 (66) | — |
| Homemaker | 11 (4) | 92 (5) | — |
| Disabled | 20 (7) | 312 (16) | — |
| Unemployed | 11 (4) | 86 (4) | — |
| Retired | 6 (2) | 143 (7) | — |
| Other | 4 (2) | 38 (2) | — |
| BMI (kg/m2) | 45.4 (41.3–51.1) | 46.8 (42.1–52.4) | 0.230 |
| MAP (mm Hg) | 125 (117–132) | 124 (115–133) | 0.395 |
| Waist circumference (cm) | 130 (121–142) | 131 (122–143) | 0.793 |
| Smoking, n (%) | 23 (9) | 73 (4) | <0.001 |
| Other drugs, n (%) | 32 (12) | 95 (5) | <0.001 |
| Sleep apnea, n (%) | 110 (40) | 979 (49) | 0.006 |
| CKD, n (%) | 35 (13) | 320 (16) | 0.180 |
| Diabetes, n (%) | 65 (24) | 725 (37) | <0.001 |
| Arterial hypertension, n (%) | 160 (60) | 1429 (73) | <0.001 |
| Dyslipidemia, n (%) | 63 (27) | 615 (38) | 0.001 |
| Metabolic syndrome, n (%) | 101 (44.1) | 899 (57) | <0.001 |
| Psychiatric medication, n (%) | 156 (58) | 1075 (55) | 0.366 |
| CVD, n (%) | 13 (5) | 193 (10) | 0.003 |
| Glucose (mg/dL) | 103.9±38 | 111±44 | 0.004 |
| Albumin (g/dL) | 4.09±0.4 | 4.12±0.4 | 0.247 |
| Platelet count (×10−3) | 289±71 | 285±71 | 0.447 |
| Leukocytes count (×10−3) | 7.9±2.2 | 8±3.2 | 0.988 |
| Bilirrubin (mg/dL) | 0.54±0.3 | 0.55±0.2 | 0.447 |
| ALT, (U/L) | 34±20 | 32±20 | 0.108 |
| AST, (U/L) | 24±12 | 25±14 | 0.506 |
| Alkaline phosphatase (U/L) | 77±20 | 79±24 | 0.811 |
| AUDIT score medial (IQR) | 2 (0–2) | 5 (4–5) | <0.001 |
Note: Data are presented as mean±SD, median (quartiles), or frequencies (%).
Other surgery procedures include sleeve gastrectomy (n=55) and biliopancreatic diversion-duodenal switch (n=17). Psychiatric medication includes treatment for depression and emotional problems.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; AUDIT, Alcohol Disorder Identification Test; BMI, body mass index; CKD, chronic kidney disease; CVD, cardiovascular disease; LAGB, laparoscopic adjustable gastric band; MAP, RYGB, Roux-en-Y gastric bypass.
Patients with BD were more frequently male (OR: 1.39 [95% CI: 1.05–1.86]), younger (OR: 0.95 [95% CI: 0.94–0.96]), smokers (OR: 2.42 [95% CI: 1.49–3.93]), and consumed other drugs (OR: 2.65 [95% CI: 1.74–4.04]). Participants without BD showed higher rates of metabolic syndrome (OR:1.40 [95% CI: 1.08–1.82]), arterial hypertension (OR:1.79 [95% CI: 1.39–2.33]), diabetes (OR:1.83 [95% CI:1.37–2.46]), dyslipidemia (OR:1.49 [95% CI:1.11–2.00]), CVD (OR: 2.15 [95% CI: 1.21–3.82]), and sleep apnea (OR: 1.43 [95% CI: 1.11–1.85]). No differences were observed between groups regarding anthropometric measurements and liver tests.
Regarding sociodemographic characteristics, individuals with a high education level who are single and currently employed were more likely to belong to the BD group (Table 1). Additionally, patients with BD had a higher prevalence of binge eating (OR: 1.35 [95% CI: 1.04–1.76]) and more frequent episodes of eating fast food per week (OR: 1.41 [95% CI: 1.07–1.85]). BD was associated with several eating behaviors, including eating when not hungry, when uncomfortably full, or working late night shifts that disrupt regular meals (Table 2).
TABLE 2.
Eating behaviors and disorders of those with binge drinking versus no binge drinking
| Eating behaviors | Binge drinking (n=272) | No binge drinking (n=1985) | p |
|---|---|---|---|
| Eat breakfast, lunch, and dinner regularly (d/wk), median (IQR) | 6 (5-7) | 6 (5–7) | 0.186 |
| Eat breakfast, lunch, and dinner regularly (6–7/wk), n (%) | 99 (37) | 826 (42) | 0.101 |
| Fast food meals/wk, median (IQR) | 2 (1–4) | 2 (0–4) | 0.008 |
| Eat fast food regularly (≥4/wk), n (%) | 87 (32) | 490 (25) | 0.015 |
| Binge eating, n (%) | 98 (36) | 586 (29) | 0.026 |
| Meals/snacks per day, n (%) | — | — | 0.110 |
| 1–4 | 100 (37) | 835 (43) | — |
| 5–6 | 136 (51) | 850 (44) | — |
| ≥7 | 32 (12) | 245 (13) | — |
| Eat in restaurant regularly (6–7/wk), n (%) | 40 (15) | 240 (13) | 0.297 |
| Restaurant meals/week, median (IQR) | 2 (1–4) | 2 (0–4) | 0.117 |
| Frequency of eating large amounts, n (%) | — | — | 0.965 |
| Less than once/week | 68 (48) | 460 (48) | — |
| Once/week | 29 (21) | 185 (20) | — |
| More than once/week | 37 (26) | 269 (28) | — |
| Nearly every day | 7 (5) | 41 (4) | — |
| Frequency of eating when not hungry, n (%) | — | — | 0.023 |
| Rarely | 43 (16) | 456 (23) | — |
| Occasionally | 98 (36) | 720 (37) | — |
| Frequently | 94 (35) | 584 (30) | — |
| Nearly every day | 36 (13) | 204 (10) | — |
| Frequency of eating when uncomfortably full, n (%) | — | — | 0.001 |
| Rarely | 60 (22) | 650 (33) | — |
| Occasionally | 114 (42) | 758 (39) | — |
| Frequently | 78 (29) | 422 (21) | — |
| Nearly every day | 19 (7) | 144 (7) | — |
| Night eating syndrome, n (%) | 43 (16) | 282 (14) | 0.520 |
| Working late nights interfere with meals, n (%) | 67 (25) | 380 (19) | 0.036 |
Note: Data are presented as median (quartiles) or frequencies (%).
Pattern of alcohol consumption during follow-up
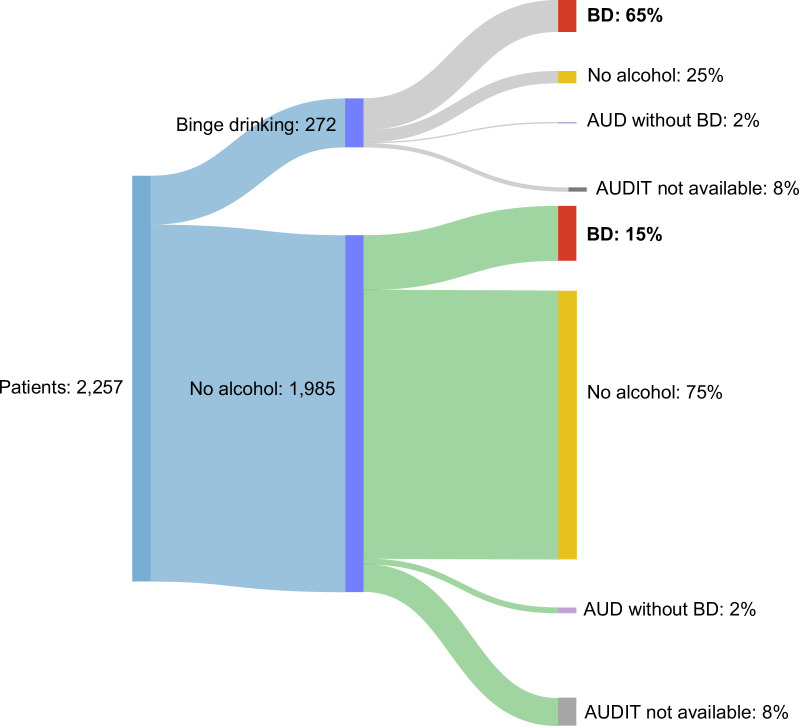
Up to 2079 (92%) patients had at least 1 AUDIT questionnaire completed during follow-up, of whom n=46 died. The prevalence of BD after surgery was 23% (n=482), twice as much as before surgery. Among patients with BD at the time of surgery, 71% (n=177) continued BD, and 1.6% (n=4) reported chronic daily alcohol consumption (AUD without BD) during follow-up. Within participants without alcohol consumption at the time of surgery, 17% (n=305) reported BD and 1.8% (n=32) AUD during follow-up (Figure 2). BD at the time of surgery was associated with a higher risk of BD after surgery (OR=10.49 [95% CI: 7.86–14.00]).
FIGURE 2.
Sankey diagram of a pattern of alcohol consumption at the time of surgery and during follow-up in all the patients included. Data are presented as numbers and proportions (%). Abbreviations: AUD, alcohol use disorder; AUDIT, alcohol disorder identification test; BD, binge drinking.
Histological evaluation
A total of 271 patients underwent liver biopsy during the bariatric procedure. Of these, liver appearance was normal in 53% (n=143), and the leading indication for liver biopsy was per study protocol (64%). Tissue samples were mainly obtained from the left lobe (80%) and had a median biopsy length of 13 (9–17) mm. Overall, steatotic droplets predominantly located in zone 3 (66%), 31% (n=84) showed features of steatohepatitis, and 48% (n=131) had any stage of fibrosis. The prevalence of patients with BD with histological information was 12% (n=33). Histological differences between patients with and without BD are summarized in Supplemental Table S2, http://links.lww.com/HC9/A997. Both groups shared similar histological features except for iron deposits (Supplemental Figure S1, http://links.lww.com/HC9/A997). Perls staining was performed in 184 patients (68%). BD had a higher iron overload (OR: 3.00 [95% CI: 1.25–7.21]), mainly due to mesenchymal deposits in the sinusoidal cells.
Survival analysis
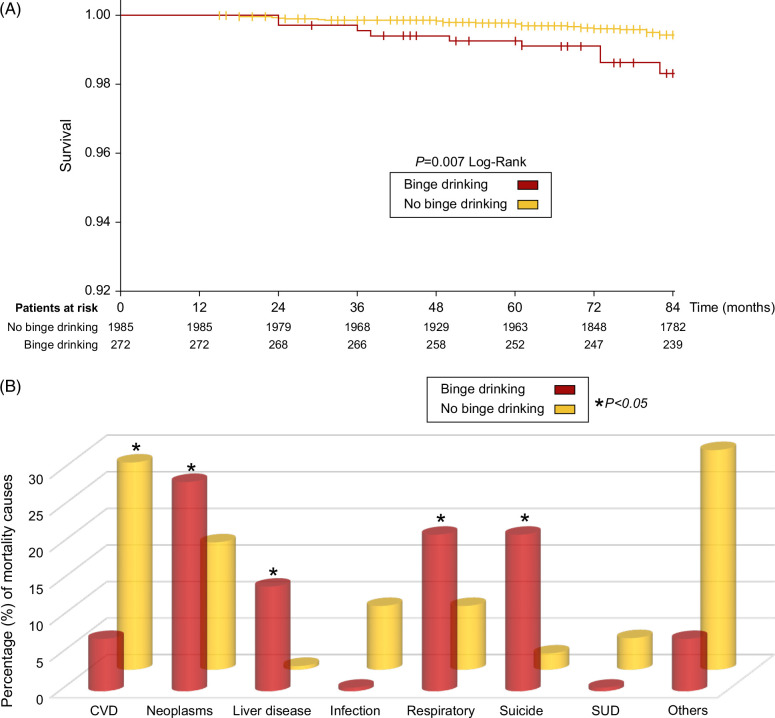
Median follow-up after bariatric surgery was 79 months (71–84). During this study period, 60 patients died, mainly due to CVD (23%) and neoplasms (19%) (Supplemental Table S2, http://links.lww.com/HC9/A997). Mortality was seen primarily in participants with Roux-en-Y gastric bypass (82%). BD at the time of surgery was associated with higher long-term mortality (5.1% vs. 2.3%, p=0.007; Figure 3A).
FIGURE 3.
(A) Kaplan-Meier plot showing the overall cumulative probability of survival in patients with binge drinking versus no binge drinking patients. The probability of survival was calculated according to KM survival analysis and compared by Log-rank. (B) Causes of mortality in patients with binge drinking versus no binge drinking. Patients with no binge drinking had a higher prevalence of mortality due to cardiovascular disease (p=0.030). Patients with binge drinking had a higher prevalence of mortality due to liver disease (p=0.020), suicide (p=0.015), neoplasms (p=0.034), and respiratory (p=0.025). Abbreviations: CVD, cardiovascular disease; SUD, substances use disorders.
The cause of death differed substantially between patients with and without BD (Figure 3B). The most common causes of death in the BD group were neoplasms (29%), suicide (21%), respiratory (21%), and liver disease (14%). Suicide prevalence in BD group was 1.1% (n=3/272), significantly higher compared to previously described rates of 0.3% in meta-analysis of bariatric surgery cohorts27 and 0.02% in the US general population, with the same age, sex, race distribution, and time period (Supplemental Figure S2, http://links.lww.com/HC9/A997).28 Participants with BD at the time of surgery had significantly more deaths due to suicide (p=0.015), liver disease (p=0.020), neoplasms (p=0.034), and respiratory (p=0.025). Supplemental Table S3, http://links.lww.com/HC9/A997, shows the subdistribution HR for the main causes of mortality.
Predictors of mortality and sensitivity analysis
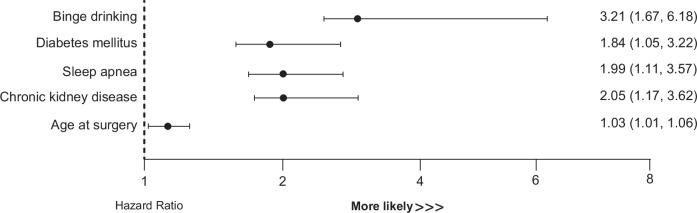
Univariate and multivariate analysis of the baseline factors predicting mortality at the time of surgery is shown in Table 3. Final adjusted multivariate model included the following variables: BD (HR: 3.21 [95% CI: 1.67–6.18]), age (HR: 1.03 [95% CI: 1.01–1.06]), diabetes (HR: 1.84 [95% CI: 1.05–3.22]), chronic kidney disease (HR: 2.05[95% CI: 1.17–3.62]), and sleep apnea (HR: 1.99 [95% CI: 1.11–3.57]) (Figure 4). All of these baseline factors were independently associated with higher long-term mortality after bariatric surgery, with BD showing the highest HR (Supplemental Figure S3, http://links.lww.com/HC9/A997). The multivariate Cox model obtained a C-index of 0.759 for mortality risk prediction. After internal validation, the model showed robustness in the results with a C-index of 0.734 and a slope of 0.823.
TABLE 3.
Baseline predictors of mortality after bariatric surgery
| Characteristics | Univariate analysis | p | Multivariate analysis | p |
|---|---|---|---|---|
| Sex (female) | 1.46 (0.83–2.55) | 0.191 | — | — |
| Surgery (RYGB) | 1.77 (0.92–3.41) | 0.080 | — | — |
| Education level (low) | 1.74 (1.01–2.99) | 0.051 | — | — |
| FIB-4 | 1.09 (0.99–1.21) | 0.082 | 1.04 (0.87–1.25) | 0.631 |
| Age at surgery | 1.06 (1.03–1.08) | <0.001 | 1.03 (1.01–1.06) | 0.019 |
| BMI (kg/m2) | 1.03 (0.99–1.06) | 0.110 | — | — |
| Binge drinking | 2.24 (1.23–4.07) | 0.001 | 3.21 (1.67–6.18) | 0.001 |
| AUD | 1.71 (0.94–3.12) | 0.082 | 0.99 (0.13–7.76) | 0.991 |
| Smoking | 1.5 (1.05–1.77) | 0.024 | 1.53 (0.90–2.60) | 0.111 |
| Ilicit drugs | 1.24 (0.45–3.41) | 0.682 | 1.20 (0.41–3.45) | 0.736 |
| Sleep apnea | 2.78 (1.58–4.87) | <0.001 | 1.99 (1.11–3.57) | 0.021 |
| CKD | 2.92 (1.72–4.96) | <0.001 | 2.05 (1.17–3.62) | 0.014 |
| Diabetes mellitus | 2.81 (1.68–4.71) | <0.001 | 1.84 (1.05–3.22) | 0.033 |
| Arterial hypertension | 1.49 (0.80–2.75) | 0.211 | — | — |
| Dyslipidemia | 2.77 (1.35–5.68) | 0.010 | — | — |
| Metabolic syndrome | 2.49 (1.33–4.66) | 0.011 | — | — |
| CVD | 2.29 (1.20–4.42) | 0.012 | — | — |
Note: Data are presented as HR and CI 95%.
Internal validation after bootstrap (500 samples); C-index 0.734; slope 0.823.
Abbreviations: AUD, alcohol use disorder; BMI, body mass index; CKD, chronic kidney disease; CVD, cardiovascular disease; FIB-4, fibrosis score 4; RYGB, Roux-en-Y gastric bypass.
FIGURE 4.
Predictive factors of mortality after bariatric surgery. The final adjusted multivariate Cox model identified the significant main factors at the time of surgery that were independent predictors of mortality. All the results are expressed as an HR with a 95% CI. The multivariate Cox model, after internal validation, showed robustness of the results with a C-index of 0.734 and a slope of 0.823.
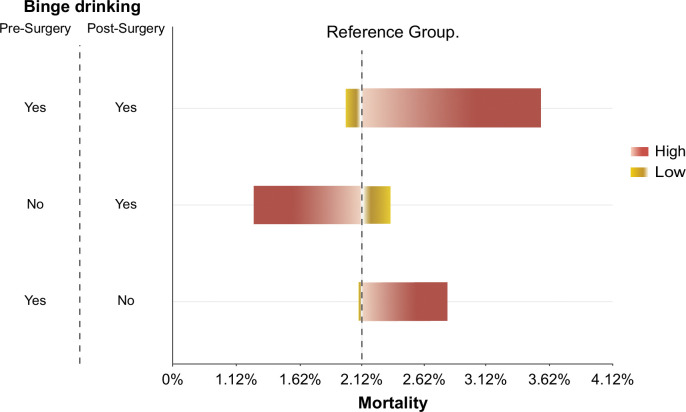
The Cox-time–depending analysis included patients with available information during follow-up (n=2,079). BD before and after surgery was independently associated with mortality (HR=2.71 [95% CI: 1.24–5.92]) with a C-index of 0.616 and a slope of 0.962 (Supplemental Table S4, http://links.lww.com/HC9/A997). There was a total of 7 deaths among patients with BD before and after surgery, n=2 due to liver disease. The sensitivity analysis illustrated with the tornado plot shows how BD before and after surgery produces the largest range of mortality (Figure 5).
FIGURE 5.
Sensitivity analysis of mortality. The sensitivity analysis has been performed using logistic regression analysis and is illustrated with a tornado plot. The specific pattern of binge drinking at baseline and follow-up on the y-axis and the range of the outcome mortality on the x-axis. The absence of binge drinking at baseline and during follow-up was taken as the reference group represented by the dashed line. In the tornado chart, each bar represents the range of the outcome. Binge drinking before and after surgery produces the largest range of mortality, and it is at the top of the graphic. The other 2 bars become smaller toward the bottom of the chart. As for the colors of the bars, the red color means the result value was produced by the upper limit (high), and the yellow light color means the result value was produced by the lower limit (low).
In the subpopulation with liver biopsy, histological features were analyzed as predictors of mortality (Supplemental Table S5, http://links.lww.com/HC9/A997). In the multivariable model, adjusted by age, sex, and surgical procedure, the following histological features were retained as independent predictors of mortality: fibrosis (HR: 3.76 [95% CI: 13.63–1.038]), portal inflammation (HR: 5.06 [95% CI: 15.35–1.67]), and mesenchymal iron overload (HR: 4.72 [95% CI: 22.23–1.00]).
DISCUSSION
The present study reveals a high prevalence and clinical impact of BD in subjects undergoing bariatric surgery. BD occurs at the time of surgery in 12%, particularly in young and single males with a high education level, consumers of cigarettes and other drugs, and with binge eating disorders. Importantly, BD prevalence increases up to 23% after surgery. Patients with BD have a higher risk of long-term mortality after surgery.
Despite the well-known beneficial effects of bariatric surgery, an increased risk of AUD after surgery has been described.2,4 Clinicians are usually unaware of this risk, probably due to under-reported alcohol consumption. Alcohol is the most frequently consumed substance among young adults, including those with severe obesity.29 Furthermore, when young adults consume alcohol, they typically drink more per occasion than adults (ie, BD), and 16% also consume other drugs.30,31 Alcohol use, and BD in particular, are primary contributors to the leading cause of death and/or unintentional injury (including attempting suicide) in young adults.32 Among patients with severe obesity, both binge behaviors (drinking and eating) frequently co-exist. Actually, it has been postulated that binge eating disorder is a precipitating factor in the onset and aggravation of excessive alcohol consumption. On the other hand, alcohol consumption can also lead to unhealthy and uncontrollable eating behavior.33 Given these behavioral similarities, we considered it clinically relevant to evaluate BD’s prevalence before bariatric surgery and recognize its impact during long-term follow-up.
The study population was obtained from the LABS-2 database. In this cohort, the prevalence of AUD at baseline was previously reported at 7%, which is almost half the prevalence of BD at the time of surgery in our study (12%).2,4 Different percentages reflect that BD is a bigger problem in young adults undergoing bariatric surgery, and special attention should be paid to identify and treat them. Our results align with other studies of bariatric cohorts reporting a rate of 14% BD prior to surgery.34 In addition, risk factors associated with BD were young males, high education level, single, currently employed, smoking, and consumption of other drugs. Other studies share these risk factors, identifying patients in whom a careful evaluation should be performed and, if appropriate, referred for substance use disorder treatment.34 Interestingly, these risk factors associated with presurgery BD are shared by those reported to increase the incidence of postsurgery AUD symptoms.2 Contrary to low education level and unfavorable socioeconomic position, which has been previously associated with NAFLD, high educational level and employment were associated with BD.16
Recently, a relationship between addictive behaviors, such as impulsive alcohol and food consumption, has been described in patients undergoing bariatric surgery.35 These addictive behaviors arise in individuals with specific psychological, cognitive, and social characteristics that make them more vulnerable to developing binge episodes. The LABS-2 cohort has a substantial proportion of patients with problematic eating behaviors, especially binge eating. Higher odds of having a binge eating disorder are related to college degree, AUD symptoms, taking psychiatric medications, and depressive symptoms.19 The relationship between alcohol and eating can be partially explained by our results, which show that BD, rather than AUD, is the condition behind the binge behavioral disorders association. This hypothesis is supported by previous studies showing that binge eating predicts incident alcohol use after bariatric surgery.36 In our study, BD was also associated with other eating behaviors and disorders, including eating fast food. Both impulsive alcohol and food consumption have been associated with depressive symptomatology, anxiety, substance abuse, and suicide attempts.37 On the other hand, patients without BD had higher rates of metabolic syndrome, arterial hypertension, diabetes, dyslipidemia, sleep apnea, and CVD, all of which are common metabolic risk factors.38
In the present study, we also found that 12% of patients with liver biopsy performed during surgery also had BD disorder. Iron overload can be present in both NAFLD and alcohol-associated liver disease.39 Patients with BD had a higher iron overload, mainly due to mesenchymal deposits in the sinusoidal cells. This association can be related to several mechanisms: (1) increased iron absorption and dysregulation of iron-related proteins mediated by alcohol consumption40; (2) ethanol exposure promoting iron absorption by downregulating hepcidin expression, and iron uptake by upregulating the expression of transferrin receptor in hepatocytes; (3) ethanol upregulating iron-dependent cell death namely ferroptosis40; (4) systemic inflammation in response to BD.41
In the LABS-2 cohort, it was previously shown that AUD was not independently associated with postoperative mortality.4,11 Despite these previous results, we evaluated mortality and BD. Strikingly, among the 60 reported deaths, 23% had BD. The presence of BD at the time of surgery was associated with a higher risk of BD during follow-up and increased long-term mortality (Figure 5). Other factors independently associated with mortality were age, diabetes, chronic kidney disease, and sleep apnea. These metabolic factors have been previously described, but BD emerges as a novel predictor of mortality.11,42 The leading causes of death in patients with BD were neoplasms, respiratory and liver disease, and suicide. The distribution of cause-specific death in these subjects is very similar to that in patients with alcohol-associated liver disease.43 Although we are aware of the low number of deaths in our cohort, they allow us to suggest that the pattern of consumption and the effect of surgery on alcohol metabolism influence the degree of liver damage5 and the existence of psychiatric comorbidity can increase the risk of suicide.44 A notable finding is the significantly higher prevalence of suicide in patients with BD compared to other US series of bariatric patients27 and the general population. These results can be explained from a psychopathological point of view since patients with binge behaviors (BD and binge eating) have predisposition to act impulsively, with an increase in the likelihood of asocial behaviors, intentional injuries, and suicide attempts.45,46 In line with other series, CVD was the leading cause of mortality in patients without BD.47 Our study implies the need to increase awareness and an adequate evaluation of mental health disorders related to alcohol in the setting of bariatric procedures before and after surgery.
Recent studies indicate that BD is associated with a rapid passage of pathogen-associated molecular patterns such as lipopolysaccharide to the circulation, leading to systemic, brain, and hepatic inflammation.41,48 The fact that bariatric surgery increases intestinal permeability and favors bacterial overgrowth49 could explain the increased susceptibility to the deleterious effects of BD among these patients.
The study has several limitations. Due to its design, there is limited capacity to verify the data quality included by all the participating centers. Although many variables were considered in the models assessing presurgery factors related to postoperative mortality, it is possible that some were missed or not always available in the clinical database. Although the availability of liver biopsies was limited and subject to the surgeon’s decision during bariatric surgery, the number of samples analyzed allows for inferring the probable pathophysiological mechanisms involved in the liver damage mediated by the pattern of alcohol consumption. Another limitation is that BD can be under-reported and under-recognized, particularly in patients being treated for medical conditions unrelated to alcohol use; however, the systematical assessment of alcohol consumption with AUDIT can help to minimize this limitation. Likewise, the type of alcohol consumed by the patients was not recorded to study the effects of different types of alcohol, which is an aspect to be clarified in future studies. Finally, the number of deaths was small and underpower for some specific causes.
In conclusion, this study shows a high prevalence of BD in patients undergoing bariatric procedures, which increases mortality risk after surgery due to suicide and liver disease. Given that BD is associated with binge eating, further studies should examine the role of psychological and pharmacological interventions and whether presurgery treatment of alcohol use and eating disorders can improve long-term outcomes.
Supplementary Material
Acknowledgments
AUTHOR CONTRIBUTIONS
Edilmar Alvarado-Tapias, David Martí-Aguado, Josepmaria Argemi, and Ramon Bataller: study concept and design. Edilmar Alvarado-Tapias, David Martí-Aguado, Concepción Gómez-Medina, Carlos Fernández-Carrillo, Meritxell Ventura-Cots, Ana Clemente, Rubén Osuna-Gómez, Clara Alfaro-Cervelló, Joaquin Cabezas, Justyna Szafranska, Albert Guinart-Cuadra, and Anna Brujats: data acquisition. Edilmar Alvarado-Tapias, David Martí-Aguado, Josepmaria Argemi, and Ramon Bataller: analysis and interpretation of data. Andreu Ferrero-Gregori: statistical analysis. Edilmar Alvarado-Tapias and David Martí-Aguado: drafting of the manuscript. Edilmar Alvarado-Tapias, David Martí-Aguado, Concepción Gómez-Medina, Andreu Ferrero-Gregori, Justyna Szafranska, Anna Brujats, Rubén Osuna-Gómez, Albert Guinart-Cuadra, Clara Alfaro-Cervelló, Elisa Pose, Meritxell Ventura-Cots, Ana Clemente, Cynthia Contreras, Joaquin Cabezas, Hugo López-Pelayo, JuanPablo Arab, Josepmaria Argemi, and Ramon Bataller: critical manuscript revision. All authors approved the final version of the article: Edilmar Alvarado-Tapias, David Martí-Aguado, Concepción Gómez-Medina, Andreu Ferrero-Gregori, Justyna Szafranska, Anna Brujats, Rubén Osuna-Gómez, Albert Guinart-Cuadra, Clara Alfaro-Cervelló, Elisa Pose, Meritxell Ventura-Cots, Ana Clemente, Cynthia Contreras, Joaquin Cabezas, Hugo López-Pelayo, JuanPablo Arab, Josepmaria Argemi, and Ramon Bataller.
FUNDING INFORMATION
LABS-2 was funded by a cooperative agreement by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Grant numbers: Data Coordinating Center -U01 DK066557; Columbia-Presbyterian—U01-DK66667 (in collaboration with Cornell University Medical Center CTSC, Grant UL1-RR024996); University of Washington—U01-DK66568 (in collaboration with CTRC, Grant M01RR-00037); Neuropsychiatric Research Institute—U01-DK66471; East Carolina University—U01-DK66526; University of Pittsburgh Medical Center—U01 DK66585 (in collaboration with CTRC, Grant UL1-RR024153); Oregon Health & Science University—U01-DK66555.
CONFLICTS OF INTEREST
Ramon Bataller received lectures fee from Abbvie and Gilead. Hugo López-Pelayo received funds for training from Lundbeck and for elaborating training materials from Advanz Pharma. Joaquin Cabezas consults, advises, is on the speakers’ bureau, and received grants from Gilead. He is on the speakers’ bureau and received grants from Abbvie. Ramon Bataller is a recipient of NIAAA grants U01AA021908, U01AA020, and NIDDK P30DK120531821. Meritxell Ventura-Cots, Carlos Fernández-Carrillo, Edilmar Alvarado-Tapias, and Ana Clemente are recipients of a scholarship grant for study extension abroad, sponsored by the Spanish Association for the Study of the Liver (AEEH). Meritxell Ventura-Cots is recipient of a Joan Rodes award from the ISCII (JR19/00015) and the PI22/01770 grant from the ISCIII—Fondos Feder. Edilmar Alvarado-Tapias is a recipient of a Joan Rodes award from the ISCII (JR20/00047) and the PI21/01995 grant from the ISCIII- Fondos Feder. David Martí-Aguado is a recipient of the Joan Rodes award ISCIII (JR22/00002) and a scholarship grant for study extension abroad, sponsored by the University of Valencia (UV-RI_MID-1528578). Elisa Pose is a recipient of the PI22/00910 grant from the ISCIII—Fondos Feder. Josepmaria Argemi is a recipient of the PI20/01663 grant from the ISCIII- Fondos Feder and an award from the “Fundación Echebano” (Pamplona, Spain). Hugo López-Pelayo is a recipient of the PI20/00760 grant from the ISCIII—Fondos Feder, 2020I004 grant from Plan Nacional sobre Drogas, and 101045870 from DG Justice-European Commission. The remaining authors have no conflicts to report.
Footnotes
Ramon Bataller and Josepmaria Argemi share senior authorship.
Edilmar Alvarado-Tapias and David Martí-Aguado contributed equally to this work.
Abbreviations: AUD, alcohol use disorder; AUDIT, Alcohol Use Disorders Identification Test; BD, binge drinking; CVD, cardiovascular disease; LABS-2, Longitudinal Assessment of Bariatric Surgery-2; NIDDK, National Institute of Diabetes and Digestive and Kidney Diseases; WHO, World Health Organization.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.hepcommjournal.com.
Contributor Information
Edilmar Alvarado-Tapias, Email: ealvaradot@santpau.cat.
David Martí-Aguado, Email: david_marti_aguado@hotmail.com.
Concepción Gómez-Medina, Email: conchin.gome@gmail.com.
Andreu Ferrero-Gregori, Email: andreu.ferrero.gregori@gmail.com.
Justyna Szafranska, Email: jszafranska@santpau.cat.
Anna Brujats, Email: abrujats@santpau.cat.
Rubén Osuna-Gómez, Email: rosuna@santpau.cat.
Albert Guinart-Cuadra, Email: aguinart@santpau.cat.
Clara Alfaro-Cervelló, Email: clara.alfaro@uv.es.
Elisa Pose, Email: EPOSE@clinic.cat.
Meritxell Ventura-Cots, Email: txell.ventura.cots@gmail.com.
Cynthia Contreras, Email: cynthiap16@gmail.com.
Joaquin Cabezas, Email: joaquin.cabezas@scsalud.es.
Hugo López-Pelayo, Email: hlopez@clinic.cat.
JuanPablo Arab, Email: jparab@gmail.com.
Josepmaria Argemi, Email: jargemi@unav.es.
Ramon Bataller, Email: bataller@clinic.cat.
REFERENCES
- 1.Adams TD, Davidson LE, Litwin SE, Kim J, Kolotkin RL, Nanjee MN, et al. Weight and metabolic outcomes 12 years after gastric bypass. N Engl J Med. 2017;377:1143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King WC, Chen J-Y, Mitchell JE, Kalarchian MA, Steffen KJ, Engel SG, et al. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA. 2012;307:2516–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mellinger JL, Shedden K, Winder GS, Fernandez AC, Lee BP, Waljee J, et al. Bariatric surgery and the risk of alcohol-related cirrhosis and alcohol misuse. Liver International. 2021;41:1012–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King WC, Chen JY, Courcoulas AP, Dakin GF, Engel SG, Flum DR, et al. Alcohol and other substance use after bariatric surgery: Prospective evidence from a U.S. multicenter cohort study. Surg Obes Relat Dis. 2017;13:1392–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvarado-Tapias E, Marti-Aguado D, Kennedy K, Fernández-Carrillo C, Ventura-Cots M, Morales-Arraez D, et al. Bariatric surgery is associated with alcohol-related liver disease and psychiatric disorders associated with AUD. Obes Surg. 2023;33:1494–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sirohi S, Richardson BD, Lugo JM, Rossi DJ, Davis JF. Impact of Roux-en-Y gastric bypass surgery on appetite, alcohol intake behaviors, and midbrain ghrelin signaling in the rat. Obesity. 2017;25:1228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orellana ER, Covasa M, Hajnal A. Neuro-hormonal mechanisms underlying changes in reward related behaviors following weight loss surgery: Potential pharmacological targets. Biochem Pharmacol. 2019;164:106–14. [DOI] [PubMed] [Google Scholar]
- 8.Carbia C, López-Caneda E, Corral M, Cadaveira F. A systematic review of neuropsychological studies involving young binge drinkers. Neurosci Biobehav Rev. 2018;90:332–49. [DOI] [PubMed] [Google Scholar]
- 9.Ivezaj V, Wiedemann AA, Grilo CM. Food addiction and bariatric surgery: A systematic review of the literature. Obes Rev. 2017;18:1386–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasin DS, O’Brien CP, Auriacombe M, Borges G, Bucholz K, Budney A, et al. DSM-5 criteria for substance use disorders: Recommendations and rationale. Am J Psychiatry. 2013;170:834–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.White GE, Courcoulas AP, King WC, Flum DR, Yanovski SZ, Pomp A, et al. Mortality after bariatric surgery: Findings from a 7-year multicenter cohort study. Surg Obes Relat Dis. 2019;15:1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jophlin LL, Singal AK, Bataller R, Wong RJ, Sauer BG, Terrault NA, et al. ACG Clinical Guideline: Alcohol-Associated Liver Disease. Am J Gastroenterol. 2024;119:30–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abuse S. Key substance use and mental health indicators in the United States: results from the 2019 National Survey on Drug Use and Health. Substance Abuse and Mental Health Services Administration. (2020). Key substance use and mental health indicators in the United States: Results from the 2019 National Survey on Drug Use and Health (HHS Publication No. PEP20-07-01-001, NSDUH Series H-55).
- 14.Åberg F, Helenius-Hietala J, Puukka P, Jula A. Binge drinking and the risk of liver events: A population-based cohort study. Liver Int. 2017;37:1373–81. [DOI] [PubMed] [Google Scholar]
- 15.U.S. Department of Health and Human Services . Longitudinal assessment of bariatric surgery (LABS). NIH Publication No 04-5573. 2010;22:1–6. [Google Scholar]
- 16.Ren Z, Bosma H, Wesselius A, Eussen SJPM, Kooi ME, van der Kallen CJH, et al. Traditional lifestyle factors partly mediate the association of socioeconomic position with intrahepatic lipid content: The maastricht study. JHEP Reports. 2023;5:100855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dwyer-Lindgren L, Bertozzi-Villa A, Stubbs RW, Morozoff C, Kutz MJ, Huynh C, et al. US county-level trends in mortality rates for major causes of death, 1980-2014. JAMA. 2016;316:2385–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ventura-Cots M, Watts AE, Bataller R. Binge drinking as a risk factor for advanced alcoholic liver disease. Liver Int. 2017;37:1281–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell JE, King WC, Courcoulas A, Dakin G, Elder K, Engel S, et al. Eating behavior and eating disorders in adults before bariatric surgery. Int J Eat Disord. 2015;48:215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleiner DE, Berk PD, Hsu JY, Courcoulas AP, Flum D, Khandelwal S, et al. Hepatic pathology among patients without known liver disease undergoing bariatric surgery: Observations and a perspective from the longitudinal assessment of bariatric surgery (LABS) study. Semin Liver Dis. 2014;34:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. [DOI] [PubMed] [Google Scholar]
- 22.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–9. [DOI] [PubMed] [Google Scholar]
- 23.Deugnier YM, Turlin B, Powell LW, Summers KM, Moirand R, Fletcher L, et al. Differentiation between heterozygotes and homozygotes in genetic hemochromatosis by means of a histological hepatic iron index: A study of 192 cases. Hepatology. 1993;17:30–4. [PubMed] [Google Scholar]
- 24.Kleiner DE, Brunt EM, Wilson LA, Behling C, Guy C, Contos M, et al. Association of histologic disease activity with progression of nonalcoholic fatty liver disease. JAMA Netw Open. 2019;2:e1912565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Núñez E, Steyerberg EW, Núñez J. Regression modeling strategies. Rev Esp Cardiol. 2011;64:501–7. [DOI] [PubMed] [Google Scholar]
- 26.Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. Springer; 2001. [Google Scholar]
- 27.Lim RBC, Zhang MWB, Ho RCM. Prevalence of all-cause mortality and suicide among bariatric surgery cohorts: A meta-analysis. Int J Environ Res Public Health. 2018;15:1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kochanek KD, Xu J, Murphy SL, Miniño AM, Kung HC. Deaths: Final data for 2009. Natl Vital Stat Rep. 2011;60:1–116. [PubMed] [Google Scholar]
- 29.Zeller MH, Washington GA, Mitchell JE, Sarwer DB, Reiter-Purtill J, Jenkins TM, et al. Alcohol use risk in adolescents 2 years after bariatric surgery. Surg Obes Relat Dis. 2017;13:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lukács A, Szabó A, Horváth E, Máté Z, Erdos C, Molnár R, et al. Students in danger: Binge drinking behaviour and associated factors in Hungary. Zdr Varst. 2021;60:244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patrick ME, Schulenberg JE. Prevalence and predictors of adolescent alcohol use and binge drinking in the United States. Alcohol Res. 2013;35:193–200. [PMC free article] [PubMed] [Google Scholar]
- 32.Patrick ME, Schulenberg JE, Martz ME, Maggs JL, O’Malley PM, Johnston LD. Extreme binge drinking among 12th-grade students in the United States: Prevalence and predictors. JAMA Pediatr. 2013;167:1019–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Escrivá-Martínez T, Herrero R, Molinari G, Rodríguez-Arias M, Verdejo-García A, Baños RM. Binge eating and binge drinking: A two-way road? An integrative review. Curr Pharm Des. 2020;26:2402–15. [DOI] [PubMed] [Google Scholar]
- 34.Spadola CE, Wagner EF, Accornero VH, Vidot DC, de la Cruz-Munoz N, Messiah SE. Alcohol use patterns and alcohol use disorders among young adult, ethnically diverse bariatric surgery patients. Subst Abus. 2017;38:82–7. [DOI] [PubMed] [Google Scholar]
- 35.Azevedo LDS, de Souza APL, Ferreira IMS, Lima DW da C, Pessa RP. Binge eating and alcohol consumption: An integrative review. Eat Weight Disord. 2021;26:759–769. [DOI] [PubMed] [Google Scholar]
- 36.Miller-Matero LR, Hamann A, LaLonde L, Martens KM, Son J, Clark-Sienkiewicz S, et al. Predictors of alcohol use after bariatric surgery. J Clin Psychol Med Settings. 2021;28:596–602. [DOI] [PubMed] [Google Scholar]
- 37.Forsén Mantilla E, Clinton D, Monell E, Levallius J, Birgegård A. Impulsivity and compulsivity as parallel mediators of emotion dysregulation in eating-related addictive-like behaviors, alcohol use, and compulsive exercise. Brain Behav. 2022;12:e2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mechanick JI, Apovian C, Brethauer S, Garvey WT, Joffe AM, Kim J, et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures—2019 update: Cosponsored by american association of clinical endocrinologists/american college of endocrinology. Endocr Pract. 2019;25:1346–59. [DOI] [PubMed] [Google Scholar]
- 39.Jesus RN, Callejas GH, Concon MM, Braga JGR, Marques RA, Chaim FDM, et al. Prevalence and factors associated with hepatic iron overload in obese individuals undergoing bariatric surgery: A cross-sectional study. Obes Surg. 2020;30:4967–73. [DOI] [PubMed] [Google Scholar]
- 40.Li LX, Guo FF, Liu H, Zeng T. Iron overload in alcoholic liver disease: Underlying mechanisms, detrimental effects, and potential therapeutic targets. Cell Mol Life Sci. 2022;79:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stankevic E, Israelsen M, Juel HB, Madsen AL, Ängquist L, Aldiss PSJ, et al. Binge drinking episode causes acute, specific alterations in systemic and hepatic inflammation-related markers. Liver Int. 2023;43:2680–91. [DOI] [PubMed] [Google Scholar]
- 42.Inaba CS, Koh CY, Sujatha-Bhaskar S, Silva JP, Chen Y, Nguyen DV, et al. One-year mortality after contemporary laparoscopic bariatric surgery: An analysis of the bariatric outcomes longitudinal database. J Am Coll Surg. 2018;226:1166–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kann AE, Jepsen P, Madsen LG, West J, Askgaard G. Cause-specific mortality in patients with alcohol-related liver disease in Denmark: A population-based study. Lancet Gastroenterol Hepatol. 2023;8:1028–34. [DOI] [PubMed] [Google Scholar]
- 44.Müller A, Hase C, Pommnitz M, de Zwaan M. Depression and suicide after bariatric surgery. Curr Psychiatry Rep. 2019;21:1–6. [DOI] [PubMed] [Google Scholar]
- 45.Hudson JI, Hiripi E, Pope HG, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61:348–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lanfredi M, Macis A, Ferrari C, Meloni S, Pedrini L, Ridolfi ME, et al. Maladaptive behaviours in adolescence and their associations with personality traits, emotion dysregulation and other clinical features in a sample of Italian students: A cross-sectional study. Borderline Personal Disord Emot Dysregul. 2021;8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee H, Lee Y ho, Kim SU, Kim HC. Metabolic dysfunction-associated fatty liver disease and incident cardiovascular disease risk: A nationwide cohort study. Clin Gastroenterol Hepatol. 2021;19:2138–47.e10. [DOI] [PubMed] [Google Scholar]
- 48.Bala S, Marcos M, Gattu A, Catalano D, Szabo G. Acute binge drinking increases serum endotoxin and bacterial DNA levels in healthy. Plos One. 2014;14:e96864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koutoukidis DA, Jebb SA, Zimmerman M, Otunla A, Henry JA, Ferrey A, et al. The association of weight loss with changes in the gut microbiota diversity, composition, and intestinal permeability: A systematic review and meta-analysis. Gut Microbes. 2022;14:2020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.