Abstract
Blood monocytes or tissue macrophages play a pivotal role in the pathogenesis of murine cytomegalovirus (MCMV) infection, providing functions beneficial to both the virus and the host. In vitro and in vivo studies have indicated that differentiated macrophages support MCMV replication, are target cells for MCMV infection within tissues, and harbor latent MCMV DNA. However, this cell type presumably initiates early, antiviral immune responses as well. In addressing this paradoxical role of macrophages, we provide evidence that the proficiency of MCMV replication in macrophages positively correlates with virulence in vivo. An MCMV mutant from which the open reading frames M139, M140, and M141 had been deleted (RV10) was defective in its ability to replicate in macrophages in vitro and was highly attenuated for growth in vivo. However, depletion of splenic macrophages significantly enhanced, rather than deterred, replication of both wild-type (WT) virus and RV10 in the spleen. The ability of RV10 to replicate in intact or macrophage-depleted spleens was independent of cytokine production, as this mutant virus was a poor inducer of cytokines compared to WT virus in both intact organs and macrophage-depleted organs. Macrophages were, however, a major contributor to the production of tumor necrosis factor alpha and gamma interferon in response to WT virus infection. Thus, the data indicate that tissue macrophages serve a net protective role and may function as “filters” in protecting other highly permissive cell types from MCMV infection. The magnitude of virus replication in tissue macrophages may dictate the amount of virus accessible to the other cells. Concomitantly, infection of this cell type initiates the production of antiviral immune responses to guarantee efficient clearance of acute MCMV infection.
Blood monocytes and tissue macrophages play a major role in the pathogenesis of cytomegalovirus (CMV) infections by serving as target cells in infected organs, as disseminators of the virus throughout the host, or as sites of CMV latency (reviewed in reference 32). The prominence of this cell lineage as a host for human CMV (HCMV) infection and replication is revealed by the frequent detection of HCMV DNA, RNA, or antigens in progenitor cells of the monocyte/macrophage lineage, in peripheral blood monocytes, or in differentiated macrophages infected in vitro or in vivo with HCMV (8, 18, 22, 25, 29, 31–33, 49, 51, 69). Infected blood monocytes, likely derived from infection of bone marrow progenitor cells, may disseminate HCMV to other permissive cells of the host (32, 65). The relatively nonpermissive monocytes and bone marrow progenitor cells harbor latent HCMV DNA (11, 22, 24), which reactivates upon cellular differentiation (25, 29, 50, 53, 54, 58).
Mouse studies using murine CMV (MCMV) corroborate findings with humans by identifying the macrophage as a major target cell. In mouse bone marrow, MCMV predominates within stromal cells (55), although hematopoietic progenitor cells may also be infected (20, 41). Circulating blood monocytes disseminate MCMV during acute infection, and their subsequent cellular differentiation into mature macrophages favors productive MCMV replication (2, 4, 16, 17, 34, 56, 66). During acute infection of the spleen, virus localizes to areas corresponding to marginal-zone macrophages (56). Since tissue macrophages are in close contact with circulating blood, they are likely one of the first cell types infected by blood-borne virus. Furthermore, macrophages serve as reservoirs of latent MCMV infection (20, 41).
In addition to their role as targets of MCMV infection, macrophages are important in the early inflammatory and innate immune responses to infection. Activated macrophages infiltrate the site of MCMV infection (17), where they may initiate a cascade of antiviral cytokines. Initially, the cytokines alpha/beta interferon (IFN-α/β), tumor necrosis factor alpha (TNF-α), interleukin (IL)-1 alpha, IL-6, and IL-12 are produced in response to early MCMV infection (14, 15, 17, 36, 37, 46, 66, 68). The former two cytokines have direct antiviral activity in that they inhibit MCMV replication (10, 17, 28, 40, 66). In addition, IFN-α/β enhances NK-cell-mediated blastogenesis and cytotoxicity, while TNF-α augments IFN-γ produced by NK cells (37). IL-12 also activates NK cells to produce IFN-γ, which directly inhibits MCMV replication (10, 28, 36–38). These two cytokines, IL-12 and IFN-γ, may also cooperate in activating cytotoxic T lymphocytes, which are pivotal in the subsequent clearing of MCMV from most target organs (23). The role of macrophages in initiating this cascade of cytokine-mediated antiviral activities has not been determined.
Macrophages therefore appear to have dual, even paradoxical, roles: one as hosts for CMV replication and another as inducers of antiviral cytokines. Growth of HCMV and MCMV in macrophages is somewhat restricted, with virus production being delayed (9) or curtailed (17, 66) compared to infection in fibroblasts. Thus, tissue macrophages may serve as an anatomical and/or functional “filter” in intrinsically or extrinsically protecting other, more-permissive cell types. We hypothesized that the magnitude of virus replication in tissue macrophages dictates the amount of virus accessible to other, more-permissive cells. Concomitantly, infection of macrophages likely initiates the production of antiviral immune responses to guarantee efficient clearance of acute infection, thereby sparing from death the host that serves as a reservoir of latent infection.
With respect to the above hypotheses, we addressed the following questions. (i) Does the ability of MCMV to replicate in macrophages influence virulence and virus titers in target organs? (ii) Does the absence of tissue macrophages enhance or deter virus replication in the spleen and liver, two major target organs for MCMV replication? (iii) What contributions do macrophages make to the production of early, antiviral cytokines? Our approach utilized a deletion mutant of MCMV which was defective for replication in mature macrophages. The highly attenuated phenotype of this mutant virus in vivo indicated that the capacity of MCMV to replicate in differentiated macrophages was a major determinant of virus virulence. However, in the absence of splenic macrophages, growth of the mutant virus in this organ was restored and replication of wild-type (WT) virus was enhanced. The induction of at least two antiviral cytokines, TNF-α and IFN-γ, required the presence of tissue macrophages and was influenced by the capacity of MCMV to replicate in this cell type. Therefore, while tissue macrophages support MCMV replication, they exert a net protective role in the pathogenesis of MCMV infection.
MATERIALS AND METHODS
Mice.
Six-week-old BALB/cAnN (Harlan Sprague Dawley, Indianapolis, Ind.) or C57BL/6J (Jackson Laboratories, Bar Harbor, Maine) mice were housed in sterile microisolator cages with sterile food, water, and bedding. CB17 severe combined immunodeficient (SCID) mice were bred and housed as previously described (17). Both BALB/cAnN and CB17 mice are of the H-2d haplotype; C57BL/6 mice are of the H-2b haplotype.
Cells.
Murine NIH 3T3 fibroblasts (ATCC CRL-1658) (American Type Culture Collection, Rockville, Md.) were propagated in Dulbecco’s modified essential medium (Mediatech, Herndon, Va.) supplemented with 10% heat-inactivated bovine calf serum (Hyclone Laboratories, Logan, Utah) and 1% l-glutamine (Gibco/BRL, Grand Island, N.Y.). IC-21 cells, a simian virus 40-transformed, C57BL/6 mouse peritoneal macrophage line (30) (ATCC TIB 186), were propagated in RPMI medium (Mediatech) supplemented with 10% heat-inactivated fetal calf serum (Gibco/BRL) and 1% l-glutamine.
Adherent peritoneal exudate cells, providing monolayers of primary macrophages, were obtained by peritoneal lavage of C57BL/6J mice. Mice received 10 μg of lipopolysaccharide (LPS) (Difco Laboratories, Detroit, Mich.) intraperitoneally (i.p.) 72 h prior to harvesting of the peritoneal exudate cells. Exudate cells were plated at 3 × 105 to 12 × 105 cells per 10 cm2 in wells or flasks containing Iscove’s medium (Mediatech) supplemented with 10% heat-inactivated fetal calf serum, 1% l-glutamine, and 50 μg of gentamicin/ml (Sigma Chemical Co., St. Louis, Mo.). After an overnight incubation, nonadherent cells were vigorously washed from the adherent monolayers. Adherent cells obtained in this manner had the morphological appearance of macrophages and were phagocytic, as determined by ingestion of latex beads (Difco Bacto latex beads; Difco Laboratories). Adherent cells from representative wells were harvested for counting, and based on this number, the remaining monolayers were infected at a multiplicity of 0.1 or 0.5 PFU of MCMV/cell.
Viruses.
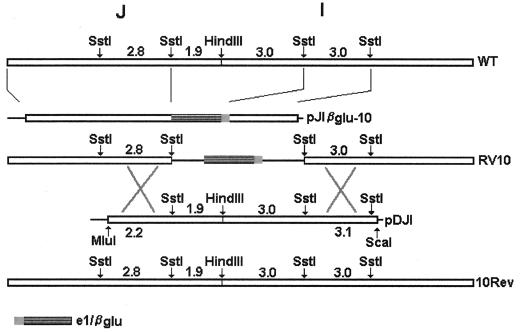
The parental WT virus used in these studies was the MCMV Smith strain (ATCC VR 194). The generation of mutant MCMV RV6, with open reading frames (ORFs) m137 and m138 deleted, was described previously (2). Mutant MCMV RV10, with ORFs M139, M140, and M141 deleted, with was generated by homologous recombination, using by our previously described methods (2). A pGEM-4Z recombination plasmid containing the first (left) two SstI fragments of MCMV HindIII-J, an e1–β-glucuronidase (β-Glu) cassette (β-glucuronidase reporter gene under the control of the MCMV e1 promoter), and the second SstI fragment within HindIII-I was constructed (pJIβglu-10) (Fig. 1). The pJIβglu-10 recombination plasmid was cotransfected with WT viral DNA to generate an MCMV mutant lacking 4.8 kb corresponding to ORFs M139, M140, and M141 (bases 193984 through 198832) (43). Revertant virus, RV10Rev, was produced by homologous recombination by using infectious, RV10 parental DNA and a recombination plasmid (pDJI) containing WT sequence from base 191764 (within m137) to base 201966 (within m143) (Fig. 1). Transfections were performed by using calcium phosphate precipitation as described previously (2). Virus RV10 was selected based on its blue plaque phenotype and was plaque purified five times. Virus RV10Rev was selected based on its white plaque phenotype as described elsewhere (21). The genotypes of the recombinant viruses were confirmed by Southern blot analyses. A genetic map of the mutant viruses is shown in Fig. 1. All virus stocks were prepared in NIH 3T3 cells and quantitated by standard plaque assay on NIH 3T3 cells. Mock virus preparations were supernatants from uninfected NIH 3T3 cells.
FIG. 1.
Construction of the MCMV mutants RV10 and RV10Rev. The open boxes indicate wild-type MCMV sequences within the HindIII J and I fragments. Solid lines denote deleted sequences. Shaded boxes indicate the locations of the e1–β-glucuronidase cassette. Plasmid pJIβglu-10 contains MCMV and e1–β-glucuronidase sequences in the vector pGem4Z; pDJI contains the denoted MCMV sequences in the vector pcDNA3. The numbers refer to sizes (in kilobases) of the indicated fragments.
Virion purification by gradient centrifugation.
In some experiments, gradient-purified virus was used to infect fibroblasts or IC-21 macrophages. MCMV was purified by gradient centrifugation on a 20 to 70% sorbitol gradient according to published protocols (5). Briefly, stock preparations of MCMV grown in NIH 3T3 cells were harvested and cellular debris was removed by centrifugation. Bacitracin (Calbiochem, La Jolla, Calif.) was added to the clarified supernatant to a final concentration of 100 μg/ml. The virus-containing supernatant was layered over a cushion of 20% sorbitol in Tris-buffered saline (TBS) (150 mM Tris, 30 mM NaCl [pH 7.5]) containing bacitracin (100 μg/ml) and then centrifuged at 26,000 × g for 1 h at 18°C. The pelleted virus was resuspended in 2 ml of TBS–bacitracin, layered onto a 20 to 70% sorbitol gradient, and centrifuged at 65,000 × g for 1 h. Low-density virions banding at the 40 to 50% interface or at the 50 to 60% interface were harvested, diluted 1:10 in TBS–bacitracin, and pelleted over a 20% sorbitol cushion for 1 h at 26,000 × g. The virus was resuspended in TBS–bacitracin and frozen, and titers were determined by standard plaque assay.
Southern blot analysis.
Southern blots were used to confirm the genomic alterations of each viral mutant. Two million NIH 3T3 cells in 100-mm-diameter tissue culture plates were infected with virus at a multiplicity of 2 PFU/ml. When the cytopathic effect reached 100%, DNA was harvested for Southern blot analysis, which was performed as described previously (2).
Northern blot analysis.
Northern blot analyses of viral RNA transcripts from RV10-, RV10Rev-, or WT virus-infected cells were performed essentially as described previously (2). For analysis of transcripts from the HindIII-J and -I regions, NIH 3T3 cells were infected at a multiplicity of 2 PFU/cell for 24 h. For analysis of immediate-early RNAs transcribed from HindIII-L or HindIII-I, 2 × 106 NIH 3T3 fibroblasts or IC-21 macrophages were infected at a multiplicity of 1 PFU/cell with virions banding at the 40 to 50% or 50 to 60% sorbitol interface. After 2 h of adsorption and penetration, virus inocula were removed, medium was added, and infection was allowed to proceed for an additional z h. Total RNA was harvested by using the RNeasy kit (Qiagen, Chatsworth, Calif.) according to the manufacturer’s instructions. Samples containing a total of 5 μg of RNA, extracted from equal numbers of cells, were loaded in each lane of a formaldehyde- agarose gel. The loading of equal amounts of RNA was confirmed by ethidium bromide staining of 18S and 28S rRNA. RNAs were first hybridized with the MCMV HindIII L fragment to detect ie1, ie2, and ie3 transcripts. The blot was then stripped by boiling for 10 min in 0.1% sodium dodecyl sulfate and hybridized with an SstI fragment from HindIII I (bases 198832 to 201744) to probe for expression of the immediate-early genes m142 and m143. Probes were labeled with digoxigenin by random priming for detection by chemiluminescence.
In vitro growth of MCMV mutants in fibroblasts and macrophages.
Mutant and WT MCMV were compared for their abilities to replicate in fibroblast and macrophage cell lines and in primary, LPS-induced peritoneal macrophages. Approximately 106 NIH 3T3 fibroblasts or IC-21 macrophages in T-25 flasks or 105 primary macrophages in 6-well plates or T-25 flasks were infected at a multiplicity of 0.1 PFU/cell (cell lines) or 0.1 to 0.5 PFU/cell (primary macrophages). At the indicated times postinfection, both intracellular virus and extracellular virus were harvested collectively and their titers were determined on NIH 3T3 cells. Growth of each virus in the three cell types was quantitated at least twice.
Southern blot analysis of cell-associated virus.
The mutant virus RV10 was compared to RV10Rev for its ability to bind to and/or penetrate IC-21 macrophages. A standard assay for herpesvirus penetration into cells (27) was modified to account for the fact that MCMV produces no cytopathic effects in IC-21 macrophages and consequently does not form plaques in this cell type. Therefore, in order to detect virus bound to or penetrated into macrophages, Southern blotting was used to detect viral genomic DNA. Initially, the validity of this method was tested by using infection of NIH 3T3 fibroblasts to compare results of the standard penetration assay, which assesses plaque formation, with those of Southern blotting for viral DNA. For the former assay, the published procedure was used (27). Briefly, 106 NIH 3T3 cells in 100-mm-diameter dishes were infected with WT virus at a multiplicity of 0.0005 PFU/cell (to yield 200 PFU/dish) at room temperature for 1 h. The virus inocula were then removed, the monolayers were washed with phosphate-buffered saline (PBS), complete medium was added to each dish, and the temperature of the dishes was shifted to 37°C for the indicated periods to allow attached virus to penetrate. The monolayers of infected cells were treated with acid-glycine saline (0.8% NaCl, 0.038% KCl, 0.01% MgCl2, 0.01% CaCl2, 0.7% glycine [pH 3]) for 1 min at 0, 15, 30, 60, 90, or 120 min after shifting to 37°C in order to inactivate and/or remove low-affinity-bound virus that was attached but had not yet penetrated. Infected monolayers were then overlaid with semisolid medium to determine the number of plaques formed in each treatment group. The percentage of bound virus which penetrated was calculated as follows: (number of plaques formed on acid-glycine-washed monolayers)/(number of plaques formed on unwashed monolayers) × 100.
For the Southern blotting method, fibroblasts or IC-21 macrophages were infected at room temperature or at 4°C at a multiplicity of 4 PFU/cell for 1 h. Monolayers were washed with PBS to remove the inocula, and complete medium was added to each dish. After the temperature shift to 37°C and acid-glycine washes at the indicated times, all infected monolayers were incubated for a total of 4 h. Cell-associated DNA was harvested and digested with HindIII and BamHI (Promega, Madison, Wis.) for Southern blotting to detect viral DNA, performed as described previously (2). The control for a maximal amount of viral DNA consisted of infected cells incubated for the entire 4 h without acid-glycine washing of the monolayers. An MCMV HindIII-I region probe was used to detect viral DNA.
In vivo growth of MCMV mutants.
Recombinant and WT viruses were compared for their abilities to replicate in mouse spleen and liver tissues. Mice received 3 × 105 PFU of tissue culture-passaged virus intravenously (i.v.) in the tail vein, and organs were harvested from three individual mice as 20% (wt/vol) homogenates on each of days 1, 2, and 3 postinfection and titered as described previously (2). Blood was also collected, in heparinized tubes, and serial 10-fold dilutions of whole blood in tissue culture media were used for virus titration. These experiments were performed at least twice.
For assessment of relative virulence, recombinant and WT viruses were compared for their lethalities for adult (6-week-old) SCID mice. The SCID mice were injected i.p. with 104 PFU of tissue culture-passaged mutant or WT virus and observed daily for survival. Lethality experiments were performed at least twice for each virus.
Macrophage depletion.
Replication of recombinants and WT viruses was compared in intact and macrophage-depleted spleens and livers of BALB/cAnN mice. Anesthetized animals were injected i.v. with a total volume of 0.5 ml containing 300 μl of PBS and 200 μl of multilamellar liposomes encapsulating dichloromethylene-bisphosphonate (Cl2MBP) or, as a control, with 0.5 ml of PBS alone. The Cl2MBP was a kind gift from Boehringer GmbH (Mannheim, Germany). When liposomes encapsulating Cl2MBP (L-Cl2MBP) are administered i.v., they are readily phagocytized by liver macrophages (Kupffer cells) and splenic red pulp macrophages, marginal-zone macrophages, marginal metallophilic macrophages, and marginal-zone dendritic cells (26, 35, 63, 64). Lysosomal enzymes present specifically within these cell populations degrade the liposomes and release the Cl2MBP, leading to apoptosis of the cells (62). Peripheral blood monocytes and other tissue macrophages (including peritoneal cells) are spared from L-Cl2MBP-induced death when the liposomes are administered i.v. For this study, use of PBS as a control for L-Cl2MBP treatment was preferred over use of empty liposomes, which may alter macrophage functions, including antiviral activities, in otherwise intact cells (63). The L-Cl2MBP method of tissue macrophage depletion efficiently and specifically depletes tissues of macrophages and macrophage-like marginal dendritic cells without toxicity for other cell types (61, 63).
Forty-eight hours after L-Cl2MBP administration, mice received 3 × 105 PFU of tissue culture-passaged WT, RV10, or RV10Rev virus i.v. The spleen, liver, and blood of four or five individual mice in each of the two treatment groups were harvested at 1, 2, or 3 days postinfection for virus titration. The efficiency of splenic macrophage depletion was verified histologically in spleens from PBS- or L-Cl2MBP-treated mice at 3 days after infection with WT virus. Frozen tissue sections were stained for detection of macrophage-specific acid phosphatase (7), as expression of this enzyme is minimally downregulated by MCMV infection (59).
Cytokine analysis.
Levels of cytokines produced by splenocytes in response to WT or mutant virus infections in mice with intact spleens or macrophage-depleted spleens were compared. Mice were injected i.v. with L-Cl2MBP or PBS and 48 h later were infected i.v. with tissue culture-passaged virus as described above. Spleens were harvested from three individual mice in each treatment group at 36 h after infection, the time at which early cytokine production in response to MCMV infection peaks (46). Suspensions containing 5 × 106 total splenocytes per ml were prepared in RPMI 1640 containing 1% low-endotoxin serum (Hyclone) according to published procedures (37). After 24 h of culture, cell-free supernatants from each individual spleen were pooled and frozen. Levels of TNF-α, IL-12 p40, IL-12 p70, IFN-γ, and IL-6 in the splenocyte-conditioned media were quantitated by sandwich enzyme-linked immunosorbent assay (ELISA) as previously described (6). Student’s t test was performed to evaluate statistical differences in cytokine values between mice treated with L-Cl2MBP or PBS and between mice infected with WT or mutant MCMV.
RESULTS
Construction and in vitro growth characteristics of RV10 and RV10Rev.
We previously described a mutant MCMV, RV7, in which ORFs m137 through M141, contained within the HindIII-J and -I regions of the viral genome, are deleted and which grows poorly in the differentiated macrophage cell line IC-21 (2). Another mutant virus, RV6, with m137 and m138 deleted, grows like WT virus in IC-21 macrophages (1), suggesting that an ORF(s) within the M139 to M141 region contributes to growth of MCMV in this cell type. Therefore, a mutant virus (RV10) with ORFs M139, M140, and M141 deleted was generated to assess the function of these ORFs in macrophage-specific growth and viral pathogenesis. A marker-rescued revertant of RV10, RV10Rev, was also generated by homologous recombination of WT sequences back into RV10. Southern blotting of viral DNA digested with HindIII and BamHI confirmed the genotypes of RV10 and RV10Rev (data not shown). Northern blot analyses of RNA isolated from RV10-infected fibroblasts during the late phase of replication revealed the expression of RNA transcripts of WT size from genes proximal to the deleted region, although RV10 m138 RNA was larger than the RNA of WT virus. Transcripts from the HindIII-J and -I region in RV10Rev-infected cells were identical to those in WT virus-infected cells (data not shown).
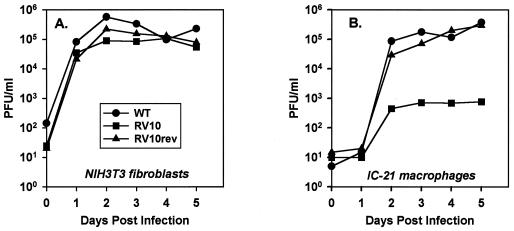
Both RV10 and RV10Rev grew like WT virus in NIH 3T3 fibroblasts (Fig. 2A). Replicate experiments consistently revealed no significant differences in growth among WT virus, RV10Rev, and RV10 in this fibroblast cell line. These data confirm the nonessential function of the M139 to M141 region in MCMV replication.
FIG. 2.
Growth of RV10 and WT MCMV in fibroblasts and macrophages in vitro. Monolayers of NIH 3T3 cells (A) or IC-21 macrophages (B) were infected at a multiplicity of 0.1 PFU/cell. Cell-free and cell-associated viruses were collectively titerate at the indicated times postinfection by plaque assay on NIH 3T3 cells. Data shown are representative of three individual experiments.
In contrast, growth characteristics of RV10 in differentiated, mature macrophages proved that deletion of these three ORFs compromised the ability of MCMV to grow in macrophages. Growth of RV10 in immortalized IC-21 macrophages was significantly reduced, with peak titers up to 1,000-fold lower than that of WT virus or RV10Rev (Fig. 2B). Likewise, peak titers of RV10 replication in primary, differentiated peritoneal macrophages obtained by lavage were 100- and 500-fold lower than those of WT virus at days 3 and 5 postinfection, respectively (data not shown). In the primary macrophages, growth of RV6 was similar to that of WT virus (data not shown), as previously reported for the IC-21 macrophage cell line (2). Thus, M139, M140, and/or M141 gene products are necessary for optimal growth of MCMV in differentiated macrophages. Importantly, the defect in growth of RV10 in the peritoneal macrophage cell line was reproducible in primary, differentiated peritoneal macrophages. This validates the use of the IC-21 cell line for further characterization of this mutant virus and confirms the biological relevance of this gene region in MCMV pathogenesis.
Viral gene expression in RV10-infected macrophages.
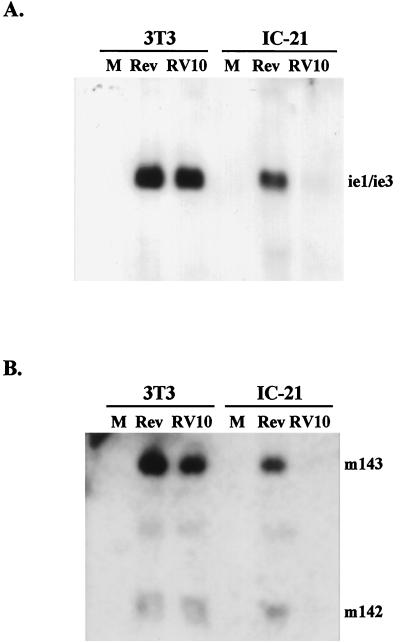
It was of interest to identify the stage of the MCMV replication cycle at which growth of RV10 in macrophages was curtailed. The growth rate of RV10 in IC-21 macrophages was similar to that previously reported for the mutant virus RV7, which lacks ORFs m137 through M141 (2). The defect in growth of RV7 was identified as a block in expression of the major immediate-early genes ie1, ie2, and ie3 (2). Therefore, immediate-early gene expression in RV10-infected IC-21 macrophages was assessed and compared to that in RV10Rev-infected cells. For these experiments, highly purified infectious virions obtained by centrifugation through a sorbitol density gradient were used for infection of fibroblasts and IC-21 macrophages. Experiments were performed by using each of the two bands (40 to 50% interface and 50 to 60% interface) which contained most of the infectious virus and which likely contained a majority of single-capsid, enveloped virus (3). Equal amounts of RNA from equal numbers of infected cells were analyzed by Northern blotting for expression of immediate-early genes. Expression from two immediate-early gene regions was assessed—the major immediate-early gene region in HindIII-L (ie1, ie2, and ie3) and immediate-early genes m142 and m143 in HindIII-I (12). These two immediate-early gene regions have different kinetics of expression and are therefore likely to be independently regulated (12).
The results indicated that the ie1, ie3, m142, and m143 genes were expressed from RV10Rev and RV10 at comparable levels in NIH 3T3 fibroblasts 4 h after infection (Fig. 3). However, ie1, ie3, m142, and m143 gene expression in RV10-infected IC-21 macrophages was significantly reduced compared to that in RV10Rev-infected macrophages and compared to that in RV10-infected fibroblasts. From these experiments, it was evident that RV10 was significantly limited in expression of at least these two immediate-early gene regions upon infection of IC-21 macrophages. Therefore, the M139, M140, and M141 gene region influences replication in this macrophage cell line at the earliest stages of infection: attachment, penetration, uncoating, or immediate-early gene expression.
FIG. 3.
Expression of RV10 and RV10Rev immediate-early genes in fibroblasts and macrophages. NIH 3T3 fibroblasts or IC-21 macrophages were mock infected (M) or were infected with RV10Rev (Rev) or RV10 at a multiplicity of 1 PFU/cell. The virus inocula were purified virions banding at the 40 to 50% sorbitol interface of a 20 to 70% sorbitol gradient. Five micrograms of total RNA harvested at 4 h after infection was run on a formaldehyde gel for Northern blot analyses. The loading of equal amounts of RNA was confirmed by ethidium bromide staining of the 18S and 28S rRNA. (A) Analysis of the major immediate-early gene region. The Northern blot was hybridized to a probe corresponding to the HindIII L region of MCMV in order to detect RNA from ie1 (2.7 kb), ie2 (1.7 kb), or ie3 (2.7 kb). (B) Analysis of the m142 and m143 immediate-early gene region. The Northern blot shown in panel A was stripped and hybridized with a probe corresponding to m142 and m143 (bases 198832 to 201744 of the MCMV genome) within HindIII-I. The faint band visible between the m142 and m143 transcripts represents incompletely stripped ie1/ie3 signal.
Attachment and penetration of RV10 during infection of macrophages.
The above-presented results indicated that expression of at least two MCMV immediate-early gene regions in infected IC-21 macrophages was compromised by deletion of the M139 to M141 region. Because of these findings and the fact that M139, M140, and M141 are expressed at early times after infection (12), we considered the possibility that these gene products influence entry of MCMV into macrophages. It is possible that specific viral gene products are required for efficient receptor-mediated attachment to and/or penetration into a phagocytic cell. Unfortunately, standard procedures to assess the rate of viral penetration could not be used because MCMV does not form plaques in IC-21 cells. Therefore, we compared, by Southern blotting, the amounts of macrophage-associated RV10 DNA and RV10Rev DNA, representing attached and/or penetrated virus.
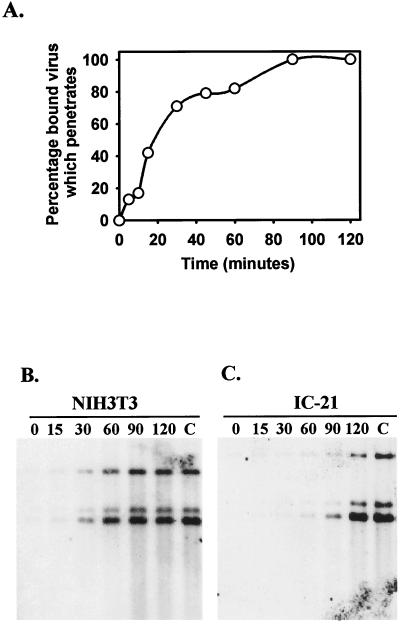
To confirm the validity of the assay as a measure of bound or penetrated virus, we first compared results obtained by using Southern blotting to those obtained by using the standard penetration assay in NIH 3T3 fibroblasts (described in Material and Methods). Figure 4A depicts the rate of penetration of WT MCMV into NIH 3T3 fibroblasts as measured by using a standard plaque assay of infected monolayers subsequent to acid–glycine washes at various time points to inactivate bound virus not yet penetrated. The results indicated that maximal penetration of MCMV into fibroblasts occurred between 60 and 90 min postinfection (after shift to 37°C). In a parallel experiment, NIH 3T3 fibroblasts infected with RV10Rev were harvested for Southern blotting at the indicated times after a shift to 37°C. The results, shown in Fig. 4B, indicate that maximal amounts of cell-associated virus stably bound or penetrated between 60 and 90 min postinfection. These results are identical to those obtained by using the more-traditional penetration assay and verify that the Southern blotting method is reliable for detecting DNA of cell-associated virus that either is stably bound or has penetrated.
FIG. 4.
Wild-type MCMV and RV10Rev attachment to or penetration into NIH 3T3 fibroblasts and IC-21 macrophages. (A) Penetration assay. NIH 3T3 cells were infected with WT MCMV at room temperature for 1 h, were shifted to 37°C, and at the indicated times after the temperature shift were washed with acid-glycine buffer. Monolayers were then overlaid with semisolid complete medium and incubated for 5 days to quantitate the number of plaques which formed in each treatment group. The percentage of bound virus which penetrated was calculated as follows: (the number of plaques formed with acid-glycine washing)/(the number of plaques formed without acid-glycine washing) × 100. (B and C) Viral DNA stably attached to or penetrated into fibroblasts and macrophages. NIH 3T3 fibroblasts (B) or IC-21 macrophages (C) were infected as described above at room temperature or at 4°C with RV10Rev (4 PFU/cell). At the indicated times after the shift to 37°C, monolayers were washed with acid–glycine buffer and returned for a total of 4 h of incubation in complete medium. Cells were then harvested for extraction of viral DNA and Southern blotting. The DNA was hybridized with a probe corresponding to HindIII-L. The C denotes control cells that were not washed with acid-glycine but were incubated for a total of 4 h after the shift to 37°C.
When RV10Rev infection of IC-21 macrophages was assessed by the Southern blotting method, maximal levels of viral DNA bound and/or penetrated between 90 and 120 min postinfection (Fig. 4C). These data suggest that entry of MCMV into macrophages is less efficient than entry into fibroblasts. Nevertheless, by 120 min postinfection, maximal amounts of WT MCMV attached to or penetrated into fibroblasts and IC-21 macrophages.
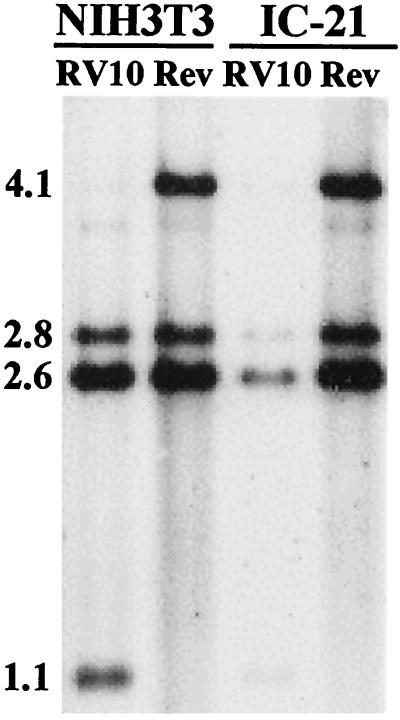
Based on the above-presented results, we compared the amounts of RV10 DNA and RV10Rev DNA associated with fibroblasts and macrophages at 120 min postinfection. Fibroblasts and IC-21 cells were infected with either type of virus at a multiplicity of 4 PFU/cell at 4°C for 1 h and then shifted to 37°C. At 120 min after the shift, monolayers were washed with acid-glycine buffer (except the controls) and returned for incubation for a total of 4 h. Viral DNA was harvested from the infected-cell monolayers and analyzed by Southern blotting. The results, presented in Fig. 5, clearly show that comparable amounts of RV10 DNA and RV10Rev DNA bound to or penetrated NIH 3T3 fibroblasts. However, RV10 was much less efficient in either binding to or penetrating IC-21 macrophages than RV10Rev. These results would explain the lower levels of immediate-early gene expression upon RV10 infection of IC-21 cells.
FIG. 5.
RV10 attachment to or penetration into NIH 3T3 fibroblasts and IC-21 macrophages. Experiments were performed essentially as described in the legend for Fig. 4B. In this experiment, monolayers of NIH 3T3 fibroblasts or IC-21 macrophages were infected with RV10 or RV10Rev (Rev), were shifted to 37°C, and at 120 min after the shift were washed with acid-glycine buffer. Infected cells were incubated for a total of 4 h in complete medium. Digestion of RV10 DNA with HindIII and BamHI yields bands of 2.8, 2.6, and 1.1 kb which hybridize to the HindIII-I probe. RV10Rev DNA digested with the same enzymes yields bands of 4.1, 2.8, and 2.6 kb which hybridize to this probe.
Virulence of RV10 in SCID mice.
If macrophages are a prominent target cell of MCMV infection in vivo, then a mutant virus that replicates poorly in macrophages, such as RV10, is likely to be attenuated for virulence in vivo. This phenotype would not be evident in normal mice, such as BALB/c mice, because in these animals tissue culture-passaged virus is highly attenuated. Stocks of virulent RV10 cannot be obtained because replication of this mutant virus was not detectable in mouse salivary glands (52), as previously shown for mutant virus RV7 (2). Therefore, we compared the virulence of RV10 and RV10Rev in SCID mice. Because these mice are devoid of mature, functional T and B lymphocytes, they are exquisitely sensitive to MCMV infection, succumbing to as few as 3 PFU of tissue culture-passaged virus (42). Thus, these mice provide minimal control of MCMV replication within macrophages and other target cell types. The numbers and function of tissue macrophages, which serve as potential target cells, are normal in these otherwise immunodeficient mice (1).
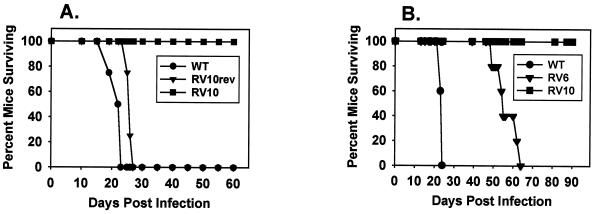
While all mice infected with 104 PFU of WT virus or RV10Rev died by day 28 postinfection, all RV10-infected mice survived for at least 90 days after infection (Fig. 6). Infection with RV6, a mutant virus that grows like WT virus in macrophages, also resulted in 100% mortality, although with delayed kinetics compared to that produced by WT virus (Fig. 6B). From these experiments, we conclude that the M139 to M141 gene region contributes to MCMV virulence and pathogenesis in its natural host.
FIG. 6.
Lethality of RV10 and WT MCMV for SCID mice. (A and B) Mice (four or five per group) were infected i.p. with 104 PFU of the indicated virus and observed daily for mortality. The experiments were performed twice.
Growth of RV10 in spleen and liver tissue.
The above-presented data revealed a correlation between the virulence of MCMV and the ability of the virus to replicate in macrophages. However, RV10 may be replication defective for several cell types within target organs, and in sum, these deficiencies contributed to its high degree of attenuation in vivo. To test this hypothesis, we assessed the ability of RV10 to replicate in two major target organs of BALB/c mice, the spleen and liver. RV10 and RV10Rev were injected i.v. to directly administer virus to these two target organs. The spleen contains a dense network of macrophages in direct contact with the circulation. The liver also contains an abundance of macrophages, but they are diffusely distributed throughout the sinusoids. In addition, hepatocytes are permissive for MCMV replication (39, 44).
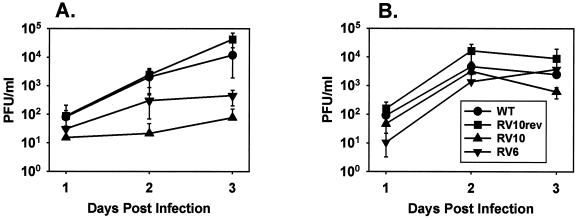
The data shown in Fig. 7A indicate that RV10 replicated poorly, if at all, in intact spleens on days 1 through 3 postinfection. Both RV10Rev and WT virus replicated substantially, and RV6 (deleted of m137 and m138) replicated to modest levels in this organ. No infectious WT or mutant viruses were detected in blood during the 3-day period (data not shown). The degree of MCMV replication in the spleen correlated with lethality in SCID mice, suggesting that MCMV replication in this organ is a major determinant of virus virulence in vivo. In contrast to the spleen, RV10 replicated in liver tissue with kinetics similar to that of WT virus and that of RV10Rev (Fig. 7B). The attenuated virus RV6 also replicated with near-WT kinetics in this organ. The data indicate that RV10 is not replication defective for all tissues. The attenuated phenotype of RV10 appears to be restricted to certain cell types in vivo.
FIG. 7.
Growth of RV10 and WT MCMV in spleen and liver tissues. BALB/c mice were inoculated i.v. with 3 × 105 PFU of the indicated virus. Spleen (A) and liver (B) tissues were harvested from three individual mice at the indicated times postinfection. Virus titers in 20% (wt/vol) tissue homogenates were determined by plaque assay on NIH 3T3 cells. Data points are the average values for three individual mice, and error bars represent standard deviations.
Growth of RV10 in macrophage-depleted tissues.
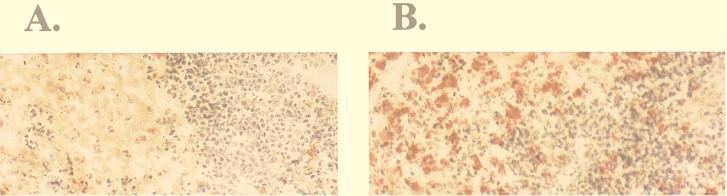
These data suggested that the ability of MCMV to replicate in macrophages may directly influence the level of virus replication in the macrophage-dense environment of the spleen. Is growth of RV10 in macrophages the limitation to replication in the spleen, or is RV10 replication defective for other cell types in this organ as well? In order to demonstrate that splenic macrophages were the limitation to efficient RV10 replication in this target organ, growth of RV10 in intact spleens was compared to that in spleens specifically depleted of tissue macrophages. Mice were depleted of splenic macrophages by i.v. injection of L-Cl2MBP prior to MCMV infection with RV10 or RV10Rev. This method is highly specific for depletion of macrophages and macrophage-like dendritic cells, is nontoxic and, when administration is i.v., is selective for spleen and liver macrophages exclusively (see Materials and Methods). Virus titers in macrophage-depleted spleens and livers were quantitated on days 1 to 3 postinfection and compared to those in control mice with intact organs. As a control for L-Cl2MBP administration, PBS, rather than empty liposomes, was chosen to ensure that control mice were normal with respect to macrophage numbers and, importantly, with respect to function as well (63). Histological examination of spleen tissue collected from WT virus-infected mice 3 days postinfection confirmed that L-Cl2MBP treatment was effective in eliminating 80 to 90% of the acid phosphatase-positive marginal zone and red pulp macrophages and in maintaining depletion of these cells even after virus infection (Fig. 8).
FIG. 8.
Depletion of splenic macrophages by L-Cl2MBP treatment. Frozen tissue sections of spleens from mice injected i.v. with L-Cl2MBP (A) or PBS (B) and infected with 3 × 105 PFU of WT MCMV were stained for the macrophage-specific marker acid phosphatase. Mice received virus 48 h after L-Cl2MBP or PBS treatment, and spleens were harvested at 3 days postinfection. Acid phosphatase-positive cells in the red pulp, marginal zone, and white pulp stain bright red.
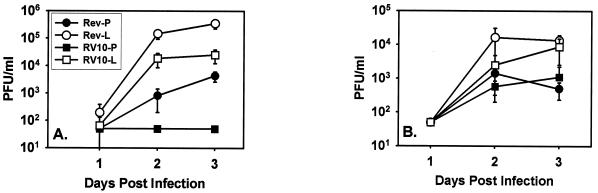
Liposome treatment significantly enhanced levels of RV10Rev replication in the spleen (Fig. 9A). More dramatically, this treatment restored replication of RV10 from undetectable levels of virus to greater than 104 PFU/ml of spleen homogenate (Fig. 9A). These data indicate that red pulp macrophages, marginal-zone macrophages, marginal-zone dendritic cells, and possibly marginal metallophilic macrophages are cellular determinants of early MCMV replication in the spleen. Most importantly, the data indicate that in the absence of macrophages, RV10 is capable of efficient replication in the spleen, thus suggesting that growth in this cell type was indeed the limitation to replication of RV10 in this organ. Depletion of Kupffer cells had a less dramatic effect on replication of RV10Rev and RV10 in the liver (Fig. 9B). This supports the evidence that other cells, such as hepatocytes, are a major target cell type in this organ (39, 44) and indicates that RV10 is not replication defective in such cells.
FIG. 9.
Growth of RV10 and WT MCMV in macrophage-depleted and intact tissues. Mice were injected i.v. with either L-Cl2MBP or PBS and 48 h later were infected with 3 × 105 PFU of the indicated virus. Spleens (A) and livers (B) were harvested from four or five individual mice at the indicated times postinfection. Virus titers in 20% (wt/vol) homogenates were determined by plaque assay on NIH 3T3 cells. Data points are the average values for individual mice, and error bars represent standard deviations. Rev designates infection with RV10Rev, the suffix -P designates treatment with PBS, and the suffix -L designates treatment with L-Cl2MBP.
Antiviral cytokine response to RV10 and RV10Rev infection of the spleen.
An explanation for the data presented above is that splenic macrophages may be an initial target cell for MCMV replication and that in this cell type in vivo, production of WT virus may be efficient yet limited while replication of RV10 is severely curtailed. In the absence of such a macrophage filter, MCMV may have direct access to more-permissive cell types that have not yet been identified. However, there is an alternative explanation for the finding that MCMV titers were higher in macrophage-depleted spleens. Macrophages are likely a major source of antiviral cytokines and regulators of NK cell activity early in MCMV infection (36, 37, 46). The antiviral activity of cytokines and NK cells acting upon growth-retarded RV10 may significantly inhibit mutant virus production, conferring the attenuated phenotype in vivo. In the absence of macrophages and thus the antiviral activity, perhaps RV10 has the opportunity to replicate to substantial levels in other types of cells that normally support only low levels of RV10 replication. If this hypothesis is correct, then RV10 infection of intact spleens should induce levels of antiviral cytokines comparable to those induced by WT viruses in this organ. In the absence of macrophages, RV10 should induce significantly lower levels of cytokines.
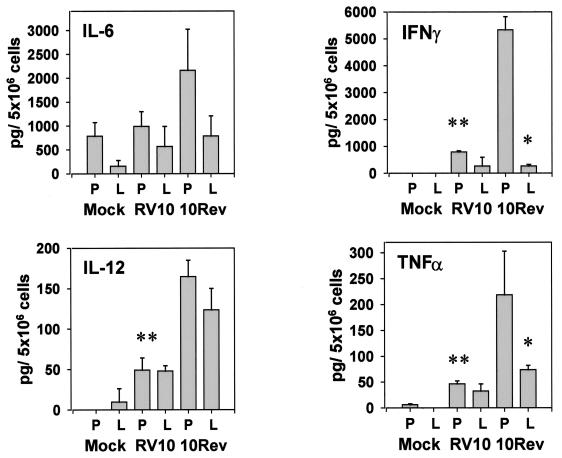
We tested this hypothesis by comparing levels of cytokines in mock-, RV10Rev-, or RV10-infected intact spleens with the levels in L-Cl2MBP-treated spleens. We quantitated the levels of IL-6, IL-12 (p40 and p70), and TNF-α, three cytokines produced early in response to MCMV infection (46). IFN-γ was also quantitated, as this potent antiviral cytokine is produced by infiltrating NK cells in response to monokines induced by MCMV infection (36). The results (see below) indicated that enhanced replication of RV10 in macrophage-depleted spleens was not due to a reduction in cytokine levels but more likely reflected the absence of a macrophage barrier. Secondarily, the results revealed several interesting findings concerning the contribution of macrophages to production of these cytokines and the influence of MCMVv’s replicative capacity on cytokine production. For clarity, the results are presented below as follows: (i) comparison of cytokines induced by RV10Rev in intact spleens with those in macrophage-depleted tissue, (ii) comparison of cytokines induced in intact spleens by RV10 and by RV10Rev, and (iii) comparison of cytokines induced by RV10 in intact spleens and those in spleens depleted of macrophages.
In response to RV10Rev infection, splenocytes from intact organs produced elevated levels of IL-6 (Fig. 10). However, the values were not statistically different from those for mock-infected animals, and no significant differences were found between normal and macrophage-depleted spleens. Because there were no statistically significant differences among IL-6 levels in any of the six treatment groups, results of IL-6 determinations are not described below.
FIG. 10.
Cytokine levels produced by splenocytes in response to RV10 or WT MCMV infection of normal or macrophage-depleted mice. Mice were administered L-Cl2MBP (L) or PBS (P) i.v., and 48 h later, they were inoculated i.v. with mock virus preparation or with RV10Rev or RV10 (3 × 105 PFU). Thirty-six hours later, spleens were harvested from three individual mice, and suspensions containing 5 × 106 splenocytes/ml were incubated overnight. After 24 h, supernatants were collected and cytokines were quantitated by sandwich ELISA assays. IL-12 data are for IL-12 p40. Values represent the mean titers and error bars denote standard deviations for three mice. A single asterisk denotes a significant difference (P ≤ 0.05) between L-Cl2MBP- and PBS-treated mice infected with RV10Rev. A double asterisk denotes a significant difference (P ≤ 0.05) between RV10- and RV10Rev-infected mice treated with PBS. The data are representative of two separate experiments.
As predicted, RV10Rev infection induced IL-12 p40 to detectable levels (Fig. 10). Liposome treatment had no significant effect on production of this cytokine, as levels of IL-12 p40 induced by RV10Rev in PBS-treated or L-Cl2MBP-treated mice were not significantly different (P > 0.05). Therefore, L-Cl2MBP-insensitive cells, such as endothelial cells, may be a significant source of this cytokine following MCMV infection. Levels of IL-12 p70 were, in general, undetectable (<1.0 pg/5 × 106 cells) in splenocyte-conditioned media from all treatment groups (data not shown).
In contrast, production of the antiviral cytokines TNF-α and IFN-γ was severely compromised by L-Cl2MBP treatment in RV10Rev-infected animals (Fig. 10). At this early time postinfection (36 h), TNF-α was likely produced by splenic macrophages, while infiltrating, activated NK cells likely produced IFN-γ. Apparently, the production of IFN-γ by NK cells and/or the infiltration of NK cells was directly or indirectly compromised by L-Cl2MBP treatment.
A comparison of cytokine levels induced in intact spleens by infection with RV10 and by infection with RV10Rev revealed that the replicative capacity of MCMV influenced production of IL-12 p40, TNF-α, and IFN-γ. RV10, which fails to replicate to detectable levels in intact spleens, induced significantly lower levels of these cytokines than did RV10Rev (Fig. 10). This finding may reflect differences in the total numbers of virus-infected cells and implies that virus replication per se, rather than mere exposure to virions, was required for optimal production of these early, antiviral cytokines.
Finally, a comparison of cytokine levels induced in PBS- and L-Cl2MBP-treated mice infected with RV10 demonstrated that L-Cl2MBP treatment had no significant effect on levels of any of the four cytokines induced by this mutant virus. Importantly, these data indicated that the enhancement of replication of RV10 in macrophage-depleted spleens compared to that in intact organs was not due to a reduction in levels of these cytokines. Thus, elevated titers of RV10 in macrophage-depleted spleens likely reflected the absence of a macrophage filter and increased access to more-permissive cell types.
DISCUSSION
Results of in vitro studies using mutant virus RV10 suggest that the products of MCMV M139, M140, and/or M141 function to regulate growth of this virus in macrophages, a biologically important target cell. Mutant RV10 is impaired in the early stages of MCMV replication in macrophages, i.e., at or preceding the time of viral immediate-early gene expression. The levels of expression of at least four immediate-early genes from two different regions of the MCMV genome, HindIII-L and HindIII-I, were significantly lower in RV10-infected macrophages than in RV10Rev-infected cells. The results of experiments performed to semiquantitatively assess the amount of virus stably bound to or penetrated into macrophages indicate that the M139, M140, and/or M141 gene product likely functions in efficient MCMV attachment to or penetration into macrophages. Products of M139, M140, or M141 apparently do not confer resistance to the antiviral effects of IFN-α/β, which is produced early after infection of macrophages, because neutralization of these type I interferons does not restore growth of RV10 in IC-21 macrophages (52). Current studies aim to (i) identify the gene(s) which bestows efficient replication of MCMV in macrophages, (ii) characterize the gene product(s), and (iii) assess the function of the gene product(s) in specific stages of MCMV entry into macrophages or as a transcriptional transactivator of immediate-early genes.
Through the use of RV10 and L-Cl2MBP, several interesting yet paradoxical findings concerning the role of tissue macrophages in MCMV pathogenesis were obtained. The fact that RV10 was defective for growth in macrophages in vitro and in spleen tissue in vivo supports the notion that efficient replication of MCMV in macrophages is required for abundant growth in at least this target organ. This cell type provided the limitation to RV10 replication in the spleen, as RV10 grew to nearly WT levels in macrophage-depleted spleens. The data on MCMV replication in L-Cl2MBP-treated compared to PBS-treated spleens, however, clearly demonstrate that splenic marginal-zone macrophages, marginal metallophilic macrophages, red pulp macrophages, and marginal-zone dendritic cells are not required for replication of MCMV in the spleen. In contrast, these cells provide a net protective effect.
The fact that efficient replication of MCMV in macrophages appeared to be a prerequisite for virus replication in the spleen even though macrophage and dendritic cell depletion enhanced virus growth seems paradoxical. However, this may be explained by the concept of macrophages/dendritic cells serving as a protective barrier or filter. In the spleen, macrophages are likely one of the first cell types exposed to blood-borne virus. The abilities of macrophages to (i) destroy, through phagocytosis, a percentage of extracellular virions, (ii) produce antiviral cytokines, and (iii) regulate growth of HCMV (8, 9, 25) or MCMV (17, 66) which enters macrophages via receptor-mediated events collectively may confer their protective function. Consequently, the ability of MCMV to replicate efficiently in this cell type and subsequently seed neighboring, perhaps more-permissive cell types may ultimately determine virus titers in the spleen.
Our data indicate that macrophages contribute directly or indirectly to production of two cytokines, TNF-α and IFN-γ, both of which inhibit MCMV replication (17, 28). The latter cytokine is produced early in MCMV infection by activated NK cells (38). Indeed, independent studies have shown that L-Cl2MBP treatment of C57BL/6 mice prior to MCMV infection eliminated NK cell infiltration in the spleen, as assessed by quantitation of NK1.1+ and/or DX5+ CD3− splenocytes and by killing of NK-sensitive YAC-1 cells (45). This is likely due to a dependence of NK cell infiltration, differentiation, or activation on cytokines or chemokines produced by macrophages or marginal-zone dendritic cells (47, 48). For example, Kupffer cells are required for the differentiation of peripheral blood NK cells into highly activated, hepatic NK cells (60).
Comparisons between the cytokine profiles of macrophage-depleted spleens and intact spleens from mice infected with RV10 indicate that differences in cytokine production cannot explain the restored growth of RV10 in macrophage-depleted animals. There were no significant differences between the two treatment groups for RV10-induced IL-6, IL-12, IFN-γ, or TNF-α levels. Although we cannot rule out the possibility that the levels of other antiviral cytokines induced by RV10 but not quantitated in this study were diminished by macrophage depletion, we conclude that the elimination of macrophages as a target cell for RV10 had the greatest influence on RV10 replication in the spleen.
The ability of MCMV to replicate in the spleen appeared to influence the overall degree of virulence, an observation previously reported (19). In this study, a direct correlation between the ability of MCMV to replicate in the spleen and lethality in SCID mice was noted. RV6, which replicated in the spleen but replicated poorly compared to WT virus or RV10Rev, killed SCID mice with delayed kinetics. We have found that other mutant MCMV which replicate in the spleen, but only poorly compared to WT virus, kill SCID mice with delayed kinetics (13). RV10, which failed to replicate to detectable levels in the spleen, did not kill SCID mice within 90 to 100 days. It is not feasible to repeat the lethality studies using L-Cl2MBP-treated SCID mice, as most all macrophage subpopulations in the spleen would be restored after L-Cl2MBP treatment by the expected time of MCMV-induced death (62), and effects of repeated L-Cl2MBP injections have not been assessed in this immunocompromised host.
Macrophages had a minor role in control of MCMV replication in the liver compared to that of replication in the spleen. The mutant virus RV10, which failed to replicate to detectable levels in the spleen, replicated to WT levels in the liver. Depletion of Kupffer cells enhanced levels of MCMV replication in the liver, but to a lesser extent than in the spleen. The liver contains large numbers of tissue macrophages, diffusely distributed throughout the sinusoids, in contrast to the highly organized, dense network of splenic macrophages. Aside from this anatomical difference, the quality or function of tissue macrophages residing in the liver and spleen may differ. For example, the peaks of the titers of type I interferons are delayed and the titers are significantly lower in the livers of MCMV-infected BALB/c mice compared to those in the spleen (67). Other antiviral immune effector cells, such as NK cells, function divergently in these two organs (57). Furthermore, there is evidence that in the liver, hepatocytes are the preferred target cell for MCMV replication (39, 44). Collectively, these differences between the liver and the spleen may explain the tissue-specific discrepancies in RV10 replication and in the effect of L-Cl2MBP treatment on MCMV replication in these tissues.
The results of these studies support the hypothesis that macrophages play a pivotal role in the pathogenesis of MCMV infections. Initially, these cells may serve as target cells in at least the spleen, and the capacity of the virus to replicate efficiently in macrophages influences, in part, the magnitude of MCMV replication in that organ. While efficient replication in macrophages may be a necessary (although not sufficient) requirement for robust virus replication in this important target organ, MCMV replication in this cell type leads to controlled growth of the virus, likely due collectively to the growth rate of the virus in this cell type and the early induction of antiviral cytokines. These two conditions may provide a net protective role for at least splenic macrophages and may favor the eventual establishment of MCMV latency in macrophages. It is likely that complex virus-macrophage interactions which are beneficial to both the virus and the host exist, ensuring persistence of CMV within the host.
ADDENDUM
A recent study using L-Cl2MBP-treated mice also revealed a protective role for tissue macrophages during the early stages of acute MCMV infection (S. Hamano, H. Yoshida, H. Takimoto, K. Sonoda, K. Osada, X. He, Y. Minamishima, G. Kimura, and K. Nomoto, Microbiol. Immunol. 42:607–616, 1998).
ACKNOWLEDGMENTS
This work was supported by PHS grant R01 CA41451 (to A.E.C. and R.M.S.) and by The Thomas F. Jeffress and Kate Miller Jeffress Memorial Trust (A.E.C.). H.W.V. was supported by PHS grant R01 AI39616. C.A.B. and M.C.R. were supported by PHS grants R01 MH47674 and T32 ES07272, respectively.
We sincerely thank Boehringer Mannheim for the generous contribution of dichloroimethylene-bisphosphonate. We appreciate the critical review of the manuscript by Victoria J. Cavanaugh.
REFERENCES
- 1.Bancroft G J, Kelly J P. Macrophage activation and innate resistance to infection in SCID mice. Immunobiology. 1994;191:424–431. doi: 10.1016/S0171-2985(11)80448-1. [DOI] [PubMed] [Google Scholar]
- 2.Cavanaugh V J, Stenberg R M, Staley T L, Virgin IV H W, MacDonald M R, Paetzold S, Farrell H E, Rawlinson W D, Campbell A E. Murine cytomegalovirus with a deletion of genes spanning HindIII-J and -I displays altered cell and tissue tropism. J Virol. 1996;70:1365–1374. doi: 10.1128/jvi.70.3.1365-1374.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chong K T, Mims C A. Murine cytomegalovirus particle types in relation to sources of virus and pathogenicity. J Gen Virol. 1981;57:415–419. doi: 10.1099/0022-1317-57-2-415. [DOI] [PubMed] [Google Scholar]
- 4.Collins T M, Quirk M R, Jordon M C. Biphasic viremia and viral gene expression in leukocytes during acute cytomegalovirus infection of mice. J Virol. 1994;68:6305–6311. doi: 10.1128/jvi.68.10.6305-6311.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Compton T, Nowlin D M, Cooper N R. Initiation of human cytomegalovirus infection requires initial interaction with cell surface heparan sulfate. Virology. 1993;193:834–841. doi: 10.1006/viro.1993.1192. [DOI] [PubMed] [Google Scholar]
- 6.Cousens L P, Orange J S, Su H C, Biron C A. Interferon-alpha/beta inhibition of interleukin 12 and interferon-gamma production in vitro and endogenously during viral infection. Proc Natl Acad Sci USA. 1997;94:634–639. doi: 10.1073/pnas.94.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eikelenboom P. Characterization of non-lymphoid cells in the white pulp of the mouse spleen: an in vivo and in vitro study. Cell Tissue Res. 1978;195:445–460. doi: 10.1007/BF00233888. [DOI] [PubMed] [Google Scholar]
- 8.Fish K N, Britt W, Nelson J A. A novel mechanism for persistence of human cytomegalovirus in macrophages. J Virol. 1996;70:1855–1862. doi: 10.1128/jvi.70.3.1855-1862.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fish K N, Depto A S, Moses A V, Britt W, Nelson J A. Growth kinetics of human cytomegalovirus are altered in monocyte-derived macrophages. J Virol. 1995;69:3737–3743. doi: 10.1128/jvi.69.6.3737-3743.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gribaudo G, Ravaglia S, Gaboli M, Gariglio M, Cavallo R, Landolfo S. Interferon-alpha inhibits the murine cytomegalovirus immediate-early gene expression by down-regulating NF-κ activity. Virology. 1995;211:251–260. doi: 10.1006/viro.1995.1398. [DOI] [PubMed] [Google Scholar]
- 11.Hahn G, Jores R, Mocarski E S. Cytomegalovirus remains latent in a common precursor of dendritic and myeloid cells. Proc Natl Acad Sci USA. 1998;95:3937–3942. doi: 10.1073/pnas.95.7.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanson, L. K., B. L. Dalton, Z. Karabekian, H. E. Farrell, W. D. Rawlinson, R. M. Stenberg, and A. E. Campbell. Transcriptional analysis of the murine cytomegalovirus HindIII-I region: identification of a novel immediate early gene region. Submitted for publication. [DOI] [PubMed]
- 13.Hanson, L. K., J. S. Slater, H. W. Virgin IV, and A. E. Campbell. Unpublished data.
- 14.He X, Yoshida H, Minamishima Y, Nomoto K. Analysis of the role of CD4+ T-cells during murine cytomegalovirus infection in different strains of mice. Virus Res. 1995;36:233–245. doi: 10.1016/0168-1702(95)00010-n. [DOI] [PubMed] [Google Scholar]
- 15.Heise M T, Connick M, Virgin H W., IV Murine cytomegalovirus inhibits interferon gamma-induced antigen presentation to CD4 T cells by macrophages via regulation of expression of major histocompatibility complex class II-associated genes. J Exp Med. 1998;187:1037–1046. doi: 10.1084/jem.187.7.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heise M T, Pollock J L, O’Guin A, Barkon M L, Bormley S, Virgin H W., IV Murine cytomegalovirus infection inhibits IFN gamma-induced MHC class II expression on macrophages: the role of type I interferon. Virology. 1998;241:331–344. doi: 10.1006/viro.1997.8969. [DOI] [PubMed] [Google Scholar]
- 17.Heise M T, Virgin H W., IV The T-cell-independent role of gamma interferon and tumor necrosis factor alpha in macrophage activation during murine cytomegalovirus and herpes simplex virus infections. J Virol. 1995;69:904–909. doi: 10.1128/jvi.69.2.904-909.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ibanez C E, Schrier R, Ghazal P, Wiley C, Nelson J A. Human cytomegalovirus productively infects primary differentiated macrophages. J Virol. 1991;65:6581–6588. doi: 10.1128/jvi.65.12.6581-6588.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katzenstein D A, Yu G S, Jordan M C. Lethal infection with murine cytomegalovirus after early viral replication in the spleen. J Infect Dis. 1983;148:406–411. doi: 10.1093/infdis/148.3.406. [DOI] [PubMed] [Google Scholar]
- 20.Koffron A J, Hummel J, Patterson B K, Yan S, Kaufman D B, Fryer J P, Stuart F P, Abecassis M I. Cellular localization of latent murine cytomegalovirus. J Virol. 1998;72:95–103. doi: 10.1128/jvi.72.1.95-103.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohler C P, Kerry J A, Carter M, Muzithras V P, Jones T R, Stenberg R M. Use of recombinant virus to assess human cytomegalovirus early and late promoters in the context of the viral genome. J Virol. 1994;68:6589–6597. doi: 10.1128/jvi.68.10.6589-6597.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kondo K, Xu J, Mocarski E S. Human cytomegalovirus latent gene expression in granulocyte-macrophage progenitors in culture and in seropositive individuals. Proc Natl Acad Sci USA. 1996;93:11137–11142. doi: 10.1073/pnas.93.20.11137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koszinowski U H, Del Val M, Reddehase M J. Cellular and molecular basis of the protective immune response to cytomegalovirus infection. Curr Top Microbiol Immunol. 1990;154:189–220. doi: 10.1007/978-3-642-74980-3_8. [DOI] [PubMed] [Google Scholar]
- 24.Larsson S, Soderberg-Naucler C, Wang F Z, Moller E. Cytomegalovirus DNA can be detected in peripheral blood mononuclear cells from all seropositive and most seronegative healthy blood donors over time. Transfusion (Bethesda) 1998;38:271–278. doi: 10.1046/j.1537-2995.1998.38398222871.x. [DOI] [PubMed] [Google Scholar]
- 25.Lathey J L, Spector S A. Unrestricted replication of human cytomegalovirus in hydrocortisone-treated macrophages. J Virol. 1991;65:6371–6375. doi: 10.1128/jvi.65.11.6371-6375.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leenen P J, Radosevic K, Voerman J S, Salomon B, van Rooijen N, Klatzmann D, van Ewijk W. Heterogeneity of mouse spleen dendritic cells: in vivo phagocytic activity, expression of macrophage markers, and subpopulation turnover. J Immunol. 1998;160:2166–2173. [PubMed] [Google Scholar]
- 27.Long D, Cohen G H, Muggeridge M I, Eisenberg R J. Cysteine mutants of herpes simplex virus type 1 glycoprotein D exhibit temperature-sensitive properties in structure and function. J Virol. 1990;64:5542–5552. doi: 10.1128/jvi.64.11.5542-5552.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lucin P, Jonjic S, Messerle M, Polic B, Hengel H, Koszinowski U H. Late phase inhibition of murine cytomegalovirus replication by synergistic action of interferon-gamma and tumour necrosis factor. J Gen Virol. 1994;75:101–110. doi: 10.1099/0022-1317-75-1-101. [DOI] [PubMed] [Google Scholar]
- 29.Maciejewski J P, Bruening E E, Donahue R E, Sellers S E, Carter C, Young N S, St. Jeor S. Infection of mononucleated phagocytes with human cytomegalovirus. Virology. 1993;195:327–336. doi: 10.1006/viro.1993.1383. [DOI] [PubMed] [Google Scholar]
- 30.Mauel J, Defendi V. Infection and transformation of mouse peritoneal macrophages by simian virus 40. J Exp Med. 1971;134:335–350. doi: 10.1084/jem.134.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mendelson M, Monard S, Sissons P, Sinclair J. Detection of endogenous human cytomegalovirus in CD34+ bone marrow progenitors. J Gen Virol. 1996;77:3099–3102. doi: 10.1099/0022-1317-77-12-3099. [DOI] [PubMed] [Google Scholar]
- 32.Michelson S. Interaction of human cytomegalovirus with monocytes/macrophages: a love-hate relationship. Pathol Biol. 1997;45:146–158. [PubMed] [Google Scholar]
- 33.Minton E J, Tysoe C, Sinclair J H, Sissons J G P. Human cytomegalovirus infection of the monocyte/macrophage lineage in bone marrow. J Virol. 1994;68:4017–4021. doi: 10.1128/jvi.68.6.4017-4021.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitchell B M, Leung A, Stevens J G. Murine cytomegalovirus DNA in peripheral blood of latently infected mice is detectable only in monocytes and polymorphonuclear leukocytes. Virology. 1996;223:198–207. doi: 10.1006/viro.1996.0468. [DOI] [PubMed] [Google Scholar]
- 35.Naito M, Nagaie H, Kawano S, Umeza H, Zhu H, Moriyama H, Yamamoto T, Takahashi H, Takei Y. Liposome-encapsulated dichloromethylene diphosphonate induces macrophage apoptosis in vivo and in vitro. J Leukoc Biol. 1996;60:337–344. doi: 10.1002/jlb.60.3.337. [DOI] [PubMed] [Google Scholar]
- 36.Orange J S, Biron C A. An absolute and restricted requirement for IL-12 in natural killer cell IFN-gamma production and antiviral defense. Studies of natural killer and T cell responses in contrasting viral infections. J Immunol. 1996;156:1138–1142. [PubMed] [Google Scholar]
- 37.Orange J S, Biron C A. Characterization of early IL-12, IFN-alpha/beta, and TNF effects on antiviral state and NK cell responses during murine cytomegalovirus infection. J Immunol. 1996;156:4746–4756. [PubMed] [Google Scholar]
- 38.Orange J S, Wang B, Terhorst C, Biron C A. Requirement for natural killer cell-produced interferon gamma in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration. J Exp Med. 1995;182:1045–1056. doi: 10.1084/jem.182.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papadimitriou J M, Shellam G R, Robertson T A. An ultrastructural investigation of cytomegalovirus replication in murine hepatocytes. J Gen Virol. 1984;65:1979–1990. doi: 10.1099/0022-1317-65-11-1979. [DOI] [PubMed] [Google Scholar]
- 40.Pavic I, Polic B, Crnkovic I, Lucin P, Jonjic S, Koszinowski U H. Participation of endogenous tumour necrosis factor alpha in host resistance to cytomegalovirus infection. J Gen Virol. 1993;74:2215–2223. doi: 10.1099/0022-1317-74-10-2215. [DOI] [PubMed] [Google Scholar]
- 41.Pollock J L, Presti R M, Paetzold S, Virgin H W., IV Latent murine cytomegalovirus infection in macrophages. Virology. 1997;227:168–179. doi: 10.1006/viro.1996.8303. [DOI] [PubMed] [Google Scholar]
- 42.Pollock J L, Virgin H W., IV Latency, without persistence, of murine cytomegalovirus in the spleen and kidney. J Virol. 1995;69:1762–1768. doi: 10.1128/jvi.69.3.1762-1768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rawlinson W D, Farrell H E, Barrell B G. Analysis of the complete DNA sequence of murine cytomegalovirus. J Virol. 1996;70:8833–8849. doi: 10.1128/jvi.70.12.8833-8849.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reynolds R P, Rahija R J, Schenkman D I, Richter C B. Experimental murine cytomegalovirus infection in severe combined immunodeficient mice. Lab Anim Sci. 1993;43:291–295. [PubMed] [Google Scholar]
- 45.Ruzek, M. C., and C. A. Biron. Unpublished data.
- 46.Ruzek M C, Miller A H, Opal S M, Pearce B D, Biron C A. Characterization of early cytokine responses and an IL-6 dependent pathway of endogenous glucocorticoid induction during murine cytomegalovirus infection. J Exp Med. 1997;185:1185–1192. doi: 10.1084/jem.185.7.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salazar-Mather T, Orange J, Biron C. Early murine cytomegalovirus (MCMV) infection induced liver natural killer (NK) cell inflammation and protection through MIP-1α-dependent pathways. J Exp Med. 1998;187:1–14. doi: 10.1084/jem.187.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salazar-Mather T P, Ishikawa R, Biron C A. NK cell trafficking and cytokine expression in splenic compartments after IFN induction and viral infection. J Immunol. 1996;157:3054–3064. [PubMed] [Google Scholar]
- 49.Sinclair J, Sissons P. Latent and persistent infections of monocytes and macrophages. Intervirology. 1996;39:293–301. doi: 10.1159/000150501. [DOI] [PubMed] [Google Scholar]
- 50.Sinclair J H, Baillie J, Bryant L A, Taylor-Wiedeman J A, Sissons J G. Repression of human cytomegalovirus major immediate early gene expression in a monocytic cell line. J Gen Virol. 1992;73:433–435. doi: 10.1099/0022-1317-73-2-433. [DOI] [PubMed] [Google Scholar]
- 51.Sinzger C, Plachter B, Grefte A, The T, Jahn G. Tissue macrophages are infected by human cytomegalovirus in vivo. J Infect Dis. 1996;173:240–245. doi: 10.1093/infdis/173.1.240. [DOI] [PubMed] [Google Scholar]
- 52.Slater, J. S., L. K. Hanson, and A. E. Campbell. Unpublished data.
- 53.Soderberg-Naucler C, Fish K N, Nelson J A. Interferon-gamma and tumor necrosis factor-alpha specifically induce formation of cytomegalovirus-permissive monocyte-derived macrophages that are refractory to the antiviral activity of these cytokines. J Clin Investig. 1997;100:3154–3163. doi: 10.1172/JCI119871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soderberg-Naucler C, Fish K N, Nelson J A. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell. 1997;91:119–126. doi: 10.1016/s0092-8674(01)80014-3. [DOI] [PubMed] [Google Scholar]
- 55.Steffens H P, Podlech J, Kurz S, Angele P, Dreis D, Reddehase M J. Cytomegalovirus inhibits the engraftment of donor bone marrow cells by downregulation of hematopoietin gene expression in recipient stroma. J Virol. 1998;72:5006–5015. doi: 10.1128/jvi.72.6.5006-5015.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stoddart C A, Cardin R D, Boname J M, Manning W C, Abenes G B, Mocarski E S. Peripheral blood mononuclear phagocytes mediate dissemination of murine cytomegalovirus. J Virol. 1994;68:6243–6253. doi: 10.1128/jvi.68.10.6243-6253.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tay C H, Welsh R M. Distinct organ-dependent mechanisms for the control of murine cytomegalovirus infection by natural killer cells. J Virol. 1997;71:267–275. doi: 10.1128/jvi.71.1.267-275.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taylor-Wiedeman J, Sissons P, Sinclair J. Induction of endogenous human cytomegalovirus gene expression after differentiation of monocytes from healthy carriers. J Virol. 1994;68:1597–1604. doi: 10.1128/jvi.68.3.1597-1604.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Bruggen I, Price P, Robertson T, Papadimitriou J. Morphological and functional changes during cytomegalovirus replication in murine macrophages. J Leukoc Biol. 1989;46:508–520. doi: 10.1002/jlb.46.6.508. [DOI] [PubMed] [Google Scholar]
- 60.Vanderkerken K, Bouwens L, van Rooijen N, van den Berg K, Baekeland M, Wisse E. The role of Kupffer cells in the differentiation process of hepatic natural killer cells. Hepatology. 1995;22:283–290. [PubMed] [Google Scholar]
- 61.van Rooijen N, Bakker J, Sanders A. Transient suppression of macrophage functions by liposome-encapsulated drugs. Trends Biotechnol. 1997;15:178–185. doi: 10.1016/s0167-7799(97)01019-6. [DOI] [PubMed] [Google Scholar]
- 62.van Rooijen N, Kors N, Kraal G. Macrophage subset repopulation in the spleen: differential kinetics after liposome-mediated elimination. J Leukoc Biol. 1989;45:97–104. doi: 10.1002/jlb.45.2.97. [DOI] [PubMed] [Google Scholar]
- 63.van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 64.van Rooijen N, Sanders A, van den Berg T K. Apoptosis of macrophages induced by liposome-mediated intracellular delivery of clodronate and propamidine. J Immunol Methods. 1996;193:93–99. doi: 10.1016/0022-1759(96)00056-7. [DOI] [PubMed] [Google Scholar]
- 65.Waldman W J, Knight D A, Huang E H, Sedmak D D. Bidirectional transmission of infectious cytomegalovirus between monocytes and vascular endothelial cells: an in vitro model. J Infect Dis. 1995;171:263–272. doi: 10.1093/infdis/171.2.263. [DOI] [PubMed] [Google Scholar]
- 66.Yamaguchi T, Shinagawa Y, Pollard R B. Relationship between the production of murine cytomegalovirus and interferon in macrophages. J Gen Virol. 1988;69:2961–2971. doi: 10.1099/0022-1317-69-12-2961. [DOI] [PubMed] [Google Scholar]
- 67.Yeow W S, Lai C M, Beilharz M W. The in vivo expression patterns of individual type I interferon genes in murine cytomegalovirus infection. Antivir Res. 1997;34:17–26. doi: 10.1016/s0166-3542(96)01018-2. [DOI] [PubMed] [Google Scholar]
- 68.Yerkovich S T, Olver S D, Lenzo J C, Peacock C D, Price P. The roles of tumor necrosis factor-α, interleukin-1, and interleukin-12 in murine cytomegalovirus infection. Immunology. 1997;91:45–52. doi: 10.1046/j.1365-2567.1997.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhuravskaya T, Maciejewski J P, Netski D M, Bruening E, Mackintosh F R, St. Jeor S. Spread of human cytomegalovirus (HCMV) after infection of human hematopoietic progenitor cells: model of HCMV latency. Blood. 1997;90:2482–2491. [PubMed] [Google Scholar]