Abstract
To investigate receptor-mediated Moloney murine leukemia virus (MoMuLV) entry, the green fluorescent protein (GFP)-tagged ecotropic receptor designated murine cationic amino acid transporter (MCAT-1) (MCAT-1-GFP) was constructed and expressed in 293 cells (293/MCAT-1-GFP). 293/MCAT-1-GFP cells displayed green fluorescence primarily at the cell membrane and supported wild-type levels of MoMuLV vector binding and transduction. Using immunofluorescence labeling and confocal microscopy, it was demonstrated that the surface envelope protein (SU) gp70 of MoMuLV virions began to appear inside cells 5 min after virus binding and was colocalized with MCAT-1-GFP. However, clathrin was not colocalized with MCAT-1-GFP, suggesting that MoMuLV entry, mediated by MCAT-1, does not involve clathrin. Double immunofluorescence labeling of SU and clathrin in 293 cells expressing untagged receptor (293/MCAT-1) gave the same results, i.e., SU and clathrin did not colocalize. In addition, we examined the transduction ability of MoMuLV vector on HeLa cells overexpressing the dominant-negative GTPase mutant of dynamin (K44A). HeLa cells overexpressing mutant dynamin have a severe block in endocytosis by the clathrin-coated-pit pathway. No significant titer difference was observed when MoMuLV vector was tranduced into HeLa cells overexpressing either wild-type or mutant dynamin, while the transduction ability of vesicular stomatitis virus glycoprotein pseudotyped vector into HeLa cells overexpressing mutant dynamin was decreased significantly. Taken together, these data suggest that MoMuLV entry does not occur through the clathrin-coated-pit-mediated endocytic pathway.
The envelope protein of ecotropic murine leukemia virus (MuLV) is composed of two different subunits, surface (SU) glycoprotein (gp70) and transmembrane (TM) protein (p15E) (72, 73). The SU subunit is responsible for virus binding to its specific receptor, murine cationic amino acid transporter (MCAT-1) (3, 32, 46, 68, 74), and the TM subunit is involved in fusion between the viral membrane and the host cell membrane (4, 16, 22, 76, 77). For the virus to infect target cells, it needs to deliver its genome into the cell either by fusion of the viral membrane with the plasma membrane or by fusion with the endosome membrane after endocytosis. Although the mechanisms of these entry pathways are poorly understood, previous studies suggest that human immunodeficiency virus (33, 38, 59), avian leukosis virus subgroup A (13), and amphotropic MuLV (40) appear to enter cells by direct fusion on the cell surface following receptor binding, while vesicular stomatitis virus (VSV) (36, 61) and influenza virus (37) enter cells by endocytosis. In the latter case, following virus binding to receptor and internalization, low pH in the endosome triggers exposure of the fusion peptide (which resides at the N terminus of TM) to mediate fusion between the viral membrane and the endosome membrane, releasing the viral core into the cytoplasm (6, 7). Low-pH-triggered fusion of the glycoprotein of VSV (VSV-G) (61) and influenza virus is inhibited by lysosomotropic agents that block endosomal acidification (28).
Several lines of evidence support the idea that ecotropic MuLV enters cells by endocytosis. Ecotropic Moloney MuLV (MoMuLV) entry into NIH 3T3, SC-1, normal rat kidney, and Rat-1 cells is sensitive to lysosomotropic agents, suggesting that the MoMuLV entry is pH dependent (40). Risco et al. (53) demonstrated by immunoelectron microscopy that both SU and TM of MoMuLV appear inside NIH 3T3 cells in different-sized vesicles after infection, which is consistent with the idea that MoMuLV infects NIH 3T3 cells through endocytic vesicles. Recently, it has been demonstrated that different cell lines require different components of host cell cytoskeleton for ecotropic MuLV entry (26). Entry into NIH 3T3 cells and XC cells is greatly diminished by the disruption of the actin cytoskeleton before, but not shortly after, virus internalization, implying a critical role for actin in both cell lines in the early steps of ecotropic MuLV entry (26). However, disruption of microtubules before and shortly after virus internalization markedly reduces entry into NIH 3T3 cells, while entry into XC cells remains efficient, suggesting that intact microtubules are required in a postpenetration step unique to efficient virus entry via endocytosis (26). Taken together, these data indicate that ecotropic MuLV infects cells by endocytosis, but the specific entry pathway may differ in different cell lines. However, transformed cell lines, such as rat XC cells and NIH 3T3/DTras, are able to form syncytia after exposure to ecotropic MuLV at neutral pH (22, 27, 71), and syncytium formation in XC cells is not inhibited by lysosomotropic agents (40). In addition, C-terminal R-peptide-truncated MoMuLV can mediate syncytium formation even in nontransformed cell lines at neutral pH (49, 51). Therefore, although the reported pH dependence and immunoelectron microscopy studies suggest an endocytic pathway for ecotropic MuLV infection, the possibility of direct membrane fusion is not excluded.
In general, receptors that are endocytosed can either be constitutively endocytosed or require ligand induction. At least five different endocytic pathways (the clathrin-mediated pathway, the caveola-mediated pathway, a clathrin- and caveolin-independent pathway, macropinocytosis, and phagocytosis) are known, and the clathrin-mediated endocytic pathway is the major and best characterized adsorptive pathway (17, 52, 57). Clathrin is a cellular protein that is involved in receptor-mediated endocytosis and vesicle transport from the trans-Golgi network to the lysosome (54). Some receptors, like transferrin receptor and low-density lipoprotein receptor, are constitutively concentrated in clathrin-coated pits, while epidermal growth factor (EGF) receptor requires ligand-induced activation for concentration in coated pits.
Several viruses have been shown to infect cells by the clathrin-mediated endocytic pathway. Semliki Forest virus (35) and VSV (61) were observed by electron microscopy infect cells by a clathrin-mediated endocytic pathway. Clathrin-coated vesicle budding from the plasma membrane and the trans-Golgi network requires dynamin (21, 39). Dynamin, another component of clathrin-coated pits, is a member of the GTPase family that helps in pinching off the clathrin-coated pits from the plasma membrane. GDP-bound dynamin is randomly distributed in the clathrin-coated pits, and after GTP exchange followed by ligand binding to receptor, GTP-bound dynamin is concentrated around the neck of coated pits (20) to sever the coated pits and release the coated vesicle from the plasma membrane by using its GTPase activity (62). In HeLa cells overexpressing the dominant-negative GTPase mutant of dynamin (lys44 to ala44 [K44A]), endocytosis of transferrin, EGF, and adenovirus by clathrin-coated pits is severely reduced (12, 70).
There has been considerable effort to engineer ecotropic MoMuLV-derived envelopes to target different cell surface molecules. To engineer an efficient vector based on MoMuLV, it is helpful to understand the MoMuLV entry pathway that is mediated by its own receptor, MCAT-1 (1). Different endocytic pathways for different cell surface molecules have been studied either by using drugs that inhibit endosome acidification, by fluorescence, or by electron microscopy techniques (14, 15, 60, 63).
To this end, we tagged MCAT-1 with green fluorescence protein (GFP) to monitor the MoMuLV receptor during virus entry. 293 cells stably expressing newly constructed MCAT-1-GFP (293/MCAT-1-GFP) and untagged MCAT-1 (293/MCAT-1) were established. Using these cells, we conducted indirect immunofluorescence labeling followed by confocal microscopy to examine colocalization of receptor and SU, receptor and clathrin, and SU and clathrin. Our microscopy study shows that clathrin is colocalized neither with MCAT-1-GFP nor with SU, while the receptor is colocalized with SU during MoMuLV entry. Wild-type or K44A mutant dynamin overexpressing HeLa cells were engineered to stably express either MCAT-1 or MCAT-1-GFP to allow us to investigate the functional consequence of overexpression of mutant dynamin on MoMuLV transduction. The transduction efficiency of MoMuLV remained the same in both cells, indicating that MoMuLV entry is not regulated by dynamin. On the basis of these results, we conclude that MoMuLV entry does not depend on the clathrin-coated-pit-mediated endocytic pathway.
MATERIALS AND METHODS
Antibodies.
Rat monoclonal antibody 83A25, which recognizes the C-terminal region of MuLV gp70 (SU), was provided by L. Evans (NIH Rocky Mountain Laboratories, Hamilton, Mont.). The following reagents were purchased: mouse monoclonal anti-clathrin immunoglobulin M (IgM) antibody CHC 5.9, which recognizes the clathrin heavy chain of coated vesicles (American Research Products, Inc., Belmont, Mass.); R-phycoerythrin (R-PE)-conjugated goat anti-rat IgG (H+L) and Cy3-conjugated streptavidin (Jackson Immunoresearch Laboratories, Inc., West Grove, Pa.); anti-hemagglutinin (HA)-peroxidase (Boehringer Mannheim, Indianapolis, Ind.); biotinylated anti-rat IgG, biotinylated anti-mouse IgM (μ chain specific), and fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgM (μ chain specific) (Vector Laboratories, Burlingame, Calif.); and tetramethylrhodamine-conjugated transferrin (Molecular Probes, Eugene, Oreg.).
Plasmids.
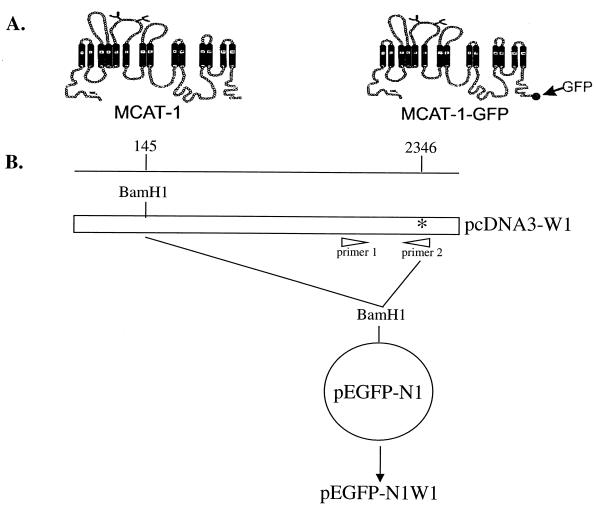
pJET and pcDNA3-W1IgG1 were provided by Lorraine M. Albritton (Memphis, Tenn.). pJET expresses an MCAT, and pcDNA3-W1IgG1 expresses an MCAT with an IgG1 epitope tag inserted into the PvuII site of the seventh extracellular loop. GFP expression plasmid pEGFP-N1 was purchased from Clontech (Palo Alto, Calif.). For the construction of MCAT-1 tagged with GFP at the C terminus (Fig. 1A), a 2-kb BamHI-EcoRI fragment encoding MCAT-1 was obtained by restriction enzyme digestion of pJET and substituted for the analogous sequence in pcDNA3-W1IgG1. The resulting vector, pcDNA3-W1, was used for the construction of GFP-tagged MCAT-1 (pEFGP-N1W1). A BamHI site was generated at the C terminus of MCAT-1 in pcDNA3-W1 by using oligonucleotide primers 1 (GGCTTTTTACCGGTAGCCGAG) and 2 (CAACCGCTGTCACCCTGGTGGGTGGCCGTGCACGCGGATCCGCTTTGCACTGGTCCAAGTTGC (underlining indicates the BamHI site) (Fig. 1B). The resulting 2-kb BamHI receptor fragment was inserted into the BamHI restriction site of the GFP expression vector (pEGFP-N1) in frame with the GFP sequence (Fig. 1B). GFP fusion with MCAT-1 was confirmed by enzyme digestion and sequencing (data not shown).
FIG. 1.
(A) Schematic representations of the receptor proteins. MCAT-1, ecotropic murine leukemia virus receptor; MCAT-1-GFP, GFP-tagged ecotropic murine leukemia virus receptor. (B) Schematic diagram of the construction of pEGFP-N1W1 (MCAT-1-GFP). pcDNA3-W1 is an MCAT-1 expression plasmid; pEGFP-N1 is a GFP expression plasmid; primer 1, GGCTTTTTACCGGTAGCCGAG; primer 2, CAACCGCTGTCACCCTGGTGGGTGGCCGTGCACGCGGATCCGCTTTGCACTGGTCCAAGTTGC (underlining indicates the BamHI site). ∗, stop codon for MCAT-1. Numbers along the map indicate amino acid positions on MCAT-1. Selected restriction enzyme sites are indicated.
Receptor mutants.
All the receptor protein point mutants were constructed in the GFP-tagged MCAT-1 expression vector pEGFP-N1W1, using the PCR-based mutagenesis kit (QuickChange Site-Directed Mutagenesis Kit; Stratagene, La Jolla, Calif.). Two complementary mutagenic oligonucleotides containing single or double mutations were used to introduce mutations. Segments of amplified sequences that contain single or double mutations were completely sequenced and digested with convenient restriction enzymes and inserted into the analogous sequences in the parental vector pEGFP-N1W1. Mutants are designated by the amino acid in the wild-type receptor protein followed by the residue number and amino acid changed in the mutant protein. The amino acid residues are numbered from the N terminus of MCAT-1 after the signal peptide is cleaved.
Cell lines.
293 cells and NIH 3T3 cells were maintained in D10 medium, which is Dulbecco’s modified essential medium (DMEM) (Cell Culture Core Facility, University of Southern California) supplemented with 10% fetal calf serum (Hyclone, Logan, Utah) and 2 mM glutamine (Gibco/BRL, Grand Island, N.Y.).
Ψ2nβg (#9) cells are Ψ2-derived ecotropic retroviral producer cells containing the pCnβg vector (18) that expresses the nuclear β-galactosidase gene. Ψ2 cells were transduced with an amphotropic retroviral supernatant (titer, 106 CFU/ml) that contains a vector plasmid encoding a nuclear β-galactosidase gene and a G418 resistance gene. G418-resistant clones were screened by growing transduced Ψ2 cells in D10 medium containing 0.55 mg of G418/ml. Selected G418-positive clones were further examined for β-galactosidase expression by X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining procedures. After selection, cells were maintained in D10.
The stable HeLa cells, wild-type HeLa and K44A HeLa, which overexpress wild-type dynamin and a dominant-negative GTPase mutant of dynamin, respectively, were provided by Sandra L. Schmid (Scripps Institute, La Jolla, Calif.). HeLa cells were maintained in D10 medium containing 400 μg of G418 (for selection for transactivator) (Gibco/BRL)/ml, 2 μg of tetracycline (to suppress dynamin expression) (Sigma, St. Louis, Mo.)/ml, and 200 ng of puromycin (for selection for dynamin) (Sigma)/ml.
Binding and titer determinations.
Binding was determined by a fluorescence-activated cell sorter (FACS) assay performed as described previously (75). Cells were suspended with trypsin-EDTA (Life Technologies, Grand Island, N.Y.), and a total of 5 × 105 cells were incubated with 1 ml of MoMuLV vector supernatant (titer, 2 × 107 CFU/ml) at 4°C for 2 h with gentle shaking. Cells were washed with 10% normal goat serum in 1× Dulbecco’s phosphate-buffered saline solution (PBS; Irvine Scientific, Santa Ana, Calif.). Cells were incubated with the antibody 83A25 at 4°C for 1 h and then with R-PE-conjugated goat anti-mouse IgG (H+L) at 4°C for 30 min as primary and secondary antibodies, respectively. Following two washes, cells were resuspended with 4% paraformaldehyde in 1× PBS and subjected to analysis by flow cytometry.
For titer determination on 293 cells, a total of 5 × 105 cells were seeded in a 6-well plate and 24 h later, medium was replaced with 1 ml of serially diluted Ψ2nβg (#9) supernatant containing Polybrene (8 μg/ml; Sigma). After overnight incubation, viral supernatant was replaced with fresh D10 medium, and cells were stained for nuclear β-galactosidase expression 24 h later. For titer determination on 293T cells expressing mutant receptor, a total of 1.5 × 106 293T cells in a 100-mm tissue culture dish were transfected with 30 μg of receptor expression plasmid by the calcium phosphate procedure as described previously (45). At 16 h after transfection, the DNA precipitate was removed and replaced with D10 medium for 24 h, after which transfected cells were replated in a 30-mm well of a 6-well tissue culture plate. Twenty-four hours later, the D10 medium was replaced with 1 ml of serially diluted Ψ2nβg (#9) supernatant containing Polybrene (8 μg/ml; Sigma). The cells were stained for nuclear β-galactosidase expression. For titer determination of HeLa cells, a total of 3 × 104 cells were plated in a 6-well plate, and cells were incubated with medium either with tetracycline (to suppress dynamin expression) or without tetracycline (to induce dynamin expression) at 37°C for ∼50 h. The cells were stained for nuclear β-galactosidase expression 24 h later.
Generation of stable 293 cells and HeLa cells.
To generate 293 cells stably expressing the wild-type untagged (pcDNA3-W1) or GFP-tagged (pEGFP-N1W1) MCAT gene, a total of 5 × 105 293 cells were seeded on a 60-mm plate and transfected with 30 μg of plasmid pcDNA3-W1 or plasmid pEGFP-N1W1 by calcium phosphate precipitation. pcDNA3-W1 and pEGFP-N1W1 encode the wild-type untagged or GFP-tagged MCAT gene (as well as a neomycin resistance gene), respectively. Transfected cells were cultured at 37°C in D10 medium containing 0.55 mg of G418/ml. G418-resistant clones were screened by FACS analysis and β-galactosidase staining for the ability to bind to and be transduced with Ψ2nβg (#9). For the generation of GFP-tagged MCAT-expressing 293 cells, positive clones were further screened for GFP expression by using fluorescence microscopy. After selection, cells were maintained in D10 medium.
To generate wild-type and K44A HeLa cells stably expressing the wild-type untagged or GFP-tagged MCAT gene, 60-mm plates seeded with 5 × 105 HeLa cells were cotransfected with a total of 30 μg of plasmid pHR5 and pcDNA3-w1 or pEGFP-N1W1 by calcium phosphate precipitation. Plasmid pHR5 encodes a hygromycin resistance gene (provided by Gene Therapy Inc., Gaithersburg, Md.). Transfected cells were cultured at 37°C in D10 medium containing 400 μg of G418/ml, 2 μg of tetracycline/ml, 200 ng of puromycin/ml, and 0.55 mg of hygromycin (Calbiochem, La Jolla, Calif.)/ml. Hygromycin-resistant clones were screened by FACS analysis and β-galactosidase staining for their ability to bind to and be transduced with Ψ2nβg (#9). For the generation of GFP-tagged MCAT-expressing HeLa cells, positive clones were further screened for GFP expression by using fluorescence microscopy. After selection, cells were maintained in D10 medium containing 400 μg of G418/ml, 2 μg of tetracycline/ml, and 200 ng of puromycin/ml.
Virus preparations using sucrose gradient centrifugation.
MoMuLV vector supernatants were purified in the following way. Vector supernatants were collected and subjected to centrifugation for 16 h at 8,000 rpm (JA14 rotor; Beckman, Palo Alto, Calif.) at 4°C. The resulting pellets were collected in D10 medium and carefully loaded onto the top of a sucrose gradient (5 to 55% sucrose [wt/wt] in 1× TNE [10 mM Tris-HCl, 100 mM NaCl, 1 mM EDTA; pH 7.2]) and centrifuged in an SW50 rotor at 30,000 rpm for 3 h at 4°C. The virus band was carefully removed by using an 18-gauge needle with a syringe and placed in a dialysis cassette (Slide-A-Lyzer 10K dialysis cassette; Pierce, Rockford, Ill.) for overnight dialysis against DMEM at 4°C with gentle agitation. For subsequent indirect immunofluorescence labeling and the transduction assay, fetal calf serum was added to the dialyzed virus to a final concentration of 10% to stabilize the recovered virus. To determine whether the virus was still intact, titer determination was carried out on NIH 3T3 cells by G418 selection (Gibco/BRL). To do so, 3 × 104 cells were plated in a 30-mm well of a 6-well tissue culture plate and 24 h later, medium was replaced with 1 ml of serially diluted purified virus containing Polybrene (8 μg/ml). After overnight incubation, cells were selected for G418 resistance by growth for 10 days in D10 medium containing G418 (0.5 mg/ml). G418-resistant colonies were counted after methylene blue staining.
Indirect immunofluorescence labeling and confocal microscopy analysis.
A 2-well glass chamber slide was coated with poly-l-lysine (Sigma) and laminin (Boehringer Mannheim). For immunofluorescence labeling of 293 cells, which stably express GFP-tagged MCAT (pEGFP-N1W1), for SU or clathrin, a total of 2 × 105 cells were seeded into each well and grown at 37°C overnight. Before labeling, 37°C D10 medium was replaced with cold D10 medium and the chamber slide was transferred to 4°C medium for 8 min. Chilled cells were rinsed with cold PBS once and incubated with MoMuLV supernatant (titer, 1 × 107 CFU/ml [multiplicity of infection, 30] versus 5 × 106 CFU/ml [multiplicity of infection, 15] for crude virus supernatant versus purified virus, respectively) at 4°C for 2 h for binding. At the end of the incubation, cells were rinsed twice with cold PBS and incubated at 37°C with 37°C prewarmed D10 medium for different time periods. At the end of each incubation, cells were fixed with 3.7% paraformaldehyde for 15 min and permeabilized with 0.1% Triton X-100 in PBS for 5 min at room temperature (RT). PBS containing 3% bovine serum albumin and 5% goat serum was used for blocking. After blocking, cells were incubated with primary and secondary antibodies for 3 h (or overnight at 4°) and 1 h, respectively. The primary antibodies used were 83A25 for SU labeling or mouse monoclonal anti-clathrin IgM antibody CHC 5.9 for clathrin labeling. Secondary antibodies were biotinylated anti-rat IgG for 83A25 and biotinylated anti-mouse IgM (μ chain specific) for mouse monoclonal anti-clathrin. Following washing with PBS five times at RT, cells were incubated with Cy3-conjugated streptavidin for 11 min at RT. After an additional 5 washings with PBS at RT, slides were mounted with mounting medium (Vectashield; Vector Laboratories).
Labeling was examined with a Zeiss LSM-2 laser scanning confocal microscope equipped with barrier filters for fluorescein and Cy3. A plan-neofluor 40× (numerical aperture, 1.3) oil immersion objective was used for the imaging of fluorescence-labeled cells. Image analysis was performed by using the standard system operating software provided with the Zeiss LSM microscope (version 2.08). Regions of colocalization were generated by a digital overlay and appear yellow. Color photomicrographs were produced with a Sony printer connected to the video output of the microscope. For double immunofluorescence labeling of SU and clathrin of 293 cells stably expressing untagged wild-type MCAT, the labeling procedure was carried out sequentially, using antibody 83A25, biotinylated anti-rat IgG, and Cy3-conjugated streptavidin for SU labeling, and then anti-clathrin, FITC-conjugated anti-mouse IgM (μ chain specific) for clathrin labeling.
Transferrin endocytosis in 293 cells.
A 2-well glass chamber slide that was coated with poly-l-lysine (Sigma) and laminin (Boehringer Mannheim) was seeded with a total of 2 × 105 293/MCAT-1-GFP cells on each well and grown at 37°C overnight. Before incubation of cells with transferrin, D10 medium at 37°C was replaced with cold D10 medium and the chamber slide was transferred to 4°C medium for 8 min. Chilled cells were rinsed with cold PBS once and incubated with tetramethylrhodamine-conjugated transferrin at 4°C for 2 h for binding. Cells were rinsed twice with cold PBS, 37°C prewarmed D10 medium was added, and the cells were incubated at 37°C for 30 min. The cells were then fixed with 3.7% paraformaldehyde for 15 min and rinsed with RT PBS, and slides were mounted with mounting medium (Vectashield). Slides were examined with a Zeiss LSM-2 laser scanning confocal microscope as described above.
Western analysis for dynamin expression.
One-hundred-millimeter-diameter plates were seeded with wild-type or K44A HeLa cells and were maintained with or without tetracycline for ∼50 h. Cells were collected by trypsinization and lysed with 250 μl of lysis buffer (100 mM Tris-HCl [pH 7.4], 1% Triton X-100, 0.05% sodium dodecyl sulfate [SDS], 5 mg of sodium deoxycholate/ml, 150 mM NaCl, 1 mM phenylmethylsulfonyl fluoride), and the protein concentration was determined by the Bradford protein assay (Bio-Rad, Hercules, Calif.). Next, 30 μg of protein was loaded onto an SDS–8 to 16% polyacrylamide gel and electrophoresed. Then the gel was transferred to Immobilon-P (Millipore Corporation, Bedford, Mass.). After blocking overnight at 4°C in 5% milk-TBST (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.1% Tween 20), the blot was incubated with 5 μg of anti-HA-peroxidase at RT for 1 h and developed by using the ECL system (Amersham, Buckinghamshire, England).
RESULTS
Characteristics of GFP-tagged MCAT-1 (MCAT-1-GFP).
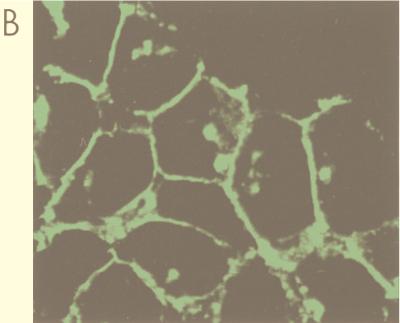
To study MoMuLV receptor (MCAT-1)-mediated MoMuLV entry, we tagged MCAT-1 with GFP at the C terminus (Fig. 1A). A BamHI site was generated at the C terminus of MCAT-1 by oligonucleotide-directed PCR, and the BamHI-BamHI receptor fragment that encodes the whole MCAT-1 was subcloned into a GFP expression vector (pEGFP-N1) in frame (Fig. 1B). To determine if the resulting GFP-tagged receptor (MCAT-1-GFP) behaves like wild-type untagged MCAT-1, MCAT-1 and MCAT-1-GFP were stably expressed in 293 cells (which do not express any endogenous MCAT-1). The resulting cell lines are called 293/MCAT-1 and 293/MCAT-1-GFP. 293/MCAT-1-GFP cells supported both wild-type binding and transduction (Fig. 2A). 293/MCAT-1-GFP cells displayed green fluorescence primarily around the cell membrane by UV absorption at 488 nm (Fig. 2B).
FIG. 2.
Functional analysis of MCAT-1 and MCAT-1-GFP. (A) 293, 293/MCAT-1, and 293/MCAT-1-GFP cells were incubated with MoMuLV at 4°C for 2 h. Binding ability of MCAT-1 and MCAT-1-GFP with MoMuLV was measured by FACS analysis after virus binding followed by incubation with anti-SU monoclonal antibody (83A25) and then R-PE-conjugated goat anti-rat IgG secondary antibody. Transduction ability was assayed on 293/MCAT-1 and 293/MCAT-1-GFP cells by measuring nuclear β-galactosidase activity after transducing cells with an ecotropic retroviral vector containing a nuclear β-galactosidase gene (Ψ2nβg#9). The titer on 3T3 cells was 4 × 106 CFU/ml. (B) 293/MCAT-1-GFP cells showed green fluorescence primarily at the cell surface under a fluorescence microscope.
MCAT-1-GFP was colocalized with MoMuLV SU during virus entry.
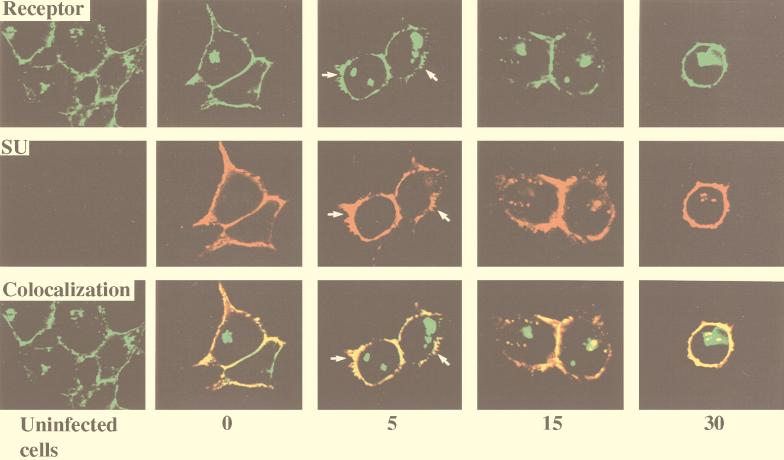
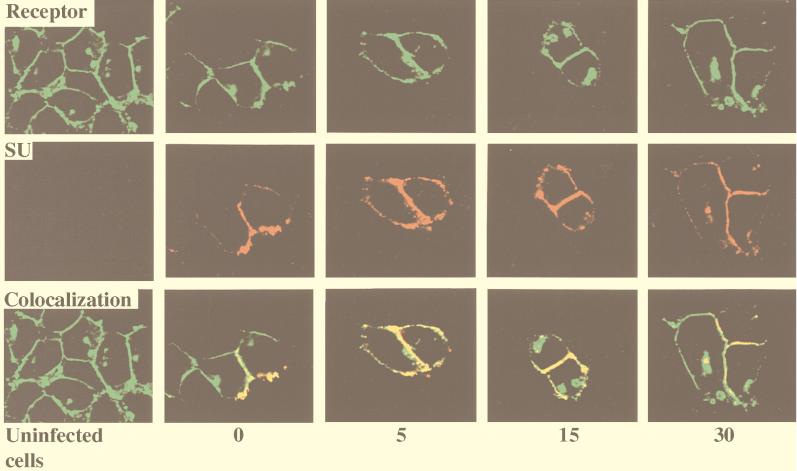
By constructing MCAT-1-GFP, we obtained an ecotropic MuLV receptor that demonstrates green fluorescence and retains wild-type receptor function. To investigate receptor-mediated MoMuLV entry, we visualized virus envelope protein and examined its colocalization with receptor during virus entry by indirect immunofluorescence labeling and confocal microscopy analysis. MoMuLV vector was bound to 293/MCAT-1-GFP cells and incubated at 37°C for different time periods (0, 5, 15, and 30 min). At the end of each incubation period, 293/MCAT-1-GFP cells were immunostained for virus envelope protein SU by using monoclonal antibody 83A25, which recognizes the C terminus of MoMuLV gp70 (SU). We then analyzed the colocalization of MCAT-1-GFP and SU, which appears yellow, by digital overlaying of both images. As indicated by the faint red staining inside the cell after a 5-min incubation, SU protein started to appear inside the cell after a 5-min incubation at 37°C (Fig. 3), and the stained SU protein was colocalized with MCAT-1-GFP inside the cell and at the cell surface. Also, significant membrane disturbance of the target cell was observed after a 5-min incubation at 37°C with MoMuLV vector (Fig. 3).
FIG. 3.
Colocalization of SU and MCAT-1-GFP in 293/MCAT-1-GFP cells after MoMuLV binding and following incubation at 37°C for different time periods. 293/MCAT-1-GFP cells were incubated with MoMuLV at 37°C for 0, 5, 15, or 30 min. Then cells were fixed, permeabilized, and stained with anti-SU (83A25), biotinylated goat anti-rat IgG secondary antibody, and Cy3-conjugated streptavidin. Color photomicrographs were produced with a Sony printer connected to the video output of the Zeiss confocal microscope. Arrows indicate significant membrane disturbance.
The control (uninfected) samples stained after incubating cells with D10 medium alone (Fig. 3) or stained only with secondary antibody and Cy3-conjugated streptavidin after incubating the cells with MoMuLV vector (data not shown) did not demonstrate staining. 293 cells (which do not express any endogenous MCAT-1) did not stain for SU protein after incubation with MoMuLV vector for the same time periods (0, 5, 15, and 30 min) (data not shown). We also immunostained 293/MCAT-1-GFP cells with antibody 83A25 after incubation with virions containing D84K mutant envelope proteins (which are defective in binding to MCAT-1) (32) or with virions containing L493V or L445E mutant envelope proteins (which are fusion defective but maintain wild-type binding ability) (76, 77). 293/MCAT-1-GFP cells incubated with virions containing the D84K mutant envelope showed no distinct or specific staining signals, even though Western analysis indicated the existence of viral particles in the viral supernatant (data not shown). On the other hand, cells incubated with virions containing L493V or L445E mutant envelope showed the same staining pattern as wild-type MoMuLV vector, i.e., SU protein colocalized with MCAT-1-GFP inside the cell and at the cell surface (data not shown). These data establish that the SU label is ascribed to the envelope proteins that bind to MCAT-1-GFP on the cell surface and inside the cell and that MoMuLV entry is specifically mediated by MCAT-1-GFP.
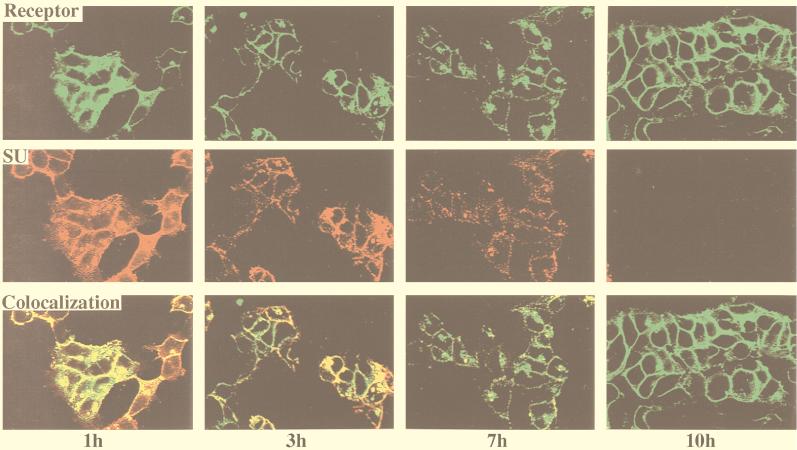
To examine the time course of virus-receptor interaction within the cell, we extended the 37°C incubation period to 10 h. SU labeling gradually disappeared with longer incubation times and completely disappeared after 7 to 10 h of incubation (Fig. 4). At all time points, the SU protein colocalized with MCAT-1-GFP.
FIG. 4.
Colocalization of SU and MCAT-1-GFP in 293/MCAT-1-GFP cells after MoMuLV binding and following incubation at 37°C for different time periods. 293/MCAT-1-GFP cells were incubated with MoMuLV at 37°C for 1, 3, 7, or 10 h. Cells were then fixed, permeabilized, and stained with anti-SU (83A25), biotinylated goat anti-rat IgG secondary antibody, and Cy3-conjugated streptavidin. Color photomicrographs were produced with a Sony printer connected to the video output of the Zeiss confocal microscope.
Colocalization of MCAT-1-GFP and SU using sucrose gradient-purified virus.
The vector supernatants used for the experiments described above were crude mixtures that contain viral particles, shed gp70, and membrane pieces with envelope proteins. To exclude the possibility that the resulting SU label was from the shed gp70 and membrane pieces with envelope proteins, we prepared virions that were purified by sucrose gradient centrifugation. Consistent with the data obtained with unpurified supernatants, SU labeling by using sucrose gradient-purified virus showed that SU protein started to appear inside the cell after 5 min of incubation at 37°C, and it was colocalized with MCAT-1-GFP inside the cell and at the cell surface (Fig. 5). To ensure that the purification process did not damage the virus’s transduction ability, we determined titers on NIH 3T3 cells by using aliquots of gradient-purified virus. The viral titers remained comparable before and after the purification procedure (1 × 107 versus 5 × 106).
FIG. 5.
Colocalization of SU and MCAT-1-GFP in 293/MCAT-1-GFP cells using purified viral particles after binding and following incubation at 37°C for different time periods. 293/MCAT-1-GFP cells were incubated with purified viral particles at 37°C for 0, 5, 15, or 30 min. Then cells were fixed, permeabilized, and stained with anti-SU (83A25), biotinylated goat anti-rat IgG secondary antibody, and Cy3-conjugated streptavidin. Color photomicrographs were produced with a Sony printer connected to the video output of the Zeiss confocal microscope.
No colocalization of clathrin and MCAT-1-GFP during MoMuLV entry.
The clathrin-mediated endocytic pathway is the best studied absorptive pathway and is needed by many cell surface molecules. It has also been demonstrated by electron microscopy and pH dependence assays that several viruses (i.e., influenza virus, VSV, and Semliki Forest virus) infect their target cells through a clathrin-mediated endocytic pathway (19, 37, 61). To investigate whether MoMuLV enters target cells by a clathrin-mediated endocytic pathway, we immunostained 293/MCAT-1-GFP cells for clathrin. 293/MCAT-1-GFP cells were bound with MoMuLV vector and incubated at 37°C for different time periods (0, 5, 15, and 30 min). Cells were stained for clathrin by using monoclonal antibody CHC 5.9, which recognizes clathrin heavy chain. Colocalization of MCAT-1-GFP and clathrin during MoMuLV entry was analyzed by digital overlaying of images. We observed membrane disturbance of the target cell after a 5-min incubation at 37°C that was similar to what we observed with SU staining (Fig. 6). However, unlike the SU staining, no significant colocalization of MCAT-1-GFP and clathrin was observed during these time periods (Fig. 6), suggesting that clathrin is not involved in MoMuLV entry. When incubated with D10 medium alone (Fig. 6), 293/MCAT-1-GFP cells showed a characteristic peripheral punctate pattern of plasma membrane clathrin-coated pits and perinuclear staining, which is consistent with the staining pattern of clathrin described in the literature (5, 14, 23).
FIG. 6.
Indirect immunofluorescence labeling of clathrin and confocal analysis of 293/MCAT-1-GFP cells after MoMuLV binding and incubation at 37°C for different time periods. 293/MCAT-1-GFP cells were bound with MoMuLV vector and incubated at 37°C for 0, 5, 15, or 30 min. Then cells were fixed, permeabilized, and stained with anti-clathrin monoclonal antibody (CHC 5.9), biotinylated goat anti-mouse IgM (μ chain specific) and Cy3-conjugated streptavidin. Color photomicrographs were produced with a Sony printer connected to the video output of the Zeiss confocal microscope. Arrows indicate significant membrane disturbance.
No colocalization of clathrin and SU during MoMuLV entry.
The above experiments demonstrate that MoMuLV entry is mediated by its receptor but suggest that clathrin is not involved. To confirm this conclusion and to test whether the GFP moiety at the C terminus of MCAT-1-GFP affects MoMuLV entry, we carried out double labeling for SU and clathrin in 293/MCAT-1 cells. Since 293/MCAT-1 cells do not express GFP, both SU and clathrin can be stained and visualized in the same cell with different colors of fluorescence, red (Cy3) and green (FITC), respectively. We analyzed the direct colocalization of SU and clathrin by digital overlaying of images. Consistent with the results obtained with 293/MCAT-1-GFP cells, SU protein began to appear inside the cell after a 5-min incubation at 37°C (Fig. 7). No colocalization of SU and clathrin was observed (Fig. 7), which is also consistent with the staining results of clathrin in 293/MCAT-1-GFP cells. These data indicate that clathrin is not involved in MCAT-1-mediated MoMuLV entry and that the C-terminal GFP moiety does not appear to influence viral entry.
FIG. 7.
Double immunofluorescence labeling of clathrin and SU in 293/MCAT-1 cells after MoMuLV vector binding and incubation at 37°C for different time periods. 293/MCAT-1 cells were bound with MoMuLV and incubated at 37°C for 0, 5, 15, or 30 min. Cells were fixed, permeabilized, and stained with anti-SU (83A25), biotinylated goat anti-rat IgG, Cy3-conjugated streptavidin for SU stain, and then with anti-clathrin monoclonal antibody (CHC 5.9), followed by goat FITC-mouse IgM (μ chain specific) for clathrin staining. Color photomicrographs were produced with a Sony printer connected to the video output of the Zeiss confocal microscope.
293 cells are competent for clathrin-mediated endocytosis.
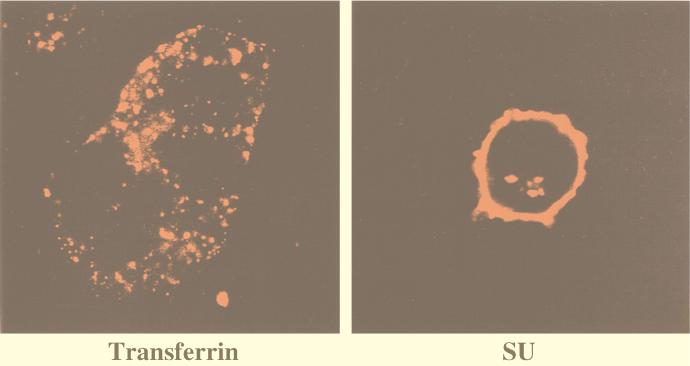
Having demonstrated that clathrin is not colocalized with MCAT-1-GFP nor with MoMuLV SU during virus entry, we next examined whether these 293 cells have a competent clathrin-mediated endocytic pathway. Transferrin is a well-studied ligand that is endocytosed by the clathrin-coated-pit pathway. We examined the entry of transferrin and compared it with that of MoMuLV SU. Tetramethylrhodamine-conjugated transferrin was efficiently internalized into 293/MCAT-1-GFP cells during a 30-min incubation at 37°C (Fig. 8, left panel). Furthermore, the entry pattern reflected the characteristic punctate pattern of clathrin (5, 14, 23). Finally, compared to the staining pattern of MoMuLV SU after a 30-min incubation (Fig. 8, right panel), a substantial amount of SU protein was still present at the cell surface, while a majority of transferrin was internalized after 30 min at 37°C (Fig. 8). 293/MCAT-1 cells were also shown to be competent for clathrin-mediated endocytosis by the transferrin entry study (data not shown).
FIG. 8.
Transferrin internalization in 293/MCAT-1-GFP cells. 293/MCAT-1-GFP cells were bound with tetramethylrhodamine-conjugated transferrin and incubated at 37°C for 30 min. Cells were fixed, and color photomicrographs were produced with a Sony printer connected to the video output of the Zeiss confocal microscope. (Left panel) Transferrin internalization at 37°C for 30 min. (Right panel) SU staining after virus incubation with 293/MCAT-1-GFP cells at 37°C for 30 min.
MoMuLV can transduce HeLa cells overexpressing a GTPase mutant of dynamin.
To further investigate whether clathrin is functionally critical for MoMuLV entry, we used HeLa cells that are defective in the clathrin-mediated endocytic pathway (K44A HeLa; lys44 to ala44) to analyze the transduction ability of the MoMuLV vector. K44A HeLa cells are defective in the clathrin-mediated endocytic pathway by overexpression of a dominant-negative GTPase mutant of dynamin (K44A) (12, 30, 70).
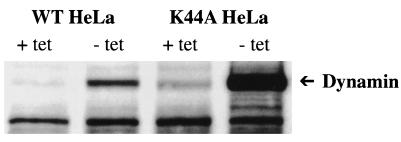
We performed Western analysis to examine the overexpression of wild-type and K44A mutant dynamins. Wild-type and mutant dynamins were detected in the corresponding HeLa cells (wild-type HeLa versus K44A HeLa) without tetracycline, as shown in Fig. 9. Then, we stably expressed MCAT-1 or MCAT-1-GFP in these HeLa cells. MCAT-1-expressing stable cells (wild-type HeLa/MCAT-1 versus K44A HeLa/MCAT-1) were selected by FACS analysis and X-Gal staining. MCAT-1-GFP-expressing cells (wild-type HeLa/MCAT-1-GFP versus K44A HeLa/MCAT-1-GFP) were selected by FACS analysis, X-Gal staining, and fluorescence microscopy analysis (data not shown). There was no significant titer difference, (2.7 ± 1.4) × 107 versus (1.8 ± 0.9) × 107, between wild-type HeLa/MCAT-1-GFP and K44A HeLa/MCAT-1-GFP cells when transduced with MoMuLV vector (Table 1). However, with VSV-G pseudotyped MoMuLV vector, the titer on K44A HeLa cells was 2.5 logs lower than that of wild-type HeLa, (7.5 ± 1.1) × 102 versus (3 ± 0.9) × 105, suggesting that the MoMuLV entry pathway is different from the VSV entry pathway and that a clathrin-coated-pit-mediated endocytic pathway is not responsible for MoMuLV transduction. We obtained similar results from wild-type HeLa/MCAT-1 and K44A HeLa/MCAT-1 cells, which also confirmed that the GFP moiety at the C terminus of MCAT-1-GFP does not affect the entry pathway of MoMuLV. Dynamin overexpression at the time of transduction by MoMuLV vector and VSV-G pseudotyped MoMuLV vector was confirmed by indirect immunofluorescence labeling of dynamin by using the same monoclonal antibody that was used for Western analysis (data not shown). We also observed no significant titer difference for either MoMuLV vector or VSV-G pseudotyped MoMuLV vector when HeLa cells were incubated with tetracycline to suppress dynamin overexpression (Table 1), which further confirms dynamin overexpression without tetracycline in HeLa cells.
FIG. 9.
Western analysis of the wild-type and the dominant-negative GTPase mutant of dynamin (K44A) by inducible expression in stably transformed HeLa cells. Wild-type dynamin-overexpressing HeLa cells (WT HeLa) and dominant-negative GTPase mutant of dynamin-overexpressing HeLa cells (K44A HeLa) were cultured in the presence (+ tet) or absence (− tet) of tetracycline for 50 h to suppress or induce dynamin expression, respectively. Cell lysates were separated on SDS–11 to 14% polyacrylamide gel under reducing conditions. After transfer to Immobilon-P, the blot was probed with anti-HA-peroxidase.
TABLE 1.
Comparison of the titers of MoMuLV and VSV-G from wild-type HeLa and K44A HeLa cells stably expressing MCAT-1 and MCAT-1-GFPa
| Virus (no. of experiments) and condition | Titer (CFU/ml) of virus in indicated cells stably expressing:
|
|||
|---|---|---|---|---|
| MCAT-1
|
MCAT-1-GFP
|
|||
| Wild-type HeLa | K44A HeLa | Wild-type HeLa | K44A HeLa | |
| MoMuLV (4) | ||||
| Without tetracycline | (3.0 ± 0.8) × 106 | (5.0 ± 2.0) × 106 | (2.7 ± 1.4) × 107 | (1.8 ± 0.9) × 107 |
| With tetracycline | (1.9 ± 0.3) × 106 | (2.2 ± 0.3) × 106 | (1.1 ± 1.1) × 107 | (1.0 ± 1.1) × 107 |
| VSV-G (3) | ||||
| Without tetracycline | (3.0 ± 0.5) × 105 | (2.0 ± 0.5) × 102 | (3.0 ± 0.9) × 105 | (7.5 ± 1.1) × 102 |
| With tetracycline | (3.6 ± 0.6) × 105 | (2.3 ± 1.2) × 105 | (2.7 ± 0.6) × 105 | (4.0 ± 1.7) × 105 |
Titers were averaged from independent experiments and are expressed as means ± standard deviations. Titers were measured with or without tetracycline at the same time in parallel experiments. The titers of MoMuLV vector and of VSV-G pseudotyped MoMuLV vector were also obtained at the same time in parallel experiments.
Disruption of putative internalization signal for clathrin-coated-pit-mediated endocytic pathway in the receptor protein does not affect transduction by MoMuLV.
Previous mutagenesis studies of transmembrane receptors suggest the existence of an “internalization signal” in the intracellular region. Various motif structures, such as NPX(1-4)Y, YXRF, YXX⊘ (⊘ is a bulky hydrophobic residue), dileucine, etc., have been identified for this role (8, 25, 31, 64, 69). We localized a putative NPX(1-4)Y motif structure (amino acids 425 to 431) and a putative YXX⊘ motif structure (amino acids 434 to 437) in the intracellular region of MCAT-1 and disrupted the motif structures by mutating tyrosine residues to alanine (Table 2). We constructed mutant receptor proteins with either a single-point mutation (Y425A or Y434A) or a double-point mutation (Y425A/Y434A). 293T cells transiently transfected with cDNA expressing mutated receptors with either a single or a double mutation were able to be transduced by ecotropic retroviral vectors, as were cells expressing wild-type MCAT-1 (Table 2).
TABLE 2.
Ecotropic virus titers on 293T cells that express mutant receptor protein
| Receptor proteina | Titer (CFU/ml)b of MoMuLV vector |
|---|---|
| Y425Ac | 3.5 × 106 |
| Y434Ac | 2 × 106 |
| Y425A/Y434A | 3 × 106 |
| Wild type | 4 × 106 |
Mutants are identified by the residue in the wild-type receptor protein, followed by its position and the substituted amino acid residue.
Titers were averaged from at least two independent experiments and are expressed as mean numbers of β-galactosidase-expressing colonies.
The locations of receptor mutants Y425A and Y434A are indicated by the first and second boldface letters, respectively, in the sequence LRYQPEQPNLVYQMARTTEELDRVDQNELVSASESQTGFL.
DISCUSSION
Data from this study indicate that MCAT-1-mediated MoMuLV entry can occur independent of the clathrin-coated-pit-mediated endocytic pathway. Based on immunofluorescence analysis, we have shown that clathrin is not involved in MoMuLV entry. Moreover, biochemical analysis demonstrated that the transduction ability of MoMuLV vector is not regulated by dynamin expression. In addition, disruption of a putative internalization signal in MCAT-1 did not affect the transduction ability of the MoMuLV vector.
The endocytic pathways of different ligands and cell surface molecules have been studied by indirect immunofluorescence labeling and confocal microscopy using antibodies that are specific to ligands, cell surface molecules, or cellular markers that reside in different stages of endocytic vesicles (14, 15, 60, 63). Since generating antibody specific to MCAT-1 or extracellular epitope-tagged receptor that retains wild-type receptor function has not been successful, we constructed GFP-tagged MCAT-1 (MCAT-1-GFP), which retains wild-type receptor function, to monitor MCAT-1 during MoMuLV entry. 293/MCAT-1-GFP cells showed bright green fluorescence primarily around the cell membrane and some inside the cell. Even though the green fluorescence inside the cell was not thoroughly analyzed, it has been shown by others (36a) that some labeled receptor protein is in the Golgi compartment.
By indirect immunofluorescence labeling followed by confocal microscopy analysis, we observed that, in both 293/MCAT-1 and 293/MCAT-1-GFP cells, small portions of MoMuLV SU label started to appear inside the cell after a 5-min incubation at 37°C and then gradually increased. When the 37°C incubation time period was prolonged, we observed that complete disappearance of SU labels inside the cell and on the cell surface took 7 to 10 h, which is consistent with the results of previous studies by Andersen and Nexø (2). They have shown that when lysates of 3T3 cells infected with B-tropic virus C57MC were separated by electrophoresis on SDS-polyacrylamide gel and analyzed by autoradiography, virus protein gp70 could be detected from the infected cells at up to 7 to 12 h of incubation at 37°C. In addition, in our study, colocalization of the receptor and the SU protein was also observed for the whole time (7 to 10 h), suggesting that the envelope protein, after binding, remains in contact with receptor inside the cell for an extended time and is recognized by specific antibody.
Even though the intracellular SU label appears to increase over the 37°C incubation time period, it is also true that substantial amounts of SU label colocalized with receptors is still present at the cell surface even after a 30-min incubation. This finding could be used to support the counterhypothesis that MoMuLV enters cells through direct membrane fusion. The significance of these populations of SU proteins in MoMuLV entry requires further investigation.
Immunofluorescence labeling and confocal microscopy analysis with purified virions (to exclude the staining of shed gp70 and membrane pieces containing envelope) gave the same results as the unpurified crude supernatants. A study by Yu et al. (75) showed that the anti-SU antibody used in our immunofluorescence labeling (83A25) could not recognize purified gp70. Taken together, these data indicate that the SU labels we observed in our study are not from shed gp70.
For biochemical analysis of MoMuLV entry, we used HeLa cells (K44A HeLa) overexpressing a dominant-negative GTPase mutant of dynamin, K44A. Dynamin, a 100-kDa GTPase, is a component of clathrin-coated pits that helps the formation of constricted clathrin-coated vesicles. Dynamin is the mammalian homologue of the Drosophila shibire gene product (9, 65). Mutations in shibire cause a defect in endocytosis, leading to the accumulation of clathrin-coated pits on the cell membrane (29, 67). Similarly, invaginated clathrin-coated pits accumulate on the surfaces of K44A HeLa cells overexpressing a dominant-negative GTPase mutant of dynamin (12, 66, 67), even though receptors on the cell surface can still bind to their ligands, establishing that dynamin is required for clathrin-coated-vesicle formation. In these HeLa cells, clathrin-coated-pit-mediated endocytosis of transferrin and EGF is blocked >80% and >60%, respectively (12). Using K44A HeLa cells, adenovirus (70) as well as ligands such as transferrin and EGF have been shown to utilize the clathrin-mediated endocytic pathway for receptor-mediated entry. In our study, both wild-type HeLa and K44A HeLa cells expressing MCAT-1 and MCAT-1-GFP demonstrated comparable binding and transduction ability by the MoMuLV vector, suggesting that dynamin is not critical for MCAT-1-mediated MoMuLV entry. Interestingly, while the mutant dynamin expression could significantly inhibit the transduction ability of VSV-G vector, the transduction ability was not completely abolished. Previous studies by Damke et al. (11) and Wang et al. (70) using the same HeLa cells to study the clathrin-mediated endocytosis of transferrin, EGF, and adenovirus infection demonstrated that ligand internalization and virus infection in these cells were not completely inhibited, suggesting the existence of an alternative pathway(s) (30). Since the transduction ability of the MoMuLV vector did not give a significant difference in HeLa cells expressing wild-type or mutant dynamin, MoMuLV may utilize this alternative pathway(s) for infection. Further studies to characterize these alternative pathways and their possible role in MoMuLV entry are necessary. Finally, we cannot exclude the possibility of an incomplete inhibition of endogenous dynamin by the overexpressed dominant-negative mutant form of dynamin in HeLa cells.
For transmembrane receptors that are endocytosed by the clathrin-coated-pit-mediated pathway either constitutively or by ligand activation, the intracellular region or C terminus of the receptors has been shown to be important for recruiting receptors into the clathrin-coated pits and for subsequent sorting to different stages of cellular trafficking within the cell (34, 42, 64). Mutagenesis studies of transferrin, EGF, and several G-protein-coupled multitransmembrane receptors support the idea of the existence of an internalization signal. Tyrosine-based motif structures are shown to bind in vitro with AP-2 protein (25, 50), a component of clathrin-coated pits. Separate domains of AP-2 protein can bind to clathrin and to some internalization signals, thereby allowing AP-2 protein to recruit transmembrane receptors and clathrin into the clathrin-coated pits. We constructed mutated receptors whose putative internalization signal for the clathrin-coated-pit-mediated endocytic pathway is disrupted. 293T cells expressing mutated receptors were able to be transduced by ecotropic retroviral vectors, as were cells expressing wild-type MCAT-1, demonstrating that the two tyrosine-based motifs in MCAT-1 do not play a significant role in MoMuLV transduction. These data further suggest that MoMuLV entry does not involve a clathrin-coated-pit-mediated endocytic pathway but the potential role of the two tyrosine-based motifs in MCAT-1 distribution or function requires further investigation. In addition, since we did not test every putative internalization signal of MCAT-1, further investigations will be needed to determine if there is an internalization signal in the intracellular region of MCAT-1 or the possibility of direct membrane fusion for MoMuLV entry.
Even though the clathrin-coated-pit-mediated endocytic pathway has been recognized as the best characterized adsorptive pathway so far, the discovery of non-clathrin-coated invaginations of the plasma membrane, caveolae, suggests that there is more than one pathway in the cell that can participate in cellular trafficking. Caveolae are specialized microdomains (55) that can also be located by immunolabeling the marker protein caveolin (43), and caveolae structures can be reconstituted in a cell-free system (55, 56). Originally, caveolae were postulated to play a role in transcytosis of molecules in polarized cells, but more recent work suggests that caveolae may also mediate receptor-mediated endocytosis. Caveolae appear to be involved in vesicular transport of toxins (44), ligands bound to GPI-anchored protein (47, 48), viruses (24), and seven-transmembrane surface proteins (55). Interestingly, immunohistochemistry of porcine pulmonary artery endothelial cells demonstrates the colocalization of cationic amino acid transporter (CAT-1) with caveolar structures (41). Therefore, although it is not clear whether CAT-1 and MCAT-1 utilize the same mechanism for amino acid transport and virus entry, respectively, it is still attractive to speculate that caveolae may play a role in ecotropic MuLV entry. However, a detectable amount of caveolin expression and morphologically identifiable caveolar structures are enriched only in certain cell types, such as lung endothelial and polarized cells (55). In addition, endothelial cells change phenotypically when isolated and grown under culture conditions that lead to a 10-fold-lower level of caveolae in vitro (55). Furthermore, it should be noted that not all non-clathrin-coated invaginations observed on the plasma membrane are caveolae. Non-clathrin-coated and non-caveola-coated invaginations that pinch off to form smooth vesicles carrying the fluid-phase marker horseradish peroxidase into cells have been extensively characterized in a number of cell types (30).
Ecotropic MoMuLV-derived retroviral vectors have been engineered to target different cell surface molecules for use in gene therapy. Modifications of the SU region of MoMuLV have been introduced by insertion or replacement with a single-chain antibody or ligand that is specific to a cell surface antigen or receptor (10, 58). Most of these targeted virions retained wild-type binding ability for their specific receptor or cognizant cell surface molecule but demonstrated very low or no transduction ability, suggesting the existence of a postbinding block in the engineered vectors (10, 58). These data indicate that retroviruses require specific properties of cell surface molecules to allow the release of viral cores into the cytoplasm. Further investigations to elucidate the details of the MCAT-1-mediated MoMuLV entry pathway are needed. These strategies will be helpful in designing better MoMuLV-based targetable retroviral vectors for gene therapy.
ACKNOWLEDGMENTS
We thank Ernesto Barron from the USC Electron Microscopy Core Facility for his assistance with confocal microscopy, Gengjie Yang for technical help, Paula M. Cannon for her critical reading of the manuscript, and Sandra L. Schmid from the Scripps Institute for providing the HeLa cells used in this study.
This work was supported by Genetic Therapy Inc. (GTI)/Novartis and by NIH grant CA 59318.
REFERENCES
- 1.Albritton L M, Tseng L, Scadden D, Cunningham J M. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell. 1989;57:659–666. doi: 10.1016/0092-8674(89)90134-7. [DOI] [PubMed] [Google Scholar]
- 2.Andersen K B, Nexø B A. Entry of murine retrovirus into mouse fibroblasts. Virology. 1983;125:85–98. doi: 10.1016/0042-6822(83)90065-x. [DOI] [PubMed] [Google Scholar]
- 3.Battini J L, Heard J M, Danos O. Receptor choice determinants in the envelope glycoproteins of amphotropic, xenotropic, and polytropic murine leukemia viruses. J Virol. 1992;66:1468–1475. doi: 10.1128/jvi.66.3.1468-1475.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berkowitz R D, Goff S P. Point mutations in Moloney murine leukemia virus envelope protein: effects on infectivity, virion association, and superinfection resistance. Virology. 1993;196:748–757. doi: 10.1006/viro.1993.1532. [DOI] [PubMed] [Google Scholar]
- 5.Bruder G, Wiedenmann B. Identification of a distinct 9S form of soluble clathrin in cultured cells and tissues. Exp Cell Res. 1986;164:449–462. doi: 10.1016/0014-4827(86)90043-1. [DOI] [PubMed] [Google Scholar]
- 6.Bullough P A, Hughson F M, Skehel J J, Wiley D C. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature. 1994;371:37–43. doi: 10.1038/371037a0. [DOI] [PubMed] [Google Scholar]
- 7.Carr C M, Kim P C. A spring-loaded mechanism for the conformational change of influenza hemagglutinin. Cell. 1993;73:823–832. doi: 10.1016/0092-8674(93)90260-w. [DOI] [PubMed] [Google Scholar]
- 8.Chen W J, Goldstein J L, Brown M S. NPXY, a sequence often found in cytoplasmic tail is required for coated pit-mediated internalization of the low density lipoprotein receptor. J Biol Chem. 1990;265:3116–3123. [PubMed] [Google Scholar]
- 9.Chen M S, Obar R A, Schroeder C C, Austin T W, Poodry C A, Wadsworth S C, Vallee R B. Multiple forms of dynamin are encoded by shibire, a Drosophila gene involved in endocytosis. Nature. 1991;351:583–586. doi: 10.1038/351583a0. [DOI] [PubMed] [Google Scholar]
- 10.Cosset F L, Morling F J, Takeuchi Y, Weiss R A, Collins M K L, Russel S J. Retroviral retargeting by envelopes expressing an N-terminal binding domain. J Virol. 1995;69:6314–6322. doi: 10.1128/jvi.69.10.6314-6322.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damke H, Baba T, Van der Bliek A M, Schmid S L. Clathrin-independent pinocytosis is induced in cells overexpressing a temperature-sensitive mutant of dynamin. J Cell Biol. 1995;131:69–80. doi: 10.1083/jcb.131.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damke H, Baba T, Warnock D E, Schmid S L. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilbert J M, Mason D, White J M. Fusion of Rous sarcoma virus with host cells does not require exposure to low pH. J Virol. 1990;64:5106–5113. doi: 10.1128/jvi.64.10.5106-5113.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodman O B, Jr, Krupnick J G, Santini F, Gurevich V V, Penn R B, Gagnon A W, Keen J H, Benovic J L. β-Arrestin acts as a clathrin adaptor in endocytosis of the β2-adrenergic receptor. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 15.Grady E F, Slice L W, Brant W O, Walsh J H, Payan D G, Bunnett N W. Direct observation of endocytosis of gastrin releasing peptide and its receptor. J Biol Chem. 1995;270:4603–4611. doi: 10.1074/jbc.270.9.4603. [DOI] [PubMed] [Google Scholar]
- 16.Gray K D, Roth M J. Mutational analysis of the envelope gene of Moloney murine leukemia virus. J Virol. 1993;67:3489–3496. doi: 10.1128/jvi.67.6.3489-3496.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gruenberg J, Maxfield F R. Membrane transport in the endocytic pathway. Curr Opin Cell Biol. 1995;7:552–563. doi: 10.1016/0955-0674(95)80013-1. [DOI] [PubMed] [Google Scholar]
- 18.Han J-H, Cannon P M, Lai K-M, Zhao Y, Eiden M V, Anderson W F. Identification of envelope protein residues required for the expanded host range of 10A1 murine leukemia virus. J Virol. 1997;71:8103–8108. doi: 10.1128/jvi.71.11.8103-8108.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helenius A, Kartenbeck J, Simons K, Fries E. On the entry of Semliki Forest virus into BKH-21 cells. J Cell Biol. 1980;84:404–420. doi: 10.1083/jcb.84.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinshaw J E, Schmid S L. Dynamin self-assembles into rings suggesting a mechanism for coated vesicle budding. Nature. 1995;374:190–192. doi: 10.1038/374190a0. [DOI] [PubMed] [Google Scholar]
- 21.Jones S M, Howell K E, Henley J R, Cao H, McNiven M A. Role of dynamin in the formation of transport vesicles from the trans-Golgi network. Science. 1998;279:573–577. doi: 10.1126/science.279.5350.573. [DOI] [PubMed] [Google Scholar]
- 22.Jones J S, Risser R. Cell fusion induced by the murine leukemia virus envelope glycoprotein. J Virol. 1993;67:67–74. doi: 10.1128/jvi.67.1.67-74.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kartenbeck J, Schmid E, Müller M, Franke W W. Immunological identification and localization of clathrin and coated vesicles in cultured cells and in tissues. Exp Cell Res. 1981;133:191–211. doi: 10.1016/0014-4827(81)90369-4. [DOI] [PubMed] [Google Scholar]
- 24.Kartenbeck J, Stuckenbrock H, Helenius A. Endocytosis of simian virus 40 into the endoplasmic reticulum. J Cell Biol. 1989;109:2721–2729. doi: 10.1083/jcb.109.6.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirchhausen T, Bonifacino J S, Riezman H. Linking cargo to vesicle formation: receptor tail interactions with coat proteins. Curr Opin Cell Biol. 1997;9:488–495. doi: 10.1016/s0955-0674(97)80024-5. [DOI] [PubMed] [Google Scholar]
- 26.Kizhatil K, Albritton L M. Requirements for different components of the host cell cytoskeleton distinguish ecotropic murine leukemia virus entry via endocytosis from entry via surface fusion. J Virol. 1997;71:7145–7156. doi: 10.1128/jvi.71.10.7145-7156.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klement V, Rowe W P, Hartley J W, Pugh W E. Mixed culture cytopathogenicity: a new test for growth of murine leukemia viruses in tissue culture. Proc Natl Acad Sci USA. 1969;63:753–759. doi: 10.1073/pnas.63.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koff W C, Knight V. Inhibition of influenza virus uncoating by rimantadine hydrochloride. J Virol. 1979;31:261–263. doi: 10.1128/jvi.31.1.261-263.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kosaka T, Ikeda K. Reversible blockage of membrane retrieval and endocytosis in the garland cell of the temperature-sensitive mutant of Drosophila melanogaster, shibiretsl. J Cell Biol. 1983;97:499–507. doi: 10.1083/jcb.97.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamaze C, Schmid S L. The emergence of clathrin-independent pinocytic pathways. Curr Opin Cell Biol. 1995;7:573–580. doi: 10.1016/0955-0674(95)80015-8. [DOI] [PubMed] [Google Scholar]
- 31.Letourneur F, Klausner R D. A novel di-leucine motif and a tyrosine-based motif independently mediate lysosomal targeting and endocytosis of CD3 chains. Cell. 1992;69:1143–1157. doi: 10.1016/0092-8674(92)90636-q. [DOI] [PubMed] [Google Scholar]
- 32.MacKrell A J, Soong N W, Curtis A M, Anderson W F. Identification of a subdomain in the Moloney murine leukemia virus envelope protein involved in receptor binding. J Virol. 1996;70:1768–1774. doi: 10.1128/jvi.70.3.1768-1774.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maddon P J, McDougal J S, Clapham P R, Dalgleish A G, Jamal S, Weiss R A, Axel R. HIV infection does not require endocytosis of its receptor, CD4. Cell. 1988;54:865–874. doi: 10.1016/s0092-8674(88)91241-x. [DOI] [PubMed] [Google Scholar]
- 34.Marks M S, Ohno H, Kirchhausen T, Bonifacino J S. Protein sorting by tyrosine-based signals: adapting to the Ys and wherefores. Trends Cell Biol. 1997;7:124–128. doi: 10.1016/S0962-8924(96)10057-X. [DOI] [PubMed] [Google Scholar]
- 35.Marsh M, Helenius A. Adsorptive endocytosis of Semliki Forest virus. J Mol Biol. 1980;142:439–454. doi: 10.1016/0022-2836(80)90281-8. [DOI] [PubMed] [Google Scholar]
- 36.Marsh M, Helenius A. Viral entry into animal cells. Adv Viral Res. 1989;36:107–151. doi: 10.1016/S0065-3527(08)60583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36a.Masuda, Mari. Personal communication.
- 37.Matlin K S, Reggio H, Helenius A, Simons K. Infectious entry pathway of influenza virus in a canine kidney cell line. J Cell Biol. 1982;91:601–613. doi: 10.1083/jcb.91.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McClure M O, Marsh M, Weiss R A. Human immunodeficiency virus infection of CD4-bearing cells occurs by a pH-independent mechanism. EMBO J. 1988;7:513–518. doi: 10.1002/j.1460-2075.1988.tb02839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McClure S J, Robinson P J. Dynamin, endocytosis and intracellular signalling (review) Mol Membr Biol. 1996;13:189–215. doi: 10.3109/09687689609160598. [DOI] [PubMed] [Google Scholar]
- 40.McClure M O, Sommerfelt M A, Marsh M, Weiss R A. The pH independence of mammalian retrovirus infection. J Gen Virol. 1990;71:767–773. doi: 10.1099/0022-1317-71-4-767. [DOI] [PubMed] [Google Scholar]
- 41.McDonald K K, Zharikov S, Block E R, Kilberg M S. A caveolar complex between the cationic amino acid transporter 1 and endothelial nitric-oxide synthase may explain the “argine paradox”. J Biol Chem. 1997;272:31213–31216. doi: 10.1074/jbc.272.50.31213. [DOI] [PubMed] [Google Scholar]
- 42.Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- 43.Monier S, Parton R G, Vogel F, Behlke J, Henke A, Kurzchalia T V. VIP21-caveolin, a membrane protein constituent of the caveolar coat, oligomerizes in vivo and in vitro. Mol Biol Cell. 1995;6:911–927. doi: 10.1091/mbc.6.7.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Montesano R, Roth J, Robert A, Orci L. Non-coated membrane invaginations are involved in binding and internalization of cholera and tetanus toxins. Nature. 1982;296:651–653. doi: 10.1038/296651a0. [DOI] [PubMed] [Google Scholar]
- 45.Morgan R A, Nussbaum O, Muenchau D D, Shu L, Couture L, Anderson W F. Analysis of the functional and host range-determining regions of the murine ecotropic and amphotropic retrovirus envelope protein. J Virol. 1993;67:4712–4721. doi: 10.1128/jvi.67.8.4712-4721.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ott D, Rein A. Basis for receptor specificity of nonecotropic murine leukemia virus surface glycoprotein gp70SU. J Virol. 1992;66:4632–4638. doi: 10.1128/jvi.66.8.4632-4638.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parton R G. Ultrastructural localization of gangliosides: GM1 is concentrated in caveolae. J Histochem Cytochem. 1994;42:155–166. doi: 10.1177/42.2.8288861. [DOI] [PubMed] [Google Scholar]
- 48.Parton R G, Joggerst B, Simons K. Regulated internalization of caveolae. J Cell Biol. 1994;127:1199–1215. doi: 10.1083/jcb.127.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ragheb J A, Anderson W F. pH-independent murine leukemia virus ecotropic envelope-mediated cell fusion: implications for the role of the R peptide and p12E TM in viral entry. J Virol. 1994;68:3220–3231. doi: 10.1128/jvi.68.5.3220-3231.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rapoport I, Miyazaki M, Boll W, Duckworth B, Cantley L C, Shoelson S, Kirchhausen T. Regulatory interactions in the recognition of endocytic sorting signals by AP-2 complexes. EMBO J. 1997;19:2240–2250. doi: 10.1093/emboj/16.9.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rein A, Mirro J, Haynes J G, Ernst S M, Nagashima K. Function of the cytoplasmic domain of a retroviral transmembrane protein: p15E-p2E cleavage activates the membrane fusion capability of the murine leukemia virus Env protein. J Virol. 1994;68:1773–1781. doi: 10.1128/jvi.68.3.1773-1781.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reizman H, Woodman P G, van Meer G, Marsh M. Molecular mechanisms of endocytosis. Cell. 1997;91:731–738. doi: 10.1016/s0092-8674(00)80461-4. [DOI] [PubMed] [Google Scholar]
- 53.Risco C, Menendez-Arias L, Copeland T D, da Silva P P, Oroszlan S. Intracellular transport of the murine leukemia virus during acute infection of NIH 3T3 cells: nuclear import of nucleocapsid protein and integrase. J Cell Sci. 1995;108:3039–3050. doi: 10.1242/jcs.108.9.3039. [DOI] [PubMed] [Google Scholar]
- 54.Schmid S L. Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu Rev Biochem. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- 55.Schnitzer J E, Oh P, Dvorak A M, Liu J, Mcintosh D P. Separation of caveolae from associated microdomains of GPI-anchored proteins. Science. 1995;269:1435–1439. doi: 10.1126/science.7660128. [DOI] [PubMed] [Google Scholar]
- 56.Schnitzer J E, Oh P, McIntosh D P. Role of GTP hydrolysis in fission of caveolae directly from plasma membrane. Science. 1996;274:239–242. doi: 10.1126/science.274.5285.239. . (Erratum, 274:1069.) [DOI] [PubMed] [Google Scholar]
- 57.Shpetner H S, Vallee R B. Identification of dynamin, a novel mechanochemical enzyme that mediates interaction between microtubles. Cell. 1989;59:421–432. doi: 10.1016/0092-8674(89)90027-5. [DOI] [PubMed] [Google Scholar]
- 58.Somia N V, Zoppe M, Verma I M. Generation of targeted retroviral vectors by using single-chain variable fragment: an approach to in vivo gene therapy. Proc Natl Acad Sci USA. 1995;92:7570–7574. doi: 10.1073/pnas.92.16.7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stein B S, Gowda S D, Lifson J D, Penhallow R C, Bensch K G, Engleman E G. pH-independent HIV entry into CD4-positive T cells via virus envelope fusion to the plasma membrane. Cell. 1987;49:659–668. doi: 10.1016/0092-8674(87)90542-3. [DOI] [PubMed] [Google Scholar]
- 60.Subtil A, Hémar A, Dautry-Varsat A. Rapid endocytosis of interleukin 2 receptors when clathrin-coated pit endocytosis is inhibited. J Cell Sci. 1994;107:3461–3468. doi: 10.1242/jcs.107.12.3461. [DOI] [PubMed] [Google Scholar]
- 61.Superti F, Seganti L, Ruggeri F M, Tinari A, Donelli G, Orsi N. Entry pathway of vesicular stomatitis virus into different host cells. J Gen Virol. 1987;68:387–399. doi: 10.1099/0022-1317-68-2-387. [DOI] [PubMed] [Google Scholar]
- 62.Sweitzer S M, Hinshaw J E. Dynamin undergoes a GTP-dependent conformational change causing vesiculation. Cell. 1998;93:1021–1029. doi: 10.1016/s0092-8674(00)81207-6. [DOI] [PubMed] [Google Scholar]
- 63.Tolbert L M, Lameh J. Human muscarinic cholinergic receptor Hm1 internalizes via clathrin-coated vesicles. J Biol Chem. 1996;271:17335–17342. doi: 10.1074/jbc.271.29.17335. [DOI] [PubMed] [Google Scholar]
- 64.Trowbridge I S, Collawn J F, Hopkins C R. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu Rev Cell Biol. 1993;7:124–128. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- 65.Urrutia R, Henley J R, Cook T, McNiven M A. The dynamins: redundant or distinct functions for an expanding family of related GTPases? Proc Natl Acad Sci USA. 1997;94:377–384. doi: 10.1073/pnas.94.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van der Blick A M, Meyerowitz E M. Dynamin-like protein encoded by the Drosophila shibire gene associated with vesicular traffic. Nature. 1991;351:411–414. doi: 10.1038/351411a0. [DOI] [PubMed] [Google Scholar]
- 67.Van der Blick A M, Redelmeier T M, Damke H, Tisdale E J, Meyerowitz E M, Schmid S L. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J Cell Biol. 1993;122:553–563. doi: 10.1083/jcb.122.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vogt M, Haggblom C, Swift S, Haas M. Specific sequences of the env gene determine the host range of two XC-negative viruses of the Rauscher virus complex. Virology. 1986;154:420–424. doi: 10.1016/0042-6822(86)90470-8. [DOI] [PubMed] [Google Scholar]
- 69.Voorhees P, Deignan E, van Donselaar E, Humphrey J, Marks M S, Peters P J, Bonifacino J S. An acidic sequence within the cytoplasmic domain of furin functions as a determinant of trans-Golgi network localization and internalization from the cell surface. EMBO J. 1995;14:4961–4975. doi: 10.1002/j.1460-2075.1995.tb00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang K, Huang S, Kapoor-Munshi A, Nemerow G. Adenovirus internalization and infection require dynamin. J Virol. 1998;72:3455–3458. doi: 10.1128/jvi.72.4.3455-3458.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilson C A, Marsh J W, Eiden M. The requirements for viral entry differ from those for virally induced syncytium formation in NIH 3T3/DTras cells exposed to Moloney murine leukemia virus. J Virol. 1992;66:7267–7269. doi: 10.1128/jvi.66.12.7262-7269.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Witte O N, Tsukamoto-Adey A, Weissman I L. Cellular maturation of oncornavirus glycoproteins: topological arrangement of precursor and product forms in cellular membrane. Virology. 1977;76:539–553. doi: 10.1016/0042-6822(77)90236-7. [DOI] [PubMed] [Google Scholar]
- 73.Witte O N, Wirth D F. Structure of the murine leukemia virus envelope glycoprotein precursor. J Virol. 1979;29:735–743. doi: 10.1128/jvi.29.2.735-743.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yoshimoto T, Yoshimoto E, Meruelo D. Identification of amino acid residues critical for infection with ecotropic murine leukemia retrovirus. J Virol. 1993;67:1310–1314. doi: 10.1128/jvi.67.3.1310-1314.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yu H, Soong N, Anderson W F. Binding kinetics of ecotropic (Moloney) murine leukemia retrovirus with NIH 3T3 cells. J Virol. 1995;69:6557–6562. doi: 10.1128/jvi.69.10.6557-6562.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao Y, Lee S, Anderson W F. Functional interactions between monomers of the retroviral envelope protein complex. J Virol. 1997;71:6967–6972. doi: 10.1128/jvi.71.9.6967-6972.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhu N-L, Cannon P M, Chen D, Anderson W F. Mutational analysis of the fusion peptide of Moloney murine leukemia virus transmembrane protein p15E. J Virol. 1998;72:1632–1639. doi: 10.1128/jvi.72.2.1632-1639.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]