Abstract
Background
Current understanding of post-COVID-19 syndrome in South Korea is primarily based on survey studies or research targeting specific patient groups, such as those hospitalized. Moreover, the majority of relevant studies have been conducted in European and North American populations, which may limit their applicability to the South Korean context. To address this gap, our study explores the one-year outcomes of COVID-19, focusing on the potential post-acute syndrome and all-cause mortality in South Korea.
Methods
This retrospective cohort study used nationwide claims data in South Korea, including adults aged >18 with records between January 20, 2020, and February 25, 2021. Patients were classified into COVID-19 and non-COVID-19 groups and matched 1:1 based on propensity scores. Primary outcomes were 12-month post-acute COVID-19 syndrome and all-cause mortality.
Results
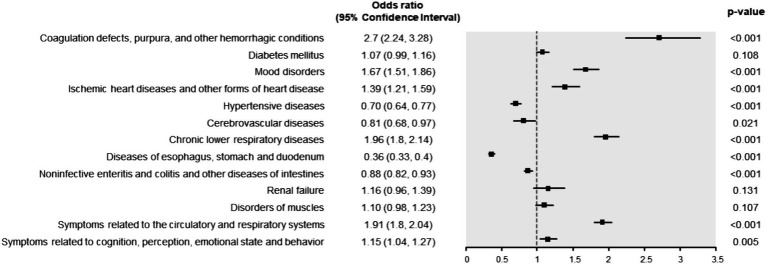
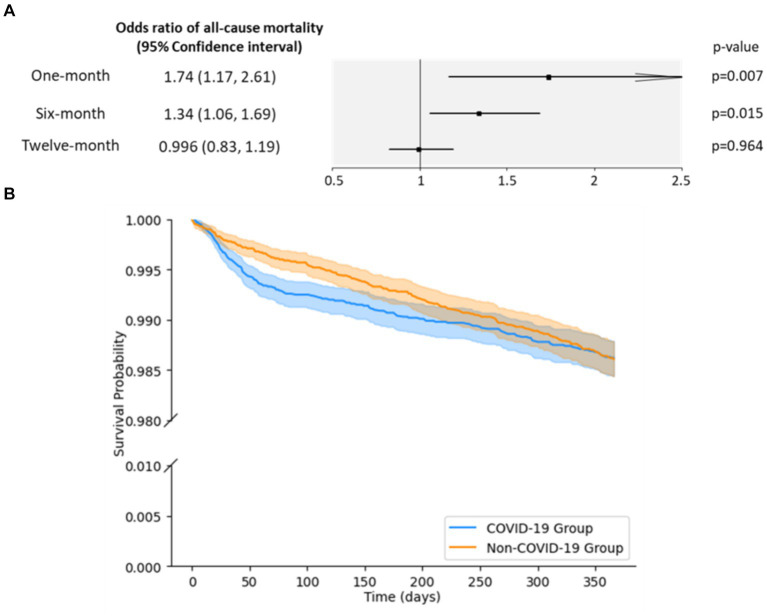
The study involved 34,802 matched patients. The COVID-19 group had significantly elevated risks of coagulopathies (OR = 2.70 [2.24, 3.28]; p < 0.001), chronic lower respiratory diseases (OR = 1.96 [1.80, 2.14]; p < 0.001), symptoms of the circulatory and respiratory systems (OR = 1.91 [1.80, 2.04]; p < 0.001), mood disorders (OR = 1.67 [1.51, 1.86]; p < 0.001), cardiac diseases (OR = 1.39 [1.21, 1.59]; p < 0.001), and symptoms of cognition, perception, emotional state, and behavior (OR = 1.15 [1.04, 1.27]; p = 0.005). All-cause mortality was higher in the COVID-19 group during the 6 months (OR = 1.34 [1.06, 1.69]; p = 0.015), but gradually decreased, reaching an OR of 0.996 ([0.83, 1.19]; p = 0.964) at 1 year.
Conclusion
In South Korea, the 12-month post-acute COVID-19 syndrome includes coagulopathies, respiratory issues, mood disorders, and cardiac diseases. The risk of all-cause mortality post-COVID-19 is heightened for up to 6 months, then significantly decreases and resolves within a year.
Keywords: COVID-19, post-acute sequelae of COVID-19, mortality, South Korea, cohort studies
1. Introduction
Post-acute COVID-19 Syndrome is characterized by a range of new, recurring, or persistent symptoms or conditions that COVID-19 survivors experience beyond the acute phase (1). The prevalence of post-acute COVID-19 syndrome ranges from 5 to 50%, depending on factors such as the definition used, the population studied, and the time period observed (2). Health complications associated with post-acute COVID-19 Syndrome include, but are not limited to, thromboembolic disorders, neuropsychiatric issues, and chronic fatigue syndrome (3).
Although progress has been made in understanding the long-term effects of COVID-19 up to one-year post-infection, knowledge gaps still remain. For example, previous investigations have been studied in specific patient groups such as hospitalized, or were conducted predominantly in European and American countries, with relatively few studies focusing on Asian populations, especially in South Korea (4–7). Furthermore, much of the existing research in Asia focused only on subjective findings based on survey or narrowly targeted specific medical conditions (8–12).
Motivated by the knowledge gap, we investigated the one-year consequences of COVID-19, focusing on the potential post-acute COVID-19 syndrome and all-cause mortality in South Korea. Leveraging the wide coverage of a nationwide population-based claims database in South Korea (13–15), we aim to understand how COVID-19 affects the general population across all demographics and varying COVID-19 severities. To this end, we identified diseases, symptoms and all-cause mortality experienced by individuals who contracted COVID-19 prior to the initiation of the COVID-19 vaccination in South Korea. We then evaluated whether the diseases, symptoms and all-cause mortality happened more frequently or less in individuals who were infected with COVID-19 than in those not in the same period.
2. Materials and methods
2.1. Data source
We used a nationwide claims database named the HIRA Covid-19 OMOP database, provided by the Health Insurance Review & Assessment Service (HIRA) in South Korea, standardized according to the Observational Medical Outcomes Partnership Common Data Model (OMOP CDM, version 5.3) (13). Maintained by the governmental institute in South Korea, the HIRA Covid-19 OMOP database contains information about COVID-19 diagnosis, managed by the Korea Disease Control and Prevention Agency, and all-cause mortality data, linked with the national death registry of Statistics Korea (13). All COVID-19 diagnoses during our study period were confirmed only by reverse transcription polymerase chain reaction (RT-PCR) testing (13).
This study was approved by the Institutional Review Board (IRB) of Seoul National University Hospital (SNUH), Seoul, South Korea. Due to the retrospective and de-identified nature of this study, the SNUH IRB waived the requirement for obtaining informed consent from study participants (IRB No: E-2207-022-1337).
2.2. Study population
Eligible patients were adults aged >18 years old and had at least one visit record in the HIRA CDM database between January 20, 2020 and February 25, 2021. These two dates were the day when the first case of COVID-19 was confirmed in South Korea and the day when the COVID-19 vaccination program started in South Korea, respectively. Eligible patients were divided into two groups based on the presence or absence of a COVID-19 diagnosis record during the study period: COVID-19 and non-COVID-19 groups, respectively.
The index date was the date of the first recorded COVID-19 diagnosis or the initial visit date within the study period for the COVID-19 and the non-COVID-19 groups, respectively. To ensure covariate balance, eligible patients were matched 1:1 based on propensity scores derived from a logistic regression model incorporating age, sex, Charlson Comorbidity Index (CCI), and index month/year. Baseline covariates were matched using data from 1 year prior to the index date to account for pre-existing conditions. We utilized the OHDSI adaptation of the CCI, which employs SNOMED CT coding and has been validated across major studies for its comparable performance to the Quan adaptation. Propensity score matching was performed using the open-source OHDSI Cohort Method packages in R (16).
2.3. Outcomes
The primary outcomes of interest were the potential 12-month post-acute COVID-19 syndrome, defined as the occurrence of pre-specified diseases and symptoms (Supplementary Table S1) observed between one-month and a year after the index date, and all-cause mortality within a year after the index date. Pre-specified diseases and symptoms were chosen based on their possible association with post-acute COVID-19 syndrome (3). We categorized potential 12-month post-acute COVID-19 syndrome according to the Korean Standard Classification of Diseases and Causes of Death, 8th edition (KCD-8). Outcomes related to external causes (e.g., injury, poisoning) or congenital anomalies were excluded from the pre-specified diseases and symptoms. The temporal trends in the primary outcomes were also assessed over a year divided into three periods: the acute phase (between the index date and 1 month after an index date), the 6-month post-acute phase (between 1 and 6 months after an index date), and the 12-month post-acute phase (between 1 and 12 months after an index date).
2.4. Statistical analyses
We estimated an odds ratio (OR) of the primary outcomes between the COVID-19 and non-COVID-19 groups using a multiple logistic regression, which incorporated age at the index date, sex, CCI, and index month/year as covariates. Kaplan–Meier Survival curves were used to visually compare the differences in survival probability between the two groups. Furthermore, we analyzed temporal trends in ORs for the primary outcomes using linear regression, where OR and numerically encoded time periods were the dependent and independent variables, respectively. All statistical analyses were performed using R (version 3.5.1; R Foundation, Vienna, Austria).
3. Results
3.1. Study population
A total of 18,278 and 5,501,604 patients initially met the eligibility criteria for the COVID-19 and non-COVID-19 groups, respectively. After 1:1 propensity score matching, the study population consisted of 34,802 patients with both the COVID-19 and non-COVID-19 groups accounting for half of the total patients (i.e., n = 17,401) (Table 1). Baseline characteristics were adequately balanced, with the average age of the study population at 49 years, and females constituting 48%. The average Charlson Comorbidity Index (CCI) score in the COVID-19 group was 1.65, slightly lower than that in the non-COVID-19 group (1.768).
Table 1.
Baseline characteristics of the COVID-19 and non-COVID-19 groups before and after propensity score matching.
| Before PS matching (overall group) | After PS matching* | |||||
|---|---|---|---|---|---|---|
| COVID-19 (N = 18,278) |
Non-COVID-19 (N = 5,501,604) |
aSD | COVID-19 (N = 17,401) |
Non-COVID-19 (N = 17,401) |
aSD | |
| Age, years | 50.0 | 51.2 | 0.068 | 48.8 | 48.6 | 0.016 |
| Charlson Comorbidity Index | 1.923 | 1.523 | 0.194 | 1.650 | 1.768 | 0.055 |
| Sex (%) | ||||||
| Male | 47.4% | 49.5% | 0.043 | 47.6% | 47.5% | 0.003 |
| Female | 52.6% | 50.5% | 0.043 | 52.4% | 52.5% | 0.003 |
| Index date (%) | ||||||
| February 2020–May 2020 | 18.0% | 47.8% | 0.298 | 18.9% | 20.1% | 0.012 |
| June 2020–September 2020 | 17.3% | 49.6% | 0.323 | 18.0% | 17.8% | 0.020 |
| October 2020–February 2021 | 66.8% | 5.3% | 0.615 | 65.3% | 64.3% | 0.010 |
Values are numbers (percentages) unless stated otherwise. PS, propensity score; aSD, absolute standardized difference.
*Balance in covariate distribution between two groups was assessed using the absolute standardized mean difference.
3.2. 12-month post-acute COVID-19 syndrome
The COVID-19 group had significantly higher risks of coagulation defects, purpura, and other hemorrhagic conditions (OR = 2.70 [2.24, 3.28]; p < 0.001), chronic lower respiratory diseases (OR = 1.96 [1.80, 2.14]; p < 0.001), symptoms related to the circulatory and respiratory systems (OR = 1.91 [1.80, 2.04], p < 0.001), mood disorders (OR = 1.67 [1.51, 1.86]; p < 0.001), ischemic heart diseases and other forms of heart disease (OR = 1.39 [1.21, 1.59]; p < 0.001), and symptoms related to cognition, perception, emotional state and behavior (OR = 1.15 [1.04, 1.27]; p = 0.005) (Figure 1). Conversely, the risks of noninfective enteritis and colitis (OR = 0.88 [0.82, 0.93]; p < 0.001), cerebrovascular diseases (OR = 0.81 [0.68, 0.97]; p < 0.001), hypertensive disorders (OR = 0.70 [0.64, 0.77]; p < 0.001), and diseases of esophagus, stomach, and duodenum (OR = 0.36 [0.33, 0.40]; p < 0.001) were significantly lower in the COVID-19 group than in the non-COVID-19 group. On the other hand, the risks of diabetes mellitus (OR = 1.07 [0.99, 1.16]; p = 0.108), muscular disorders (OR = 1.10 [0.98, 1.23]; p = 0.107), and renal failure (OR = 1.16 [0.96, 1.39]; p = 0.131) did not differ significantly between the two groups.
Figure 1.
Odds ratio (OR) of 12-month post-acute COVID-19 syndrome. An OR greater than one indicates a higher risk of post-acute COVID-19 syndrome in the COVID-19 group than in the non-COVID-19 group.
3.3. All-cause mortality
In the acute phase, the COVID-19 group had significantly higher odds of all-cause mortality than the non-COVID-19 group (OR = 1.74 [1.17, 2.61]; p = 0.007, Figure 2A). However, this increased risk disappeared by a year after the index date (OR = 0.996 [0.83, 1.19]; p = 0.964), with a statistically significant 0.6-fold decrease in the ORs over a year (Supplementary Table S3). While the COVID-19 group had a slightly lower survival probability than the non-COVID-19 group (Figure 2B), this difference was not statistically significant and the largest observed difference in survival probability between the two groups was 0.33 percentage points at day 82.
Figure 2.
Odds ratio (OR) and survival probabilities for all-cause mortality. (A) ORs of all-cause mortality by period. An OR greater than one indicates a higher risk of all-cause mortality in the COVID-19 group than in the non-COVID-19 group. (B) Kaplan–Meier survival curves for all-cause mortality. The curves represent the survival probabilities in days for the COVID-19 group (blue) and the non-COVID-19 group (orange). The shaded regions correspond to the lower and upper 95% confidence bounds.
3.4. Temporal changes in post COVID-19 syndrome
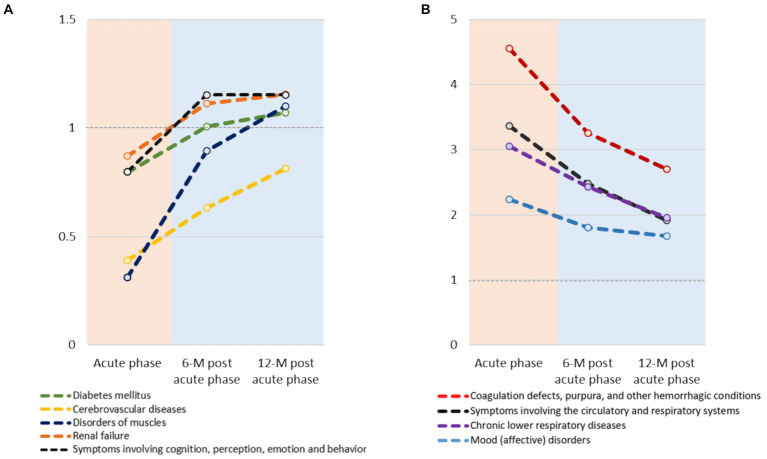
Although statistically not significant, consistent increases in the ORs were noted for disorders of muscles (3.5-fold increase), cerebrovascular diseases (2.1-fold increase), symptoms related to cognition, perception, emotional state and behavior (1.4-fold increase), diabetes mellitus (1.3-fold increase) and renal failure (1.3-fold increase) (Figure 3; Supplementary Table S3). Conversely, a significant decrease in OR was observed for chronic lower respiratory diseases (0.6-fold decrease). Furthermore, consistent but statistically insignificant decreases in the ORs for mood disorders (0.7-fold decrease), coagulation defects, purpura, and other hemorrhagic conditions (0.6-fold decrease).
Figure 3.
Temporal trends in odds ratios (OR) of post COVID-19 syndrome over a year between COVID-19 and non-COVID-19 groups: (A) increasing trend and (B) decreasing trend. Only those with a difference of OR ≥ 0.2 between the acute and 12-month post-acute phases, and a fold change in OR of ≥1.2 or ≤0.7 between these phases are shown.
4. Discussion
Using a nationwide claims database in South Korea, we identified several diseases that were more frequently experienced by patients contracted with COVID-19 than those not, which could be collectively referred to as post-acute COVID-19 syndrome. Notably, the COVID-19 group had a significantly higher risk of developing coagulation defects, purpura, and other hemorrhagic conditions for a year post-infection (OR = 2.70 [2.24, 3.28], p < 0.001). A probable mechanism for the heightened risk of hemorrhagic conditions is the cytokine storm triggered by the SARS-CoV-2 virus (17–21). Furthermore, the interaction of SARS-CoV-2 with endothelial cells in the lung, particularly those with overexpressed angiotensin-converting enzyme 2 (ACE2), could exacerbate the risk of coagulopathies by inducing a pro-coagulative and inflammatory state (17–21). Moreover, we noted a significantly higher risk of ischemic heart diseases and other forms of heart diseases in the COVID-19 group (OR = 1.39 [1.21, 1.59], p < 0.001). Our findings suggest that the impact of SARS-CoV-2 on hematological and inflammatory functions extends beyond the acute phase, with potential long-term implications, including the development of cardiovascular diseases such as ischemic heart diseases (22–24).
A significantly elevated risk of chronic lower respiratory diseases (OR = 1.96 [1.80, 2.14], all p < 0.001) was observed in COVID-19 patients, potentially exacerbated by the virus-induced inflammatory environment, which may accelerate the progression and worsen symptoms of pre-existing conditions such as chronic obstructive pulmonary disease (COPD) (25–28). These findings underscore the long-term respiratory implications of COVID-19, contributing to the emergence of new chronic respiratory diseases and aggravating existing conditions. Likewise, there was a sustained increase in the risk of mood disorders among COVID-19 patients for up to a year (OR = 1.67 [1.51, 1.86], p < 0.001). Factors such as pandemic-induced stress, uncertainty, and strict quarantine measures, including social distancing and isolation, highlight the necessity for comprehensive, long-term mental health care strategies for COVID-19 survivors (29–31).
Moreover, COVID-19 patients had a higher risk of all-cause mortality during both the acute phase (OR = 1.74 [1.17, 2.61], p = 0.007) and at 6 months (OR = 1.34 [1.06, 1.69], p = 0.015), which diminished by 1 year (OR = 0.996 [0.83, 1.19], p = 0.964). Supporting these observations, another study demonstrated that the COVID-19 pandemic did not significantly affect the overall national mortality rate in South Korea (32). Furthermore, South Korea has maintained a low COVID-19 death rate at 0.7 (per 100,000) since the start of the pandemic (0.2 since May 2020; 0.2 since June 2020), which was much lower than that in other countries such as the United States, i.e., 60.3 since the start of the pandemic; 36.9 since May 2020; 27.2 since June 2020 (33). Several factors contribute to this low mortality rate, including South Korea’s robust public health preparedness, effective management protocols, and the demographic characteristics of our study population.
South Korea was ranked as the fifth best country globally for disaster preparedness and management protocols aimed to reduce COVID-19 mortality (34). In addition, the government provided COVID-19 diagnoses and treatments free of charge to all COVID-19 patients supporting patient recovery (29, 35). These comprehensive healthcare and public health strategies in South Korea may have mitigated the long-term mortality risks associated with COVID-19. Moreover, the demographic characteristics of the study population, with an average age of 48.8 years and a moderate comorbidity burden (CCI score of 1.65), may have influenced the observed all-cause mortality. While these factors collectively contribute to our findings, the precise reasons for the low mortality in COVID-19 patients in South Korea remain unclear.
However, the risk for diseases of the digestive system remained significantly lower in the COVID-19 group over a year than in the non-COVID-19 group (noninfective enteritis and colitis, OR = 0.88 [0.82, 0.93]); diseases of esophagus, stomach, and duodenum (OR = 0.36 [0.33, 0.40]; all p < 0.001) in the COVID-19 group than in the non-COVID-19 group. This observation may be attributed to the paradoxical dual role of the ACE2 receptor in the digestive system, where its overexpression increases susceptibility to SARS-CoV-2 but its anti-inflammatory effects could potentially protect against severe digestive complications (36, 37). Additionally, many physicians in South Korea often prescribe gastrointestinal medications such as rebamapide and famotidine along with other drugs, particularly in patients with common respiratory illness (38–40). This prescription practice, which became more widespread after the South Korean government began covering the full cost of medications for COVID-19 patients (40), could have contributed to the lower risks for diseases of the digestive system in the COVID-19 group in our findings. On the other hand, several studies have proposed potential therapeutic advantages of gastrointestinal medications as COVID-19 treatments (41–43). However, the direct impact of these medications on digestive diseases in COVID-19 patients remains unclear.
This study had two major limitations. First, potential confounders such as socioeconomic status and vaccination status that could have affected health outcomes were not fully adjusted (35). However, we adjusted for various factors to minimize the impact of potential confounders. Additionally, by using nationwide data, our study ensured a broad and representative sample. Furthermore, we included only those study participants whose index dates were before the start of the COVID-19 vaccination program in South Korea (35). In addition, it is unlikely, if not impossible, that our study population included patients vaccinated during the follow-up period. Although the COVID-19 vaccination program in South Korea began on February 26, 2021, its roll-out has been seriously hampered by the failure in securing a sufficient number of vaccine doses to cover the population until the end of 2021. Therefore, the early vaccination program in South Korea was strictly prioritized to people over 70 years old. Additionally, those confirmed with COVID-19 (all testing results were PCR-based) were excluded from the vaccination program at least until mid-2022. Given the average age of our study population was 50 and the one-year follow-up period ends February 2022, it is unlikely, if not impossible, that our study population included patients vaccinated during the follow-up period.
Secondly, the utilization of claims data may have introduced bias in disease reporting and diagnosis. The claims database in South Korea includes primary and additional diagnoses that are recorded as the main reasons for treatment or prescription. However, claims data often prioritize specific diagnoses for billing purposes, potentially overlooking other health issues. This issue was particularly pronounced during the COVID-19 pandemic, when there was heightened attention on reporting severe respiratory illnesses and commonly prioritized illnesses related to COVID-19. Consequently, this may have led to an underreporting of less severe or secondary conditions not closely associated with COVID-19, skewing our understanding of the prevalence and diversity of health conditions (44–46).
We observed a slightly lower risk of hypertensive disorders (OR = 0.70 [0.64, 0.77], p < 0.001) and cerebrovascular diseases (OR = 0.81 [0.68, 0.97], p = 0.021) in the COVID-19 group compared to the non-COVID-19 group. This finding differs from other studies that reported insignificant associations between COVID-19 and these conditions (47–49). This discrepancy may have been caused by the underreporting or deprioritization of those conditions by physicians in South Korea, particularly during the peak of the COVID-19 pandemic.
Additionally, changes in public behavior during the COVID-19 pandemic may explain the lower odds ratios for certain diseases. For example, healthcare utilization for hypertension increased among the general population in South Korea during the pandemic (50, 51). This suggests that while COVID-19 patients might have experienced delays in care for certain conditions, non-COVID-19 patients continued to seek and receive care, possibly to address health concerns proactively before any healthcare service disruptions could occur. Therefore, the lower risk of certain conditions in the COVID-19 patients observed in this study should not be misconstrued as a protective effect of COVID-19. Instead, it is more likely to reflect changes in healthcare utilization and physician reporting patterns during the pandemic, which differently impacted access to healthcare and the management of COVID-19 and non-COVID-19 patients. Conversely, diseases that showed an increased risk in the COVID-19 group may have actually had a lower risk, influenced by similar biases.
While we used comprehensive list of predefined diseases and symptoms covered a wide range of health conditions to reduce the risk of missing less common health issues, further studies using electronic medical records (EMR), which provide a more accurate and details of patient health status and clinical outcomes with physician-confirmed diagnoses and laboratory details, may further validate our findings. Such studies could also address potential biases introduced by the prioritization of specific diagnoses during the pandemic.
In conclusion, post-acute COVID-19 syndrome in South Korea comprises coagulopathies, lower respiratory diseases, mood disorders, and ischemic heart diseases. All-cause mortality is also increased after infection with COVID-19 for up to 6 months, after which the risk significantly decreases and eventually resolves within a year. However, these results should be interpreted with caution, considering the changes in healthcare delivery and reporting biases specific to the Korean healthcare system during the pandemic.
Data availability statement
This study was conducted using anonymized data in a retrospective analysis, adhering to the guidelines set by the Institutional Review Board (IRB). In compliance with IRB regulations, the anonymized data used in this study cannot be shared. This restriction is in place to uphold the privacy and confidentiality standards required by the IRB.
Ethics statement
The studies involving humans were approved by the Institutional Review Board (IRB) of Seoul National University Hospital (SNUH), Seoul, South Korea (IRB No: E-2207-022-1337). The studies were conducted in accordance with local legislation and institutional requirements. Written informed consent for participation was not required from the participants or their legal guardians/next of kin because the database was fully anonymized.
Author contributions
J-HW: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Visualization, Writing – original draft. YH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – review & editing, Funding acquisition. SK: Conceptualization, Investigation, Methodology, Validation, Writing – review & editing. HL: Conceptualization, Funding acquisition, Investigation, Methodology, Supervision, Writing – review & editing.
Acknowledgments
This study used an OMOP-CDM data provided by the Health Insurance Review & Assessment Service (HIRA), South Korea (i.e., HIRA CDM). The views expressed in this paper are solely those of the author(s) and none of the HIRA or the Korea Ministry of Health and Welfare (MOHW), South Korea.
Funding Statement
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by the BK21FOUR Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education (5120200513755).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2024.1403153/full#supplementary-material
References
- 1.Cha C, Baek G. Symptoms and management of long COVID: a scoping review. J Clin Nurs. (2024) 33:11–28. doi: 10.1111/jocn.16150 [DOI] [PubMed] [Google Scholar]
- 2.Ledford H. How common is long COVID? Why studies give different answers. Nature. (2022) 606:852–3. doi: 10.1038/d41586-022-01702-2, PMID: [DOI] [PubMed] [Google Scholar]
- 3.Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. (2023) 21:133–46. doi: 10.1038/s41579-022-00846-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han Q, Zheng B, Daines L, Sheikh A. Long-term sequelae of COVID-19: a systematic review and meta-analysis of one-year follow-up studies on post-COVID symptoms. Pathogens. (2022) 11:269. doi: 10.3390/pathogens11020269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang Q, Jia M, Sun Y, Jiang B, Cui D, Feng L, et al. One-year temporal changes in long COVID prevalence and characteristics: a systematic review and meta-analysis. Value Health. (2023) 26:934–42. doi: 10.1016/j.jval.2022.11.011, PMID: [DOI] [PubMed] [Google Scholar]
- 6.Huarcaya-Victoria J, Alarcon-Ruiz CA, Barzola-Farfán W, Cruzalegui-Bazán C, Cabrejos-Espinoza M, Aspilcueta-Montoya G, et al. One-year follow-up of depression, anxiety, and quality of life of Peruvian patients who survived COVID-19. Qual Life Res. (2023) 32:139–49. doi: 10.1007/s11136-022-03208-w, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.González J, Zuil M, Benítez ID, de Gonzalo-Calvo D, Aguilar M, Santisteve S, et al. One year overview and follow-up in a post-COVID consultation of critically ill patients. Front Med. (2022) 9:897990. doi: 10.3389/fmed.2022.897990, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim Y, Bae S, Chang H-H, Kim S-W. Long COVID prevalence and impact on quality of life 2 years after acute COVID-19. Sci Rep. (2023) 13:11207. doi: 10.1038/s41598-023-36995-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim Y, Kim S-W, Chang H-H, Kwon KT, Bae S, Hwang S. Post-acute COVID-19 syndrome in patients after 12 months from COVID-19 infection in Korea. BMC Infect Dis. (2022) 22:93. doi: 10.1186/s12879-022-07062-6, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raman B, Bluemke DA, Lüscher TF, Neubauer S. Long COVID: post-acute sequelae of COVID-19 with a cardiovascular focus. Eur Heart J. (2022) 43:1157–72. doi: 10.1093/eurheartj/ehac031, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elseidy SA, Awad AK, Vorla M, Fatima A, Elbadawy MA, Mandal D, et al. Cardiovascular complications in the post-acute COVID-19 syndrome (PACS). IJC Heart Vasc. (2022) 40:101012. doi: 10.1016/j.ijcha.2022.101012, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W, Wang C-Y, Wang S-I, Wei JC-C. Long-term cardiovascular outcomes in COVID-19 survivors among non-vaccinated population: a retrospective cohort study from the TriNetX US collaborative networks. EClinicalMedicine. (2022) 53:101619. doi: 10.1016/j.eclinm.2022.101619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kyoung D-S, Kim H-S. Understanding and utilizing claim data from the Korean National Health Insurance Service (NHIS) and Health Insurance Review & Assessment (HIRA) database for research. J Lipid Atheroscler. (2022) 11:103–10. doi: 10.12997/jla.2022.11.2.103, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Health Insurance Review & Assessment Service . HIRA common data model (CDM) release (the first release) application guide. (2022). Available at: https://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA020002000100&brdScnBltNo=4&brdBltNo=9695#none.
- 15.Kim J-W, Kim C, Kim K-H, Lee Y, Yu DH, Yun J, et al. Scalable infrastructure supporting reproducible Nationwide healthcare data analysis toward FAIR stewardship. Sci Data. (2023) 10:674. doi: 10.1038/s41597-023-02580-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuemie M, Suchard M, Ryan P. CohortMethod: new-user cohort method with large scale propensity and outcome models. (2018). 2017.
- 17.Terpos E, Ntanasis-Stathopoulos I, Elalamy I, Kastritis E, Sergentanis TN, Politou M, et al. Hematological findings and complications of COVID-19. Am J Hematol. (2020) 95:834–47. doi: 10.1002/ajh.25829, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savla SR, Prabhavalkar KS, Bhatt LK. Cytokine storm associated coagulation complications in COVID-19 patients: pathogenesis and management. Expert Rev Anti-Infect Ther. (2021) 19:1397–413. doi: 10.1080/14787210.2021.1915129, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin Y, Ji W, Yang H, Chen S, Zhang W, Duan G. Endothelial activation and dysfunction in COVID-19: from basic mechanisms to potential therapeutic approaches. Signal Transduct Target Ther. (2020) 5:293. doi: 10.1038/s41392-020-00454-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giannis D, Ziogas IA, Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. (2020) 127:104362. doi: 10.1016/j.jcv.2020.104362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Minno A, Ambrosino P, Calcaterra I, Di Minno MND. COVID-19 and venous thromboembolism: A meta-analysis of literature studies. Semin Thromb Hemost. (2020) 46:763–71. doi: 10.1055/s-0040-1715456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lou M, Yuan D, Liao S, Tong L, Li J. Potential mechanisms of cerebrovascular diseases in COVID-19 patients. J Neurovirol. (2021) 27:35–51. doi: 10.1007/s13365-021-00948-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saeed S, Tadic M, Larsen TH, Grassi G, Mancia G. Coronavirus disease 2019 and cardiovascular complications: focused clinical review. J Hypertens. (2021) 39:1282–92. doi: 10.1097/HJH.0000000000002819, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan H, Tang X, Song Y, Liu P, Chen Y. Influence of COVID-19 on cerebrovascular disease and its possible mechanism. Neuropsychiatr Dis Treat. (2020) 16:1359–67. doi: 10.2147/NDT.S251173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tiotiu A, Chong Neto H, Bikov A, Kowal K, Steiropoulos P, Labor M, et al. Impact of the COVID-19 pandemic on the management of chronic noninfectious respiratory diseases. Expert Rev Respir Med. (2021) 15:1035–48. doi: 10.1080/17476348.2021.1951707, PMID: [DOI] [PubMed] [Google Scholar]
- 26.Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA. (2020) 323:2329–30. doi: 10.1001/jama.2020.6825 [DOI] [PubMed] [Google Scholar]
- 27.Fraser E. Long term respiratory complications of covid-19. BMJ. (2020) 370:m3001. doi: 10.1136/bmj.m3001 [DOI] [PubMed] [Google Scholar]
- 28.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. (2020) 8:420–2. doi: 10.1016/S2213-2600(20)30076-X, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi JY. COVID-19 in South Korea. Postgrad Med J. (2020) 96:399–402. doi: 10.1136/postgradmedj-2020-137738, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaw A. Potential mechanisms of COVID-19-related psychological problems and mental disorders. Adv Exp Med Biol. (2021) 1318:727–35. doi: 10.1007/978-3-030-63761-3_40 [DOI] [PubMed] [Google Scholar]
- 31.Zhu C, Zhang T, Li Q, Chen X, Wang K. Depression and anxiety during the COVID-19 pandemic: epidemiology, mechanism, and treatment. Neurosci Bull. (2023) 39:675–84. doi: 10.1007/s12264-022-00970-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shin MS, Sim B, Jang WM, Lee JY. Estimation of excess all-cause mortality during COVID-19 pandemic in Korea. J Korean Med Sci. (2021) 36:e280. doi: 10.3346/jkms.2021.36.e280, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bilinski A, Emanuel EJ. COVID-19 and excess all-cause mortality in the US and 18 comparison countries. JAMA. (2020) 324:2100–2. doi: 10.1001/jama.2020.20717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salihu HM, Dongarwar D, Aliyu MH, Azuine RE. Global ranking of COVID-19-related mortality by country using a novel pandemic efficiency index (PEI). Int J Mater Child Health AIDS. (2020) 9:182–5. doi: 10.21106/ijma.378, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwon SL, Oh J. COVID-19 vaccination program in South Korea: a long journey toward a new normal. Health Policy Technol. (2022) 11:100601. doi: 10.1016/j.hlpt.2022.100601, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong SH, Lui RN, Sung JJ. Covid-19 and the digestive system. J Gastroenterol Hepatol. (2020) 35:744–8. doi: 10.1111/jgh.15047 [DOI] [PubMed] [Google Scholar]
- 37.Potdar AA, Dube S, Naito T, Li K, Botwin G, Haritunians T, et al. Altered intestinal ACE2 levels are associated with inflammation, severe disease, and response to anti-cytokine therapy in inflammatory bowel disease. Gastroenterology. (2021) 160:809–822.e7. e7. doi: 10.1053/j.gastro.2020.10.041, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim M, Je NK. Potentially inappropriate gastrointestinal medication for patients with the common cold. Res Clin Pharm. (2023) 1:100–14. doi: 10.59931/rcp.23.020 [DOI] [Google Scholar]
- 39.Byeon JJ. Prescription of digestive system drugs to the patients with no digestive symptoms. J Korean Acad Fam Med. (1997) 18:78–84. [Google Scholar]
- 40.Choi YJ, Sohn J, Kim TH. Changes in expenditures of the National Health Insurance of Korea during the COVID-19 pandemic and the financial implications thereof. Yonsei Med J. (2023) 64:71–5. doi: 10.3349/ymj.2022.0481, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwon R, Kim HJ, Lee SW, Koyanagi A, Shin JI, Song T-J, et al. Effectiveness of famotidine on the risk of poor prognosis in patients with COVID-19: a nationwide cohort study in Korea. Heliyon. (2023) 9:e16171. doi: 10.1016/j.heliyon.2023.e16171, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Freedberg DE, Conigliaro J, Wang TC, Tracey KJ, Callahan MV, Abrams JA, et al. Famotidine use is associated with improved clinical outcomes in hospitalized COVID-19 patients: a propensity score matched retrospective cohort study. Gastroenterology. (2020) 159:1129–1131.e3. e3. doi: 10.1053/j.gastro.2020.05.053, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janowitz T, Gablenz E, Pattinson D, Wang TC, Conigliaro J, Tracey K, et al. Famotidine use and quantitative symptom tracking for COVID-19 in non-hospitalised patients: a case series. Gut. (2020) 69:1592–7. doi: 10.1136/gutjnl-2020-321852, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.London JW, Fazio-Eynullayeva E, Palchuk MB, Sankey P, McNair C. Effects of the COVID-19 pandemic on cancer-related patient encounters. JCO Clin Cancer Informat. (2020) 4:657–65. doi: 10.1200/CCI.20.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pifarré I Arolas H, Vidal-Alaball J, Gil J, López F, Nicodemo C, Saez M. Missing diagnoses during the COVID-19 pandemic: a year in review. Int J Environ Res Public Health. (2021) 18:5335. doi: 10.3390/ijerph18105335, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Papautsky EL, Rice DR, Ghoneima H, McKowen ALW, Anderson N, Wootton AR, et al. Characterizing health care delays and interruptions in the United States during the COVID-19 pandemic: internet-based, cross-sectional survey study. J Med Internet Res. (2021) 23:e25446. doi: 10.2196/25446, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gallo G, Calvez V, Savoia C. Hypertension and COVID-19: current evidence and perspectives. High Blood Press Cardiovasc Prev. (2022) 29:115–23. doi: 10.1007/s40292-022-00506-9, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hong K, Kisiju T, Kim J, Chun BC. Cardio-cerebrovascular complications in COVID-19 patients: a retrospective cohort study. Front Med. (2022) 9:1045274. doi: 10.3389/fmed.2022.1045274, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khalili N, Haseli S, Bahrami-Motlagh H, Keshavarz E, Khalili N, Langroudi TF, et al. Neurologic involvement in COVID-19: radiologists’ perspective. Acad Radiol. (2020) 27:1051–3. doi: 10.1016/j.acra.2020.04.035, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim Y, Park S, Oh K, Choi H, Jeong EK. Changes in the management of hypertension, diabetes mellitus, and hypercholesterolemia in Korean adults before and during the COVID-19 pandemic: data from the 2010-2020 Korea National Health and nutrition examination survey. Epidemiol Health. (2023) 45:45. doi: 10.4178/epih.e2023014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kang T, Lee Y, Kang M. Impact of COVID-19 on healthcare utilization among chronic disease patients in South Korea. Prev Med Rep. (2024) 41:102680. doi: 10.1016/j.pmedr.2024.102680, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study was conducted using anonymized data in a retrospective analysis, adhering to the guidelines set by the Institutional Review Board (IRB). In compliance with IRB regulations, the anonymized data used in this study cannot be shared. This restriction is in place to uphold the privacy and confidentiality standards required by the IRB.