Abstract
Kaposi’s sarcoma (KS)-associated herpesvirus (KSHV) encodes a G-protein-coupled receptor (GCR) homolog. This protein is a potent, constitutively active signalling molecule that can influence both proliferation and angiogenesis when ectopically expressed in fibroblasts in vitro. Here we have examined the expression of the KSHV GCR gene in virus-infected lymphoid cells and in KS tumors. Our results show that in both situations the gene is expressed primarily during lytic replication; its transcription is unaffected by inhibition of viral DNA synthesis, indicating that it is expressed in the early phases of the lytic program. The major transcript bearing GCR sequences is bicistronic, harboring coding sequences for another viral gene, K14, at its 5′ end. Extensive searches for monocistronic GCR mRNAs using nuclease mapping and reverse transcription-PCR failed to detect such species. The 5′ end of K14/GCR mRNA maps to nucleotide (nt) 127848, and its poly(A) addition site maps to nt 130546; a 149-nt intron is present in the K14/GCR intergenic region. These results suggest that the KSHV GCR is translated by unconventional mechanisms involving either translational reinitiation, internal ribosomal entry, or leaky ribosomal scanning. The restriction of GCR expression to the lytic cycle has important implications for the potential role(s) of the GCR in KS pathogenesis.
Kaposi’s sarcoma (KS), an endothelial tumor with neoangiogenic and inflammatory components, is a common neoplasm of AIDS patients. The epidemiology of KS strongly implicates a sexually transmitted cofactor other than human immunodeficiency virus in its pathogenesis (3). In recent years, a novel human herpesvirus, KS-associated herpesvirus (KSHV; also called human herpesvirus 8), has emerged as the leading candidate for this cofactor (8; reviewed in references 13 and 37). KSHV DNA is found in all KS tumors, localized to the endothelial (spindle) cells of the lesion (5, 40). Infection is found at high rates in groups at high KS risk and much lower rates in the general population; in individual subjects, infection precedes KS development and is strongly correlated with increased KS risk (14, 15, 21, 26, 45). KSHV is a member of the lymphotropic gammaherpesvirus subfamily, which includes herpesvirus saimiri and Epstein-Barr virus. In keeping with this classification, KSHV has been linked to at least two lymphoproliferative disorders: primary effusion lymphoma (PEL) and multicentric Castleman’s disease (6, 39). In the majority of KS spindle cells and PEL cells, the KSHV genome exists in a latent state, with only a small subpopulation of cells in either tumor displaying lytic viral replication (28, 34, 35, 40).
The viral gene products responsible for KS pathogenesis remain to be defined. Genes known to be expressed in latency (9, 10) include those encoding (i) LANA, the latency-associated nuclear antigen (20, 22, 33); (ii) v-cyclin, a homolog of cellular D-type cyclins (16, 24, 41); (iii) v-FLIP, a protein whose homologs impair caspase activation and programmed cell death (30, 42); and (iv) products of the K12 locus (46), whose biochemical activities are unknown but which may influence cell proliferation in vitro (29). In addition, KSHV harbors numerous additional genes that bear homology to cellular functions involved in growth control, signal transduction, and other regulatory processes (4, 13, 30–32, 36). One of these, open reading frame (ORF) 74, encodes a protein with homology to known G-protein-coupled receptors (GCRs), in particular several human chemokine receptors and an interleukin-8 receptor homolog encoded by herpesvirus saimiri (7). The KSHV GCR has been demonstrated to be a constitutively active receptor that signals through the phosphoinositide-inositoltrisphosphate-protein kinase C pathway (1). Mouse fibroblasts stably transfected with the KSHV GCR gene form foci, and these foci are tumorigenic in nude mice (2). Furthermore, expression of the KSHV GCR in such fibroblasts has been shown to induce secretion of vascular endothelial growth factor, a known mediator of angiogenesis (2). Based on these findings, it has been proposed that the KSHV GCR may be a key mediator of both the proliferative and angiogenic components of KS (2).
Here we examine the structure and expression of the mRNAs encoding ORF 74, which is located immediately rightward of a previously described cluster of latently expressed genes (Fig. 1A) (10). We show that in the PEL cell line BCBL-1, ORF 74 is expressed as an early lytic gene. In keeping with this, in situ hybridization of BCBL-1 cells and KS tumors reveals that ORF 74 is transcribed predominantly in the subpopulation of cells that express lytic cycle genes. A detailed analysis of the fine structure of this transcript reveals that ORF 74 is the downstream gene of a bicistronic message whose 5′ gene encodes ORF K14, a homolog of the cellular OX-2 protein (36). The implications of these findings for the potential role(s) of GCR in KS are discussed.
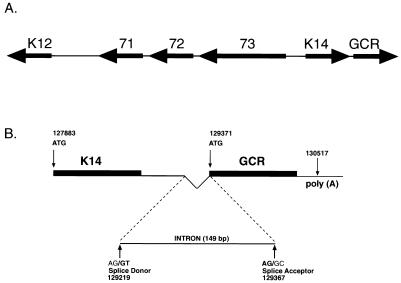
FIG. 1.
(A) Diagram of KSHV ORF 74 genomic location and mRNA structure. The ORFs for GCR (ORF 74) and K14 are adjacent and located immediately to the right of the cluster of latency genes (ORFs 73, 72, and 71 and K12); arrows indicate direction of transcription. (B) Locations of the intron in the K14/GCR intergenic region and of the poly(A) signal. Also shown are the start codon positions for both ORFs, as well as the positions for the consensus splice donor and acceptor sites. Numbers below the splice sites refer to the first (nt 129219) and last (nt 129367) nucleotides of the intron indicated by the two bold G’s. Nucleotide positions are according to the sequence of Russo et al. (36).
MATERIALS AND METHODS
Cell lines, plasmids, and probes.
BCBL-1 cells, a B-cell lymphoma line latently infected with KSHV (23, 35), were cultured in RPMI 1640 supplemented with 10% fetal bovine serum, 2 mM penicillin, streptomycin and l-glutamine, 1 mM sodium bicarbonate, and 0.05 mM 2-mercaptoethanol at 37°C in 5% CO2. BJAB cells, an Epstein-Barr virus-negative (25) and KSHV-negative B-cell lymphoma line, were cultured in the same medium and conditions as BCBL-1 cells.
The KSHV GCR gene was subcloned from phage lambda 4, a genomic KSHV clone derived from a pulmonary KS tumor (46). A 1-kb BamHI fragment from clone lambda 4 (nucleotide [nt] 129211 to 130212, based on the sequence of Russo et al. [36]) was subcloned into the BamHI site of pBluescript SKII+ (Stratagene) to create plasmid pML14. pML14 was linearized with EcoRI within the polylinker and transcribed with T3 RNA polymerase and [32P]UTP to produce an antisense riboprobe for ORF 74. For a double-stranded DNA probe for ORF 74, the 1-kb BamHI fragment from pML14 was gel purified and labeled with DNA polymerase by using a random priming procedure (Rediprime; Amersham). The ORF K14-specific probe was made by cutting clone lambda 4 with SmaI (at nt 127909) and NdeI (nt 128320) to release a ∼400-bp fragment; following gel purification, this fragment was labeled by random priming as above. A probe for the K14/ORF 74 intergenic region was created by cutting a cDNA clone that contained the 5′ untranslated region of ORF 74 with AvrII (within KSHV sequences) and EcoRI (within the plasmid backbone) to release a fragment containing KSHV nt 128948 to 129214; following gel purification, the fragment was labeled by random priming as described above. To create a GCR expression vector for the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, the AvrII (nt 129215)-SacII (nt 130405) fragment from lambda 4 containing the entire GCR ORF and 156 bp upstream of the ATG was cloned into the XbaI-SacII sites of pBluescript (Stratagene) to create pML16. The insert of pML16 was subsequently cloned into an expression plasmid under the control of the SRα promoter (composed of the simian virus 40 [SV40] early promoter and part of the human T-lymphotropic virus type 1 [HTLV-1] long terminal repeat) to create plasmid pML17.
RNA preparation and Northern blotting.
BCBL-1 cells were diluted to 2.5 × 105 cells/ml 12 h prior to treatment with 0.7 mM phosphonoformic acid (PFA) (19, 27) or mock treatment; 12 h later, half were treated with 12-O-tetradecanoylphorbol-13-acetate (TPA; 20 ng/ml), and half were mock treated for 12 h. At that point, cells were washed and then maintained at a constant PFA level for another 48 h, at which time RNA was isolated by using RNAzol B (Tel-Test, Inc., Friendswood, Tex.) as recommended by the manufacturer. A 50-μg aliquot of each RNA sample was used for poly(A) enrichment using the Oligotex mRNA purification system (Qiagen) as recommended by the manufacturer; 1.5 μg of polyadenylated RNA that resulted from this purification was separated on a 1% agarose–17% formaldehyde gel and transferred to Hybond-N membrane for 12 h in 10× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). The blot was then UV cross-linked and hybridized with the ORF 74 riboprobe in hybridization buffer (0.5 M NaCl, 5% sodium dodecyl sulfate [SDS], 6% polyethylene glycol 8000, 250 μg of salmon sperm DNA per ml, 50% formamide) at 72°C. After overnight hybridization, the blot was rinsed and then washed with 2× SSC–0.1% SDS for 15 min, with 0.5× SSC–0.1% SDS for 15 min, and finally with 0.1× SSC–0.1% SDS for 15 min. Subsequently, the blot was exposed to Kodak XAR5 film for 72 h.
cDNA cloning.
The cDNA clones containing ORF 74 were isolated from a poly(dT)-primed library made by R. Renne from induced BCBL-1 cells in Lambda Zap (Stratagene). Screening was performed with the genomic ORF 74 BamHI fragment described above.
RNase protection assay.
For ORF 74, plasmids to make riboprobes were constructed by using PCR to amplify fragments of genomic DNA from the lambda 4 subclone. For probe G1, primers KG1 (5′ GCGGTGCATCACCTACTTCAG) and GK2 (5′ CTCACACACGCTCSCTTCTAGGC) were created. For probe G2, the primers used were GCR2 (5′ CCCCTTCTGATTCTGACAGACAAC) and GK2 (see above). The PCR products were cloned into pCR2.1 (Invitrogen). The plasmids were linearized with the HindIII site from the polylinker and transcribed with T7 RNA polymerase in the presence of radiolabeled [32P]UTP. The riboprobes were purified on a 4% denaturing acrylamide gel and eluted from the gel for 12 h at 37°C in elution buffer (2.5 M sodium acetate, 0.5 mM EDTA, 0.5% SDS). The probes were precipitated with ethanol and resuspended in 10 μl of Tris-EDTA, and specific activity was determined. The RNase protection assay was performed essentially as described in reference 1a. In brief, 5 × 105 cpm of each probe was added to 10 μg of total RNA that had been precipitated and resuspended in hybridization buffer. Hybridization was carried out overnight at 37°C, and then 300 μl of RNase solution (10 mM Tris [pH 7.6], 5 mM EDTA, 300 mM NaCl, 40 μg of RNase A per ml, 0.4 U of RNase T1 per μl) was added for 1 h at 60°C. The reactions were stopped with SDS and proteinase K addition, and the products were purified by phenol-chloroform extraction and ethanol precipitation. The samples were separated on an 5% denaturing acrylamide gel and exposed to film for 72 h.
5′ RACE.
5′ rapid amplification of cDNA ends (5′ RACE) was performed on 2 μg of 24-h TPA-treated BCBL-1 total RNA. Primer KR5 (5′ CTGCAAAGCAGACACGCCTTCTT) was used for cDNA synthesis; primers K5-2 (5′ GATATAACTCCGCCCTCCACTACG) and 7306 (5′ CGCGGCGCCCGGGACAATC) were used for subsequent PCR steps as instructed by the manufacturer (Boehringer Mannheim Inc.). RACE products generated after two rounds of PCR were cloned into pCR2.1 (Invitrogen) and sequenced.
Primer extension.
Primer extension was performed as described by Zhong et al. (46); 10 μg of total RNA from BCBL-1 cells that were either uninduced or induced for 24 h with TPA were used with the primer K5-1 (5′ GGCACCAATCAGAAAGTAGCTTG); 10 μg of yeast RNA was used as a control. The reaction mixtures were loaded on a 6% denaturing acrylamide gel and then exposed to Kodak Biomax MS film for 5 days.
S1 nuclease assay.
S1 nuclease mapping was performed with an S1 mapping kit from Ambion (Austin, Tex.) according to the manufacturer’s instructions; 10 μg of total RNA from BCBL-1 cells or BJAB cells treated with TPA for 24 h was used with the end-labeled primer SK3 (5′ GGAGGCAGCTGCGCCACGAAGCAGTCACGTCCCCAAGAGCAGCAGCTTGGTCCATT). Hybridization (12 h) and S1 nuclease digestion (30 min) were performed at 37°C. After the reactions were complete, the samples were separated on a 6% denaturing acrylamide gel and exposed to Kodak Biomax MS film for 5 days.
Reverse transcription-PCR (RT-PCR).
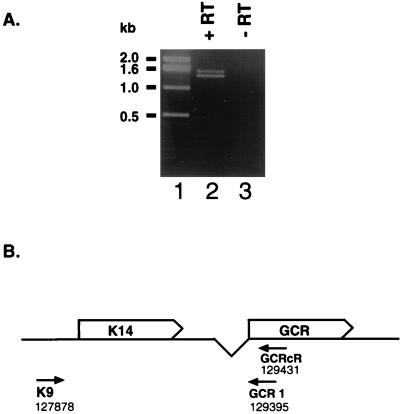
Total RNA (0.5 μg) from TPA-treated BCBL-1 cells was first treated with RQ1 DNase (Promega) as recommended by the manufacturer. The RNA was then reverse transcribed with 10 pmol of primer GCRcR (5′ GAGTTTCATTCCAGGATTCATCATC) and 200 U of Moloney murine leukemia virus reverse transcriptase (Gibco BRL) for 35 min at 42°C as described by Dittmer et al. (10). The cDNA was then purified and resuspended in 100 μl, using a High Pure PCR purification kit (Boehringer Mannheim) according to the manufacturer’s instructions; 10 μl of this cDNA was used in a PCR with primers K9 (5′ GCAGCTGCCTCCAAATGATACACAC) and GCR1 (5′ GAAGATGGTTAGGAAATCCTCGGC) plus 1 U of Taq polymerase and buffer (Perkin-Elmer). The amplification conditions were 30 cycles of 1 min at 94°C, 1 min at 65°C, and 1.5 min at 72°C followed by 10 min at 72°C. The amplification products were cloned into pCR2.1 (Invitrogen) and sequenced.
In situ hybridization.
Riboprobes were generated by runoff transcription of linearized plasmid DNA containing ORF 74 (pML14), K12 (T0.7) (46), or nut-1 (46) inserts. Radiolabeled (to a specific activity of ∼109 dpm/μg) and nonisotopic probes were synthesized with 35S- and digoxigenin-labeled UTP, respectively. In situ hybridization protocols described in detail by Haase (18) were used for both tissue specimens and cultured cells. Briefly, 6-μm sections of a formalin-fixed paraffin-embedded dermal KS lesion from an HIV-infected individual were deparaffinized and pretreated in a series of solutions containing 0.2 N HCl, 0.15 M triethanolamine (pH 7.4), 0.005% digitonin, and 5 μg of proteinase K per ml. Slides with sections of BCBL-1 cells were pretreated with 0.2 N HCl–2× SSC at 70°C plus 1 μg of proteinase K per ml. The slides were then acetylated and hybridized for 16 to 18 h at 45°C with a solution containing 10% dextran sulfate, 50% deionized formamide, 20 mM HEPES (pH 7.4), 1 mM EDTA, 1× Denhardt’s medium, poly(A) (1 mg/ml), 0.6 M NaCl, 100 mM dithiothreitol, yeast RNA (250 μg/ml), and 35S-labeled riboprobe (105 cpm/μl) or digoxigenin-labeled riboprobe (0.15 ng/μl). The slides were then treated with RNase, washed under conditions of increasing stringency, dehydrated, coated with photographic emulsion, and exposed at 4°C (40). For double labeling, slides were hybridized with a mixture of radiolabeled and nonisotopic probes. Following the posthybridization wash, digoxigenin was detected with alkaline phosphatase-conjugated antibody and nitroblue tetrazolium–5-bromo-4-chloro-3-indolylphosphate substrate (Boehringer Mannheim). The slides were then coated with photographic emulsion and exposed at 4°C to develop the radiolabel signal. The slides were stained with standard hematoxylin-and-eosin protocols or, in the case of the double label, briefly with hematoxylin alone.
MTT assay.
Subconfluent Cos7 cells were transfected with a GCR expression plasmid (pML17) or the empty vector, using Lipofectamine (Gibco BRL) according to the manufacturer’s instructions. At 48 h posttransfection, cells were trypsinized and resuspended in medium; MTT (0.3 mg/ml) was added with occasional mixing for 2.5 h. Cells were pelleted and lysed in 10% Triton X-100–0.1 N HCl in isopropanol. The A570 and A690 were determined spectrophotometrically. The assay was performed on two separate transfections, and the results are displayed as the means and standard deviations of these measurements.
RESULTS
Size and expression of ORF 74 mRNA.
Because of the proposed role(s) of the ORF 74 gene product in the pathogenesis of KS (1, 2), we sought to determine the structure and temporal class of its mRNA. To characterize the expression of this mRNA, we examined polyadenylated RNA from BCBL-1 cells by Northern blotting with a probe specific for ORF 74. BCBL-1 is a B-cell line derived from an AIDS-related body cavity-based lymphoma that is latently infected with KSHV (23, 35); it can be induced to undergo lytic replication upon addition of phorbol esters such as TPA (35). The drug PFA is known to selectively inhibit viral DNA synthesis, leading to suppression of late gene transcription while immediate early and early genes remain unaffected (19, 27). BCBL-1 cells were mock treated or treated with TPA alone, PFA alone, or TPA plus PFA, and RNA was isolated from these cells 48 h later; 1.5 μg of RNA poly(A)-purified from each of the samples was separated on a 1.2% agarose–17% formaldehyde gel and transferred to a Hybond-N membrane. An antisense riboprobe for the coding region of ORF 74 was used to detect the ORF 74-containing mRNA. Figure 2A shows a major mRNA species of approximately 2.8 kb hybridizing to the ORF 74 probe, as well as a much less abundant larger species of about 9 kb. The 2.8-kb mRNA is strongly upregulated by TPA (lane 3), suggesting that it is a lytic gene, and is unaffected by treatment with PFA (lane 4), indicating that it is not a late gene but rather an immediate-early or early gene. The same kinetics were observed when a duplicate filter was probed for a known early gene, the thymidine kinase gene (data not shown). The small amount of mRNA that hybridizes to ORF 74 in the absence of TPA (lane 1) comes from the 1 to 3% of cells in the BCBL-1 culture in which KSHV is spontaneously reactivated to lytic replication. The larger 9-kb mRNA is temporally coregulated with the major 2.8-kb species. We speculate that it may be derived by readthrough of the 2.8-kb RNA past its poly(A) site into 3′ sequences, since the poly(A) signal of the 2.8-kb species is a variant (AUUAAA) from the consensus AAUAAA (see below) that is known to result in a modestly reduced efficiency of polyadenylation (38). However, we have not characterized this RNA in detail, and other models for its structure are not excluded.
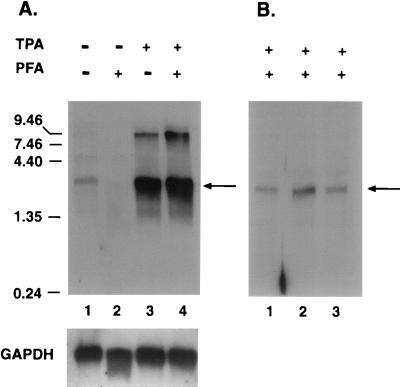
FIG. 2.
Analysis of GCR mRNA expression. (A) Top, autoradiogram of a Northern blot containing poly(A)-purified mRNA from BCBL-1 cells either mock treated (lane 1), PFA treated (lane 2), TPA treated (lane 3), or TPA and PFA treated (lane 4). The probe used was an antisense ORF 74 riboprobe, and kilobase size markers are shown on the left. The blot was reprobed with a glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-specific probe as a loading control (bottom). The arrow indicates the major mRNA at 2.8 kb. The slight degradation of the RNA in lane 2 may account for the reduced ORF 74 signal in this lane. (B) Autoradiogram of a Northern blot of total RNA from BCBL-1 cells treated with TPA and PFA and hybridized with probes for K14 (lane 1), the intergenic region (lane 2), and ORF 74 (lane 3). The arrow indicates the 2.8-kb mRNA species.
Since the ORF 74 coding region is only 1.0 kb, the 2.8-kb transcript was presumed to contain sequences for an adjacent gene. The best candidate for this gene is ORF K14, which encodes an OX-2 homolog, as it is the only adjacent ORF whose coding region has the same polarity as ORF 74 (Fig. 1A). To determine whether K14 is in fact part of the 2.8-kb mRNA, 5 μg of total RNA from TPA- and PFA-treated BCBL-1 cells was examined by Northern blotting using probes for K14 and for the K14/ORF 74 intergenic region. Figure 2B shows that the same 2.8-kb transcript anneals with probes for the K14 gene (lane 1), the intergenic region (lane 2), and the ORF 74 coding region (lane 3). The low-abundance 9-kb band is not seen here, presumably owing to the lower amount of RNA used in this experiment and the lower specific activity of the probes used. These results suggest that K14 and ORF 74 mRNAs are contained in a single 2.8-kb lytic transcript that is expressed early in viral replication.
In situ hybridization confirms that ORF 74 is a lytic cycle gene.
The above population analyses were performed with RNA extracted from the PEL cell line BCBL-1. To determine the profile of ORF 74 transcription at the single-cell level, we performed in situ hybridization with antisense riboprobes. We found that only a few percent of the cells in uninduced BCBL-1 cultures contained detectable levels of ORF 74 RNAs (Fig. 3E). These cells, which colabel with probes for lytic genes (e.g., nut-1 and the major capsid protein gene [not shown]), are the source of the low levels of ORF 74 mRNAs seen in the Northern analysis of comparable uninduced cultures (Fig. 2). As expected, TPA treatment induces ORF 74 transcription, consistent with lytic expression in these cells (Fig. 3F). To explore the relevance of these findings for KS, we examined primary KS tumors. Figure 3A shows that few cells in the tumor express ORF 74 mRNA, and these cell numbers are comparable to those expressing the known lytic gene, nut-1 (Fig. 3B). In contrast, hybridization to a subjacent section with probe for the viral K12 (T0.7) gene that is transcribed in both lytically and latently infected cells reveals the large number of infected cells within the lesion (Fig. 3C). Double labeling with radiolabeled ORF 74 probe and digoxigenin-labeled nut-1 (whose expression is confined to lytic infection) probe confirmed that the ORF 74 transcripts are produced predominantly in those cells which are in the lytic cycle (Fig. 3D). This result accords well with that for BCBL-1 and suggest that most of the spindle cells in KS tumors do not express ORF 74.
FIG. 3.
In situ localization of KSHV GCR gene expression to lytically infected cells in KS and BCBL-1 cell culture. In situ hybridization of 35S-labeled riboprobes specific for KSHV RNAs to thin sections of a dermal KS lesion reveals that few cells are transcribing ORF 74 (visualized as silver grains that have developed in a photographic emulsion coating the specimen) (A, arrow; 7-day exposure). The frequency of ORF 74-positive cells is similar to that of cells transcribing the lytic gene, nut-1 (B; 4-h exposure) and represents only a fraction of the total population of infected cells that are revealed by hybridization with probe to detect the viral K12 (T0.7) gene, which transcribed in latent as well as lytically infected cells (C; 3-day exposure). Double-label in situ hybridization with 35S-labeled ORF 74 riboprobe (yellow-green silver grains as viewed with epipolarized illumination) and digoxigenin-labeled nut-1 riboprobe (dark purple nuclei) shows colocalization to the same subpopulation of spindle tumor cells (D; 7-day exposure). Hybridization of the ORF 74 probe to thin sections of paraffin-embedded BCBL-1 cells before TPA treatment (E, arrows; 3-day exposure) and 2 days after treatment with TPA (F; 3-day exposure) reveals that uninduced cultures contain a minor subset (1 to 3%) of cells expressing ORF 74 and that GCR transcription is turned on in cells induced to undergo lytic replication. (A to C, E, F) Counterstained with hematoxylin and eosin; (D) lightly counterstained with hematoxylin.
Fine structure of the 3′ portion of K14/GCR RNA.
To begin the study of the fine structure of the GCR transcript, we screened an oligo(dT)-primed cDNA library prepared from TPA-induced BCBL-1 cells, using a DNA probe specific for ORF 74. Six clones that contained ORF 74 sequences were isolated and sequenced; none was full length. The two longest clones (1.6 kb) were identical and included the entirety of the GCR coding region, together with additional 5′ and 3′ sequences. At the 3′ end of the clones was a run of T residues corresponding to the poly(A) tail; 29 nt 5′ to this poly(A) tail was a likely poly(A) addition signal, AUUAAA, at nt 130517. At the 5′ end of the two clones, just upstream of the initiator AUG codon for GCR, comparison of the cDNA sequence with the genomic sequence revealed the presence of a small intron of 149 nt, with a splice donor at nt 129219 and splice acceptor at nt 129367 (Fig. 1B). The splice donor and acceptor sites conform to consensus splice sites found in higher eukaryotes. (Of the four remaining cDNA clones, one was similarly polyadenylated but shorter, while the others bore evidence of 3′ deletions most likely due to rearrangements during cloning.)
To confirm the presence of this intron and to see if any unspliced RNAs were also generated from this region, we performed an RT-PCR analysis. Primers spanning the putative intron were used to amplify randomly primed cDNAs from both total and oligo(dT)-selected RNA from TPA treated BCBL-1 cells. Sequencing of the major RT-PCR product revealed that it was indeed spliced and confirmed the splice donor and acceptor sites determined by cDNA cloning (not shown). No products corresponding to unspliced RNA were detected.
Searching for monocistronic GCR mRNAs.
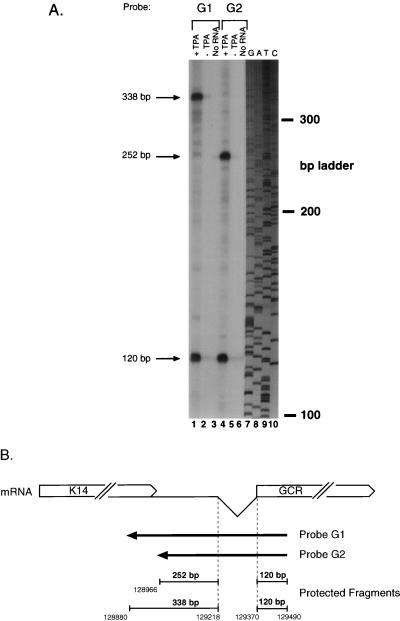
Although the major RNA containing GCR sequences appears to be a K14/GCR bicistronic transcript, we were concerned that a minor monocistronic GCR mRNA might initiate within the K14/ORF 74 intergenic region. To search for such an RNA, we performed RNase protection across this region, using the antisense riboprobes (G1 and G2) depicted in Fig. 4B. Both were transcribed from genomic KSHV DNA and shared a common 5′ end within GCR sequences, but they terminated at different sites within ORF K14 or the intergenic region. Each probe was hybridized to total RNA from BCBL-1 cells that were either untreated or treated with PFA and TPA. After hybridization, the samples were treated with RNases A and T1 for 1 h at 37°C and separated on a 6% denaturing acrylamide gel. As shown in Fig. 4A (lanes 1 and 4), hybridization with both probes G1 and G2 resulted in protection of a common 120-nt fragment representing the sequence 3′ to the intron. Each probe also generated a larger protected fragment (Fig. 4A); as depicted in Fig. 4B, these correspond exactly to the sizes predicted from protection by RNA segments arising from the spliced RNA in the region 5′ to the splice donor. No other protected species were detected in this analysis. Additional RNase mapping studies of this region, including studies using cDNA probes bearing the 149-nt splice (not shown), similarly yielded no evidence for any transcripts other than the spliced bicistronic RNA discussed above. In particular, we detected no start sites corresponding to the 5′ end of the 1.6-kb ORF 74-containing cDNAs that emerged from our cDNA cloning analyses; these are therefore incomplete cDNAs derived by premature termination during cDNA synthesis.
FIG. 4.
RNase protection analysis to identify a potential monocistronic GCR mRNA. Locations of the antisense riboprobes used and sizes of the resultant protected fragments are diagrammed in panel B. (A) Autoradiogram of a 6% denaturing acrylamide gel containing the protected species. Total RNA from either TPA-treated (lanes 1 and 4) or untreated (lanes 2 and 5) BCBL-1 cells or no RNA (lanes 3 and 6) was hybridized to probe G1 (lanes 1 to 3) or probe G2 (lanes 4 to 6), digested with RNase, denatured, and electrophoresed. A sequencing ladder was run on the same gel and used for sizing (lanes 7 to 10).
The 5′ end of K14/GCR mRNA.
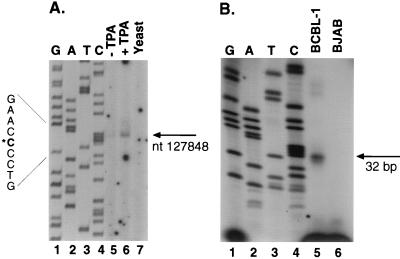
Given the location of the poly(A) addition site at nt 130546 and the approximate size of the transcript (2.8 kb), we estimated that the 5′ end of the RNA would lie just upstream of the K14 ORF. To search for the start site of the 2.8-kb transcript, we used primer extension, 5′ RACE, and S1 nuclease analysis. For the primer extension analysis, we used a labeled oligonucleotide (K5-1) located 81 nt downstream of the ATG of K14 (Fig. 5B). Following annealing of this end-labeled primer to RNA from TPA-induced BCBL-1 cells and extension with reverse transcriptase, a single product of 116 bp was detected (Fig. 5A, lane 6); as expected, the amount of this extension product was greater with RNA from TPA-induced cells than with RNA from uninduced cells (Fig. 5A, lanes 5 and 6). There was no extension product in the control reaction with yeast RNA (Fig. 5A, lane 7). The size of this product, determined by comparison to the sequencing ladder generated by using the same primer, indicates a potential start site at nt 127848. A confirmatory 5′ RACE analysis (see Materials and Methods for details) yielded four clones, two of which terminated precisely at nt 127848, in excellent agreement with the primer extension (the sequences of the remaining two clones indicated that they resulted from mispriming).
FIG. 5.
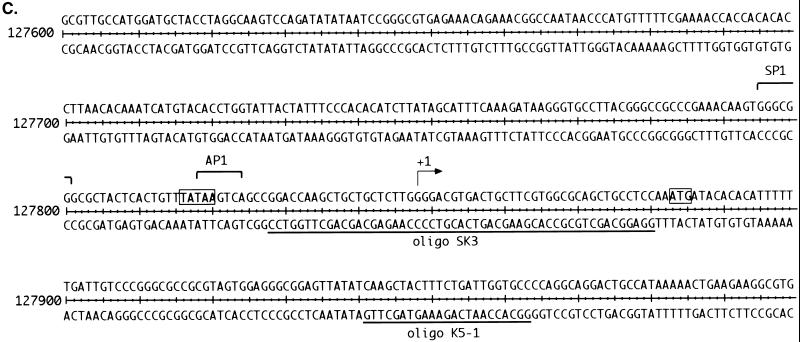
Mapping of the 5′ end of the 2.8-kb transcript, using primer extension and S1 nuclease protection. (A) Primer extension was performed with labeled oligonucleotide K5-1 (diagrammed in panel C) annealed to RNA from either untreated (lane 5) or TPA-treated (lane 6) BCBL-1 cells or to yeast RNA (lane 7). A sequencing ladder generated by using the same primer on genomic DNA was run on the gel (lanes 1 to 4) for size comparison. The arrow points to the extension product and its corresponding position, also indicated by the asterisk on the left. (B) S1 nuclease protection was performed on RNA from TPA-treated BCBL-1 cells (lane 5) or TPA-treated BJAB cells (lane 6). A sequencing ladder was run on the same gel to provide size standards (lanes 1 to 4). The primer used for the sequencing ladder was positioned 2 bp 5′ to oligonucleotide (oligo) SK3. The arrow points to the protected fragment of 32 bp. (C) Sequence of the region surrounding the start site for the 2.8-kb mRNA. Shown are the start site, binding sites for transcription factors, and positions of oligonucleotides used for S1 analysis and primer extension. The start site is indicated by +1. The arrows below the sequence represent the oligonucleotide directionality, and the transcription factor binding sites are indicated by brackets above the sequence. The TATA box and ATG start codon are indicated by boxes.
Since both primer extension and 5′ RACE rely on the use of reverse transcriptase, which can prematurely terminate at RNA secondary structures, we used S1 nuclease analysis to further confirm the mapping. This was done by using an oligonucleotide of 53 nt (probe SK3 [Fig. 5C]) that spanned the putative start site at nt 127848. RNA from TPA-induced BCBL-1 and BJAB (a KSHV-negative Burkitt’s lymphoma cell line) was hybridized to the 53-bp 5′-end-labeled probe and then treated with S1 nuclease. The resulting 32 ± 1-nt protected fragment, seen only in the BCBL-1 sample (Fig. 5B), confirms nt 127848 as the presumed start site and implies that the 5′ noncoding region upstream of ORF K14 is only ca. 35 nt. Transcripts initiating from this start site and polyadenylated at nt 130546 would result in a mRNA of 2,698 bp, which accords well with the estimated size of the transcript by Northern blotting. Importantly, this start site is 30 bp downstream of a recognizable TATA box. By scanning the region 5′ to this start site, we found several potential transcription factor binding sites, including an Sp1 site at nt 127755 and an AP-1 site overlapping the TATA box (Fig. 5C). Interestingly, the latency promoter directing ORF 73 (LANA) expression (10) lies in the body of the K14 ORF on the opposite strand. This finding raises the possibility that lytic cycle K14/GCR mRNAs have the potential to serve as antisense RNA regulators of the latency transcripts from the ORF 71–73 cluster, and tests of this hypothesis are in progress.
Searching for alternatively spliced GCR RNAs from the K14 promoter.
Another potential way to generate a monocistronic mRNA for ORF 74 would be by alternative splicing from a putative splice donor within the K14 5′ noncoding region to a splice acceptor site 5′ to ORF 74 (for instance, the known splice site at nt 129368, just 5′ to the GCR AUG). To search for such spliced RNAs, we used a sensitive RT-PCR assay. cDNA was prepared from TPA-induced BCBL-1 RNA by using a primer in the body of the GCR coding region. PCR amplification from this template was carried out with an upstream primer from the K14 5′ noncoding region (K9) and a downstream primer just 3′ to the GCR AUG (GCR1). As shown in Fig. 6, the only PCR products detected were of a size expected from amplification of the full-length, bicistronic RNA species; no smaller species reflecting the splicing out of K14 coding sequences was observed. Sequencing of these products confirmed that they corresponded to the intact sequence between the primers. The longer species corresponded to the genomic sequence and may have resulted from the presence of nuclear pre-mRNA; the shorter species is identical in sequence except for the excision of the 149-nt intron previously described.
FIG. 6.
RT-PCR analysis of K14 and GCR to search for alternatively spliced GCR RNAs. (A) PCR products resulting from reverse transcription and subsequent amplification of BCBL-1 TPA-treated RNA (lanes 2 and 3). RNA was annealed to primer GCRcR (B), and cDNA synthesis was undertaken in the presence (lane 2) or absence (lane 3) of Moloney murine leukemia virus reverse transcriptase (RT). PCR amplification of this cDNA with the primers K9 and GCR1 (B) was performed; the products were electrophoresed on a 1% agarose gel and stained with ethidium bromide. Molecular weight standards are shown in lane 1. (B) Diagram of the K14-GCR region and the primers used in the experiment represented in panel A.
Consequences of GCR overexpression.
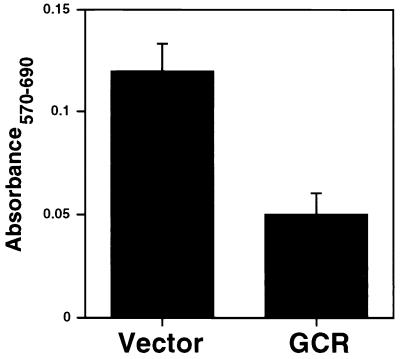
The positioning of GCR coding sequences at the 3′ end of a bicistronic mRNA would seem designed to ensure their inefficient expression. To examine what would occur if the GCR gene was strongly expressed, we constructed an expression vector in which the SRα promoter (composed of the SV40 early promoter and part of the HTLV-1 long terminal repeat) was used to drive transcription of a monocistronic GCR message. This plasmid was transfected into Cos7 cells, which allow for additional amplification of expression based on SV40 DNA replication. Cell viability was assayed by the MTT assay, which measures the ability of viable cells to metabolize the compound MTT to formazan, which is detected spectrophotometrically (see Materials and Methods). As shown in Fig. 7, substantial cell death was observed when GCR was overexpressed.
FIG. 7.
MTT assay of GCR-transfected Cos7 cells. Cos7 cells were transfected with a GCR expression plasmid or the empty vector, exposed to MTT, and lysed 48 h posttransfection. The A570 and A690 were measured, and A570 − A690 was plotted.
DISCUSSION
These studies demonstrate that the principal KSHV mRNA bearing GCR coding sequences is a lytic transcript composed of the coding regions of both ORF K14 and ORF 74, with the K14 gene located in the 5′ position. Extensive searches (by sensitive RNase mapping and RT-PCR approaches) for alternatively initiated or alternatively spliced RNAs in which ORF 74 would be the sole (or at least the 5′) coding region revealed no evidence for such transcripts, though of course the existence of such RNAs at levels below the detection thresholds of our methods cannot be formally excluded. The evidence points instead to the conclusion that the 2.8-kb RNA is likely functionally bicistronic, with the K14 gene translated by conventional initiation mechanisms and the GCR gene translated by nonclassical strategies, e.g., modified ribosomal scanning, translational reinitiation, or internal ribosome entry. Although unusual, this arrangement may not be without logic. Published data indicate that the KSHV GCR is a powerful and constitutively active signalling molecule (1). Presumably even modest levels of KSHV GCR would suffice to carry out its signalling functions, and overexpression of such a potent activity might even be deleterious. Indeed, in our hands, overexpression of the GCR in transiently transfected COS cells triggers cell death (Fig. 7). Thus, relegation of the GCR to the 3′ position in the transcript, where its translation would be expected to be inefficient, may be a strategy for ensuring levels of expression commensurate with cell survival.
The finding that KSHV GCR is a lytic cycle gene is consistent with observations in other herpesvirus infections. For example, cytomegalovirus encodes several GCR homologs as lytic genes (43, 44). The limitation of KSHV GCR expression to the lytic cycle has implications for its potential role(s) in KS tumorigenesis. Since most lytically infected cells eventually die, it is unlikely that cells expressing detectable GCR transcripts in KS will go on to proliferate—consistent with the observation that most spindle cells in KS tumors do not express GCR mRNA. Of course, it is formally possible that abortively infected cell types express some lytic cycle genes yet escape destruction; but even assuming that every spindle cell expressing GCR in a KS lesion is abortively infected (a proposition for which there is no evidence), this would still represent only a tiny fraction of proliferating spindle cells in the tumor. Still another model for how GCR expression could be linked to proliferation involves the induction and secretion of growth-inducing factors from GCR-expressing cells. However, proliferative effects from this mechanism would be expected to operate on uninfected as well as infected cells. As such, this model would fail to explain one of the central features of KS: that most proliferating spindle cells are latently infected with KSHV (5, 40). We therefore find it improbable that the viral GCR is principally responsible for spindle cell proliferation in KS tumors, despite its demonstrated ability to deregulate growth in 3T3 cells (1).
On the other hand, expression of GCR in lytically infected cells could have numerous and profound pathologic consequences for the two other components of a KS lesion: neoangiogenesis and inflammatory cell infiltration. Bais et al. (2) have clearly shown that GCR signalling initiates a program of cellular gene expression that culminates in synthesis and release of vascular endothelial growth factor. Clearly, paracrine release of such potent angiogenic factors from even a small subpopulation of cells could trigger dramatic neovascularization, a hallmark of KS histology. It is also conceivable that paracrine mediators released under the influence of GCR signalling have effects on inflammatory cell infiltration. We think it much more likely that GCR plays its role in KS pathogenesis by these paracrine mechanisms than by cell-autonomous effects of GCR expression.
Finally, regardless of any potential role in KS pathogenesis, lytic cycle GCR expression likely plays additional roles in the KSHV life cycle—a cycle which in normal immunocompetent hosts is largely played out in lymphoid rather than endothelial cells. The cellular chemokine receptor, Burkitt’s lymphoma receptor 1, is known to regulate the migration of B cells both systemically and within lymphoid organ microenvironments (11, 12, 17). If the product of ORF 74 shares this function, then perhaps its expression during lytic reactivation would provide a mechanism to home virus-laden cells to lymphoid organs, where the released viral progeny could more readily infect other susceptible B cells. Alternatively, GCR-activated signalling pathways may result in the activation or induction of host and/or viral proteins that upregulate lytic growth. Decisive tests of the role of the GCR in viral growth and KS pathogenesis will likely require the development of genetic systems for constructing and propagating mutations in individual viral genes.
ACKNOWLEDGMENTS
We thank Dirk Dittmer and Rolf Renne for technical assistance and the remainder of the Ganem lab for helpful discussions.
J.R.K. was supported by funds from the National Institutes of Health (grant T32GM08568). D.G. is an investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Arvanitakis L, Geras-Raaka E, Varma A, Gershengorn M C, Cesarman E. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature. 1997;385:347–350. doi: 10.1038/385347a0. [DOI] [PubMed] [Google Scholar]
- 1a.Ausubel F M, Brent R, Kingston R E, Moore D D, Smith J A, Seidman J G, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1994. [Google Scholar]
- 2.Bais C, Santomasso B, Coso O, Arvanitakis L, Raaka E G, Gutkind J S, Asch A S, Cesarman E, Gershengorn M C, Mesri E A. G-protein-coupled receptor of Kaposi’s sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature. 1998;391:86–89. doi: 10.1038/34193. . (Erratum, 392:210.) [DOI] [PubMed] [Google Scholar]
- 3.Beral V, Peterman T A, Berkelman R L, Jaffe H W. Kaposi’s sarcoma among persons with AIDS: a sexually transmitted infection? Lancet. 1990;335:123–128. doi: 10.1016/0140-6736(90)90001-l. [DOI] [PubMed] [Google Scholar]
- 4.Boshoff C, Endo Y, Collins P D, Takeuchi Y, Reeves J D, Schweickart V L, Siani M A, Sasaki T, Williams T J, Gray P W, Moore P S, Chang Y, Weiss R A. Angiogenic and HIV-inhibitory functions of KSHV-encoded chemokines. Science. 1997;278:290–294. doi: 10.1126/science.278.5336.290. [DOI] [PubMed] [Google Scholar]
- 5.Boshoff C, Schulz T F, Kennedy M M, Graham A K, Fisher C, Thomas A, McGee J O, Weiss R A, O’Leary J J. Kaposi’s sarcoma-associated herpesvirus infects endothelial and spindle cells. Nat Med. 1995;1:1274–1278. doi: 10.1038/nm1295-1274. [DOI] [PubMed] [Google Scholar]
- 6.Cesarman E, Chang Y, Moore P S, Said J W, Knowles D M. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 7.Cesarman E, Nador R G, Bai F, Bohenzky R A, Russo J J, Moore P S, Chang Y, Knowles D M. Kaposi’s sarcoma-associated herpesvirus contains G protein-coupled receptor and cyclin D homologs which are expressed in Kaposi’s sarcoma and malignant lymphoma. J Virol. 1996;70:8218–8223. doi: 10.1128/jvi.70.11.8218-8223.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang Y, Cesarman E, Pessin M S, Lee F, Culpepper J, Knowles D M, Moore P S. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 9.Davis M A, Sturzl M A, Blasig C, Schreier A, Guo H G, Reitz M, Opalenik S R, Browning P J. Expression of human herpesvirus 8-encoded cyclin D in Kaposi’s sarcoma spindle cells. J Natl Cancer Inst. 1997;89:1868–1874. doi: 10.1093/jnci/89.24.1868. [DOI] [PubMed] [Google Scholar]
- 10.Dittmer D, Lagunoff M, Renne R, Staskus K, Haase A, Ganem D. A cluster of latently expressed genes in Kaposi’s sarcoma-associated herpesvirus. J Virol. 1998;72:8309–8315. doi: 10.1128/jvi.72.10.8309-8315.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forster R, Emrich E, Voss C, Lipp M. Expression of the G protein-coupled receptor BLR1 defines mature recirculating B cells and a subset of T-helper memory cells. Blood. 1994;84:830–840. [PubMed] [Google Scholar]
- 12.Forster R, Mattis A E, Kremmer E, Wolf E, Brem G, Lipp M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1037–1047. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- 13.Ganem D. KSHV and Kaposi’s sarcoma: the end of the beginning? Cell. 1997;91:157–160. doi: 10.1016/s0092-8674(00)80398-0. [DOI] [PubMed] [Google Scholar]
- 14.Gao S J, Kingsley L, Hoover D R, Spira T J, Rinaldo C R, Saah A, Phair J, Detels R, Parry P, Chang Y, Moore P S. Seroconversion to antibodies against Kaposi’s sarcoma-associated herpesvirus-related latent nuclear antigens before the development of Kaposi’s sarcoma. N Engl J Med. 1996;335:233–241. doi: 10.1056/NEJM199607253350403. [DOI] [PubMed] [Google Scholar]
- 15.Gao S J, Kingsley L, Li M, Zheng W, Parravicini C, Ziegler J, Newton R, Rinaldo C R, Saah A, Phair J, Detels R, Chang Y, Moore P S. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcoma. Nat Med. 1996;2:925–928. doi: 10.1038/nm0896-925. [DOI] [PubMed] [Google Scholar]
- 16.Godden-Kent D, Talbot S J, Boshoff C, Chang Y, Moore P, Weiss R A, Mittnacht S. The cyclin encoded by Kaposi’s sarcoma-associated herpesvirus stimulates cdk6 to phosphorylate the retinoblastoma protein and histone H1. J Virol. 1997;71:4193–4198. doi: 10.1128/jvi.71.6.4193-4198.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gunn M D, Ngo V N, Ansel K M, Ekland E H, Cyster J G, Williams L T. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt’s lymphoma receptor-1. Nature. 1998;391:799–803. doi: 10.1038/35876. [DOI] [PubMed] [Google Scholar]
- 18.Haase A T. Analysis of viral infections by in situ hybridization. In: Valentino K L, Eberwine J H, Barchas J D, editors. Symposium on in situ hybridization: applications to neurobiology. New York, N.Y: Oxford University Press; 1987. pp. 197–219. [Google Scholar]
- 19.Kedes D H, Ganem D. Sensitivity of Kaposi’s sarcoma-associated herpesvirus replication to antiviral drugs. Implications for potential therapy. J Clin Investig. 1997;99:2082–2086. doi: 10.1172/JCI119380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kedes D H, Lagunoff M, Renne R, Ganem D. Identification of the gene encoding the major latency-associated nuclear antigen of the Kaposi’s sarcoma-associated herpesvirus. J Clin Investig. 1997;100:2606–2610. doi: 10.1172/JCI119804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kedes D H, Operskalski E, Busch M, Kohn R, Flood J, Ganem D. The seroepidemiology of human herpesvirus 8 (Kaposi’s sarcoma-associated herpesvirus): distribution of infection in KS risk groups and evidence for sexual transmission. Nat Med. 1996;2:918–924. doi: 10.1038/nm0896-918. . (Erratum, 2:1041.) [DOI] [PubMed] [Google Scholar]
- 22.Kellam P, Boshoff C, Whitby D, Matthews S, Weiss R A, Talbot S J. Identification of a major latent nuclear antigen LNA-1 in the human herpesvirus 8 genome. J Hum Virol. 1997;1:19–29. [PubMed] [Google Scholar]
- 23.Komanduri K V, Luce J A, McGrath M S, Herndier B G, Ng V L. The natural history and molecular heterogeneity of HIV-associated primary malignant lymphomatous effusions. J Acquired Immune Defic Syndr Hum Retrovirol. 1996;13:215–226. doi: 10.1097/00042560-199611010-00003. [DOI] [PubMed] [Google Scholar]
- 24.Li M, Lee H, Yoon D W, Albrecht J C, Fleckenstein B, Neipel F, Jung J U. Kaposi’s sarcoma-associated herpesvirus encodes a functional cyclin. J Virol. 1997;71:1984–1991. doi: 10.1128/jvi.71.3.1984-1991.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marchini A, Longnecker R, Kieff E. Epstein-Barr virus (EBV)-negative B-lymphoma cell lines for clonal isolation and replication of EBV recombinants. J Virol. 1992;66:4972–4981. doi: 10.1128/jvi.66.8.4972-4981.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin J N, Ganem D E, Osmond D H, Page-Shafer K A, Macrae D, Kedes D H. Sexual transmission and the natural history of human herpesvirus 8 infection. N Engl J Med. 1998;338:948–954. doi: 10.1056/NEJM199804023381403. [DOI] [PubMed] [Google Scholar]
- 27.Medveczky M M, Horvath E, Lund T, Medveczky P G. In vitro antiviral drug sensitivity of the Kaposi’s sarcoma-associated herpesvirus. AIDS. 1997;11:1327–1332. doi: 10.1097/00002030-199711000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Miller G, Heston L, Grogan E, Gradoville L, Rigsby M, Sun R, Shedd D, Kushnaryov V M, Grossberg S, Chang Y. Selective switch between latency and lytic replication of Kaposi’s sarcoma herpesvirus and Epstein-Barr virus in dually infected body cavity lymphoma cells. J Virol. 1997;71:314–324. doi: 10.1128/jvi.71.1.314-324.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muralidhar S, Pumfery A M, Hassani M, Sadaie M R, Azumi N, Kishishita M, Brady J N, Doniger J, Medveczky P, Rosenthal L J. Identification of kaposin (open reading frame K12) as a human herpesvirus 8 (Kaposi’s sarcoma-associated herpesvirus) transforming gene. J Virol. 1998;72:4980–4988. doi: 10.1128/jvi.72.6.4980-4988.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neipel F, Albrecht J C, Fleckenstein B. Cell-homologous genes in the Kaposi’s sarcoma-associated rhadinovirus human herpesvirus 8: determinants of its pathogenicity? J Virol. 1997;71:4187–4192. doi: 10.1128/jvi.71.6.4187-4192.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicholas J, Ruvolo V, Zong J, Ciufo D, Guo H G, Reitz M S, Hayward G S. A single 13-kilobase divergent locus in the Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genome contains nine open reading frames that are homologous to or related to cellular proteins. J Virol. 1997;71:1963–1974. doi: 10.1128/jvi.71.3.1963-1974.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicholas J, Ruvolo V R, Burns W H, Sandford G, Wan X, Ciufo D, Hendrickson S B, Guo H G, Hayward G S, Reitz M S. Kaposi’s sarcoma-associated human herpesvirus-8 encodes homologues of macrophage inflammatory protein-1 and interleukin-6. Nat Med. 1997;3:287–292. doi: 10.1038/nm0397-287. [DOI] [PubMed] [Google Scholar]
- 33.Rainbow L, Platt G M, Simpson G R, Sarid R, Gao S J, Stoiber H, Herrington C S, Moore P S, Schulz T F. The 222- to 234-kilodalton latent nuclear protein (LNA) of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) is encoded by orf73 and is a component of the latency-associated nuclear antigen. J Virol. 1997;71:5915–5921. doi: 10.1128/jvi.71.8.5915-5921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Renne R, Lagunoff M, Zhong W, Ganem D. The size and conformation of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) DNA in infected cells and virions. J Virol. 1996;70:8151–8154. doi: 10.1128/jvi.70.11.8151-8154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Renne R, Zhong W, Herndier B, McGrath M, Abbey N, Kedes D, Ganem D. Lytic growth of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat Med. 1996;2:342–346. doi: 10.1038/nm0396-342. [DOI] [PubMed] [Google Scholar]
- 36.Russo J J, Bohenzky R A, Chien M C, Chen J, Yan M, Maddalena D, Parry J P, Peruzzi D, Edelman I S, Chang Y, Moore P S. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8) Proc Natl Acad Sci USA. 1996;93:14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schulz T F. Kaposi’s sarcoma-associated herpesvirus (human herpesvirus-8) J Gen Virol. 1998;79:1573–1591. doi: 10.1099/0022-1317-79-7-1573. [DOI] [PubMed] [Google Scholar]
- 38.Sheets M D, Ogg S C, Wickens M P. Point mutations in AAUAAA and the poly (A) addition site: effects on the accuracy and efficiency of cleavage and polyadenylation in vitro. Nucleic Acids Res. 1990;18:5799–5805. doi: 10.1093/nar/18.19.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d’Agay M F, Clauvel J P, Raphael M, Degos L, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995;86:1276–1280. [PubMed] [Google Scholar]
- 40.Staskus K A, Zhong W, Gebhard K, Herndier B, Wang H, Renne R, Beneke J, Pudney J, Anderson D J, Ganem D, Haase A T. Kaposi’s sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J Virol. 1997;71:715–719. doi: 10.1128/jvi.71.1.715-719.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swanton C, Mann D J, Fleckenstein B, Neipel F, Peters G, Jones N. Herpes viral cyclin/Cdk6 complexes evade inhibition by CDK inhibitor proteins. Nature. 1997;390:184–187. doi: 10.1038/36606. [DOI] [PubMed] [Google Scholar]
- 42.Thome M, Schneider P, Hofmann K, Fickenscher H, Meinl E, Neipel F, Mattmann C, Burns K, Bodmer J L, Schroter M, Scaffidi C, Krammer P H, Peter M E, Tschopp J. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 43.Vieira J, Schall T J, Corey L, Geballe A P. Functional analysis of the human cytomegalovirus US28 gene by insertion mutagenesis with the green fluorescent protein gene. J Virol. 1998;72:8158–8165. doi: 10.1128/jvi.72.10.8158-8165.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Welch A R, McGregor L M, Gibson W. Cytomegalovirus homologs of cellular G protein-coupled receptor genes are transcribed. J Virol. 1991;65:3915–3918. doi: 10.1128/jvi.65.7.3915-3918.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whitby D, Howard M R, Tenant-Flowers M, Brink N S, Copas A, Boshoff C, Hatzioannou T, Suggett F E, Aldam D M, Denton A S, et al. Detection of Kaposi sarcoma associated herpesvirus in peripheral blood of HIV-infected individuals and progression to Kaposi’s sarcoma. Lancet. 1995;346:799–802. doi: 10.1016/s0140-6736(95)91619-9. [DOI] [PubMed] [Google Scholar]
- 46.Zhong W, Wang H, Herndier B, Ganem D. Restricted expression of Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genes in Kaposi sarcoma. Proc Natl Acad Sci USA. 1996;93:6641–6646. doi: 10.1073/pnas.93.13.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]