Abstract
Introduction
Migraine and endometriosis are chronic disabling pain conditions. There is evidence for a shared genetic background. Migraine phenotype and course in patients with the comorbidity are insufficient investigated. Both conditions can be treated with progestins.
Methods
For this observational study we included women with migraine and endometriosis, visiting our clinic from 2015 to 2021. We collected available information from charts and complemented these data by a structured phone interview to collect more specific information on migraine and the course of both diseases.
Results
From 344 patients fulfilling the inclusion criteria, 94 suffered from both, endometriosis and migraine. Migraine with aura was reported by 41% of the patients and was associated with earlier onset of migraine (age < 17 years (OR 6.54) and with a history of medication overuse headache (OR 9.9, CI 1.6–59.4). Present monthly migraine frequency (1.5 ± 2.6) was significantly lower than five years before the interview (2.9 ± 4.64). There was a correlation between medication overuse headache and use of analgesics more than 3 days/months for dysmenorrhoea (p < 0.03). ASRM endometriosis score was not associated with migraine characteristics.
Conclusions
We conclude that the comorbidity of endometriosis is highly linked to migraine with aura. Migraine onset in these patients was earlier. Further studies are needed to explore, if the observed decrease in migraine frequency can be attributed to recent endometriosis surgery and to understand if early diagnosis and treatment of both conditions may contribute to improve the course of both conditions.
Trial registration BASEC Nr. 2021-00285.
Keywords: Migraine, Endometriosis, Aura, Dysmenorrhea
Introduction
Both, endometriosis and migraine are chronic inflammatory disorders with estrogens playing a pivotal role in the pathophysiology [1–4]. Both conditions are associated with chronic pain and a high grade of disability in women during the reproductive years. Typically, symptoms decrease, when women approach menopause [5, 6]. Today there is high evidence that both conditions might share a common genetic background and that polymorphisms of sex hormones play a crucial role [3, 4, 7]. In a large case–control study, Yang et al. found among 20,220 patients with endometriosis a 1.7-fold higher prevalence of migraines than in controls.[8]. Survey-based case–control studies in endometriosis patients reported migraine in 29–69% of the affected women, which is far higher than the expected prevalence in the general female population [1, 2, 9]. Both conditions respond to treatment with the synthetic progestin desogestrel, which is used continuously and inhibits ovulation [10–15].
Severe dysmenorrhea without response to pain killers is one of the typical early symptoms in adolescents with endometriosis [16]. Early diagnosis of both conditions and specific treatment is highly relevant to reduce the probability of developing chronic pelvic pain and possibly corresponding alterations of pain response in the brain [17]. Women with comorbid migraine and endometriosis might be at increased risk for medication overuse headache (MOH), as they need to use painkillers for menstrual and non-menstrual pelvic pain and in addition for their headaches. While several studies investigated the prevalence of migraine in women with endometriosis, there is only minimal knowledge on migraine characteristics in women with the comorbidity. Theoretically, endometriosis patients might be more prone to suffer from chronic migraine or a higher migraine frequency. It is unknown if women with this comorbidity suffer rather from migraine with aura (MA). There seems to be a genetic component [18]. Women during the reproductive years tend to suffer more from menstrual migraine without aura (MO). With the present study, we aimed to identify migraine phenotypes in women with migraine and endometriosis and the dynamics of migraine in these patients. In particular we focused on aura, monthly migraine frequency (MMF) at present during puberty and 5 years ago, age at migraine onset, history of medication overuse headache, use of prophylactic agents and quality of life were in our focus.
Materials and methods
Study design
This observational study was conducted at the Clinic for Reproductive Endocrinology in the Department of Gynaecology of the University Hospital of Zurich. Data were collected from patient records and supplemented through telephone interviews. The presented data is part of a broader study investigating the characteristics of endometriosis in women with different comorbidities.
Data collection
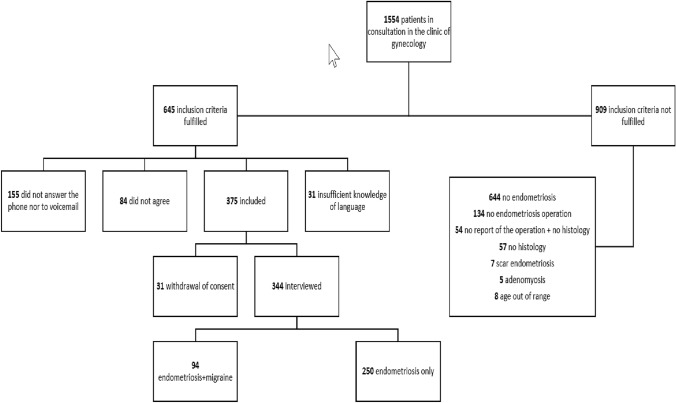
Potential participants with the diagnosis of endometriosis and migraine were preselected by searching charts from all endometriosis patients treated in our outpatient clinic from January 2015 to July 2021. Included were all premenopausal patients aged > 18 years with biopsy-confirmed endometriosis, who in addition suffered from migraine (Fig. 1). Diagnosis of migraine and migraine with aura were evaluated in telephone interviews using the criteria of the International Classification of Headache Disorders (3rd version, ICHD-3) of the International Headache Society to ensure the correct differentiation between migraine and other types of headaches [19]. In order to increase reliability, both interviewers were trained with 50 interviews prior to start of the study. Postmenopausal women and those with adenomyosis or scar endometriosis were excluded. During a phone contact, we informed patients about the study and asked if they were interested in participating. Those willing to participate received an information sheet and provided consent. Details about the inclusion process are presented in Fig. 1. The interview questions were adapted from a structured questionnaire developed from the World Endometriosis Research Foundation “Women’s Health Symptom Survey Questionnaire” [20]. The questionnaire covers demographics, height, weight, medical conditions, operations, family history, menstruation, pregnancies, deliveries, potential symptoms of endometriosis, and medication. In addition 30 headache-specific questions, were added, including age at migraine onset, migraine frequency in the past (at age < 20 years and 5 years ago) and at present, aura, triggers, acute and prophylactic medication and disability, using the Migraine Disability Assessment questionnaire (MIDAS) [21]. From the surgery reports, we had access to information about endometriosis score (ASRM), infiltration depth, number of affected compartments, and localization [22].
Fig. 1.
The flowchart containing the recruiting, inclusion and data collection process
Statistical analyses
We performed all statistical analyses using IBM SPSS version 27.0.1.0. We presented categorical variables as frequencies and continuous variables as means with standard deviations. For comparison of subgroups we calculated independent sample t-tests for normally distributed variables and Wilcoxon-Mann–Whitney test for not normally distributed variables. Further, we compared categorical variables with Chi-Square or Fisher’s exact test. Spearman`s rank correlation was used to test dependence of nonparametric measures. Significance level was set at p = 0.05.
Ethical approval
The study was approved by the ethics committee of Zürich (BASEC Nr. 2021-00285) and registered at clinical Trials.gov (NCT04816357).
Results
Baseline characteristics of the 94 women with migraine and EM are demonstrated in Table 1. Mean age at migraine onset was 19.7 ± 8.4 years and mean age at menarche 12.7 ± 1.6 years. Migraine features, including the current frequency, MMF five years ago and at age younger than 20 years, migraine type and MIDAS score and grade are listed in Table 2. Altogether, the MMF was rather low at present and at age < 20 years, while it was substantially higher 5 years ago (p < 0.01 vs frequency now; p < 0.03 vs frequency at age < 20 years) (Table 2). Currently, 59% of the patients did not use any prophylactic agents, 37% used magnesium or riboflavin on a regular basis and 3.1% used Botox or b-blockers. Pain medication was prescribed from neurologists in 18.5%. and from GCPs in 35.1%. Another 35.1% of the participants bought the medication in the pharmacy without prescription and 11.3% received it from other sources. Migraine with aura (MA) had been reported form 41% of the women. Women with MA reported more frequently unilateral attacks (p < 0.045. In our setting MA was associated with younger age at migraine onset and with a history of medication overuse headache (MOH) (Table 3). No associations were found with ASRM stage (p < 0.37) or depth of infiltration of lesions (p < 0.45 for infiltration ≥ 3 cm).). MMF in the past did not differ between MA and MO patients. Women with a history of MOH suffered more often from more than 2 monthly attacks not now, but 5 years ago (p = 0.05), were more often triptan users (p = 0.05) and were more often using more than 3 days/months analgesics for dysmenorrhoea (p < 0.04) (Table 3). MOH was not associated with a personal history of depression (p < 0.25). Endometriosis ASRM score was not associated with MA, higher frequency of migraine or MOH. Patients with a history of MOH were also not affected by deeper infiltration of endometriosis lesions, what is known to be associated with more pain (p = 1.0 for infiltration ≥ 3 cm).
Table 1.
Baseline and endometriosis characteristics
| Patients’ characteristics: | Number of patients n (%)/mean ± standard deviation (min–max) | N |
|---|---|---|
| Age (years) | 36.4 ± 7.6 (20–53) | 94 |
| Height (cm) | 167.3 ± 6.0 (153–184) | 94 |
| Weight (kg) | 65.2 ± 11.2 (40–120) | 94 |
| Body mass index (kg/m2) | 23.3 ± 3.8 (17–43) | 94 |
| Age at first manifestation of migraine (years) | 19.7 ± 8.4 (6–45) | 88 |
| Depression ever | 37 (39.4) | 94 |
| Mean age at menarche | 12.8 ± 1.6 (8–17) | 93 |
| Combined pill/patch/ring use at present | 9 (9.6) | 94 |
| ASRM stage | 94 | |
| 1 | 25 (26.6) | |
| 2 | 20 (21.3) | |
| 3 | 21 (22.3) | |
| 4 | 28 (29.8) | |
| Depth of infiltration > 3 cm | 21 (22.3) | 94 |
| Dysmenorrhea score 2/3 yes | 72 (77.4) | 93 |
| > 2 days of analgesics during menstruation | 44 (46.8) | 94 |
Table 2.
Migraine features in women with comorbid endometriosis
| Characteristics | Number of patients n (%)/mean ± SD (min–max) | N | |
|---|---|---|---|
| Migraine with aura | (Yes) | 39 (41.5) | 94 |
| Migraine onset < 17 years | (Yes) | 39 (44.3) | 94 |
| Migraine frequency at age under 20: | < 1 migraine attack/month | 50 (56.2) | 89 |
| 1 or 2 migraine attacks/month | 18 (20.2) | 89 | |
| > 2 migraine attacks/month | 21 (23.6) | 89 | |
| Migraine frequency at age under 20 | (Attacks/month) | 1.6 ± 2.78 (0–15) | 89 |
| Migraine frequency five years ago: | (Attacks/month) | 2.9 ± 4.64 (0–25) | 90 |
| Migraine frequency five years ago: | > 2 migraine attacks/month | 28 (31.1) | 90 |
| Current migraine frequency: | (Attacks/month) | 1.5 ± 2.65 (0–15) | 93 |
| Current migraine frequency: | > 2 migraine attacks/month | 20 (21.5) | 93 |
| Mean duration of migraine attacks | (Hours) | 16.6 ± 19.1 (0.5–72) | 91 |
| Ø migraine attack duration over 9 h | (Yes) | 50 (54.9) | 91 |
| Status migraenosus ever | (Yes) | 22 (23.4) | 94 |
| Migraine symptoms | |||
| Unilateral migraine | (Yes) | 63 (67.0) | 94 |
| Sensitivity to light | (Yes) | 91 (96.8) | 94 |
| Sensitivity to noise | (Yes) | 63 (67.0) | 94 |
| Nausea | (Yes) | 83 (88.3) | 94 |
| Emesis | (Yes) | 44 (46.8) | 94 |
| Triptan for acute migraine treatment | (Yes) | 22 (23.7) | 93 |
| Ever MOH | (Yes) | 8 (8.6) | 93 |
| MIDAS score | 6.8 (13.0) | 94 | |
| MIDAS grade (migraine disability assessment score) | Grade 1 (little or no disability) | 60 (63.8) | 94 |
| Grade 2 (mild disability) | 15 (16.0) | 94 | |
| Grade 3 (moderate disability) | 4 (4.3) | 94 | |
| Grade 4 (severe disability) | 15 (16.0) | 94 | |
aNumber of participants from 94 included patients
bsevere pain: pain score 3 in a pain scale from 0 to 3
Table 3.
Correlations for MA and MOH
| MA | Correlation coefficient | n | 95% confidence interval of odds ratio | p-value |
|---|---|---|---|---|
| First manifestation of migraine under 17 years old | 0.331 | 88 | 0.124, 0.510 | 0.002 |
| Current migraine frequencya | − 0.069 | 93 | − 0.275, 0.143 | 0.511 |
| Medication overuse headache | 0.206 | 93 | − 0.004, 0.398 | 0.048 |
| MOH | ||||
| More than 2 attacks/month | 0.033 | 92 | − 0.179, 0.242 | 0.754 |
| More than 2 attacks/month five years ago | 0.220 | 89 | 0.006, 0.415 | 0.038 |
| Triptan use | 0.224 | 92 | 0.014, 0.415 | 0.032 |
| More than 3 days/months analgesics for dysmenorrhoea | 0.226 | 94 | 0.017, 0.416 | 0.029 |
aMigraine frequency in migraine days per month
Discussion
There is little knowledge about the course and features of migraine in women who additionally suffer from comorbid endometriosis. Previous studies mainly focused on migraine prevalence in endometriosis patients, and cycle characteristics in patients with both, endometriosis and migraine [1, 2, 9, 23, 24]. Our sample included women in the middle of their reproductive years, recruited from an endometriosis clinic with an average BMI and typical age of migraine onset [25]. Mean age (36.4 years) was comparable with that in other endometriosis-migraine trials, but lower than in the CAMEO study, which focused on non-gynaecologic comorbidities. This needs consideration when interpreting the results [1, 2, 24, 26, 27]. Interestingly we found an MA rate of 41% in patients with endometriosis. MMF was low (1.5 attacks), potentially related to the selection of patients from a gynaecology outpatient clinic. It is surprising, that the reported MMF five years before the interview was significantly higher (2.9 attacks/month). Few women had ever experienced MOH (8.6%) and none of the participants suffered from chronic migraine at the time of the study. Mean age at menarche was 12.7 years and migraine onset before age 17 was reported by 44.3% of participants. Endometriosis ASRM score was not associated with MA, higher frequency of migraine or MOH. As a higher ASRM stage is not typically associated with more pelvic pain, we also tested for associations with depth of infiltration of the endometriosis lesions [28]. We also did not find an association here.
MMF in our trial was lower than in population-based studies [26, 27, 29]. We expected higher frequencies in women with two disabling pain comorbidities, especially as we see in our clinic for hormonal migraines rather patients highly affected by both conditions. The CAMEO study reported a MMF of 3.5 days for slightly older patients with other types of comorbidities in a huge sample representative for the US population [26]. For MMF at age < 20 years, our data are in accordance with findings from a diary -based study including migraine patients without endometriosis using combined hormonal contraceptives in a setting in Switzerland. In the latter study, MMF rose to 4.2 monthly attacks until mean age 26.5 years [30].
The decline of MMF during the reproductive years in our study is unusual [26, 31]. This decline compared to the frequency 5 years ago was probably not related to the new start of prophylactic agents, as the number of women using pharmaceutical products for headache prevention at the time of the interview was very low (3.2%). Therefore, we suggest that recent endometriosis surgery and treatment might play a pivotal role in the observed reduction. Improvement of one pain condition might exert a positive effect on the course of the second pain condition [17]. Long-term studies in migraine patients suffering from endometriosis are necessary to improve our understanding of the impact of endometriosis surgery and treatment on the course of migraine. Up to date, most studies addressing comorbidities of migraine focus on cardiovascular conditions, psychic conditions, and autoimmune disease but not on endometriosis [26, 32, 33].
Our patients reported a relatively long duration of migraine attacks with 16.6 h. In a recent Italian multicenter study, around 50% of the migraine patients suffered from migraine episodes lasting less than 24 h, while the mean duration in the CAMEO setting was 27.7 h [26, 34]. The duration of attacks might be related to insufficient response to acute medication. Only 18% of the patients in our setting had ever seen a neurologist to receive a prescription, and triptan use in this setting of an endometriosis clinic was rather low. Triptans would not reduce pain associated with endometriosis, what could contribute to the preferred use of other types of analgesics in patients with both conditions.
Two trials with patients recruited from a gynaecologic center did not report MA data and typical migraines features for the subgroup of patients with both, endometriosis and migraine patients [2, 35]. In comparison to the MA prevalence in population-based studies (5–6%), we found a much higher prevalence (41.5%) [34, 36, 37]. This finding is very much in line with the results from Ferrero, who also studied migraine features in more detail in women recruited from an endometriosis-clinic [38]. The strengths of our trial with a smaller sample size is the inclusion of only patients with surgically confirmed endometriosis and collection of migraine data not with a questionnaire but in interviews performed by neurologists. Ferrero et al. found a MA prevalence of 35% and a slightly higher migraine frequency with 44.7 attacks/year [38]. The higher MMF in this trial, performed more than 15 years ago, could be related to the high percentage of combined hormonal contraceptive (CHC) users (25.6%). Today it is better known among neurologists and gynaecologists, that CHCs should not be used in MA patients. In addition, CHC use for endometriosis treatment is less common. Considering the high genetic background with sex hormone receptor polymorphisms in endometriosis-migraine patients, we suggest that we have a special subgroup of migraineurs, with different migraine features, especially more frequent aura [3, 4]. Factors associated with MA in our trial were migraine onset at an earlier age ( 17 years) and a history of MOH. Earlier age at migraine onset might also be an indicator for a stronger genetic background. The pathophysiology of MA is by far not complete understood. One of the leading theories is that aura phenomena are linked to cortical spreading depression (CSD). Hormone fluctuations during the menstrual cycle, especially estrogen fluctuations might play a role in the development of CSD [39]. Endometriosis lesions may release estradiol. Sandweiss et al. found, that rats, after a 17-β-estradiol injection, developed significantly more CSD episodes over a 12-h recording period. Pre-administration of an estrogen receptor antagonist blocked CSD events and pain behaviors [40]. However, the low amounts of estrogen released locally from endometriosis lesions are minimal in comparison to the cyclic estrogen production in the ovaries. Endometriosis patients have regular cycles and do not differ from other healthy young women with regard to estrogen levels or fluctuations. Therefore, it seems unlikely, that the high prevalence of aura in endometriosis patients is generated from the estrogen release from endometriosis lesions.
None of our patients suffered from chronic migraine, which would have an expected prevalence in the female population of around 1.3% and might be even higher in women with comorbidities [26, 41]. We cannot exclude that with age some of our participants might develop chronic migraine in the future. In a small sample of endometriosis patients recruited from a tertiary headache center who were of similar age, Tietjen et al. found chronic migraine in 40% of women [35]. The reason for such differences is unclear but might be explained with differing recruitment-strategies.
Chronic pain conditions, more severe headache, psychic conditions and triptan use have been shown to be associated with a higher risk or medication overuse headaches (MOH) [32, 42–44]. MOH prevalence in the general population of migraineurs is 1–2%. It was 11.9% in a study including patients with non-gynaecologic comorbidities and much higher in trials conducted in headache clinics (50–72%) [26, 42, 45–47]. In our trial 8.6% of the participants reported to have ever experienced MOH. Surprising for us in this setting of women with two disabling pain conditions was, that at present none of the participants suffered from MOH. Special features of a history of MOH in our trial include more migraine attacks 5 years ago, migraine with aura, use of triptans (OR 4.9) and use of more pain killers to treat pelvic pain during the menstrual bleeding (Table 3). Use of pain killers for other conditions than migraine may contribute to the risk of developing MOH [48]. Again, we raise the theory, that the low MOH rate could be related to the improvement of endometriosis pain after surgery and treatment. Future prospective studies should focus on this point. The location for recruitment, i.e. headache clinic, gynaecologic setting, or general population, has to be taken into account when comparing results with other studies.
MIDAS score in our trial did not differ substantially from the CAMEO study (6.0 and 6.8). A MIDAS grade indicating moderate or severe disability in 20% of the women is of concern and reflects severe suffering in a subgroup of our sample.
Altogether, we observed in contrast to the normal course of migraine in women during the reproductive years a decrease in migraine frequency in our sample. If this improvement is causally related to endometriosis surgery, has to be investigated in further prospective studies. We confirm that the prevalence of MA is higher than expected from the general population. Migraine with aura, early migraine onset and episodic migraine have been shown to be associated with a positive family history [18, 49]. Therefore and based on the knowledge that endometriosis-migraine patients share common sex-hormone specific polymorphisms, we postulate a potential shared genetic background as reason for the high prevalence of MA. In comparison to studies with endometriosis-migraine patients recruited from headache clinics, we found much lower MMF, a lower MOH rate and no patients with chronic migraine. This might indicate that there are two subtypes and phenotypes of women with both conditions. On the one hand, those with endometriosis pain as the major burden and less migraines and on the other hand patients with migraine as the major burden, who would suffer from a higher MMF and more often from chronic migraines.
Strength and limitations
Migraine features might differ between patients recruited from a gynaecologic clinic or a headache center. Our results apply to women who visited a specialised endometriosis clinic. Women recruited from a gynaecologic center with endometriosis pain as their major burden might suffer from less severe migraines than those recruited at headache centers. Only a subset had ever consulted a neurologist for their headache problem. Endometriosis was the more disabling condition for our participants. A limitation of this trial is the lack of a control group. Furthermore, we cannot exclude that there might be some recall bias related to interview data addressing events very long ago like age at menarche or migraine onset. Strength of the study include the sample size and the collection of migraine data through personal telephone interviews performed by specialists. In addition, the diagnosis of endometriosis was histologic confirmed and localization and ASRM score provided detailed information on the stage and phenotype of the condition.
Conclusion
We conclude that the comorbidity of endometriosis maybe linked to MA, while MMF is rather low. Further studies are needed to explore, if the observed decrease in MMF at present in comparison to MMF 5 years ago, can be attributed to recent endometriosis surgery. ASRM score was not associated with any of the migraine features, nor was deep infiltration of the lesions.
Acknowledgements
We thank Chiara Knobel and Lea Portmann for data collection and performance of the interviews. Further we thank all patients who agreed to be part of this project.
Abbreviations
- MO
Migraine without aura
- MA
Migraine with aura
- MO
Migraine without aura
- MOH
Medication overuse headache
- MMF
Monthly migraine frequency
- ASRM
American society for reproductive medicine
Authors contributions
GSM: Conceptualized and organized the study. Performed the acquisition of analysis and interpretation the data, wrote the the abstract and introduction and main parts of the discussion; revised the final manuscript. HD: Contributed to development of the interview, supervised correct data entry, supported statistical analyses and interpretation of the data. PI: Contributed to data interpretation and critical discussion of the data. PS: Contributed to data interpretation and critical discussion of the data. AG: Contributed to data interpretation and critical discussion of the data. CS: Contributed to statistics and interpretation of the data and main parts of the discussion. All authors have approved the submitted version of the manuscript and have agreed to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Funding
Open access funding provided by University of Zurich. No funding.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
GSM: Reports fee for advisory board and presentations for/from Novartis, Eli Lilly, Almirall Lundbeck. CJS: Reports fees for consulting, advisory boards, presentations, and travel support for/from Novartis, Eli Lilly, TEVA Pharmaceuticals, Pfizer, Allergan, Abbvie, Almirall, Amgen, Lundbeck, MindMed, Grünenthal. He is part-time-employee at Zynnon. He has received research support from Swiss Heart Foundation, Eye on Vision Foundation, Baasch-Medicus Foundation, German Migraine and Headache Society, Visual Snow Syndrome Germany e.V. ARG has received honoraria for speaking or consulting from Amgen, Abbvie, Allergan, Biomed, Curatis, EliLilly, Lundbeck, Novartis, Sanofi and TEVA. PS has received honoraria for advisory boards from Novartis, Eli Lilly, Almirall. HD declares no CO. PI declares no COI.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wu Y, Wang H, Chen S, Lin Y, Xie X, Zhong G, Zhang Q (2021) Migraine is more prevalent in advanced-stage endometriosis, especially when co-occuring with adenomoysis. Front Endocrinol (Lausanne) 12:814474 10.3389/fendo.2021.814474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maitrot-Mantelet L, Hugon-Rodin J, Vatel M, Marcellin L, Santulli P, Chapron C, Plu-Bureau G (2020) Migraine in relation with endometriosis phenotypes: results from a French case-control study. Cephalalgia 40(6):606–613 10.1177/0333102419893965 [DOI] [PubMed] [Google Scholar]
- 3.Adewuyi EO, Sapkota Y, International Endogene Consortium I, andMe Research T, International Headache Genetics Consortium I, Auta A, Yoshihara K, Nyegaard M, Griffiths LR, Montgomery GW et al (2020) Shared molecular genetic mechanisms underlie endometriosis and migraine comorbidity. Genes (Basel) 11(3) [DOI] [PMC free article] [PubMed]
- 4.van der Vaart JF, Merki-Feld GS (2022) Sex hormone-related polymorphisms in endometriosis and migraine: a narrative review. Womens Health (Lond) 18:17455057221111316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mattsson P (2003) Hormonal factors in migraine: a population-based study of women aged 40 to 74 years. Headache 43(1):27–35 10.1046/j.1526-4610.2003.03005.x [DOI] [PubMed] [Google Scholar]
- 6.Wang SJ, Fuh JL, Lu SR, Juang KD, Wang PH (2003) Migraine prevalence during menopausal transition. Headache 43(5):470–478 10.1046/j.1526-4610.2003.03092.x [DOI] [PubMed] [Google Scholar]
- 7.Nyholt DR, Gillespie NG, Merikangas KR, Treloar SA, Martin NG, Montgomery GW (2009) Common genetic influences underlie comorbidity of migraine and endometriosis. Genet Epidemiol 33(2):105–113 10.1002/gepi.20361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang MH, Wang PH, Wang SJ, Sun WZ, Oyang YJ, Fuh JL (2012) Women with endometriosis are more likely to suffer from migraines: a population-based study. PLoS ONE 7(3):e33941 10.1371/journal.pone.0033941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller JA, Missmer SA, Vitonis AF, Sarda V, Laufer MR, DiVasta AD (2018) Prevalence of migraines in adolescents with endometriosis. Fertil Steril 109(4):685–690 10.1016/j.fertnstert.2017.12.016 [DOI] [PubMed] [Google Scholar]
- 10.Merki-Feld GS, Imthurn B, Langner R, Sandor PS, Gantenbein AR (2013) Headache frequency and intensity in female migraineurs using desogestrel-only contraception: a retrospective pilot diary study. Cephalalgia 33(5):340–346 10.1177/0333102412473373 [DOI] [PubMed] [Google Scholar]
- 11.Merki-Feld GS, Imthurn B, Langner R, Seifert B, Gantenbein AR (2015) Positive effects of the progestin desogestrel 75 mug on migraine frequency and use of acute medication are sustained over a treatment period of 180 days. J Headache Pain 16:522 10.1186/s10194-015-0522-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sacco S, Merki-Feld GS, Karen L, Aegidius E, Bitzer J, Canonico M, Gantenbein AR, Kurth T, Lampl C, Lidegaard O, Anne MacGregor E et al (2018) Effect of exogenous estrogens and progestogens on the course of migraine during reproductive age: a consensus statement by the European headache federation (EHF) and the European society of contraception and reproductive health (ESCRH). J Headache Pain 19(1):76 10.1186/s10194-018-0896-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casper RF (2017) Progestin-only pills may be a better first-line treatment for endometriosis than combined estrogen-progestin contraceptive pills. Fertil Steril 107(3):533–536 10.1016/j.fertnstert.2017.01.003 [DOI] [PubMed] [Google Scholar]
- 14.Nappi RE, Sances G, Allais G, Terreno E, Benedetto C, Vaccaro V, Polatti F, Facchinetti F (2011) Effects of an estrogen-free, desogestrel-containing oral contraceptive in women with migraine with aura: a prospective diary-based pilot study. Contraception 83(3):223–228 10.1016/j.contraception.2010.07.024 [DOI] [PubMed] [Google Scholar]
- 15.Morotti M, Remorgida V, Venturini PL, Ferrero S (2014) Progestogen-only contraceptive pill compared with combined oral contraceptive in the treatment of pain symptoms caused by endometriosis in patients with migraine without aura. Eur J Obstet Gynecol Reprod Biol 179:63–68 10.1016/j.ejogrb.2014.05.016 [DOI] [PubMed] [Google Scholar]
- 16.Liakopoulou MK, Tsarna E, Eleftheriades A, Arapaki A, Toutoudaki K, Christopoulos P (2022) Medical and behavioral aspects of adolescent endometriosis: a review of the literature. Children (Basel) 9(3):384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karp BI, Sinaii N, Nieman LK, Silberstein SD, Stratton P (2011) Migraine in women with chronic pelvic pain with and without endometriosis. Fertil Steril 95(3):895–899 10.1016/j.fertnstert.2010.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Boer I, Terwindt GM, van den Maagdenberg A (2020) Genetics of migraine aura: an update. J Headache Pain 21(1):64 10.1186/s10194-020-01125-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38(1):1–211 [DOI] [PubMed]
- 20.World Endometriosis Research Foundation. Women`s Health Survey Questionniare
- 21.Stewart WF, Lipton RB, Dowson AJ, Sawyer J (2001) Development and testing of the migraine disability assessment (MIDAS) questionnaire to assess headache-related disability. Neurology 56(6 Suppl 1):S20-28 [DOI] [PubMed] [Google Scholar]
- 22.Revised American Society for Reproductive Medicine classification of endometriosis (1996) Fertil Steril, 67(5):817–821 [DOI] [PubMed]
- 23.Spierings EL, Padamsee A (2015) Menstrual-cycle and menstruation disorders in episodic vs chronic migraine: an exploratory study. Pain Med 16(7):1426–1432 10.1111/pme.12788 [DOI] [PubMed] [Google Scholar]
- 24.Tietjen GE, Bushnell CD, Herial NA, Utley C, White L, Hafeez F (2007) Endometriosis is associated with prevalence of comorbid conditions in migraine. Headache 47(7):1069–1078 10.1111/j.1526-4610.2007.00784.x [DOI] [PubMed] [Google Scholar]
- 25.Buse DC, Loder EW, Gorman JA, Stewart WF, Reed ML, Fanning KM, Serrano D, Lipton RB (2013) Sex differences in the prevalence, symptoms, and associated features of migraine, probable migraine and other severe headache: results of the American migraine prevalence and prevention (AMPP) study. Headache 53(8):1278–1299 10.1111/head.12150 [DOI] [PubMed] [Google Scholar]
- 26.Lipton RB, Fanning KM, Buse DC, Martin VT, Hohaia LB, Adams AM, Reed ML, Goadsby PJ (2019) Migraine progression in subgroups of migraine based on comorbidities: results of the CaMEO Study. Neurology 93(24):e2224–e2236 10.1212/WNL.0000000000008589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aegidius K, Zwart JA, Hagen K, Schei B, Stovner LJ (2006) Oral contraceptives and increased headache prevalence: the Head-HUNT Study. Neurology 66(3):349–353 10.1212/01.wnl.0000196481.57994.09 [DOI] [PubMed] [Google Scholar]
- 28.Fauconnier A, Chapron C, Dubuisson JB, Vieira M, Dousset B, Breart G (2002) Relation between pain symptoms and the anatomic location of deep infiltrating endometriosis. Fertil Steril 78(4):719–726 10.1016/S0015-0282(02)03331-9 [DOI] [PubMed] [Google Scholar]
- 29.Katsarava Z, Mania M, Lampl C, Herberhold J, Steiner TJ (2018) Poor medical care for people with migraine in Europe—evidence from the Eurolight study. J Headache Pain 19(1):10 10.1186/s10194-018-0839-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merki-Feld GS, Sandor PS, Nappi RE, Pohl H, Schankin C (2022) Clinical features of migraine with onset prior to or during start of combined hormonal contraception: a prospective cohort study. Acta Neurol Belg 122(2):401–409 10.1007/s13760-021-01677-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burch RC, Buse DC, Lipton RB (2019) Migraine: epidemiology, burden, and comorbidity. Neurol Clin 37(4):631–649 10.1016/j.ncl.2019.06.001 [DOI] [PubMed] [Google Scholar]
- 32.Katsarava Z, Buse DC, Manack AN, Lipton RB (2012) Defining the differences between episodic migraine and chronic migraine. Curr Pain Headache Rep 16(1):86–92 10.1007/s11916-011-0233-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicodemo M, Cevoli S, Giannini G, Cortelli P (2012) Comorbidity in perimenstrual migraine. Curr Pain Headache Rep 16(5):477–483 10.1007/s11916-012-0281-z [DOI] [PubMed] [Google Scholar]
- 34.Barbanti P, Egeo G, Aurilia C, Fiorentini G, Proietti S, Tomino C, Bonassi S (2022) Italian migraine registry study G: the first report of the Italian migraine registry (I-GRAINE). Neurol Sci 43(9):5725–5728 10.1007/s10072-022-06214-5 [DOI] [PubMed] [Google Scholar]
- 35.Tietjen GE, Conway A, Utley C, Gunning WT, Herial NA (2006) Migraine is associated with menorrhagia and endometriosis. Headache 46(3):422–428 10.1111/j.1526-4610.2006.00290.x [DOI] [PubMed] [Google Scholar]
- 36.Kurth T, Rist PM, Ridker PM, Kotler G, Bubes V, Buring JE (2020) Association of migraine with aura and other risk factors with incident cardiovascular disease in women. JAMA 323(22):2281–2289 10.1001/jama.2020.7172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rasmussen BK (1995) Epidemiology of migraine. Biomed Pharmacother 49(10):452–455 10.1016/0753-3322(96)82689-8 [DOI] [PubMed] [Google Scholar]
- 38.Ferrero S, Pretta S, Bertoldi S, Anserini P, Remorgida V, Del Sette M, Gandolfo C, Ragni N (2004) Increased frequency of migraine among women with endometriosis. Hum Reprod 19(12):2927–2932 10.1093/humrep/deh537 [DOI] [PubMed] [Google Scholar]
- 39.Grafstein B (1956) Mechanism of spreading cortical depression. J Neurophysiol 19(2):154–171 10.1152/jn.1956.19.2.154 [DOI] [PubMed] [Google Scholar]
- 40.Sandweiss AJ, Cottier KE, McIntosh MI, Dussor G, Davis TP, Vanderah TW, Largent-Milnes TM (2017) 17-beta-estradiol induces spreading depression and pain behavior in alert female rats. Oncotarget 8(69):114109–114122 10.18632/oncotarget.23141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lipton RB, Silberstein SD (2015) Episodic and chronic migraine headache: breaking down barriers to optimal treatment and prevention. Headache 55(Suppl 2):103–122 10.1111/head.12505_2 [DOI] [PubMed] [Google Scholar]
- 42.Diener HC, Dodick D, Evers S, Holle D, Jensen RH, Lipton RB, Porreca F, Silberstein S, Schwedt T (2019) Pathophysiology, prevention, and treatment of medication overuse headache. Lancet Neurol 18(9):891–902 10.1016/S1474-4422(19)30146-2 [DOI] [PubMed] [Google Scholar]
- 43.Park HK, Chu MK, Oh SY, Moon HS, Song TJ, Lee MJ, Kang JJ, Hong Y, Cho SJ, Investigators R (2022) Interim analysis of the registry for load and management of medication overuse headache (RELEASE): a multicenter, comprehensive medication overuse headache registry. Cephalalgia 42(6):455–465 [DOI] [PubMed]
- 44.Schwedt TJ, Alam A, Reed ML, Fanning KM, Munjal S, Buse DC, Dodick DW, Lipton RB (2018) Factors associated with acute medication overuse in people with migraine: results from the 2017 migraine in America symptoms and treatment (MAST) study. J Headache Pain 19(1):38 10.1186/s10194-018-0865-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jonsson P, Hedenrud T, Linde M (2011) Epidemiology of medication overuse headache in the general Swedish population. Cephalalgia 31(9):1015–1022 10.1177/0333102411410082 [DOI] [PubMed] [Google Scholar]
- 46.Calhoun A, Ford S (2008) Elimination of menstrual-related migraine beneficially impacts chronification and medication overuse. Headache 48(8):1186–1193 10.1111/j.1526-4610.2008.01176.x [DOI] [PubMed] [Google Scholar]
- 47.Bigal ME, Rapoport AM, Sheftell FD, Tepper SJ, Lipton RB (2004) Transformed migraine and medication overuse in a tertiary headache centre–clinical characteristics and treatment outcomes. Cephalalgia 24(6):483–490 10.1111/j.1468-2982.2004.00691.x [DOI] [PubMed] [Google Scholar]
- 48.Schmid CW, Maurer K, Schmid DM, Alon E, Spahn DR, Gantenbein AR, Sandor PS (2013) Prevalence of medication overuse headache in an interdisciplinary pain clinic. J Headache Pain 14:4 10.1186/1129-2377-14-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pelzer N, Louter MA, van Zwet EW, Nyholt DR, Ferrari MD, van den Maagdenberg AM, Haan J, Terwindt GM (2019) Linking migraine frequency with family history of migraine. Cephalalgia 39(2):229–236 10.1177/0333102418783295 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.