Abstract
Background
Peritoneal dialysis (PD) catheter related infections continue to be a major cause of morbidity and transfer to hemodialysis (HD) in PD patients. The treatment of tunnel infection (TI) could be challenging, especially when the infection involves the superficial cuff requiring the removal of the catheter. To spare the patient the loss of the catheter and the transfer to HD, several mini-invasive surgical techniques have been proposed as rescue therapy. Furthermore, nowadays, the rapid growth of digital technology has enormously increased the diagnostic sensibility of the echo signal allowing to accurately defines the extent of the infectious process along the PD catheter tunnel.
Methods
Between 1st January 2020 and 31st December 2021 seven patients who underwent exit-site relocation by external splicing and cuff removal at our institution due to refractory TI were included in the study. All patients were followed until 12 months after the procedure. As soon as TI was defined refractory to the medical therapy, an ultrasonographic examination of the catheter tunnel was performed to define the extent of the infectious episode.
Results
Among the 7 infectious episodes, 4 were caused by P. aeruginosa, and 3 by S. aureus. Around the superficial cuff the hypo/anechoic collections detected by ultrasounds showed a mean diameter of 3.05 ± 0.79 mm. The exit-site relocation by external splicing and cuff removal was successful in all cases (7/7, 100%).
Conclusions
In our experience the use of exit site relocation by external splicing and cuff removal as rescue therapy for TI with positive ultrasounds for TI limited to superficial cuff involvement and without secondary peritonitis, yielded to promising results with a success rate of 100%. This preliminary experience underlines the paramount usefulness of tunnel echography in accurately defining the extent of TI and, consequently, guiding the choice of the therapeutical approach in refractory TI.
Keywords: Peritoneal dialysis, Exit-site infection, Tunnel infection, Peritonitis, Ultrasonography, Ultrasound, Cuff shaving, Cuff removal, Partial reimplantation, Splicing
Introduction
Peritoneal dialysis (PD) catheter related infections can lead to peritonitis, hospitalization, shift to hemodialysis (HD) and death [1–3]. Nearly 20% of the exit site infections may arise in conjunction with or develop into tunnel infection (TI), while up to 60% of TI are refractory to medical therapy requiring the removal of the PD catheter [4].
In fact, the spread of the infective process to the superficial cuff makes the infection hardly responsive to the antibiotic therapy since the microorganisms can infiltrate the Dacron of the catheter cuffs and form a biofilm in this area that facilitates their proliferation [4, 5]. The creation of this layer becomes a barrier for antibiotic agents [6, 7]. As a consequence, TI with involvement of the cuffs is unlikely to respond to antibiotic therapy, requiring often the removal of the catheter [1, 8–10]. However, this management necessitates the interruption of PD and the transition to HD via the placement of a temporary central venous catheter.
Conversely, the optimal therapeutic approach should allow continuation of PD with an uninfected catheter. Thus, several noninvasive surgical techniques, such as the cuff-shaving, the partial reimplantation of the PD catheter or the exit-site relocation by external splicing and superficial cuff removal have been suggested [11, 12].
Furthermore, the rapid growth of digital technology has generated a solid background for the rapid development of ultrasonographic techniques allowing an increasingly refined analysis of the diagnostic information of the echo signal [13]. Thus, nowadays the ultrasound evaluation of the PD catheter tunnel represents a non-invasive, easily tolerated and readily available method with high sensitivity in experienced hands [14]. However, pre-operative ultrasonographical examination has never been used as a tool to select the more appropriate approach in case of refractory TI. Recently, our group has proposed a specific algorithm that, combining anatomical and ultrasonographical features, can guide the treatment choice [11, 13]. Thus, in order to obtain preliminary data that could initially validate this approach and corroborate the ultrasonographical diagnostic criteria, we exploited part of this algorithm to treat seven cases of refractory TI by external splicing and cuff removal procedure.
Methods
Participants and study design
This is a single center retrospective cohort study carried out at Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico between January 1st 2020 and December 31st 2021. Adults (> 18 years) who showed a TI resistant to antibiotic therapy and positive ultrasonographic findings of superficial cuff involvement with no signs of infection beyond this point, absence of secondary peritonitis, and exit-site/superficial cuff distance greater than 2 cm, were included in the study. Those patients were candidate for cuff removal and were followed until 12 months after the intervention. The data were collected by reviewing an electronic database (Galenus®, Infogramma s.r.l., Milan, Italy).
Clinical management
In every patient a straight double-cuffed Tenckhoff catheter was placed with a modified double purses string technique around the inner-cuff either in semi-surgical or surgical procedure, as described elsewhere [15, 16]. The creation of the subcutaneous part was achieved by using a piercing tunneller in a direction able to minimize shear forces with the superficial cuff located at least 4 cm from the exit of the skin [4]. As far as routinely exit-site care is concerned, patients were instructed to apply hydrogen peroxide followed by 5% sodium hypochlorite solution to the skin surface three to four times per week.
Tunnel infection was diagnosed in the presence of clinical inflammation (erythema, swelling, tenderness or induration) along the catheter tunnel [1].
Empirical oral antibiotic with cephalexin 500 mg bid was initiated in all patients at the clinical diagnosis of TI. Subsequently, the antibiotic therapy was adjusted according to the antibiogram results. In the case of methicillin-sensitive S. aureus cephalexin was continued. Differently, when P. aeruginosa was isolated, the patients were shifted to oral ciprofloxacin 250 mg bid while if methicillin-resistant S. aureus was identified, they were treated with intravenous vancomycin associated to oral rifampicin 300 mg bid.
All the episodes were defined refractory to the medical therapy in case of no improvement after two weeks of targeted antibiotic treatment or 3-weeks for TI due to P. aeruginosa [1]. At this time, a careful ultrasound examination of the catheter tunnel was performed. Then, provided a positive ultrasonographic findings of superficial cuff involvement (hypo/anechoic area with a diameter > 2 mm), but no signs of infection beyond this point as well as absence of secondary peritonitis, exit-site relocation by external splicing and cuff removal was employed as a catheter rescue therapy in case of an exit-site/superficial cuff distance greater than 2 cm (Fig. 1). After the surgical procedure, the antibiotic treatment was continued for further 3 weeks.
Fig. 1.
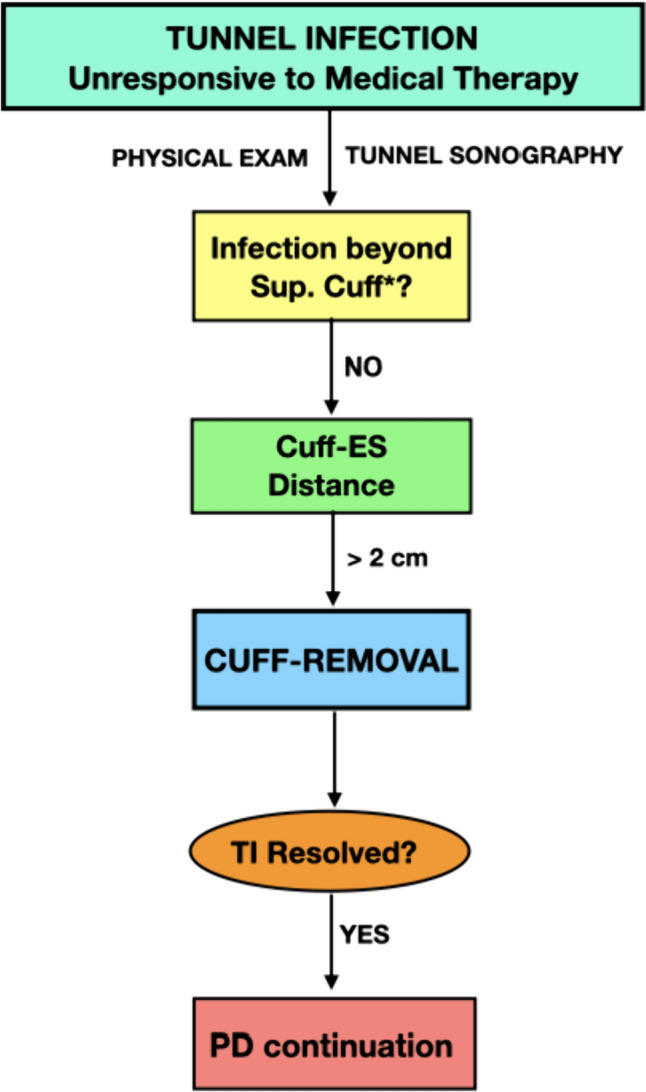
Flowchart regarding the management of tunnel infection unresponsive to medical therapy with concomitant superficial cuff infection, but without ultrasonographic evidence of infection spreading beyond the superficial cuff and an exit-site/superficial cuff distance greater than 2 cm. CUFF-REMOVAL exit-site relocation by external splicing and cuff removal, ES exit-site, PD peritoneal dialysis, Sup. Cuff superficial cuff, TI tunnel infection. *The extent of the infectious process should be assessed by physical exam (searching for erythema, swelling, induration, or tenderness over the subcutaneous pathway) and by ultrasonographic evaluation (detection of hypoechogenic area between the tube/cuff of the catheter and the surrounding tissues with a diameter > 2 mm) [modified by [11]]
Surgical procedure
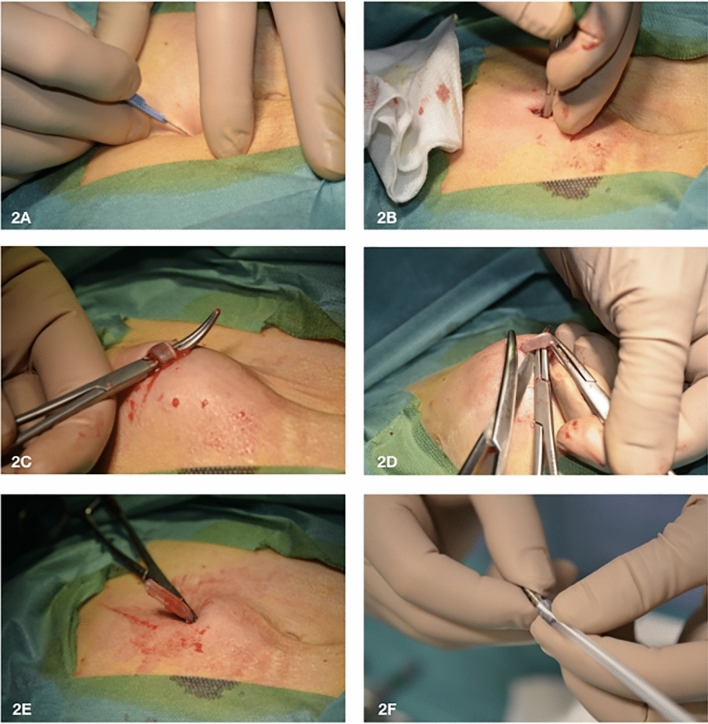
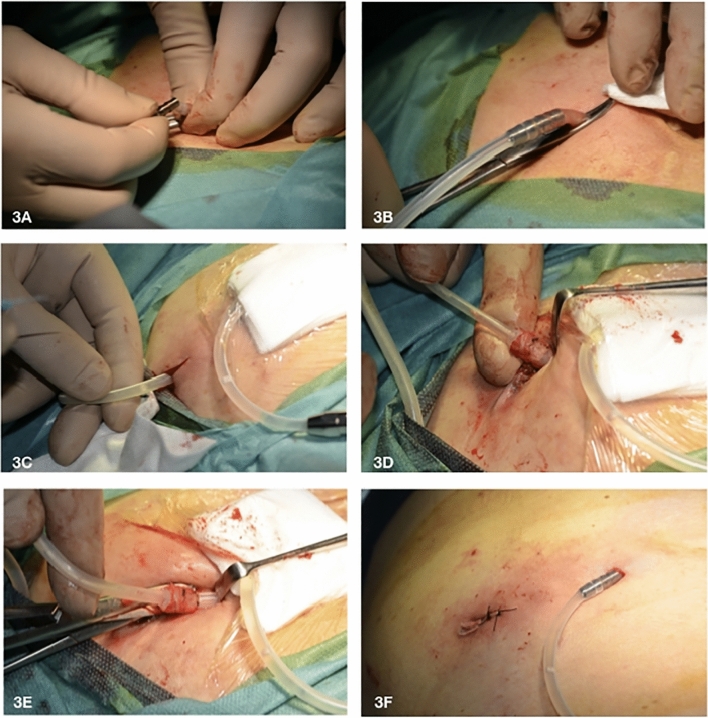
The exit-site relocation by external splicing and cuff removal consists in creating a skin incision of approximately 5 mm at 1–2 cm distance from the superficial cuff along the subcutaneous catheter course towards the deep cuff (Fig. 2A). Then, through this tiny opening, the peritoneal catheter is retrieved by a blunt dissection (Fig. 2B), lifted out from the skin (Fig. 2C), and cut 1 cm distance from the superficial cuff (Fig. 2D). After dragging out the free end of the catheter from the skin (Fig. 2E), it is externally lengthened by connecting a double-barbed titanium extender that was previously attached to new catheter piece (Figs. 2F, 3A, B). Hence, a skin incision of 2 cm is performed in proximity of the infected superficial cuff which is, then, isolated by a dissection of the surrounding tissue and, eventually, removed through the old exit-site (Fig. 3C–F) [11, 12]. The superficial cuff was conserved and sent for microbiological evaluation.
Fig. 2.
A–F Exit-site relocation by external splicing and cuff removal—first part. A Skin incision of approximately 5 mm at 1–2 cm distance from the superficial cuff in the direction of the deep cuff; B through the tiny opening the peritoneal catheter is retrieved from the subcutaneous tissue; C then it is lifted out from the skin; D and is cut 1 cm distance from the superficial cuff; E now the free end of the catheter is dragged out from the s subcutaneous layer; F subsequently, part of a new catheter is taken and one of the two free ends is attached to a double-barbed titanium extender
Fig. 3.
A–F Exit-site relocation by external splicing and cuff removal—second part. A the catheter is externally lengthened by connecting its remaining free end to the second edge of the double-barbed titanium extender; B the adapter used to extend the catheter remains outside the new exit-site to not hinder its maturation; C skin incision of about 1–2 cm at the level of the old exit-site sinus is carried out; D then, the adipose and scar tissue adjacent to the infected superficial cuff is removed; E eventually, the infected superficial cuff connected to the distal part of the old catheter is taken out through the old exit-site; F the new-exit site is distant 4–5 cm from the skin incision performed to remove the old exit-site
Ultrasound examination
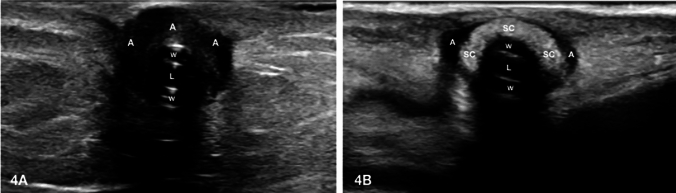
Before starting the ultrasonographic examination, the exit-site was carefully disinfected by applying hydrogen peroxide followed by 5% sodium hypochlorite solution and covered with a transparent sterile film dressing to avoid its contamination. Then, using a 7–13 MHz frequency linear probe (Affiniti 70G, Philips®, Amsterdam, Netherlands), the tunnel of the catheter was evaluated maintaining the patient in supine decubitus. Once the catheter was identified, it was followed, by both long and short-axis visualization, along the whole subcutaneous path from the exit-site until the peritoneal cavity. The examination was aimed at detecting the presence of pathological hypo/anechoic collection surrounding either the catheter walls or the Dacron cuffs. A hypo/anechoic area with a diameter > 2 mm located between the catheter walls and the surrounding tissues was acknowledged as a positive exam (Fig. 4A). The involvement of either the superficial or deep cuff by the infectious process was defined as the detection of the previous ultrasonographic criteria around the hyperechoic signal of the Dacron cuff (Fig. 4B).
Fig. 4.
A, B Positive ultrasonographic findings of tunnel infection with superficial cuff involvement. A Presence of hypo/anechoic area with a diameter > 2 mm located between the catheter walls and the surrounding tissues; B Presence of hypo/anechoic area with a diameter > 2 mm around the hyperechoic signal of the Dacron cuff indicating the involvement of the superficial by the infectious process. A hypo/anechoic area (abscess), L lumen of the catheter, SC superficial cuff, W catheter walls
Statistical analysis
Normally distributed variables are presented as mean ± standard deviation, while nonparametric data are presented as median with interquartile range. Categorical variables are expressed as frequency and percentage.
Results
Population characteristics and causative organisms
The demographic and clinical details of the 7 participants are shown in Table 1. Most patients were male (57.1%) with a mean age of 63.7 ± 11.3 (standard deviation [SD]) years. The median time of continuous ambulatory PD treatment at TI diagnosis was 15.7 months (interquartile range 7.9–21.8). Among the 7 episodes of TI, 4 were caused by Pseudomonas aeruginosa (P. aeruginosa), while 3 by Staphylococcus aureus (S. aureus).
Table 1.
Baseline characteristics and clinical details regarding the seven patients who underwent exit site relocation by external splicing and cuff removal as rescue catheter treatment for refractory tunnel infection
| ID-1 | ID-2 | ID-3 | ID-4 | ID-5 | ID-6 | ID-7 | |
|---|---|---|---|---|---|---|---|
| Sex (M = male; F = female) | F | M | F | M | F | M | M |
| Age (years) | 49 | 69 | 81 | 70 | 54 | 55 | 68 |
| Kidney disease | ADPKD | Unknown | Unknown | Diabetic Neph | Cardiorenal type II | IgA Neph | Membranous Neph |
| Time on PD (months) | 11.4 | 4.3 | 17.4 | 26.2 | 3.2 | 15.7 | 34.4 |
| PRE ATB Therapy | Cepha– > cipro | Cefa– > vanco | Cepha– > cipro | Cephalexin | Cepha– > cipro | Cephalexin | Cepha– > cipro |
| Microorganism | P. aeruginosa | S. aureus | P. aeruginosa | S. aureus | P. aeruginosa | S. aureus | P. aeruginosa |
| DUR PRE ATB therapy (days) | 28 | 21 | 25 | 19 | 27 | 18 | 24 |
| DIM AREA SUP CUFF (mm) | 2.22 | 2.45 | 2.31 | 3.66 | 2.82 | 3.81 | 4.11 |
| MAX DIM AREA PERICAT (mm) | 3.24 | 3.64 | 3.43 | 7.82 | 3.55 | 4.13 | 5.43 |
| POST ATB THERAPY | Ciprofloxacin | Vancomycin | Ciprofloxacin | Cephalexin | Ciprofloxacin | Cephalexin | Ciprofloxacin |
| DUR POST ATB THERAPY (days) | 21 | 21 | 21 | 21 | 21 | 21 | 21 |
| Relapse at 12 months | No | No | No | No | No | No | No |
ADPKD autosomal dominant polycystic kidney disease, cepha cephalexin, cipro ciprofloxacin, DIM dimension, DUR duration, Neph nephropathy, ID patient identification number, PRE ATB THERAPY antibiotic therapy prescribed before exit site relocation by external splicing and cuff removal (– > = shift to other molecule during the antibiotic course), POST ATB THERAPY antibiotic therapy prescribed after the exit site relocation by external splicing and cuff removal, PD peritoneal dialysis, PERICAT pericatheter, vanco vancomycin
Clinical outcomes
Around the superficial cuff the hypo/anechoic collections detected by ultrasonographic examination showed a mean diameter of 3.05 mm ± SD 0.79 mm (Table 1). However, the greatest dimension of the peri-catheter liquid collection was always found in a portion between the exit-site and the superficial cuff (mean value 4.46 mm ± SD 1.65 mm; Table 1). No signs of infection beyond the superficial cuff either by physical or ultrasonographic examination were identified.
In every case, the microbiological specimen of the superficial cuff, collected during the operation, identified the presence of the same microorganisms which had been previously isolated from the purulent discharge of the exit-site.
The exit-site relocation by external splicing and cuff removal was successful in all cases (7/7, 100%). In fact, after 12 months from the surgical procedure, physical and ultrasonographic examination of the tunnel resulted negative as well as the microbiological swab performed both at the old and new exit-site (Table 1). No patient needed to interrupt the peritoneal exchanges.
Discussion
The available evidence has shown that tunnel infections with concomitant involvement of the cuffs are unlikely to respond efficaciously to antibiotic therapy, thereby requiring in most cases the removal of the catheter [8–10]. However, via ultrasonographic examination is possible to accurately determine the extent of the infectious process and to identify with high sensibility any liquid collection around the PD catheter, despite specific ultrasonographical criteria have not been precisely established yet.
The presence of an abscess surrounding the cuff corresponds to the dissemination of the infectious process in the Dacron material; hence, we decided to employ as diagnostic criteria for a positive ultrasonographical exam the presence of hypo/anechoic area with a diameter > 2 mm located between the cuff and the adjacent tissue.
In order to spare the patient the loss of the peritoneal catheter and the change of dialysis modality, our group has recently proposed a specific approach for the treatment of refractory TI [11, 13]. Within this algorithm, provided the absence of infection beyond the superficial cuff as well as secondary peritonitis, we suggested to consider the distance between the exit-site and the superficial cuff. If this distance was less than or equal to 2 cm, cuff-shaving was proposed, otherwise (distance > 2 cm) it was suggested to relocate the exit-site by external splicing and cuff removal or by partial reimplantation of the catheter [11].
According to our algorithm, in the seven cases reported the distance between the exit-site and the superficial cuff was greater than 2 cm, thus the exit-site relocation by external splicing and cuff removal was employed to treat the episodes of refractory TI without secondary peritonitis due to P. aeruginosa or S. aureus (Fig. 1). The procedure was successful in all cases (7/7, 100%). Indeed, after 12 months from the surgical procedure we did not observe any relapsing episodes.
In a prior analysis with the use of cuff removal technique, we obtained a less satisfying success rate of approximately 70% (15 out of 21 cases vs 7/7 of this series) in both Gram-positive (12 out of 17 cases) and Gram-negative infections (3 out of 4 cases) [11]. This outcome discrepancy might be due to the absence of pre-operative ultrasonographical evaluation in the previous experience. In fact, the ultrasonographic examination of the tunnel has proved to be as specific as the clinical parameters, but with a considerably greater sensitivity, especially in case of TI involving the portion of the tunnel comprised between the superficial and deep cuff [9, 10, 17, 18].
Thus, at that time, the surgical procedure could have been also carried out in cases in which the infection had been already spread beyond the superficial cuff making the mini-invasive surgical approach ineffective.
Conversely, upon the new protocol we always performed a pre-procedure ultrasonographic examination to define the extent of the infectious process that, eventually, guided the choice of the surgical technique [13]. Therefore, the precise localization via ultrasounds of the infection process could reasonably explain the significative outcome improvement observed in this series. It is worth of mentioning that, to the best of our knowledge, this is the first study in which the type of surgical procedure was decided on the basis of the findings observed by a pre-operative ultrasonographic examination of the subcutaneous tunnel.
In comparison to the cuff-shaving, the exit-site relocation by external splicing and cuff removal has the advantage of being less traumatic [19]. In fact, it is performed through a small incision that corresponds to the location of the new exit-site. This procedure does not require the removal of the fatty tissue around the infected cuff, while also limiting bleeding and wound pain [11, 12]. Contrary to the partial reimplantation technique, a shorter incision is performed [20–23]. In addition, the catheter is extended outside the skin allowing a rapid diagnosis and a prompt resolution in case of disconnection [11, 12].
However, besides the extent of the infection (progression deeper than the superficial cuff or presence of concurrent peritonitis) further contraindications of our procedure might be represented either by a long distance between the catheter and the skin or by a poor exit-site location.
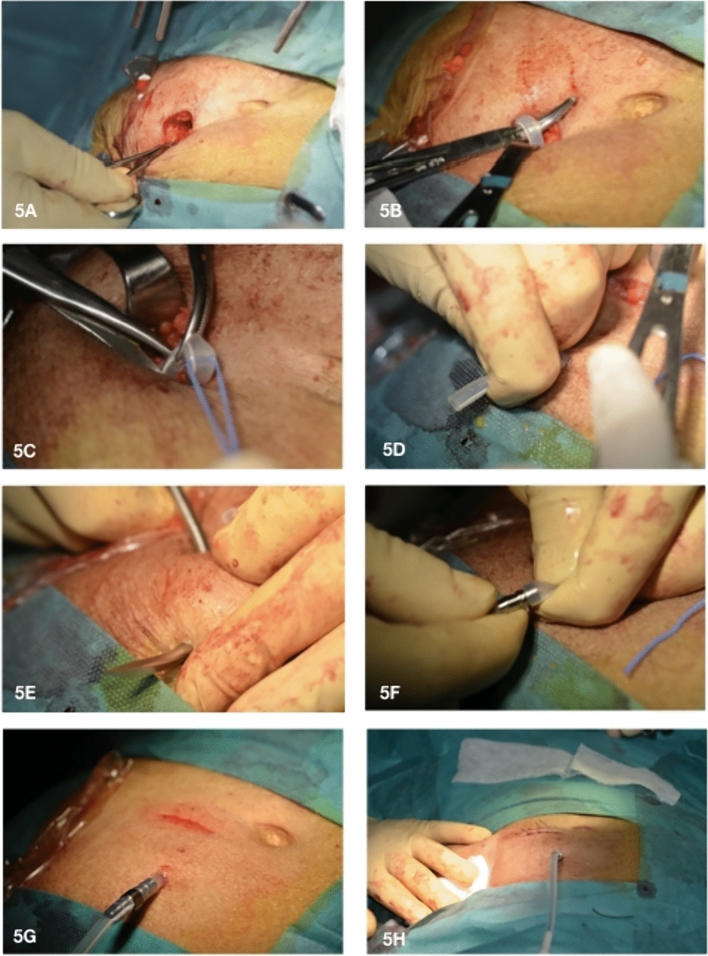
In fact, in the first case the depth of the catheter complicates the isolation of the device trough a tiny cutaneous incision. While in the second case, the poor exit-site location constricts the creation of the new exit-site few centimeters from the old one (approximately a distance between the previous exit-site and the superficial cuff) often leading to unsatisfactory results. Thus, in these cases the partial reimplantation of the catheter may represent the elective technique. This procedure consists in isolating the portion of the catheter between the two cuffs and splicing it in proximity of the deep cuff. Thereafter, the device is extended via the portion of another catheter and tunneled subcutaneously in the contralateral hemi-abdomen with the creation of a new exit-site. Thus, this approach would allow to move the new exit-site far from the old one and to facilitate the isolation of the catheter since the skin incision is inevitably larger [11]. Another feasible option in the previous conditions (long distance between the catheter and the skin or a poor exit-site location) is represented by a modified technique of our procedure. The variation consists in carrying out a wider incision (approximately 3–4 cm) at 1–2 cm distance from the superficial cuff along the catheter path in the direction of the deep cuff that permits to isolate a longer portion of the subcutaneous device. Once the catheter is cut off at the proximity of the superficial cuff, the free end is attached to a metal bend rod and the isolated part of the catheter is dragged in the subcutis to create a new exit-site away from the old one. Eventually, once the catheter is taken out from the skin, it is externally lengthened by connecting the double-barbed titanium extender (Fig. 5A–H).
Fig. 5.
A–H Modified cuff removal technique by additional tunneling of the PD catheter. A skin incision of approximately 3–4 cm 1 at 1–2 cm distance from the superficial cuff along the catheter path in the direction of the deep cuff; B through the opening the peritoneal catheter is retrieved from the subcutaneous tissue C the catheter is cut 1 cm distance from the superficial cuff; D then, the free end of the catheter is lifted out from the skin; E subsequently, the catheter free end is attached to a metal bend rod and the isolated part of the catheter is dragged in the subcutis to create a new exit-site; F-G Once the catheter is taken out from the skin, it is externally lengthened by connecting a double-barbed titanium extender that was previously attached to new catheter piece; H now the new exit-site is located far away from the old one
In conclusion, these preliminary results underline the usefulness of the integration of the physical examination with the ultrasound evaluation when deciding the therapeutic approach in the treatment of the refractory tunnel infection. In particular, in PD patients affected by TI with positive ultrasonographic findings of superficial cuff involvement (presence of hypo/anechoic area with a diameter > 2 mm between the cuff and the adjacent tissue), but no signs of infection beyond this point, and absence of secondary peritonitis, exit-site relocation by external splicing and cuff removal, as a rescue therapy for refractory TI, showed a success rate of 100%. Certainly, additional data collected on a larger population is needed to validate both the proposed ultrasonographical diagnostic criteria and the therapeutical algorithm.
Funding
Open access funding provided by Università degli Studi di Milano within the CRUI-CARE Agreement.
Data availability
The data that support the findings of this study are not openly available, but are obtainable from the corresponding author upon reasonable request. Data are located in controlled access data storage at University of Milan.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chow KM, Li PK-T, Cho Y et al (2023) ISPD Catheter-related infection recommendations: 2023 update. Perit Dial Int 2023(43):201–219 10.1177/08968608231172740 [DOI] [PubMed] [Google Scholar]
- 2.van Diepen ATN, Tomlinson GA, Jassal SV (2012) The association between exit site infection and subsequent peritonitis among peritoneal dialysis patients. Clin J Am Soc Nephrol 7:1266–1271 10.2215/CJN.00980112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scalamogna A, Nardelli L, Zubidat D, Castellano G (2023) Simultaneous replacement and removal of the peritoneal catheter is effective in patients with refractory tunnel infections sustained by S. aureus. Int Urol Nephrol 55:151 10.1007/s11255-022-03288-0 [DOI] [PubMed] [Google Scholar]
- 4.Nardelli L, Scalamogna A, Messa P (2021) The impact of the superficial cuff position on the exit site and tunnel infections in CAPD patients. J Nephrol 34:493–501 10.1007/s40620-020-00788-z [DOI] [PubMed] [Google Scholar]
- 5.Ciofu O, Moser C, Jensen PØ, Høiby N (2022) Tolerance and resistance of microbial biofilms. Nat Rev Microbiol 20:621–635 10.1038/s41579-022-00682-4 [DOI] [PubMed] [Google Scholar]
- 6.Costerton J, Stewart P, Greenberg E (1979) Bacterial biofilms: a common cause of persistent Infections. Science 1999(284):1318–1322 [DOI] [PubMed] [Google Scholar]
- 7.Donlan RM, Costerton JW (2002) Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15:167–193 10.1128/CMR.15.2.167-193.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vychytil A, Lorenz M, Schneider B, Hörl W, Haag-Weber M (1998) New criteria for management peritoneal dialysis patients of catheter infections using ultrasonography. J Am Soc Nephrol 9:290–296 10.1681/ASN.V92290 [DOI] [PubMed] [Google Scholar]
- 9.Plum J, Sudkamp S, Grabensee B (1994) Results of ultrasound-assisted diagnosis of tunnel infections in continuous ambulatory peritoneal dialysis. Am J Kidney Dis 23:99–104 10.1016/S0272-6386(12)80818-3 [DOI] [PubMed] [Google Scholar]
- 10.Kwan T, Tong MK, Siu Y, Leung K (2004) Ultrasonography in the management of exit site infections in peritoneal dialysis patients. Nephrology 9:348–352 10.1111/j.1440-1797.2004.00331.x [DOI] [PubMed] [Google Scholar]
- 11.Scalamogna A, Nardelli L, Castellano G (2022) The use of mini-invasive surgical techniques to treat refractory exit-site and tunnel infections in peritoneal dialysis patients: a clinical approach. J Nephrol 36(7):1743–1749 10.1007/s40620-022-01479-7 [DOI] [PubMed] [Google Scholar]
- 12.Scalamogna A, Nardelli L, Cosa F, Pisati S, Messa P (2021) Mini-invasive surgical techniques for the peritoneal catheter rescue in refractory tunnel infections. G Ital Nefrol 1(3) [PubMed]
- 13.Nardelli L, Scalamogna A, Castellano G (2023) Utility of ultrasonographic examination in catheter-related infections in peritoneal dialysis: a clinical approach. J Nephrol 36:1751–1761 10.1007/s40620-023-01589-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nardelli L, Scalamogna A, Zeiler M, Messa P (2020) Use of ultrasounds in PD catheter related infections: indications and clinical implications. G Ital Nefrol 37(Suppl 75):S75 [PubMed] [Google Scholar]
- 15.Scalamogna A, Nardelli L, Zanoni F, Messa P (2020) Double purse-string around the inner cuff of the peritoneal catheter: a novel technique for an immediate initiation of continuous peritoneal dialysis. Int J Artif Organs 43:365–371 10.1177/0391398819891735 [DOI] [PubMed] [Google Scholar]
- 16.Scalamogna A, Nardelli L, Cicero E, Castellano G (2022) Analysis of mechanical complications in urgent-start peritoneal dialysis. J Nephrol 35:1489–1496 10.1007/s40620-022-01294-0 [DOI] [PubMed] [Google Scholar]
- 17.Holley JL, Foulks CJ, Moss AH, Willard D (1989) Ultrasound as a tool in the diagnosis and management of exit-site infections in patients undergoing continuous ambulatory peritoneal dialysis. Am J Kidney Dis 14:211–216 10.1016/S0272-6386(89)80073-3 [DOI] [PubMed] [Google Scholar]
- 18.Korzets Z, Erdberg A, Golan E, Verner M, Rathaus V, Bernheim J (1996) Frequent involvement of the internal cuff segment in CAPD peritonitis and exit-site infection—an ultrasound study. Nephrol Dial Transplant 11:336–339 10.1093/oxfordjournals.ndt.a027263 [DOI] [PubMed] [Google Scholar]
- 19.Meng C, Beco A, Oliveira A, Pereira L, Pestana M (2019) Peritoneal dialysis cuff-shaving—a salvage therapy for refractory exit-site infections. Perit Dial Int 39:276–281 10.3747/pdi.2018.00193 [DOI] [PubMed] [Google Scholar]
- 20.Oki R, Hamasaki Y, Komaru Y et al (2020) Catheter diversion procedure with exit-site renewal promotes peritoneal dialysis catheter survival. Kidney Int Rep 6:325–332 10.1016/j.ekir.2020.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chao S, Tsai T (1996) Partial replantation of Tenckhoff catheters to treat intractable exit-site/tunnel infection. J Am Soc Nephrol 7:1085–1087 10.1681/ASN.V771085 [DOI] [PubMed] [Google Scholar]
- 22.Cheung AHS, Wheeler MS, Limm WML, Wong LL, Fan F, Wong LMF (1995) A salvage technique for continuous ambulatory peritoneal dialysis catheters with exit-site infections. Am J Surg 170:60–61 10.1016/S0002-9610(99)80253-0 [DOI] [PubMed] [Google Scholar]
- 23.Clouatre Y, Cartier P, Charbonneau R, Deziel C, Allard M, Madore F (2000) Outpatient CAPD catheter salvage for persistent exit-site/tunnel infection. Nephrol Dial Transplant 15:231–234 10.1093/ndt/15.2.231 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are not openly available, but are obtainable from the corresponding author upon reasonable request. Data are located in controlled access data storage at University of Milan.