Abstract
Introduction
Once-daily fixed-dose combinations (FDC) containing abacavir (ABC), dolutegravir (DTG), and lamivudine (3TC) have been approved in the US for adults and children with HIV weighing ≥ 6 kg. This analysis assessed the ability of previously developed ABC, DTG, and 3TC pediatric population pharmacokinetic (PopPK) models using multiple formulations to describe and predict PK data in young children using dispersible tablet (DT) and tablet formulations of ABC/DTG/3TC FDC in the IMPAACT 2019 study.
Methods
IMPAACT 2019 was a Phase I/II study assessing the PK, safety, tolerability, and efficacy of ABC/DTG/3TC FDC in children with HIV-1. Intensive and sparse PK samples were collected over 48 weeks. Existing drug-specific pediatric PopPK models for ABC (2-compartment), DTG (1-compartment), and 3TC (1-compartment) were applied to the IMPAACT 2019 drug concentration data without re-estimation (external validation) of PopPK parameters. Drug exposures were then simulated across World Health Organization weight bands for children weighing ≥ 6 to < 40 kg for each drug and compared with pre-defined exposure target ranges.
Results
Goodness-of-fit and visual predictive check plots demonstrated that the previously developed pediatric PopPK models sufficiently described and predicted the data. Thus, new PopPK models describing the IMPAACT 2019 data were unnecessary. Across weight bands, the predicted geometric mean (GM) for ABC AUC0–24 ranged from 14.89 to 18.50 μg*h/ml, DTG C24 ranged from 0.74 to 0.95 μg/ml, and 3TC AUC0–24 ranged from 10.50 to 13.20 μg*h/ml. These exposures were well within the pre-defined target ranges set for each drug.
Conclusion
This model-based approach leveraged existing pediatric data and models to confirm dosing of ABC/DTG/3TC FDC formulations in children with HIV-1. This analysis supports ABC/DTG/3TC FDC dosing in children weighing ≥ 6 kg.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-024-01008-y.
Keywords: Dolutegravir, Abacavir, Lamivudine, Population pharmacokinetics, HIV-1, Pediatrics
Key Summary Points
| Child-friendly single-tablet fixed dose combination (FDC) regimens are critical for improved adherence and treatment outcomes in HIV-1. There are very few FDC options available for children weighing < 14 kg. |
| The present study modeled the pharmacokinetics (PK) of abacavir (ABC), dolutegravir (DTG), and lamivudine (3TC) after administering a once-daily ABC/DTG/3TC FDC dose (dispersible tablet or tablet) to pediatric participants with HIV-1 in IMPAACT 2019 study. |
| ABC, DTG, and 3TC PK exposures following once-daily ABC/DTG/3TC dispersible tablet and tablet dosing were comparable to those observed in pediatrics and adults with single entities, supporting extrapolation of adult efficacy data. |
Introduction
Approximately 1.54 million children aged 0–14 were living with HIV globally in 2022. Unfortunately, only 57% of the children with HIV were receiving life-saving antiretroviral therapy (ART). Children under the age of 15 represent a small portion of all people living with HIV (about 4%), but they represent a significant portion of new HIV infections (10%) and deaths due to AIDS (13%) [1–3]. Immediate ART is recommended for everyone diagnosed with HIV, including children and adolescents. Current guidelines recommend initiating ART using three drugs in children, primarily composed of a dual nucleoside/nucleotide reverse transcriptase inhibitor (NRTI) backbone, along with an anchor drug such as an integrase strand transfer inhibitor (INSTI), a non-nucleoside reverse transcriptase inhibitor (NNRTI), or a boosted protease inhibitor (PI). The introduction of fixed dose combination tablets (FDCs) revolutionized pediatric ART [4]. These formulations combine multiple antiretroviral (ARV) drugs into one pill, simplifying treatment for adolescents and young children. FDCs address common challenges, such as swallowing multiple pills or remembering multiple doses, by offering a single, manageable option. The significant reduction in the pill burden also helps alleviate the burden on their caregivers, who often manage multiple medications. Finally, it improves adherence, another major factor with FDCs, leading to better viral suppression, slower disease progression, and enhanced overall treatment outcomes for pediatric HIV patients [5].
A once-daily FDC that combines two NRTIs, abacavir (ABC) and lamivudine (3TC), and the HIV INSTI dolutegravir (DTG), has been approved in many global markets. This FDC tablet contains ABC 600 mg/DTG 50 mg/3TC 300 mg (marketed as Triumeq) and is indicated for treating HIV-1 infection in adults and pediatric patients weighing at least 40 kg [6]. DTG is approved for use with other ART agents for treatment-naive and -experienced patients at least 4 weeks of age and weighing at least 3 kg [7]. ABC is a guanosine nucleoside analog [8], and 3TC is a cytidine nucleoside analog [9]. Both are approved for use in pediatric patients starting from 3 months of age. The World Health Organization (WHO) also recommends using DTG-based regimens as the preferred first- and second-line treatment for all populations, including pregnant women and those of childbearing potential [10].
To expand the application of the ABC/DTG/3TC FDC to children with HIV-1 infection, a new child-friendly FDC formulation of DTG 5 mg/ABC 60 mg/3TC 30 mg was developed as a dispersible tablet (DT) [11]. The safety and pharmacokinetics of this new ABC/DTG/3TC FDC DT formulation along with tablet formulation was evaluated in the IMPAACT 2019 study in children < 12 years old with HIV-1 (≥ 6 to < 40 kg) [12]. As stated earlier, each of the individual components of ABC/DTG/ 3TC FDC has already been approved for use in pediatric patients weighing at least 6 kg. Within each weight band, doses administered in IMPAACT 2019 using the ABC/DTG/3TC FDC formulations (DT & tablet) were the same or similar to those US-FDA approved for individual components (Table S1). This ABC/DTG/3TC FDC regimen aims to balance a simplified dosing scheme while ensuring safe and efficacious exposure for all individual drug components across a wide range of body weight.
The current analysis aimed to determine the ability of the established ABC, DTG, and 3TC pediatric population pharmacokinetic (PopPK) models to describe and predict the observed ABC/DTG/3TC PK data in the IMPAACT 2019 study. This PopPK analysis supported the pediatric dosing regimens for ABC/DTG/3TC FDC (DT and tablet) based on the WHO-defined weight bands. The aim was to assess whether the new formulation is able to achieve drug exposure levels in children across the relevant weight bands similar to those observed in children and adults when these medicines are taken individually. These results were intended to support the regulatory approval of ABC/DTG/3TC FDC (DT and tablet) for children weighing at least 6 kg.
Methods
Ethics
The IMPAACT 2019 study (ClinicalTrials gov identifier NCT03760458) was designed in accordance with the International Council for Harmonization, Good Clinical Practice guidelines [13], and applicable country-specific requirements and abided by the ethical principles of the Declaration of Helsinki. Informed consent was obtained from all the participants before screening. Institutional review boards and national authorities reviewed and approved the trial protocol, amendments, and informed consent forms.
Study Design and Pharmacokinetic Analysis
IMPAACT 2019 (Fig. 1) was an international Phase I/II, multisite, open-label study investigating the PK, safety, tolerability, and efficacy of ABC/DTG/ 3TC FDC (tablets and DT) in pediatric patients < 12 years of age living with HIV-1. Pediatric participants were enrolled concurrently based on five separate weight bands (≥ 6 to < 10 kg, ≥ 10 to < 14 kg, ≥ 14 to < 20 kg, ≥ 20 to < 25 kg, and ≥ 25 to < 40 kg). Intensive PK sampling was performed in the first 5–7 pediatric participants enrolled in each weight band at the Week 1 (Days 5–10) study visit for dose confirmation [14]. Intensive plasma PK samples were collected up to 24 h after dosing (pre-dose, 1, 2, 3, 4, 6, 8, and 24 h post-dose). Sparse PK samples were collected across all participants at Weeks 1 (in participants not undergoing intensive PK sampling at Week 1) pre-dose and at least 2 h post-dose, and at weeks 4, 12, 24, 36, and 48. Among participants with known Met184Val mutations, additional sparse PK samples were collected at weeks 8, 16, and 20. Dose confirmation for each weight band was based on achieving the pre-defined geometric mean (GM) PK targets [area under the concentration–time curve over 24 h (AUC0–24) and concentration at the end of the 24-h dosing interval (C24) for DTG and AUC0–24 for ABC and 3TC] for each drug along with safety outcomes. The GM C24 target range for DTG was 0.697–2.26 µg/ml, and the target GM AUC0–24 was 37–134 µg*h/ml. This DTG target range was selected based on adult PK data and is similar to ranges used in prior pediatric studies [15]. For ABC, the GM AUC0–24 target range was 6.3–50.4 µg*h/'ml, and the 3TC GM AUC0–24 target range was 6.3–26.5 µg*h/ml. These ABC and 3TC targets were selected based on the lower and upper bound of the 90% CIs for predicted exposures with administration once per day in children with HIV [14].
Fig. 1.
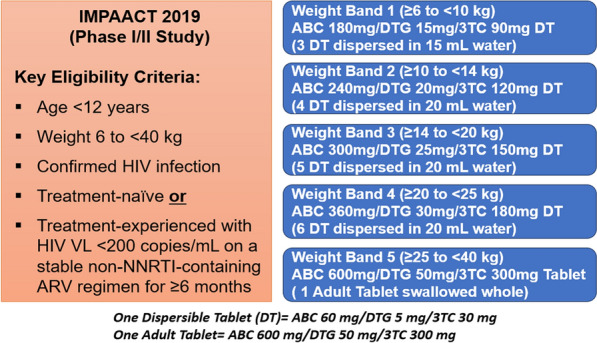
IMPAACT 2019 study design
Plasma PK samples were analyzed using validated reverse-phase high-performance liquid chromatography paired with tandem mass spectrometry (MS/MS). The lower limit of quantification (LOQ) in plasma was 5 ng/ml, and the upper limit of quantification (ULOQ) was 10,000 ng/ml for all the three components (DTG, ABC, 3TC). In addition, ABC samples below LOQ (5 ng/ml) were further analyzed using a separate ultra-sensitive method with an LOQ in plasma of 50 pg/ml and a ULOQ of 10,000 pg/ml.
PK Modeling Methods
Description of Established Pediatric PopPK Models for ABC, DTG, and 3TC
The PopPK analysis was performed using NONMEM software, version 7.3.0 (ICON Development Solutions), and run management was performed using Pirana (version 2.9.7). All post-processing diagnostic plots, simulations, post hoc analysis dataset preparation, calculations of secondary PK parameters, and summary statistics were performed using R (version 3.5.1) with RStudio (version 1.1 463). The established pediatric PopPK models for each single entity (ABC, DTG, and 3TC) were previously developed with pooled rich pediatric data. These existing PopPK models were previously used to support the current dosing of each constituent compound in pediatrics. [15, 16]. A brief description of each of the models is provided in the following sections.
ABC Pediatric PopPK Model
The existing ABC PopPK model was a pooled analysis combining six clinical studies[16]. PopPK parameters from this model are presented in Table 1. Plasma ABC exposure following oral dosing in pediatric participants (n = 169) aged 5 months to 13 years of age (4.6–61.3 kg) was well described by a two-compartment model, with IIV on (CL/F), apparent volume of distribution of the central compartment (V2/F) and apparent peripheral compartment volume of distribution (V3/F), intercompartmental clearance (Q/F), and IOV on CL/F. Weight was a significant covariate on CL/F and V/F. A study-specific relative bioavailability term (F1) was included in the model to describe substantially higher observed exposure in the ARROW Substudy Part 2 compared to other studies despite administration of similar doses/formulations [16].
Table 1.
Previously reported ABC pediatric PopPK parameter estimates
| Parameters | Point estimate (%RSE) |
|---|---|
| Apparent clearance, CL/F [L/h] | 16.3 (3.62) |
| Apparent central volume of distribution, V2/F [l] | 10.1(7.56) |
| Absorption rate constant, KA [h−1] | 0.85 (2.31) |
| Intercompartment clearance, Q/F [l/h] | 1.69 (7.87) |
| Apparent peripheral compartment volume of distribution, V3/F [l] | 23.0 (17.4) |
| F, tablet ARROW PK Substudy Part 2 | 1.62 (8.02) |
| F, solution ARROW PK Substudy Part 2 | 1.75 (8.23) |
| CL/F ~ (WT/15.6) | 0.794 FIX |
| V/2F ~ (WT/15.6) | 0.698 FIX |
| Interindividual variability | CV% |
| Q/F | 67.9 |
| V2/F | 51.9 |
| V3/F | 91.9 |
| CL/F | 36.3 |
| Interoccasion variability | |
| IOV-CL/F | 29.2 |
| Residual error | |
| Proportional error | 37.5% |
Further details about covariance matrix and full model can be found in [16]
%RSE percent relative standard error; IOV inter-occasion variability
DTG Pediatric PopPK Model
The existing DTG model was a pooled analysis combining two clinical studies [15]. PopPK parameters from this model are presented in Table 2. Plasma DTG exposure in pediatric participants (n = 239) aged 2 months to 18 years of age (3.9–91.0 kg) was well described by a one-compartment PK model with first-order absorption and first-order elimination, with interindividual variability (IIV) on apparent clearance (CL/F), apparent volume of distribution (V/F), and absorption rate constant (Ka) [15]. The PopPK model accounts for differences in bioavailability across formulations and the impact of food on DTG exposures. CL/F and V/F were allometrically scaled for body weight, respectively, with exponent estimates of 0.455 and 0.556. Inter-occasion variability (IOV) was added to CL/F and Ka. In addition, an enzyme maturation function was applied to CL/F, where half-maximal maturation was 52 weeks post-menstrual age (12 weeks post-natal age) [17]. No other covariates were identified in the model.
Table 2.
Previously reported DTG pediatric PopPK parameter estimates
| Parameters | Point estimate (%RSE) |
|---|---|
| Apparent clearance, CL/F [l/h] | 1.03 (2.31) |
| Apparent central volume of distribution, V/F [l] | 13.6 (2.42) |
| Absorption rate constant, KA, FCT [h−1] | 0.854 (11.2) |
| Absorption rate constant, KA ~ DT and granules [h−1] | 2.04 (15.7) |
| F, fasted FCT | 1.00 |
| F, without regard to food FCT | 1.10 (3.03) |
| F, fasted DT/granules | 1.53 (3.26) |
| CL/F ~ (WT/70) | 0.455 (4.15) |
| V/F ~ (WT/70) | 0.556 (3.87) |
| CL/F ~ maturation function (FMAT) | |
| Maturation half time, TM50 [PMA weeks] | 52.2 FIX |
| Hill coefficient related to the slope of the enzyme maturation process | 3.43 FIX |
| Interindividual variability | CV% |
| CL/F | 29.4 |
| V/F | 26.4 |
| KA | 107 |
| Interoccasion variability | |
| IOV-CL/F | 33.9 |
| IOV-KA | 91.7 |
| Residual error | |
| Proportional error study P1093 | 28.6% |
| Additive error (μg/ml), study P1093 | 0.00164 |
| Proportional error ODYSSEY | 11.1% |
| Additive error study (μg/ml), ODYSSEY | 0.090 |
DT dispersible tablet; FCT film coated tablet; PMA post-natal age; PMA post-menstrual age
FMAT = (PMAHILL/(PMAHILL + TM50HILL); PMA (weeks) = PNA (years)*52 (weeks) + 40 (weeks). Further details about covariance matrix and full model can be found in the reference [15]
3TC Pediatric PopPK Model
The existing 3TC model was a pooled analysis combining six clinical studies [16]. PopPK parameters from this model are presented in Table 3. The plasma 3TC exposure after oral administration in pediatric participants (n = 209) aged 4 months to 19 years of age (3.1–66.4 kg) was well described by a one-compartment model with first-order absorption and first-order elimination. A lag time (ALAG1) for absorption also was included. IIV and IOV were estimated for CL/F, V/F, and Ka [16]. Weight was a significant covariate on CL/F and V/F. A higher absolute bioavailability (F1) estimate was identified for solid dosage forms (tablet and capsule) than for the oral solution, consistent with the results of a 3TC relative bioavailability study conducted in children [18].
Table 3.
Previously reported 3TC pediatric PopPK parameter estimates
| Parameters | Point estimate (%RSE) |
|---|---|
| Absorption rate constant, KA [h−1] | 2.08 (9.76) |
| Lag time ALAG1 (h) | 0.297 (12.1) |
| Apparent central volume of distribution, V/F [l] | 23.1 (4.68) |
| Apparent clearance, CL/F [l/h] | 9.16 (4.49) |
| Absolute bioavailability (F1) solution PO | 0.496 (5.36) |
| Absolute bioavailability (F1) tablet PO | 0.609 (5.35) |
| CL/F ~ (WT/18.5) | 0.758 (7.07) |
| VL/F ~ (WT/18.5) | 0.677 (8.98) |
| Weighing factor for residual error | 4.72 (7.22) |
| Interindividual variability | CV% |
| CL/F | 28.6 |
| V/F | 32.7 |
| KA | 76.5 |
| Interoccasion variability | |
| IOV-CL/F | 24.9 |
| IOV-V/F | 60.0 |
| IOVKA | 19.7 |
| Residual error | |
| Additive error [mg/l] | 0.003 |
Further details about covariance matrix and full model can be found in [16]
External Model Validation Approach
An external model validation was applied to evaluate the adequacy of the existing pediatric PopPK models to describe the PK and variability in pediatric participants receiving ABC/DTG/3TC DT and tablet in the IMPAACT 2019 study. A sequential modeling approach was employed. The existing pediatric PopPK models were applied to the PK dataset from the IMPAACT 2019 study without re-estimating PopPK parameters (using the MAXEVAL = 0 option in NONMEM). The predictive performance of these existing pediatric PopPK models was evaluated using standard goodness-of-fit (GOF) plots. Simulation-based diagnostics were also used, including visual predictive checks (VPC) and normalized prediction distribution error (NPDE).
Assessment of Predictive Performance of Existing Models
The previously developed individual pediatric PopPK models were used to compute individual predictions of AUC0–24, maximum observed concentration (Cmax), and C24 for each of the individual components of ABC/DTG/ 3TC FDC (DT and tablet) following a steady-state dose for each participant included in the IMPAACT 2019 PopPK analysis. The individual estimates of all model parameters were obtained from the final models by an empirical Bayes estimation method (post hoc estimates from NONMEM). Individual estimates of AUC0–24, (Cmax), and C24 were obtained by simulating the steady-state concentration-time profiles (concentrations simulated at 0, 1, 2, 3, 4, 6, 8, and 24 h) using post hoc PK parameter estimates for each individual. The PK parameters AUC0–24 (calculated by linear up/log down trapezoidal rule), Cmax, and C24, and their summary statistics were computed and stratified by weight bands (all weights bands ≥ 6 to < 10 kg, ≥ 10 to < 14 kg, ≥ 14 to < 20 kg, ≥ 20 to < 25 kg, ≥ 25 to < 40 kg) using R version 3.2.5. These mode1-based, steady-state, post hoc PK parameters were compared with NCA PK parameters [14] to assess the predictive performance of the previous pediatric PopPK models.
Clinical Trial Simulations
Model simulations were performed to evaluate the appropriateness of ABC/DTG/3TC DT and tablet dosing regimen in the pediatric population. The simulations aimed at confirming the anticipated exposure across a wider range of body weights and age ranges. The DTG, ABC, and 3TC concentrations were predicted at 0, 1, 2, 3, 4, 6, 8, and 24 h following steady-state weight-based once-daily doses of ABC/DTG/3TC DT and tablet. Based on the CDC growth charts, pediatric population distributions corresponding to weight bands were constructed. The simulated clinical trial population consisted of 1000 participants, with 200 participants in each weight band, ensuring even distribution of the weights and ages in the simulation [15]. In total, 1000 replicate trials were simulated of 1000 participants with the once-daily ABC/DTG/3TC dosing regimen, and plasma AUC0–24, Cmax, and C24 were calculated for each of the individual drugs.
Results
Summary of Samples and Demographics
There were 57 participants enrolled in the IMPAACT 2019 study, and 35 participants (7 in each weight band) provided dose-evaluable intensive PK samples. Of these 57 participants, 2 (one in the ≥ 6 to < 10 kg weight band and the other in the ≥ 10 to < 14 kg weight band) discontinued the study during the first week because of palatability issues. A total of 55 participants contributed 590 ABC, 598 DTG, and 597 3TC intensive and sparse PK samples in the current population PK analysis. A summary of the demographics of the entire dataset is provided in Table S2. The median (min–max) baseline age across the 55 participants was 6.0 (1.00–11.0) years, and weight was 17.00 (8.15–39.30) kg, with an approximately similar proportion of the population being female (45.5%) and male (54.5%). Most of the population was Black (67%), with 31% Asians and the remaining 2% from all remaining race groups.
Evaluation of ABC Pediatric PopPK Model and Simulations
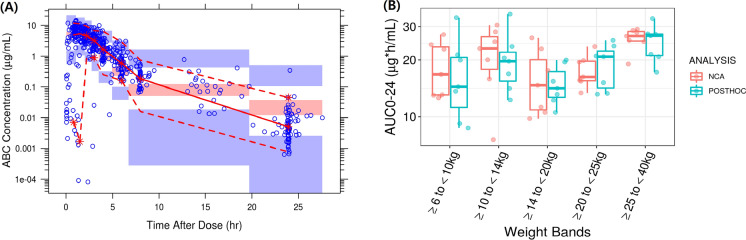
The previously developed ABC pediatric PopPK model successfully predicted the IMPAACT 2019 ABC PK data without re-estimating PopPK parameters. The VPC (Fig. 2A) and GOF plots (Figure S1) for the ABC model showed good agreement between the observed and predicted concentrations. The plots showed some overprediction of variability, likely due to the lower number of participants in each weight band in the IMPAACT 2019 study. There was a slight model misspecification, especially with some of the sparse PK data (Fig. S1). The observed ABC PK data in the IMPAACT 2019 study fell mostly within the 95% prediction interval (Fig. 2A). There was some overprediction of ABC trough concentrations. However, as the absolute difference was relatively small, this was not expected to have any significant impact on AUC0–24, which is the primary marker for the antiviral activity of ABC [19–21]. Overall, the previously developed pediatric ABC PopPK model captured the central tendency of data, and its performance was considered acceptable for the current analysis.
Fig. 2.
A Visual predictive check for the ABC model and B non-compartmental analysis (NCA) calculated vs. model predicted individual post hoc ABC AUC0–24 values with once daily ABC/DTG/3TC doses for pediatric patients (intensive PK population group only). A Blue circles: observed concentrations. Red solid and red dotted lines: median and 95% quantile of observed concentrations respectively; red and blue shaded areas: 95% confidence intervals of prediction median and 95% prediction intervals. B Boxes represent median (central horizontal line), first quartile, and third quartile of the data. Vertical solid line through the middle of the boxes (whiskers) represents 1.5*IQR. Red circles represent NCA, and cyan circles represent model predicted AUC0–24 values
The individual AUC0–24 post hoc estimates obtained from the model by an empirical Bayes estimation method showed parameters were comparable to the observed NCA PK parameters and were within the variability of the parameter estimates for each weight band (Fig. 2B). While the model accurately predicted AUC0–24 and Cmax, trough (C24) concentrations were slightly overpredicted (Table S3) [14]. However, this was considered not to be clinically relevant for NRTI drugs such as ABC since the plasma AUC0–24 is a significant PK parameter that correlates with HIV viral RNA suppression [19–21]. In addition, the ABC exposures observed in IMPAACT 2019 are comparable to the historical ABC pediatric and adult PK data and were within the pre-defined target range. Overall, the ABC PopPK model predicted the observed data well, and it was deemed appropriate for the purpose of clinical trial simulations. The predicted ABC exposures with the proposed doses of ABC/DTG/3TC (DT and tablet formulations) across different weight bands based on the final PopPK model are provided in Table 4. The PK parameter estimates were comparable for all the weight bands and marginally increased in the ≥ 25 to < 40 kg weight band. This could be due to the fact that most participants in this weight band (≥ 25 to < 40 kg) were concentrated between 25 to 30 kg weight range. The higher ABC dosage they received (~ 20 mg/kg) might have led to slightly higher geometric means. However, the predicted GM AUC0–24 ranges were consistent across the weight bands (14.89–18.50 µg*h/ml) for both DT and tablet formulations and were above the minimum target AUC0–24 GM exposure of 6.3 µg*h/ml. These proposed ABC/DTG/3TC FDC doses are expected to provide similar efficacy as observed with the ABC single entity.
Table 4.
Summary of simulated steady state PK parameters by weight band for ABC/DTG/3TC FDC dosing
| Weight bands | Proposed daily dose |
Cmax (µg/ml) |
AUC0–24 (µg*h/ml) |
C24 (µg/ml) |
|---|---|---|---|---|
| ABC | ||||
| ≥ 6 to < 10 kg | 180 mg DT | 6.63 (2.84–14.96) | 17.36 (6.66–43.70) | 0.039 (0.003–0.344) |
| ≥ 10 to < 14 kg | 240 mg DT | 6.66 (2.85–15.04) | 16.66 (6.43–41.62) | 0.027 (0.003–0.246) |
| ≥ 14 to < 20 kg | 300 mg DT | 6.41 (2.71–14.71) | 15.47 (5.94–39.12) | 0.020 (0.003–0.178) |
| ≥ 20 to < 25 kg | 360 mg DT | 6.31 (2.65–14.43) | 14.89 (5.72–37.49) | 0.016 (0.003–0.138) |
| ≥ 25 kg to < 40 kg | 600 mg tablet | 8.06 (3.31–18.85) | 18.50 (7.00–47.70) | 0.014 (0.003–0.136) |
| DTG | ||||
| ≥ 6 to < 10 kg | 15 mg DT | 6.99 (3.83–13.05) | 70.55 (30.5–166.4) | 0.94 (0.13–4.60) |
| ≥ 10 to < 14 kg | 20 mg DT | 6.88 (3.90–12.13) | 65.36 (29.69–142.6) | 0.74 (0.10–3.54) |
| ≥ 14 to < 20 kg | 25 mg DT | 7.12 (4.03–12.59) | 68.57 (31.33–150.4) | 0.81 (0.11–3.78) |
| ≥ 20 to < 25 kg | 30 mg DT | 7.42 (4.21–13.15) | 72.36 (33.03–158.3) | 0.88 (0.13–4.03) |
| ≥ 25 kg to < 40 kg | 50 mg tablet | 6.24 (3.41–11.38) | 66.75 (30.49–146.1) | 0.95 (0.15–4.11) |
| 3TC | ||||
| ≥ 6 to < 10 kg | 90 mg DT | 2.83 (1.43–5.48) | 11.57 (5.82–22.36) | 0.012 (0.003–0.132) |
| ≥ 10 to < 14 kg | 120 mg DT | 2.84 (1.45–5.44) | 11.40 (5.79–21.66) | 0.0118 (0.003–0.127) |
| ≥ 14 to < 20 kg | 150 mg DT | 2.74 (1.39–5.28) | 10.78 (5.49–20.6) | 0.0114 (0.003–0.121) |
| ≥ 20 to < 25 kg | 180 mg DT | 2.70 (1.37–5.20) | 10.50 (5.34–19.96) | 0.0112 (0.003–0.119) |
| ≥ 25 kg to < 40 kg | 300 mg tablet | 3.48 (1.73–6.80) | 13.20 (6.59–25.65) | 0.0115 (0.003–0.125) |
AUC0–24, Cmax and C24 presented as a GM (90% prediction interval). The GM C24 target range for DTG was 0.697–2.26 µg/ml, and the target GM AUC0–24 was 37–134 µg*h/ml. For ABC, the GM AUC0–24 target range was 6.3–50.4 µg*h/'ml, and the 3TC GM AUC0–24 target range was 6.3–26.5 µg*h/ml
Evaluation of DTG Pediatric PopPK Model and Simulations
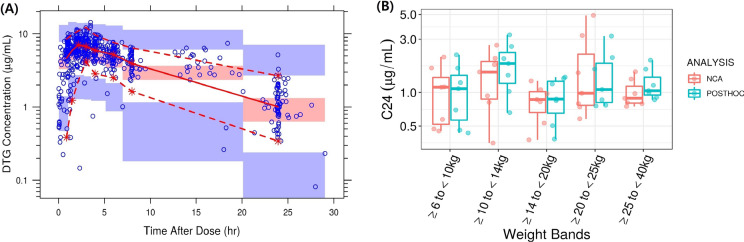
The previously developed DTG pediatric PopPK model successfully predicted the IMPAACT 2019 PK data without re-estimating PopPK parameters. The VPC (Fig. 3A) and GOF (Fig. S2) for the DTG model showed good agreement between observed and predicted concentrations. The plot showed that the data also were well predicted over time. However, there was some overprediction of variability, likely due to the relatively lower number of participants in each weight band in the IMPAACT 2019 study. Similar to the ABC model, there was a slight model misspecification (Fig. S2). The observed DTG PK data in the IMPACT 2019 study fell well within the 95% prediction interval (Fig. 3A). Overall, the model accurately predicted the data and was deemed appropriate for the current analysis. As a result, no model refitting was attempted for the DTG dataset.
Fig. 3.
A Visual predictive check for the DTG model and B NCA calculated vs model predicted individual post hoc DTG C24 values with once-daily ABC/DTG/3TC doses for pediatric patients (intensive PK population group only). A Blue circles: observed concentrations. Red solid and red dotted lines: median and 95% quantile of observed concentrations respectively; red and blue shaded areas: 95% confidence intervals of prediction median and 95% prediction intervals. B Boxes represent median (central horizontal line), first quartile, and third quartile of the data. Vertical solid line through the middle of the boxes (whiskers) represents 1.5*IQR. Red circles represent NCA, and cyan circles represent model predicted C24 values
The individual post hoc DTG C24 estimates were comparable to those observed from the NCA and were within the variability of the parameter estimates for all the weight bands (Fig. 3B) [14]. In addition, the DTG exposures observed in IMPAACT 2019 are comparable to historical DTG pediatric and adult PK data and within the pre-defined target range. Overall, the previously developed DTG pediatric PopPK model successfully predicted the observed data and was deemed appropriate for the subsequent clinical trial simulations (Table S4). The predicted DTG exposures with the proposed doses of ABC/DTG/3TC (DT and tablet formulations) across different weight bands based on the DTG pediatric PopPK model are provided in Table 4. The predicted GM C24 ranges were consistent across the weight bands (0.74–0.95 µg /ml) for both DT and tablet formulations and were above the minimum target GM concentration of 0.697 µg/ml. Similarly, GM AUC0–24 ranges ranged from 65.36 to 72.36 µg *h/ml across the weight bands, well above the minimum target GM exposure of 37 µg*h/ml [14]. These ABC/DTG/3TC FDC doses are expected to provide similar efficacy as observed with DTG single entity since exposures are similar.
Evaluation of 3TC Pediatric PopPK Model and Simulations
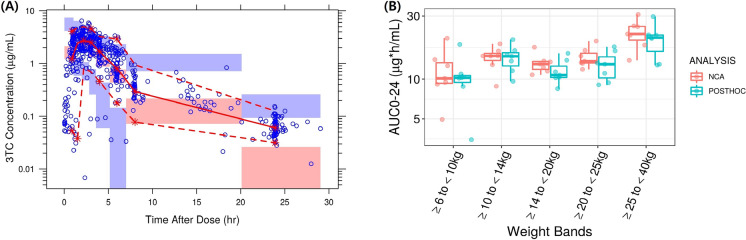
The previously developed 3TC pediatric PopPK model successfully predicted the IMPAACT 2019 PK data without re-estimating PopPK parameters. The VPC (Fig. 4A) and GOF plots (Fig. S3) for 3TC show good agreement between observed and predicted concentrations. There was some mismatch in the lower concentration range at the end of the dosing interval. However, as this only occurs at the end of the dosing interval where concentrations are low, this does not significantly affect the predictions of the overall exposure. Similar to the other two components (ABC and DTG), there was a slight model misspecification, especially with some of the sparse PK data. As the VPC plot shows, overall observed 3TC PK data in the IMPAACT 2019 study mainly fell within the 95% prediction interval (Fig. 4A). This indicates that the model can reliably be used for making predictions and considered adequate for this analysis.
Fig. 4.
A Visual predictive check for the 3TC model and B NCA calculated vs. model predicted individual post hoc 3TC AUC0–24 values with once daily ABC/DTG/3TC doses for pediatric patients (intensive PK population group only). A Blue circles: observed concentrations. Red solid and red dotted lines: median and 95% quantile of observed concentrations, respectively; red and blue shaded areas: 95% confidence intervals of prediction median and 95% prediction intervals. B Boxes represent median (central horizontal line), first quartile, and third quartile of the data; vertical solid line through the middle of the boxes (whiskers) represents 1.5*IQR, red circles represent NCA, and cyan circles represent model predicted AUC0–24 values
The individual 3TC AUC0–24 post hoc estimates were comparable to the observed NCA data and were within the variability of the parameter estimates for all the weight bands (Fig. 4B). While the model accurately predicted AUC0–24 and Cmax, some deviations were observed for trough (C24) concentrations, as it was slightly underpredicted (Table S5) [14]. However, this was considered not clinically relevant because for NRTI drug such as 3TC, AUC0–24 is the best predictor of antiviral activity similar to ABC [19–21]. In addition, the 3TC exposures observed in IMPAACT 2019 are comparable to the historical 3TC pediatric and adult PK data and were within the pre-defined target range. Overall, the previously developed 3TC pediatric PopPK model successfully predicted the observed data and was deemed appropriate for subsequent clinical trial simulation. Based on the final model, the predicted 3TC exposures with the proposed doses of DTG/ABC/3TC (DT and tablet formulations) across different weight bands are provided in Table 4. The PK estimates were consistent across the different weight bands and demonstrated that the previously developed 3TC pediatric PopPK model could predict PK exposure in IMPAACT 2019 participants with ABC/DTG/3TC dosing. The 3TC PK exposures observed in IMPAACT 2019 were higher compared to the simulated data, primarily in the ≥ 25 to < 40 kg weight band, which might be because actual participants enrolled in this weight band were concentrated between the 25–30 kg weight range. The higher dosage they received (~ 10 mg/kg) might have led to slightly higher exposure. However, the predicted GM AUC0–24 ranges were consistent across the weight bands (10.50–13.20 µg*h/ml) for both DT and tablet formulations. They exceeded the minimum target AUC0–24 GM exposure of 6.3 µg*h/ml. These proposed ABC/DTG/3TC FDC doses are expected to provide similar efficacy as observed with 3TC single entity.
Discussion
Pediatric programs for HIV center around the primary objective of exposure matching through PK analysis. This IMPAACT 2019 study included PK as a primary objective. Since the sample size was small, the anticipated safety and efficacy in children can be extrapolated from adults based on exposure matching. In addition, respective single entities have already been approved in pediatrics in the same weight group, establishing expected efficacy and safety at the given target exposures. Thus, not only can the exposure be matched with those observed as safe and efficacious in adults but also with those that have already been observed to be safe and efficacious in pediatrics is critical. Given the smaller sample size in each of the weight bands and having the majority of participants provide sparse PK samples, the used model-based approach was essential to achieve an understanding of the potential variability in PK and reduced the uncertainty of predicting exposures in a larger number of pediatric patients as provided in the clinical trial simulations, as opposed to direct extrapolation of observed exposures.
The present study modeled the PK of ABC, DTG, and 3TC after administering a once-daily FDC dose (DT or tablet) to pediatric participants with HIV-1. Its purpose was to leverage existing pediatric ABC, DTG, and 3TC PK models which have been used previously for regulatory approval of the individual drugs. These models were developed using extensive pediatric data across different weight ranges (4.6–61.3 kg for ABC, 3.90–91.0 kg for DTG, and 5.1–66.4 kg for 3TC) and using several different formulations. These models were used to predict observed concentrations in pediatric participants (≥ 6 to < 40 kg) receiving ABC/DTG/3TC DT and tablet formulations in the IMPAACT 2019 study. These models were applied to the individual IMPAACT 2019 data without re-estimation of PopPK parameters. The GOF and VPC plots demonstrated that the models sufficiently describe and predict the plasma drug concentration data. As the models could adequately predict the observed data and PK parameters, it was unnecessary to re-evaluate covariate relationships or develop new PopPK models specifically for the IMPAACT 2019 data.
The post hoc PK parameter estimates were comparable to the NCA PK parameter estimates. They were also within the target exposure range for the most critical exposure metrics defined in the study protocol based on observed PK of the single entity in adult and pediatric populations (DTG: C24 and AUC0–24, ABC + 3TC: AUC0–24).
ABC, DTG, and 3TC PK exposures following once-daily ABC/DTG/3TC DT and tablet dosing were comparable to those observed in children and adults with single entities, supporting extrapolation of adult efficacy data [16, 22–26]. This is further supported by weight band-based PK simulations (Table 4). The predicted exposures for each of the single entities were also within the target exposure range in pediatrics, providing further support for the use of FDC formulation in a larger pediatric population. The ABC/DTG/3TC FDC doses evaluated in IMPAACT 2019 are expected to provide safety and efficacy similar to those observed in the single-entity studies.
Conclusions
This model-based approach allowed leveraging existing pediatric data and models, which included the weight range (≥ 6 to < 40 kg) to confirm the ABC/DTG/3TC doses in the pediatric population for the DT and tablet formulations. Overall, the modeling and simulation, along with IMPAACT 2019 data, support the once-daily administration of ABC/DTG/3TC DTs to children living with HIV-1 weighing ≥ 6 to < 25 kg and ABC/DTG/3TC tablets to pediatric patients weighing at least 25 kg.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
All listed authors meet the criteria for authorship set forth by the International Committee of Medical Journal Editors. The authors thank the study participants, all investigators, and site staff who participated in the study.
Medical Writing, Editorial, and Other Assistance
Medical writing and editorial assistance was provided by Vivek Dadhania, PhD, DABT, ERT (SciQra LLC) and funded by ViiV Healthcare.
Author Contributions
Hardik Chandasana and Sven van Dijkman wrote the manuscript; Hardik Chandasana, Rashmi Mehta, Mark Bush, Helena Rabie, Patricia Flynn, Tim Cressey, Edward Acosta, and Kristina M Brooks designed the research; Hardik Chandasana and Sven van Dijkman performed the analysis; Hardik Chandasana, Sven van Dijkman, Rashmi Mehta, Mark Bush, Helena Rabie, Patricia Flynn, Tim Cressey, Edward Acosta, and Kristina M Brooks interpreted data. All authors contributed to critically revising the manuscript for important intellectual content and approve the manuscript for publication.
Funding
The IMPAACT 2019 study was funded by ViiV Healthcare. Overall support for the IMPAACT network was provided by the National Institute of Allergy and Infectious Diseases (NIAID) with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH), all components of the National Institutes of Health (NIH), under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), and by NICHD contract number HHSN275201800001I. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The journal's Rapid Service fee was funded by GSK.
Data Availability
Per IMPAACT policy, the data cannot be made publicly available due to the ethical restrictions in the study’s informed consent documents and in the IMPAACT Network’s approved human subjects protection plan; public availability may compromise participant confidentiality. However, data are available to all interested researchers upon request to the IMPAACT Statistical and Data Management Center’s data access committee (e-mail address: sdac.data@fstrf.org) with the agreement of the IMPAACT Network.
Declarations
Conflict of Interest
Hardik Chandasana, Sven van Dijkman, and Rashmi Mehta are employee of GSK and owns stock in GSK. Mark Bush is an employee of ViiV Healthcare and own stock in GSK. Kristina M Brooks received consulting fees from ViiV Healthcare. Helena Rabie, Patricia Flynn, Tim Cressey, and Edward Acosta have nothing to declare.
Ethical Approval
The IMPAACT 2019 study (ClinicalTrials gov identifier NCT03760458) was designed in accordance with the International Council for Harmonization, Good Clinical Practice guidelines [13], and applicable country-specific requirements and abided by the ethical principles of the Declaration of Helsinki. Informed consent was obtained from all the participants before screening. Institutional review boards and national authorities reviewed and approved the trial protocol, amendments, and informed consent forms.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.UNICEF data., Paediatric care and treatment; 2023. https://data.unicef.org/topic/hivaids/paediatric-treatment-and-care/. Accessed 22 Aug 2023.
- 2.UNICEF data. Global and regional trends; 2023. https://data.unicef.org/topic/hivaids/global-regional-trends/. Accessed 22 Aug 2023.
- 3.HIV treatment guidelines for the use of antiretroviral agents in pediatric HIV infection; 2023. https://clinicalinfo.hiv.gov/en/guidelines/pediatric-arv/whats-new. Accessed 31 Aug 2023.
- 4.Guidelines for the use of antiretroviral agents in pediatric HIV infection; 2023. https://clinicalinfo.hiv.gov/en/guidelines/pediatric-arv/regimens-recommended-initial-therapy-antiretroviral-naive-children. Accessed 31 Aug 2023.
- 5.Ramjan R, Calmy A, Vitoria M, Mills EJ, Hill A, Cooke G, et al. Systematic review and meta-analysis: patient and programme impact of fixed-dose combination antiretroviral therapy. Trop Med Int Health. 2014;19(5):501–13. 10.1111/tmi.12297 [DOI] [PubMed] [Google Scholar]
- 6.TRIUMEQ (abacavir, dolutegravir, and lamivudine) tablets, for oral use TRIUMEQ PD (abacavir, dolutegravir, and lamivudine) tablets for oral suspension. Research Triangle Park, NC, USA: ViiV Healthcare and GlaxoSmithKline; 2014.
- 7.TIVICAY (dolutegravir) tablets, for oral use TIVICAY PD (dolutegravir) tablets for oral suspension. In: Research Triangle Park N, USA, editor. Research Triangle Park, NC, USA: ViiV Healthcare and GlaxoSmithKline; 2013.
- 8.ZIAGEN (abacavir sulfate) Tablets, for oral use ZIAGEN (abacavir sulfate) oral solution. In: Research Triangle Park N, USA, editor. Research Triangle Park, NC, USA: ViiV Healthcare and GlaxoSmithKline; 1998.
- 9.EPIVIR (lamivudine) tablets for oral use EPIVIR (lamivudine) oral solution. Research Triangle Park, NC, USA: ViiV Healthcare and GlaxoSmithKline; 1995.
- 10.WHO recommends dolutegravir as preferred HIV treatment option in all populations; 2019. https://www.who.int/news/item/22-07-2019-who-recommends-dolutegravir-as-preferred-hiv-treatment-option-in-all-populations. Accessed 31 Aug 2023.
- 11.Singh RP, Adkison KK, Baker M, Parasrampuria R, Wolstenholme A, Davies M, et al. Development of dolutegravir single-entity and fixed-dose combination formulations for children. Pediatr Infect Dis J. 2022;41(3):230–7. 10.1097/INF.0000000000003366 [DOI] [PubMed] [Google Scholar]
- 12.The International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Network. 2020. https://www.impaactnetwork.org/studies/impaact2019. Accessed 31 Aug 2023. [DOI] [PMC free article] [PubMed]
- 13.International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) guideline for good clinical practice E6(R2); 2016.
- 14.Brooks KM, Kiser JJ, Ziemba L, Ward S, Rani Y, Cressey TR, et al. Pharmacokinetics, safety, and tolerability of dispersible and immediate-release abacavir, dolutegravir, and lamivudine tablets in children with HIV (IMPAACT 2019): week 24 results of an open-label, multicentre, phase 1–2 dose-confirmation study. Lancet HIV. 2023;10(8):e506–17. 10.1016/S2352-3018(23)00107-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandasana H, Thapar M, Hayes S, Baker M, Gibb DM, Turkova A, et al. Population pharmacokinetic modeling of dolutegravir to optimize pediatric dosing in HIV-1-infected infants, children, and adolescents. Clin Pharmacokinet. 2023;62(10):1445–59. 10.1007/s40262-023-01289-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.USFDA. Clinical pharmacology review for ziagen (abacavir sulfate) and epivir (lamivudine). https://fda.report/media/92051/N20-977S027-Abacavir-sulfate-Clinpharm-PREA.pdf. Accessed 31 August 2023.
- 17.Anderson BJ, Holford NH. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet. 2009;24(1):25–36. 10.2133/dmpk.24.25 [DOI] [PubMed] [Google Scholar]
- 18.Kasirye P, Kendall L, Adkison KK, Tumusiime C, Ssenyonga M, Bakeera-Kitaka S, et al. Pharmacokinetics of antiretroviral drug varies with formulation in the target population of children with HIV-1. Clin Pharmacol Ther. 2012;91(2):272–80. 10.1038/clpt.2011.225 [DOI] [PubMed] [Google Scholar]
- 19.Fletcher CV, Kawle SP, Kakuda TN, Anderson PL, Weller D, Bushman LR, et al. Zidovudine triphosphate and lamivudine triphosphate concentration–response relationships in HIV-infected persons. AIDS. 2000;14(14):2137–44. 10.1097/00002030-200009290-00010 [DOI] [PubMed] [Google Scholar]
- 20.Wang LH, Begley J, St ClaireHarris RLJ, Wakeford C, Rousseau FS. Pharmacokinetic and pharmacodynamic characteristics of emtricitabine support its once daily dosing for the treatment of HIV infection. AIDS Res Hum Retroviruses. 2004;20(11):1173–82. 10.1089/aid.2004.20.1173 [DOI] [PubMed] [Google Scholar]
- 21.Weller S, Radomski KM, Lou Y, Stein DS. Population pharmacokinetics and pharmacodynamic modeling of abacavir (1592U89) from a dose-ranging, double-blind, randomized monotherapy trial with human immunodeficiency virus-infected subjects. Antimicrob Agents Chemother. 2000;44(8):2052–60. 10.1128/AAC.44.8.2052-2060.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.USFDA. Clinical pharmacology review. Tivicay (dolutegravir) PD. https://www.fda.gov/media/140939/download. Accessed 31 August 2023.
- 23.Bollen PDJ, Moore CL, Mujuru HA, Makumbi S, Kekitiinwa AR, Kaudha E, et al. Simplified dolutegravir dosing for children with HIV weighing 20 kg or more: pharmacokinetic and safety substudies of the multicentre, randomised ODYSSEY trial. Lancet HIV. 2020;7(8):e533–44. 10.1016/S2352-3018(20)30189-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruel TD, Acosta EP, Liu JP, Gray KP, George K, Montanez N, et al. Pharmacokinetics, safety, tolerability, and antiviral activity of dolutegravir dispersible tablets in infants and children with HIV-1 (IMPAACT P1093): results of an open-label, phase 1–2 trial. Lancet HIV. 2022;9(5):e332–40. 10.1016/S2352-3018(22)00044-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waalewijn H, Chan MK, Bollen PDJ, Mujuru HA, Makumbi S, Kekitiinwa AR, et al. Dolutegravir dosing for children with HIV weighing less than 20 kg: pharmacokinetic and safety substudies nested in the open-label, multicentre, randomised, non-inferiority ODYSSEY trial. Lancet HIV. 2022;9(5):e341–52. 10.1016/S2352-3018(21)00292-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J, Hayes S, Sadler BM, Minto I, Brandt J, Piscitelli S, et al. Population pharmacokinetics of dolutegravir in HIV-infected treatment-naive patients. Br J Clin Pharmacol. 2015;80(3):502–14. 10.1111/bcp.12639 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Per IMPAACT policy, the data cannot be made publicly available due to the ethical restrictions in the study’s informed consent documents and in the IMPAACT Network’s approved human subjects protection plan; public availability may compromise participant confidentiality. However, data are available to all interested researchers upon request to the IMPAACT Statistical and Data Management Center’s data access committee (e-mail address: sdac.data@fstrf.org) with the agreement of the IMPAACT Network.