Abstract
Introduction
Efficient epidemiological monitoring of virus diseases is crucial in evaluating general public health, the prevalence of specific diseases, the pattern of spread, and implementing preventative and control strategies into action.
Methods
This study analyzed data obtained from the Field Epidemiology Program (FETP) which is part of the Ministry of Health (MOH) in Saudi Arabia, which contained reported cases of infectious diseases over four years, from January 2018 to December 2021, to investigate and highlight the significant trend and incidence rate for each viral infectious disease.
Results
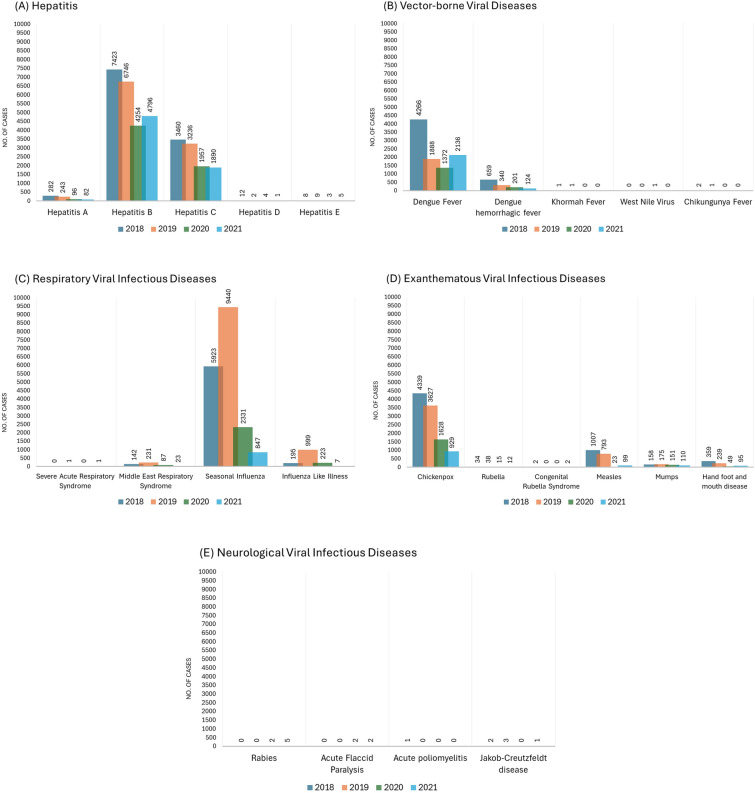
Of the reported viral infectious diseases, hepatitis B and C, dengue fever (DF), influenza, chickenpox, and measles were the highest reported viral cases over four years. For the aforementioned diseases, males were often more susceptible to viral infections than females. Except for DF, this viral infection was more common in Saudi citizens. Viral illnesses like hand, foot, and mouth disease were less prevalent, while neurological viral disorders such as acute flaccid paralysis were rarely detected. There was an overall reduction in viral cases recorded during 2020–2021, which may be attributed to the implementation of preventive measures during the Coronavirus Disease 2019 (COVID–19) pandemic or an underreporting of cases during the lockdown of that time.
Conclusion
The prevalence of these common viral infections in the Saudi population suggests that understanding the mechanisms influencing changes in these viruses, methods of transmission, and the burden of these diseases is a priority for health policy. This understanding is necessary to develop effective intervention and preventive strategies.
Keywords: Epidemiology, Surveillance, Viral infections, Ministry of Health, Saudi Arabia
Key Summary Points
| Why carry out this study? |
| Viral infections are recognized as one of the leading causes of illness and death globally. |
| Epidemiological surveillance is considered a vital approach to monitoring, preventing, and controlling infection outbreaks. |
| This study demonstrates the current Saudi epidemiological surveillance for viral infections that were reported from 2018 to 2021. |
| What was learned from the study? |
| The results revealed that hepatitis B and C, dengue fever; influenza illness, influenza-like illness, chickenpox and measles were the highest reported viral cases over the four years duration, while hand, foot, and mouth disease and neurological viral disorders such as acute flaccid paralysis were less prevalent. |
| The study revealed a clear lack of reporting and representation of some viral infections, implementing an improved public health surveillance system is necessary to overcome challenges associated with underreporting of communicable diseases. |
Introduction
Viral infectious diseases can have a serious impact on mortality and morbidity over many years. Before vaccinations were developed, chicken pox, for example, caused thousands of fatalities and is one of the viral illnesses with a higher fatality rate. A recent example which caused a terrible impact on individuals’ lives and economies all across the world is the Coronavirus Disease 2019 (COVID–19) pandemic, which is only one of several significant viral infectious disease epidemics that have occurred in recent years [1]. However, there have been notable achievements in public health over the past few decades that contributed to the reduction in mortality and morbidity, especially in childhood mortality, improved accessibility of treatments, better sanitation, and the development of vaccination programs [2].
On the other hand, air pollution, international transportation systems that raised the possibility of pathogen transmission, and high population density all contributed to the continuous development of viral infectious disease [1]. According to the United States Centers for Disease Control and Prevention (CDC), there were approximately 41,917 deaths caused by influenza and pneumonia in 2021 [3], compared to other, more widespread viral infections like hepatitis B virus (HBV), which is estimated to be the cause of 820,000 deaths annually [4]. According to the CDC, epidemiological surveillance is “the ongoing, systematic collection, analysis, and interpretation of health-related data essential to planning, implementation, and evaluation of public health practice” [5]. National authorities are often responsible for the execution of such surveillance, which includes reporting, monitoring, and evaluating specific cases.
On a global level, various surveillance-related details are quickly and easily accessible on global websites, such as those from the CDC (www.cdc.gov/mmwr) and the World Health Organization (WHO; www.who.int/csr). Another significant website is the Global Burden of Disease (https://www.healthdata.org/research-analysis/gbd) which provides a complete picture of mortality and disability across nations, periods, ages, and sex presented in this epidemiological analysis. In Saudi Arabia, infectious disease cases are reported in the Field Epidemiology Program (FETP), which is part of the Deputy Ministry for Public Health at the Ministry of Health (MOH).
In addition to the significance of epidemiological monitoring, assessments of the burden of disease can help health policy in at least five important ways: assessing performance, generating forums for informed debate of values and priorities, identifying national control priorities, creating knowledge, and allocating resources across health interventions [6]. This study has analyzed data obtained from the FETP, which contained reported cases of infectious diseases over 4 years, from January 2018 to December 2021, to investigate and highlight the significant trend and incidence rate for each viral infectious disease.
Methods
The Saudi National Authorities have developed an epidemiology surveillance system to investigate and control the prevalence rate of infectious diseases. In this study, the FETP’s surveillance data and epidemiology reports were obtained and assessed to show the varying epidemiology of viral infectious diseases in Saudi Arabia from January 2018 to December 2021, a period for which data were available. The total number of cases was calculated along with their demography, as represented by sex and nationality. The cumulative case data obtained were employed to calculate the incidence rates (IR) per 100,000 population with a 95% confidence interval (CI). Demographic data were used to compare between males and females, as well as the nationality categories for Saudis and non-Saudis, and were analyzed using Student’s t test, since the data were normally distributed, and results are presented as means. To estimate trends in viral infectious disease rates over time, the cumulative cases reported during the specified period were analyzed using regression analysis which includes P values and the F statistic (F value) to test the overall significance of the regression model, and the high F value indicates that the regression model provides a better fit than a model with no predictors. The P value has been associated with the F value to assess the significance of relationships in this study. The analysis of reported cases was performed using Microsoft Excel software (version 16.75.2), and a P value ≤ 0.05 was considered statistically significant for all statistical analyses in this epidemiology study. The data presented in this study are based on previously collected data and no new data of human participants were involved by any of the authors; hence, no ethical approval was required.
Results
The Saudi monthly epidemiology reports showed the number of cases for each disease and reflected the trend of infection from 2018 to 2021. In Saudi Arabia, the hepatitis IR varies from high to low based on the type. For example, in 2018, the IR of HBV was 24.6 (95% CI 24.52–24.68) compared to the IR of hepatitis E (HEV) which was 0 (95% CI 0–0). Based on the results of the total number of cases reported, the prevalence rates of HBV and C were higher than hepatitis A (HAV), HEV, and D. However, the reported cases reduced in the period of 2018–2021, such as the total number of hepatitis C (HCV) cases in 2018 was 3460, whereas in 2021 the cases had reduced to 1890 (Table 1; Fig. 1A). The mean values for sex and nationality were different for each group category. Based on the mean P value, there was no significant difference between males and females, as shown in Table 2. Nevertheless, HBV (P value = 0.00) and HCV (P value = 0.02) showed significant differences between the nationality groups (Table 2). Moreover, according to the regression test, there was a significant decreasing trend in HAV (F = 18.23, P ≤ 0.05) (Table 3).
Table 1.
The incidence rate (IR) per 100,000 population, with a confidence interval (CI), for the total number of reported viral infections between 2018 and 2021 in Saudi Arabia
| Viral infectious disease | Total in 2018 (IR) | 95% CI | Total in 2019 (IR) | 95% CI | Total in 2020 (IR) | 95% CI | Total in 2021 (IR) | 95% CI |
|---|---|---|---|---|---|---|---|---|
| Hepatitis A | 282 (0.9) | 0.88–0.92 | 243 (0.8) | 0.78–0.82 | 96 (0.3) | 0.29–0.31 | 82 (0.3) | 0.29–0.31 |
| Hepatitis B | 7423 (24.6) | 24.52–24.68 | 6746 (22.4) | 22.23–22.57 | 4254 (13.5) | 13.24–13.76 | 4796 (15.6) | 15.41–15.79 |
| Hepatitis C | 3460 (11.5) | 11.43–11.57 | 3236 (10.8) | 10.67–10.93 | 1957 (6.2) | 6.08–6.32 | 1890 (6.1) | 6.04–6.16 |
| Hepatitis D | 12 (0.0) | 0.00–0.00 | 2 (0.0) | 0.00–0.00 | 4 (0.0) | 0.00–0.00 | 1 (0.0) | 0.00–0.00 |
| Hepatitis E | 8 (0.0) | 0.00–0.00 | 9 (0.0) | 0.00–0.00 | 3 (0.0) | 0.00–0.00 | 5 (0.0) | 0.00–0.00 |
| Dengue fever | 4266 (14.1) | 13.42–14.78 | 1888 (6.3) | 6.17–6.43 | 1372 (4.3) | 4.11–4.49 | 2136 (6.9) | 6.67–7.13 |
| Dengue hemorrhagic fever | 659 (2.2) | 2.10–2.30 | 340 (1.1) | 1.04–1.16 | 201 (0.6) | 0.55–0.65 | 124 (0.4) | 0.38–0.42 |
| Alkhumra fever | 1 (0.00) | 0.00–0.00 | 1 (0.00) | 0.00–0.00 | 0 (0.00) | 0.00–0.00 | 0 (0.00) | 0.00–0.00 |
| Chikungunya fever | 2 (0.01) | 0.01–0.01 | 1 (0.00) | 0.00–0.00 | 0 (0.00) | 0.00–0.00 | 0 (0.00) | 0.00–0.00 |
| West Nile virus | 0 (0.00) | 0.00–0.00 | 0 (0.00) | 0.00–0.00 | 1 (0.00) | 0.00–0.00 | 0 (0.00) | 0.00–0.00 |
| Severe acute respiratory syndrome | 0 (0.00) | 0.00–0.00 | 1 (0.00) | 0.00–0.00 | 0 (0.00) | 0.00–0.00 | 1 (0.00) | 0.00–0.00 |
| Middle East respiratory syndrome | 142 (0.5) | 0.49–0.51 | 231 (0.8) | 0.76–0.84 | 87 (0.3) | 0.28–0.32 | 23 (0.1) | 0.10–0.10 |
| Influenza (seasonal) | 5923 (19.61) | 18.81–20.41 | 9440 (31.40) | 30.44–32.36 | 2331 (7.39) | 6.78–8.00 | 847 (2.75) | 2.50–3.00 |
| Influenza-like illness | 195 (0.6) | 0.57–0.63 | 999 (3.3) | 3.17–3.43 | 223 (0.7) | 0.64–0.76 | 7 (0.00) | 0.00–0.00 |
| Chickenpox | 4339 (14.4) | 14.22–14.58 | 3627 (12.1) | 11.98–12.22 | 1628 (5.16) | 4.90–5.42 | 929 (3.02) | 2.98–3.06 |
| Measles | 1007 (3.33) | 3.21–3.45 | 793 (2.64) | 2.45–2.83 | 23 (0.07) | 0.06–0.08 | 99 (0.32) | 0.29–0.35 |
| Mumps | 158 (0.52) | 0.51–0.53 | 175 (0.58) | 0.57–0.59 | 151 (0.48) | 0.47–0.49 | 110 (0.36) | 0.35–0.37 |
| Rubella | 34 (0.1) | 0.10–0.10 | 38 (0.13) | 0.13–0.13 | 15 (0.05) | 0.05–0.05 | 12 (0.04) | 0.04–0.04 |
| Congenital rubella syndrome | 2 (0.01) | 0.01–0.01 | 0 (0.00) | 0.00–0.00 | 0 (0.00) | 0.00–0.00 | 2 (0.01) | 0.01–0.01 |
| Hand, foot, and mouth diseases | 359 (1.19) | 1.15–1.23 | 239 (0.79) | 0.75–0.83 | 49 (0.16) | 0.15–0.17 | 95 (0.31) | 0.30–0.32 |
| Rabies | 0 (0.00) | 0.00–0.00 | 0 (0.00) | 0.00–0.00 | 2 (0.01) | 0.01–0.01 | 5 (0.02) | 0.02–0.02 |
| Acute flaccid paralysis | 0 (0.00) | 0.00–0.00 | 0 (0.00) | 0.00–0.00 | 2 (0.01) | 0.01–0.01 | 2 (0.01) | 0.01–0.01 |
| Acute poliomyelitis | 1 (0.00) | 0.00–0.00 | 0 (0.00) | 0.00–0.00 | 0 (0.00) | 0.00–0.00 | 0 (0.00) | 0.00–0.00 |
| Creutzfeldt–Jakob disease | 2 (0.01) | 0.01–0.01 | 3 (0.01) | 0.01–0.01 | 0 (0.00) | 0.00–0.00 | 1 (0.00) | 0.00–0.00 |
Fig. 1.
Epidemiological trends of infectious viral diseases in Saudi Arabia during 2018–2021, showing the total reported cases of: A hepatitis; B vector-borne viral diseases; C respiratory viral infectious disease; D exanthematous viral infectious disease; and E neurological viral infectious disease
Table 2.
The differences between demographic categories (sex and nationality) based on the mean values of the total number of reported viral infections between 2018 and 2021 in Saudi Arabia
| Viral infectious disease | Reported sex | Reported nationality | ||||
|---|---|---|---|---|---|---|
| Male (mean) | Female (mean) | P value | Saudi (mean) | Non-Saudi (mean) | P value | |
| Hepatitis A | 107.25 | 68.25 | 0.34 | 110 | 64.5 | 0.27 |
| Hepatitis B | 3554 | 2246.25 | 0.06 | 4405 | 1346.75 | 0.00 |
| Hepatitis C | 1543.75 | 1091.25 | 0.20 | 1850.75 | 757 | 0.02 |
| Hepatitis D | 2.5 | 2.25 | 0.90 | 3.5 | 0.5 | 0.27 |
| Hepatitis E | 4.5 | 1.75 | 0.18 | 3 | 3.25 | 0.87 |
| Dengue fever | 1992.75 | 422.5 | 0.07 | 738.25 | 1662.25 | 0.19 |
| Dengue hemorrhagic fever | 264.75 | 66 | 0.13 | 117.25 | 210 | 0.42 |
| Alkhumra fever | 0.5 | 0 | 0.18 | 0.25 | 0.25 | 1.00 |
| Chikungunya fever | 0.5 | 0.25 | 0.68 | 0.5 | 0.25 | 0.67 |
| West Nile virus | 0.00 | 0.25 | 0.39 | 0.25 | 0.00 | 0.39 |
| Severe acute respiratory Syndrome | 0.25 | 0.25 | 1.00 | 0.25 | 0.25 | 1.00 |
| Middle east respiratory syndrome | 92.5 | 28.25 | 0.14 | 94.25 | 26.25 | 0.12 |
| Influenza (seasonal) | 2364.75 | 2269.5 | 0.95 | 3194.75 | 1396.5 | 0.28 |
| Influenza like illness | 180.25 | 175.75 | 0.98 | 236 | 91.75 | 0.43 |
| Chickenpox | 1688.25 | 941.5 | 0.26 | 1770 | 817 | 0.20 |
| Measles | 244 | 236 | 0.96 | 380.5 | 99 | 0.28 |
| Mumps | 85.5 | 63 | 0.06 | 115.25 | 31.5 | 0.00 |
| Rubella | 11.25 | 13.5 | 0.66 | 20.25 | 3.75 | 0.07 |
| Congenital rubella syndrome | 0.00 | 1 | 0.18 | 1 | 0.00 | 0.18 |
| Hand, foot and mouth diseases | 103.25 | 82.25 | 0.69 | 137 | 47.75 | 0.14 |
| Rabies | 1.25 | 0.5 | 0.49 | 1 | 0.5 | 0.58 |
| Acute flaccid paralysis | 0.5 | 0.5 | 1.00 | 0.75 | 0.25 | 0.39 |
| Acute poliomyelitis | 0 | 0.25 | 0.39 | 0.00 | 0.25 | 0.39 |
| Creutzfeldt–Jakob disease | 1 | 0.5 | 0.55 | 1.25 | 0.25 | 0.21 |
Bolded values statistically significant (P ≤ 0.05)
Table 3.
Regression analysis based on the cumulative reported cases of viral infections between 2018 and 2021 in Saudi Arabia
| Viral infectious disease | Correlation coefficient | R2 value | Standard error | F value | P value |
|---|---|---|---|---|---|
| Hepatitis A | 0.94 | 0.90 | 39.11 | 18.23 | 0.05 |
| Hepatitis B | 0.88 | 0.77 | 879.52 | 6.95 | 0.11 |
| Hepatitis C | 0.93 | 0.87 | 362.71 | 13.63 | 0.06 |
| Hepatitis D | 0.80 | 0.64 | 3.65 | 3.59 | 0.19 |
| Hepatitis E | 0.70 | 0.49 | 2.39 | 1.95 | 0.29 |
| Dengue fever | 0.69 | 0.48 | 1114.67 | 1.91 | 0.30 |
| Dengue hemorrhagic fever | 0.95 | 0.90 | 87.57 | 19.83 | 0.04 |
| Alkhumra fever | 0.89 | 0.80 | 0.31 | 8.00 | 0.10 |
| Chikungunya fever | 0.94 | 0.89 | 0.38 | 16.33 | 0.05 |
| West Nile virus | 0.25 | 0.06 | 0.59 | 0.14 | 0.74 |
| Severe acute respiratory syndrome | 0.44 | 0.20 | 0.63 | 0.50 | 0.55 |
| Middle East respiratory syndrome | 0.73 | 0.53 | 73.31 | 2.33 | 0.26 |
| Influenza (seasonal) | 0.74 | 0.56 | 3119.07 | 2.56 | 0.25 |
| Influenza-like illness | 0.39 | 0.15 | 494.50 | 0.36 | 0.60 |
| Chickenpox | 0.97 | 0.95 | 409.06 | 44.68 | 0.02 |
| Measles | 0.91 | 0.83 | 244.23 | 10.23 | 0.08 |
| Mumps | 0.78 | 0.16 | 20.85 | 3.24 | 0.21 |
| Rubella | 0.87 | 0.76 | 7.83 | 6.45 | 0.12 |
| Congenital rubella syndrome | 0.00 | 0.00 | 1.41 | 0.00 | 1.00 |
| Hand, foot, and mouth diseases | 0.89 | 0.80 | 76.06 | 8.33 | 0.10 |
| Rabies | 0.92 | 0.86 | 1.07 | 12.56 | 0.07 |
| Acute flaccid paralysis | 0.89 | 0.80 | 0.63 | 8.00 | 0.10 |
| Acute poliomyelitis | 0.77 | 0.60 | 0.38 | 3.00 | 0.22 |
| Creutzfeldt–Jakob disease | 0.60 | 0.36 | 1.26 | 1.12 | 0.40 |
Bolded values statistically significant (P ≤ 0.05)
In Saudi Arabia, several of the viral diseases reported according to the FETP are vector-borne. Dengue fever (DF) and dengue hemorrhagic fever (DHF) represent the most common diseases, with total number of cases in 2018 of 4266 and 659, respectively. On the other hand, the IR of chikungunya, Alkhumra, and West Nile Virus (WNV) diseases are rarely observed (Table 1; Fig. 1B). Based on sex variation, there is no significant difference, although the mean of DF infection in female cases is 422.5, which is less than the male cases, 1992.75. Also, nationality variation has shown no significant difference even when there are differences in the number of cases between Saudi and non-Saudi (Table 2). From 2018 to 2021, the trend of DHF significantly decreased (F = 19.83, P ≤ 0.04), in addition to chikungunya fever (F = 16.33, P ≤ 0.05), as observed in Table 3.
According to the data, the most common respiratory viral infection in Saudi Arabia from 2018 to 2021 was seasonal influenza, which reached 9440 cases. Additionally, there are rarely occurring respiratory viral diseases, such as severe acute respiratory syndrome, Middle East respiratory syndrome, and influenza-like illness (ILI) with IR of (0, 0.1, and 0, respectively) in 2021, as shown in Table 1 and Fig. 1C. The mean of infected male and female cases is similar in most respiratory viral diseases, with no significant differences between sex categories or in the nationality categories (Table 2). Even though all respiratory viral infectious diseases can cause pandemics or epidemics, our data showed no significant trend through an outbreak (Table 3).
The most common exanthematous viral infectious diseases in Saudi Arabia are chickenpox and measles, whereas the less common are mumps, rubella, congenital rubella syndrome, and hand, foot, and mouth diseases. For example, in 2018, the total number of chickenpox cases reported was 4339, and the total number of measles cases was 1007, compared to the total cases reported for mumps and rubella, which represented 158 and 34 cases, respectively, as shown in Table 1 and Fig. 1D. According to the mean value for mumps, there is a significant difference between Saudi and non-Saudi categories (P value = 0.00), and the mean number of cases were 115.25 and 31.5, respectively (Table 2). Based on the regression test, chickenpox has shown a significant decrease in trend over time (F = 44.68, P ≤ 0.02) (Table 3). Interestingly, in the period of 2018–2021, the total number of cases reduced from 4339 to 929, as shown in Table 1 and Fig. 1D.
Neurological viral infectious diseases are rarely found in Saudi Arabia. From 2018 to 2021, reported cases of Creutzfeldt–Jakob disease, acute flaccid paralysis, acute poliomyelitis, and rabies were all less than five, based on FETP data, with a lower percentage than other viral infectious diseases reported. According to this finding, the IR results for all neurological viral infectious diseases was ≥ 0.02 (Table 1; Fig. 1E).
Discussion
Viral infectious diseases play critical roles in causing high mortality and morbidity for humanity. To evaluate the success of the vaccination program and to assess the efficacy of preventive measures, it is important to monitor the spread of some significant viral infections. Hepatitis diseases are one of the most critical infections worldwide due to their lethality level, transmission path, disease progression, patient age, and sex. In Saudi Arabia, the total number of hepatitis-infected cases decreased over the years, which shows the significance of the infection control guidelines followed in the country. The HBV infection rate is high among adults aged over 30–59 years compared to younger individuals, with 23,219 cases reported, despite the implementation of a mandatory immunization program in Saudi Arabia. This may be due to a decline in the seroprotection rate over time [7], necessitating an additional booster dose for each previously vaccinated person to raise the immune protection against this viral disease. Moreover, HAV infected males more than females, and the incidence was higher in the Riyadh region than in any other region, a finding which agrees with the reported study [8]. Most likely, this difference is attributed to spontaneous clearance in females and to sex hormones [9]. On the other hand, the risk of cross-border transmission of food-borne HAV infection increases with increased trade and travel between countries, so infection control requires international cooperation and safer practices. Additionally, the prevalence rate has decreased as a result of improved preventive measures and living conditions [8]. Recent vaccination studies against HAV in both Saudi Arabia and Turkey during childhood have shown significant prevention strategies and vaccine application policies in other countries [10]. Based on the data collected from the Saudi Arabia MOH, hepatitis vaccination and other infection prevention and control have led to a reduction in the prevalence rate among the population.
Moreover, vector-borne viral diseases, which include DF, DHF, AF, chikungunya fever, and WNV usually occur in tropical areas that have a massive number of insects leading to a high risk of infection. The prevalence rate of DF has been controlled in Saudi Arabia by using fogging and applying a biocontrol approach of the Wolbachia, a bacteria which is a natural and environmentally friendly method that does not involve the use of chemicals or genetic modification of the vector-borne replacement technology [11] that is taking place in Jeddah, which is an endemic city in Saudi Arabia [12], as an attempt to reduce and eradicate dengue prevalence. The Wolbachia-based biocontrol strategy was implemented in 2021, proposing an alternative strategy for reducing DF. This strategy has been lauded by the WHO and the CDC of the United States as one of the most effective and efficient methods for controlling DF [13, 14]. According to the WHO, the region of South American dengue outbreaks had almost 3 million suspected, confirmed, and reported cases in 2023, with Brazil, Bolivia, and Preu, which are tropical countries, having the highest number of dengue cases [15]. Based on the MOH reports, the total number of infected cases in Saudi Arabia is expected to reduce, based on the prevention, control, and vaccination strategies being followed.
Respiratory viral infectious diseases pose a constant threat to human life, initially presenting as a mild illness that may progress to respiratory failure. Respiratory viral infectious diseases are highly contagious and can be deadly due to their transmission and spreading through the air, and through direct and indirect contact with the infected person or contaminated surfaces. Based on the period of 2018–2021, the reported infected cases in Saudi Arabia have decreased due to the application of prevention and treatment. Globally, it was estimated that the seasonal influenza is responsible for 294,000 to 518,000 deaths annually [16]. Worldwide, preventive measures have been implemented to limit the spread of COVID-19, and investigations have shown the effectiveness of these measures in limiting the spread of several respiratory viruses [17]. Yearly in the United States, the reported ILI cases reached 50 million [18], whereas, in Saudi Arabia, the reported cases did not exceed 1000 in the period of 2018–2021 (Table 1; Fig. 1C). In general, the prevalence rate of respiratory viral diseases internationally is high through outbreaks or seasonal changes due to symptoms similarities and transmission. Applying restrictions such as social distancing significantly affects and reduces the spreading of many respiratory viral infectious diseases and, in some countries, such as South Korea, the reduction reaches 100% [19]. In Saudi Arabia, a recent study has shown that the pandemic COVID-19 has affected and impacted the number of influenza cases [20], which meets and agrees with this study’s findings. In the case of respiratory viruses that have an impact on public health, proper ventilation, cleanliness, and immunization against flu viruses are essential, especially for elderly and chronic illness patients.
Exanthematous infections such as chickenpox and measles are common in Saudi Arabia; however, the total number of cases has been reduced and controlled by the mandatory vaccines included in the childhood vaccination schedule. Noteworthy, the chickenpox vaccination program in the United States has impacted the prevalence rate and shown a significant reduction in the reported cases [21]. China has reported a decrease in the cases of rubella, measles, and chickenpox during the COVID-19 pandemic, and this significant reduction in infection rate was due to preventive measures implemented during the COVID-19 pandemic [22]. This report agrees with our findings that, from 2018 to 2021, the total number of cases has been reduced and controlled by vaccination and prevention approaches, even for other exanthematous infections that require early detection and isolation to reduce the infection rate.
Neurological viral infectious diseases are rarely found in Saudi Arabia. According to this finding, rabies cases were rarely reported from 2018 to 2021. However, a study examined 199 animals suspected of rabies in Saudi Arabia during 2010 and 2017 where 158 of them were infected with rabies, and most of them were dogs and cats. The most common cases are in the Qassim region, followed by the Eastern region. The study recommended implementing measures to eradicate rabies, which may pose a risk to farmers and veterinarians, as dogs, camels, and livestock animals are considered reservoir animals [23]. Interestingly in Western Europe, rabies disease has been eradicated for about 15–20 years [24], opposite to the situation in Nigeria, where the risk factors for rabies transmission are high due to the high rates of dog bites [25]. From 2018 to 2021, neurological viral infectious diseases had less than five reported cases according to the MOH database, which was a lower percentage than for other reported viral infectious diseases (Fig. 1E). Due to the preventive strategies that are followed in Saudi Arabia, which include high infant and children immunization, and vaccination for adults at high risk of infection, travelers from endemic countries are tested and follow the implemented programs [26].
Furthermore, based on the results of this current study, sex variations were observed in several viral diseases, such as HBV, HCV, DF, and chickenpox, which may occur due to several reasons, including that females are generally greater in adaptive and innate immune responses than men, the innate immune cell activity in females is greater than in males, and females have greater thymus cell helper (T cell) counts and ratios compared to males [27]. Another reason is that some women may have an asymptomatic infection [28].
Despite this study demonstrating epidemiology data of viral infections reported in Saudi Arabia, numerous cases were unreported, which may be due to the challenges involved in the epidemiological surveillance process. The three key challenges facing epidemiological surveillance globally: interface challenges (i.e., the method used to show data to a consumer for consumption), data format challenges (i.e., the read and write processes for the data); however, these averages may include individual items influenced by various factors, such as age or geographical region. The statistical data reflect information from recent years between 2018 and 2021 where the number of cases were provided by the FETP every month and not beyond this duration. These results might also be impacted by the COVID-19 pandemic due to the strict precautionary measures imposed by the MoH, including curfews, which could lead to underreporting of viral infections. Additionally, measures like wearing masks and social distancing contributed to reducing the spread of respiratory viruses. Even so, working on the previous challenges will result in a more comprehensive reporting system, which will be the basis for burden planning and health priority. All the abovementioned factors could serve as study limitations that may open the door for improvements in future epidemiology studies.
Conclusion
For emergency preparation and response, understanding disease development through a community, and developing statistical and mechanistic disease models that enable planning, the availability of epidemiological data which can be readily accessed is extremely crucial. This current epidemiological study investigated and highlighted the incidence of viral infectious diseases in Saudi Arabia that were reported between January 2018 and December 2021, and provided by the FETP at the MOH. The most prevalent viral cases were hepatitis B and C, dengue fever, influenza, chickenpox, and measles. Noteworthy is the impact of preventive measures implemented during the COVID-19 pandemic on the viral infectious disease trends over 4 years (2018–2021) in Saudi Arabia, which led to their decline. Interestingly, males were more likely to develop the reported viral infections than females. Prevention and intervention strategies such as sanitation and hygiene facilities along with the vaccination programs implemented during the study duration could contribute to controlling the transmission of such viral diseases and enhance public awareness towards them. An enhanced disease surveillance reporting system, such as including different variables like the source of infection, hospitalization, and mortality rates, may improve the sensitivity and specificity of surveillance data in future epidemiological studies.
Acknowledgements
The authors would like to thank the Field Epidemiology Training Program, Deputy Ministry for Public Health, Ministry of Health, Saudi Arabia for sharing the epidemiological data.
Author Contributions
Conceptualization, Essam Tawfik; Formal analysis, Areej Alajmi, Nada Alosaimi and Maryam Alotaibi; Investigation, Munirah Aleyiydi, Noura Alshiban, Majed Nassar and Nada Alhumaid; Methodology, Nada Alhumaid and Essam Tawfik; Project administration, Munirah Aleyiydi and Noura Alshiban; Resources, Abdulaziz Almutairi; Supervision, Atef Shibl and Essam Tawfik; Validation, Thamer Almangour, Ziad Memish, Abdulwahab Binjomah, Saeed Algarni, Ahmed Al-Jedai, Abdulaziz Almutairi and Atef Shibl; Visualization, Areej Alajmi; Writing—original draft, Munirah Aleyiydi, Noura Alshiban and Majed Nassar; Writing—review & editing, Thamer Almangour, Ziad Memish, Abdulwahab Binjomah, Saeed Algarni, Ahmed Al-Jedai, Abdulaziz Almutairi, Atef Shibl and Essam Tawfik.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors and the rapid service fee was funded by the authors.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article.
Declarations
Conflict of Interest
Munirah S. Aleyiydi, Noura M. Alshiban, Areej M. Alajmi, Nada F. Alosaimi, Maryam Alotaibi, Majed S. Nassar, Nada K. Alhumaid, Thamer A. Almangour, Ziad A. Memish, Abdulwahab Z. Binjomah, Saeed Algarni, Ahmed Al-Jedai, Abdulaziz S. Almutairi, Atef Shibl, and Essam A. Tawfik declare that they have no financial or non-financial conflicts of interest.
Ethical Approval
The data presented in this study is based on previously collected data and no new data of human participants was involved by any of the authors; hence no ethical approval was required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Munirah S. Aleyiydi and Noura M. Alshiban have contributed equality to this study.
References
- 1.Baker RE, et al. Infectious disease in an era of global change. Nat Rev Microbiol. 2022;20(4):4. 10.1038/s41579-021-00639-z. 10.1038/s41579-021-00639-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Metcalf CJE, et al. Transport networks and inequities in vaccination: remoteness shapes measles vaccine coverage and prospects for elimination across Africa. Epidemiol Infect. 2015;143(7):1457–66. 10.1017/S0950268814001988. 10.1017/S0950268814001988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.‘Influenza | CDC’, The United States Centers for Disease Control and Prevention (CDC). https://www.cdc.gov/nchs/fastats/flu.htm. Accessed: 30 Sep 2023.
- 4.‘Hepatitis B | CDC’, The United States Centers for Disease Control and Prevention (CDC). https://www.cdc.gov/globalhealth/immunization/diseases/hepatitis-b/data/fast-facts. Accessed 30 Sep 2023.
- 5.‘Introduction to Public Health Surveillance | CDC’, The United States Centers for Disease Control and Prevention (CDC). https://www.cdc.gov/training/publichealth101/surveillance.html. Accessed 30 Sep 2023.
- 6.Lopez AD, Mathers CD, Ezzati M, Jamison DT, MurrayCJL. Measuring the global burden of disease and risk factors, 1990–2001. In: Global burden of disease and risk factors, Washington (DC): The International Bank for Reconstruction and Development/The World Bank, 2006. http://www.ncbi.nlm.nih.gov/books/NBK11817/. Accessed 30 Sep 2023. [PubMed]
- 7.AlAteeq MA, AlEnazi LM, AlShammari MS, AlAnazi EE, Al-Hababi FH, Alateeq AM. Long-term immunity against hepatitis B virus after routine immunization among adults visiting primary care centers in Riyadh Saudi Arabia. Cureus. 2022;14(1): e21266. 10.7759/cureus.21266. 10.7759/cureus.21266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharaheeli J, Alibrahim B. Confirmed foodborne Hepatitis A in Saudi Arabia, 2005–2015. Cureus. 2022;14(1): e20878. 10.7759/cureus.20878. 10.7759/cureus.20878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdel-Gawad M, Nour M, El-Raey F, Nagdy H, Almansoury Y, El-Kassas M. Gender differences in prevalence of hepatitis C virus infection in Egypt: a systematic review and meta-analysis. Sci Rep. 2023;13(1):1. 10.1038/s41598-023-29262-z. 10.1038/s41598-023-29262-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Badur S, Öztürk S, Ozakay A, Khalaf M, Saha D, Van Damme P. A review of the experience of childhood hepatitis A vaccination in Saudi Arabia and Turkey: implications for hepatitis A control and prevention in the Middle East and North African region. Hum Vaccin Immunother. 2021;17(10):3710–28. 10.1080/21645515.2021.1920871. 10.1080/21645515.2021.1920871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ross PA, et al. Developing Wolbachia-based disease interventions for an extreme environment. PLoS Pathog. 2023;19(1): e1011117. 10.1371/journal.ppat.1011117. 10.1371/journal.ppat.1011117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Nefaie H, Alsultan A, Abusaris R. Temporal and spatial patterns of dengue geographical distribution in Jeddah, Saudi Arabia. J Infect Public Health. 2022;15(9):1025–35. 10.1016/j.jiph.2022.08.003. 10.1016/j.jiph.2022.08.003 [DOI] [PubMed] [Google Scholar]
- 13.‘Mosquitoes with Wolbachia | CDC’, Centers for Disease Control and Prevention. https://www.cdc.gov/mosquitoes/mosquito-control/community/emerging-methods/wolbachia.html. Accessed 15 Jul 2023.
- 14.‘Dengue control: three-year Indonesia trial shows promising results’, World Health Organization. https://www.who.int/news/item/07-09-2020-dengue-control-three-year-indonesia-trial-shows-promising-results. Accessed 15 Jul 2023.
- 15.‘Dengue – the Region of the Americas’. https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON475. Accessed 04 Sep 2023.
- 16.Ryabkova VA, Churilov LP, Shoenfeld Y. Influenza infection, SARS, MERS and COVID-19: cytokine storm—the common denominator and the lessons to be learned. Clin Immunol. 2021;223: 108652. 10.1016/j.clim.2020.108652. 10.1016/j.clim.2020.108652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cortes AA, Zuñiga JM. The use of copper to help prevent transmission of SARS-coronavirus and influenza viruses. A general review. Diagn Microbiol Infect Dis. 2020;98(4): 115176. 10.1016/j.diagmicrobio.2020.115176. 10.1016/j.diagmicrobio.2020.115176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spencer JA, et al. Distinguishing viruses responsible for influenza-like illness. J Theor Biol. 2022;545: 111145. 10.1016/j.jtbi.2022.111145. 10.1016/j.jtbi.2022.111145 [DOI] [PubMed] [Google Scholar]
- 19.Park S, Michelow IC, Choe YJ. Shifting patterns of respiratory virus activity following social distancing measures for coronavirus disease 2019 in South Korea. J Infect Dis. 2021;224(11):1900–6. 10.1093/infdis/jiab231. 10.1093/infdis/jiab231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bin-Saleh K, et al. Assessing the influence of COVID-19 on influenza prevalence: a multicenter time series study. J Infect Public Health. 2023;16(12):1989–93. 10.1016/j.jiph.2023.09.018. 10.1016/j.jiph.2023.09.018 [DOI] [PubMed] [Google Scholar]
- 21.CDC, ‘Chickenpox for HCPs | CDC’, Centers for Disease Control and Prevention. https://www.cdc.gov/chickenpox/hcp/index.html. Accessed 19 Aug 2023.
- 22.Wu D, Liu Q, Wu T, Wang D, Lu J. The impact of COVID-19 control measures on the morbidity of varicella, herpes zoster, rubella and measles in Guangzhou, China. Immun Inflamm Dis. 2020;8(4):844–6. 10.1002/iid3.352. 10.1002/iid3.352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasem S, et al. Rabies among animals in Saudi Arabia. J Infect Public Health. 2019;12(3):445–7. 10.1016/j.jiph.2018.10.005. 10.1016/j.jiph.2018.10.005 [DOI] [PubMed] [Google Scholar]
- 24.Delcourt J, Brochier B, Delvaux D, Vangeluwe D, Poncin P. Fox Vulpes vulpes population trends in Western Europe during and after the eradication of rabies. Mammal Rev. 2022;52(3):343–59. 10.1111/mam.12289. 10.1111/mam.12289 [DOI] [Google Scholar]
- 25.Okeme SS, Kia GS, Mshelbwala PP, Umoh JU, Magalhães RJS. Profiling the public health risk of canine rabies transmission in Kogi state, Nigeria. One Health. 2020;10: 100154. 10.1016/j.onehlt.2020.100154. 10.1016/j.onehlt.2020.100154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hazazi A, Wilson A. Noncommunicable diseases and health system responses in Saudi Arabia: focus on policies and strategies. A qualitative study. Health Res Policy Syst. 2022;20:63. 10.1186/s12961-022-00872-9. 10.1186/s12961-022-00872-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobsen H, Klein SL. Sex differences in immunity to viral infections. Front Immunol. 2021;12: 720952. 10.3389/fimmu.2021.720952. 10.3389/fimmu.2021.720952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Hajjar S, Memish ZA, McIntosh K. Middle east respiratory syndrome coronavirus (MERS-CoV): a perpetual challenge. Ann Saudi Med. 2013;33(5):427–36. 10.5144/0256-4947.2013.427. 10.5144/0256-4947.2013.427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fairchild G, et al. Epidemiological data challenges: planning for a more robust future through data standards. Front Public Health. 2018. 10.3389/fpubh.2018.00336. 10.3389/fpubh.2018.00336 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.