Abstract
Introduction
Respiratory syncytial virus (RSV) and influenza pose major disease burdens in older adults due to an aging immune system and comorbidities; seasonal overlap exists between these infections. In 2023, the RSV prefusion protein F3 older adult (RSVPreF3 OA) vaccine was first approved in the USA as a single dose for prevention of lower respiratory tract disease due to RSV in adults aged ≥ 60 years. The vaccine has since been approved in the European Union and elsewhere. RSVPreF3 OA and FLU-QIV-HD could be coadministered if immunogenicity, safety, and reactogenicity are not affected.
Methods
This open-label, randomized (1:1), controlled, phase 3 study in 1029 adults aged ≥ 65 years in the USA evaluated the immunogenicity (up to 1 month after last vaccine dose) and safety (up to 6 months after last vaccine dose) of RSVPreF3 OA coadministered with FLU-QIV-HD (co-ad group) versus FLU-QIV-HD alone followed by RSVPreF3 OA at a separate visit 1 month later (control group). Non-inferiority criterion was defined as an upper limit of the two-sided 95% confidence interval of the geometric mean titer (GMT) group ratio (control/co-ad) ≤ 1.5. Secondary endpoints included safety and reactogenicity.
Results
Proportions of participants across age categories between groups and proportions of male (50.4%) and female (49.6%) participants were well balanced; most participants were white (68.7%). Group GMT ratios for RSV-A neutralizing titers, hemagglutination inhibition titers for four influenza vaccine strains, and RSV-B neutralizing titers were non-inferior in the co-ad group versus the control group. No clinically meaningful differences in local or systemic solicited and unsolicited adverse events (AEs), serious AEs, and potential immune-mediated diseases were identified. The most common solicited AEs in both groups were injection-site pain and myalgia.
Conclusion
In adults aged ≥ 65 years, coadministration of RSVPreF3 OA and FLU-QIV-HD was immunogenically non-inferior to the sequential administration of both vaccines 1 month apart, and had clinically acceptable safety and reactogenicity profile.
Trial Registration
ClinicalTrials.gov identifier, NCT05559476.
Graphical Abstract

Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-024-00985-4.
Keywords: High-dose, quadrivalent influenza vaccine (FLU-QIV-HD); Immunogenicity; Older adults; Randomized controlled trial; Reactogenicity; Respiratory syncytial virus (RSV); RSV prefusion protein F3 older adult (RSVPreF3 OA) vaccine; Safety
Plain Language Summary
Adults aged 65 years or older are vulnerable to infections caused by influenza and respiratory syncytial viruses, due to an aging immune system and other underlying conditions. Infections with both viruses increase during autumn and winter seasons in temperate climates. In 2023, a vaccine against respiratory syncytial virus, called RSVPreF3 OA, was first approved for use in adults aged 60 years or older in the USA; the vaccine has since also been approved in the European Union and elsewhere. Giving RSVPreF3 OA in the same vaccination visit (coadministration) with a high-dose influenza vaccine, called FLU-QIV-HD, which is given to adults aged 65 years or older, could help protect against both respiratory syncytial virus and influenza. This article reports the results of a phase 3 trial comparing coadministration of the RSVPreF3 OA and FLU-QIV-HD vaccines with sequential administration (FLU-QIV-HD followed by RSVPreF3 OA 1 month later) in 1029 adults aged 65 years or older in the USA. Proportions of participants across age categories between groups, and the proportions of male (50.4%) and female (49.6%) participants were well balanced; most participants were white (68.7%). Immune response to both the vaccines among participants in the coadministration arm was non-inferior to that in the sequential arm. Coadministration was well tolerated, with no meaningful differences in adverse reactions to the vaccines compared with sequential administration. The most common adverse reactions were pain at the injection site and muscle aches. This study supports the coadministration of RSVPreF3 OA and FLU-QIV-HD in adults aged 65 years or older.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-024-00985-4.
Key Summary Points
| Why carry out the study? |
| Respiratory syncytial virus (RSV) and influenza pose major disease burdens in adults aged ≥ 65 years, due to an aging immune system and comorbidities. |
| There is seasonal overlap between these infections, which predictably causes autumn–winter epidemics. |
| Coadministration has the potential to improve vaccine coverage and help protect older adults against both RSV and influenza with a single vaccination visit. |
| What was learned from the study? |
| In this phase 3 study, coadministration of RSVPreF3 OA and FLU-QIV-HD was immunogenically non-inferior to sequential administration of RSVPreF3 OA 1 month after FLU-QIV-HD vaccination and had a clinically acceptable safety and reactogenicity profile in adults aged ≥ 65 years. |
Digital Features
This article is published with digital features, including a graphical abstract, to facilitate understanding of the article. To view digital features for this article, go to 10.6084/m9.figshare.25663728.
Introduction
Respiratory syncytial virus (RSV), a single-stranded ribonucleic acid virus with two antigenically distinct subgroups (RSV-A and RSV-B) [1], is a highly contagious pathogen that causes respiratory tract infections in people of all ages [2–6]. Older adults (typically defined as adults aged ≥ 65 years) have a high disease burden from RSV because of weakened immunity, due to aging and comorbidities [7–9]. In temperate climates, RSV typically demonstrates a distinct seasonality, predictably causing autumn–winter epidemics partially overlapping with influenza epidemics [1, 10–12]. In tropical and subtropical regions, RSV season is typically longer and outbreaks are less predictable [1, 10]. Of note, nonpharmaceutical measures introduced during the coronavirus disease 2019 (COVID-19) pandemic (i.e., mask wearing, social distancing, domiciliary confinement) altered the patterns of RSV transmission and circulation [13].
As the global population ages, morbidity and mortality from respiratory infections appear to be steadily increasing in older adults [8, 9, 14, 15]. On the basis of Global Burden of Disease data, influenza caused an estimated 145,000 deaths in 2017; the mortality rate was high in adults aged ≥ 70 years (16.4 deaths per 100,000 population) [16]. Corresponding data for RSV pneumonia showed an estimated 188,000 deaths, including a mortality rate of 11.9 deaths per 100,000 individuals among adults aged ≥ 70 years [16]. Among 320 nasopharyngeal samples (from 15 countries) positive for any virus on reverse transcriptase–polymerase chain reaction testing, influenza A was detected in 32.5% of samples, rhinovirus/enterovirus in 25.6%, and RSV in 12.8% [17]. However, RSV may be underdiagnosed in older adults as a result of undertesting, atypical presentation, difficulty obtaining appropriate samples for testing, reliance on a single (usually upper respiratory tract) specimen type, or suboptimal sensitivity of some diagnostic tests [9, 18].
In 2015, an estimated 1.5 million episodes of RSV-related acute respiratory illness occurred in adults aged ≥ 65 years in industrialized countries; approximately 14.5% of these episodes involved a hospital admission [9]. A systematic review reported the US direct cost burden resulting from RSV hospitalizations as USD1.5–4.0 billion in adults aged ≥ 60 years [19]. Another recent systematic review and meta-analysis demonstrated that RSV accounted for 7.8% of symptomatic respiratory infections (in seasonal studies) and had a case-fatality rate of 8.2% in adults aged ≥ 60 years in developed countries (as defined by the United Nations [20]) [21]. Overall, the disease burden posed by RSV in older adults is high, and likely underestimated, thus highlighting the important need for RSV prophylaxis [8, 18].
The RSV prefusion protein F3 older adult (RSVPreF3 OA) vaccine (Arexvy®; GSK, Durham, NC, USA) was first approved in the USA in May 2023 as a single dose for the prevention of lower respiratory tract disease caused by RSV (subtypes A and B) in adults aged ≥ 60 years [22]. To date, it is approved in the European Union and elsewhere [23–25]. The RSVPreF3 OA vaccine contains 120 μg of recombinant RSVPreF3 antigen and AS01E adjuvant (containing monophosphoryl lipid A [MPL], saponin Quillaja saponaria Molina fraction 21 [QS-21; Agenus Inc., Lexington, MA, USA], and liposome [25 µg MPL and 25 µg QS-21]). In June 2023, the US Advisory Committee on Immunization Practices advocated that, on the basis of shared decision-making between healthcare providers and patients, adults ≥ 60 years of age may be given a single dose of an RSV vaccine [26].
Because of the overlapping seasonality of both RSV and influenza infections [19], coadministration of RSV and influenza vaccines may help optimize vaccine coverage, provided that immunogenicity, safety, and reactogenicity of the coadministered vaccines are not affected compared with sequential administration. In a phase 3 study (NCT04841577) in adults aged ≥ 60 years in New Zealand, Panama, and South Africa, RSVPreF3 OA coadministered with a quadrivalent influenza vaccine (Fluarix Quadrivalent [FLU-QIV]; GSK) demonstrated non-inferiority of immune response compared with the control group that received FLU-QIV on day 1 followed by RSVPreF3 OA on day 31 [27, 28].
The high-dose, quadrivalent, inactivated, non-adjuvanted, split-virion influenza (FLU-QIV-HD) vaccine (Fluzone® High-Dose Quadrivalent; Sanofi Pasteur, Inc., Swiftwater, PA, USA) is indicated in the USA in adults aged ≥ 65 years for the prevention of disease caused by the influenza A and B subtype viruses contained in the vaccine [29]. Furthermore, it is also preferentially recommended for older adults aged ≥ 65 years in a number of countries, including the USA, UK, and Australia [30–32]. In this study, the immunogenicity, safety, and reactogenicity of the RSVPreF3 OA vaccine were evaluated when coadministered with a FLU-QIV-HD vaccine in adults aged ≥ 65 years.
Methods
Study Design and Participants
This was a phase 3, open-label, randomized (1:1) controlled study conducted (from October 20, 2022 to August 15, 2023) at 48 centers in the USA and involving 1037 randomized adults aged ≥ 65 years, including 1029 participants who received at least one vaccine dose (exposed set [ES]). An automated, internet-based system (Source Data Base for Internet Randomization, Version 8.1, Belgium) was used for randomization and assignment of study interventions. The randomization algorithm used a minimization procedure accounting for age (65–69, 70–79, or ≥ 80 years) and study center. The randomization of study supplies, within blocks, was performed by the study sponsor using MATerial Excellence (Version 4.07, SAS Institute, Inc., Cary, NC, USA). Entire blocks of study supplies were then shipped to the relevant study centers or warehouses.
Enrolled participants were those who could comply with the requirements of the study protocol in the opinion of the investigator; and were living in the general community or in an assisted-living facility that provided minimal assistance, such that participants were primarily responsible for self-care and activities of daily living. In addition, participants with chronic, stable medical conditions, with or without receipt of specific treatment, were allowed to enroll if they were considered medically stable by the investigator.
The principal exclusion criteria comprised participants with any confirmed or suspected immunosuppressive or immunodeficient condition resulting from disease or immunosuppressive/cytotoxic therapy; with a history of dementia or any medical condition that moderately or severely impaired cognition; with recurrent or uncontrolled neurologic disorders or seizures; with any significant underlying illness that, in the opinion of the investigator, might prevent study completion; with known hypersensitivity to any vaccine component; with a known history of Guillain–Barré syndrome or anaphylaxis; who received (or were planning to receive) any vaccine other than the study interventions during the period from 30 days before the first study vaccination until 30 days after the last study vaccination (with the exception of COVID-19 vaccines); who received any investigational or non-registered product (drug, vaccine, or medical device) other than the study interventions during the period from 30 days before the first study vaccination or during the study period; or who were previously vaccinated with an RSV vaccine at any time and/or had received an influenza vaccine in the 6 months before the study FLU-QIV-HD vaccination. Refer to the Supplementary Online Material for full exclusion criteria.
FLU-QIV-HD contains 60 μg of hemagglutinin per influenza strain per dose. The four influenza strains included in the FLU-QIV-HD vaccine are Flu A/Darwin/6/2021 (H3N2), Flu A/Victoria/2570/2019 (H1N1), Flu B/Austria/1359417/2021 (Victoria lineage), and Flu B/Phuket/3073/2013 (Yamagata lineage).
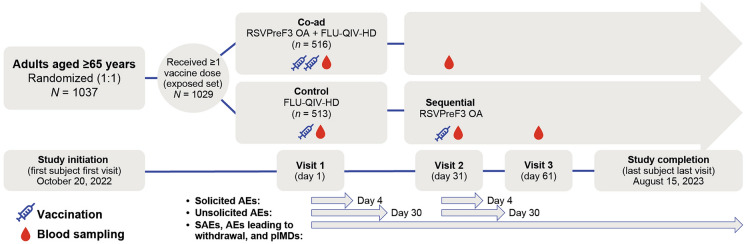
On day 1, study participants in the coadministration group (co-ad group) received a single 0.5 mL dose of RSVPreF3 OA and a single 0.7 mL dose of FLU-QIV-HD (n = 516) in different arms; study participants in the control group received a single 0.7 mL dose of FLU-QIV-HD (n = 513). On day 31, study participants in the control group received a single 0.5 mL dose of RSVPreF3 OA (Fig. 1). The vaccines were administered by intramuscular injection into the upper deltoid of either the dominant arm (FLU-QIV-HD in the co-ad group) or non-dominant arm (FLU-QIV-HD in the control group; RSVPreF3 OA in both groups).
Fig. 1.
Study design. Of 1037 adults randomized, 1029 received ≥ 1 dose of the study intervention(s). In the exposed set, 516 were randomized to coadministration of RSVPreF3 OA and FLU-QIV-HD, and 513 were randomized to sequential administration of FLU-QIV-HD and RSVPreF3 OA. The modified safety set comprised 440 participants in the co-ad group and 434 in the control group. AE adverse event, co-ad group coadministration group, FLU-QIV-HD high-dose, quadrivalent influenza vaccine, pIMD potential immune-mediated disease, RSVPreF3 OA respiratory syncytial virus prefusion protein F3 older adult vaccine, SAE serious adverse event
In the co-ad group, a blood sample (approximately 10 mL) was collected from all study participants prior to vaccination (day 1) and 1 month after vaccination (day 31). In the control group, a blood sample (approximately 10 mL) was collected from all study participants prior to vaccination (day 1), 1 month after the FLU-QIV-HD vaccination (day 31), and 1 month after the RSVPreF3 OA vaccination (day 61).
Study Objectives
The primary study objectives were to demonstrate non-inferiority based on group geometric mean titer (GMT) ratios (control group divided by co-ad group), for:
RSVPreF3 OA, measured as RSV-A neutralizing titers.
FLU-QIV-HD, measured as hemagglutination inhibition (HI) titers for each of the four influenza vaccine strains.
RSVPreF3 OA, measured as RSV-B neutralizing titers.
The study also evaluated:
The non-inferiority of FLU-QIV-HD (co-ad group vs. control group) in terms of HI seroconversion status, expressed as seroconversion rate, 1 month after the FLU-QIV-HD dose.
The humoral immune response to RSVPreF3 OA (co-ad group vs. control group) measured as RSV-A and RSV-B neutralizing titers and expressed as mean geometric increase (MGI) 1 month after the RSVPreF3 OA dose.
The humoral immune response to FLU-QIV-HD (co-ad group vs. control group) in terms of HI titers against each of the four influenza strains, expressed as GMT (days 1 and 31) or MGI (1 month after the FLU-QIV-HD dose), or in terms of HI seroconversion and seroprotection status, expressed as seroconversion and seroprotection rates, respectively (days 1 and 31). Seroconversion rates were calculated as the percentage of vaccinees with either an HI predose titer < 1:10 and a postdose titer ≥ 1:40, or a predose titer ≥ 1:10 and at least a fourfold increase in the postdose titer. Seroprotection rates were calculated as the percentage of vaccinees with a serum HI titer ≥ 1:40.
The safety and reactogenicity of RSVPreF3 OA (co-ad group vs. control group) measured as the incidence (% of study participants) of solicited adverse events (AEs), unsolicited AEs, serious AEs (SAEs), and potential immune-mediated disease (pIMDs).
Immunogenicity Assays
RSV-A and RSV-B neutralization assays were used to measure the ability of serum antibodies to neutralize RSV entry and replication in a host cell line. More detail on RSV-A and RSV-B neutralization assays can be found in the Supplementary Online Material. Serum neutralizing antibody titers were expressed as the estimated dilution (ED) 60, which corresponded to the inverse of the interpolated serum dilution yielding a 60% reduction in the number of plaques compared with the virus control wells [33, 34]. Secondary standards calibrated against the international reference (National Institute for Biological Standards and Control 16/284) were included in every run to allow conversion to international units.
HI antibody titers were determined using the method derived from the World Health Organization Manual on Animal Influenza Diagnosis and Surveillance [35]. Measurements were conducted on thawed frozen serum samples with a standardized and comprehensively validated micro-method using four hemagglutinating units of the appropriate antigens and a 0.50% fowl erythrocyte suspension. Nonspecific serum inhibitors were removed by heat treatment and receptor-destroying enzymes. Starting with an initial dilution of 1:10, a dilution series (by a factor of 2) was prepared, up to an end dilution of 1:10,240. The titration endpoint was taken as the highest dilution step that showed complete inhibition of hemagglutination. All assays were performed in duplicate, and the standard cutoff value was 10 × 1/dilution. Assay cutoff values for all immunogenicity assays used in the study are listed in Supplementary Material Table S1. Tertiary endpoint assessment of responses to the RSVPreF3 antigen were evaluated by an indirect enzyme-linked immunosorbent assay, described in more detail in the Supplementary Online Material.
Safety and Reactogenicity
Safety and reactogenicity was measured as the incidence (% of study participants) of:
- Solicited AEs (within 4 days of vaccine administration)
- Local AEs: Erythema, pain at injection site, and swelling
- Systemic AEs: Arthralgia, fatigue, fever ≥ 38 °C, headache, and myalgia
Unsolicited AEs (within 30 days of vaccine administration)
SAEs, from day 1 to study end (6 months after last vaccine dose)
pIMDs, from day 1 to study end
Data for solicited AEs and unsolicited AEs were collected using participant paper diary cards, which were returned at visit 2 (day 31; co-ad and control groups) and visit 3 (day 61; control group only) and reviewed by a physician. Participant interviews were conducted to collect data on SAEs and pIMDs, from first dose up to study end, during study visits 2 and 3 or whenever a participant contacted the site to report an SAE. Follow-up interviews were also conducted 6 months after the last vaccine dose to collect data on SAEs and pIMDs. Results from the safety analyses were descriptive, and safety analyses were performed on the ES. An additional safety analysis was conducted using the modified safety set (mSS).
Statistical Analyses
The target study enrollment was 1028 participants (514 in the co-ad group, and 514 in the control group). Please refer to the Supplementary Online Material for details on sample size calculation. The enrolled set comprised all study participants who agreed to participate in the trial after completion of the informed consent process. The safety set (the ES) comprised all study participants who received at least one dose of a study intervention. The mSS comprised all study participants who received a study intervention, excluding study participants whose diary card was not completed personally or by an authorized caregiver and/or participants who failed to demonstrate that the diary card had been completed personally or by an authorized caregiver.
The per-protocol set (PPS) comprised all study participants who received at least one study intervention, per protocol, in the control group, and all study interventions in the co-ad group; had predose–postdose immunogenicity results for at least one antigen; and complied with blood draw intervals. Participants in the PPS did not have intercurrent medical conditions that could have interfered with immunogenicity and had not received prohibited concomitant medication/vaccination. Primary and secondary immunogenicity analyses were performed on the PPS. However, as the percentage of vaccinated participants with serological results excluded from the PPS for the analysis of immunogenicity was > 5% for at least one visit and at least one group, a second analysis based on the ES was performed to complement the PPS analysis.
All statistical analyses were performed using Statistical Analysis Systems software (SAS Institute, Inc., Cary, NC, USA). In the primary endpoint analysis, two-sided 95% confidence intervals (CIs) for group GMT ratios were derived from an analysis of covariance model based on log10-transformed titers. The model included treatment group and age category (age at vaccination: 65–69, 70–79, or ≥ 80 years) as fixed effects and predose log10-transformed titers as covariates. Missing data were not replaced. All between-group analyses of secondary endpoints were descriptive. More detail on how non-inferiority testing was performed can be found in the Supplementary Online Material.
Study Ethics
The study protocol and amendments, the informed consent documentation, and other pertinent data were reviewed and approved by relevant national, regional, and investigational center-based independent ethics committees or institutional review boards (IRBs) and regulatory agencies. A centralized IRB (Advarra, Columbia, MD, USA) also conducted a final review and approved all ethical information pertaining to the study. The study was conducted in accordance with ethical principles in the Declaration of Helsinki, the principles of Good Clinical Practice, and all applicable regulatory requirements. All study participants provided signed, written, or witnessed informed consent to participate in the trial.
Results
Study Participants
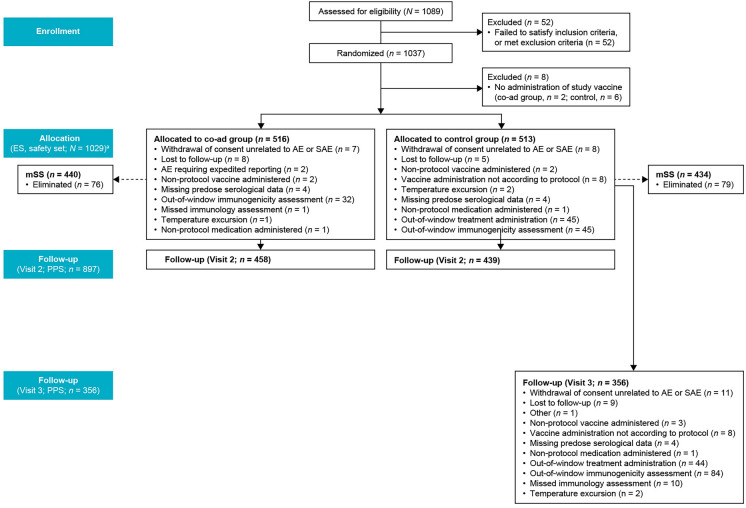
A total of 1089 participants were enrolled in the study, of whom 1037 were randomized and 1029 received at least one dose of the study intervention(s) (ES and safety set: co-ad group, n = 516; control group, n = 513). Of the total participants administered the study intervention(s), 898 (87.3%) were included in the PPS for visit 2 for FLU-QIV-HD analysis (day 31; co-ad group, n = 458; control group, n = 440), and 897 (87.2%) were included in the PPS for visit 2 RSVPreF3 OA analysis (day 31; co-ad group, n = 458; control group n = 439). A total of 356 (69.4%) participants were included in the PPS for visit 3 (day 61; control group only) for RSVPreF3 OA analysis. From the ES, 440 (85.3%) participants in the co-ad group and 434 (84.6%) in the control group were included in the mSS (Fig. 2). Up to study end, a total of 51 (5.0%) participants had withdrawn from the study (23 [4.5%] participants in the co-ad group and 28 [5.5%] in the control group). The main reasons for study discontinuation were factors unrelated to an AE or SAE (22 participants), or loss to follow-up (25 participants) (Fig. 2).
Fig. 2.
Study flow. aData from allocation to follow-up (visit 2, PPS) shown for the RSVPreF3 OA analysis. AE adverse event, co-ad group coadministration group, ES exposed set, mSS modified safety set (diary card not completed by study participant or authorized caregiver), PPS per-protocol set, RSVPreF3 OA respiratory syncytial virus prefusion protein F3 older adult vaccine, SAE serious adverse event
In the ES, the median age at visit 1 was 70 years in both groups, and the proportions of participants across age categories were similar in both groups (Table 1). A total of 519 (50.4%) participants were male and 510 (49.6%) were female. Most of the study participants were white and not of Hispanic or Latino ethnicity (Table 1). Altogether, the distribution of demographic and baseline characteristics for the PPS for immunogenicity, for the RSVPreF3 OA and FLU-QIV-HD vaccine analyses, was similar to the distribution in the ES.
Table 1.
Summary of demographic and baseline characteristics (exposed set)
| Characteristic | Coadministration group (n = 516) | Control group (n = 513) | Total (n = 1029) |
|---|---|---|---|
| Age at first dose, median (range), years | 70 (65–91) | 70 (65–92) | 70 (65–92) |
| Age group, number (%) | |||
| 65–69 years | 243 (47.1) | 241 (47.0) | 484 (47.0) |
| 70–79 years | 230 (44.6) | 231 (45.0) | 461 (44.8) |
| ≥ 70 years | 273 (52.9) | 272 (53.0) | 545 (53.0) |
| ≥ 80 years | 43 (8.3) | 41 (8.0) | 84 (8.2) |
| Sex, number (%) | |||
| Male | 249 (48.3) | 270 (52.6) | 519 (50.4) |
| Female | 267 (51.7) | 243 (47.4) | 510 (49.6) |
| Ethnicity, number (%) | |||
| Hispanic or Latino | 169 (32.8) | 161 (31.4) | 330 (32.1) |
| Not Hispanic or Latino | 347 (67.2) | 352 (68.6) | 699 (67.9) |
| Race, number (%) | |||
| American Indian or Alaska Native | 4 (0.8) | 6 (1.2) | 10 (1.0) |
| Asian | 4 (0.8) | 4 (0.8) | 8 (0.8) |
| Black or African American | 72 (14.0) | 68 (13.3) | 140 (13.6) |
| Native Hawaiian or other Pacific Islander | 0 (0.0) | 1 (0.2) | 1 (0.1) |
| White | 355 (68.8) | 352 (68.6) | 707 (68.7) |
| Other | 81 (15.7) | 82 (16.0) | 163 (15.8) |
Immunogenicity
All the primary objectives of the immunogenicity analyses were met. In the PPS, RSV-A neutralizing titers (ED60; expressed as adjusted GMT ratio) 1 month after RSVPreF3 OA administration in the co-ad group were non-inferior compared to the control group: the adjusted GMT ratio for RSV-A neutralizing titers (control group divided by co-ad group) was 1.18 (95% CI 1.04, 1.35) (Fig. 3). At 1 month after FLU-QIV-HD administration, HI titers (expressed as adjusted GMT ratios) for the four influenza vaccine strains were non-inferior in the co-ad group versus control group: GMT ratios for HI titers (control group divided by co-ad group) were 0.98 (95% CI 0.84, 1.14) for Flu A/Darwin/6/2021 (H3N2), 0.93 (95% CI 0.80, 1.08) for Flu A/Victoria/2570/2019 (H1N1), 0.95 (95% CI 0.88, 1.03) for Flu B/Austria/1359417/2021 (Victoria lineage), and 0.92 (95% CI 0.84, 1.02) for Flu B/Phuket/3073/2013 (Yamagata lineage) (Fig. 3). RSV-B neutralizing titers 1 month after RSVPreF3 OA also demonstrated non-inferiority in the co-ad group versus the control group: the adjusted GMT ratio for RSV-B neutralizing titers (control group divided by co-ad group) was 1.02 (95% CI 0.89, 1.16) (Fig. 3). Across all primary endpoints, the results in the PPS were consistent with those in the ES (Supplementary Material Table S2).
Fig. 3.
Non-inferiority of RSVPreF3 OA + FLU-QIV-HD versus control regarding GMT ratios of RSV-A and RSV-B neutralizing titers (estimated dilution 60) and HI titers for the four influenza vaccine strains. Non-inferiority criterion: Upper limit of the two-sided 95% CI of the GMT ratio (control group divided by co-ad group) ≤ 1.5 for RSV-A, each of the four influenza vaccine strains, and RSV-B. ANCOVA model applied to the log10-transformed titers. The model included treatment group and age at vaccination (65–69, 70–79, or ≥ 80 years) as fixed effects and predose log10-transformed titer as a covariate. ANCOVA analysis of covariance, CI confidence interval, co-ad group coadministration group, FLU-QIV-HD high-dose, quadrivalent influenza vaccine, GMT geometric mean titer, HI hemagglutination inhibition, RSV respiratory syncytial virus, RSVPreF3 OA RSV prefusion protein F3 older adult vaccine
The descriptive objective of non-inferiority for the co-ad group versus control group in terms of HI seroconversion rate was met for all four influenza strains (the reference criterion was that the upper limit of the two-sided 95% CI on the group difference in seroconversion rate was ≤ 10% for anti-HI antibodies). For each of the four influenza strains, geometric mean HI titer in the co-ad group was similar to that in the control group; this was true for both the PPS and ES (Supplementary Material Table S2).
The US Center for Biologics Evaluation and Research criteria for vaccine efficacy, in terms of HI seroconversion and seroprotection rates, were met for all influenza strains, except for HI seroconversion rate for the Flu B/Victoria strain in the control group. The European Medicines Agency Committee of Human Medicinal Products criteria for vaccine efficacy, in terms of seroconversion and seroprotection rates and MGIs, were met for all four influenza vaccine strains in both groups (Supplementary Material Table S3). More information on these vaccine efficacy criteria can be found in the Supplementary Online Material.
At 1 month after vaccination (day 31 for the co-ad group and day 61 for the control group), the RSV-A neutralizing titer (ED60) MGI over baseline was 5.59 (95% CI 5.02, 6.23) in the co-ad group and 6.75 (95% CI 5.93, 7.68) in the control group. Corresponding values for RSV-B neutralizing titer (ED60) MGI over baseline were 5.19 (95% CI 4.69, 5.75) in the co-ad group and 5.29 (95% CI 4.65, 6.03) in the control group. At 1 month post-vaccination (day 31), the MGI in the HI titers (1/DIL) for Flu A/H3N2, Flu A/H1N1, Flu B/Victoria, and Flu B/Yamagata strain was 6.20 (95% CI 5.49, 7.00), 5.57 (95% CI 4.81, 6.46), 2.92 ( 95% CI 2.69, 3.18), and 3.13 (95% CI 2.85, 3.44) in the co-ad group and 5.87 (95% CI 5.20, 6.62), 4.53 (95% CI 3.94, 5.21), 2.71 (95% CI 2.51, 2.93), and 2.87 (95% CI 2.62, 3.14) in the control group, respectively.
Safety and Reactogenicity
Anomalies in handwriting were observed in a subset of participant diaries during an audit performed at one site, and findings were confirmed during a for-cause audit, which revealed that 46 of the 232 (19.8%) paper diaries were at least partially completed by a member of the site staff, rather than by a study participant or authorized caregiver, without any explanation documented. Therefore, an additional safety analysis was performed on the mSS. Results for solicited AEs are based on the mSS and results for unsolicited AEs are based on the ES. No handwriting anomalies were observed at any other site.
Solicited AEs
In the mSS, a total of 264 (62.4%) participants in the co-ad group and 251 (60.2%) in the control group reported at least one solicited local AE within 4 days of any dose. A total of 264 (62.4%) participants in the co-ad group and 184 (44.4%) in the control group reported at least one solicited local AE within 4 days of visit 1, and 189 (47.3%) participants in the control group did so within 4 days of visit 2. Grade 3 solicited local AEs were reported by 13 (3.1%) participants in the co-ad group, 3 (0.7%) participants in the control group after visit 1, and 2 (0.5%) participants in the control group after visit 2. The most frequently reported solicited local AE was pain at injection site: 199 (47.0%) participants in the co-ad group versus 176 (42.5%) in the control group for the FLU-QIV-HD vaccine at visit 1; 235 (55.6%) participants in the co-ad group for the RSVPreF3 OA vaccine at visit 1; and 183 (45.8%) participants in the control group for the RSVPreF3 OA vaccine at visit 2 (Fig. 4). In the mSS, no participants in the co-ad group reported at least one medically attended solicited local AE compared with 1 (0.2%) in the control group after visit 1 and no participants in the control group after visit 2.
Fig. 4.
Proportion of study participants with at least one solicited administration-site AE or solicited AE (modified safety set). Data are presented as % of participants with a diary card. Grade 3 pain prevents normal everyday activities. Grade 3 fever > 39 °C. AE adverse event, co-ad coadministration, FLU-QIV-HD high-dose, quadrivalent influenza vaccine, RSVPreF3 OA respiratory syncytial virus prefusion protein F3 older adult vaccine
Overall, 229 (54.1%) participants in the co-ad group and 253 (60.7%) in the control group reported at least one solicited systemic AE. A total of 229 (54.1%) participants in the co-ad group and 178 (43.0%) in the control group reported at least one systemic AE at visit 1, and 186 (46.5%) participants in the control group did so at visit 2. Grade 3 solicited systemic AEs were reported by 13 (3.1%) participants in the co-ad group, 9 (2.2%) participants in the control group after visit 1, and 5 (1.3%) participants in the control group after visit 2. The most frequently reported systemic solicited AE was myalgia: 170 (40.2%) participants in the co-ad group; 141 (34.1%) in the control group for the FLU-QIV-HD vaccine at visit 1; and 125 (31.3%) in the control group for the RSVPreF3 OA vaccine at visit 2 (Fig. 4). In the mSS, no participants in the co-ad group reported at least one medically attended solicited systemic AE, compared with 2 (0.5%) in the control group after visit 1 and no participants in the control group after visit 2.
Unsolicited AEs
In the ES, 63 (12.2%) and 69 (13.5%) participants reported at least one unsolicited AE within 30 days of any dose in the co-ad and control group, respectively. The most frequently reported unsolicited AE, within 30 days of any dose, in the co-ad group was diarrhea (7 [1.4%] participants vs 4 [0.8%] in the control group) and COVID-19 in the control group (11 [2.1%] participants vs 2 [0.4%] in the co-ad group).
In the co-ad versus control group, 12 (2.3%) versus 15 (2.9%) participants had at least one SAE, of which 1 (0.2%) in the co-ad group was assessed by the investigator as FLU-QIV-HD-related (influenza and exacerbation of asthma). One (0.2%) participant in the co-ad group had an SAE associated with a fatal outcome (congestive cardiac failure; not considered vaccine-related). Up to study end, no participants in the co-ad group and 2 (0.4%) participants in the control group reported AEs defined as pIMD (brachial radiculitis and polymyalgia rheumatica). The event of brachial radiculitis was identified as a pIMD by the pre-defined list of preferred terms only and was considered by the investigator to be related to FLU-QIV-HD vaccine.
No trends or imbalances were observed in reported unsolicited AEs when the vaccines were co-administered; see Supplementary Online Material for further details.
Discussion
All the primary objectives of this phase 3 study were met; the data showed that 1 month after vaccination, the immunogenicity of RSVPreF3 OA coadministered with FLU-QIV-HD was non-inferior to RSVPreF3 OA administered sequentially 1 month after the FLU-QIV-HD vaccine in adults ≥ 65 years old. At 1 month after vaccination (day 31 in the co-ad group, and day 61 in the control group): the neutralizing titer (ED60) adjusted GMT ratio was 1.18 (95% CI 1.04, 1.35) for RSV-A; for Flu/A/Darwin/6/2021 (H3N2), Flu A/Victoria/2570/2019 (H1N1), Flu B/Austria/1359417/2021 (Victoria lineage), and Flu B/Phuket/3073/2013 (Yamagata lineage) adjusted GMT ratios were 0.98 (95% CI 0.84, 1.14), 0.93 (95% CI 0.80, 1.08), 0.95 (95% CI 0.88, 1.03), and 0.92 (95% CI 0.84, 1.02), respectively; and for RSV-B, the neutralizing titer (ED60) adjusted GMT ratio was 1.02 (95% CI 0.89, 1.16). A similarly designed study evaluating RSVPreF3 OA coadministered with FLU-QIV in older adults (NCT04841577) also demonstrated non-inferiority of coadministration compared to sequential administration [28]. In the present study, the RSV-A neutralizing titer (ED60) MGI over baseline was 5.59 (95% CI 5.02, 6.23) in the co-ad group and 6.75 (95% CI 5.93, 7.68) in the control group. MGI in the HI titers (1/DIL) for Flu A/H3N2, Flu A/H1N1, Flu B/Victoria, and Flu B/Yamagata strain was 6.20 (95% CI 5.49, 7.00), 5.57 (95% CI 4.81, 6.46), 2.92 (95% CI 2.69, 3.18), and 3.13 (95% CI 2.85, 3.44) in the co-ad group and 5.87 (95% CI 5.20, 6.62), 4.53 (95% CI 3.94, 5.21), 2.71 (95% CI 2.51, 2.93), and 2.87 (95% CI 2.62, 3.14) in the control group, respectively.
The secondary descriptive analyses should be interpreted with caution, as there were no adjustments for multiplicity testing in these comparisons and the analyses were not statistically powered. Nonetheless, the analyses suggested non-inferiority for the coadministration of FLU-QIV-HD and RSVPreF3 OA vaccines, compared with FLU-QIV-HD administered alone in the control arm, in terms of HI seroconversion rates for all four influenza strains (the lower limit of the 95% CI being ≥ 30% in participants ≥ 65 years of age).
The data for per-visit analyses showed a slight increase in solicited systemic events when the RSVPreF3 OA vaccine was coadministered with FLU-QIV-HD, compared to when the vaccines were administered at sequential visits. However, no clinically meaningful differences in solicited events (local events and systemic events) were observed, in terms of duration or severity of events, between both the co-ad and the control groups. The reported percentages of unsolicited AEs, SAEs, and pIMDs up to the end of the study were generally balanced between groups and did not alter the benefit–risk profile of the RSVPreF3 OA vaccine, which remains favorable.
Our study was limited by being conducted in only one country (albeit at 48 distinct centers). Strengths included the large sample size (N = 1089) which allowed investigators to detect vaccine effects, use of a standardized protocol and centralized laboratory testing, and inclusion of a control group. These study findings are of considerable relevance to clinical practice, given the major disease burdens posed by RSV infection and influenza in older adults [7, 19, 36–39], the seasonal overlap of these two infections [40], and the opportunity to vaccinate concomitantly, conveniently, and effectively against both RSV and influenza in the at-risk age group of older adults [37, 40].
Conclusion
This study demonstrates that coadministration was immunogenically non-inferior to sequential administration (FLU-QIV-HD then RSVPreF3 OA) and had clinically acceptable safety and reactogenicity profiles and supports coadministration of RSVPreF3 OA and FLU-QIV-HD in individuals aged ≥ 65 years. Coadministration of RSVPreF3 OA and FLU-QIV-HD can improve vaccine coverage and help protect older adults against both RSV and influenza infection with a single vaccination visit.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Paul Wakefield, of Summit Medical Group, Norwood Family Medicine, Knoxville, TN, USA, for his contributions to data acquisition
Medical Writing, Editorial, and Other Assistance
Medical writing support was provided by David Murdoch, a contract writer working on behalf of Apollo, and Silvia Pregnolato of Apollo, OPEN Health Communications, funded by GSK, in accordance with Good Publication Practice 3 (GPP) guidelines (http://www.ismpp.org/gpp-2022).
Author Contributions
Study concept or design: Sofia Valenciano. Data acquisition: Robert Buynak, Kevin Cannon, David DeAtkine, John Kirby, and Lisa Usdan. Data analysis: Sofia Valenciano, Amit Bhavsar, Nadia Meyer, Amulya Jayadev, Hiwot Amare, Anastasia Kuznetsova, and Catherine Gérard. Data interpretation: David DeAtkine, Amit Bhavsar, Nadia Meyer, Amulya Jayadev, Hiwot Amare, and Anastasia Kuznetsova. All authors provided final approval of the version to be published. All authors provided agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
Sponsorship for this study and the journal’s Rapid Service fee were funded by GSK.
Data Availability
The study report, including the protocol, will be made available on the GSK Clinical Study Register (https://www.gsk-studyregister.com). Anonymized individual participant data and study documents can be requested for further research from: www.clinicalstudydatarequest.com (study ID 214489).
Declarations
Conflict of Interest
Sofia Valenciano, Hiwot Amare, Amit Bhavsar, Catherine Gérard, Amulya Jayadev, Anastasia Kuznetsova, and Nadia Meyer are employees of GSK and may hold stock options. Robert Buynak received funding from GSK to conduct clinical research. David DeAtkine is a medical director at Central Research Assoc Inc. and receives payment as Principal Investigator for different studies. Kevin Cannon, John Kirby, and Lisa Usdan declare no conflicts of interest. AS01 is a trademark owned by or licensed to the GSK group of companies.
Ethical Approval
This study was conducted in accordance with the ethical principles that have their origins in the Declaration of Helsinki and the principles of Good Clinical Practice, and was approved by all applicable regulatory agencies, investigational center ethics committees, or institutional review boards. The study protocol and amendments, the informed consent, and other relevant information were reviewed by a national, regional, or investigational center ethics committees, or institutional review boards.
Footnotes
Prior Presentation: Some data from this manuscript were presented previously at the Ninth European Scientific Working Group on Influenza (ESWI) Conference, September 17–20, 2023, Valencia, Spain.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Borchers AT, Chang C, Gershwin ME, Gershwin LJ. Respiratory syncytial virus—a comprehensive review. Clin Rev Allergy Immunol. 2013;45(3):331–79. 10.1007/s12016-013-8368-9. 10.1007/s12016-013-8368-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Domachowske JB, Anderson EJ, Goldstein M. The future of respiratory syncytial virus disease prevention and treatment. Infect Dis Ther. 2021;10(Suppl 1):47–60. 10.1007/s40121-020-00383-6. 10.1007/s40121-020-00383-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chatterjee A, Mavunda K, Krilov LR. Current state of respiratory syncytial virus disease and management. Infect Dis Ther. 2021;10(Suppl 1):5–16. 10.1007/s40121-020-00387-2. 10.1007/s40121-020-00387-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, Wang X, Blau DM, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. 2022;399(10340):2047–64. 10.1016/S0140-6736(22)00478-0. 10.1016/S0140-6736(22)00478-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simoes EAF. Respiratory syncytial virus disease in young children and older adults in europe: a burden and economic perspective. J Infect Dis. 2022;226(Suppl 1):S1–9. 10.1093/infdis/jiac252. 10.1093/infdis/jiac252 [DOI] [PubMed] [Google Scholar]
- 6.Soni A, Kabra SK, Lodha R. Respiratory syncytial virus infection: an update. Indian J Pediatr. 2023;90(12):1245–53. 10.1007/s12098-023-04613-w. 10.1007/s12098-023-04613-w [DOI] [PubMed] [Google Scholar]
- 7.Branche AR, Saiman L, Walsh EE, et al. Incidence of respiratory syncytial virus infection among hospitalized adults, 2017–2020. Clin Infect Dis. 2022;74(6):1004–11. 10.1093/cid/ciab595. 10.1093/cid/ciab595 [DOI] [PubMed] [Google Scholar]
- 8.Savic M, Penders Y, Shi T, Branche A, Pircon JY. Respiratory syncytial virus disease burden in adults aged 60 years and older in high-income countries: a systematic literature review and meta-analysis. Influenza Other Respir Viruses. 2023;17(1):e13031. 10.1111/irv.13031. 10.1111/irv.13031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi T, Denouel A, Tietjen AK, et al. Global disease burden estimates of respiratory syncytial virus-associated acute respiratory infection in older adults in 2015: a systematic review and meta-analysis. J Infect Dis. 2020;222(Suppl 7):S577–83. 10.1093/infdis/jiz059. 10.1093/infdis/jiz059 [DOI] [PubMed] [Google Scholar]
- 10.Noyola DE, Mandeville PB. Effect of climatological factors on respiratory syncytial virus epidemics. Epidemiol Infect. 2008;136(10):1328–32. 10.1017/S0950268807000143. 10.1017/S0950268807000143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neumann G, Kawaoka Y. Seasonality of influenza and other respiratory viruses. EMBO Mol Med. 2022;14(4):e15352. 10.15252/emmm.202115352. 10.15252/emmm.202115352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chadha M, Hirve S, Bancej C, et al. Human respiratory syncytial virus and influenza seasonality patterns-Early findings from the WHO global respiratory syncytial virus surveillance. Influenza Other Respir Virus. 2020;14(6):638–46. 10.1111/irv.12726. 10.1111/irv.12726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosscrop LG, Williams TC, Tregoning JS. Respiratory syncytial virus after the SARS-CoV-2 pandemic—what next? Nat Rev Immunol. 2022;22(10):589–90. 10.1038/s41577-022-00764-7. 10.1038/s41577-022-00764-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee N, Lui GC, Wong KT, et al. High morbidity and mortality in adults hospitalized for respiratory syncytial virus infections. Clin Infect Dis. 2013;57(8):1069–77. 10.1093/cid/cit471. 10.1093/cid/cit471 [DOI] [PubMed] [Google Scholar]
- 15.Binder W, Thorsen J, Borczuk P. RSV in adult ED patients: do emergency providers consider RSV as an admission diagnosis? Am J Emerg Med. 2017;35(8):1162–5. 10.1016/j.ajem.2017.06.022. 10.1016/j.ajem.2017.06.022 [DOI] [PubMed] [Google Scholar]
- 16.GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736–88. 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed]
- 17.Falsey AR, McElhaney JE, Beran J, et al. Respiratory syncytial virus and other respiratory viral infections in older adults with moderate to severe influenza-like illness. J Infect Dis. 2014;209(12):1873–81. 10.1093/infdis/jit839. 10.1093/infdis/jit839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rozenbaum MH, Begier E, Kurosky SK, et al. Incidence of respiratory syncytial virus infection in older adults: limitations of current data. Infect Dis Ther. 2023;12(6):1487–504. 10.1007/s40121-023-00802-4. 10.1007/s40121-023-00802-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grace M, Colosia A, Wolowacz S, Panozzo C, Ghaswalla P. Economic burden of respiratory syncytial virus infection in adults: a systematic literature review. J Med Econ. 2023;26(1):742–59. 10.1080/13696998.2023.2213125. 10.1080/13696998.2023.2213125 [DOI] [PubMed] [Google Scholar]
- 20.United Nations Economic Analysis & Policy Division of the Department of Economic and Social Affairs of the United Nations Secretariat. Country classification. 2014. www.un.org/en/development/desa/policy/wesp/wesp_current/2014wesp_country_classification.pdf. Accessed 13 Sep 2023.
- 21.Nguyen-Van-Tam JS, O’Leary M, Martin ET, et al. Burden of respiratory syncytial virus infection in older and high-risk adults: a systematic review and meta-analysis of the evidence from developed countries. Eur Respir Rev. 2022;31(166): 220105. 10.1183/16000617.0105-2022. 10.1183/16000617.0105-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.GSK. US FDA approves GSK’s Arexvy, the world’s first respiratory syncytial virus (RSV) vaccine for older adults. 2023. https://www.gsk.com/en-gb/media/press-releases/us-fda-approves-gsk-s-arexvy-the-world-s-first-respiratory-syncytial-virus-rsv-vaccine-for-older-adults/. Accessed 29 June 2023.
- 23.GSK. European Commission authorises GSK’s Arexvy, the first respiratory syncytial virus (RSV) vaccine for older adults. 2023. https://www.gsk.com/en-gb/media/press-releases/european-commission-authorises-gsk-s-arexvy-the-first-respiratory-syncytial-virus-rsv-vaccine-for-older-adults/#:~:text=The%20European%20Commission%20has%20authorised%20Arexvy%20for%20active%20immunisation%20for,in%20accordance%20with%20official%20recommendations. Accessed 13 Sep 2023.
- 24.GSK. Medicines and Healthcare products Regulatory Agency authorises GSK’s Arexvy, the first respiratory syncytial virus (RSV) vaccine for older adults. 2023. https://www.gsk.com/en-gb/media/press-releases/medicines-and-healthcare-products-regulatory-agency-authorises-gsk-s-arexvy-the-first-respiratory-syncytial-virus-rsv-vaccine-for-older-adults/. Accessed 13 Sep 2023.
- 25.GSK. GSK’s Arexvy, the first respiratory syncytial virus (RSV) vaccine for older adults approved in Canada. 2023. https://ca.gsk.com/en-ca/media/press-releases/gsk-s-arexvy-the-first-respiratory-syncytial-virus-rsv-vaccine-for-older-adults-approved-in-canada/. Accessed 13 Sep 2023.
- 26.Melgar M, Britton A, Roper LE, et al. Use of respiratory syncytial virus vaccines in older adults: recommendations of the advisory committee on immunization practices—United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72(29):793–801. 10.1558/mmwr.mm7229a4. 10.1558/mmwr.mm7229a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.ClinicalTrials.gov. NCT04841577. A study on the immune response and safety elicited by a vaccine against respiratory syncytial virus (RSV) when given alone and together with a vaccine against influenza in adults aged 60 years and above. https://www.clinicaltrials.gov/study/NCT04841577. Accessed 28 Aug 2023.
- 28.Chandler R, Montenegro N, Llorach C, et al. Immunogenicity, reactogenicity, and safety of AS01E-adjuvanted RSV Prefusion F protein-based candidate vaccine (RSVPreF3 OA) when co-administered with a seasonal quadrivalent influenza vaccine in older adults: results of a phase 3, open-label, randomized controlled trial. Clin Infect Dis. 2024. 10.1093/cid/ciad786. 10.1093/cid/ciad786 [DOI] [PubMed] [Google Scholar]
- 29.Sanofi Pasteur Inc. 522 Fluzone® High-Dose Quadrivalent. Prescribing Information. 2019. https://www.fda.gov/media/132238/download#:~:text=Fluzone%C2%AE%20High%2DDose%20Quadrivalent%20is%20a%20vaccine%20indicated%20for,years%20of%20age%20and%20older. Accessed 13 Sep 2023.
- 30.Australian Government, Department of Health and Aged Care. Australian Immunisation Handbook. https://immunisationhandbook.health.gov.au/recommendations/adults-aged-65-years-are-recommended-to-receive-influenza-vaccine-every-year. Accessed 4 Apr 2024.
- 31.Joint Committee on Vaccination and Immunisation. Advice on influenza vaccines for 2022/23. https://www.nitag-resource.org/sites/default/files/2021-10/JCVI%20Statement%20on%20Influenza%20Vaccines%202022-23.pdf. Accessed 4 Apr 2024.
- 32.Centers for Disease Control and Prevention. Fluzone high-dose seasonal influenza vaccine. https://www.cdc.gov/flu/prevent/qa_fluzone.htm. Accessed 4 Apr 2024.
- 33.Bates JT, Keefer CJ, Slaughter JC, Kulp DW, Schief WR, Crowe JE Jr. Escape from neutralization by the respiratory syncytial virus-specific neutralizing monoclonal antibody palivizumab is driven by changes in on-rate of binding to the fusion protein. Virology. 2014;454–455:139–44. 10.1016/j.virol.2014.02.010. 10.1016/j.virol.2014.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barbas CF 3rd, Crowe JE Jr, Cababa D, et al. Human monoclonal Fab fragments derived from a combinatorial library bind to respiratory syncytial virus F glycoprotein and neutralize infectivity. Proc Natl Acad Sci USA. 1992;89(21):10164–8. 10.1073/pnas.89.21.10164. 10.1073/pnas.89.21.10164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization. Department of Communicable Disease Surveillance and Response. WHO Global Influenza Programme. WHO Manual on Animal Influenza Diagnosis and Surveillance. https://apps.who.int/iris/bitstream/handle/10665/68026/WHO_CDS_CSR_NCS_2002.5.pdf?sequence=1&isAllowed=y. Accessed 29 Aug 2023.
- 36.Papi A, Ison MG, Langley JM, et al. Respiratory syncytial virus prefusion F protein vaccine in older adults. N Engl J Med. 2023;388(7):595–608. 10.1056/NEJMoa2209604. 10.1056/NEJMoa2209604 [DOI] [PubMed] [Google Scholar]
- 37.Falsey AR, Walsh EE, Scott DA, et al. Phase 1/2 randomized study of the immunogenicity, safety, and tolerability of a respiratory syncytial virus prefusion F vaccine in adults with concomitant inactivated influenza vaccine. J Infect Dis. 2022;225(12):2056–66. 10.1093/infdis/jiab611. 10.1093/infdis/jiab611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mao Z, Li X, Korsten K, et al. Economic burden and health-related quality of life of respiratory syncytial virus and influenza infection in European community-dwelling older adults. J Infect Dis. 2022;226(Suppl 1):S87–94. 10.1093/infdis/jiac069. 10.1093/infdis/jiac069 [DOI] [PubMed] [Google Scholar]
- 39.Mazur NI, Terstappen J, Baral R, et al. Respiratory syncytial virus prevention within reach: the vaccine and monoclonal antibody landscape. Lancet Infect Dis. 2023;23(1):e2–21. 10.1016/S1473-3099(22)00291-2. 10.1016/S1473-3099(22)00291-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sadoff J, De Paepe E, Haazen W, et al. Safety and immunogenicity of the Ad26.RSV.preF investigational vaccine coadministered with an influenza vaccine in older adults. J Infect Dis. 2021;223(4):699–708. 10.1093/infdis/jiaa409. 10.1093/infdis/jiaa409 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study report, including the protocol, will be made available on the GSK Clinical Study Register (https://www.gsk-studyregister.com). Anonymized individual participant data and study documents can be requested for further research from: www.clinicalstudydatarequest.com (study ID 214489).