Abstract
Severe pneumonia results in high morbidity and mortality despite advanced treatments. This study investigates thoracic muscle mass from chest CT scans as a biomarker for predicting clinical outcomes in ICU patients with severe pneumonia. Analyzing electronic medical records and chest CT scans of 778 ICU patients with severe community-acquired pneumonia from January 2016 to December 2021, AI-enhanced 3D segmentation was used to assess thoracic muscle mass. Patients were categorized into clusters based on muscle mass profiles derived from CT scans, and their effects on clinical outcomes such as extubation success and in-hospital mortality were assessed. The study identified three clusters, showing that higher muscle mass (Cluster 1) correlated with lower in-hospital mortality (8% vs. 29% in Cluster 3) and improved clinical outcomes like extubation success. The model integrating muscle mass metrics outperformed conventional scores, with an AUC of 0.844 for predicting extubation success and 0.696 for predicting mortality. These findings highlight the strong predictive capacity of muscle mass evaluation over indices such as APACHE II and SOFA. Using AI to analyze thoracic muscle mass via chest CT provides a promising prognostic approach in severe pneumonia, advocating for its integration into clinical practice for better outcome predictions and personalized patient management.
Keywords: Intensive care unit, Pneumonia, Muscle, Skeletal, Machine learning, Airway extubation, Mortality
Subject terms: Prognostic markers, Computed tomography, Respiratory tract diseases
Introduction
Pneumonia represents a significant public health burden, accounting for considerable morbidity and mortality globally. Despite advancements in therapeutic strategies and critical care interventions, severe pneumonia, which necessitates intensive care unit (ICU) admission, is still associated with high morbidity and mortality1,2. Conventional prognostic models such as CURB-65, the Pneumonia Severity Index (PSI), Quick Sequential Organ Failure Assessment (qSOFA), Simplified Acute Physiology Score 3 (SAPS-3), and Acute Physiology and Chronic Health Evaluation II (APACHE II) have shown limited predictive accuracy for patients with severe pneumonia upon their admission to the ICU or emergency department3–6. Recent studies have indicated that machine learning-based prediction models may offer improved predictive performances for clinical outcomes in ICU patients with severe pneumonia3,7.
Emerging evidence from diagnostic imaging techniques, notably computed tomography (CT), has illuminated the potential of CT-based muscle assessment as an innovative marker for evaluating muscle mass and identifying sarcopenia8,9. Sarcopenia, characterized by substantial muscle loss, has profound implications for patient recovery and survival10, suggesting that muscle mass evaluation could offer critical insights into patient resilience against severe infections, including pneumonia.
Despite known correlations between sarcopenia and increased mortality in various patient populations, a substantial gap persists in our understanding of how muscle mass specifically influences outcomes in severe pneumonia. This knowledge gap hinders optimal integration of muscle assessment in prognostic evaluation of pneumonia patients. The potential of muscle mass indicators derived from routine chest CT scans in ICU settings as prognostic tools necessitate further exploration11,12. Elucidating the relationship between muscle parameters and pneumonia outcomes may catalyze a shift in patient management paradigms, facilitating the adoption of personalized treatment strategies that consider physiological resilience in conjunction with infection severity.
Thus, this study aimed to investigate the potential of thoracic muscle mass as determined by chest CT scans to serve as a biomarker for predicting clinical outcomes of ICU patients with severe community-acquired pneumonia. By categorizing patients into clusters based on their thoracic muscle mass profiles from CT scans, this study aimed to determine if these distinct groups exhibited different risks for extubation failure or in-hospital mortality, thus providing insights into the prognostic value of muscle mass in critical care settings.
Methods
Study design and eligibility criteria
Electronic medical records of patients with severe community-acquired pneumonia who were admitted to the ICU of a teaching hospital from January 2016 to December 2021 were retrospectively analyzed. Inclusion criteria were: patients aged ≥ 18 years presenting with respiratory failure attributable to severe pneumonia necessitating ICU admission. Diagnosis of pneumonia relied on chest CT findings, blood C-reactive protein (CRP) levels ≥ 4 mg/dL13, and administration of pneumonia-specific antibiotics. Severe pneumonia was defined as meeting at least one major or three minor criteria outlined in the guidelines of the Infectious Disease Society of America/American Thoracic Society14. Individuals transferred to the general ward within 3 days of ICU admission and those with medical conditions deemed more critical than pneumonia were excluded. Eligible patients were observed during hospital stay for up to 30 days from the ICU admission date.
Clinical variables
Baseline information including age, sex, body mass index, smoking history, and respiratory and non-respiratory comorbidities was obtained. Clinical conditions included conventional severity-of-illness scores such as Acute Physiology and Chronic Health Evaluation (APACHE) II, Sequential Organ Failure Assessment (SOFA), and SAPS II, initial vital signs, laboratory findings, and treatments for pneumonia. Clinical outcomes included high-flow nasal cannula (HFNC), mechanical ventilation (MV), prolonged MV, Extracorporeal Membrane Oxygenation (ECMO), extubation, tracheostomy, and in-hospital mortality.
Thoracic muscle mass measurements
Thoracic muscle mass was evaluated using chest CT scans conducted upon ICU admission of included patients. The thoracic muscle mass measurements include a comprehensive set of 60 variables that describe various aspects of muscle quantity and density within the thoracic region. The variables are categorized into three main groups: mean muscle area, mean muscle attenuation, and muscle volume. Each group provides specific insights into muscle characteristics.
Mean muscle area (cm2/cm) represents the average cross-sectional area of the muscle normalized by the length of the thoracic region. The mean muscle area is calculated as the total muscle volume divided by the length of the region of interest (ROI). This normalization accounts for individual differences in body size.
Mean muscle attenuation, measured in Hounsfield Units (HU), reflects the density and quality of muscle tissue. It is determined by averaging the HU values of all pixels within the segmented muscle area. The formula for mean muscle attenuation involves summing the HU values of all pixels within the muscle and dividing by the number of pixels.
Muscle volume (cm3) represents the total volume of muscle tissue within the specified region. It is calculated by summing the volumes of all slices within the ROI. For each slice, the volume is determined by multiplying the cross-sectional area by the slice thickness.
The total muscle area in the thoracic region is the sum of all muscle areas normalized by the length of the thoracic spine. Specific muscles, such as the right and left pectoralis major and minor, as well as the right and left erector spinae, are measured individually at different thoracic levels and along the entire thoracic spine. For measurements specific to the erector spinae muscle group at T1–T12 levels, attenuation values ranged from − 29 to 150 HU15. This specific range captures the density of muscle tissue, excluding fat and other non-muscular tissues, and is useful in assessing the quality and composition of the muscle.
For precise analysis, we utilized 3D segmentation on chest CT scans to assess muscle mass of the thoracic region, focusing on specific muscles such as the pectoralis major, pectoralis minor, and erector spinae. Initial segmentation was performed using a radiological workstation (MEDIP, MEDICALIP, Seoul, South Korea. https://medicalip.com/medip/), which combined 3D volume rendering and multiplanar image reformatting. This workstation employs a semi-automatic segmentation method using a graph cut algorithm16. It is known for having precision in defining muscle boundaries with the guidance of an experienced radiologist. To further enhance the accuracy and efficiency of muscle segmentation, we incorporated a custom-developed deep learning AI model tailored for 3D convolutional neural network-based segmentation. This model is specifically trained to identify and delineate targeted muscle groups. Detailed information about the muscle segmentation network, including the training data, training pipeline, and test performance, has been summarized in Supplementary Information S1.
Moreover, AI software (DeepCatch, MEDICALIP, Seoul, South Korea. https://medicalip.com/DeepCatch/) was used for measuring total muscle mass at various segments of the spine, specifically at T4, T12, and from T10 to T1217. A comprehensive measurement from T1 through T12 was also performed. This AI software excels in providing detailed visualizations and quantifications of muscle mass through advanced imaging analysis.
Clustering stability
To evaluate the reproducibility and stability of our clustering analysis technique, we conducted a sensitivity analysis using random subsampling. We generated 100 random subsets of the data, each containing 80% of the original sample size. For each subset, we standardized the data and performed k-means clustering with k = 3, maintaining the same parameters as the original analysis. We calculated the Adjusted Rand Index (ARI) for each pair of clustering results across the subsets to assess the similarity of the clustering structures.
Outcomes
The primary outcome was extubation. The secondary outcome was in-hospital mortality over a 30-day observation period.
Predictive model assessment
We employed a logistic regression model to predict successful extubation and in-hospital mortality based on thoracic muscle mass measurements. The entire dataset was analyzed without splitting it into training and testing sets. This approach was chosen to perform an exploratory analysis for initial model assessment and to maximize data utilization, particularly in evaluating the feasibility of using thoracic muscle mass data for predictive modeling.
Statistical analysis
K-means clustering was performed with 60 variables representing profiles of thoracic muscle mass. Clusters were visually depicted through uniform manifold approximation and projection (UMAP) and principal component analysis (PCA) plots. Categorical variables were analyzed using the chi-squared test, while continuous variables were analyzed using the independent t-test or Mann–Whitney U test. A chi-square test for linear trend was conducted to evaluate clinical outcomes according to clusters. Kaplan–Meier curves were employed to analyze time to extubation and in-hospital mortality events. Hazard ratios for extubation and in-hospital mortality were estimated using Cox proportional hazards models. We assessed the overall performance using the area under the receiver operating characteristic curve (AUC) and compared models using the Delong method18. Additionally, accuracy, specificity, sensitivity, precision, and recall were evaluated. Statistical significance was set at p < 0.05, alongside 95% confidence intervals. Statistical analyses were conducted using R statistical software version 4.1.2 (R Foundation, Vienna, Austria).
Ethics
This study followed ethical guidelines outlined in the Declaration of Helsinki of 1975. The Institutional Review Board (IRB) of Boramae Medical Center approved the study protocol and waived the requirement for informed consent from study participants to access their electronic medical records (IRB No. 10-2021-110).
Results
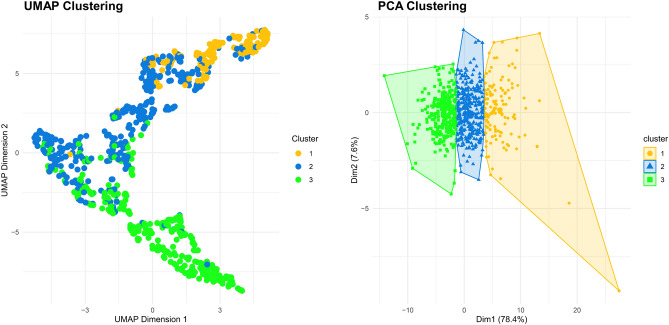
We identified a total of 795 patients admitted to the ICU with a primary diagnosis of pneumonia confirmed by chest CT scans (Supplementary Information S2). After excluding 17 patients who were transferred to the general ward within three days or presented with medical conditions more critical than pneumonia, our study included 778 patients requiring ICU admission for severe pneumonia. A comprehensive set of 60 variables related to thoracic muscle mass in the included patients is summarized in Supplementary Information S3. Using the profiles of thoracic muscle mass, three distinct clusters were derived, with 130, 351, and 297 patients allocated to these three clusters. They were visualized through UMAP and PCA plots (Fig. 1). Detailed results of PCA analysis of thoracic muscle mass profiles are provided in Supplementary Information S4. The mean ARI value obtained from 100 random subsets was 0.97, indicating a very high degree of reproducibility and stability in the clustering results.
Figure 1.
UMAP and PCA clustering based on the profiles of thoracic muscle mass. UMAP uniform manifold approximation and projection, PCA principal component analysis.
We observed significant differences in thoracic muscle mass profiles across the three clusters, with Cluster 1 group exhibiting higher values than Clusters 2 and 3 for all indicators, including mean muscle area, musculoskeletal index, and muscle volume (Supplementary Information S5, all p-value < 0.001).
Demographic and clinical features
Baseline characteristics of each cluster are outlined in Table 1. Cluster 1 was characterized by middle-aged males with metabolic concerns. Cluster 2 consisted of elderly males with respiratory comorbidities. Cluster 3 consisted of elderly patients with sarcopenic conditions and cognitive impairment. Upon thorough examination of conventional severity-of-illness scores, APACHE II, SOFA, SAPS II, and PaO2/FiO2 (PF) ratio showed no significant differences among clusters (Table 2). Significant differences in several clinical parameters including initial vital signs, urine output, neutrophil count, lymphocyte count, creatinine levels, aspartate aminotransferase (AST), alanine aminotransferase, and total bilirubin were observed among clusters. Although no differences in the use of antibiotics and systemic corticosteroids were observed between clusters, vasopressors were more frequently used in Cluster 3.
Table 1.
Baseline characteristics of ICU patients with severe pneumonia.
| Cluster 1 group (n = 130) | Cluster 2 group (n = 351) | Cluster 3 group (n = 297) | P-value | |
|---|---|---|---|---|
| Age, year, mean (SD) | 67.57 (13.61) | 74.83 (11.54) | 75.52 (13.40) | < 0.001 |
| < 65 | 50 (38.5) | 60 (17.1) | 58 (19.5) | < 0.001 |
| 65–75 | 25 (19.2) | 83 (23.6) | 58 (19.5) | 0.361 |
| > 75 | 55 (42.3) | 208 (59.3) | 181 (60.9) | < 0.001 |
| Male, n (%) | 126 (96.9) | 277 (78.9) | 157 (52.9) | < 0.001 |
| BMI, kg/m2 mean (SD) | 24.70 (4.53) | 21.18 (3.45) | 18.03 (3.69) | < 0.001 |
| Smoking history | ||||
| Current smoking, n (%) | 69 (53.1) | 218 (62.1) | 225 (75.8) | < 0.001 |
| Ex-smoker, n (%) | 53 (40.8) | 111 (31.6) | 61 (20.5) | < 0.001 |
| Never smoker, n (%) | 8 (6.2) | 22 (6.3) | 11 (3.7) | 0.307 |
| Pack year, mean (SD) | 13.66 (19.22) | 11.76 (19.16) | 7.16 (15.45) | < 0.001 |
| Comorbidities | ||||
| Respiratory comorbidities, n (%) | 20 (15.4) | 110 (31.3) | 69 (23.2) | 0.001 |
| COPD, n (%) | 9 (6.9) | 32 (9.1) | 16 (5.4) | 0.189 |
| Asthma, n (%) | 3 (2.3) | 13 (3.7) | 7 (2.4) | 0.536 |
| ILD, n (%) | 6 (4.6) | 18 (5.1) | 4 (1.3) | 0.029 |
| NTM, n (%) | 0 (0.0) | 6 (1.7) | 2 (0.7) | 0.190 |
| TB-destroyed lung, n (%) | 8 (6.2) | 61 (17.4) | 46 (15.5) | 0.008 |
| Lung cancer, n (%) | 1 (0.8) | 6 (1.7) | 3 (1.0) | 0.623 |
| Non-respiratory comorbidities, n (%) | 102 (78.5) | 265 (75.5) | 206 (69.4) | 0.083 |
| HTN, n (%) | 69 (53.1) | 209 (59.5) | 147 (49.5) | 0.035 |
| DM, n (%) | 63 (48.5) | 131 (37.3) | 110 (37.0) | 0.056 |
| CKD, n (%) | 18 (13.8) | 64 (18.2) | 33 (11.1) | 0.037 |
| CLD, n (%) | 12 (9.2) | 18 (5.1) | 16 (5.4) | 0.211 |
| CVD, n (%) | 2 (1.5) | 6 (1.7) | 5 (1.7) | 0.991 |
| CHF, n (%) | 3 (2.3) | 15 (4.3) | 13 (4.4) | 0.562 |
| CVA, n (%) | 2 (1.5) | 2 (0.6) | 4 (1.3) | 0.508 |
| Dementia, n (%) | 1 (0.8) | 25 (7.1) | 36 (12.1) | < 0.001 |
| HIV, n (%) | 1 (0.8) | 1 (0.3) | 2 (0.7) | 0.714 |
| Rheumatic disease, n (%) | 0 (0.0) | 1 (0.3) | 0 (0.0) | 0.544 |
| Metastatic cancer, n (%) | 0 (0.0) | 3 (0.9) | 6 (2.0) | 0.154 |
| Hematologic malignancy, n (%) | 0 (0.0) | 1 (0.3) | 1 (0.3) | 0.811 |
BMI body mass index, CHF congestive heart failure, CKD chronic kidney disease, CLD chronic liver disease, COPD chronic obstructive pulmonary disease, CVA cerebrovascular accident, CVD cardiovascular disease, DM diabetes mellitus, HIV human immunodeficiency virus, HTN hypertension, ICU intensive care unit, ILD interstitial lung disease, NTM non-tuberculous mycobacteria, TB tuberculosis.
Table 2.
Clinical conditions of ICU patients with severe pneumonia.
| Cluster 1 group (n = 130) | Cluster 2 group (n = 351) | Cluster 3 group (n = 297) | P-value | |
|---|---|---|---|---|
| Conventional severity-of-illness scoring systems | ||||
| APACHE II, mean (SD) | 23.78 (11.07) | 24.06 (9.93) | 24.32 (9.79) | 0.917 |
| SOFA, mean (SD) | 11.18 (8.50) | 10.07 (7.72) | 10.62 (7.66) | 0.512 |
| SAPS II, mean (SD) | 50.06 (20.15) | 52.17 (20.95) | 54.00 (20.67) | 0.333 |
| Initial vital signs | ||||
| GCS, mean (SD) | 11.26 (3.52) | 10.49 (3.51) | 9.91 (3.45) | 0.001 |
| Initial MBP, mmHg, mean (SD) | 86.83 (20.29) | 88.04 (21.67) | 81.56 (18.12) | < 0.001 |
| Initial PR, mmHg, mean (SD) | 99.23 (23.79) | 106.99 (26.44) | 104.72 (24.82) | 0.012 |
| Initial RR, mmHg, mean (SD) | 22.29 (5.81) | 22.97 (5.48) | 22.52 (5.26) | 0.382 |
| PF ratio, mean (SD) | 129.32 (68.16) | 145.59 (74.19) | 138.94 (73.37) | 0.087 |
| > 200 | 24 (18.5) | 89 (25.4) | 73 (24.6) | 0.273 |
| 100–200 | 50 (38.5) | 135 (38.5) | 106 (35.7) | 0.740 |
| < 100 | 56 (43.1) | 127 (36.2) | 73 (24.6) | 0.346 |
| Urine output, mL/h, mean (SD) | 74.48 (51.37) | 66.04 (49.77) | 61.52 (45.51) | 0.039 |
| Laboratory findings | ||||
| pH, mean (SD) | 7.35 (0.14) | 7.36 (0.14) | 7.35 (0.16) | 0.845 |
| PaCO2, mmHg, mean (SD) | 39.12 (14.40) | 37.24 (13.22) | 38.39 (14.17) | 0.339 |
| PaO2, mmHg, mean (SD) | 76.77 (36.65) | 75.35 (36.02) | 74.35 (35.29) | 0.810 |
| HCO3, mmol/L, mean (SD) | 21.24 (5.33) | 21.04 (6.56) | 21.19 (7.07) | 0.939 |
| Lactate, mmol/L, mean (SD) | 3.72 (4.97) | 3.89 (4.67) | 4.49 (5.18) | 0.197 |
| WBC, 103/mL, mean (SD) | 11.79 (6.57) | 12.24 (6.86) | 13.36 (7.96) | 0.058 |
| Neutrophil, %, mean (SD) | 77.77 (15.33) | 78.45 (15.21) | 82.35 (12.00) | < 0.001 |
| Lymphocyte, %, mean (SD) | 15.24 (12.11) | 14.21 (11.32) | 12.30 (9.90) | 0.017 |
| CRP, mg/dL, mean (SD) | 13.16 (11.32) | 15.15 (10.81) | 15.46 (10.27) | 0.110 |
| Procalcitonin, ng/mL, mean (SD) | 2.41 (6.29) | 3.00 (9.77) | 1.90 (5.36) | 0.576 |
| BUN, mg/dL, mean (SD) | 33.79 (25.35) | 35.67 (30.10) | 34.84 (26.07) | 0.796 |
| Creatinine, mg/dL, mean (SD) | 1.99 (1.83) | 1.91 (1.77) | 1.51 (1.27) | 0.002 |
| AST, IU/L, mean (SD) | 103.51 (143.87) | 75.83 (106.50) | 75.37 (114.54) | 0.046 |
| ALT, IU/L, mean (SD) | 60.30 (90.07) | 41.37 (69.51) | 36.96 (63.74) | 0.007 |
| Total bilirubin, mg/dL, mean (SD) | 1.54 (1.48) | 1.32 (1.37) | 1.12 (1.10) | 0.007 |
| Troponin I, ng/mL, mean (SD) | 7.90 (25.52) | 10.14 (29.55) | 10.49 (30.83) | 0.690 |
| NT-proBNP, pg/mL, mean (SD) | 5856.02 (9296.15) | 8025.16 (11,058.18) | 7502.44 (10,698.28) | 0.276 |
| Treatment | ||||
| Antibiotics, n (%) | 130 (100.0) | 348 (99.1) | 294 (99.0) | 0.532 |
| Systemic corticosteroid, n (%) | 87 (66.9) | 255 (72.6) | 194 (65.3) | 0.116 |
| Vasopressor, n (%) | 108 (83.1) | 292 (83.2) | 270 (90.9) | 0.010 |
ALT alanine aminotransferase, APACHE II acute physiology and chronic health evaluation II, AST aspartate aminotransferase, BUN blood urea nitrogen, CRP C-reactive protein, FiO2 fraction of inspired oxygen, GCS Glasgow Coma Scale, HCO3 bicarbonate, ICU intensive care unit, IU international unit, MBP mean blood pressure, NT-proBNP N-terminal pro-B-type natriuretic peptide, PaCO2 partial pressure of carbon dioxide in arterial blood, PaO2 partial pressure of oxygen in arterial blood, PF ratio ratio of partial pressure of oxygen to fraction of inspired oxygen, PR pulse rate, RR respiratory rate, SAPS II simplified acute physiology score II, SOFA sequential organ failure assessment, WBC white blood cell count.
Clinical outcomes
MV was more frequently applied in Cluster 1, while ECMO was more commonly used in Cluster 2 than in other clusters (Table 3). However, there was no significant linear trend across clusters. Cluster 1 had higher rates of extubation, lower rates of prolonged MV, lower rates of tracheostomy, and lower rates of in-hospital mortality than Cluster 3. A significant linear trend across clusters was noted.
Table 3.
Clinical outcomes during a 30-day observation period.
| Cluster 1 group (n = 130) | Cluster 2 group (n = 351) | Cluster 3 group (n = 297) | P-value | P for trend | |
|---|---|---|---|---|---|
| HFNC, n (%) | 29 (22.3) | 81 (23.1) | 65 (21.9) | 0.935 | 0.918 |
| MV, n (%) | 112 (86.2) | 265 (75.5) | 230 (77.4) | 0.041 | 0.647 |
| ECMO, n (%) | 0 (0.0) | 9 (2.6) | 2 (0.7) | 0.041 | 0.930 |
| Extubation, n (%) | 90 (69.2) | 199 (56.7) | 154 (51.9) | 0.004 | 0.012 |
| Prolonged MV, n (%) | 4 (3.1) | 16 (4.6) | 25 (8.4) | 0.039 | 0.010 |
| Tracheostomy, n (%) | 17 (13.1) | 63 (17.9) | 74 (25.0) | 0.009 | 0.001 |
| Inhospital mortality, n (%) | 40 (30.8) | 152 (43.3) | 145 (48.8) | 0.002 | < 0.001 |
Prolonged MV was defined as greater than 21 days of MV.
ECMO extracorporeal membrane oxygenation, HFNC high-flow nasal cannula, MV mechanical ventilation.
In the Kaplan–Meier curve, Cluster 3 had higher risks of extubation failure and in-hospital mortality (Fig. 2). In each comparison, Cluster 3 showed a prolonged time to extubation than Cluster 1 (p-value < 0.001) and Cluster 2 (p-value < 0.001). Meanwhile, Cluster 3 showed a shortened time to in-hospital mortality than Cluster 2 (p-value = 0.002), although there was no significant difference between Cluster 1 and Cluster 3.
Figure 2.
Time to extubation and in-hospital mortality.
Clinical factors related to extubation and in-hospital mortality
In unadjusted Cox regression analyses for extubation, Cluster 3 was associated with a decreased probability of successful extubation compared to Cluster 1 and Cluster 2 (Table 4). Additionally, COPD, initial PR, low PF ratio, systemic corticosteroid usage, and vasopressor administration were linked to a decreased probability of successful extubation. Higher APACHE II or SAPS II scores among conventional severity-of-illness measures were also correlated with a reduced likelihood of extubation (Supplementary information S6). In adjusted Cox regression analyses, Cluster 3 demonstrated a lower hazard ratio (HR) of extubation compared to Cluster 1 (HR: 1.373 [95% confidence interval (CI) 1.050–1.796]) and Cluster 2 (HR: 1.406 [95% CI 1.136–1.741]).
Table 4.
Hazard ratio for extubation.
| Unadjusted hazard ratio (95% CI) | P-value | Adjusted hazard ratio (95% CI) | P-value | |
|---|---|---|---|---|
| Cluster (reference: Cluster 3 group) | – | – | – | – |
| Cluster 1 group | 1.481 (1.140–1.924) | 0.003 | 1.373 (1.050–1.796) | 0.020 |
| Cluster 2 group | 1.457 (1.179–1.801) | < 0.001 | 1.406 (1.136–1.741) | 0.002 |
| COPD | 0.576 (0.390–0.849) | 0.005 | 0.611 (0.412–0.906) | 0.014 |
| Initial PR | 0.994 (0.991–0.998) | 0.002 | 0.996 (0.992–1.000) | 0.031 |
| PF ratio (reference: > 200) | – | – | – | – |
| 100–200 | 0.717 (0.561–0.917) | 0.008 | 0.797 (0.622–1.021) | 0.073 |
| < 100 | 0.552 (0.429–0.710) | < 0.001 | 0.619 (0.480–0.799) | < 0.001 |
| Systemic corticosteroid | 0.623 (0.511–0.761) | < 0.001 | 0.702 (0.573–0.860) | < 0.001 |
| Vasopressor | 0.625 (0.472–0.826) | < 0.001 | 0.725 (0.544–0.968) | 0.029 |
CI confidence interval, COPD chronic obstructive pulmonary disease, PF ratio ratio of partial pressure of oxygen to fraction of inspired oxygen, PR pulse rate.
In unadjusted Cox regression analyses for in-hospital mortality, Cluster 1 exhibited a lower HR of in-hospital mortality compared to Cluster 2 and Cluster 3 (Table 5). Among comorbidities, asthma, chronic kidney disease, and congestive heart failure were associated with a higher HR of in-hospital mortality. Initial mean blood pressure, initial respiratory rate, PF ratio, urine output, PaO2, HCO3, lactate, AST, troponin I, and vasopressor usage were correlated with the risk of in-hospital mortality. Moreover, higher APACHE II, SOFA, and SAPS II scores among conventional severity-of-illness measures were associated with an increased likelihood of in-hospital mortality (Supplementary Information S7). In adjusted Cox regression analyses, Cluster 1 demonstrated a lower HR of in-hospital mortality than Cluster 2 (HR: 0.616 [95% CI 0.433–0.876]) and Cluster 3 (HR: 0.601 [95% CI 0.419–0.861]).
Table 5.
Hazard ratio for in-hospital mortality.
| Unadjusted hazard ratio (95% CI) | P-value | Adjusted hazard ratio (95% CI) | P-value | |
|---|---|---|---|---|
| Cluster (reference: cluster 1 group) | – | – | – | – |
| Cluster 2 group | 1.456 (1.028–2.063) | 0.035 | 1.623 (1.142–2.308) | 0.007 |
| Cluster 3 group | 1.719 (1.211–2.441) | 0.002 | 1.663 (1.161–2.384) | 0.006 |
| Asthma | 1.801 (1.011–3.207) | 0.046 | 2.275 (1.256–4.119) | 0.007 |
| CKD | 1.523 (1.152–2.015) | 0.003 | 1.421 (1.048–1.925) | 0.024 |
| CHF | 2.349 (1.508–3.657) | < 0.001 | 2.221 (1.401–3.522) | < 0.001 |
| Initial MBP | 0.986 (0.980–0.992) | < 0.001 | 0.991 (0.985–0.997) | 0.002 |
| Initial RR | 1.033 (1.013–1.053) | 0.001 | 1.021 (1.001–1.041) | 0.039 |
| PF ratio | 0.993 (0.992–0.995) | < 0.001 | 0.992 (0.990–0.994) | < 0.001 |
| Urine output, mL/h | 0.994 (0.992–0.997) | < 0.001 | 0.995 (0.993–0.998) | < 0.001 |
| PaO2 | 0.995 (0.992–0.999) | 0.009 | 1.006 (1.001–1.010) | 0.011 |
| HCO3 | 0.965 (0.949–0.981) | < 0.001 | 0.970 (0.953–0.988) | 0.001 |
| Lactate | 1.081 (1.064–1.098) | < 0.001 | 1.056 (0.035–1.077) | < 0.001 |
| AST | 1.001 (1.001–1.002) | < 0.001 | 1.001 (1.000–1.002) | 0.034 |
| Troponin I | 1.005 (1.002–1.009) | 0.001 | 1.006 (1.003–1.009) | < 0.001 |
| Vasopressor | 2.564 (1.632–4.030) | < 0.001 | 1.868 (1.170–2.982) | 0.009 |
AST aspartate aminotransferase, CHF congestive heart failure, CKD chronic kidney disease, CI confidence interval, HCO3 bicarbonate, MBP mean blood pressure, PF ratio ratio of partial pressure of oxygen to fraction of inspired oxygen, RR respiratory rate, PaO2 partial pressure of oxygen in arterial blood.
Predictive performances of extubation and in-hospital mortality
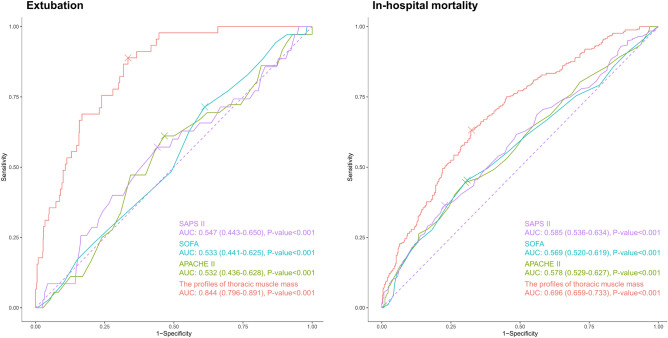
The prediction model for successful extubation using all the variables representing profiles of thoracic muscle mass showed a higher AUC (0.844 [0.796–0.891]) than APACHE II (Delong test p-value < 0.001), SOFA (Delong test p-value < 0.001), and SAPS II (Delong test p-value < 0.001) (Fig. 3). Sensitivity, specificity, positive predictive value, negative predictive value, and accuracy for predicting successful extubation are summarized in Supplementary Information S8.
Figure 3.
Prediction of extubation and in-hospital mortality.
The prediction model for in-hospital mortality employing all the variables representing profiles of thoracic muscle mass demonstrated a higher AUC (0.696 [0.659–0.733]) than APACHE II (Delong test p-value < 0.001), SOFA (Delong test p-value < 0.001), and SAPS II (Delong test p-value < 0.001) (Fig. 3). Sensitivity, specificity, positive predictive value, negative predictive value, and accuracy for predicting in-hospital mortality are summarized in Supplementary Information S9.
Discussion
Our study underscores the crucial role of thoracic muscle mass in determining clinical outcomes for ICU patients with severe pneumonia. The categorization into distinct clusters based on muscle mass profiles highlights the potential for differential treatment approaches. Patients possessing greater muscle mass (Cluster 1) demonstrated superior clinical outcomes, including higher extubation success and lower mortality rates. Patients in Cluster 1 were mainly middle-aged males with metabolic concerns, while Cluster 3 with worse outcomes consisted of older patients with sarcopenic conditions and cognitive impairments. Severity-of-illness scores such as APACHE II, SOFA, and SAPS II showed no significant differences across clusters. Prediction models using thoracic muscle mass profiles showed higher accuracy in predicting extubation success and in-hospital mortality than conventional severity scores.
Unsupervised clustering analysis serves as a robust methodology for evaluating the prognostic relevance in ICU patients19,20. The strength of clustering analysis resides in its ability to manage and interpret complex datasets, thereby facilitating a nuanced understanding of patient characteristics that might be obscured in broader analyses. A previous study has evaluated the effectiveness of a clustering approach based on clinical and echocardiographic parameters, showing that novel cardiovascular phenotypes in septic shock patients are closely associated with prognosis21. A recent study has reported that in ICU settings, clustering analysis enables provision of individualized mobilization strategies for different patient groups, significantly improving discharge success rates22. In numerous studies, sarcopenia identified through CT-measured muscle mass cutoffs has been linked to outcomes of ICU patients23–25. However, a significant limitation of these studies was the lack of a universally accepted gold standard for these cutoff values, which could vary widely depending on factors such as ethnicity, specific disease conditions, and imaging modalities. Despite the absence of a validated cut-off value for reduced muscle volume, clustering analysis can provide a unique perspective for examining intricate relationships between muscle mass and clinical outcomes. Our study categorized patients based on comprehensive profiles of thoracic muscle mass and enhanced our understanding of impacts of muscle volume profiles on clinical outcomes in critically ill patients. Our findings advocate for a broader application of clustering analysis in further studies on ICU patients.
Reduced skeletal muscle mass detected via CT scans has been associated with prognosis of critically ill ICU patients26. However, the precise mechanisms through which reduced muscle mass or sarcopenia can worsen prognosis in ICU patients remain poorly understood. There have been several hypotheses suggesting the relationship between skeletal muscle and prognosis in ICU. First, diminished skeletal muscle mass might indicate depleted protein reserves essential for responding to stress and infections, leading to an altered immune response27,28. Second, this reduction in muscle mass may significantly correlate with respiratory muscle weakness and reduce cough strength and lung function, thus increasing risks of respiratory failure and mortality29. Thirdly, reduced skeletal muscle mass can contribute to reduced mobility linked to poorer outcomes of hospitalized patients30. Indeed, a decrease in muscle thickness of the quadriceps has been associated with prolonged ICU stays31. This decreased mobility can lead to a higher incidence of complications such as pressure ulcers, deep vein thrombosis, and falls.
Our clustering analysis identified the association between decreased skeletal muscle mass and impaired cognitive function. A higher prevalence of cognitive impairments was found in the group with the lowest thoracic skeletal muscle index. This finding can be explained by a bidirectional relationship between pneumonia and cognitive function32. A recent study has shown that lower muscle mass is correlated with decreased cognitive function in older patients33. A decreased cognitive function can hinder rehabilitation and slow return to daily activities in ICU patients. One current study has shown that a lower skeletal muscle index is correlated with reduced success in weaning from a nasogastric tube, potentially affecting long-term outcomes34.
Conventional sarcopenia assessment methods such as DEXA and BIA face practical challenges in the ICU, where they are often impractical due to the need for specialized equipment and limited mobility of patients35. This situation necessitates the development of alternative strategies that utilize existing clinical data. Routine performance of chest CT scans for pneumonia diagnosis and treatment monitoring in the ICU present an opportunity for opportunistic imaging36. This method allows for the assessment of muscle mass directly from these scans, offering a streamlined, non-invasive approach to evaluating muscle health. The integration of muscle mass analysis into standard patient care protocols can significantly improve assessment of sarcopenia, enhance patient management, and potentially improve outcomes of critically ill individuals37.
Prognostic models such as APACHE II, SOFA, and SAPS II commonly used in ICU settings have their limitations, especially in older patient cohorts who exhibit unique health characteristics and needs38. In our study, we employed 3D segmentation to individually analyze pectoralis major and minor muscles (known to play a critical role in respiratory mechanics) and erector spinae muscles essential for maintaining posture and stability, particularly in patients with prolonged bed rest due to pneumonia. Our integrated analysis of these specific muscle groups enhanced by deep-learning algorithms demonstrated a prognostic accuracy superior to traditional measures. This comprehensive muscle assessment offers valuable insights into the severity of respiratory conditions and guides personalized care, highlighting the functional importance of each muscle group in patient outcomes.
While our study highlights the prognostic superiority of integrating muscle mass analysis using advanced AI-driven 3D segmentation techniques over traditional severity scores such as APACHE II and SOFA, it has several limitations. First, the retrospective design of our research introduced inherent constraints such as potential selection bias and the inability to establish causality. Second, generalizability of our findings might be limited as this study was conducted in a single teaching hospital. Third, relying solely on chest CT scans to assess thoracic muscle mass might overlook subtle physiological changes associated with conditions such as sarcopenia and other critical illnesses. Fourth, our focus on short-term clinical outcomes did not allow for evaluation of long-term impacts of muscle mass variations on patient recovery and quality of life. Fifth, despite statistical adjustments for known confounders, there remained a risk of residual confounding from unmeasured or inadequately measured variables such as nutritional status and pre-existing functional impairments. These factors could significantly influence observed associations between muscle mass and patient outcomes.
In conclusion, ICU patients with severe community-acquired pneumonia can be stratified into three distinct groups based on different profiles of thoracic muscle mass, each exhibiting unique clinical characteristics. Our findings underscore the potential role of thoracic muscle mass as a predictor of extubation success and in-hospital mortality in this patient population. To confirm our results and investigate long-term impacts of muscle health on patient outcomes, further multicentric studies are needed.
Supplementary Information
Abbreviations
- APACHE II
Acute physiology and chronic health evaluation II
- AST
Aspartate aminotransferase
- AUC
Area under the receiver operating characteristic curve
- AI
Artificial intelligence
- APACHE II
Acute physiology and chronic health evaluation II
- BIA
Bioelectrical impedance analysis
- COPD
Chronic obstructive pulmonary disease
- CRP
C-reactive protein
- CURB-65
Confusion, urea, respiratory rate, blood pressure, age ≥ 65 years
- CT
Computed tomography
- DEXA
Dual-energy X-ray absorptiometry
- ECMO
Extracorporeal membrane oxygenation
- HFNC
High-flow nasal cannula
- HCO3
Bicarbonate
- HU
Hounsfield units
- ICU
Intensive care unit
- IDSA/ATS
Infectious disease society of America/American thoracic society
- IRB
Institutional review board
- MV
Mechanical ventilation
- PCA
Principal component analysis
- PF ratio
PaO2/FiO2 ratio
- PR
Pulse rate
- PSI
Pneumonia severity index
- qSOFA
Quick sequential organ failure assessment
- SAPS-3
Simplified acute physiology score 3
Author contributions
Study concept and design: Y.H.C., D.H.K., H.W.L. Acquisition of data: D.H.K., H.J.L., T.Y.P., H.W.L. Analysis and interpretation of data: D.H.K., E.T.J., K.N.J., S.H.Y., H.W.L. Drafting the manuscript: Y.H.C., D.H.K., H.W.L. Critical revision of the manuscript and important intellectual content: E.T.J., H.J.L., T.Y.P., K.N.J., S.H.Y. Obtained funding: Y.H.C., D.H.K. Study supervision: H.W.L.
Funding
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HI21C1074). This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2020R1C1C1007280). This study was supported by the Soonchunhyang University Research Fund.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to privacy and confidentiality concerns but are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yoon-Hee Choi and Dong Hyun Kim.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-67625-2.
References
- 1.Almirall, J., Serra-Prat, M., Bolibar, I. & Balasso, V. risk factors for community-acquired pneumonia in adults: A systematic review of observational studies. Respiration94, 299–311. 10.1159/000479089 (2017). 10.1159/000479089 [DOI] [PubMed] [Google Scholar]
- 2.Cilloniz, C., Torres, A. & Niederman, M. S. Management of pneumonia in critically ill patients. BMJ375, e065871. 10.1136/bmj-2021-065871 (2021). 10.1136/bmj-2021-065871 [DOI] [PubMed] [Google Scholar]
- 3.Jeon, E. T. et al. Machine learning-based prediction of in-ICU mortality in pneumonia patients. Sci. Rep.13, 11527. 10.1038/s41598-023-38765-8 (2023). 10.1038/s41598-023-38765-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carmo, T. A. et al. Derivation and validation of a novel severity scoring system for pneumonia at intensive care unit admission. Clin. Infect. Dis.72, 942–949. 10.1093/cid/ciaa183 (2021). 10.1093/cid/ciaa183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joya-Montosa, C., Delgado-Amaya, M., Trujillo-García, E. & Curiel-Balsera, E. J. C. C. Assessment of specific risk scores for patients admitted to the ICU for severe community-acquired pneumonia.19, P9 (2015). [Google Scholar]
- 6.Richards, G. et al. CURB-65, PSI, and APACHE II to assess mortality risk in patients with severe sepsis and community acquired pneumonia in PROWESS. J. Intensive Care Med.26, 34–40. 10.1177/0885066610383949 (2011). 10.1177/0885066610383949 [DOI] [PubMed] [Google Scholar]
- 7.Cilloniz, C. et al. Machine-learning model for mortality prediction in patients with community-acquired pneumonia: Development and validation study. Chest163, 77–88. 10.1016/j.chest.2022.07.005 (2023). 10.1016/j.chest.2022.07.005 [DOI] [PubMed] [Google Scholar]
- 8.Chun, S. Y., Cho, Y. S. & Kim, H. B. Association between reduced muscle mass and poor prognosis of biliary sepsis. Sci. Rep.14, 1857. 10.1038/s41598-024-52502-9 (2024). 10.1038/s41598-024-52502-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moon, S. W. et al. Thoracic skeletal muscle quantification using computed tomography and prognosis of elderly ICU patients. Sci. Rep.11, 23461. 10.1038/s41598-021-02853-4 (2021). 10.1038/s41598-021-02853-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cruz-Jentoft, A. J. et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing48, 16–31. 10.1093/ageing/afy169 (2019). 10.1093/ageing/afy169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Branea, O. E. et al. Assessment of the diaphragm thickness decrease in critically ill COVID-19 patients: Could computed tomography be of aid regarding diaphragm muscle mass?. Cureus15, e47195. 10.7759/cureus.47195 (2023). 10.7759/cureus.47195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yokosuka, R. et al. Pectoralis muscle mass on chest CT at admission predicts prognosis in patients with pneumonia. Can. Respir. J.2021, 3396950. 10.1155/2021/3396950 (2021). 10.1155/2021/3396950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flanders, S. A. et al. Performance of a bedside C-reactive protein test in the diagnosis of community-acquired pneumonia in adults with acute cough. Am. J. Med.116, 529–535. 10.1016/j.amjmed.2003.11.023 (2004). 10.1016/j.amjmed.2003.11.023 [DOI] [PubMed] [Google Scholar]
- 14.Mandell, L. A. et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis.44(Suppl 2), S27-72. 10.1086/511159 (2007). 10.1086/511159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim, D. W. et al. Reliability of skeletal muscle area measurement on CT with different parameters: A phantom study. Korean J. Radiol.22, 624–633. 10.3348/kjr.2020.0914 (2021). 10.3348/kjr.2020.0914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jimenez-Carretero, D. et al. A graph-cut approach for pulmonary artery-vein segmentation in noncontrast CT images. Med Image Anal52, 144–159. 10.1016/j.media.2018.11.011 (2019). 10.1016/j.media.2018.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong, J. H. et al. CT analysis of thoracolumbar body composition for estimating whole-body composition. Insights Imaging14, 69. 10.1186/s13244-023-01402-z (2023). 10.1186/s13244-023-01402-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeLong, E. R., DeLong, D. M. & Clarke-Pearson, D. L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics44, 837–845 (1988). 10.2307/2531595 [DOI] [PubMed] [Google Scholar]
- 19.Hyun, S., Kaewprag, P., Cooper, C., Hixon, B. & Moffatt-Bruce, S. Exploration of critical care data by using unsupervised machine learning. Comput. Methods Programs Biomed194, 105507. 10.1016/j.cmpb.2020.105507 (2020). 10.1016/j.cmpb.2020.105507 [DOI] [PubMed] [Google Scholar]
- 20.Castela Forte, J., Perner, A. & van der Horst, I. C. C. The use of clustering algorithms in critical care research to unravel patient heterogeneity. Intensive Care Med45, 1025–1028. 10.1007/s00134-019-05631-z (2019). [DOI] [PubMed]
- 21.Geri, G. et al. Cardiovascular clusters in septic shock combining clinical and echocardiographic parameters: A post hoc analysis. Intensive Care Med.45, 657–667. 10.1007/s00134-019-05596-z (2019). 10.1007/s00134-019-05596-z [DOI] [PubMed] [Google Scholar]
- 22.Fuest, K. E. et al. Clustering of critically ill patients using an individualized learning approach enables dose optimization of mobilization in the ICU. Crit. Care27, 1. 10.1186/s13054-022-04291-8 (2023). 10.1186/s13054-022-04291-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang, X. M. et al. Sarcopenia as a predictor of mortality among the critically ill in an intensive care unit: A systematic review and meta-analysis. BMC Geriatr.21, 339. 10.1186/s12877-021-02276-w (2021). 10.1186/s12877-021-02276-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang, T. et al. Prevalence and prognostic value of preexisting sarcopenia in patients with mechanical ventilation: A systematic review and meta-analysis. Crit. Care26, 140. 10.1186/s13054-022-04015-y (2022). 10.1186/s13054-022-04015-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kou, H. W. et al. Sarcopenia is an effective predictor of difficult-to-wean and mortality among critically ill surgical patients. PLoS One14, e0220699. 10.1371/journal.pone.0220699 (2019). 10.1371/journal.pone.0220699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang, H. et al. Prevalence and mortality risk of low skeletal muscle mass in critically ill patients: An updated systematic review and meta-analysis. Front. Nutr.10, 1117558. 10.3389/fnut.2023.1117558 (2023). 10.3389/fnut.2023.1117558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salucci, S. Nutrition and regulation of muscle protein synthesis. Nutrients15. 10.3390/nu15184017 (2023). [DOI] [PMC free article] [PubMed]
- 28.Rogeri, P. S. et al. Crosstalk between skeletal muscle and immune system: Which roles do IL-6 and glutamine play?. Front. Physiol.11, 582258. 10.3389/fphys.2020.582258 (2020). 10.3389/fphys.2020.582258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sklar, M. C. et al. Association of low baseline diaphragm muscle mass with prolonged mechanical ventilation and mortality among critically ill adults. JAMA Netw. Open3, e1921520. 10.1001/jamanetworkopen.2019.21520 (2020). 10.1001/jamanetworkopen.2019.21520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown, C. J., Friedkin, R. J. & Inouye, S. K. Prevalence and outcomes of low mobility in hospitalized older patients. J. Am. Geriatr. Soc.52, 1263–1270. 10.1111/j.1532-5415.2004.52354.x (2004). 10.1111/j.1532-5415.2004.52354.x [DOI] [PubMed] [Google Scholar]
- 31.Silva-Gutierrez, A. et al. Characterization of muscle mass, strength and mobility of critically ill patients with SARS-CoV-2 pneumonia: Distribution by sex, age, days on mechanical ventilation, and muscle weakness. Front. Physiol.14, 1095228. 10.3389/fphys.2023.1095228 (2023). 10.3389/fphys.2023.1095228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah, F. A. et al. Bidirectional relationship between cognitive function and pneumonia. Am. J. Respir. Crit. Care Med.188, 586–592. 10.1164/rccm.201212-2154OC (2013). 10.1164/rccm.201212-2154OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tessier, A. J., Wing, S. S., Rahme, E., Morais, J. A. & Chevalier, S. Association of low muscle mass with cognitive function during a 3-year follow-up among adults aged 65 to 86 years in the Canadian longitudinal study on aging. JAMA Netw. Open5, e2219926. 10.1001/jamanetworkopen.2022.19926 (2022). 10.1001/jamanetworkopen.2022.19926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee, H. W. et al. Association between successful weaning from nasogastric tube feeding and thoracic muscle mass in patients with aspiration pneumonia. Medicine (Baltimore)102, e34298. 10.1097/MD.0000000000034298 (2023). [DOI] [PMC free article] [PubMed]
- 35.Achamrah, N. et al. Comparison of body composition assessment by DXA and BIA according to the body mass index: A retrospective study on 3655 measures. PLoS One13, e0200465. 10.1371/journal.pone.0200465 (2018). 10.1371/journal.pone.0200465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaitovich, A. et al. ICU admission muscle and fat mass, survival, and disability at discharge: A prospective cohort study. Chest155, 322–330. 10.1016/j.chest.2018.10.023 (2019). 10.1016/j.chest.2018.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Islam, S. et al. Fully automated deep-learning section-based muscle segmentation from CT images for sarcopenia assessment. Clin. Radiol.77, e363–e371. 10.1016/j.crad.2022.01.036 (2022). 10.1016/j.crad.2022.01.036 [DOI] [PubMed] [Google Scholar]
- 38.Bruno, R. R. et al. The Clinical Frailty Scale for mortality prediction of old acutely admitted intensive care patients: A meta-analysis of individual patient-level data. Ann. Intensive Care13, 37. 10.1186/s13613-023-01132-x (2023). 10.1186/s13613-023-01132-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to privacy and confidentiality concerns but are available from the corresponding author on reasonable request.