Abstract
Human T-cell leukemia virus type 1 (HTLV-1) has been shown to be the etiologic agent of adult T-cell leukemia (ATL), but the in vivo mechanism by which the virus causes the malignant transformation is largely unknown. In order to investigate the mechanisms of HTLV-1 leukemogenesis, we developed a rat model system in which ATL-like disease was reproducibly observed, following inoculation of various rat HTLV-1-immortalized cell lines. When previously established cell lines, F344-S1 and TARS-1, but not TART-1 or W7TM-1, were inoculated, systemic multiple tumor development was observed in adult nude (nu/nu) rats. FPM1 cells, newly established from a heterozygous (nu/+) rat syngeneic to nu/nu rats, caused transient tumors only at the injection site in adult nu/nu rats, but could progressively grow in newborn nu/nu rats and metastasize in lymph nodes. The derivative cell line (FPM1-V1AX) serially passed through newborn nu/nu rats acquired the potency to grow in adult nu/nu rats. These results indicated that only some with additional changes but not all of the in vitro HTLV-1-immortalized cell lines possessed in vivo tumorigenicity. Using the syngeneic system, we further showed the inhibition of tumor development by transferring splenic T cells from immunized rats, suggesting the involvement of T cells in the regression of tumors. This novel and reproducible nude rat model of human ATL would be useful for investigation of leukemogenesis and antitumor immune responses in HTLV-1 infection.
Human T-cell leukemia virus type 1 (HTLV-1) is etiologically associated with human adult T-cell leukemia (ATL), a chronic progressive neurological disorder termed HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) (7, 12, 32, 34), and various other human diseases (10, 24, 26, 30). Examination of the viral nucleotide sequences among different disease groups has not revealed any specific determinants that distinguish a particular HTLV-1-associated disease (4, 22, 48). Thus, it is speculated that a primary determinant of HTLV-1-associated disease is host related. HTLV-1 has been shown to activate and immortalize human T cells in vitro, resulting in polyclonal proliferation of infected cells and subsequent oligoclonal or monoclonal growth (6, 47). Several lines of evidence suggest that the viral transcription factor Tax contributes to the immortalization of T cells in vitro and in vivo (8, 39). Moreover, transgenic animals carrying the tax gene develop several types of tumors (9, 11, 45). These findings suggest that Tax plays an important role in HTLV-1-associated leukemogenesis.
Despite the apparent transforming ability of HTLV-1 under experimental conditions, most HTLV-1 carriers are asymptomatic. One explanation for this is that HTLV-1 is controlled by host immunity in most carriers, as is the case in many other viruses. It has been noticed that the response of cytotoxic T lymphocytes (CTLs) to HTLV-1 is extremely high in HAM/TSP patients but low in ATL patients (16, 18, 19, 33). Since HTLV-1-specific CTLs can recognize HTLV-1 Tax antigen and lyse ATL cells in vitro (17), it is reasonable to assume that the low CTL activity in ATL patients may result in uncontrolled proliferation of ATL cells in vivo. Another explanation for the low prevalence of ATL among HTLV-1 carriers is the in vivo evolution of HTLV-1-infected cells, since various mutations are observed in ATL cells (3, 35).
ATL exhibits a variety of clinical forms, including acute, chronic, smoldering, and lymphoma types, suggesting that there are several steps in the development of ATL (21, 46). Such multistep tumor development in HTLV-1 infection may not only reflect naturally occurring mutations but may also be influenced by the interplay between the proliferative ability of virus-infected cells and host immune response. Therefore, to investigate HTLV-1-mediated leukemogenesis, it is important to develop a suitable animal model in which a reproducible growth of leukemic cells can be achieved, which in turn can be monitored by immunological analysis.
HTLV-1 can immortalize simian, feline, rat, and rabbit lymphocytes in vitro (1, 13, 29). It is also known that HTLV-1 can infect experimental animals, such as rabbits, monkeys, and rats (1, 28, 31, 37). Using these susceptible animals, several animal models have been developed to study HTLV-1-associated diseases. The HAM/TSP-like disease model in rats of the WKA strain is well established and has been used to dissect the pathogenic mechanisms of the disease (14, 23). On the other hand, a few ATL model systems have been established so far by using rabbits and rats, but their utility is limited. For instance, the rabbit ATL model shows a reproducible development of ATL-like disease in adult animals (36), but few immunological studies can be performed with this animal, mainly because of the difficulty in obtaining inbred strains of rabbits. As for the rat models, the development of ATL-like disease was observed only in newborn animals with a very short period of disease onset (43), making it difficult to perform oncological and immunological studies at the same time. Variability in the incidence of the disease may also limit its utility (31).
In this study, we investigated the in vivo growth ability of HTLV-1-immortalized rat cell lines inoculated into nude rats. Our results showed that depending on the cell line, the nude rat exhibited distinct features of leukemic cell growth and that some of these cell lines showed persistent in vivo tumor growth in adult nude rats. We further demonstrated that splenocytes from immunocompetent syngeneic rats that had been immunized with HTLV-1-infected cells inhibited the growth of tumor cells in nude rats, indicating the importance of T cells in the rejection of leukemic cells. Our nude rat model of human ATL would be useful for investigation of leukemogenesis as well as antitumor immune responses in HTLV-1 disease.
MATERIALS AND METHODS
Animals.
Female F344/N Jcl-rnu/rnu (nu/nu) rats and F344/N Jcl-rnu/+ (nu/+) rats were purchased from Clea Japan, Inc. (Tokyo, Japan). Female F344/Slc (F344) rats and WKAH/HKmSlc (WKA) rats were purchased from SLC Japan (Shizuoka, Japan). All rats were maintained at the experimental animal facilities of Tokyo Medical and Dental University. The experimental protocol was approved by the Animal Ethics Review Committee of our university.
Cell lines.
An HTLV-1-immortalized cell line, FPM1, was established in our laboratory by cocultivating thymocytes of a nu/+ rat with HTLV-1-producing human cell line MT-2, which was treated with mytomicin (50 μg/ml) for 30 min at 37°C. The cells were maintained in RPMI 1640 with 10% heat-inactivated fetal calf serum (FCS; Whittaker, Walkersville, Md.), penicillin, and streptomycin. Ten units of interleukin 2 (IL-2) per milliliter (Shionogi, Osaka, Japan) was added at the beginning of coculture. Cells were eventually freed from exogenous IL-2. An HTLV-1-negative simian virus 40 (SV40)-transformed rat kidney cell line (FPM-SV) was established from kidney cells of a nu/+ rat in our laboratory. Briefly, kidney cells cultured for 1 week were infected with SV40 at 37°C for 1 h and then washed and cultured for 3 weeks, with replacement of culture medium twice a week. A focus growing in the culture was picked up and sequentially expanded up to a stable line. SV40 was kindly provided by S. Sugano (University of Tokyo, Tokyo, Japan). TARS-1, TART-1, and F344-S1 are rat lymphoid cell lines previously established from WKA or F344 rats (14, 43). An HTLV-1-infected rat cell line, W7TM-1, was also established from thymocytes of a WKA rat, as described previously (41). An HTLV-1-producing human cell line, MT-2, was also used (27). These cells were maintained in RPMI 1640 containing 10% FCS and antibiotics. A total of 2 × 106 of the cells described above were inoculated subcutaneously or intraperitoneally into newborn rats within 24 h of birth. Furthermore, 107 cells of each cell line were subcutaneously, intraperitoneally, or intravenously inoculated into 4-week-old rats.
YAC1 and P815 cell lines were used as a positive and negative control targets, respectively, in a natural killer (NK) assay.
Measurement of growth of subcutaneously inoculated HTLV-1-immortalized cells.
The growth of a subcutaneous tumor was measured once per week and recorded as the longest surface length (a [millimeters]) and width (b [millimeters]). Tumor volume (V [cubic millimeters]) was calculated according to the formula V = (a × b2) × 1/2, as described previously (5).
51Cr-release cytotoxicity assay.
NK activities against various rat cell lines were measured by a 6-h 51Cr-release assay at various effector/target (E/T) ratios as described previously (2). Nylon wool-passed splenocytes from nu/nu rats were used as NK effector cells. Specific cytotoxicity was calculated as [(experimental 51Cr release − spontaneous 51Cr release)/(maximum 51Cr release − spontaneous 51Cr release)] × 100%. CTL activities of splenic T cells in FPM1-immunized rats were also examined with 51Cr-labeled FPM1-V1AX or FPM-SV cells as a target.
Histological examination of metastases of HTLV-1-immortalized cells.
Rats were sacrificed after 10 weeks of inoculation, and different organs were excised. In some cases, these organs were excised within 24 h after natural death of the animal. Tumor nodules in these organs were first inspected macroscopically. The excised organs were stored as paraffin blocks following formalin fixation or as freshly frozen blocks with Tissue-Tek O.C.T. compound (Sakura Finetechnical Co., Ltd., Tokyo, Japan) at −80°C. Thinly sliced specimens of paraffin blocks were stained with hematoxylin and eosin and examined under the microscope. Immunohistologic staining was performed with thinly sliced specimens from the frozen blocks and the Envision system (DAKO, Glostrup, Denmark) with anti-rat IL-2 receptor α-chain monoclonal antibody (MAb) (Chemicon International, Inc., Temecula, Calif.), anti-rat CD4 MAb, or anti-HTLV-1 Tax MAb Lt-4 (42) as the primary antibody.
PCR for detection of HTLV-1 provirus.
Genomic DNA was isolated from various organs, and 1 μg of DNA was subjected to PCR for the amplification of the px region of HTLV-1 provirus as described previously (20). The following primers were used: px1 (5′-CCCACTTCCCAGGGTTTGGACAGAGTCTTC-3′), px2 (5′-CGGATACCCAGTCTACGTGTTTGGAGACTGT-3′), px3 (5′-GAGCCGATAACGCGTCCATCGATGGGGTCC-3′), and px4 (5′-GGGGAAGGAGGGGAGTCGAGGGATAAGGAA-3′). To identify the genomic sequence flanking the 3′ end of HTLV-1 provirus, we performed inverse PCR as described previously (38). Briefly, 1 μg of genomic DNA from FPM1 was digested with Sau3AI (Takara, Kyoto, Japan) and then ligated with T4 DNA ligase (New England Biolabs, Beverly, Mass.) to induce self-ligation. Ligated DNA was then digested with SacII (Takara) to eliminate the circular DNA that originated from 5′ proviral DNA. Using this DNA as a template, first-step PCR was performed with the primer pair U5-1 (5′-AAGCCGGCAGTCAGTCGTGA-3′) and U5-2 (5′-AAGTACCGGCAACTCTGCTG-3′) followed by the second-step PCR with the primer pair U5-3 (5′-GAAAGGGAAAGGGGTGGAAC-3′) and U5-4 (5′-CCAGCGACAGCCCATTCTAT-3′). The amplified fragments were subjected to sequence analysis by the dideoxy method with the DNA Sequence kit (Applied Biosystems, Foster City, Calif.) and automatic sequencer 377 (Applied Biosystem). Based on the sequence flanking the 3′ end of HTLV-1 provirus, a primer (FPM1-Gen1 [5′-TGCCCTGGTCATGGTGTCTC-3′]) was designed to amplify the integration site of the virus in FPM1 cells. PCR amplification was performed with the primer set FPM1-Gen1 and U5-4.
Transplantation of splenic T cells into nude rats.
Four-week-old nu/+ rats were intraperitoneally inoculated with 2 × 107 FPM1 cells. After 4 weeks, 107 T-cell-enriched splenocytes were isolated by passage through a nylon wool column and then were intraperitoneally injected into 4-week-old nu/nu rats that were simultaneously inoculated subcutaneously with 2 × 107 FPM1-V1AX cells. The nu/nu rats inoculated with FPM1-V1AX alone or with splenocytes from age-matched naive nu/+ rats served as a control. The size of each subcutaneous tumor was measured every week.
RESULTS
In vivo tumorigenicity of established HTLV-1-infected cell lines.
To assess the in vivo growth ability of five previously established cell lines infected with HTLV-1, including F344-S1, TARS-1, TART-1, W7TM-1, and MT-2, we inoculated 2 × 106 or 1 × 107 cells of each line into newborn or 4-week-old rats, respectively. As shown in Table 1, F344-S1 and TARS-1 cells progressively and systemically grew and were distributed in adult nu/nu rats irrespective of the route of inoculation.
TABLE 1.
In vivo tumorigenicity of established HTLV-1-transformed cell lines
| Cell line | Origin | Recipient (age) | No. of rats | Inoculation routea | Finding(s)b |
|---|---|---|---|---|---|
| F344-S1 | F344 | F344 (NBc) | 4 | i.p. | NS |
| F344 (4 wk) | 2 | s.c. | NS | ||
| nu/nu (4 wk) | 5 | s.c. | Progressive s.c. tumor with multiple metastases | ||
| nu/nu (4 wk) | 1 | i.p. | Progressive i.p. multiple tumors | ||
| nu/nu (4 wk) | 1 | i.v. | Progressive systemic multiple tumors | ||
| TARS-1 | WKAH | WKAH (4 wk) | 5 | s.c. | Transient s.c. tumor (1 wk)d |
| WKAH (4 wk) | 1 | i.p. | NS | ||
| nu/nu (4 wk) | 3 | s.c. | Progressive s.c. tumor with lung metastasis | ||
| nu/nu (4 wk) | 1 | i.p. | Progressive i.p. multiple tumors | ||
| nu/nu (4 wk) | 1 | i.v. | Progressive systemic multiple tumors | ||
| TART-1 | WKAH | WKAH (NB) | 9 | s.c. | NS |
| nu/nu (4 wk) | 1 | s.c. | NS | ||
| W7TM-1 | WKAH | WKAH (NB) | 7 | s.c. | NS |
| nu/nu (4 wk) | 1 | s.c. | NS | ||
| MT-2 | Human | nu/+ (4 wk) | 4 | i.v. | NS |
| nu/nu (4 wk) | 4 | i.v. | NS | ||
| F344 (4 wk) | 4 | i.p. | NS |
i.p., intraperitoneal; s.c., subcutaneous; i.v., intravenous.
Autopsy was performed 4 to 10 weeks after inoculation. NS, no symptoms.
NB, newborn.
Tumor cell growth was observed for the first week after inoculation.
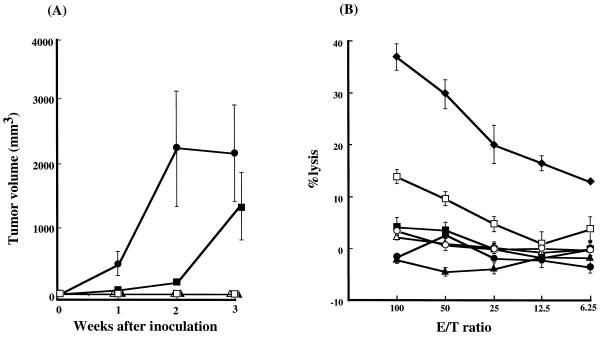
In case of subcutaneous inoculation, a continuous growth of subcutaneous tumors was observed in rats inoculated with F344-S1 or TARS-1 cells (Fig. 1A). Out of five F344-S1-inoculated rats, one rat died after 3 weeks of inoculation, while euthanasia was induced in the other four rats after 3, 4, 7, and 8 weeks of inoculation due to the generalized severe weakness. One of these rats suffered from dysbasia, while another showed severe jaundice. On the other hand, all TARS-1-inoculated rats survived. They were sacrificed at 10 weeks after inoculation and subjected to autopsy. A massive growth of inoculated cells was observed in T-cell-deficient nu/nu rats (Fig. 2a and d). In contrast, F344-S1 and TARS-1 cells did not grow in either newborn or 4-week-old adult syngeneic rats, suggesting the involvement of T cells in the inhibition of tumor cell growth. As summarized in Table 1, two other rat cell lines, TART-1 and W7TM-1, were not tumorigenic in either nu/nu rats or newborn syngeneic rats. Furthermore, human MT-2 cells did not grow in nu/nu rats or immune-competent F344 rats. We also assessed the NK cell sensitivity of the above cell lines. As shown in Fig. 1B, none of the rat cell lines used were significantly lysed by splenocytes derived from nu/nu rats, whereas NK-sensitive YAC1 and MT-2 cells were effectively killed by the same effector cells. These results indicated that NK cells could be responsible for the rejection of MT-2 cells, but are less likely to be responsible for the rejection of TART-1 and W7TM-1 cells in nu/nu rats.
FIG. 1.
Cell line differences in tumorigenicity in adult nu/nu rats and susceptibility of NK cells. (A) Four-week-old female nu/nu rats were subcutaneously inoculated with 107 F344-S1 (●) or TARS-1 (■), W7TM-1(▴), TART1(▵), and MT-2(□) cells. The tumor size was measured once every week and expressed in cubic millimeters by the formula described in Materials and Methods. Results are indicated as means ± standard deviations in each group of two or three rats. Similar results were obtained in two independent experiments. (B) F344-S1(●), TARS-1(■), W7TM-1(▴), TART1(▵), MT-2(□), P815(○), and YAC1 (⧫) cells were labeled with 51Cr for 1 h and used as target cells. Nylon wool-passed splenocytes from nu/nu rats were used as effectors at various E/T ratios, as indicated. Results are indicated as mean percent lysis ± standard deviation.
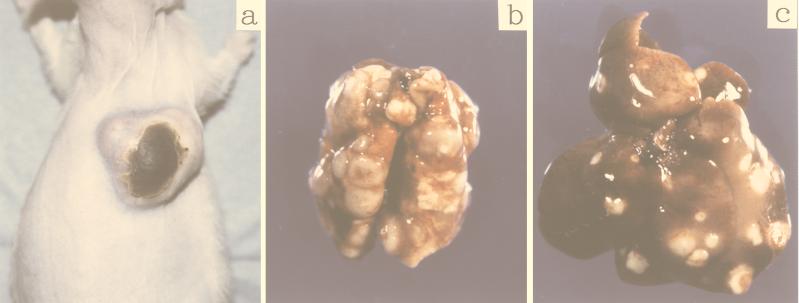
FIG. 2.
Macroscopic examination of a representative 7-week-old nu/nu rat after 3 weeks of subcutaneous inoculation of F344-S1 cells (a to c) and another representative 14-week-old nu/nu rat after 10 weeks of subcutaneous inoculation of TARS-1 cells (d and e). (a) Note the large tumor at the site of inoculation (solid arrow) and the appearance of several skin rashes (open arrow). (b) Note the hypertrophied axillary lymph node (closed arrow) and several other nodules at the site of skin rashes (open arrow). (c) Metastatic tumors in the lungs (arrow). (d) Note the progressive growth of subcutaneous tumor cells (arrow). (e) Metastatic tumors in the lungs (arrow).
Metastasis of tumor cells in adult nude rats.
At autopsy of rats inoculated subcutaneously with F344-S1 cells, we observed tumor nodules in the lungs, liver, spleen, spinal cord, ovaries, and lymph nodes and found multiple spotty subcutaneous metastases in the skin (Fig. 2a, b, and c). Histological examination showed a massive infiltration of tumor cells in the lungs and lymph nodes (Fig. 3). In one rat inoculated with TARS-1 cells subcutaneously, we found a number of tumor nodules in the lung (Fig. 2e). However, there were no visible metastases in other two rats inoculated with TARS-1 except for nodules at the injection site (Fig. 2d). We also assessed the tissue distribution of HTLV-1 provirus DNA in rats inoculated with these two cell lines by using nested PCR with pairs of primers that amplified fragments in the px region. As shown in Table 2, in three F344-S1-inoculated rats, HTLV-1 provirus DNA was detected in all tissues examined, except in the submandibular gland of one rat. In TARS-1-inoculated rats, HTLV-1 provirus DNA was detected in the heart (2 of 3 rats), lungs (3 of 3), livers (3 of 3), spinal cord (2 of 3), bone marrow (3 of 3), and peripheral blood (2 of 3). These results indicated that the inoculated tumor cells and/or secondarily infected recipient cells could distribute even in tissues without visible metastases.
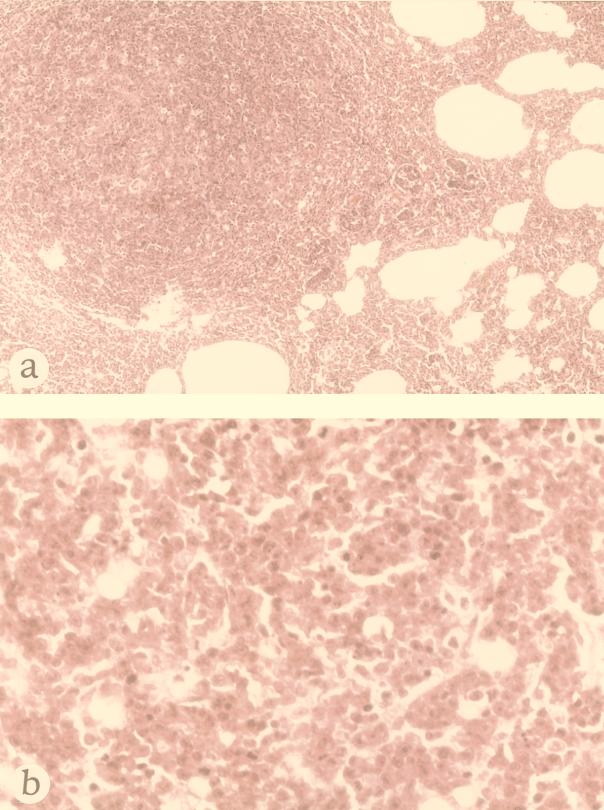
FIG. 3.
Histological examination of a representative tumor detected in a 7-week-old nu/nu rat after 3 weeks of subcutaneous inoculation of F344-S1 cells. Hematoxylin-eosin staining. (a) Low magnification of a tumor in the lung (×75). (b) High magnification of tumor cells in the lymph node. Note the polygonal tumor cells which contain ample cytoplasm and a large nucleus with a prominent nucleolus (×300).
TABLE 2.
Tissue distribution of HTLV-I provirus in rats inoculated with the virus-infected cell linesa
| Cell linesb | No. of rats with HTLV-1/no. tested
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cerebrum | Cerebellum | Submandibular gland | Heart | Lung | Liver | Spleen | Kidney | Spinal cord | Bone marrow | Peripheral blood | |
| F344-S1 | 3/3 | 3/3 | 2/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 | 3/3 |
| TARS-1 | 0/3 | 1/3 | 1/3 | 2/3 | 3/3 | 3/3 | 1/3 | 1/3 | 2/3 | 3/3 | 2/3 |
| FPM1 | 0/3 | 3/3 | 0/3 | 0/3 | 2/3 | 1/3 | 3/3 | 2/3 | 2/3 | 0/3 | 0/3 |
Purified DNA from the indicated tissue was subjected to PCR amplification at 3 to 10 weeks after inoculation.
A total of 107 cells of each cell line were subcutaneously inoculated into nu/nu rats.
Establishment of syngeneic rat HTLV-1-tumor system.
The preferential growth of F344-S1 and TARS-1 cells in T-cell-deficient nu/nu rats suggested that T cells play an important role in the rejection of the tumor. For further analysis of in vivo immune responses against HTLV-1 tumor in nu/nu rats, we attempted to establish a syngeneic experimental system. First, we established an HTLV-1-immortalized cell line from thymocytes of a nu/+ rat, which is syngeneic with nu/nu rats. This cell line, FPM1, expressed rat CD4, CD5, CD25, major histocompatibility class I (MHC-I), and MHC-II (data not shown). This phenotype resembles that of human ATL cells.
In the next step, FPM1 cells were subcutaneously inoculated into 4-week-old adult nu/nu rats followed by evaluation of the growth of tumor cells at the inoculation site. Although we observed a growth of subcutaneous nodules in the first 2 weeks, these lesions diminished in size afterward and eventually disappeared. Furthermore, no apparent distant metastastic tumors were detected in these rats. We next assessed the tissue distribution of HTLV-1 provirus DNA in these rats by the px-specific PCR method. The provirus was detected in the cerebellum (3 of 3 rats), lungs (2 of 3), spleen (3 of 3), kidneys (2 of 3), and spinal cord (2 of 3) (Table 2). These results suggested that FPM1 cells were able to reach several organs, although visible metastases were not evident.
In vivo tumorigenicity of a series of FPM1 cells.
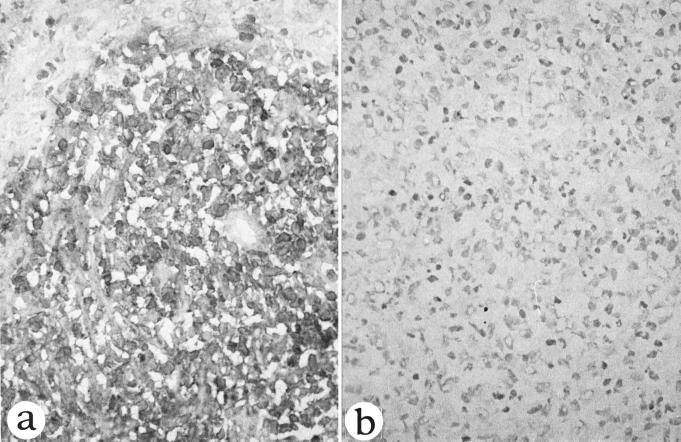
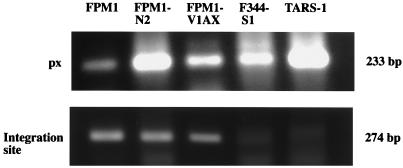
Since FPM1 cells did not form metastatic lesions in any organ in adult nu/nu rats, we next inoculated these cells subcutaneously in newborn nu/+ and nu/nu rats. In newborn nu/+ rats, no growth of tumor cells was noted at the inoculation site. In contrast, inoculated cells formed solid nodules in newborn nu/nu rats and tumor lesions continued to grow for 2 weeks. After that period, the rats were sacrificed because they became very weak. Immunohistological analysis showed that infiltrated tumor cells strongly expressed rat IL-2 receptor (Fig. 4). These tumor cells were weakly positive for rat CD4 and HTLV-1 tax (data not shown). In the next step, we established a cell line (FPM1-N2) from the subcutaneous masses and inoculated these cells subcutaneously into newborn nu/+ and nu/nu rats. Although the cells did not grow in any of five newborn nu/+ rats, all four nu/nu rats developed subcutaneous nodules. Three of the rats died within 2 weeks of inoculation, and the remaining rat was sacrificed. At autopsy, we found a massive hypertrophy of systemic lymph nodes and isolated the cell lines from axillary lymph nodes (FPM1-V1AX). These results are summarized in Table 3. PCR analyses of the cellular flanking region of HTLV-1 confirmed that the integration site in these cell lines was similar to that of FPM1 (Fig. 5).
FIG. 4.
Immunohistological staining of a subcutaneous tumor in a 3-week-old nu/nu rat subcutaneously inoculated with FPM1 cells within 24 h after birth. (a) Most tumor cells are positive for IL-2 receptor α (×300). (b) The same tissue stained with normal mouse serum (×300).
TABLE 3.
In vivo tumorigenicity of a series of FPM1 cell lines
| Cell line | Origin | Recipient (age) | No. of rats | Inoculation routea | Finding(s)b |
|---|---|---|---|---|---|
| FPM1 | In vitro-transformed thymocytes | nu/+ (NBc) | 2 | s.c. | NS |
| nu/nu (NB) | 2 | s.c. | Progressive tumor | ||
| nu/+ (NB) | 5 | i.p. | NS | ||
| nu/nu (NB) | 3 | i.p. | Death (2 wk)d | ||
| nu/nu (4 wk) | 4 | s.c. | Transient tumor | ||
| nu/nu (4 wk) | 2 | i.p. | NS | ||
| FPM1-N2 | s.c. tumor | nu/+ (NB) | 5 | s.c. | NS |
| nu/nu (NB) | 4 | s.c. | Progressive s.c. tumor with metastasis in lymph nodes | ||
| FPM1-V1AX | Axillary lymph node | nu/nu (4 wk) | 6 | s.c. | Progressive s.c. tumor with multiple systemic metastasis |
s.c., subcutaneous; i.p., intraperitoneal.
Autopsy was performed 2 to 10 weeks after inoculation. NS, no symptoms.
NB, newborn.
The rats died within 2 weeks after inoculation.
FIG. 5.
Detection of the unique flanking region of HTLV-1 provirus in a series of FPM1 cells. Genomic DNA (0.5 μg) was obtained from rat HTLV-1-infected cell lines and was subjected to PCR amplification with primer pair px1 and px4 (px) or U5-4 and Gen1 (the integration site). The amplified products were separated on 2% agarose gel and stained with ethidium bromide.
Since FPM1-V1AX cells were isolated from metastatic lymph nodes, we examined in the next step if these cells formed metastases in adult nude rats. For this purpose, we inoculated FPM1-V1AX cells subcutaneously in six adult nu/nu rats. All six rats developed subcutaneous tumors (Fig. 6a). Two rats died within 4 weeks of inoculation, while the other four were sacrificed at 3 or 4 weeks. At autopsy, most rats developed metastases, preferably in the liver (5 of 6), lymph nodes (5 of 6), and lungs (4 of 6), as shown in Fig. 6. Furthermore, we also found metastases in the kidneys (1 of 6 rats) and in the spleens (2 of 6).
FIG. 6.
Macroscopic examination of nu/nu rats inoculated subcutaneously with FPM1-V1AX cells. (a) Growth of subcutaneous tumors in an 8-week-old nu/nu rat after 4 weeks of inoculation. (b and c) Metastasis of the tumor cells observed in lungs (b) and liver (c) of the same rat.
Regression of growth of FPM1-V1AX by splenocytes from FPM1-immunized rats.
Since the FPM1-V1AX cell line provided us with a syngeneic HTLV-1 tumor system that could be evaluated macroscopically, we next assessed the in vivo significance of T-cell immunity against HTLV-1 tumor. For this purpose, we examined if spleen cells from immunized rats can inhibit the growth of FPM1-V1AX cells in nu/nu rats. T cells were isolated from spleens of nu/+ rats that had been intraperitoneally inoculated with FPM1. These T cells were injected intraperitoneally into nu/nu rats at the same time as subcutaneous inoculation of FPM1-V1AX cells. As shown in Fig. 7A, significant suppression of tumor growth was observed in these rats in the first week of inoculation, compared with other groups of FPM1-V1AX-inoculated rats which were untreated or treated with naive T cells. After 2 weeks of inoculation, tumors in the immunized T-cell-inoculated rats were completely diminished. At the same period, significant tumor regression was also observed in rats treated with naive T cells, whereas the subcutaneous tumors continued to grow in untreated rats. There were no metastatic lesions in the tissues of the rats with tumor regression, in contrast to the visible nodules in the lungs and livers of tumor-bearing rats. Thus, both immunized and naive T cells induced tumor regression, but the immunized cells acted earlier and more efficiently. We also determined whether the T cells isolated from the immunized rats have CTL activities specific to FPM1-V1AX cells by using the 51Cr release assay. Our results showed that the uncultured T cells from immunized rats effectively lysed 51Cr-labeled FPM1-V1AX cells, but not FPM-SV cells (Fig. 7B). Splenic T cells from naive nu/+ rats did not have detectable levels of CTL activity against FPM1-V1AX. These results suggested that T-cell populations, especially the CTLs expanded by immunization, played critical roles in the regression of HTLV-1-infected cells.
FIG. 7.
Regression of the growth of FPM1-V1AX cells induced by FPM1-immunized T cells. (A) T cells were isolated from nu/+ rats that had been inoculated with FPM1 or age-matched naive nu/+ rats. Four-week-old nu/nu rats were subcutaneously inoculated with 2 × 107 FPM1-V1AX cells alone (■) or simultaneously with intraperitoneal inoculation of 107 of the immunized (●) or naive (○) T cells. The tumor size was measured once every week and expressed in cubic millimeters by the formula described in Materials and Methods. Results are indicated as means ± standard deviations in each group of two or three rats. Similar results were obtained in three independent experiments. (B) FPM1-V1AX (circles) or FPM-SV (squares) cells were labeled with 51Cr for 1 h and used as target cells. Nylon wool-passed splenocytes from FPM1-immunized nu/+ rats (open symbols) or naive nu/+ rats (closed symbols) were used as effectors at various E/T ratios, as indicated. Results are indicated as mean percent lysis ± standard deviation.
DISCUSSION
In this study, we established a reproducible ATL animal model by using HTLV-1-immortalized rat T-cell lines and T-cell-deficient nude rats. In adult nu/nu rats, we demonstrated that a previously established T-cell line, F344-S1, induced severe clinical manifestations characterized by multiple systemic metastasis of tumor cells within 2 weeks of inoculation. It is noteworthy that F344-S1 induced cutaneous erythema associated with the subcutaneous infiltration of tumor cells, similar to ATL cells, which also often exhibit affinity to the skin (44). Among the rat cell lines utilized in the present study, F344-S1, TARS-1, and FPM1 caused visible tumor development, whereas W7TM-1 and TART-1 did not. It is not clear what determined the in vivo tumorigenicity of these cell lines. Previous reports by others and our present results indicated the involvement of NK cells in the rejection of MT-2 cells (15). However, this is not the case in the rat cell lines we used, because these cells were minimally susceptive to NK cells regardless of in vivo tumorigenicity (Fig. 1B). The ability to cause metastasis also varied among cell lines. In contrast to F344-S1, TARS-1 only formed limited metastatic lesions, although these cells grew at the site of inoculation. Interestingly, the original and later reports of studies with TARS-1 cells demonstrated multiple metastases in newborn syngeneic rats, but the frequency of the disease in later reports was markedly decreased (23, 31). The discrepancy between the results of the previous and present studies may be due to clonal diversity of the original cell line or the use of different rat strains or rats of different ages.
The newly established FPM1 cell line grew in newborn nude rats, and the derivative subclone (FPM1-V1AX) of this cell line formed metastatic tumors in adult nu/nu rats. FPM1-V1AX cells may acquire certain genetic mutations that are important for in vivo growth of HTLV-1-infected cells. Similar phenotypic changes were reported in TARS-1 and rabbit HTLV-1-transformed cell lines (31, 49). In this regard, Mahana et al. recently reported constitutive phosphorylation of the Vav proto-oncogene in a rabbit cell line with in vivo leukemogenic capability (25). Using our system, we are currently investigating whether the difference between FPM1 and FPM1-V1AX cells could be explained by genetic differences. These studies are important to fully understand the multistep leukemogenesis in HTLV-1 infection.
In addition to studying the mechanisms of leukemogenesis in vivo, our animal model also offers the advantage of investigating the immunologic response against HTLV-1-infected cells in vivo. Tumors developed only in athymic rats, but not in immunocompetent rats, suggesting the importance of T-cell immunity in HTLV-1 tumor formation. Furthermore, we provided evidence for the antitumor effect of T cells obtained from FPM1-immunized nu/+ rats against FPM1-V1AX tumor in nu/nu rats. Since the genetic background of nu/+ rats is identical to that of nu/nu rats, cells derived from nu/+ rats exhibit their antitumor function in nu/nu rats without any allogeneic reaction. The immune T cells effective for tumor regression contained CTL activity against tumor cells. Since previous reports indicated that the HTLV-1-specific CTLs were isolated from the virus-infected rats similarly inoculated with HTLV-1-infected cells (40, 41), such CTL cells could be the main mediators of the rejection of tumor cells in nude rats in the present study. The CTL epitopes important for such rejection of HTLV-1-infected tumors remain to be clarified.
In conclusion, we have established a novel animal model of ATL-like disease, in which lymphoproliferative disease can be reproducibly induced in adult nude rats. The model allows evaluation of the effects of immunological approaches against HTLV-1-associated tumor development. Our model is also useful for dissecting the multistep leukemogenic process of HTLV-1, to analyze anti-HTLV-1 tumor immunity, and to develop effective immunotherapies for HTLV-1-related tumors.
ACKNOWLEDGMENTS
We thank Masao Matsuoka and Ken-ichiro Etoh (Kumamoto University, Kumamoto, Japan) for technical help with the inverse PCR method and Sachiko Seki for excellent technical assistance with histological examinations. We are grateful to Mitsuhiko Yanagisawa and Shu Endo for cooperation with the maintenance of animals at the P3 level facilities. We also thank F. G. Issa (University of Sydney) for careful reading and editing of the manuscript.
This work was supported in part by grants from the Agency of Science and Technology of Japan and the Japan Science and Technology Corporation.
REFERENCES
- 1.Akagi T, Takeda I, Oka T, Ohtsuki Y, Yano S, Miyoshi I. Experimental infection of rabbits with human T-cell leukemia virus type I. Jpn J Cancer Res. 1985;76:86–94. [PubMed] [Google Scholar]
- 2.Brunner K T, Mauel J, Cerottini J C, Chapuis B. Quantitative assay of the lytic action of immune lymphoid cells on 51-Cr-labelled allogeneic target cells in vitro: inhibition by isoantibody and by drugs. Immunology. 1968;14:181–196. [PMC free article] [PubMed] [Google Scholar]
- 3.Cesarman E, Chadburn A, Inghirami G, Gaidano G, Knowles D M. Structural and functional analysis of oncogenes and tumor suppressor genes in adult T-cell leukemia/lymphoma shows frequent p53 mutations. Blood. 1992;80:3205–3216. [PubMed] [Google Scholar]
- 4.Daenke S, Nightingale S, Cruickshank J K, Bangham C R. Sequence variants of human T-cell lymphotropic virus type I from patients with tropical spastic paraparesis and adult T-cell leukemia do not distinguish neurological from leukemic isolates. J Virol. 1990;64:1278–1282. doi: 10.1128/jvi.64.3.1278-1282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enomoto A, Kato K, Yagita H, Okumura K. Adoptive transfer of cytotoxic T lymphocytes induced by CD86-transfected tumor cells suppresses multi-organ metastases of C1300 neuroblastoma in mice. Cancer Immunol Immunother. 1997;44:204–210. doi: 10.1007/s002620050374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gazzolo L, Duc Dodon M. Direct activation of resting T lymphocytes by human T-lymphotropic virus type I. Nature. 1987;326:714–717. doi: 10.1038/326714a0. [DOI] [PubMed] [Google Scholar]
- 7.Gessain A, Barin F, Vernant J C, Gout O, Maurs L, Calender A, de The G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;ii:407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- 8.Grassmann R, Dengler C, Muller-Fleckenstein I, Fleckenstein B, McGuire K, Dokhelar M C, Sodroski J G, Haseltine W A. Transformation to continuous growth of primary human T lymphocytes by human T-cell leukemia virus type I X-region genes transduced by a herpesvirus saimiri vector. Proc Natl Acad Sci USA. 1989;86:3351–3355. doi: 10.1073/pnas.86.9.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grossman W J, Kimata J T, Wong F H, Zutter M, Ley T J, Ratner L. Development of leukemia in mice transgenic for the tax gene of human T-cell leukemia virus type I. Proc Natl Acad Sci USA. 1995;92:1057–1061. doi: 10.1073/pnas.92.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall W W, Liu C R, Schneewind O, Takahashi H, Kaplan M H, Roupe G, Vahlne A. Deleted HTLV-I provirus in blood and cutaneous lesions of patients with mycosis fungoides. Science. 1991;253:317–320. doi: 10.1126/science.1857968. [DOI] [PubMed] [Google Scholar]
- 11.Hinrichs S H, Nerenberg M, Reynolds R K, Khoury G, Jay G. A transgenic mouse model for human neurofibromatosis. Science. 1987;237:1340–1343. doi: 10.1126/science.2888191. [DOI] [PubMed] [Google Scholar]
- 12.Hinuma Y, Nagata K, Hanaoka M, Nakai M, Matsumoto T, Kinoshita K I, Shirakawa S, Miyoshi I. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci USA. 1981;78:6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoshino H, Tanaka H, Shimotohno K, Miwa M, Nagai M, Shimoyama M, Sugimura T. Immortalization of peripheral blood lymphocytes of cats by human T-cell leukemia virus. Int J Cancer. 1984;34:513–517. doi: 10.1002/ijc.2910340414. [DOI] [PubMed] [Google Scholar]
- 14.Ishiguro N, Abe M, Seto K, Sakurai H, Ikeda H, Wakisaka A, Togashi T, Tateno M, Yoshiki T. A rat model of human T lymphocyte virus type I (HTLV-I) infection. 1. Humoral antibody response, provirus integration, and HTLV-I-associated myelopathy/tropical spastic paraparesis-like myelopathy in seronegative HTLV-I carrier rats. J Exp Med. 1992;176:981–989. doi: 10.1084/jem.176.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishihara S, Tachibana N, Okayama A, Murai K, Tsuda K, Mueller N. Successful graft of HTLV-I-transformed human T-cells (MT-2) in severe combined immunodeficiency mice treated with anti-asialo GM-1 antibody. Jpn J Cancer Res. 1992;83:320–323. doi: 10.1111/j.1349-7006.1992.tb00108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobson S, Shida H, McFarlin D E, Fauci A S, Koenig S. Circulating CD8+ cytotoxic T lymphocytes specific for HTLV-I pX in patients with HTLV-I associated neurological disease. Nature. 1990;348:245–248. doi: 10.1038/348245a0. [DOI] [PubMed] [Google Scholar]
- 17.Kannagi M, Matsushita S, Harada S. Expression of the target antigen for cytotoxic T lymphocytes on adult T-cell-leukemia cells. Int J Cancer. 1993;54:582–588. doi: 10.1002/ijc.2910540411. [DOI] [PubMed] [Google Scholar]
- 18.Kannagi M, Sugamura K, Kinoshita K, Uchino H, Hinuma Y. Specific cytolysis of fresh tumor cells by an autologous killer T cell line derived from an adult T cell leukemia/lymphoma patient. J Immunol. 1984;133:1037–1041. [PubMed] [Google Scholar]
- 19.Kannagi M, Sugamura K, Sato H, Okochi K, Uchino H, Hinuma Y. Establishment of human cytotoxic T cell lines specific for human adult T cell leukemia virus-bearing cells. J Immunol. 1983;130:2942–2946. [PubMed] [Google Scholar]
- 20.Kato H, Koya Y, Ohashi T, Hanabuchi S, Takemura F, Fujii M, Tsujimoto H, Hasegawa A, Kannagi M. Oral administration of human T-cell leukemia virus type 1 induces immune unresponsiveness with persistent infection in adult rats. J Virol. 1998;72:7289–7293. doi: 10.1128/jvi.72.9.7289-7293.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawano F, Yamaguchi K, Nishimura H, Tsuda H, Takatsuki K. Variation in the clinical courses of adult T-cell leukemia. Cancer. 1985;55:851–856. doi: 10.1002/1097-0142(19850215)55:4<851::aid-cncr2820550424>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 22.Kinoshita T, Tsujimoto A, Shimotohno K. Sequence variations in LTR and env regions of HTLV-I do not discriminate between the virus from patients with HTLV-I-associated myelopathy and adult T-cell leukemia. Int J Cancer. 1991;47:491–495. doi: 10.1002/ijc.2910470403. [DOI] [PubMed] [Google Scholar]
- 23.Kushida S, Mizusawa H, Matsumura M, Tanaka H, Ami Y, Hori M, Yagami K-I, Kameyama T, Tanaka Y, Yoshida A, Nyunoya H, Shimotohno K, Iwasaki Y, Uchida K, Miwa M. High incidence of HAM/TSP-like symptoms in WKA rats after administration of human T-cell leukemia virus type 1-producing cells. J Virol. 1994;68:7221–7226. doi: 10.1128/jvi.68.11.7221-7226.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LaGrenade L, Hanchard B, Fletcher V, Cranston B, Blattner W. Infective dermatitis of Jamaican children: a marker for HTLV-I infection. Lancet. 1990;336:1345–1347. doi: 10.1016/0140-6736(90)92896-p. [DOI] [PubMed] [Google Scholar]
- 25.Mahana W, Zhao T M, Teller R, Robinson M A, Kindt T J. Genes in the pX region of human T cell leukemia virus I influence Vav phosphorylation in T cells. Proc Natl Acad Sci USA. 1998;95:1782–1787. doi: 10.1073/pnas.95.4.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mann D L, DeSantis P, Mark G, Pfeifer A, Newman M, Gibbs N, Popovic M, Sarngadharan M G, Gallo R C, Clark J, Blattner W. HTLV-I-associated B-cell CLL: indirect role for retrovirus in leukemogenesis. Science. 1987;236:1103–1106. doi: 10.1126/science.2883731. [DOI] [PubMed] [Google Scholar]
- 27.Miyoshi I, Kubonishi I, Yoshimoto S, Akagi T, Ohtsuki Y, Shiraishi Y, Nagata K, Hinuma Y. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981;294:770–771. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura H, Hayami M, Ohta Y, Ishikawa K, Tsujimoto H, Kiyokawa T, Yoshida M, Sasagawa A, Honjo S. Protection of cynomolgus monkeys against infection by human T-cell leukemia virus type-I by immunization with viral env gene products produced in Escherichia coli. Int J Cancer. 1987;40:403–407. doi: 10.1002/ijc.2910400320. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura H, Tanaka Y, Komuro-Tsujimoto A, Ishikawa K, Takadaya K, Tozawa H, Tsujimoto H, Honjo S, Hayami M. Experimental inoculation of monkeys with autologous lymphoid cell lines immortalized by and producing human T-cell leukemia virus type-I. Int J Cancer. 1986;38:867–875. doi: 10.1002/ijc.2910380614. [DOI] [PubMed] [Google Scholar]
- 30.Nishioka K, Maruyama I, Sato K, Kitajima I, Nakajima Y, Osame M. Chronic inflammatory arthropathy associated with HTLV-I. Lancet. 1989;i:441. doi: 10.1016/s0140-6736(89)90038-x. [DOI] [PubMed] [Google Scholar]
- 31.Oka T, Sonobe H, Iwata J, Kubonishi I, Satoh H, Takata M, Tanaka Y, Tateno M, Tozawa H, Mori S, Yoshiki T, Ohtsuki Y. Phenotypic progression of a rat lymphoid cell line immortalized by human T-lymphotropic virus type I to induce lymphoma/leukemia-like disease in rats. J Virol. 1992;66:6686–6694. doi: 10.1128/jvi.66.11.6686-6694.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A, Matsumoto M, Tara M. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;i:1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- 33.Parker C E, Daenke S, Nightingale S, Bangham C R. Activated, HTLV-1-specific cytotoxic T-lymphocytes are found in healthy seropositives as well as in patients with tropical spastic paraparesis. Virology. 1992;188:628–636. doi: 10.1016/0042-6822(92)90517-s. [DOI] [PubMed] [Google Scholar]
- 34.Poiesz B J, Ruscetti F W, Gazdar A F, Bunn P A, Minna J D, Gallo R C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakashita A, Hattori T, Miller C W, Suzushima H, Asou N, Takatsuki K, Koeffler H P. Mutations of the p53 gene in adult T-cell leukemia. Blood. 1992;79:477–480. [PubMed] [Google Scholar]
- 36.Simpson R M, Zhao T M, Hubbard B S, Sawasdikosol S, Kindt T J. Experimental acute adult T cell leukemia-lymphoma is associated with thymic atrophy in human T cell leukemia virus type I infection. Lab Investig. 1996;74:696–710. [PubMed] [Google Scholar]
- 37.Taguchi H, Sawada T, Fukushima A, Iwata J, Ohtsuki Y, Ueno H, Miyoshi I. Bilateral uveitis in a rabbit experimentally infected with human T-lymphotropic virus type I. Lab Investig. 1993;69:336–339. [PubMed] [Google Scholar]
- 38.Takemoto S, Matsuoka M, Yamaguchi K, Takatsuki K. A novel diagnostic method of adult T-cell leukemia: monoclonal integration of human T-cell lymphotropic virus type I provirus DNA detected by inverse polymerase chain reaction. Blood. 1994;84:3080–3085. [PubMed] [Google Scholar]
- 39.Tanaka A, Takahashi C, Yamaoka S, Nosaka T, Maki M, Hatanaka M. Oncogenic transformation by the tax gene of human T-cell leukemia virus type I in vitro. Proc Natl Acad Sci USA. 1990;87:1071–1075. doi: 10.1073/pnas.87.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanaka Y, Isobe A, Masuda M, Tozawa H, Koyanagi Y, Yamamoto N, Shida H. Immunogenicity of human T cell leukemia virus type-I (HTLV-I) antigens for cytotoxic T lymphocytes in the rat system. J Immunol. 1991;147:3646–3652. [PubMed] [Google Scholar]
- 41.Tanaka Y, Tozawa H, Koyanagi Y, Shida H. Recognition of human T cell leukemia virus type I (HTLV-I) gag and pX gene products by MHC-restricted cytotoxic T lymphocytes induced in rats against syngeneic HTLV-I-infected cells. J Immunol. 1990;144:4202–4211. [PubMed] [Google Scholar]
- 42.Tanaka Y, Yoshida A, Takayama Y, Tsujimoto H, Tsujimoto A, Hayami M, Tozawa H. Heterogeneity of antigen molecules recognized by anti-tax1 monoclonal antibody Lt-4 in cell lines bearing human T cell leukemia virus type I and related retroviruses. Jpn J Cancer Res. 1990;81:225–231. doi: 10.1111/j.1349-7006.1990.tb02554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tateno M, Kondo N, Itoh T, Chubachi T, Togashi T, Yoshiki T. Rat lymphoid cell lines with human T cell leukemia virus production. I. Biological and serological characterization. J Exp Med. 1984;159:1105–1116. doi: 10.1084/jem.159.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50:481–492. [PubMed] [Google Scholar]
- 45.Yamada S, Ikeda H, Yamazaki H, Shikishima H, Kikuchi K, Wakisaka A, Kasai N, Shimotohno K, Yoshiki T. Cytokine-producing mammary carcinomas in transgenic rats carrying the pX gene of human T-lymphotropic virus type I. Cancer Res. 1995;55:2524–2527. [PubMed] [Google Scholar]
- 46.Yamaguchi K, Nishimura H, Kohrogi H, Jono M, Miyamoto Y, Takatsuki K. A proposal for smoldering adult T-cell leukemia: a clinicopathologic study of five cases. Blood. 1983;62:758–766. [PubMed] [Google Scholar]
- 47.Yamamoto N, Okada M, Koyanagi Y, Kannagi M, Hinuma Y. Transformation of human leukocytes by cocultivation with an adult T cell leukemia virus producer cell line. Science. 1982;217:737–739. doi: 10.1126/science.6980467. [DOI] [PubMed] [Google Scholar]
- 48.Yoshida M, Osame M, Usuku K, Matsumoto M, Igata A. Viruses detected in HTLV-I-associated myelopathy and adult T-cell leukaemia are identical on DNA blotting. Lancet. 1987;i:1085–1086. doi: 10.1016/s0140-6736(87)90506-x. [DOI] [PubMed] [Google Scholar]
- 49.Zhao T M, Robinson M A, Sawasdikosol S, Simpson R M, Kindt T J. Variation in HTLV-I sequences from rabbit cell lines with diverse in vivo effects. Virology. 1993;195:271–274. doi: 10.1006/viro.1993.1373. [DOI] [PubMed] [Google Scholar]