Summary
Background
Childhood Cancer Survivors (CCSs) are more likely to report sexual dysfunction than people without cancer history. Sexual functioning encompasses more than just sexual dysfunction. The scarcity of information regarding the status and influencing factors of sexual functioning in CCSs, hampers to devise suitable screening or interventions. This review aims to summarize research progress on sexual functioning and associated factors among CCSs.
Methods
This review protocol is registered in PROSPERO(CRD42023427939) and performed according to PRISMA guidelines. From inception to November 15, 2023, a comprehensive search was conducted in PubMed, EMBASE, CINAHL, Web of Science, SCOPUS, PsycINFO, CNKI Database, Wanfang of Chinese Database, SinoMed Database and Cochrane Library on sexual functioning and childhood cancer survivors. Inclusion criteria were English or Chinese studies focusing on sexual functioning and related factors of cancer survivors, who diagnosed with cancer before 18 years old, and were adult and disease-free when participating in the study. Studies were excluded if the focus was on adult cancer patients or without age information.
Findings
395 records were retrieved, and 22 studies were finally included in this review. Results suggest that CCSs experience a substantial burden of sexual issues, including delayed psychosexual development, low satisfaction, and high prevalence of dysfunction. Underlying factors related to sexual functioning of CCSs were identified, including demographic, cancer treatment-related, psychological, and physiological factors. The historical change in research on sexual functioning was summarized.
Interpretation
Research on sexual functioning among CCSs is limited. The extent to which cancer and related treatments affect sexual functioning remains largely unknown. The relationships between various factors and mechanisms underlying sexual functioning need to be confirmed by more rigorous studies to enable effective interventions to be developed.
Funding
None.
Keywords: Psychosexual, Sexual functioning, Sexual dysfunction, Childhood cancer survivors, Review
Research in context.
Evidence before this study
Sexuality in adulthood for CCSs needs more medical attention. To summarize the research progress on sexual functioning and associated factors among CCSs, we performed a systematic search in PubMed, EMBASE, CINAHL, Web of Science, SCOPUS, PsycINFO, CNKI Database, Wanfang of Chinese Database, SinoMed Database and Cochrane Library, using the following search terms “child”/“pediatrics”/“child∗”/“Adolescent” AND “carcinoma”/“cancer"/“neoplasms”/“oncology”/“leukemia∗” AND “Survivors” AND “sexual function"/“Sexual Dysfunction”/“psychosexual function” AND “Risk Factors”. The search yielded only 395 reports. Additionally, a comprehensive review targets sexual functioning among CCSs is scarce, which limits healthcare professionals making appropriate therapeutic decisions for CCSs.
Added value of this study
This review comprehensively summarizes the research evidence related to sexual functioning in CCSs, especially the historical research change, assessment tools of sexual functioning, milestones of psychosexual development, common sexual problems, and prevalence of sexual dysfunction among CCSs. This review also enriched factors on sexual functioning, categorized into four categories of associated factors. Especially the identified psychological factors and psychosexual development characteristics can guide healthcare professionals to design more systematic screening programs and target interventions for CCSs who are at risk of sexual dysfunction.
Implications of all the available evidence
The findings of this review provide detailed information on the historical research change of sexual functioning, as well as the variety, complexity, and severity of sexual functioning among CCSs. We suggest that healthcare professionals should provide more information to children with cancer and their caregivers, including potential risks and adverse effects of treatment on sexual functioning. Additionally, a comprehensive screening program and appropriate interventions are also urgent needs for sexual functioning in CCSs, especially psychological support to address the sexual needs of CCSs.
Introduction
Based on the 2019 data of Global Burden of Disease Study, 291,319 new cases from childhood cancer were documented in 2019 around the world.1 Advances in cancer diagnosis and treatment have led to improved 5-year survival for children with cancer and approximately 80% of these patients will become long-term survivors.2,3 However, as a result of cancer and/or its treatment, childhood cancer survivors (CCSs) are at risk of recurrence, subsequent primary cancers, long-term treatment effects, chronic diseases, various social and socioeconomic consequences, and poor psychological well-being,4,5 all of which demand more attention in survivorship research.
Sexual dysfunction is a common late effect of cancer for CCS. The International Classification of Diseases (ICD-11) and the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) both specify criteria for the classification and diagnosis of sexual dysfunction. Previous studies suggested that as normative physiological and psychological developments are interrupted by cancer and its treatment, CCSs are more likely to report sexual dysfunction, both in male survivors6, 7, 8, 9 and female survivors,7,8,10 compared to people without cancer history. The Children's Oncology Group (COG) Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers delineate risk of sexual dysfunction,11 but do not clearly define associated factors and screening programs for sexual dysfunction.
However, disruptions to development in addition to late effects mean that there are broader implications for sexual functioning in CCSs. Sexual functioning is an important part of general health, influencing an individual's physical, psychosocial, developmental, and emotional well-being.6,12,13 Unlike sexual dysfunction, sexual functioning refers to an individual's physiological and psychological performance in sexual behavior, including sexual desire, ability to reach orgasm, arousal, sexual pleasure, and satisfaction.14 When CCSs enter adolescence and adulthood, they gradually develop romantic relationships, resulting in different sexual and reproductive issues.15 Given the unique nature of CCSs, it is also essential to focus on the psychosexual developmental journey. Disruption of one or more of these components can lead to sexual dysfunction that can negatively impact the quality life of survivors.16
Sexual functioning is also complex and requires normative interaction of multiple components including physiology, psychosexual development, romantic relationship, body image, and desire.17,18 Physiological factors mainly include gonadal tissue damage and endocrine disorders. These issues will interrupt puberty, resulting in various endocrine complications and deficiency of secondary sexual characteristics, which contribute to sexual dysfunction and can even lead to infertility.19 Likewise, a cancer diagnosis in childhood and adolescence coincides with rapid cognitive and psychological developments which should warrant special attention.12,13 Previous studies have shown that up to 25%, 30%, 40% and 70% of CCSs experience global distress,20 anxiety,21,22 depression,21 and post-traumatic stress22 respectively, which are all closely linked to sexual problems.23 Moreover, social difficulties and setbacks in schooling and employment12,24 may impact survivors’ ability to develop intimate relationships and achieve sexual satisfaction. Poor body image also leads to a feeling of dissatisfaction with outward appearance and a perception of reduced attractiveness,25, 26, 27 further contributing to sexual dysfunction in CCS. However, possibly due to a lack of ICD codes for sexual functioning, this topic is often neglected, despite cancer harming their sexual functioning which may last for a lifetime.28
Although previous studies have investigated associated factors of sexual dysfunction among CCSs, however heterogeneity across the studies limits interpretation.7,29 Regarding the associated factors, for example, some studies found that cancer diagnosis of CCSs30 correlated with sexual functioning, but other studies showed no association.23,29 The synthesis of findings from existing studies is crucial to evaluate research progress of sexual functioning in CCSs and summarize the evidence regarding related factors.
At present, three reviews have focused on sexual functioning among CCSs.6,16,17 However, previous review studies included both CCS as well as young adults diagnosed after age 18, limiting their generalizability to CCSs. Besides, two reviews16,17 just focused on sexual dysfunction instead of sexual functioning, possibly because only sexual dysfunction is included in ICD-11 and DSM-5. To address this research gap, we conducted a scoping review to identify and summarize the research evidence on sexual functioning and related factors among CCSs. In particular, this review only targeted CCSs and focused sexual functioning as an outcome to capture different sexual concerns.
Methods
The present review protocol was registered in the PROSPERO (reference number: CRD42023427939) and performed according to the PRISMA guidelines.
Identifying the research questions
The primary research questions influenced by a paucity of current evidence were.
-
1)
What published research exists on sexual functioning in CCSs?
-
2)
What is the status of sexual functioning in CCSs?
-
3)
What factors affect sexual functioning in CCSs?
Two researchers (FNY and QL) independently searched, screened, and extracted data from the included studies between November and December 2023.
Identifying relevant studies
A comprehensive search, from inception of the database to November 15, 2023, was conducted in the following databases: PubMed, EMBASE, CINAHL, Web of Science, SCOPUS, PsycINFO, CNKI Database, Wanfang of Chinese Database, SinoMed Database and Cochrane Library. The search strategy combined MeSH terms, Emtree terms and keywords that were according to each database, shown in Table 1. Further searching included checking the reference lists of selected studies and previous related systematic review articles on sexual functioning, to identify additional relevant articles.
Table 1.
Search Strategy (Taking Pubmed search as an example).
| #1 | “pediatrics" [Mesh] OR “paediatric∗" OR “child" [Mesh] OR “Adolescent" [Mesh] OR “child∗" [Title/Abstract] |
| #2 | (“neoplasms” [Mesh] OR “carcinoma” [Mesh] OR “neoplasms”[Mesh] OR “cancer” [Title/Abstract] OR “oncology” [Title/Abstract] OR “neoplasm∗” [Title/Abstract] OR “carcinoma∗” [Title/Abstract] OR “tumo∗” [Title/Abstract] OR “malignan∗” [Title/Abstract] OR “melanoma” [Title/Abstract] OR “sarcoma” [Title/Abstract] OR “adenocarcinoma∗” [Title/Abstract] OR “glioma∗” [Title/Abstract] OR “lymphoma∗” [Title/Abstract] OR “myeloma∗" [Title/Abstract] OR “leukemia∗” [Title/Abstract] OR “leucaemia∗” [Title/Abstract]) AND (“Survivors” [Mesh] OR “Cancer Survivors” [Mesh] OR “Adult Survivors of Child Adverse Events” [Mesh] OR “long-term survivors” [Title/Abstract] OR “adult survivors” [Title/Abstract]) |
| #3 | “Risk Factors” [Mesh] OR “Risk Assessment” [Mesh] OR “associated factors” [Title/Abstract] OR “relevant factor”[Title/Abstract] OR “factors” [Title/Abstract] OR “Prognosis” [Mesh] OR “predictor∗” [Title/Abstract] OR “prediction” [Title/Abstract] OR “Prevalence” [Mesh] |
| #4 | “Sexual Health” [Mesh] OR “Orgasm” [Mesh] OR “Erectile Dysfunction” [Mesh] OR “Sexual Dysfunction, Physiological” [Mesh] OR “Sexual Dysfunctions, Psychological” [Mesh] OR “sexual function” [Title/Abstract] OR “sexual problem" [Title/Abstract] OR “sexual abnormality” [Title/Abstract] OR “sexual intercourse problem" [Title/Abstract] OR “Ejaculatory disorder∗” [Title/Abstract] OR “Premature ejaculation” [Title/Abstract] OR “Arousal Disorder∗” [Title/Abstract] OR “Hypoactive sexual desire” [Title/Abstract] OR “Orgasmic disorder∗” [Title/Abstract] OR “Sexual pain” [Title/Abstract] OR “psychosexual function” [Title/Abstract] |
| #5 | #1 AND #2 AND #3 AND #4 |
The two research members separately retrieved studies according to the search strategy, screened the list of studies by their titles and abstracts, and read full text of potential studies. Studies that met the eligibility criteria were included in data extraction and analysis. Disagreements and ambiguities were resolved through discussion and consultation with a senior investigator (KYH). Endnote 21 reference manager software was used to collect and organize the search results from all included databases and to remove duplicate articles.
Studies were included according to the eligibility criteria: 1) the study focused on sexual functioning and related factors; 2) original research, including observational studies like cross-sectional, case–control or cohort studies, qualitative studies, mixed methodology, or other research designs; 3) patients were diagnosed with any type of cancer before the age of 18 years, and be an adult and be disease-free at the time when they took part in the study; and 4) the published language was English or Chinese. Studies were excluded if the focus was on adult cancer patients or without age information.
Data extraction
Data was extracted according to the pre-designed standardized data extraction form, including first author, publication time, journal name, study duration, study design, country/location, sample size, age at diagnosis, age at assessment, and measurement tool. Authors of the included studies were contacted where there were inconsistencies or missing information.
Data analysis and presentation
The extracted data were analyzed using descriptive statistics; qualitative data were synthesized using content analysis.31 The main findings were organized and reported in a narrative summary based on the review questions of this scoping review. The extracted data were also presented in a tabular format. We used the Sankey flow diagram to visualize the historical change in research on sexual functioning, which was drawn using the author-made modules in MS Excel 2023. The width of the curve represents the magnitude of the flow, i.e., the number of relevant studies.
Role of the funding source
There was no funding source for this study.
Results
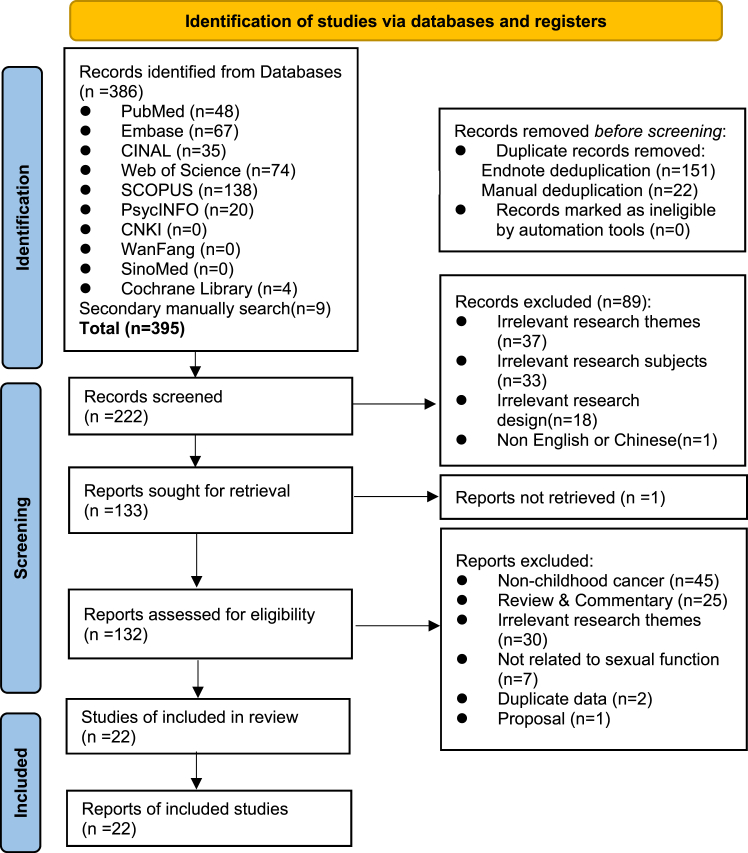
A total of 395 records were retrieved from the databases, of which 22 studies were included in this review. The PRISMA flow diagram presenting the screening and selection process is shown in Fig. 1.
Fig. 1.
PRISMA∗ flow diagram (∗PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses).
Study characteristics
The 22 included studies were published from 2000 to 2022, of which two were qualitative studies,32,33 six were cohort studies,10,23,28,34, 35, 36 remaining were cross-sectional studies. The included studies were conducted in a range of countries, including the United States (n = 13),9,10,23,28,29,32, 33, 34, 35,37, 38, 39, 40 Sweden (n = 3),7,30,41 Finland (n = 2),8,36 Netherlands (n = 1),42 Korea (n = 1),43 Hong Kong (n = 1),44 and Germany (n = 1).45 Sample sizes ranged from 21 to 2546 participants. Five studies focused on male survivors,8,9,28,36,41 two studies focused on female survivors,10,34 and the remaining included both male and female survivors. Except for three studies targeting childhood craniopharyngioma,35 acute lymphoblastic leukemia (ALL)8 and hematopoietic stem cell transplantation (HSCT),36 the remaining included participants diagnosed with all types of cancer. The characteristics of 22 included studies are described in Table 2.
Table 2.
The characteristics of 22 included studies.
| Author | Journal | Country/Study design | Title | Subjects | Sample Size | Age at diagnosis (years) | Age at assessment (years) | Assessment tools |
|---|---|---|---|---|---|---|---|---|
| Relander et al. (2000)41 | Medical and Pediatric Oncology | Sweden; Cross-sectional study | Gonadal and sexual function in men treated for childhood cancer | Male survivors treated during the period 1970–1989, disease-free and off treatment for at least 1 year | 77 male survivors (35% leukemia/lymphoma, 31% brain tumors, 34% others) | Mean:11; Range: 10 months to 17 years | Mean:23.6; Range: 18.6–38.5 | Self-administered questionnaire |
| Van Dijk et al. (2008)42 | Psycho-Oncology | Netherlands; Cross-sectional study | Psychosexual functioning of childhood cancer survivors | Finished treatment at least 5 years ago; between 16 and 40 years old at study | 60 survivors (31 males, 29 females; Acute lymphoblastic leukemia = 27, Acute myeloid leukemia = 5, non-Hodgkin lymphoma = 15, Solid tumors = 11, Brain tumors = 2) | Mean (SD): 8.3 (4.5); Range: 1-16 | Mean (SD): 24.6 (5.3); Range: 18–39 | Psychosexual and Social Functioning Questionnaire |
| Zebrack et al. (2010)37 | Psycho-oncology | Southern California, US; Cross-sectional study | Sexual functioning in young adult survivors of childhood cancer | Off-treatment and disease-free at the study | 599 survivors (282 males, 316 females; Leukemia = 225, Hodgkins' disease = 98, non-Hodgkin's Lymphoma = 54, CNS/Brain Tumors = 79, Solid tumors/soft tissue tumors = 73, Kidney = 25, Other = 43) | Mean (SD): 11.0 (6.0); Range: NR | Mean (SD): 27.0 (5.5); Range: 18-19 | The MOS Sexual Functioning scale |
| Sundberg et al. (2011)30 | European Journal of Cancer | Sweden; Cross-sectional study | Sexual function and experience among long-term survivors of childhood cancer | Diagnosed at ages 0–18 during the period 1985–1999, at least 5 years beyond diagnosis; at least 18 years of age at the study | 224 survivors (108 males, 116 females; 25% CNS tumors, 22% leukemia, 19% lymphoma, and 34% other tumors) vs. 283 general participants | Mean: 9; Range: NR | Mean: 24; Range: 18-37 | A 30 items self-reported questionnaire |
| Bober et al. (2013)23 | Journal of Sexual Medicine | US; cohort study | Sexual function in childhood cancer survivors: A report from project REACH | Survivors of a malignancy other than nonmelanoma skin cancer; 2 years from cancer diagnosis; 1 year after treatment | 291 survivors (141 males, 150 females; Brain tumor = 92, Hodgkins' lymphoma = 67, Leukemia = 64, Sarcoma = 34, Other = 34) | NR | Mean: 27; Range: 18–57 | Swedish Health-Related Quality of Life Survey (Swed-QUAL) |
| Ford et al. (2014)34 | Journal of Clinical Oncology | US; Multicenter cohort study | Psychosexual functioning among adult female survivors of childhood cancer: A report from the childhood cancer survivor study | Diagnosed with cancer between 1970 and 1986; survival at least 5 years since diagnosis | 2178 female survivors (Hodgkin lymphoma = 335, CNS tumor = 206, Non-Hodgkin lymphoma = 116, Leukemia = 723, Bone cancer = 227, Neuroblastoma = 138, Kidney cancer = 241, Soft tissue sarcoma = 192) vs. 408 siblings | NR | Mean: NR; Range: NR | SFQ, Women's Health Questionnaire (WHQ), Sexual Self-Schema (SSS), the Medical Outcomes Survey Short Form-36 |
| Lehmann et al. (2016)38 | Psycho-oncology | US; Cross-sectional | Body issues, sexual satisfaction, and relationship status satisfaction in long-term childhood cancer survivors and healthy controls | 20–40 years old at study; 5–18 years old at diagnosis; non-CNS malignancies; at least 5 years post-diagnosis and off treatment | 87 survivors of non-CNS malignancies (39 males, 48 females, Leukemia = 38, Lymphoma = 27, Solid tumors = 22) vs. 87 healthy control | Mean (SD): 12.1 (3.8); Range: 5–18 | Mean (SD): 27.8 (5.1); Range: 20–40 | The 10-item BIS; Body dissociation subscale of the Scale of Body Connection; GMSEX |
| Haavisto et al. (2016)8 | Cancer | Finland; Cross-sectional study | Sexual function in male long-term survivors of childhood acute lymphoblastic leukemia | Males diagnosed with ALL when they were boys younger than 16 years | 52 male survivors vs. an age and sex-matched control group recruited from the occupational health services | Mean (SD): 4.5 (5.8); Range: 0–15 | Mean (SD): 28.5 (5.8); Range: 25-38 | The Derogatis Interview for Sexual Functioning self-report (DISF-SR) |
| Ritenour et al. (2016)28 | Journal of Sexual Medicine | US and Canada retrospectively; cohort study | Erectile dysfunction in male survivors of childhood cancer-A report from the childhood cancer survivor study | Diagnosis and initial treatment of leukemia, CNS malignancy, Hodgkin's lymphoma, non-Hodgkin's lymphoma, neuroblastoma, soft tissue sarcoma, kidney cancer, or bone cancer; 5 years from diagnosis; resident of the United States or Canada at the time of follow up | 1441 male survivors (Leukemia = 535, CNS tumors = 138, Hodgkin lymphoma = 259, Non-Hodgkin lymphoma = 179, Kidney = 132, Neuroblastoma = 81, Soft tissue sarcoma, Bone cancer = 153) vs. 274 siblings | Mean (SD): NR; Range: 0–21 | Mean (SD): 37.2 (7.3); Range: NR | IIEF |
| Lehmann et al. (2017)39 | Cancer | US; Cross-sectional study | Psychosexual development and satisfaction in long-term survivors of childhood cancer: neurotoxic treatment intensity as a risk indicator | aged 20–40 years old at the study; diagnosed with any malignancy between ages 5–18 years; 5 years after diagnosis | 144 survivors (female = 77, male = 67, Brain tumors = 47, Leukemia = 42, Lymphoma = 31, Solid tumor = 24) vs. 144 US residents' control | Mean (SD): 11.7 (3.8); Range: 5–18 | Mean (SD): 28 (5.3); Range: 20-40 | GMSEX; The Satisfaction with Relationship Status Scale |
| Yoon et al. (2017)43 | Cancer research treatment | Korea; Cross-sectional study | Gonadal and sexual dysfunction in childhood cancer survivors | More than 2 years since treatment; no evidence of recurrence | 105 survivors (57 males, 48 females; Leukemia = 23, Lymphoma = 17, Brain tumors = 18, Solid tumors = 56, Histiocytosis = 1) | Mean: 13.3; Range: 0.9–22.6 | Mean: 19.7; Range: 18–26.5 | Korean version of the IIEF; Korean version of the FSFI |
| Laura et al. (2018)9 | JAMA Oncology | St Jude, US; Cross-sectional study | Erectile dysfunction in male survivors of childhood cancer | Male CCSs, 18 years or older, 10 years or more from diagnosis of childhood cancer | 1021 male survivors (Not reported the type of childhood cancer) | Mean (SD): 8.4 (5.5); Range: NR | Mean (SD): 32.1 (8.4); Range: NR | 6-item version of the IIEF |
| Lehmann et al. (2018)40 | Psycho-Oncology | US; Cross-sectional study | Psychosexual development and satisfaction with timing of developmental milestones among adult survivors of childhood cancer | Aged 20 to 40 at the study; diagnosed at ages 5 to 18; ≥5 years post-diagnosis | 90 survivors (56 females, 34 males; Leukemia = 25, Brain tumor = 24, Lymphoma = 22, Other solid tumors = 18) | NR | Mean (SD): 29.8 (5.2); Range: 22-43 | The psychosexual development subscale of the Course of Life Questionnaire |
| Ng et al. (2019)44 | Hong Kong Medical Journal | HK; Cross-sectional study | Sexual function, self-esteem, and general well-being in Chinese adult survivors of childhood cancers: a cross-sectional survey | Diagnosed at age <18 years; aged 18–40 years at the study; not undergoing treatment; disease-free >3 years after treatment | 200 survivors (91 females, 109 males; Haematological cancer = 133, Acute lymphoid leukaemia = 92, Acute myeloid leukaemia = 15, Hodgkin lymphoma = 10, Other = 16) | Mean (SD): 7.8 (5.09); Range: NR | Mean (SD): 25.4 (5.57); Range: NR | The MOS Sexual Functioning scale |
| Greenberget al. (2020)29 | Journal of Sexual Medicine | US; Cross-sectional study | Male and female sexual dysfunction in pediatric cancer survivors | With a previous cancer diagnosis <18 years of age, evidence of cancer cure or complete remission, and to be sexually active in the last 6 months | 57 survivors (28 males and 29 females; Bone cancer = 8, Leukemia = 27, Lymphoma = 12, Other cancer = 10) | Mean (SD): 8.9 (5.0); Range: NR | Mean (SD): 23.7 (4.1); Range: NR | FSFI; IIEF-5 |
| Hidalgo et al. (2020)35 | Child's Nervous System | US; Retrospective cohort study | Quality of life, hypothalamic obesity, and sexual function in adulthood two decades after primary gross-total resection for childhood craniopharyngioma | Underwent gross total, curative resection for primary craniopharyngioma ≤18 years; ≥18 years or older at the time of this study; ≥10 years post-operative follow-up | 22 survivors (13 males and 9 females) | NR | NR | The MOS Sexual Functioning Scale |
| Haavisto et al. (2020)36 | Cancers | Finland and Denmark; Cohort study | Male sexual function after allogeneic hematopoietic stem cell transplantation in childhood: A multicenter study | Male adult survivors of childhood hematopoietic stem cell transplantation (HSCT) | 97 HSCT male survivors (Acute lymphoblastic leukemia = 45; Acute myeloid leukemia = 9; Non-Hodgkin lymphoma = 5; Severe aplastic anemia = 14, other cancer = 24) compared to 56 healthy control | Mean (SD): 8.7 (4.4); Range: 0.2–16.4 | Mean (SD): 28.8 (7.3), Range: 18.5–47.0 | Self-reported sexual functioning |
| Bjornard et al. (2020)10 | Journal of Sexual Medicine | US; Cohort study | Psychosexual functioning of female childhood cancer survivors: a report from the St. Jude lifetime cohort study | Females at least 10 years from diagnosis, ≥18 years of age at the study | 712 female survivors (Leukemia = 260, Lymphoma = 127, CNS tumor = 51, Soft tissue tumor = 57, Renal Tumor = 68, Osteosarcoma = 24, Other = 125) vs. 122 community controls | Mean (SD): 8.05 (5.58) | Mean (SD): 31.21 (7.71) | SFQ |
| Hoven et al. (2021)7 | European Journal of Cancer | Sweden; Cross-sectional study | Sexual dysfunction in young adult survivors of childhood cancer: A population-based study | Diagnosed between ages 0 and 17 and were 19–40 years of age and residents in Sweden at the time of enrolment | 2546 survivors (1213 males and 1333 females; Haematological cancers = 1218, CNS tumours = 577, solid tumours = 748, other and unspecified malignant neoplasms = 3) vs. 819 comparison group | Mean (SD): ((male:7.8 (5.4); female:7.4 (5.4)); Range: NR | Mean (SD): ((male:29.2 (6.1); female:28.8 (6.1)); Range: NR | The PROMIS Sexual Function and Satisfaction Measure (SexFS) version 2.0; The Swedish version of BIS |
| Lehmann et al. (2022)45 | J Sex Med | Germany; Cross-sectional study | Psychosexual development and sexual functioning in young adult survivors of childhood cancer | Diagnosed with any type of cancer before age 18; ≥5 years postdiagnosis | 492 survivors (296 females and 196 males; leukemia = 195, lymphoma = 101, CNS tumor = 94, other cancer types = 102) | Mean (SD): 7.9 (4.8); Range: 0-17 | Mean (SD): 23.3 (2.5); Range: 21-26 | The psychosexual development subscale of the Course of Life Questionnaire; GMSEX; The MOS Sexual Functioning Scale |
| Frederick et al. (2016)32 | Pediatric Blood Cancer | US; Qualitative study | Sexual dysfunction in young adult survivors of childhood cancer | Between the ages 18 and 39 at the time of interview; ≥2 years from cancer diagnosis; ≥1 year since treatment; reported ≥2 sexual problems screened with the five-question general sexual functioning subscale within the Swedish Health-Related Quality-of-Life Survey | 22 survivors (10 males, 12 females; Leukemia = 6, Hodgkin lymphomas = 5, Non-Hodgkin lymphoma = 2, Bone tumors = 3, Rhabdomyosarcoma = 1, Neuroblastoma = 1, Germ cell tumor = 1, Other = 1) | Mean (SD): 13.0 (4.6); Range: 1-20 | Mean (SD): 22.6 (3.5) Range: 18-31 | Semi-structured interview exploring participants' experiences with sexual dysfunction and clinical care needs |
| Nahata et al. (2020)33 | Journal of Adolescent And Young Adult Oncology | US; Qualitative study | Romantic relationships and physical intimacy among survivors of childhood cancer | Young adult survivors of childhood cancer with Lymphoma, Leukemia, Brain tumor, and Other solid tumors; 20–40 years old at the time of initial recruitment; diagnosed between 5 and 18 years of age; ≥5 years postdiagnosis; seen in clinic within the previous 2 years | 40 survivors (25 females and 15 males; Lymphoma = 12, Leukemia = 11, Brain tumor = 4, and Other solid tumors = 13) | Mean (SD): 11.1 (3.2); Range: 5-17 | Mean (SD): 29.8 (4.8); Range: 23-42 | Semi-structured phone interview exploring the impact of cancer on romantic relationships and sexual/physical intimacy |
Note: MOS, medical outcomes study; CNS, central nervous system; IIEF, International Index of Erectile Function; FSFI, Female Sexual Function Index; ALL, Acute Lymphoblastic Leukemia; HSCT, hematopoietic stem cell transplantation; PROMIS, Patient- Reported Outcomes Measurement Information System; BIS, Body Image Scale; SFQ, Sexual Functioning Questionnaires; GMSEX, Global Measure of Sexual Satisfaction; NR, Not reported.
Historical change of research on sexual functioning in CCSs
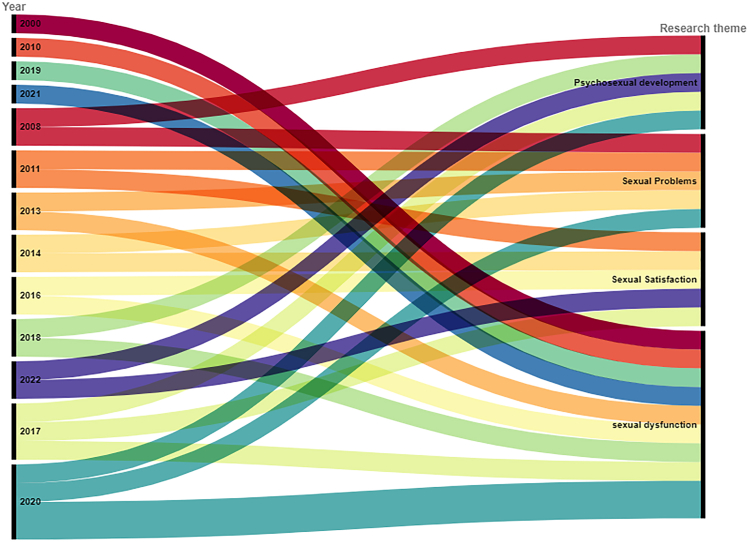
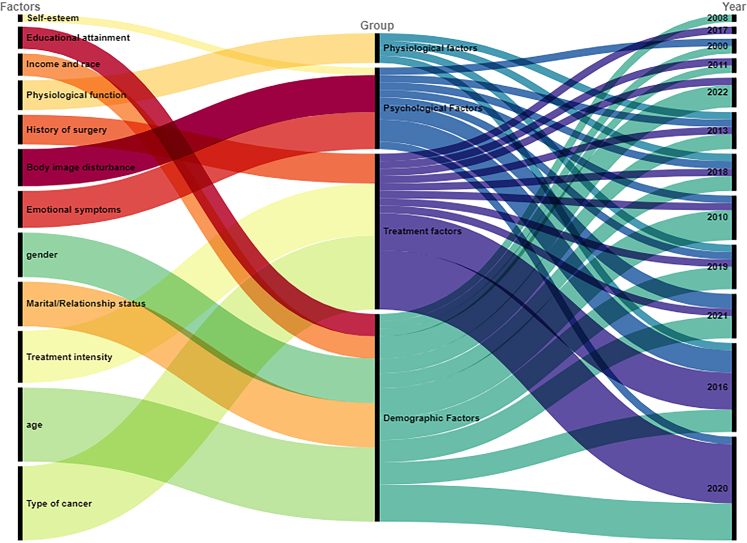
In this review, 72.72% of the studies were published after 2016, and the highest number of publications were in 2017 and 2020. The historical change in research topics on sexual functioning in CCSs is shown in Fig. 2. In brief, the first publication related to sexual functioning was published in 2000, focusing on sexual dysfunction. After 2008, another research theme, sexual problems emerged. Since 2013, there has an increasing attention to sexual satisfaction. Although psychosexual development in CCSs was first studied in 2008, this topic has been neglected up until 2017, with some publications found from 2017 to 2020. Fig. 3 presents the historical change in research on factors influencing sexual functioning in CCSs. Research on factors influencing sexual functioning is focused on the years 2021, 2016, and 2020. The first study examining the influencing factors was published in 2000 and focused on both demographic and psychological factors. Concerning the impact of demographics on sexual functioning in CCS, it remained the core research focus. In contrast, the impact of psychological factors has been neglected up until 2010, with more studies being published. The impact of physiological factors on sexual functioning in CCSs was first published in 2013 and this topic has been occasionally studied to 2019. For treatment-related factors, their impact was first examined in 2010 and has been continuously examined until 2022.
Fig. 2.
Historical research change on sexual functioning.
Fig. 3.
Historical change of factors on sexual functioning.
Assessment tools of sexual functioning
In terms of assessment tools for sexual functioning, several questionnaires were commonly adopted, including the Medical Outcome Study Sexual Functioning Scale (MOF-SF), the International Index of Erectile Function (IIEF), the Female Sexual Function Index (FSFI), the Global Measure of Sexual Satisfaction (GMSEX), the Sexual Functioning Questionnaires (SFQ), the Derogatis Interview for Sexual Functioning (DISF/DISF-SR), the PROMIS Sexual Function and Satisfaction measure (PROMIS SexFS), and the Health-related Quality of Life Survey. Three studies applied a self-developed scale. Overall, these assessment tools for sexual functioning were not validated for CCSs.
Status of sexual functioning in CCSs
Psychosexual development among CCSs
Five out of 22 included studies focused on the psychosexual development among CCSs, the key findings are shown in Table 3. Lehmann et al.40 reported a delay in psychosexual development among CCSs, where most survivors reached all milestones of psychosexual development at an average age of 29.8 years. Taking sexual debut as an example, females were 1.6 years older and males were 1.5 years older than US healthy peers to experience their sexual debut.40 Even when CCS experienced milestones at an older age, most cancer survivors perceived that the milestones were achieved at the right time.40,45 CCSs preferred to delay rather than reach milestones earlier, including first physical intimacy, sexual debut, and falling in love.40 In contrast, Van Dijk et al.42 found that there was no difference in age in achieving different milestones of psychosexual development among CCSs, including sexual fantasies, kissing, male masturbation, and oral sex. In addition, female survivors were slightly more likely than male survivors to have experienced their first relationship, first kiss, and experience with physical intimacy relative.45 Two studies39,40 also explored the relationship between neurotoxicity and psychosexual development and identified that rates of sexual debut were lower with increased neurotoxic treatment intensity. For example, Lehmann et al.39 showed that the high-dose neurotoxic group was less likely to experience sexual debut and being partnered than survivors in the low-dose and non-neurotoxic group. Additionally, the type of diagnosis was correlated with psychosexual development, in which survivors with brain tumors39 and leukemia45 were least likely to be sexually experienced and to be partnered. These results were similar to Lehmann et al.,40 who concluded that survivors diagnosed with brain tumors or leukemia in childhood normally received more neurotoxic treatments. One qualitative study33 including 40 CCSs explored their development of romantic and sexual relationships. A total of 22 participants reported negative impacts of cancer on their romantic relationships, including fertility-related concerns, physical effects, feeling emotionally self-protected, delayed dating, poor body image, and physical dysfunction; all of which affected sexual functioning directly or indirectly. Approximately half of the participants also perceived positive experiences or no impact on their sexual functioning. These areas included creating new perspectives, increased maturity, and stronger bonds with partners.
Table 3.
The key findings in psychosexual development of CCSs.
| Key findings | Detailed information or examples | References |
|---|---|---|
|
|
40 |
|
|
42 |
|
|
40,45 |
|
|
45 |
|
|
39,40 |
|
|
39,45 |
|
|
33 |
Sexual problems among CCSs
Five included studies reported some common sexual problems among CCSs, the common problems and prevalence are shown in Table 4. Van Dijk et al.42 conducted a cross-sectional study of 60 survivors, aged from 17 to 39, and found that one-third of participants had never experienced sexual intercourse, 41.4% experienced no sexual attraction, 44.8% seldom or never satisfied with their sexual lives, 23.3% reported seldom or never feeling a strong sense of really female or male. Additionally, 44.2% of the survivors reported rarely or never feeling sexually attractive towards others. Bober et al.23 found that the most commonly endorsed items of sexual problems in 291 CCSs included a lack of interest in sex (30%), difficulties enjoying sex (24%), and difficulties being aroused (23%). A cross-sectional study30 of 224 survivors showed that difficulties with erections were reported by 19% of men, and 29% of women reported problems achieving orgasm. Another study10 of 712 sexually active female survivors pointed out that the general sexual problems including lack of interest/desire (18.4%), inability to achieve orgasm (16.5%), and physical discomforts such as vaginal dryness (15.7%), and vaginal tightness (18.0%). However, this study included only female survivors. A multicenter cohort study34 including females also reported that survivors had significantly lower sexual interest, desire, arousal, and activity compared with siblings. However, it is difficult to categorize, compare, and analyze sexual problems due to the lack of uniform criteria for sexual problems and the heterogeneity across studies in terms of age, gender, and assessment methods.
Table 4.
The key findings in sexual problems of CCSs.
| References | Sample | Key findings |
|---|---|---|
| 42 | Cross-sectional study of 60 CCSs |
|
| 23 | Cross-sectional study of 291 CCSs |
|
| 30 | Cross-sectional study of 224 survivors |
|
| 10 | Cohort study of 712 female CCSs |
|
| 34 | Multicenter cohort study of 2178 female CCSs |
|
Sexual satisfaction among CCSs
Sexual satisfaction is a crucial but easily overlooked aspect. Lehmann et al.45 pointed out that sexual satisfaction was positively related to sexual functioning. The other four included studies on sexual satisfaction presented conflicting results. Lehmann et al.38,39 compared CCSs with healthy controls in 2016 (n = 87) and 2017 (n = 144) and suggested no profound difference in sexual satisfaction between the survivors and controls. In contrast, another study30 included a larger number of survivors (n = 224) and found lower sexual satisfaction in male, but not female survivors when compared to healthy controls. However, Ford et al.34 focused on female CCSs and found a statistically significant difference in sexual satisfaction between 2178 female survivors and 408 sibling controls.
Prevalence of sexual dysfunction among CCSs
Nine publications defined sexual dysfunction and reported the incidence of sexual dysfunction among CCSs, the detailed information is shown in Table 5. Relander et al.41 adopted a questionnaire of six questions reflecting sexual function in 66 CCSs and found that 30.3% of patients reported one or more sexual problems. Zebrack et al.37 used the MOS-SF to assess sexual functioning in young adult CCSs and found 42.7% of the entire sample (52% of females and 32% of males) reported at least one problematic symptom and hence were classified to have sexual dysfunction. In addition, a large population-based (n = 2546) study,7 which also focused on young adult CCSs, reported that 57% of female and 35% of male survivors reported a dysfunction in at least one domain, and 22% of females and 13% of males reported dysfunction in at least two domains by the PROMIS SexFS. Bober et al.23 applied the classification criteria of reporting 2 items on the Swed-QUAL sexual functioning measure, and identified 29% out of 291 participants were sexual dysfunction cases, of which 37.3% in males and 19.9% in females. Two large-sample studies9,28 focused on erectile dysfunction in male CCSs by IIEF-EF and reported 12.3% and 29.0% of survivors suffering from sexual dysfunction respectively, which were significantly higher than siblings. Meanwhile, one large-sample cohort study10 focused on sexual dysfunction in female CCSs by Sexual Functioning Questionnaires (SFQ), which classified survivors with scores <10th percentile of controls as sexual dysfunction and identified 19.9% of females experienced sexual dysfunction. Another cross-sectional study44 of Chinese survivors showed that 24.0% of patients had experienced sexual dysfunction by the MOS-SF which defined sexual dysfunction as having at least one sexual problem. Although another study29 also reported the prevalence of sexual dysfunction, of which erectile dysfunction was 25.0% by IIEF-5 in 28 males, and the rates of sexual issues among 29 females was 52.4% by FSFI, due to the limitations of the assessment tool and sample size, this comparison is not very clinically meaningful. Overall, the prevalence of sexual dysfunction in CCSs varied widely, ranging from 12.30% to 46.54%, and that in males ranged from 12.30% to 54.00%, while in females ranged from 19.90% to 57.00%. Although most studies have indicated a statistically significantly higher prevalence of sexual dysfunction among CCSs than in the general population7,37 and a higher prevalence in females than in males in several studies,7,29,37 it is not possible to pool data for meta-analysis due to methodological heterogeneity, such as differences in sample size, diagnoses, definitions of sexual dysfunction, and assessment tools of sexual dysfunction.
Table 5.
The key findings in prevalence of sexual dysfunction of CCSs.
| References | Sample | Assessment tools | Key findings |
|---|---|---|---|
| 41 | Cross-sectional study of 66 male CCSs | a self-administered questionnaire of six questions |
|
| 37 | Cross-sectional study of 599 young adult CCSs | the MOS Sexual Functioning scale |
|
| 7 | Large population-based cross-sectional of 2546 young adult CCSs | the PROMIS Sexual Function and Satisfaction Measure |
|
| 23 | Cohort study of 291 CCSs | the Swed-QUAL sexual functioning measure |
|
| 28, 9 | Cohort study of 1441 males; cross-sectional of 291 males | IIEF |
|
| 10 | Cohort study of 712 females | SFQ |
|
| 44 | Cross-sectional study of 200 CCSs | the MOS Sexual Functioning scale |
|
| 29 | Cross-sectional study of 57 CCSs | IIEF-5, FSFI |
|
Note: MOS, medical outcomes study; PROMIS, Patient-Reported Outcomes Measurement Information System; IIEF, International Index of Erectile Function; SFQ, Sexual Functioning Questionnaires; FSFI, Female Sexual Function Index.
Associated factors of sexual functioning in CCSs
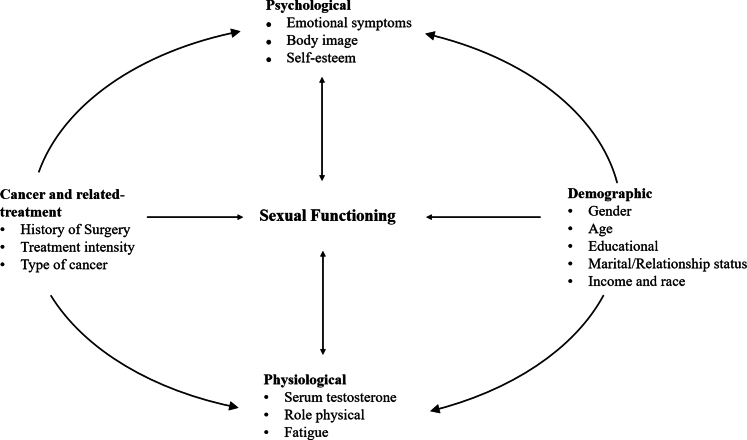
Based on the included studies, we identified four categories of associated factors: demographic-related, treatment-related, psychological, and physiological (Fig. 4). The summary of associated factors is shown in Table 6.
Fig. 4.
Associated factors of sexual functioning among CCSs∗ (∗CCSs, Childhood Cancer Survivors).
Table 6.
The associated factors on sexual functioning of CCSs.
| Risk factors | Key findings | Detailed information or examples | References |
|---|---|---|---|
| Demographic-related factors | |||
| Gender |
|
|
23,37 |
|
|
30,44 | |
|
|
7 | |
|
|
45 | |
|
|
30 | |
| Age |
|
|
42 |
|
|
9,10,28,44 | |
|
|
45 | |
|
29,38,41 | ||
| Educational attainment |
|
|
7,10 |
|
37 | ||
| Marital/Relationship status |
|
|
10,37,44 |
|
|
7,38,45 | |
| Income and race |
|
|
37 |
|
|
9 | |
|
10 | ||
| Treatment-related factors | |||
| History of surgery |
|
|
10,28,29,44 |
| Treatment intensity |
|
|
7,28,39,40 |
|
|
10,29,38 | |
| Type of cancer |
|
|
10,30 |
|
23,29,37,38,45 | ||
| Psychological-related factors | |||
| Emotional symptoms |
|
|
7,29,37 |
|
|
32,37 | |
|
|
10,23 | |
| Body image |
|
|
7,9,32,41 |
|
38 | ||
| Self-esteem |
|
44 | |
| Physiological-related factors |
|
|
9,23,32,44 |
Note: CCSs, childhood cancer survivors; CNS, central nervous systerm.
Demographic-Related Factors
Demographic-related factors on sexual functioning include gender, age, educational attainment, marital/relationship status, income level, and race.
Gender
Six studies7,23,30,37,44,45 compared the occurrence of sexual problems or dysfunction by gender. Zebrack et al.37 conducted a cross-sectional study among 599 survivors and found that the overall mean sexual symptom score for females was more than twice that of males. This implies that female survivors experienced more severe sexual dysfunction symptoms and poorer sexual functioning compared to male survivors. This finding is also supported by another cohort study23 of 291 CCSs. On the contrary, Sundberg et al.30 and Ng et al.44 reported that male survivors more frequently reported sexual dysfunction and felt sexually less attractive than female survivors and healthy male controls. In addition, the common sexual problems in females are different from those in males. A population-based study of 2546 CCSs7 found that sexual dysfunction among female survivors was most common in the domains of interest in sexual activity, orgasm ability, and vulvar discomfort labial, while males were often concerned about sexual satisfaction, interest in sexual activity, and erectile dysfunction. It is interesting to note that about half of survivors were willing to seek advice when experiencing sexual problems, with males preferring a physician and females more likely to consult with a friend.30 The psychosexual development also differed between male and female survivors. A survey of 492 German CCSs45 found that female survivors were somewhat more likely than male survivors to have had their first relationship, first kiss, and experience with physical intimacy relative at a relatively later age. Importantly, the correlation between sexual satisfaction and sexual functioning also varied by gender with a previous study indicating a stronger correlation among female survivors compared to male survivors.45
Age
Particularly the age at cancer diagnosis, age at assessment, and time since diagnosis, were found to be closely linked to sexual experience in nine studies.9,10,28,29,38,41,42,44,45 For example, results from a quantitative analysis42 showed that, compared with survivors treated in childhood, survivors treated in adolescence had a delay in achieving sexual milestones such as dating, touching under clothes, female masturbation, and sexual intercourse. Similarly, those diagnosed in childhood reported better sexual functioning than those diagnosed in adolescence.45 In terms of age at assessment, five studies confirmed that the survivors with older age had significantly less sexual experience, poorer sexual functioning, and higher incidence of erectile dysfunction compared to the general population.9,10,28,42,44 For example, Ritenour et al.28 reported that the older age (50+ years vs. 20–29 years) was statistically significantly associated with erectile dysfunction among male survivors. Bjornard et al.10 explored the associated factors among 936 female survivors and found that older age (45–54 years vs. 18–24 years) exhibited higher levels of sexual dysfunction. Besides, Bober et al.23 compared sexual dysfunction and non-sexual dysfunction cases and revealed that sexual dysfunction cases were statistically significantly older than non-cases. Also, one study45 reported that a longer time since diagnosis was weakly related to better sexual functioning, possibly explained by a better adjustment over time. Some studies found a contradictory finding which reported that age at cancer diagnosis or time since diagnosis neither influences psychosexual development, nor sexual functioning.29,38,41
Educational attainment
Two studies similarly found a relationship between educational attainment and sexual functioning among CCSs. Hoven et al.7 revealed that higher education was less likely to report dysfunction in certain sexual domains in females and the domain of interest sexual activity in males. Bjornard et al.10 also revealed that the risk of sexual dysfunction in female CCSs who had a college degree was 0.56 times lower than those without a college degree. However, Zebrack et al.37 found that there was no statistically significant difference in sexual functioning by different education levels in young adult survivors.
Marital/relationship status
Marital or relationship status was found to be an associated factor of sexual dysfunction and satisfaction among CCSs in six studies.7,10,37,38,44,45 Out of six studies, three reported that married survivors experienced significantly higher rates of sexual dysfunction compared to survivors who were unmarried or single.10,37,44 Furthermore, two of these studies10,37 highlighted that this trend was particularly evident among female survivors, suggesting that marriage had a more pronounced impact on sexual dysfunction in women than in men. In examining the impact of relationship status on sexual functioning, Lehmann et al.38 examined 87 survivors and discovered that those in partnerships reported superior sexual functioning and greater sexual satisfaction compared to single participants. This finding was echoed by two other studies,7,45 which also found that survivors in relationships experienced higher satisfaction and lower rates of sexual dysfunction than those who were single.
Income and race
One study37 noted the relationship between income and sexual functioning, which focused on the survivors from Southern California and found that male survivors with income less than $25,000 reported significantly more sexual symptoms. Only one cross-sectional study9 of 956 males found a correlation between race and erectile dysfunction and reported that the Hispanic ethnicity and Black race were independent factors for erectile dysfunction in male survivors. However, another study10 reported there were no statistically significant differences between survivors with and without sexual dysfunction regarding household income, or race/ethnicity.
Treatment-related factors
The included studies reported some treatment factors that may relate to sexual functioning among CCSs. These factors included history of surgery, treatment intensity, and type of cancer.
History of surgery
Four studies10,28,29,44 reported a relationship between the history of surgery and sexual functioning but is limited to a few specific surgical procedures, surgical sites, and post-surgical effects. Ritenour et al.28 focused on erectile dysfunction in 1441 male survivors and showed that a history of surgery involving the spinal cord or sympathetic nerves, history of prostate surgery, and pelvic surgery were associated with erectile dysfunction. Pelvic surgery as an associated factor for female sexual dysfunction was also confirmed in another study by Bjornard et al.,10 which focused on 936 female CCSs. Meanwhile, Greenberg et al.29 also reported that female pediatric cancer survivors who underwent surgery or radiation to the pelvis had significantly lower sexual satisfaction and pain domain scores than patients who did not undergo this treatment modality. In addition, Ng et al.44 focused on 109 male and 91 female CCSs in Hong Kong, emphasizing that the history of surgery with external effects was closely related to sexual functioning.
Treatment intensity
Seven studies7,10,28,29,38, 39, 40 mentioned an association between treatment intensity and sexual functioning, but the results were inconsistent. Ritenour et al.28 showed that if the testicular radiation dose was more than 10 Gy, there was a positive correlation with sexual dysfunction (RR 3.55; 95% CI 1.53–8.24). Hoven et al.7 using the Intensity of Treatment Rating scale, also reported that females who had received more intensive treatment were more likely to report dysfunction in two or more sexual domains, whereas males with a more intensive treatment were more likely to report dysfunction related to orgasm pleasure. Neurotoxic treatment intensity was also found to affect psychosexual development, CCSs with high-dose neurotoxic treatment showed less sexual experience, bad relationship status, and even less likely to have children.39,40 For the three remaining studies,10,29,38 none of them found a relationship between treatment intensity and sexual functioning. For example, Greenberg et al.29 found that survivors who had radiation therapy as part of their oncologic treatment showed a similar trend in sexual satisfaction scores when compared with patients who did not have radiation therapy. Moreover, Bjornard et al.10 compared survivors with and without sexual dysfunction and showed that there was no statistically significant difference in exposures to any chemotherapy, including alkylating agents, or radiation therapy, except oophorectomy.
Type of cancer
Seven studies10,23,29,30,37,38,45 mentioned the relationship between cancer type and sexual functioning. Bjornard et al.10 reported that CCSs with a diagnosis of germ cell tumors, renal tumors diagnosis, and leukemia had a higher risk of sexual dysfunction in female CCSs. Sundberg et al.30 showed that those diagnosed with a CNS tumor more frequently reported sexual arousal problems, low sexual satisfaction, low frequency of sexual activity during the past 12 months, and having fewer sexual partners compared with other diagnoses. However, some studies confirmed that there were no differences between sexual functioning and type of diagnosis.23,29,37,38,45 For example, Lehmann et al.38 compared leukemia and lymphoma with other solid tumors (except CNS malignancies), and found that survivors did not differ in body image, sexual satisfaction, and relationship status satisfaction.
Three studies8,35,36 included in this review investigated sexual functioning in distinct cancer types, namely ALL, childhood craniopharyngioma, and HSCT in childhood cancer cases. Notably, survivors of ALL8 and HSCT36 exhibited varying degrees of sexual functioning impairment compared to the general population. For instance, Haavisto et al.36 enrolled 97 male CCSs undergone HSCT and identified significant testicular damage in this group. This was evidenced by reduced testosterone levels, decreased testicular volumes, and lower sperm counts. Furthermore, these survivors experienced compromised sexual functioning, including difficulties with sexual arousal, orgasm, and sexual drive, a higher likelihood of being without a partner, and socioeconomic disadvantages.
Psychological-related factors
Some studies reported psychological factors related to sexual functioning among CCSs, including emotional distress, body image disturbance, and self-esteem.
Emotional symptoms
Six studies7,10,23,29,32,37 reported that sexual functioning was correlated with emotional distress, including nervousness during sexual intercourse, anxiety, and depression, and this issue was particularly prominent among female survivors. Zebrack et al.37 identified that sexual functioning was significantly correlated with all subscale and global measures of distress for both males and females. Also, those reporting more sexual dysfunction reported greater depressive symptoms, somatization, anxiety, and mental health functioning of SF-36 scales, as well as a greater overall symptom index score. Greenberg et al.29 pointed out that patients who were difficult to relax during sexual intercourse exhibited higher levels of sexual dysfunction. Hoven et al.7 found that survivors with greater emotional distress were more likely to report sexual dysfunction, both males and females. Similarly, female survivors with depression symptoms reported more sexual dysfunction in the study of Bjornard et al.10 Besides, survivors experiencing sexual dysfunction also reported clinically higher levels of anxiety and depression, limitations associated with emotions in role performance, and mental health problems. For example, females with sexual dysfunction demonstrated significant limitations on emotional functioning, mental health, and social functioning.23 Besides, one qualitative study32 of 22 patients with sexual problems found that 91% of the participants reported psychological distress which affected their sexual activity, including concern about their ability to perform sexual activity and worry about partners’ reactions. Also, the participants experienced general anxiety, which interfered with their ability to relax and engage in sex.
Body image disturbance
Although body image is recognized to be important in sexual functioning, the relationship between body image and sexual functioning in CCSs is conflicting among the included four studies.7,9,32,41 One study7 involving 2546 patients stated that body image disturbance was a risk factor for sexual dysfunction, and reported that survivors with greater body image disturbance were more likely to report sexual dysfunction. This phenomenon is similarly observed in two cross-sectional studies in which one investigated 956 male survivors and showed the survivors with greater body image dissatisfaction were more likely to report ED in both sexually active and general groups,9 another study showed that the group with no sexual problem had statistically significantly better body image scores.43 Conversely, Lehmann et al.38 reported that body image disturbance did not correlate with sexual satisfaction, a key aspect of sexual functioning. This finding is contradictory to the results of two previous studies that showed body image disturbance was associated with sexual dysfunction. In a qualitative study,32 most participants with sexual dysfunction described concern about the perceptions of other people, particularly their intimate partners on their altered body image due to cancer and its treatment.
Self-esteem
Only one cross-sectional survey44 addressed the relationship between self-esteem and sexual functioning. This study involved 200 Chinese CCSs divided into three groups based on their sexual functioning scores. This study reported that the group reporting no sexual problem had statistically significantly higher Rosenberg self-esteem scale scores.
Physiological-related factors
Four included studies9,23,32,44 identified some physiological factors may related to sexual functioning among CCSs, with detailed information in Table 5. Iersel et al.46 conducted a study exploring erectile dysfunction in 956 male CCSs and identified that low serum testosterone levels and low lean muscle mass increased the risk of sexual dysfunction. Bober et al.23 similarly showed that survivors experiencing sexual dysfunction also reported poorer functioning across all subscales of the SF-12 including physical functioning, role physical, and fatigue. A consistent result was found by Ng et al.,44 which focused on the association between physical function and sexual dysfunction among Chinese CCSs. They found that CCSs with higher physical component scores were more likely to show no sexual problem. This associated factor has also been observed in the results of a qualitative study,32 which conducted semi-structured interviews with 22 patients with sexual problems. A total of 77% of participants in the qualitative study described physical problems, which were mostly related to the late side effects of surgery, chemotherapy, and radiation therapy, such as vaginal dryness, pain, and fatigue.
Discussion
Sexual functioning is an important part of overall health and is often overlooked in CCSs, although the negative impact of cancer on sexual functioning can last a lifetime. As sexual functioning includes a wide range of conditions and assessments, this scoping review was carried out to fully capture the diversity of studies. This review focuses on CCSs and summarizes the research progress and available evidence on sexual functioning, thus identifying knowledge gaps in the literature to guide future research initiatives. Given the broadness of the concept of sexual functioning, we qualitatively summarized the sexual functioning of CCSs and its associated factors. We anticipated a paucity of current evidence, therefore were inclusive of all study designs yet still only included 22 studies for analysis.
From the historical review of research on sexual functioning, we identified that an increasing number of studies have been published since 2016. This could be explained by the issuance of a guideline on sexual functioning for cancer survivors in 2016 by NCCN.47 Despite psychosexual development in CCSs was first studied in 2008, this research topic has been overlooked until 2017. Even though there has been increasing attention to this research topic since 2017, only five related studies have been published up until now. Given the importance of psychosexual development, more studies should be done to address this research area in CCSs. Our historical review also found that examining the impact of demographic and treatment-related factors on sexual functioning in CCSs remains the current trend. Nevertheless, these factors, e.g. gender, educational attainment, and types of cancer, are mostly unmodifiable which may not be very useful in the intervention development. In contrast, the impact of psychological and physiological factors has received less attention, with more related studies being published since 2010. Given the modifiable nature of psychological factors, continuous attention should be given to this topic to assist healthcare professionals in identifying appropriate interventions for CCSs.
This review identified that the prevalence of sexual dysfunction in CCSs ranged from 12.30% to 54.00% in males and 19.90%–57.00% in females, such a wide range of prevalence will limit clinical implications. The difference in prevalence across the included studies could be explained by the heterogeneity of studies in terms of participant characteristics, sample size, assessment tools, and statistical analysis. For example, participants in the included studies had a wide variety of diagnoses and treatments including chemotherapy, radiation therapy, surgery, and bone marrow transplant. The other explanation is that there is no consensus on the evaluation criteria for sexual dysfunction. Although most studies have reported levels of sexual functioning, only nine have made a diagnosis of sexual dysfunction and reported its prevalence. For example, Greenberg et al.29 used the IIEF which defined sexual dysfunction as scores ≤25 or FSFI which defined sexual dysfunction as scores <26.55. Zebrack et al.37 reported sexual dysfunction as having “a little of a problem” in one or more areas of sexual functioning assessed by MOS-SF. To advance research in this field, a clear and universally accepted definition of sexual dysfunction is needed to be established in the future.
Although psychosexual development was less frequently evaluated than sexual dysfunction, it plays a key role in earlier ages during the construction of gender identity and sexual orientation,14 which are strongly associated with sexual functioning.33,45 However, the existing findings about the effect of cancer and its treatment on psychosexual development among CCSs are inconsistent. Perhaps, such effects on patients' psychosexual development are largely influenced by the patients’ own perception. As illustrated in two included studies,40,45 some participants were satisfied with their psychosexual development and considered themselves achieving milestones at the right time regardless of a perceived delay in achievement. Another qualitative study33 also highlighted that cancer and its treatment could bring positive outcomes to sexual functioning by creating new perspectives for CCSs, increasing their maturity, and strengthening the bonds with their partners. Additionally, the review found poorer psychosexual development in survivors with brain tumors39 and leukemia,45 which may be related to the fact that survivors of these two diagnoses receive more neurotoxic treatments.40 The effect of neurotoxic treatment on psychosexual development is currently unclear, and future studies are expected to clarify this issue. Notably, there also exists a significant gap in studies examining the influence of cancer treatment on the sexual orientation and gender identity of CCS. Future research should be developed in this area to better understand the psychosexual development of CCS across diverse identities. Moreover, it is imperative to develop and implement sexual functioning assessment tools that are inclusive of all genders and sexual orientations. Such non-heteronormative tools will ensure a comprehensive representation of CCS, allowing for interventions that are sensitive to the unique experiences of each individual.
Concerning demographic-related factors, our review found that sexual functioning differed by gender with most studies generally supporting that female survivors had greater impairment in sexual functioning. This phenomenon may be explained by some psychological characteristics specific to females. A previous qualitative study32 found that psychological issues, such as anxiety, fear of partner rejection, fear of being pitied, infertility concern, and poor self-esteem, played an important role in predicting sexual dysfunction. Since females are known to be more likely to experience posttraumatic psychological symptoms and emotional sequelae than males,23,48,49 it is understandable that the degree of impairment in sexual dysfunction among females is higher. Another possible explanation is that females above 45 years old may enter menopause.50 In addition, before the age of 45, the risk of premature menopause, a common side effect of cancer treatment,51 can negatively impact the sexual functioning of female CCSs. Pathways and mechanisms that explain why females are more susceptible to sexual impairment remain to be tested.
Age, including age at diagnosis, age at assessment, and time since diagnosis were associated with sexual functioning. First, survivors diagnosed in childhood reported better sexual functioning than those diagnosed in adolescence. This may be related to “catch-up growth”, which refers to a period of accelerated growth experienced by children after a period of slowed or stunted growth due to a variety of factors, such as cancer and its related treatment.52 Previous studies highlighted that survivors diagnosed in childhood have more chance for “catch-up growth”, thus reducing the effects of cancer and its treatment on their physical functions, including sexual functioning.53 Also, sexual organs are under rapid development during adolescence,54 and are more vulnerable to the effects of cancer and its treatment. Adolescence is the gold period for social development, sexual identity, and exploration of sexuality with peers,42,49 diagnosed with cancer during this period will affect psychosexual development notably. Second, older age is associated with poor sexual functioning. Given that declining sex organs and declining sex hormone levels with age, like menopause, can themselves contribute to declining sexual functioning, it is uncertain whether the declining sexual functioning is associated with cancer and related treatments or a natural recessionary trajectory. Interestingly, this review also found an inconsistent finding, which concluded that age at cancer diagnosis or time since diagnosis did not influence sexual functioning.23,29,38 These studies included CCSs who were younger than 27 years old. The differences in birth cohorts and medical cancer treatment may be potential factors accounting for these contradictory findings. Finer age-stratified studies and lifetime cohort studies may be able to clarify this contradiction.
Despite the contradictory results, marital/relationship status may be another potential factor associated with sexual functioning in CCSs, which was not found in adolescent and young adult survivors.16,17 The discordance may be explained by heterogeneity in the gender of research subjects and the difference in definitions between marital status and partner status. Marital status refers to an individual's legal or official standing regarding marriage, while partner status refers to the nature of a person's current romantic or intimate relationship. Since different definitions were applied in existing studies, discordances might occur. Educational attainment was also found to be an associated factor which is not mentioned in the previous review.16,17 Particularly, females with a college/university degree or higher had a lower risk for sexual dysfunction. This association was not obvious in males, which may be due to the limited number of studies. The last demographic-related factors identified were race and income. Although there is no clear explanation, the differences in cancer treatment55 and cultural beliefs towards sexual functioning56 in different geographical and economic locations may be possible to address the effect of these factors on sexual functioning.
Three treatment-related factors were identified associated with sexual functioning among CCSs. The first associated factor was the history of surgery, but the effect is limited to some specific surgical procedures and sites, particularly spinal cord or sympathetic nerves surgery, prostate surgery, and pelvic surgery.10,28 In addition, surgery with external effects, i.e., scar was also an associated factor for sexual dysfunction in CCSs.44 A possible explanation is that these types of surgery could damage the neurovascular bundles near the sex organs, leading to severe impairment of the ejaculatory and/or erectile function in males.57 Scars may also influence patients' sexual functioning by affecting their perceptions of physical aesthetics and sexual attractiveness. This is supported by the study conducted by Olsson et al.,58 which found that survivors perceived themselves to be less sexually attractive due to scars on their bodies and hence were less satisfied with their sexual functioning. Treatment intensity is potentially negatively correlated with sexual functioning. This finding is in line with other studies,4,59 which found that testicular radiation ≥10 Gy, cranial radiation≥30 Gy with central hypogonadism, and high doses of alkylating agents were statistically significantly related to sexual dysfunction. Nevertheless, some included studies did not find any significant difference in sexual functioning and sexual satisfaction, thus thresholds of therapeutic intensity for effects on sexual functioning may be a point to be explored in the future. The third associating factor was cancer type, including germ cell tumor, renal tumor, and leukemia. Notably, only three included studies8,35,36 focused on a specific type of cancer. However, the level and predictors of sexual functioning for some other common pediatric cancer diagnoses, like lymphoma, bone cancer, and CNS tumor, have not been reported, and future research on different cancer diagnoses is urgently needed to ensure support is directed to those who most need it. Again, due to the limited number of studies exploring the relationship between cancer type and sexual functioning in CCSs, more studies targeted at specific cancer types are necessary for clarification.
Additionally, psychological-related factors are important contributing to sexual dysfunction among CCS, especially among female survivors. Although previous studies have suggested that physiological and psychological factors can interact with each other to influence sexual functioning,10,60,61 the mechanism is not clear. Besides, emotional symptoms can either strengthen or weaken a person's feelings of sexual arousal and desire,62,63 which are closely related to sexual functioning.64 The evidence for the effects of body image on sexual functioning is relatively clear. Just as the findings of a qualitative study, poor self-image hampered the development of intimate relationships among CCSs.65 Furthermore, self-image is closely linked with self-esteem which is known to be a very important factor in recovery,66, 67, 68 particularly enabling cancer survivors to return to their daily activities. One thing worth noting is that sexual activity is an act of interaction and communication between two partners, partners of individuals with sexual dysfunction are more likely to experience sexual problems.69 One's own sexual functioning could be significantly impacted by partner's response via psychological mechanisms.70 To date, most researchers have included only one member of the couple in studies and little is known about dyadic influences, no published studies have reported the impact of partners on sexual functioning among CCSs. Studies on this topic would be interesting. Overall, caution should be taken in interpreting the relationship between psychological factors and sexual functioning. Previous studies have suggested that there may be a complex interrelationship between these two variables.71 Hence, we cannot conclude whether psychological factors were causes or products of sexual dysfunction in CCSs. Notwithstanding the difficulty in concluding a cause-and-effect relationship, our findings showed that psychological factors were significantly associated with sexual functioning among CCSs. Therefore, appropriate interventions are needed to address the psychological needs of CCSs with sexual dysfunction.
In our review, some physiological factors, including testosterone level and physical function, were identified to be associated with sexual functioning. In fact, their impacts on sexual functioning were attributable to cancer and its treatment.72 For example, alkylating agents in chemotherapy, testicular radiation, and surgery or radiation to the genitourinary organs and/or hypothalamic-pituitary region can bring different physiological and endocrine disorders; all these will subsequently contribute to sexual dysfunction. Currently, studies assessing sexual functioning through pituitary-hypothalamic-gonadal axis and neuroendocrine pathways are lacking. In this review, only four studies attempted to present sexual functioning of CCSs using an endocrine perspective, particularly in terms of testicular volume, analysis of semen, and serum endocrine such as follicle-stimulating hormone, luteinizing hormone, testosterone, and inhibin B. To address this under-researched area, more longitudinal research should incorporate some hormonal markers to clarify the underlying pathophysiology of sexual dysfunction. A better understanding would strengthen our ability to screen CCS for issues in survivorship to identify those at risk.
This study had some limitations. Firstly, the focus of this review was cancer survivors diagnosed under the age of 18. Some studies focused on both adolescents and young adult cancer survivors were excluded, because they did not differentiate the two groups. Meanwhile, our review cannot reflect the sexual development of childhood cancer patients who are on active cancer treatment. Besides, there is variation in the measurement of sexual functioning across studies. Hence, this hindered us in making a direct comparison of sexual dysfunction among the included studies. Although this review identified several measures for assessing sexual functioning in the general population, only one specific measurement is tailored for cancer survivors, which is PROMIS SexFS.7 A standardized tool tailored to CCSs does not exist. The absence of a CCS-specific assessment tool may contribute to the under-recognition of sexual functioning issues and the underestimation of sexual dysfunction prevalence among CCSs. The development of a standardized self-report tool could facilitate the collection of sensitive sexual information by medical professionals, breaking down communication barriers and enabling patients to discuss sensitive sexual matters as part of their care comfortably.73 It is crucial to customize the assessment items to capture any sexual-related impact resulting from cancer and its treatment in CCSs. This information can be used to guide clinicians about the treatment options for these sexual-related impacts. Therefore, future research is needed to develop appropriate tools for CCSs to assess sexual functioning.
To conclude, this review comprehensively summarizes the research evidence related to sexual functioning in CCSs, especially the historical research change, assessment tools of sexual functioning, milestones of psychosexual development, common sexual problems, and prevalence of sexual dysfunction among CCSs. Findings of this review address a higher prevalence of sexual dysfunction than healthy peers, especially female survivors, the sexual problems are diverse by gender. However, these findings are not yet definitive due to insufficient evidence on the topic to date and the heterogeneity of included research. In addition, there are no CCSs-specific multidimensional sexual functioning scales, which greatly limits the comparison and integration of findings across studies, and future research is expected to address this issue. The underlying etiology of sexual problems is often multifactorial and complex among CCSs. This review also enriched factors on sexual functioning, categorized into four categories of associated factors. Especially the identified psychological factors and psychosexual development characteristics can guide healthcare professionals to design more systematic screening programs and target interventions for CCSs who are at risk of sexual dysfunction. Although some identified factors have a unified influence on sexual functioning, most factors are inconsistent or even contradictory, such as marital/relationship status, age at cancer diagnosis, treatment intensity, and time since diagnosis. In the future, large-sample, high-quality study designs in this field, such as population-based cohort studies and mixed studies, should be conducted to explore in depth the relationship and mechanisms between influencing factors and sexual functioning. The heterogeneity of studies should also be reduced by standardizing measurement criteria, study subjects, and disease diagnosis, thereby improving the integration of studies.
Contributors
Funa Yang, Ka Yan Ho, Frances-Kam-Yuet Wong designed the study. Funa Yang, Qi Liu and Ting Mao conducted the literature search and searched the articles. Funa Yang, Qi Liu contributed to the data extraction process. Funa Yang, Ka Yan Ho, Frances-Kam-Yuet Wong analyzed the data and interpretation of data. Funa Yang and Ka Yan Ho drafted the manuscript. Janelle Yorke and Kate Law contributed to interpretation of the included studies and interpretation and presentation of results, manuscript preparation. Chiu Sau Ying, Godfrey Chan Chi Fung, Xiaoxia Xu, Hongying Shi, Lanwei Guo, NG Chi Fai, Pak Yin Anthony Liu, John Yuen, Getaneh Mulualem Belay, Katherine Ka Wai Lam and Lanwei Guo reviewed and provided expert opinions. Janelle Yorke and Kate Law contributed to interpretation of the included studies and interpretation and presentation of results, manuscript preparation. Ka Yan Ho accessed and verified the data, and was responsible for the decision to submit the manuscript. All the authors contributed to the article and approved the submitted version.
Data sharing statement
Review protocol is available on the PROSPERO website. All calculated and extracted calculated data are available upon requests by email to the first author.
Declaration of interests
All authors declare that they have no conflict of interest.
References
- 1.Wu Y., Deng Y., Wei B., et al. Global, regional, and national childhood cancer burden, 1990-2019: an analysis based on the Global Burden of Disease Study 2019. J Adv Res. 2022;40:233–247. doi: 10.1016/j.jare.2022.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhakta N., Force L.M., Allemani C., et al. Childhood cancer burden: a review of global estimates. Lancet Oncol. 2019;20(1):e42–e53. doi: 10.1016/S1470-2045(18)30761-7. [DOI] [PubMed] [Google Scholar]
- 3.Siegel D.A., Richardson L.C., Henley S.J., et al. Pediatric cancer mortality and survival in the United States, 2001-2016. Cancer. 2020;126(19):4379–4389. doi: 10.1002/cncr.33080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erdmann F., Frederiksen L.E., Bonaventure A., et al. Childhood cancer: survival, treatment modalities, late effects and improvements over time. Cancer epidemiology. 2021;71(Pt B) doi: 10.1016/j.canep.2020.101733. [DOI] [PubMed] [Google Scholar]
- 5.Landier W., Skinner R., Wallace W.H., et al. Surveillance for late effects in childhood cancer survivors. J Clin Oncol. 2018;36(21):2216–2222. doi: 10.1200/JCO.2017.77.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crist N., Egert M.C., Bernie H.L. Sexual dysfunction in male childhood cancer survivors and adolescent and young adult survivors of hematologic malignancies. Sex Med Rev. 2023;11(2):106–113. doi: 10.1093/sxmrev/qeac013. [DOI] [PubMed] [Google Scholar]
- 7.Hoven E., Fagerkvist K., Jahnukainen K., et al. Sexual dysfunction in young adult survivors of childhood cancer - a population-based study. Eur J Cancer. 2021;154:147–156. doi: 10.1016/j.ejca.2021.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Haavisto A., Henriksson M., Heikkinen R., Puukko-Viertomies L.R., Jahnukainen K. Sexual function in male long-term survivors of childhood acute lymphoblastic leukemia. Cancer. 2016;122(14):2268–2276. doi: 10.1002/cncr.29989. [DOI] [PubMed] [Google Scholar]
- 9.van Iersel L., Li Z., Chemaitilly W., et al. Erectile dysfunction in male survivors of childhood cancer. JAMA Oncol. 2018;4(11):1613–1616. doi: 10.1001/jamaoncol.2018.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bjornard K.L., Howell C.R., Klosky J.L., et al. Psychosexual functioning of female childhood cancer survivors: a report from the St. Jude lifetime cohort study. J Sex Med. 2020;17(10):1981–1994. doi: 10.1016/j.jsxm.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Children's Oncology Group . Children's Oncology Group; Monrovia, CA: 2018. Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers. [Google Scholar]
- 12.Tonorezos E.S., Cohn R.J., Glaser A.W., et al. Long-term care for people treated for cancer during childhood and adolescence. Lancet. 2022;399(10334):1561–1572. doi: 10.1016/S0140-6736(22)00460-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brinkman T.M., Recklitis C.J., Michel G., Grootenhuis M.A., Klosky J.L. Psychological symptoms, social outcomes, socioeconomic attainment, and health behaviors among survivors of childhood cancer: current state of the literature. J Clin Oncol. 2018;36(21):2190–2197. doi: 10.1200/JCO.2017.76.5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Segarra-Echebarría R., Fernández-Pérez I., García-Moncho J.M., Delarze-Carrillo L. In: Psychopathology in women: incorporating gender perspective into descriptive psychopathology. Sáenz-Herrero M., editor. Springer International Publishing; Cham: 2015. Psychosexual development and sexual dysfunctions; pp. 25–51. [Google Scholar]
- 15.Lehmann V., Laan E.T.M., den Oudsten B.L. Sexual health-related care needs among young adult cancer patients and survivors: a systematic literature review. J Cancer Survi. 2022;16(4):913–924. doi: 10.1007/s11764-021-01084-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cherven B., Sampson A., Bober S.L., et al. Sexual health among adolescent and young adult cancer survivors: a scoping review from the Children's Oncology Group Adolescent and Young Adult Oncology Discipline Committee. CA Cancer J Clin. 2021;71(3):250–263. doi: 10.3322/caac.21655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sopfe J., Gupta A., Appiah L.C., Chow E.J., Peterson P.N. Sexual dysfunction in adolescent and young adult survivors of childhood cancer: presentation, risk factors, and evaluation of an underdiagnosed late effect: a narrative review. J Adolesc Young Adult Oncol. 2020;9(5):549–560. doi: 10.1089/jayao.2020.0025. [DOI] [PubMed] [Google Scholar]
- 18.Murphy D., Klosky J.L., Reed D.R., Termuhlen A.M., Shannon S.V., Quinn G.P. The importance of assessing priorities of reproductive health concerns among adolescent and young adult patients with cancer. Cancer. 2015;121(15):2529–2536. doi: 10.1002/cncr.29466. [DOI] [PubMed] [Google Scholar]
- 19.Anazodo A.C., Choi S., Signorelli C., et al. Reproductive care of childhood and adolescent cancer survivors: a 12-year evaluation. J Adolesc Young Adult Oncol. 2021;10(2):131–141. doi: 10.1089/jayao.2020.0157. [DOI] [PubMed] [Google Scholar]
- 20.Michel G., Brinkman T.M., Wakefield C.E., Grootenhuis M. Psychological outcomes, health-related quality of life, and neurocognitive functioning in survivors of childhood cancer and their parents. Pediatr Clin. 2020;67(6):1103–1134. doi: 10.1016/j.pcl.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Prasad P.K., Hardy K.K., Zhang N., et al. Psychosocial and neurocognitive outcomes in adult survivors of adolescent and early young adult cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2015;33(23):2545–2552. doi: 10.1200/JCO.2014.57.7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDonnell G.A., Salley C.G., Barnett M., et al. Anxiety among adolescent survivors of pediatric cancer. J Adolesc Health. 2017;61(4):409–423. doi: 10.1016/j.jadohealth.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bober S.L., Zhou E.S., Chen B., Manley P.E., Kenney L.B., Recklitis C.J. Sexual function in childhood cancer survivors: a report from Project REACH. J Sex Med. 2013;10(8):2084–2093. doi: 10.1111/jsm.12193. [DOI] [PubMed] [Google Scholar]
- 24.Frederiksen L.E., Mader L., Feychting M., et al. Surviving childhood cancer: a systematic review of studies on risk and determinants of adverse socioeconomic outcomes. Int J Cancer. 2019;144(8):1796–1823. doi: 10.1002/ijc.31789. [DOI] [PubMed] [Google Scholar]
- 25.Gerstl B., Signorelli C., Wakefield C.E., et al. Sexual and reproductive complications and concerns of survivors of childhood, adolescent and adult cancer. J Cancer Surviv. 2023 doi: 10.1007/s11764-023-01349-6. 10.1007/s11764-023-01349-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olsson M., Enskär K., Steineck G., Wilderäng U., Jarfelt M. Self-perceived physical attractiveness in relation to scars among adolescent and young adult cancer survivors: a population-based study. J Adolesc Young Adult Oncol. 2018;7(3):358–366. doi: 10.1089/jayao.2017.0089. [DOI] [PubMed] [Google Scholar]
- 27.Robertson E.G., Sansom-Daly U.M., Wakefield C.E., et al. Sexual and romantic relationships: experiences of adolescent and young adult cancer survivors. J Adolesc Young Adult Oncol. 2016;5(3):286–291. doi: 10.1089/jayao.2015.0061. [DOI] [PubMed] [Google Scholar]
- 28.Ritenour C.W.M., Seidel K.D., Leisenring W., et al. Erectile dysfunction in male survivors of childhood cancer-a report from the childhood cancer survivor study. J Sex Med. 2016;13(6):945–954. doi: 10.1016/j.jsxm.2016.03.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenberg D.R., Khandwala Y.S., Bhambhvani H.P., Simon P.J., Eisenberg M.L. Male and female sexual dysfunction in pediatric cancer survivors. J Sex Med. 2020;17(9):1715–1722. doi: 10.1016/j.jsxm.2020.05.014. [DOI] [PubMed] [Google Scholar]
- 30.Sundberg K.K., Lampic C., Arvidson J., Helstrom L., Wettergren L. Sexual function and experience among long-term survivors of childhood cancer. Eur J Cancer. 2011;47(3):397–403. doi: 10.1016/j.ejca.2010.09.040. [DOI] [PubMed] [Google Scholar]
- 31.Elo S., Kyngäs H. The qualitative content analysis process. J Adv Nurs. 2008;62(1):107–115. doi: 10.1111/j.1365-2648.2007.04569.x. [DOI] [PubMed] [Google Scholar]
- 32.Frederick N.N., Recklitis C.J., Blackmon J.E., Bober S. Sexual dysfunction in young adult survivors of childhood cancer. Pediatr Blood Cancer. 2016;63(9):1622–1628. doi: 10.1002/pbc.26041. [DOI] [PubMed] [Google Scholar]
- 33.Nahata L., Morgan T.L., Lipak K.G., Olshefski R.S., Gerhardt C.A., Lehmann V. Romantic relationships and physical intimacy among survivors of childhood cancer. J Adolesc Young Adult Oncol. 2020;9(3):359–366. doi: 10.1089/jayao.2019.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ford J.S., Kawashima T., Whitton J., et al. Psychosexual functioning among adult female survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2014;32(28):3126. doi: 10.1200/JCO.2013.54.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hidalgo E.T., Orillac C., Kvint S., et al. Quality of life, hypothalamic obesity, and sexual function in adulthood two decades after primary gross-total resection for childhood craniopharyngioma. Child's Nerv Syst. 2020;36(2):281–289. doi: 10.1007/s00381-019-04161-9. [DOI] [PubMed] [Google Scholar]
- 36.Haavisto A., Mathiesen S., Suominen A., et al. Male sexual function after allogeneic hematopoietic stem cell transplantation in childhood: a multicenter study. Cancers. 2020;12(7):1786. doi: 10.3390/cancers12071786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zebrack B.J., Foley S., Wittmann D., Leonard M. Sexual functioning in young adult survivors of childhood cancer. Psycho Oncol. 2010;19(8):814–822. doi: 10.1002/pon.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lehmann V., Hagedoorn M., Gerhardt C.A., et al. Body issues, sexual satisfaction, and relationship status satisfaction in long-term childhood cancer survivors and healthy controls. Psycho Oncol. 2016;25(2):210–216. doi: 10.1002/pon.3841. [DOI] [PubMed] [Google Scholar]
- 39.Lehmann V., Tuinman M.A., Keim M.C., et al. Psychosexual development and satisfaction in long-term survivors of childhood cancer: neurotoxic treatment intensity as a risk indicator. Cancer. 2017;123(10):1869–1876. doi: 10.1002/cncr.30513. [DOI] [PubMed] [Google Scholar]
- 40.Lehmann V., Keim M.C., Ferrante A.C., Olshefski R.S., Gerhardt C.A. Psychosexual development and satisfaction with timing of developmental milestones among adult survivors of childhood cancer. Psycho Oncol. 2018;27(8):1944–1949. doi: 10.1002/pon.4746. [DOI] [PubMed] [Google Scholar]
- 41.Relander T., Cavallin-Ståhl E., Garwicz S., Olsson A.M., Willén M. Gonadal and sexual function in men treated for childhood cancer. Med Pediatr Oncol. 2000;35(1):52–63. doi: 10.1002/1096-911x(200007)35:1<52::aid-mpo9>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 42.Van Dijk E., Van Dulmen-den Broeder E., Kaspers G., Van Dam E., Braam K., Huisman J. Psychosexual functioning of childhood cancer survivors. Psycho Oncol. 2008;17(5):506–511. doi: 10.1002/pon.1274. [DOI] [PubMed] [Google Scholar]
- 43.Yoon J.Y., Park H.J., Ju H.Y., et al. Gonadal and sexual dysfunction in childhood cancer survivors. 2017;49(4):1057–1064. doi: 10.4143/crt.2016.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ng C.F., Hong C.Y.L., Lau B.S.Y., et al. Sexual function, self-esteem, and general well-being in Chinese adult survivors of childhood cancers: a cross-sectional survey. Hong Kong Med J. 2019;25(5):372–381. doi: 10.12809/hkmj197913. [DOI] [PubMed] [Google Scholar]
- 45.Lehmann V., Gerhardt C.A., Baust K., Kaatsch P., Hagedoorn M., Tuinman M.A. Psychosexual development and sexual functioning in young adult survivors of childhood cancer. J Sex Med. 2022;19(11):1644–1654. doi: 10.1016/j.jsxm.2022.07.014. [DOI] [PubMed] [Google Scholar]
- 46.Van Iersel L., Li Z., Chemaitilly W., et al. Erectile dysfunction in male survivors of childhood cancer: a report from the St. jude lifetime cohort study. Endocr Rev. 2018;39(2) [Google Scholar]
- 47.Melisko M.E., Narus J.B. Sexual function in cancer survivors: updates to the NCCN guidelines for survivorship. J Natl Compr Cancer Netw. 2016;14(5S):685–689. doi: 10.6004/jnccn.2016.0193. [DOI] [PubMed] [Google Scholar]
- 48.Lehmann V., Ferrante A.C., Winning A.M., Gerhardt C.A. The perceived impact of infertility on romantic relationships and singlehood among adult survivors of childhood cancer. Psycho Oncol. 2019;28(3):622–628. doi: 10.1002/pon.4999. [DOI] [PubMed] [Google Scholar]
- 49.Stinson J.N., Jibb L.A., Greenberg M., et al. A qualitative study of the impact of cancer on romantic relationships, sexual relationships, and fertility: perspectives of Canadian adolescents and parents during and after treatment. J Adolesc Young Adult Oncol. 2015;4(2):84–90. doi: 10.1089/jayao.2014.0036. [DOI] [PubMed] [Google Scholar]
- 50.Khalesi Z.B., Jafarzadeh-Kenarsari F., Mobarrez Y.D., Abedinzade M. The impact of menopause on sexual function in women and their spouses. Afr Health Sci. 2020;20(4):1979–1984. doi: 10.4314/ahs.v20i4.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Im C., Lu Z., Mostoufi-Moab S., et al. Development and validation of age-specific risk prediction models for primary ovarian insufficiency in long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study and St Jude Lifetime Cohort. Lancet Oncol. 2023;24(12):1434–1442. doi: 10.1016/S1470-2045(23)00510-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wit J.-M., Boersma B. Catch-up growth: definition, mechanisms, and models. J Pediatr Endocrinol Metabol. 2002;15:1229–1242. [PubMed] [Google Scholar]
- 53.Crawshaw M.A., Sloper P. ‘Swimming against the tide’–the influence of fertility matters on the transition to adulthood or survivorship following adolescent cancer. Eur J Cancer Care. 2010;19(5):610–620. doi: 10.1111/j.1365-2354.2009.01118.x. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y., Li T., Yao R., et al. Comparison of body-image dissatisfaction among Chinese children and adolescents at different pubertal development stages. Psychol Res Behav Manag. 2020;13:555–562. doi: 10.2147/PRBM.S242645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morshed N., Haskard-Zolnierek K., Zhan F.B. Geographic variations of racial/ethnic disparities in late-stage diagnosis of childhood cancer in Texas. South Med J. 2020;113(5):224–231. doi: 10.14423/SMJ.0000000000001097. [DOI] [PubMed] [Google Scholar]
- 56.Atallah S., Johnson-Agbakwu C., Rosenbaum T., et al. Ethical and sociocultural aspects of sexual function and dysfunction in both sexes. J Sex Med. 2016;13(4):591–606. doi: 10.1016/j.jsxm.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 57.Sukhu T., Ross S., Coward R.M. Urological survivorship issues among adolescent boys and young men who are cancer survivors. Sex Med Rev. 2018;6(3):396–409. doi: 10.1016/j.sxmr.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 58.Olsson M., Steineck G., Enskär K., Wilderäng U., Jarfelt M. Sexual function in adolescent and young adult cancer survivors-a population-based study. J Cancer Surviv. 2018;12(4):450–459. doi: 10.1007/s11764-018-0684-x. [DOI] [PubMed] [Google Scholar]
- 59.Frederick N.N., Lehmann V., Ahler A., et al. Pediatric blood & cancer; 2021. Psychosexual functioning in cancer survivorship: what the pediatric oncologist needs to know. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Greaves P., Sarker S.J., Chowdhury K., et al. Fertility and sexual function in long-term survivors of haematological malignancy: using patient-reported outcome measures to assess a neglected area of need in the late effects clinic. Br J Haematol. 2014;164(4):526–535. doi: 10.1111/bjh.12651. [DOI] [PubMed] [Google Scholar]
- 61.Pastuszak A.W., Badhiwala N., Lipshultz L.I., Khera M. Depression is correlated with the psychological and physical aspects of sexual dysfunction in men. Int J Impot Res. 2013;25(5):194–199. doi: 10.1038/ijir.2013.4. [DOI] [PubMed] [Google Scholar]
- 62.Fischer V.J., Andersson G., Billieux J., Vögele C. The relationship between emotion regulation and sexual function and satisfaction: a scoping review. Sex Med Rev. 2022;10(2):195–208. doi: 10.1016/j.sxmr.2021.11.004. [DOI] [PubMed] [Google Scholar]
- 63.Graham C.A. The DSM diagnostic criteria for female sexual arousal disorder. Arch Sex Behav. 2010;39(2):240–255. doi: 10.1007/s10508-009-9535-1. [DOI] [PubMed] [Google Scholar]
- 64.Mitchell K.R., Wellings K.A., Graham C. How do men and women define sexual desire and sexual arousal? J Sex Marital Ther. 2014;40(1):17–32. doi: 10.1080/0092623X.2012.697536. [DOI] [PubMed] [Google Scholar]
- 65.Thompson A.L., Long K.A., Marsland A.L. Impact of childhood cancer on emerging adult survivors' romantic relationships: a qualitative account. J Sex Med. 2013;10(Suppl 1):65–73. doi: 10.1111/j.1743-6109.2012.02950.x. [DOI] [PubMed] [Google Scholar]
- 66.Tonsing K.N., Ow R. Quality of life, self-esteem, and future expectations of adolescent and young adult cancer survivors. Health Soc Work. 2018;43(1):15–21. doi: 10.1093/hsw/hlx047. [DOI] [PubMed] [Google Scholar]
- 67.Tarkowska M., Głowacka-Mrotek I., Nowikiewicz T., et al. Sexual functioning and self-esteem in women after mastectomy - a single-centre, non-randomised, cross-sectional study. Contemp Oncol. 2020;24(2):106–111. doi: 10.5114/wo.2020.95876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vidthya S., Sherina M.S., Rampal L., Fadhilah S.I., Ummavathy P. Self-esteem among cancer patients receiving chemotherapy in selected. Med J Malaysia. 2019;74(5):405–412. [PubMed] [Google Scholar]
- 69.Pascoal P.M., Byers E.S., Alvarez M.-J., et al. A dyadic approach to understanding the link between sexual functioning and sexual satisfaction in heterosexual couples. J Sex Res. 2018;55(9):1155–1166. doi: 10.1080/00224499.2017.1373267. [DOI] [PubMed] [Google Scholar]
- 70.Velten J., Brailovskaia J., Margraf J. Exploring the impact of personal and partner traits on sexuality: sexual excitation, sexual inhibition, and big five predict sexual function in couples. J Sex Res. 2019;56(3):287–299. doi: 10.1080/00224499.2018.1491521. [DOI] [PubMed] [Google Scholar]
- 71.Flynn K.E., Lin L., Bruner D.W., et al. Sexual satisfaction and the importance of sexual health to quality of life throughout the life course of U.S. Adults. J Sex Med. 2016;13(11):1642–1650. doi: 10.1016/j.jsxm.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Voznesensky M., Annam K., Kreder K.J. Understanding and managing erectile dysfunction in patients treated for cancer. J Oncol Pract. 2016;12(4):297–304. doi: 10.1200/JOP.2016.010678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tounkel I., Nalubola S., Schulz A., Lakhi N. Sexual health screening for gynecologic and breast cancer survivors: a review and critical analysis of validated screening tools. Sex Med. 2022;10(2) doi: 10.1016/j.esxm.2022.100498. [DOI] [PMC free article] [PubMed] [Google Scholar]