Abstract
The Mahoney strain of poliovirus type 1 (OM) is generally unable to cause paralysis in mice. We isolated a mouse-adapted mutant, PV1/OM-SA (SA), from the spinal cord of a mouse that had been intracerebrally inoculated with OM. SA showed mouse neurovirulence only with intraspinal inoculation, and the infected mice developed a flaccid paralysis, which was indistinguishable from that observed in poliovirus-sensitive transgenic mice inoculated with OM. SA antigens were detected in neurons of the spinal cords of the infected mice. Nucleotide (nt) sequence analysis revealed 9 nt changes on the SA genome, resulting in three amino acid (a.a.) substitutions, i.e., one each in the capsid proteins VP4 and VP1 and in the noncapsid protein 2C. To identify the key mutation site(s) for the mouse neurovirulence, virus recombinants between OM and SA were constructed by using infectious cDNA clones of these two viruses and tested for their mouse neurovirulence after inoculation via an intraspinal route. The results indicated that a mutation at nt 928 (replacement of A with G), resulting in a substitution of Met for Ile at a.a. 62 within VP4, was responsible for conferring the mouse neurovirulence phenotype of the mutant SA. The mutation in VP4 may render the virus accessible to a molecule that acts as a virus receptor and is located on the surfaces of neurons of the mouse spinal cord. This molecule appears not to be expressed in the mouse brain.
Poliovirus (PV), the causative agent of poliomyelitis, is a human enterovirus that belongs to the Picornaviridae family and is classified into three stable serotypes, 1, 2, and 3. The poliovirion is an icosahedral, nonenveloped particle that is composed of 60 copies each of four capsid proteins, VP1, VP2, VP3, and VP4, and a single-stranded RNA genome of positive polarity. The study of the three-dimensional structure has revealed that the three larger capsid proteins, VP1, VP2, and VP3, form the outer surface; the smallest one, VP4, is confined to the inner surface and is in contact with the viral RNA (11). A deep depression, called a canyon (30), on the surface of the virion has been suggested to be an attachment site for the PV receptor (PVR) on the surface of permissive cells.
PV infection is initiated by oral ingestion of the virus followed by its multiplication in the alimentary mucosa. After extensive multiplication in tissues including tonsils and Peyer’s patches, the virus moves into the blood. If viremia becomes established, the virus invades the central nervous system (CNS) and paralytic poliomyelitis occurs as a result of destruction of neurons in the CNS, especially motor neurons in the anterior horn of the spinal cord (3).
Strains of PV infect only primates and cannot infect mice, with the exceptions of the PV type 2 (PV2) wild strains, the Lansing and MEF-1 strains, which were isolated by serial passages in cotton rats (2, 32). These PV2 strains, however, do not cause the typical syndrome of poliomyelitis, although they may kill mice when injected intraspinally or intracerebrally (9). The predominant molecular determinant of species specificity of PV is the PVR, a cell surface molecule with three immunoglobulin (Ig)-like domains expressed only by primate cells (14, 20). Transgenic (Tg) mice expressing the human PVR are susceptible to infection with all three serotypes of PV, and when these mice are inoculated with PV they show clinical symptoms similar to those observed in humans and monkeys (13, 29).
After PV infection of the CNS of Tg mice, PV antigens or genomic RNA are detected only in neurons and not in vascular endothelial cells or glial cells (12, 13, 15, 27). In situ hybridization experiments using Tg mice have demonstrated that the PVR mRNAs are also detected only in neurons of the CNS. Thus, the expression of human PVR may confer cell tropism to PV in the CNS of Tg mice. However, Western blot analyses have shown that PVR are expressed in a wide range of human and Tg mouse tissues, including tissues that are not targets of PV infection (7, 14, 15, 20, 24). These observations indicate that PV tissue tropism is governed neither merely by expression of the PVR gene nor solely by accessibility of cells to the virus.
To clarify the molecular basis of PV tropism, attempts have been made to obtain mouse-adapted PV type 1 (PV1) mutants by serial passages in the mouse CNS (5, 22), by genetic exchange of gene segments between PV1 and mouse-virulent PV2 (18, 19, 23), and by selection of persistently infectious PVs in human neuroblastoma (6). The molecular studies of mouse-adapted mutants have revealed that determinants of mouse neurovirulence phenotype are located either at the surface of the viral capsid, i.e., at residues 94 to 102 (BC loop) and residue 160 of VP1, residue 142 of VP2, and residue 60 of VP3, or inside the viral capsid, i.e., at residues 22, 40, and 54 of VP1, residue 31 of VP2, and residue 62 of VP4 (5, 6, 18, 22). All these mutant viruses show mouse neurovirulence after inoculation via an intracerebral route.
Here we isolated a mouse spinal cord-specific mutant of the PV1 Mahoney strain. Non-Tg mice intraspinally inoculated with this mutant developed paralysis, which was indistinguishable from that in Tg mice inoculated with wild-type PV, but the mice intracerebrally inoculated with this mutant did not show any symptoms under the conditions used. The genomic determinant was found to be nucleotide (nt) 928, located within the VP4 capsid protein-coding region. Our results suggest that PV with this mutation recognizes a surface molecule of neurons in the mouse spinal cord, which is possibly not expressed in the brain, as a receptor.
MATERIALS AND METHODS
Cells, viruses, and mice.
African green monkey kidney (AGMK) cells were grown in Dulbecco modified Eagle’s medium supplemented with 5% newborn calf serum and used for the transfection experiment with infectious cDNA clones, plaque purification of viruses, and plaque assays. Suspension-cultured HeLa S3 cells were grown in RPMI 1640 medium supplemented with 5% newborn calf serum and used for preparation of PV.
The PV1 Mahoney virus strain (OM) was recovered from AGMK cells transfected with RNA transcribed from the infectious cDNA clone, pOM (33). The light-sensitive OM virus was prepared by using neutral red as described previously (17).
The Tg mouse line ICR-PVRTg21 (13), at the hemizygous stage, and mice of the IQI line, an inbred strain of ICR mice, at the age of 6 to 10 weeks, were used for mouse neurovirulence tests. Other inbred mouse strains (C3H/he, C57BL/6, BALB/c, and DBA/2) were purchased from Japan SLC, Inc. All mice used had been maintained under specific-pathogen-free condition before use.
Antibodies.
Hyperimmune anti-PV1 serum was prepared by injecting OM virus into a rabbit and used for tissue staining. Rabbit hyperimmune sera specific for PV1/Mahoney, PV2/Lansing, and PV3/Leon, generous gifts from the Japan Poliomyelitis Research Institute, were used for virus type identification tests. Anti-glial fibrillary acidic protein (GFAP) monoclonal antibody (clone 2.2B10) was purchased from Zymed Laboratories, Inc. Goat anti-rabbit IgG conjugated with fluorescein isothiocyanate (FITC) and goat anti-rat IgG (H+C) conjugated with Texas red were purchased from Medical & Biological Laboratories, Co., Ltd., and Vector Laboratories, Inc., respectively.
Viral RNA preparation.
Genomic RNA of mutant virus was isolated from DEAE Sepharose CL-6B-purified virions (16) by three extractions with 1 volume each of phenol and chloroform–isoamyl alcohol (24:1) followed by one extraction with chloroform–isoamyl alcohol. RNA was then precipitated with ethanol.
Construction of infectious cDNA clones.
The cDNAs corresponding to the 5′ proximal portion of the genome of the mutant PV1/OM-SA (SA) were prepared by reverse transcriptase (RT) PCR of the mutant genome RNA; oligonucleotides 5′-CTGAGAATTCGTAATACGACTCACTATAGGTTAAAACAGCTCTGGGGTTG-3′ (nucleotide sequence of EcoRI site, T7 phage 10 promoter, and nt 1 to 20 of PV RNA) and 3′-CCACCACCTTCAACGGACTA-5′ (antisense sequence for nt 1182 to 1201 of PV RNA) were used as sense and antisense primers, respectively. The RT PCR product was digested with EcoRI and SphI and inserted into the EcoRI and SphI sites of pBR322. This plasmid was named pBR5′T7.
Double-stranded cDNA complementary to the genome of the mutant was prepared by using the cDNA synthesis system Plus (Amersham) (33). The cDNA with EcoRI adapters at both ends was inserted into the EcoRI site of the plasmid vector pSVA14 (10). Plasmid carrying cDNA nearly equivalent in length to full-length PV RNA was chosen. This plasmid was called pSVA-SA. An infectious cDNA clone of mutant SA was constructed by replacing the AatII fragments of pSVA-SA with the corresponding fragments from pBR5′T7. The resulting clone was designated pSA.
Nucleotide sequence analysis.
To identify the key mutation site(s) in the genome of SA virus, the nucleotide sequence of cDNA containing the entire SA genome was determined by using a Pharmacia LKB ALFRed DNA sequencer. The sequence determination was also performed to confirm the mutation sites of various recombinants made by exchange between OM and SA viruses.
Construction of recombinant cDNAs.
Infectious recombinants of cDNAs from OM and from SA were constructed by using infectious cDNA clones of these viruses, pOM and pSA. Allele replacement experiments were carried out on these two plasmids with the restriction enzymes BanII, PvuI, NheI, NruI, and SpeI under conditions recommended by the manufacturers. The digestion products were separated by gel electrophoresis on 1% agarose. Ligations and transformations were performed by standard methods (31). The plasmid designation pOM/SA-cap denotes a plasmid that carries the capsid protein-coding region of pSA in the background of the pOM sequence. Other recombinants were also named by using similar terminology.
Construction of single point mutant.
A single point mutation at nt 926 (replacement of A with G), resulting in substitution of Val for Ile at amino acid (a.a.) 62 within the VP4 capsid protein, was introduced into the OM genome by RT PCR by using oligonucleotides 5′-CTGAGAATTCGTAATACGACTCACTCATAGGTTAAAACAGCTCTGGGGTTC-3′ (corresponding to nt 900 to 940; the site of substitution [nt 926] is underlined) and 3′-CCACCACCTTCAACGGACTA-5′ (nt 1182 to 1201) as sense and antisense primers. The 267-bp BanII-NruI fragment was used to substitute the corresponding fragment of pOM. The infectious cDNA clone thus obtained was designated pMah-VP4V62.
Transfection.
RNA transcripts were synthesized from PvuI-linearized cDNAs. AGMK cells on a 60-mm-diameter dish were transfected with 1 to 5 μg of RNA by a DEAE–dextran method (10, 33). The viruses recovered from the cells transfected with pSA, pOM/SA-cap, pSA/OM-cap, pOM/SA-VP4, pSA/OM-VP4, pOM/SA-VP1, pSA/OM-VP1, pOM/SA-VP3, pSA/OM-VP3, and pMah-VP4V62 were designated SA, OM/SA-cap, SA/OM-cap, OM/SA-VP4, SA/OM-VP4, OM/SA-VP1, SA/OM-VP1, OM/SA-VP3, SA/OM-VP3, and Mah-VP4V62, respectively.
Mouse neurovirulence test.
Mice, at the age of 6 to 10 weeks, were intracerebrally and intraspinally inoculated with 30 and 5 μl of PV suspensions, respectively, and the animals were observed daily for 14 days for paralysis and mortality. The 50% lethal dose (LD50) of each virus was calculated by the method of Reed and Muench (26).
Recovery of viruses from tissues.
The spinal cords and brains were removed from paralyzed mice and homogenized in phosphate-buffered saline (PBS) (10 mM phosphate buffer [pH 7.0], 137 mM NaCl, and 2.6 mM KCl) to prepare a 10% emulsion of the tissue. The homogenates were centrifuged to remove the debris, and the supernatant containing virus was subjected to plaque purification or a plaque assay.
Preparation, storage, and sectioning of tissues.
Mice were intraspinally inoculated with PV suspensions as described above. Mice that became moribund after infection were anesthetized and perfused with PBS through the left ventricle. The spinal cords were removed by dissection, and 1-cm-long pieces were immediately frozen in O.C.T. compound (Miles, Inc., Elkhart, Ind.) in a dry ice–acetone–cold hexane bath. The blocks were stored in plastic bags, to prevent dehydration, at −80°C. Sections (thickness, 8 μm) were prepared by using a Jung CM 3000 cryostat (Leica Instruments GmbH), mounted on 3-aminopropyltriethoxysilane (APS)-coated slides (Matsunami Glass Ind., Ltd.), and air dried. As a negative control, frozen sections of the spinal cords collected from IQI mice 5 days after the intraspinal inoculation with OM virus were prepared.
Immunofluorescence assay.
All the reactions for immunofluorescence assay were carried out at room temperature except for the primary antibody incubation. Sections were fixed in 2% paraformaldehyde for 10 min and rehydrated in PBS. Nonspecific staining was blocked by incubation in 10% normal goat serum for 30 min. Primary antibodies were overlaid on the slides at 4°C overnight, and secondary antibodies conjugated with FITC or Texas red were overlaid on the slide for 1 h. The sections were examined with a confocal laser scanning microscope (Bio-Rad).
RESULTS
Isolation of a mouse-adapted PV1 mutant.
To investigate the molecular basis for host restriction of PV1 in mice, we isolated a mouse-adapted PV1 mutant. A total of 53 IQI mice were inoculated intracerebrally with 107 PFU of light-sensitive OM virus, in which mutations should accumulate during the repeated replication in a medium containing neutral red. Of these, 6 mice showed signs of paralysis. The spinal cord and brain were removed from one of these paralyzed mice and homogenized as described in Materials and Methods. The homogenates were used for plaque assay. Plaques of at least two size ranges were detected, although these plaques were much smaller than those of OM virus. The smaller plaques were detected mainly in homogenates of the spinal cord, and the larger ones were detected mainly in those of the brain. These results suggested the presence of distinctive variants within the CNS homogenates. Three rounds of plaque purification were performed on these viruses in AGMK cells. Viruses from smaller plaques, but not those from larger plaques, caused paralysis in IQI mice following intraspinal inoculation. A smaller plaque variant was propagated in HeLa S3 suspension cells and designated PV1/OM-SA (SA) (Fig. 1). As shown in Fig. 1, a small population of viruses that formed larger plaques appeared during the propagation, suggesting low stability of the SA phenotype in in vitro-cultured cells.
FIG. 1.
Plaque phenotypes of OM and SA. AGMK cells grown in the 60-mm-diameter dishes were infected with OM or SA, respectively. Cells in the dishes were incubated at 37°C for 72 h, fixed, and stained with 1% crystal violet.
To verify that the mutant was a PV1, we carried out neutralization tests by using rabbit hyperimmune sera specific for PV1/Mahoney, PV2/Lansing, or PV3/Leon. Only the anti-PV1/Mahoney serum could neutralize the mutant. Therefore, the mutant was serotypically identical to PV1 (data not shown). The smaller-plaque phenotype may be indicative that the SA infection cycle in AGMK cells is less efficient than that of the parental OM virus. In fact, the titers of virus harvested from AGMK cells infected with SA virus were about 10 times lower than those of virus harvested from cells infected with the parental virus (data not shown).
An infectious cDNA clone, pSA, was prepared as described in Materials and Methods. The viruses recovered from AGMK cells transfected with RNA transcribed from the infectious cDNA clone showed SA phenotypes, that is, very small plaque phenotype and mouse neurovirulence phenotype, after the intraspinal inoculation. Accordingly, the virus derived from pSA was used as SA virus in the following experiments.
Mouse spinal cord specificity of mutant SA.
IQI mice were infected with OM or its mutant SA via various inoculation routes (Table 1). Interestingly, SA caused paralysis in IQI mice only when delivered by the intraspinal inoculation route. IQI mice intraspinally injected with SA developed a flaccid paralysis and death in a dose-dependent manner, and the LD50 was calculated to be 1.25 × 103 PFU. The first clinical symptoms observed were those affecting their limbs, at 3 to 5 days after the intraspinal inoculation, and progression to death occurred within 5 to 7 days. Some mice survived with paralysis in one or both hind limbs. These clinical signs were indistinguishable from those for Tg mice injected with OM virus. Intracerebral, intravenous, and intramuscular inoculation of 107 PFU of SA did not produce neurovirulence for IQI mice (Table 1). OM virus did not cause paralysis of mice when delivered by any inoculation routes used. The neurovirulence of SA was also tested by using other inbred mouse strains, such as C3H/he, C57BL/6, BALB/c and DBA/2. Intraspinal inoculation with 105 PFU of SA caused paralysis and death in 8 to 10 of 10 injected mice of these strains (data not shown). Furthermore, no neurovirulence was observed for any inbred mouse strains used after intracerebral inoculation of 107 PFU of mutant SA. Thus, the manifestation of neurovirulence only with intraspinal inoculation of mutant SA appears to be a phenomenon common in these mouse strains.
TABLE 1.
Neurovirulence test of PV1 in IQI mice
| Virus | LD50 (PFU) of PV1 inoculated via various routes:
|
|||
|---|---|---|---|---|
| Intraspinal | Intracerebral | Intravenous | Intramuscular | |
| OM | >106 | >107 | >107 | >107 |
| SA | 1.25 × 103 | >107 | >107 | >107 |
To confirm that the SA virus does not replicate in the mouse brain, virus titers in the brains of IQI mice were measured at various times after the intracerebral inoculation (Fig. 2). The time profile of virus titers of mutant SA is almost identical to that of the parental OM virus in IQI mice, suggesting that SA virus does not replicate in the brain. Thus, SA virus appears to adapt only to the mouse spinal cord.
FIG. 2.
Time course of virus titers in the mouse brain. IQI mice were intracerebrally inoculated with 106 PFU of virus suspensions (30 μl) of OM and SA, respectively. Cerebrums of infected mice were removed at the indicated times and homogenized as described in Materials and Methods. The homogenates were centrifuged at low speeds, and the supernatants containing viruses were subjected to plaque assays. Error bars represent the standard errors of three experiments.
PV antigens in the spinal cord.
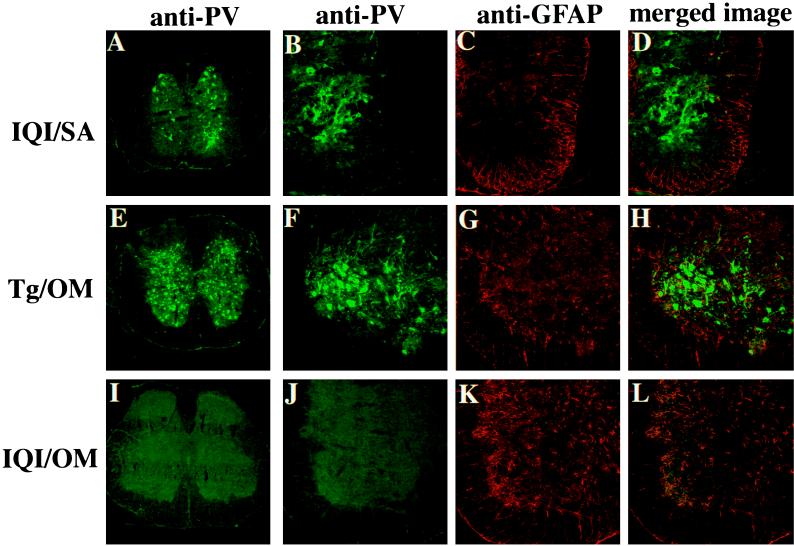
The cervical, thoracic, and lumbar spinal cords of PV-infected mice were examined for the presence of viral antigens. Frozen sections of the spinal cord were reacted with rabbit anti-PV1 hyperimmune serum followed by FITC-conjugated second antibody. In both the ventral and dorsal horns of the lumbar spinal cords of SA-infected IQI mice PV antigens were seen only in neurons (Fig. 3A). Similar observations were obtained for the cervical and thoracic spinal cords (data not shown). These results resemble those obtained for OM-infected Tg mice (Fig. 3E), suggesting that in IQI mice mutant SA, like OM virus in the Tg mice, multiplies to a level of approximately 107 PFU in the spinal cord. PV antigens were scarcely detected in the spinal cords of OM-infected IQI mice (Fig. 3I).
FIG. 3.
Distribution of PV antigens in spinal cords of mice intraspinally infected with PV1. Frozen sections were prepared from the spinal cords of IQI mice infected with 104 PFU of SA virus (A through D), Tg mice infected with 102 PFU of OM virus (E through H), and IQI mice infected with 105 PFU of OM virus (I through L). Sections were stained with anti-PV antibodies (green), and the astrocyte marker GFAP antibody (red). Lumbar spinal cords of SA-infected IQI mice and OM-infected Tg mice are shown in panels A and E, respectively. PV antigens were detected in both the ventral and dorsal horns. Panels B through D show the left dorsal horns of the lumbar spinal cords of SA-infected IQI mice. Overlap of staining for anti-PV antibodies (green) with that for GFAP antibody (red) was not observed in the sections. Panels F through H show left ventral horns of lumbar spinal cords of OM-infected Tg mice. PV antigens were detected both in the cell body and in axonal and dendritic processes. Staining for anti-PV antibodies (green) did not overlap with that for GFAP antibody (red). Panels I through L show lumbar spinal cords of OM-infected IQI mice. PV antigen was not detected in the sections.
To investigate whether glial cells also have PV antigens, rat anti-GFAP antibody (Fig. 3C, G, and K), which reacts only with astrocytic cells, and rabbit anti-PV1 hyperimmune serum (Fig. 3B, F, and J) were employed to stain frozen sections of the spinal cords as described in Materials and Methods. PV antigens appeared not to be present in astrocytic cells (Fig. 3D, H, and L). This further supports the idea that both the OM virus and its mutant virus SA replicate mainly in neurons and not in astrocytes.
Mutation site determining SA phenotype.
To identify a mutation site(s) that influences the mouse neurovirulence phenotype, we determined the entire nucleotide sequence of the SA genome. Comparison with the nucleotide sequence of the genome of OM virus disclosed a total of 9 nt changes. The mutation sites are shown in Fig. 4. Three of them appeared to result in amino acid substitutions, one each located within the capsid proteins VP4 (Ile62 to Met) and VP1 (Ala232 to Thr) and within the non-capsid protein 2C (Lys270 to Asn), respectively.
FIG. 4.
Nucleotide and amino acid substitutions in the genomes of SA compared with the sequences of OM. The PV genome is shown at the top of the panel. P1 denotes the viral capsid protein region. P2 and P3 denote the viral non-capsid protein regions. UTR, untranslated region of RNA. Nucleotide and amino acid differences between OM (closed boxes) and SA (open boxes) are indicated in boldface.
It has been reported that mutations in the capsid protein-coding region could confer PV1 mouse neurovirulence phenotype (5, 6, 22). We also considered that mutations found in the capsid protein-coding region might be responsible for the mouse virulence phenotype of SA virus. Accordingly, allele replacement was carried out on the capsid protein-coding regions of the OM and mutant SA viruses. The recombinant viruses were designated OM/SA-cap and SA/OM-cap, respectively (Fig. 5). The phenotypes of OM/SA-cap and SA/OM-cap were compared with those of OM and SA mutant. OM/SA-cap showed a small-plaque phenotype in AGMK cells (data not shown) and a neurovirulence phenotype in IQI mice (Fig. 5). Thus, OM/SA-cap has phenotypes similar to those of mutant SA. On the other hand, SA/OM-cap showed phenotypes similar to those of OM. These results suggest that the mouse virulence phenotype of mutant SA is due to mutations in the capsid-coding region of the viral genome.
FIG. 5.
Structures of the genomes of recombinant viruses. The genome structures of the recombinant viruses are shown as combinations of OM (closed boxes) and SA (open boxes) sequences. Nucleotide sequence differences that resulted in amino acid differences between OM and SA are shown on the OM and SA genomes. Cleavage sites of restriction enzymes used for allele replacement experiments are denoted by the names of the enzymes with the relevant nucleotide positions. The nomenclature for virus strains indicated on the left is explained in Materials and Methods. The LD50 (PFU) of each virus obtained from mouse neurovirulence testing following inoculation via the intraspinal route is shown on the right. P1, P2, P3, and UTR are defined in the legend for Fig. 4.
To identify the key mutation(s) in the capsid region, the sites of mutation of the VP4-, VP1-, or VP3-coding regions in the OM and SA viruses were replaced with each other. The nomenclature for and structures of the resulting recombinant viruses are shown in Fig. 5. The neurovirulence of these recombinant viruses was tested by using IQI mice. All the recombinant viruses that had a mutation at nt 928 showed a neurovirulence phenotype in IQI mice similar to that of the mutant SA virus (Fig. 5). These results indicate that a mutation at nt 928 (replacement of A with G) (Ile62 to Met) within the region coding for the capsid protein VP4 is the key mutation for the mouse neurovirulence phenotype shown by the mutant virus SA.
A mutation at nt 926 (A to G), resulting in substitution of Val for Ile at a.a. 62 within the capsid protein VP4, has been identified in the genome of the mouse-neurovirulent PV1 mutant (6). We constructed a single-point mutant of OM carrying a G at nt 926, Mah-VP4V62, as described in Materials and Methods, and the mouse neurovirulence was compared with that of the mutant SA (Fig. 6). When 106 PFU of Mah-VP4V62 was inoculated intraspinally, three of five IQI mice showed mild paralysis 6 to 8 days after the inoculation. The mild paralysis did not progress toward severe paralysis or death. On the other hand, IQI mice inoculated with 105 PFU of SA showed severe paralysis and died. Thus, the degree of mouse neurovirulence of Mah-VP4V62 delivered via an intraspinal inoculation appears to be much less than that of mutant SA. Intracerebral inoculation of 2.25 × 107 PFU of Mah-VP4V62 did not cause paralysis in IQI mice (Fig. 6), although 107 PFU of intracerebrally inoculated virus caused paralysis in three of six OF1 mice (6). This discrepancy may be due to the different mouse strains used or possible differences in the nucleotide sequences of the genomes of PV1/Mahoney laboratory strains between the two laboratories. In any event, a.a. residue 62 of VP4 must play an important role in host range determination of PV, and mutation of Ile to Met at a.a. 62 within VP4 seems to be especially important for the mouse spinal cord-specific neurovirulence of SA.
FIG. 6.
Neurovirulence test of Mah-VP4V62 in mice. IQI mice were inoculated intraspinally (A) or intracerebrally (B) with the Mah-VP4V62 virus or intraspinally with SA (C) as described in Materials and Methods. The amount of virus inoculated per animal is expressed in log10 PFU at the bottom of each graph. The height of each vertical bar represents the survival period of a mouse. Hatched bars and filled bars indicate portions of the survival period without any clinical symptoms and with paralysis, respectively.
DISCUSSION
A mouse-adapted PV1 mutant (SA), that replicates specifically in the spinal cord, was isolated. This is the first report describing a mouse-adapted PV that shows mouse neurovirulence only when delivered via an intraspinal inoculation route. Mutants of this type might have previously been missed, because an intracerebral neurovirulence test has usually been employed to assess mouse adaptation of PVs. In fact, the replication rate of SA in the mouse brain was similar to that of the parental OM virus (Fig. 2). Our data suggest that PV replication in the brain is required for spread of the virus from the brain to the spinal cord. Since the mutant SA was isolated from the spinal cord of a mouse intracerebrally inoculated with OM virus, it is likely that a certain mutant(s) in a light-sensitive OM virus preparation replicates in the brain first and then spreads to the spinal cord, where an additional mutation(s) occurs during the viral replication, resulting in loss of the capacity for replication in the brain.
Mutant SA can replicate in the brain of the PV-sensitive Tg mouse (data not shown). The observation indicates that all the processes of replication of mutant SA are active after the virus entry into the brain cells and that the defective replication of SA in the brains of non-Tg mice is due to the lack of a cell surface molecule that serves as a receptor of the mutant virus SA. This, in turn, leads to a possibility that mutant SA becomes a mouse-adapted virus by acquiring the capacity to recognize a surface molecule of neurons of the spinal cord as the virus receptor. This molecule may not be expressed in the mouse brain.
The key mutation for the mouse adaptation of mutant SA was identified as a single mutation at nt 928 (A to G) of the genome, that results in substitution of Met for Ile at a.a. 62 of the viral capsid protein VP4. Mutation of Ile to Val at residue 62 of VP4 has been identified as a determinant for mouse adaptation, although this mutant showed mouse neurovirulence when via an intracerebral inoculation route (6) and via an intraspinal inoculation route (Fig. 6). These observations suggest that the a.a. residue 62 of VP4 has an important role in determination of host range of PV. It is not known at present how the amino acid substitution within VP4 functions in mouse adaptation of PV. It is possible, however, that Ile62 of VP4 contributes to maintenance of the canyon structure on the virion surface. Substitution of the amino acid residue, therefore, may result in recognition of molecules in addition to PVR. The mutation may also affect the efficiency of release of VP4 from the virion particle during receptor-mediated virion conformational change (1, 4, 21). Thus, mutant SA may be able to recognize a new mouse molecule in the spinal cord as the receptor, allowing the virion to undergo conformational alteration.
The mutant virus SA showed smaller plaques in AGMK cells (Fig. 1) and less-efficient growth in the brains of Tg mice compared with those of OM virus (data not shown). The efficiencies of early events in PV infection, such as PVR recognition and the virus uncoating process, are possibly altered in SA virus infection. As a result, in AGMK cells, the particle-to-PFU ratio of SA may be higher than that of the parental strain, OM. Indeed, the titer of SA virus recovered from the infected AGMK cells was lower than that of OM virus, as mentioned above. It is also possible that a mutation in VP4 lowers the efficiencies of virus replication processes, such as virus assembly and protein processing. The RNA sequence itself around nt 928 may also have an unknown important role in PV replication.
It is of interest that IQI mice are resistant to 107 PFU of intramuscularly inoculated SA (Table 1). Intramuscularly inoculated PV was shown to be transported through the axon as an intact 160S particle and cause paralysis of Tg mice (8, 25, 28). This pathway is known to be the neural pathway which is dependent on PVR expression. Although the precise mechanisms of development of paralysis mediated by the neural pathway are not known, PVR is thought to have roles in processes including PV incorporation at synapses, PV transportation through the axon, and PV uncoating in the neural cell body (25, 28). Our data on the mutant virus SA, therefore, suggest that a putative new receptor molecule for SA present on the surfaces of neurons in the spinal cord is defective in functions involved in at least one of the processes required for development of the disease by the neural pathway.
Viruses that form larger plaques are readily obtained from preparations of SA during the viral replication in cultured cells. These viruses are no longer mouse neurovirulent (data not shown). To obtain insight into the molecular mechanisms of PV adaptation to mice, several revertants are now being characterized.
ACKNOWLEDGMENTS
We are grateful to S. Kuge, K. Shiroki, and H. Toyoda for helpful suggestions and discussions. We thank Y. Sasaki for expert technical assistance and E. Suzuki and M. Watanabe for help in preparation of the manuscript.
This work was supported by grants from The Ministry of Education, Science, Sports, and Culture of Japan; The Ministry of Health and Welfare of Japan; and The Science and Technology Agency of Japan.
REFERENCES
- 1.Arita M, Koike S, Aoki J, Horie H, Nomoto A. Interaction of poliovirus with its purified receptor and conformational alteration in the virion. J Virol. 1998;72:3578–3586. doi: 10.1128/jvi.72.5.3578-3586.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong C. Successful transfer of the Lansing strain of poliomyelitis virus from the cotton rat to the white mouse. Public Health Rep. 1939;54:2302–2305. [Google Scholar]
- 3.Bodian D. Emerging concept of poliomyelitis infection. Science. 1955;122:105–108. doi: 10.1126/science.122.3159.105. [DOI] [PubMed] [Google Scholar]
- 4.Couderc T, Delpeyroux F, Le Blay H, Blondel B. Mouse adaptation determinants of poliovirus type 1 enhance viral uncoating. J Virol. 1996;70:305–312. doi: 10.1128/jvi.70.1.305-312.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Couderc T, Hogle J, Le Blay H, Horaud F, Blondel B. Molecular characterization of mouse-virulent poliovirus type 1 Mahoney mutants: involvement of residues of polypeptides VP1 and VP2 located on the inner surface of the capsid protein shell. J Virol. 1993;67:3808–3817. doi: 10.1128/jvi.67.7.3808-3817.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Couderc T, Guédo N, Calvez V, Pelletier I, Hogle J, Colbère-Garapin F, Blondel B. Substitutions in the capsids of poliovirus mutants selected in human neuroblastoma cells confer on the Mahoney type 1 strain a phenotype neurovirulent in mice. J Virol. 1994;68:8386–8391. doi: 10.1128/jvi.68.12.8386-8391.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freistadt M S, Kaplan G, Racaniello V R. Heterogeneous expression of poliovirus receptor-related proteins in human cells and tissues. Mol Cell Biol. 1990;10:5700–5706. doi: 10.1128/mcb.10.11.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gromeier M, Wimmer E. Mechanism of injury-provoked poliomyelitis. J Virol. 1998;72:5056–5060. doi: 10.1128/jvi.72.6.5056-5060.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gromeier M, Lu H-H, Wimmer E. Mouse neuropathogenic poliovirus strains cause damage in the central nervous system distinct from poliomyelitis. Microb Pathog. 1995;18:253–267. doi: 10.1016/S0882-4010(05)80002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hagino-Yamagishi K, Nomoto A. In vitro construction of poliovirus defective interfering particles. J Virol. 1989;63:5386–5392. doi: 10.1128/jvi.63.12.5386-5392.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hogle J M, Chow M, Filman D J. Three-dimensional structure of poliovirus at 2.9 Å resolution. Science. 1985;229:1358–1365. doi: 10.1126/science.2994218. [DOI] [PubMed] [Google Scholar]
- 12.Horie H, Koike S, Kurata T, Sato-Yoshida Y, Ise I, Ota Y, Abe S, Hioki K, Kato H, Taya C, Nomura T, Hashizume S, Yonekawa H, Nomoto A. Transgenic mice carrying the human poliovirus receptor: new animal model for study of poliovirus neurovirulence. J Virol. 1994;68:681–688. doi: 10.1128/jvi.68.2.681-688.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koike S, Taya C, Kurata T, Abe S, Ise I, Yonekawa H, Nomoto A. Transgenic mice susceptible to poliovirus. Proc Natl Acad Sci USA. 1991;88:951–955. doi: 10.1073/pnas.88.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koike S, Horie H, Ise I, Okitsu A, Yoshida M, Iizuka N, Takeuchi K, Takegami T, Nomoto A. The poliovirus receptor protein is produced both as membrane-bound and secreted forms. EMBO J. 1990;9:3217–3224. doi: 10.1002/j.1460-2075.1990.tb07520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koike S, Aoki J, Nomoto A. Transgenic mouse for the study of poliovirus pathogenicity. In: Wimmer E, editor. Cellular receptors for animal viruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. pp. 463–480. [Google Scholar]
- 16.Li T, Zhang A, Iizuka N, Nomoto A, Arnold E. Crystallization and preliminary X-ray diffraction studies of coxsackievirus B1. J Mol Biol. 1992;223:1171–1175. doi: 10.1016/0022-2836(92)90268-o. [DOI] [PubMed] [Google Scholar]
- 17.Mandel B. The relationship between penetration and uncoating of poliovirus in HeLa cells. Virology. 1967;31:702–712. doi: 10.1016/0042-6822(67)90198-5. [DOI] [PubMed] [Google Scholar]
- 18.Martin A, Wychowski C, Couderc T, Crainic R, Hogle J, Girard M. Engineering a poliovirus type 2 antigenic site on a type 1 capsid results in a chimaeric virus which is neurovirulent for mice. EMBO J. 1988;7:2839–2847. doi: 10.1002/j.1460-2075.1988.tb03140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin A, Benichou D, Couderc T, Hogle J M, Wychowski C, Van Der Werf S, Girard M. Use of type 1/type 2 chimeric polioviruses to study determinants of poliovirus type 1 neurovirulence in a mouse model. Virology. 1991;180:648–658. doi: 10.1016/0042-6822(91)90078-p. [DOI] [PubMed] [Google Scholar]
- 20.Mendelsohn C L, Wimmer E, Racaniello V R. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989;56:855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- 21.Moscufo N, Yafal A G, Rogove A, Hogle J, Chow M. A mutation in VP4 defines a new step in the late stages of cell entry by poliovirus. J Virol. 1993;67:5075–5078. doi: 10.1128/jvi.67.8.5075-5078.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moss E G, Racaniello V R. Host range determinants located on the interior of the poliovirus capsid. EMBO J. 1991;10:1067–1074. doi: 10.1002/j.1460-2075.1991.tb08046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murray M G, Bradley J, Yang X-F, Wimmer E, Moss E G, Racaniello V R. Poliovirus host range is determined by a short amino acid sequence in neutralization antigenic site I. Science. 1988;241:213–215. doi: 10.1126/science.2838906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nomoto A, Koike S, Aoki J. Tissue tropism and species specificity of poliovirus infection. Trends Microbiol. 1994;2:47–51. doi: 10.1016/0966-842x(94)90125-2. [DOI] [PubMed] [Google Scholar]
- 25.Ohka S, Yang W-X, Terada E, Iwasaki K, Nomoto A. Retrograde transport of intact poliovirus through the axon via the fast transport system. Virology. 1998;250:67–75. doi: 10.1006/viro.1998.9360. [DOI] [PubMed] [Google Scholar]
- 26.Reed L J, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 27.Ren R, Racaniello V R. Human poliovirus receptor gene expression and poliovirus tissue tropism in transgenic mice. J Virol. 1992;66:296–304. doi: 10.1128/jvi.66.1.296-304.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren R, Racaniello V R. Poliovirus spread from muscle to the central nervous system by neural pathways. J Infect Dis. 1992;166:747–752. doi: 10.1093/infdis/166.4.747. [DOI] [PubMed] [Google Scholar]
- 29.Ren R, Costantini F, Gorgacz E J, Lee J J, Racaniello V R. Transgenic mice expressing a human poliovirus receptor: a new model for poliomyelitis. Cell. 1990;63:353–362. doi: 10.1016/0092-8674(90)90168-e. [DOI] [PubMed] [Google Scholar]
- 30.Rossmann M G, Arnold E, Erickson J W, Frankenberger E A, Griffith J P, Hecht H-J, Johnson J E, Kamer G, Luo M, Mosser A G, Rueckert R R, Sherry B, Vriend G. Structure of a human common cold virus and functional relationship to other picornaviruses. Nature. 1985;317:145–153. doi: 10.1038/317145a0. [DOI] [PubMed] [Google Scholar]
- 31.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 32.Schlesinger R W, Morgan I M, Olitsky P K. Transmission to rodents of Lansing type poliomyelitis virus originating in the Middle East. Science. 1943;98:452–454. doi: 10.1126/science.98.2551.452. [DOI] [PubMed] [Google Scholar]
- 33.Shiroki K, Ishii T, Aoki T, Kobashi M, Ohka S, Nomoto A. A new cis-acting element for RNA replication within the 5′ noncoding region of poliovirus type 1 RNA. J Virol. 1995;69:6825–6832. doi: 10.1128/jvi.69.11.6825-6832.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]