Abstract
Musk is an important animal product, but the musk secretion mechanism of forest musk deer (Moschus berezovskii) is still unclear. The musk synthesis process in forest musk deer is extremely complex, and many raw materials are directly or indirectly derived from forest musk deer blood. In this study, metabolomics was used to analyze the blood of forest musk deer in secretory and non-secretory phases for the first time, aim at explaining the secretion mechanism from the perspective of blood metabolism. We found that P450-related, choline-related, axonal regeneration and other pathways and related metabolites were significantly enriched during the musk secretion of forest musk deer. These pathways and metabolites related to P450 and choline in blood may have important implications for the mechanism of musk secretion in forest musk deer, because blood components were closely related to musk components and could provide raw materials for musk synthesis in musk gland cells.
Keywords: Blood components, Metabolomics, Musk secretion
Subject terms: Metabolomics, Biochemistry, Metabolomics
Introduction
Forest musk deer (Moschus berezovskii) (FMD), also known as musk deer, are the smallest musk deer species and are mainly found in China. Musk deer is a relatively primitive group of deer animals, small in size, solitary, strong territorial; Natural populations successfully utilize a variety of habitat types; wide range of food, strong adaptability; The twin rate was higher and the larval development was faster. Therefore, it can be considered that musk deer is the type of deer that prefers r strategy1,2. They inhabit forests at an altitude of 2400‒3800 m, mainly broad-leaved, coniferous, and mixed coniferous and broad-leaved forests in low mountainous areas. FMD prefers to live in mixed coniferous and broadleaved forests with high relative humidity. They have independent activities and strong attachment to the original habitat. Musk is a precious Chinese herbal remedy and a premium raw ingredient. Throughout history, the population of FMD has been at risk due to the unrestricted exploitation of musk3. Habitat reduction and indiscriminate hunting have endangered wild FMD populations4. Owing to the sharp decline in the number of wild FMD in China, on October 24, 2002, all FMDs were reclassified from national second-class to national first-class protected wild animals5.
During the estrous season, FMD attract females by secreting musk2. Secreted musk can help male FMD maintain relationships with specific female FMD, thereby providing opportunities for proliferation of the population and improving their quality of life. In addition, these secreted musks can inhibit the entry of other species, establish certain territories, resist attacks by hostile species, and communicate socially to deter other populations, thereby maintaining the balance of the population6. Secreted musks also provide considerable physiological information, such as the sex, age, and physical state of FMD, as well as the local environmental conditions2.
Musk is secreted by the musk glands of male FMD and contains extremely complex components, mainly macrocyclic ketones, pyridines, steroids, and other alkaloids, as well as polypeptide proteins, fatty acids, esters, and inorganic acids7. The synthesis mechanism of various components in musk is unclear, which greatly affects our understanding of the musk formation process. The mechanism of musk synthesis has been studied from several perspectives, including the anatomical and tissue structure of the musk sac8, the regulation of sex hormones on musk secretion9, and genetic diversity of musk-related genes in FMD10. However, studies on the regulatory mechanisms of musk synthesis are limited. Blood is difficult to obtain because of the stress caused to FMDs. For FMD, research has been conducted examining genomics, transcriptomics, and proteomics11–13. Upon comparing its genome with genomes of nine other cloven-hoofed animals, it was discovered that eight positively selected genes (PSGs) within the FMD were identified in three KEGG pathways: steroid hormone biosynthesis, terpenoid backbone biosynthesis, and aldosterone-regulated sodium reabsorption12. These pathways are linked to musk metabolism and synthetic processes. Another Illumina-based study revealed significant enrichment of aldosterone metabolic process, flavone metabolic process, and aldosterone biosynthetic process in musk glands11. These findings align with our own discoveries. To the best of our knowledge, with the aim of understanding FMD secretion physiological condition, this is the first analysis of FMD blood metabolites that fills an important knowledge gap in this field.
The raw materials required for musk synthesis are mainly obtained from the blood supply; therefore, blood metabolites in the secretion phase are critical for musk synthesis. Metabolomics can qualitatively and quantitatively analyze all the low-molecular-weight metabolites in certain organisms,tissues, and cells14. In this study, the differentially metabolites identified via plasma metabolomics were analyzed during musk secretion and non-secretion periods.
Several pathways and metabolites with the highest proportion of expression were annotated to determine the complex metabolic network between blood metabolites and the musk synthesis process in FMD, thereby providing a basis for exploring the related mechanism of blood metabolites in the musk formation process.
Methods
Blood sample collection
The 10 male FMD used in the experiment are from the Hebei Huailai Breeding Base. The base is located in Xinglinbao Village, Wangjialou, Huailai County, Hebei Province. There are more than 300 closed shelters and more than 200 FMD are kept in captivity. The main diets of FMD are leaves, including apricot leaves, elm leaves, ailanthus leaves, birch leaves, as well as dandelions and Houttuynia cordata. All 10 FMDs involved in our research were maintained in the same captive environment with the same diet and sanitation standards. As only male musk deer produce musk, all individuals involved in the study were male.
This study was approved by the Ethics Committee of Beijing Forestry University, Beijing, China; Zhiyangtianbao Biological Science and Technology Ltd, Huailai, China (which managed the sampled FMD). The study was conducted in accordance with the recommendations of the Institutional Animal Ethics and Care Committee of the Beijing Forestry University. All experimental procedures were performed with the assistance of the local veterinarians. This blood collection method has been proven to be simple, safe and reliable, and has little impact on the animals after the blood collection.
The FMD involved in this study are all kept in captivity and are privately owned by operators in the artificial breeding industry, but their trades and breeding licenses are supervised by the Forestry Bureau. Male FMD secretes musk from May to July every year15, in this period many of its daily behaviors and habits tend to decrease or disappear, such as food intake being reduced or even stopped16, and the amount of time spent on lying and resting is increased, but it is still active during peak activity hours. During activities, FMD moves less, stands more, looks numb, and stands blankly. There are occasional defecation movements, but no feces are discharged, it also licks the vagina more frequently17. In terms of physiological manifestations, the testicles and scrotum of male FMD are swollen and drooping, the scent glands are enlarged and clearly visible, and the musk fragrance overflows and has a fragrant smell16. Based on the above status, it can be judged that the FMD has entered the stage of secretion.
We conducted blood sampling on 10 male FMD during the non-secretion phase from April 14–17, 2023, and then repeated the procedure on the same 10 FMD during the secretory phase from June 2–5, 2023. Prior to the blood collection, the 10 FMDs were herded into a small enclosure 1–2 h in advance. Subsequently, the FMDs were guided into a capture wooden box through a specially designed false door with holes. A veterinarian should then securely grasp the FMD's hind legs with both hands, lifting the lower leg below the joint, twisting the FMD to the left side, and holding the front leg below the wrist joint with the right hand. The FMD should be positioned flat on the veterinarian's thigh with attention to avoid compressing the heart and rumen while the veterinarian is seated on a small wooden stool 30–40 cm high. An assistant should hold the FMD's head to prevent any harm from its fangs during moments of agitation. Simultaneously, the assistant should cover the FMD's eyes with a cloth, ensuring not to obstruct its nose to maintain proper breathing.
We collected blood samples from 10 FMDs at the Hebei Huailai Breeding Base during musk secretion and non- secretion periods in April and June in 2022. FMD are wild animals, but the blood collected in this study came from captive populations. The blood collector collects blood from the veins of the forelimbs of FMD. the assistant holds the FMD, then the blood collector straightens the lower limb of the FMD with his left hand to fill the veins. Or use the pulse as an indicator and insert a syringe into the blood vessel with your right hand.
One to two milliliters of venous blood was extracted from each FMD using disposable sterile syringes. All fresh blood samples were collected in test tubes containing ethylenediaminetetraacetic acid then stored in liquid nitrogen. After centrifugation, the plasma metabolites were utilized for metabolomics analysis.
Metabolite extraction
The liquid chromatography tandem-mass spectrometry (LC–MS/MS) system for the metabolomic analysis consisted of a Waters Acquity I-Class PLUS ultra-high performance liquid tandem Waters Xevo G2-XS QTof high-resolution mass spectrometer. The column was purchased from Waters Acquity UPLC HSS (T3 column, 1.8 μm, 2.1 × 100 mm).
The following solutions were used for the positive ion mode: mobile phase A: 0.1% formic acid aqueous solution; mobile phase B: 0.1% formic acid acetonitrile. For negative ion mode the following solutions were used: mobile phase A: 0.1% formic acid aqueous solution; mobile phase B: 0.1% formic acid acetonitrile. The injection volume was 1 μL.
LC–MS/MS analysis
We used a Waters Xevo G2-XS QTOF high-resolution mass spectrometer to collect primary and secondary mass spectrometry data in MSe mode under the control of acquisition software (MassLynx V4.2, Waters). In each data acquisition cycle, we performed dual-channel data acquisition simultaneously at both low and high collision energies. The low collision energy range was 2 V, the high collision energy range was 10‒40 V, the scanning frequency for a mass spectrum was 0.2 s. The parameters of the electrospray ionization source were as follows: cone voltage: 30 V; Capillary voltage: 2000 V (positive ion mode) or − 1500 V (negative ion mode); ion source temperature: 150 °C; backflush gas flow rate: 50 L/h; desolventizing gas flow rate: 800 L/h; desolvent gas temperature 500 °C.
Data preprocessing and annotation
For peak extraction, peak alignment, and other data processing operations, the raw data collected by MassLynx V4.2 were processed using Progenesis QI software. Based on Progenesis QI software, online METLIN database and Biomark’s self- built library were used for identification. The theoretical fragment identification and mass deviation were within 100 ppm.
Data analysis
The original peak area information was normalized to the total peak area for subsequent analysis. We used principal component analysis and Spearman’s correlation analysis to assess the repeatability of the samples within the group and the quality control samples. All the identified compounds were classified and searched for pathway information in the Kyoto Encyclopedia of Genes and Genomes (KEGG), Human Metabolome Database (HMDB), and LipidMaps databases. The difference multiples were calculated and compared, and the statistical significance (p-value) of the difference of each compound was calculated by the t-test. The orthogonal projections structure-discriminant analysis (OPLS-DA) modeling was orthogonally projected using the R language package “ropls,” and 200 permutation tests were performed to verify the reliability of the model. We used multiple cross-validations to calculate the variable importance in the projection (VIP) value of the model. The differential metabolites were screened by the combination of OPLS-DA model, p-value, and VIP value. The screening criteria were p-value < 0.05, fold change (FC) > 1, and VIP > 1. The hypergeometric distribution test was used to calculate the differences in metabolites with significant KEGG pathway enrichment.
Results
Metabolomics analysis
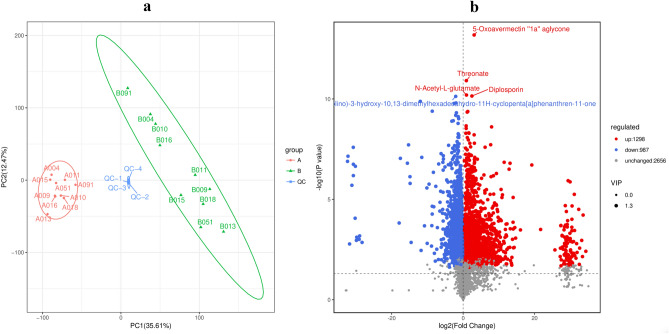
In the default mode, we identified 16,847 peaks, from which 4941 annotated metabolites were extracted. Figure 1(a),(b) show that two groups of samples generally showed significant differences in their characteristics. The metabolite contents during the non-secretion period were relatively similar, while the metabolite contents during the secretion period varied significantly.
Figure 1.
(a) Principal component analysis diagram of difference grouping. Note: the green data points in group B represent the secreted category, the red data points in group A represent the non-secreted category. (b) Volcano plot of sample metabolites. Note: each point in the volcano map represents a metabolite; the scatter size represents the VIP value of the OPLS‒DA model.
In the secretion period, 1298 metabolites were upregulated and 987 metabolites were downregulated significantly; the increase was generally greater than the decrease (which showed in Fig. 1(b)). The reliability of the results was determined because the FC values were generally high. Figure 1(b) shows the annotation characteristics of the five most important significantly upregulated compounds in the volcanic map, i.e., 5-oxoavermectin "1a" aglycone, threonate, N-acetyl-l-glutamate, diplosporin, which increased significantly from the non-secretion period to the secretion period.
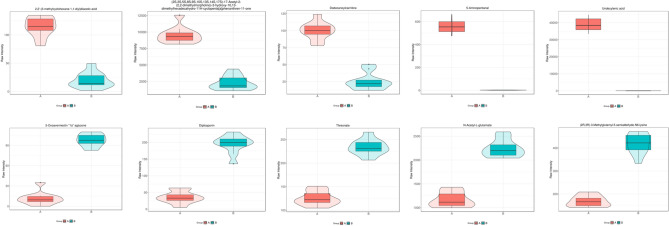
Figure 2 shows the top 10 metabolites with the smallest p value. Among the up-regulated metabolites, N-Acetyl-l-glutamate has the most significant increase; among the down-regulated metabolites, undecylenic acid has the most significant decrease.
Figure 2.
Violin plot of the top 10 metabolites with the smallest p-value. Note: the x-axis represents the grouping, and the y-axis is the metabolite content. The middle is the box diagram, and the thin black line extending from it represents the 95% confidence interval, the minimum value, and the maximum value, forming spacing that can reflect the degree of variation in the data.
Pathway analysis
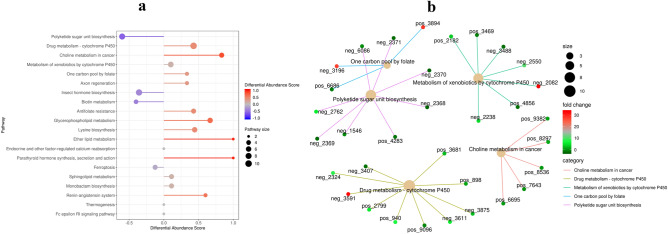
The metabolic pathways most strongly related to the differences between the musk non-secretion and secretion periods of FMD are shown in Fig. 3(a). The relationships between the five most significant metabolic pathways and annotated differential metabolites are shown in Fig. 3(b).
Figure 3.
(a) Differential metabolite differential abundance score plot. The 10 pathways with the most significant metabolic differences. Red indicates up-regulation and purple indicates down-regulation. The size of the circle represents pathway size, and the length of the color line represents differential abundance score. (b) Enrichment network diagram. The figure shows the specific metabolites involved in the five most significant pathways, and the color of the metabolite circle represents fold change.
Among the pathways with the highest up-regulated abundance, we mainly focus on the following three categories of pathways: P450-related pathways, choline-related pathways and axon regeneration, because these three categories of pathways are most obviously related to the musk secretion mechanism of FMD.
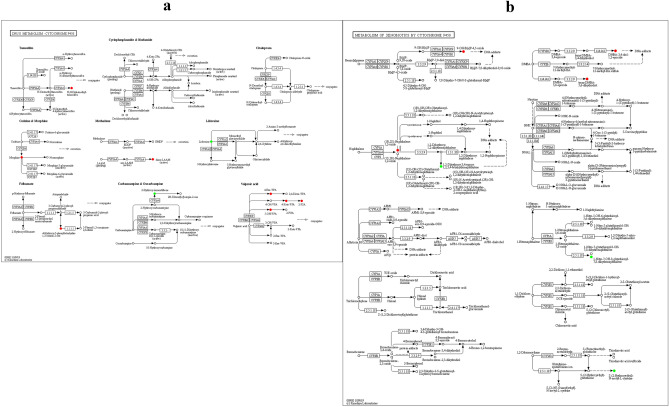
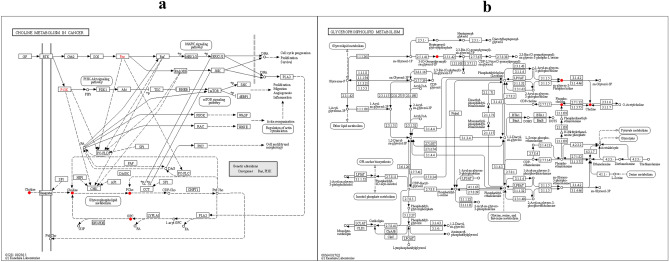
P450 related pathways include the following two: drug metabolism—cytochrome P450, which involves several significant metabolites (5-Hydroxyvalproic acid, etc. see in Fig. 4(a)), another one is metabolism of xenobiotics by cytochrome P450, in which the important metabolite (1aalpha,2beta,3alpha,11calpha)-1a,2,3,11c-Tetrahydro-6,11-dimethylbenzo[6,7]phenanthro[3,4-b]oxirene-2,3-diol) upregulated significantly (Fig. 4(b)). Despite P450-related pathway, another two choline-related pathways also worth attention: choline metabolism in cancer, in which choline, choline phosphate, and glycerophosphocholine are significantly up-regulated (Fig. 5(a)), these metabolites are also enriched in pathway glycerophospholipid metabolism (Fig. 5(b)). Finally, there is axon regeneration, in which Anandamide and 5-Hydroxy-L-tryptophan are significantly increased (Fig. 6).
Figure 4.
(a) KEGG annotation results of differential metabolites in drug metabolism—cytochrome P450. (b) KEGG annotation results of differential metabolites in metabolism of xenobiotics by cytochrome P450. Red indicates that the metabolite content is significantly up-regulated, and green indicates that the metabolite content is significantly down-regulated.
Figure 5.
(a) KEGG annotation results of differential metabolites in choline metabolism in cancer. (b) KEGG annotation results of differential metabolites in glycerophospholipid metabolism.
Figure 6.
KEGG annotation results of differential metabolites in axon regeneration.
Discussion
Analysis of the physiological and chemical bases of musk secretion mechanisms has become an important issue in musk research; however, current understanding of related factors remains limited, particularly the connection between musks and blood metabolites18. Based on the hypothesis that musk secretion by FMD is related to a series of different metabolic processes9, metabolomics may be a valuable tool for improving our understanding of the complex mechanism of musk secretion. FMD is categorized as a national first-class protected species and is also known for its high stress levels. Considering the biological traits of the research subjects and the minimum sample size essential for the study, we conclude that utilizing 10 male musk deer would be adequate to mitigate the influence of random errors. To the best of our knowledge, this is the first research to use high-throughput metabolomics to study musk secretion from FMD.
According to the KEGG functional annotation and differential metabolite enrichment analysis, we found that the following significantly enriched pathways deserve focused discussion. We focus on the two pathways related to P450, drug metabolism—cytochrome P450 and metabolism of xenobiotics by cytochrome P450, because Cytochrome P450 plays an important role in the secretion of FMD19. Two choline-related pathways also worth discussion, because choline plays an important role in cancer-related pathways, and the similarity between cancer and musk secretion has been focused on10. Axon regeneration also worth discussion, because we think it is potentially related to the abnormal behavior of FMD when secreting musk20.
Cytochrome P450 (CYP450) belongs to a large family of self-oxidizing heme proteins19. Drug metabolism-cytochrome P450 and the metabolism of xenobiotics by cytochrome P450 pathways were significantly enriched in the serum of musk deer during the musk secretion period, indicating that in the synthesis of male hormones (i.e., C19 steroids) in musk components, P450 in the blood during the musk secretion period may have played a role in the further modification or transformation of its precursors.
As a terminal oxygenase, P450 is involved in sterol synthesis. We found that in the drug metabolism-cytochrome P450 metabolic pathway, there was significant enrichment of several branches, such as methadone, tamoxifen, felbamate, and valproic acid. The basic function of eukaryotic class I enzymes in the P450 family is related to the mitochondrial inner membrane, which catalyzes several steps in steroid hormone and vitamin D biosynthesis19. In animals, it has many physiological functions in the biosynthesis and catabolism of signaling molecules, lipid oxides, and steroid hormones19. Previous studies have shown that P450 plays a role in the metabolism of xenobiotics and endogenous substrates involved in the metabolism of steroids, fatty acids, prostaglandins, and even ketones21. It can be speculated that the high P450 pathway activity of musk deer during the musk secretion period may be closely related to the synthesis of musk ketone, cyclopentadecanone, cholesterol, 3a-hydroxy-5b-androstane-17-one, cholesterol, 1,2-cyclododecanediol, and other musk components.Previous studies have found that several important steroid oxidation reactions occur in mitochondria, including the steroid oxidation reaction catalyzed by cholesterol side chain lyase (now known as P450 11A1), which initiates the entire process of steroid production22. This suggests that the synthesis of steroids, the most important major component of musk, may be significantly affected by P450-related pathways (Fig. 4).
Notably, several studies have examined the role of cytochrome P450 in herbal plants. Plants and animals share common metabolic pathways and secondary metabolites at the cellular level. Using in vitro and in vivo methods, previous studie have identified many herbs and natural compounds isolated from herbs as substrates, inhibitors, and/or inducers of cytochrome P450 enzymes23. Moreover, the regulation of CYPs in animals by herbal products appears to be complex, depending on the type, dosage, administration route, target organs, and species24. Recent studies have shown that feeding Chinese herbal medicines to FMD can supplement their nutrition25. Therefore, the association between herbal medicine and P450 may provide an optimization strategy for the nutrition of FMD and improving their aroma secretion ability.
Owing to the rapid proliferation of aromatic cells during the rapid growth period of the gland, this process is similar to that in malignant tumors. Therefore, some researchers have regarded it as cancer-like growth10. The gradual growth of musk glands from the non-secretion period to the secretion period requires cytokines to closely regulate cell growth. We found that the Choline metabolism in cancer (Fig. 5a) and glycerophospholipid metabolism (Fig. 5b) were significantly enriched during aroma secretion. Among these, the concentrations of choline, phosphocholine, sn-glycero-3-Phosphocholine, and 3-(O-geranylgeranyl)-sn-glycerol 1-phosphate metabolites were significantly increased (Fig. 5), which may have been positively correlated with gland growth. Choline is an essential nutrient that plays important roles in cellular metabolism and normal function24,26. The liver is the central organ responsible for choline metabolism. Many studies have emphasized the importance of glycerophosphocholine in cancer27. Active enzymes in the GPC degradation pathway are often overexpressed in cancer cells, and may cause cancer cell proliferation, migration, and invasion28. The decrease in the activity of the ferroptosis pathway observed in this study (Fig. 3a) may also have been related to this phenomenon.
Upregulation of the axon regeneration pathway can explain the musk-secreting behavior of FMD. Our experiments revealed that metabolites such as anandamide and oxitriptan were significantly enriched (Fig. 6). Anandamide affects sleep and eating patterns, enhances happiness, and relieves pain29. Oxitriptan reduces food intake in rats, and slows food intake and improves exercise performance in dogs20. Previous studies have shown that estrogen induces precocious axonogenesis in the developing rat brain30. It can be speculated that these neuromodulatory metabolites increase the degree of stress during the estrous period in FMD, which is closely related to high alertness and reduced eating behavior during the secretion period31. Previous studies have shown that bile volume in male musk deer is negatively correlated with musk secretion; the greater the stress, the smaller the bile volume of the FMD32. Therefore, it can be speculated that the axon regeneration pathway promotes musk secretion by enhancing stress and reducing the bile volume in FMD.
Due to the protection level of FMD and the vulnerability to panic stress, it is difficult to take blood samples, so the amount of samples we obtained is small, which is not enough to support more in-depth analysis. On the one hand, metabolomics studies on blood only locate the pathways vaguely with abnormal expression in the secretory phase and some metabolic components. Revealing the secretion mechanism requires more targeted localization experiments for specific metabolites in these metabolic pathways. On the other hand, due to the wide range of labeled metabolites and pathways in metabolomics, we could not explain the correlation between all the significantly changed pathways and musk secretion of FMD. However, we still present these results in the text, rather than omitting them in order to prevent induced argument. It is very important to reveal the secretion mechanism of FMD, because it helps to alleviate the protection pressure caused by the use of musk on FMD by restoring the incense production process in vitro. If a library of musk components of FMD can be established, and then a transformation path library can be established, and then compared with the metabolomics components involved in this work, then, even through the enumeration method, a complex interactive relationship between the blood metabolite group and the musk component group can be accurately established.
Conclusion
Non-targeted metabolomics provide valuable information on metabolic pathways, which may be an important supplement for explaining the mechanisms of musk secretion in FMD. Drug metabolism-cytochrome P450 and metabolism of xenobiotics by cytochrome P450 pathways appeared to play central roles in musk secretion. Pathways such as choline metabolism in cancer have verified the commonality between scent gland growth and cancer. Axon regeneration may be related to the abnormal behavior of FMD in secretory phase. In the future, it will be necessary to conduct joint omics of the transcriptome, genome, and proteome, as well as target experiments on the identified compound types, to more accurately verify the metabolic pathways and metabolites that were the focus of this study. The current omics analyses have explored the FMD from various angles. In the future, a comprehensive meta-analysis should consolidate these dispersed studies, followed by functional validation through in vitro cell culture based on the deduced outcomes.
Acknowledgements
Special thanks are extended to the Hebei Huailai Breeding Base. Their collaboration and granting us access to collect the biological maternal (blood) specimens used in this study have been invaluable.
Author contributions
Yufan Wang (First Author): conceptualization, methodology, software, investigation, formal analysis, writing—original draft; Pengcheng Yang: data curation, writing—original draft; Xian An: visualization, investigation; Jingyao Hu: resources, supervision; Taoyue Chen: software, validation; Liancheng Xu: software, investigation; Yuli Xu: visualization; Shuqiang Liu* (corresponding author): conceptualization, funding acquisition, resources, supervision, writing—review and editing; Congxue Yao (second corresponding author): funding acquisition, resources.
Funding
This work was supported by the Beijing Municipal Natural Science Foundation (Grant No. 5242015), Huailai Zhiyangtianbao Technical development Co., Ltd. (2021HXFWBH-LSQ-02) and Zhangzhou Pien Tze Huang Pharmaceutical Co., Ltd. (2021HXFWBH-LSQ-01).
Data availability
The data used to support the finding of this study are available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Liu, Z. Dispersing or contracting: A perspective on the evolutionary history and population conservation of musk deer. Am. J. Life Sci.4, 20. 10.11648/j.ajls.20160402.12 (2016). 10.11648/j.ajls.20160402.12 [DOI] [Google Scholar]
- 2.Song, X., Yang, F. & Xing, X. Species, distribution, value and conservation strategies of musk deer in China [J]. Spec. Econ. Anim. Plants9, 5–7 (2008). [Google Scholar]
- 3.Zhongxian, X. et al. Chemical composition and microbiota changes across musk secretion stages of forest musk deer. Front. Microbiol.1, 1. 10.3389/fmicb.2024.1322316 (2024). 10.3389/fmicb.2024.1322316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ling, L. et al. Characterization of major histocompatibility complex DRA and DRB genes of the forest musk deer (Moschus berezovskii) [J]. Chin. Sci. Bull.58(18), 2191–2197 (2013). 10.1007/s11434-012-5581-5 [DOI] [Google Scholar]
- 5.Hu, X. Quantitative study on the dynamics of intestinal parasites and flora in forest musk deer and its health indication function. Beijing Forestry University, PhD dissertation. 10.26949/d.cnki.gblyu.2017.000093 (2017).
- 6.Ning, W. Study on the main behaviors of captive male forest musk deer during estrus and non-estrus [D] (Beijing Forestry University, 2014).
- 7.Zhang, T. et al. Study of compositions of musks in different types secreted by forest musk deer (Moschus berezovskii). PLoS One.16(3), e0245677. 10.1371/journal.pone.0245677 (2021). 10.1371/journal.pone.0245677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fangdong, Z. Study on cytogenetics and molecular marker microsatellites in forest musk deer [D] (Sichuan University, Chengdu, 2005).
- 9.Tang, Z. S. et al. Quality markers of animal medicinal materials: Correlative analysis of musk reveals distinct metabolic changes induced by multiple factors. Phytomedicine44, 258–269. 10.1016/j.phymed.2018.03.008 (2018). 10.1016/j.phymed.2018.03.008 [DOI] [PubMed] [Google Scholar]
- 10.Zhou, C. et al. Genomic evidence sheds light on the genetic mechanisms of musk secretion in muskrats. Int. J. Biol. Macromol.145, 1189–1198. 10.1016/j.ijbiomac.2019.10.045 (2020). 10.1016/j.ijbiomac.2019.10.045 [DOI] [PubMed] [Google Scholar]
- 11.Xu, Z. et al. Illumina-based de novo transcriptome sequencing and analysis of Chinese forest musk deer. J. Genet.96, 1033–1040. 10.1007/s12041-017-0872-x (2017). 10.1007/s12041-017-0872-x [DOI] [PubMed] [Google Scholar]
- 12.Zhou, C. et al. Comparative genomics reveals the genetic mechanisms of musk secretion and adaptive immunity in chinese forest musk deer. Genome Biol. Evol.11(4), 1019–1032. 10.1093/gbe/evz055 (2019). 10.1093/gbe/evz055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang, J. et al. ITRAQ-based quantitative proteomics analysis of forest musk deer with pneumonia. Front. Vet. Sci.26(9), 1012276. 10.3389/fvets.2022.1012276 (2022). 10.3389/fvets.2022.1012276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schrimpe-Rutledge, A. C. et al. Untargeted metabolomics strategies-challenges and emerging directions. J. Am. Soc. Mass Spectrom.27(12), 1897–1905. 10.1007/s13361-016-1469-y (2016). 10.1007/s13361-016-1469-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han, H. et al. Behavioral characteristics and influencing factors of captive forest musk deer (Moschus berezovskii) [J]. J. Sichuan Agric. Univ.37(01), 116–121. 10.16036/j.issn.1000-2650.2019.01.018 (2019). 10.16036/j.issn.1000-2650.2019.01.018 [DOI] [Google Scholar]
- 16.Bai, R. et al. Relationship between behavioral diversity and musk secretion of captive forest musk deer [J]. J. Zhejiang A&F Univ.36(2), 343–348. 10.11833/j.issn.2095-0756.2019.02.016 (2019). 10.11833/j.issn.2095-0756.2019.02.016 [DOI] [Google Scholar]
- 17.Dayong, F. et al. Some behavioral performance of captive male musk deer during the musk-secreting period [J]. Agric. Technol.40(09), 126–130. 10.19754/j.nyyjs.20200515046 (2020). 10.19754/j.nyyjs.20200515046 [DOI] [Google Scholar]
- 18.Yang, J. et al. Characteristics of steroidogenesis-related factors in the musk gland of Chinese forest musk deer (Moschus berezovskii). J. Steroid Biochem. Mol. Biol.212, 105916. 10.1016/j.jsbmb.2021.105916 (2021). 10.1016/j.jsbmb.2021.105916 [DOI] [PubMed] [Google Scholar]
- 19.Werck-Reichhart, D. & Feyereisen, R. Cytochromes P450: A success story. Genome Biol.1(6), 3003. 10.1186/gb-2000-1-6-reviews3003 (2000). 10.1186/gb-2000-1-6-reviews3003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maffei, M. E. 5-Hydroxytryptophan (5-HTP): Natural occurrence, analysis, biosynthesis, biotechnology, physiology and toxicology. Int. J. Mol. Sci.22(1), 181. 10.3390/ijms22010181 (2020). 10.3390/ijms22010181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schenkman, J. B. Steroid metabolism by constitutive cytochromes P450. J. Steroid Biochem. Mol. Biol.43(8), 1023–1030. 10.1016/0960-0760(92)90329-H (1992). 10.1016/0960-0760(92)90329-H [DOI] [PubMed] [Google Scholar]
- 22.Guengerich, F. P. Cytochrome P450 research. J. Biol. Chem.294(5), 1671–1680. 10.1074/jbc.TM118.004144 (2019). 10.1074/jbc.TM118.004144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou, S. et al. Interactions of herbs with cytochrome P450. Drug Metab. Rev.35(1), 35–98. 10.1081/dmr-120018248 (2003). 10.1081/dmr-120018248 [DOI] [PubMed] [Google Scholar]
- 24.Hollenbeck, C. B. The importance of being choline. J. Am. Diet Assoc.110(8), 1162–1165. 10.1016/j.jada.2010.05.012 (2010). 10.1016/j.jada.2010.05.012 [DOI] [PubMed] [Google Scholar]
- 25.Jiang, Y. et al. Changes in the gut microbiota of forest musk deer (Moschus berezovskii) during ex situ conservation. Front. Microbiol.13, 969593. 10.3389/fmicb.2022.969593 (2022). 10.3389/fmicb.2022.969593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeisel, S. H. et al. Choline, an essential nutrient for humans. FASEB J.5, 2093–2098 (1991). 10.1096/fasebj.5.7.2010061 [DOI] [PubMed] [Google Scholar]
- 27.Sonkar, K. et al. Focus on the glycerophosphocholine pathway in choline phospholipid metabolism of cancer. NMR Biomed.1, e4112. 10.1002/nbm.4112 (2019). 10.1002/nbm.4112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Danishad, K. K. A. et al. Assessment of therapeutic response of locally advanced breast cancer (LABC) patients undergoing neoadjuvant chemotherapy (NACT) monitored using sequential magnetic resonance spectroscopic imaging (MRSI). NMR Biomed.23, 233–241 (2010). 10.1002/nbm.1436 [DOI] [PubMed] [Google Scholar]
- 29.Biringer, R. G. The rise and fall of anandamide: Processes that control synthesis, degradation, and storage. Mol. Cell Biochem.476(7), 2753–2775. 10.1007/s11010-021-04121-5 (2021). 10.1007/s11010-021-04121-5 [DOI] [PubMed] [Google Scholar]
- 30.Hung, A. J. et al. Estrogen, synaptic plasticity and hypothalamic reproductive aging. Exp. Gerontol.38(1–2), 53–59. 10.1016/s0531-5565(02)00183-3 (2003). 10.1016/s0531-5565(02)00183-3 [DOI] [PubMed] [Google Scholar]
- 31.Meng, X. et al. Relationship between estrus cycles and behavioral durations of captive female alpine musk deer. Integr. Zool.3(2), 143–148. 10.1111/j.1749-4877.2008.00082 (2008). 10.1111/j.1749-4877.2008.00082 [DOI] [PubMed] [Google Scholar]
- 32.Xiaobing, L. et al. Bile level of captive musk deer in non-breeding season and its relationship with musk secretion and reproductive performance [J ]. Appl. Ecol.30(02), 661–667. 10.13287/j.1001-9332.201902.038 (2019). 10.13287/j.1001-9332.201902.038 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the finding of this study are available from the corresponding author upon request.