Abstract
Introduction
Our objective was to conduct a systematic review and meta‐analysis of studies evaluating the oncological and reproductive outcomes of patients with endometrial atypical hyperplasia (AH) and endometrioid endometrial cancer (EEC) undergoing conservative therapy with hysteroscopic resection (HR).
Material and methods
This study followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement for systematic reviews and meta‐analyses. The study strictly followed the methodological framework proposed by the Cochrane Handbook and was retrospectively registered in PROSPERO (CRD42023469986). Searches were conducted in PubMed, Embase, and the Cochrane Library, from inception to October 10, 2023. A checklist based on items of the Newcastle–Ottawa Scale and the Methodological Index for Non‐randomized Studies was used for quality assessment. The primary end points for this meta‐analysis were complete response (CR), pregnancy, and live birth rates following HR‐based therapy in patients with EEC or AH. The secondary end point was the recurrence rate (RR).
Results
Twenty‐one articles involving 407 patients with clinical stage IA, low or intermediate grade, EEC, and 444 patients with AH managed with HR‐based conservative treatment were included for this systematic review. CR to HR‐based conservative therapy was achieved in 88.6% of patients with EEC and 97.0% of patients with AH. Of these, 30.6% and 24.2%, respectively, had live births. The overall pooled disease RR was 18.3% and 10.8% in patients with EEC and AH, respectively. Further subset analyses revealed that EEC patients with body mass index (BMI) ≤28 kg/m2 had higher CR rates as well as higher chances of pregnancy and live birth (91.6% CR, 32.9% pregnancy, 31.1% live birth) compared with patients with BMI >28 kg/m2 (86.4% CR, 28.4% pregnancy, 23.0% live birth). The HR followed by oral progestogen subgroup had higher CR rates and higher chances of pregnancy and live birth (91.8% CR, 36.3% pregnancy, 28.2% live birth) than the HR followed by the levonorgestrel intrauterine system subgroup (82.5% CR, 25.3% pregnancy, 16.3% live birth).
Conclusions
Hysteroscopic resection followed by progestins appears to be a promising choice for fertility‐sparing treatment in young patients with AH and EEC, with effective and safe responses. The live birth rate remains to be improved by providing medical guidance and encouragement.
Keywords: conservative therapy, endometrial atypical hyperplasia, endometrial cancer, fertility‐sparing, hysteroscopic resection
HR‐based fertility‐sparing therapy showed an effective and safe response. Patients with EEC and a BMI ≤28 kg/m2 had higher CR, pregnancy, and live birth rates. Live birth rates remain to be improved by providing medical guidance and encouragement.
Abbreviations
- AH
atypical hyperplasia
- BMI
body mass index
- CR
complete response
- EC
endometrial cancer
- EEC
endometrioid endometrial cancer
- GnRHa
gonadotropin‐releasing hormone agonist
- G1
grade 1
- G2
grade 2
- HR
hysteroscopic resection
- PROSPERO
International Prospective Register of Systematic Reviews
- LNG‐IUS
levonorgestrel intrauterine system
- OP
oral progestins
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses
- RR
recurrence rate
Key message.
Hysteroscopic resection‐based fertility‐sparing therapy showed an effective and safe response. Patients with endometrioid endometrial cancer and a BMI ≤28 kg/m2 had higher complete response, pregnancy, and live birth rates. Live birth rates remain to be improved by providing medical guidance and encouragement.
1. INTRODUCTION
Endometrial cancer (EC) is the third most common gynecological cancer. The global incidence of EC has increased by 132% over the past 30 years. 1 , 2 In addition, the number of patients under the age of 40 years has risen persistently in recent years, 3 and the incidence of early‐stage, low‐grade EC has increased from 2.2 to 4.0 per 100 000 in women aged 35–39 years and from 0.7 to 2.0 per 100 000 in women aged 30–34 years from 2000 to 2017. 4
The standard therapy for early‐stage EC is total extra‐fascial hysterectomy and bilateral salpingo‐oophorectomy, with or without lymphadenectomy 3 ; however, this treatment may not be acceptable or suitable for young women who have a strong desire to maintain their fertility. Therefore, fertility‐sparing management of EC and precursor lesions of EC, such as endometrial atypical hyperplasia (AH), should be considered.
The main conservative therapy for early EC or AH is oral or intrauterine progestins. In one study, the complete response (CR) rate was 65.8% for AH and 48.2% for low‐grade early‐stage EC, while the recurrence rate (RR) was 23.2% for AH and 35.4% for low‐grade early‐stage EC. 5 Hysteroscopic resection (HR) followed by oral or intrauterine progestins has been reported as a method for improving therapeutic efficacy in many recent studies. 6 Previous reviews or meta‐analyses have reported that the CR rates for HR conservative therapy in EC/AH and EC, are approximately 90%–95.3% (EC), and 98.06% (AH and EC), respectively. 7 , 8 , 9 However, reproductive outcomes, including pregnancy and live birth rates, which are the ultimate goals of fertility‐sparing treatment, are limited and vary widely (pregnancy rate for EC: 34%–47.8%, live birth rate for EC: 25.5%–30.7%, live birth rate for EC and AH: 52.57%). 7 , 8 , 9 , 10 The purpose of our article is to systematically analyze the oncological and reproductive outcomes among AH and EC patients undergoing conservative therapy with HR.
2. MATERIAL AND METHODS
2.1. Identification of literature
This study followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement for systematic reviews and meta‐analyses. The methodological framework proposed in the Cochrane Handbook was strictly followed, 11 and the study was retrospectively registered in PROSPERO (CRD42023469986). The articles were searched from PubMed, Embase, and the Cochrane Library from inception to October 10, 2023, using combinations of free terms, with variations, and controlled vocabulary (eg MeSH terms/descriptors), based on the following themes: endometrial cancer, endometrial atypical hyperplasia, premalignant endometrial, hysteroscopes, fertility sparing, and conservative treatment (Table S1). Searches were conducted independently by two investigators (SZ and LT).
2.2. Study selection
The searched articles were saved in a reference manager (Endnote 20; Clarivate, Philadelphia, PA, USA) and duplicate articles were removed. Studies were selected according to the following criteria:
Presence of a group or subgroup of women with clinical stage IA, low or intermediate grade (G1 or G2) endometrioid EC (EEC), or AH.
Inclusion of patients with fertility‐sparing desires.
Presence of a group or subgroup of patients undergoing conservative therapy with HR.
Presence of reported oncological and/or reproductive outcomes, including disease regression, relapse, and/or pregnancy and live births.
The following exclusion criteria were applied:
Original full texts were unavailable.
Impossibility to isolate or extract outcome data of interest.
Case reports and small studies with fewer than five patients in total.
Articles with the same or duplicated patient information.
Articles not written in English or Chinese.
Preliminary screening based on the title, abstract, and full text was performed by two independent reviewers (SZ and JZ). Second, a thorough examination of the full text was performed by two independent reviewers (SZ and YY), and a third reviewer (LC) was consulted if there was disagreement. For articles with the same or duplicate patient information, the most recent or complete publication was selected.
2.3. Data extraction
Data were extracted by two independent reviewers (YS and XZ), including study population (country, year of publication, number of participants, age, body mass index [BMI], and pathological and histological characteristics), study design, treatment protocol, method and timing of interval endometrial re‐evaluation, follow‐up length, oncological outcomes (i.e. disease regression and relapse), and reproductive outcomes (i.e. pregnancy and live birth).
2.4. Quality assessment
Quality assessment was performed by two independent reviewers (SZ and JZ), according to the checklist developed by Herrera Cappelletti et al. 10 (Table S2 and Appendix S1), which is based on items of the Newcastle–Ottawa Scale 12 and the Methodological Index for non‐randomized studies. 13 A third reviewer (LT) was consulted if consensus was not reached.
2.5. Statistical analyses
For the quantitative synthesis of primary outcomes, the CR rate (the numerator was the number of patients who achieved CR and the denominator was the number of patients with EEC or AH undergoing conservative therapy with HR) and the rates of pregnancy or live birth (the numerator was the number of patients who became pregnant or had live births during the follow‐up period, and the denominator was the number of all AH or EEC patients undergoing conservative therapy with HR, complete responders, or complete responders who attempted to conceive during the follow‐up period) were estimated by pooling data from individual studies in the meta‐analysis of proportions. For secondary outcomes, the RR (the numerator was the number of patients who experienced recurrence during the follow‐up period, and the denominator was the number of patients who achieved CR during the follow‐up period) was estimated by pooling data from individual studies in a meta‐analysis of proportions.
The above‐mentioned meta‐analysis of proportions was performed using a random‐ or fixed‐effects model to combine the data. The heterogeneity of the effects was analyzed statistically using the I 2 test. A random effects model was used when I 2 statistics >50% and the fixed‐effect model was used when I 2 statistics ≤50%. Forest plots were used to directly demonstrate the meta‐analyses.
Sensitivity analyses were performed using the leave‐one‐out strategy, and further subset meta‐analyses were defined by criteria related to BMI (≤28 kg/m2, >28 kg/m2) and type of HR‐based combination therapy (HR + oral progestins [OP], HR + levonorgestrel intrauterine system [LNG‐IUS]). Publication bias was assessed by constructing funnel plots and by using Egger's test for plot asymmetry (at least 10 studies). All values of p were two‐sided, and the level of significance was <0.05. The above analyses were carried out in R with packages of “meta” and “metaprop” (R Foundation for Statistical Computing, Vienna, Austria).
3. RESULTS
3.1. Selection and characteristics of included studies
A total of 264 studies were identified (66 duplicates) (Figure 1). After screening based on titles and abstracts, 163 articles were excluded, and 35 were retained for full‐text review, reporting conservative treatment for EC/EC and AH. Finally, 21 articles (407 patients with clinical stage IA, G1–G2, EEC, and 444 patients with AH managed with HR‐based conservative treatment) published up until October 10, 2023, were eligible for this systematic review (Table 1). Six studies were prospective and 15 were retrospective. These studies were conducted in Asia (11/21) and Europe (10/21). Nine studies included only patients with EC, and 12 studies included both patients with EC and AH.
FIGURE 1.
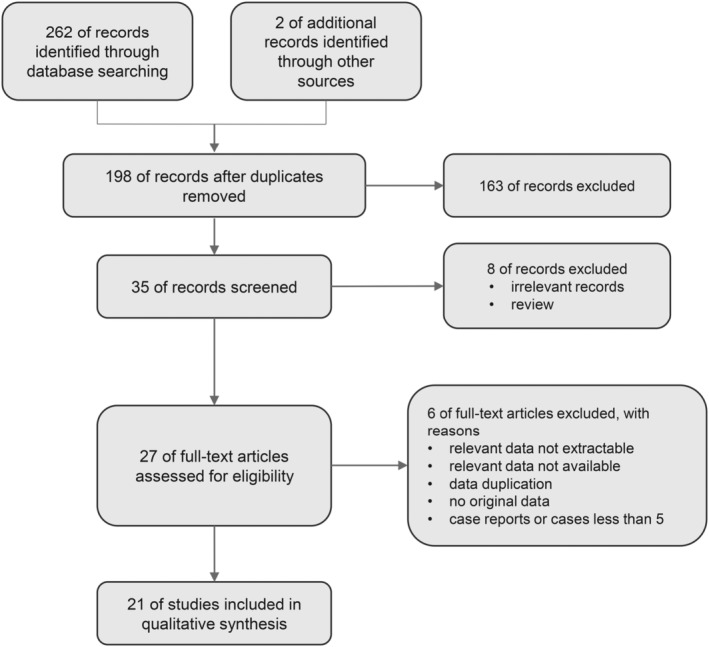
Study flow diagram.
TABLE 1.
The basic characteristics of included studies.
| Author | Country | Publication year | Study design | Number of patients, n | Mean age (range), year | Mean BMI (range), kg/m2 | Treatment | Median follow up in months (range) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AH | EEC | G1 | G2 | ||||||||
| Atallah et al. 14 | Lebanon | 2021 | R | 3 | 8 | 7 | 1 | 33.0 (NK) | 28.0 (NK) | HR + OP+GnRHa | 40.0 (12.0–108.0) |
| Ayhan et al. 20 | Turkey | 2020 | R | 27 | 30 | 30 | 0 |
AH: 34.0 b (20.0–43.0) EC: 32.0 b (20.0–45.0) |
AH: 28.8 (22.0–41.0) EC: 30.8 (23.0–40.0) |
HR + OP |
AH: 50.3 (11.0–100.0) EC:55.5 (6.0–133.0) |
| Casadio et al. 21 | Italy | 2020 | R | 46 | 36 | 36 a | 0 |
AH: 32.2 (NK) EC: 33.1 (NK) |
AH: 27.0 (NK) EC: 29.1 (NK) |
HR + OP |
AH: 36.0 (24.0–60.0) EC: 30.0 (24.0–60.0) |
| Falcone et al. 15 | Italy | 2017 | P | 0 | 28 | 27 | 1 | 36.1 (25.0–40.0) | 27.9 (20.9–53.5) | HR + OP/L | 92.0 (6.0–172.0) |
| 0 | 6 | 6 | 0 | NK | NK | HR + OP:6 | NK | ||||
| 0 | 22 | 21 | 1 | NK | NK | HR + L:22 | NK | ||||
| Falcone et al. 18 | Italy | 2020 | R | 0 | 17 | 0 | 17 | 34.0 (28.0–44.0) | 28.9 (19.8–42.0) | HR + OP/L | 35.0 (15.0–139.0) |
| 0 | 5 | 0 | 5 | NK | NK | HR + OP | NK | ||||
| 0 | 12 | 0 | 12 | NK | NK | HR + L | NK | ||||
| Giampaolino et al. 22 | Italy | 2019 | R | 55 | 14 | 14 | 0 | 35.1 (20.0–44.0) | 25.9 (20.2–44.8) | HR + L | 24 (NK) |
| Jing et al. 23 | China | 2022 | R | 31 | 48 | 48 | 0 |
AH: 30.0 b (NK) EC: 29.0 b (NK) |
AH: 23.4 b (NK) EC: 23.6 b (NK) |
HR + OP |
AH: 49.4 (NK) EC: 38.3 (NK) |
| Laurelli et al. 24 | Italy | 2011 | P | 0 | 14 | 14 | 0 | 36.6 (26.0–40.0) | 29.3 (23.0–53.0) | HR + OP/L | NK |
| 0 | 6 | 6 | 0 | 38.0 (36.0–40.0) | 26.3 (24.0–31.0) | HR + OP | 65.0 (50.0–79.0) | ||||
| 0 | 8 | 8 | 0 | 35.6 (26.0–40.0) | 31.5 (23.0–53.0) | HR + L | 27.0 (13.0–43.0) | ||||
| Laurelli et al. 16 | Italy | 2016 | P | 0 | 21 | 20 | 1 | 35.9 (25.0–40.0) | 28.6 (23.2–53.5) | HR + L | 85.0 (30.0–114.0) |
| Mazzon et al. 25 | Italy | 2020 | R | 0 | 6 | 6 | 0 | 32.5 (27.0–39.0) | 22.2 (18.0–25.0) | HR + OP | 194.0 (164.0–228.0) |
| De Marzi et al. 26 | Italy | 2015 | R | 20 | 3 | 3 | 0 | 36.6 (23.0–43.0) | 23.3 (18.5–32.0) | HR + OP/L | 25.0 (8.0–37.0) |
| Raffone et al. 27 | Italy | 2021 | R | 37 | 6 | 6 | 0 |
AH: 36.1 (NK) EC: 35.5 (NK) |
AH: 27.9 (NK) EC: 32.4 (NK) |
HR + L | NK |
| Shan et al. 28 | China | 2013 | P | 12 | 14 | 14 | 0 |
AH: 29.3 (24.0–36.0) EC: 30.1 (18.0–39.0) |
AH: 25 (18.1–37.9) EC: 21.9 (7.4–30.5) |
HR + OP |
AH: 31.8 (17.0–54.0) EC: 30.4 (15.0–66.0) |
| Tock et al. 17 | Belgium | 2018 | R | 9 | 9 | 8 | 1 | NK | NK | HR + GnRHa | NK |
| 9 | 1 | 34.3 (27.0–41.0) | NK | AH:58.3(7.0–180.0) | |||||||
| 9 | 8 | 1 | 30.8 (18.0–38.0) | NK | EC: 23.1 (5.0–72.0) | ||||||
| Wang et al. 29 | China | 2017 | R | 0 | 11 | 11 | 0 | 28.7 (25.0–39.0) | 22.9 (18.1–28.6) | HR + OP | 82.3 (15.0–152.0) |
| Wang et al. 30 | China | 2015 | P | 0 | 6 | 6 | 0 | 28.0 (25.0–34.0) | 20.4 (17.9–22.9) | HR + OP | 46.5 (26.0–91.0) |
| Wang et al. 31 | China | 2014 | R | 0 | 37 | 37 | 0 | 32.0 b (18.0–40.0) | 24.9 b (17.9–44.9) | HR + OP | 78.6 (19.1–252.8) |
| Xi et al. 32 | China | 2023 | P | 25 | 16 | 16 | 0 | 34.0 b (25.0–40.0) | 30.0 b (24.5–42.9) | HR + OP/L | 32.0 (8.0–65.0) |
| Xu et al. 33 | China | 2020 | R | 59 | 37 | 37 | 0 | NK | NK | HR + OP/L/L + OP | NK |
| 18 | 14 | 14 | 0 | 33.3 (NK) | 28.8 (NK) | HR + L | NK | ||||
| 19 | 13 | 13 | 0 | 32.6 (NK) | 29.5 (NK) | HR + OP | NK | ||||
| 22 | 10 | 10 | 0 | 32.2 (NK) | 28.3 (NK) | HR + L + OP | NK | ||||
| Yang et al. 34 | China | 2019 | R | 120 | 40 | 40 | 0 |
AH: 33.0 b (22.0–47.0) EC: 31.0 b (23.0–42.0) |
AH: 23.9 b (16.4–44.1) EC: 24.4 b (18.3–36.4) |
HR + OP/L |
AH:13.5 (1.0–36.0) EC: 9.0 (3.0–53.0) |
| Yang et al. 35 | China | 2019 | R | 0 | 6 | 6 | 0 | 33.7 (30.0–36.0) | 29.9 (23.6–36.9) | HR + OP | 32.0 (4.0–49.0) |
Abbreviations: AH, atypical hyperplasia; BMI, body mass index; EEC, endometrioid endometrial cancer; G1, grade 1; G2, grade 2; GnRHa, gonadotropin‐releasing hormone agonist; HR + GnRHa, combined treatment with HR and GnRHa; HR + L + OP, combined treatment with HR, LNG‐IUS and OP; HR + L, combined treatment with HR and LNG‐IUS; HR + OP, combined treatment with HR and OP; HR + OP/L, combined treatment with HR and OP/HR and LNG‐IUS; HR + OP/L/L + OP, combined treatment with HR and OP/HR and LNG‐IUS/HR, LNG‐IUS and OP; HR + OP+GnRHa, combined treatment with HR, OP, and GnRHa; HR, hysteroscopic resection; LNG‐IUS, levonorgestrel intrauterine system; NK, not known; OP, oral progesterone; P, prospective; R, retrospective.
Five patients with myometrial invasion.
Median value.
The mean age of patients with EC ranged from 28.0 to 38.0 years and the mean age of patients with AH ranged from 29.3 to 36.1 years. The median follow‐up length of patients with EC ranged from 9.0 to 194.0 months and the median follow‐up length of patients with AH ranged from 13.5 to 58.3 months. The mean BMI of patients with EC ranged from 20.4 to 32.4 kg/m2 and the mean BMI of patients with AH ranged from 23.4 to 28.8 kg/m2.
Five studies involved G2 EEC 14 , 15 , 16 , 17 , 18 and one involved EEC with myometrial infiltration (<3 mm). 19 The treatment regimens in this review included HR followed by OP (megestrol acetate [160–320 mg/day], medroxyprogesterone acetate [250–500 mg/day], and norethisterone acetate [10 mg/day]), LNG‐IUS insertion regimen, OP + LNG‐IUS, gonadotropin‐releasing hormone agonist (GnRHa), and OP + GnRHa.
3.2. Primary outcome
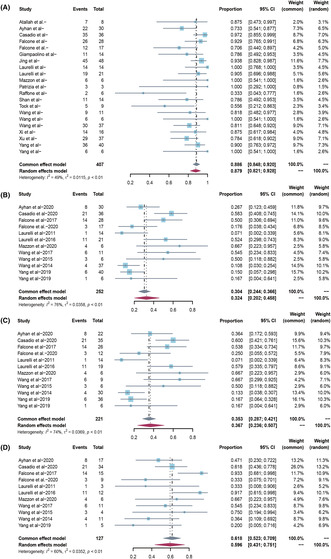
No patients who underwent conservative therapy were reported to have died in any of the included studies. Outcomes are shown in Table 2. Of the 407 patients with EEC, 88.6% (95% confidence interval [CI] 84.8–92.0; I 2 48.7%) achieved CR (Figure 2A). The chance of pregnancy for all treated patients with EC was 32.4% (95% CI 20.2–45.9; I 2 76.2%) based on a meta‐analysis of 12 studies (252 patients) (Figure 2B). The chance of pregnancy for complete responders was 36.7% (95% CI 23.6–50.8; I 2 74.4%) based on a meta‐analysis of 12 studies (221 patients) (Figure 2C). The chance of pregnancy for complete responders who attempted to conceive during follow up was higher, 59.6% (95% CI 43.1–75.2; I 2 59.8%) based on a meta‐analysis of 11 studies (127 patients) (Figure 2D; Table 3).
TABLE 2.
Outcomes in women with atypical hyperplasia or endometrial cancer of included studies.
| Author, Publication Year | Number of patients (n) | Number of patients (n) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AH | EEC | Treatment | CR | CR‐AH | CR‐EEC | Re (*) | AH‐Re (*) | EEC‐Re (*) | Pregnancy | Birth | Birth‐AH | Birth‐EEC | |
| Atallah 2021 14 | 3 | 8 | HR + OP + GnRHa | 10 | 3 | 7 | 1 | 0 | 1 (12) | 6 | 6 | 2 | 4 |
| Ayhan 2020 20 | 27 | 30 | HR + OP | 47 | 25 | 22 | 7 | 2 | 5 |
AH:4 EC:8 |
8 | 3 | 5 |
| Casadio 2020 21 | 46 | 36a | HR + OP | 81 | 46 | 35 | 8 | 4 (24.0, 3.0–36.0) | 4 (48, 3–60) |
AH:31 EC:21 |
35 | 21 | 14 |
| Falcone 2017 15 | 0 | 28 | HR + OP/L | 26 | 0 | 26 | 2 | 2 (24.5, 8–41) | 14 | 13 | 13 | ||
| 0 | 6 | HR + OP | 6 | 0 | 6 | 0 | 0 | 3 | 3 | 3 | |||
| 0 | 22 | HR + L | 20 | 0 | 20 | 2 | 2 | 11 | 10 | 10 | |||
| Falcone 2020 18 | 0 | 17 | HR + OP/L | 12 | 0 | 12 | 3 | 0 | 3 | 3 | 3 | 0 | 3 |
| 0 | 5 | HR + OP | 4 | 0 | 4 | 1 | 0 | 1 (28.0) | 2 | 2 | 0 | 2 | |
| 0 | 12 | HR + L | 8 | 0 | 8 | 2 | 0 | 2 (5.0, 4.0–6.0) | 1 | 1 | 0 | 1 | |
| Giampaolino 2019 22 | 55 | 14 | HR + L | 62 | 51 | 11 | 4 | 2 (24) | 2 (12) | 10 | 10 | 10 | 0 |
| Jing 2022 23 | 31 | 48 | HR + OP | 76 | 31 | 45 | 26 | 11 (17.6, 14.1–48.7) | 15 (18.0, 11.3–35.0) | 29 | 29 | 14 | 15 |
| Laurelli 2011 24 | 0 | 14 | HR + OP/L | 14 | 0 | 14 | 1 | 1 | 1 | 1 | 1 | ||
| 0 | 6 | HR + OP | 6 | 0 | 6 | 0 | 0 | 0 | 1 | 1 | 1 | ||
| 0 | 8 | HR + L | 8 | 0 | 8 | 1 | 0 | 1 (5.0) | 0 | 0 | 0 | ||
| Laurelli 2016 16 | 0 | 21 | HR + L | 19 | 0 | 19 | 2 | 0 | 2 (24.5, 8–41) | 11 | 10 | 10 | |
| Mazzon 2020 25 | 0 | 6 | HR + OP | 6 | 0 | 6 | 3 | 3 (75.6, 35.4–102.6) | 4 | 4 | 4 | ||
| De Marzi 2015 25 | 20 | 3 | HR + OP/L | 23 | 20 | 3 | 1 | 0 | 1 (6.0) | 6 | 5 | ||
| Raffone 2021 27 | 37 | 6 | HR + L | 39 | 37 | 2 | 10 | 9 | 1 | 10 | |||
| Shan 2013 28 | 12 | 14 | HR + OP | 21 | 10 | 11 | 6 | 3 (9.0,6.0–18.0) | 3 (12.0,10.0–24.0) | 2 | 1 | 0 | 1 |
| Tock 2018 16 | 9 | 9 | HR + GnRHa | 12 | 7 | 5 | 3 | 2 | 1 | 4 | 4 | 2 | 2 |
| 9 | 7 | 7 | 2 | 2 (9.5, 9.0–10.0) | 2 | 2 | |||||||
| 9 | 5 | 5 | 1 | 1 (3) | 2 | 2 | |||||||
| Wang 2017 29 | 0 | 11 | HR + OP | 9 | 0 | 9 | 0 | 6 | 6 | 0 | 6 | ||
| Wang 2015 30 | 0 | 6 | HR + OP | 6 | 0 | 6 | 0 | 3 | 3 | 3 | |||
| Wang 2014 31 | 0 | 37 | HR + OP | 30 | 0 | 30 | 15 | 0 | 15 (20.0, 13.0–155.0) | 4 | 4 | 0 | 4 |
| Xi 2022 31 | 25 | 16 | HR + OP/L | 37 | 23 | 14 | 6 | 4 (21.5,10.0–33.0) | 2 (27.0, 24.0–30.0) | 10 | 4 | 2 | 2 |
| Xu 2020 33 | 18 | 14 | HR + L | 26 | 5 | 14 | 9 | ||||||
| 19 | 13 | HR + OP | 29 | 3 | 17 | 13 | |||||||
| 22 | 10 | HR + L + OP | 28 | 3 | 15 | 8 | |||||||
| 59 | 37 | HR + OP/L/L + OP | 83 | 54 | 29 | 11 20.2, (9.0–46.0) | 7 | 4 | 46 | 30 | 17 | 13 | |
| Yang 2019 34 | 120 | 40 | HR + OP/L | 148 | 112 | 36 | 8 | 4 (8.0, 3.0–26.0) | 4 (7.0,4.0–28.0) |
AH:21 EC:6 |
15 | ||
| Yang 2019 35 | 0 | 6 | HR + OP | 6 | 0 | 6 | 0 | 1 | 1 | 1 | |||
Abbreviations: AH, atypical hyperplasia; AH‐Re, number of AH patients who experienced recurrence; Birth, number of patients who had live birth; Birth‐AH, number of AH patients who had live birth; Birth‐EEC, number of EEC patients who had live birth; CR, complete response; CR‐AH, number of AH patients who achieved CR; CR‐EEC, number of EEC patients who achieved CR; EEC, endometrioid endometrial cancer; EEC‐Re, number of EEC patients who experienced recurrence; GnRHa, gonadotropin‐releasing hormone agonist; HR + GnRHa, combined treatment with HR and GnRHa; HR + L + OP, combined treatment with HR, LNG‐IUS and OP; HR + L, combined treatment with HR and LNG‐IUS; HR + OP, combined treatment with HR and OP; HR + OP/L, combined treatment with HR and OP/HR and LNG‐IUS; HR + OP/L/L + OP, combined treatment with HR and OP/HR and LNG‐IUS/HR, LNG‐IUS and OP; HR + OP+GnRHa, combined treatment with HR, OP, and GnRHa; HR, hysteroscopic resection; LNG‐IUS, levonorgestrel intrauterine system; OP, oral progesterone; Pregnancy, number of patients who became pregnancy; Re, number of patients who experienced recurrence.
Median time to recurrence after complete response, range.
FIGURE 2.
(A) Overall complete response rate in all endometrial cancer patients treated with hysteroscopic resection‐based conservative therapy. (B) Overall pregnancy rate in all endometrial cancer patients treated with hysteroscopic resection‐based conservative therapy. (C) Overall pregnancy rate in endometrial cancer complete responders treated with hysteroscopic resection‐based conservative therapy. (D) Overall pregnancy rate in endometrial cancer complete responders who attempted to conceive.
TABLE 3.
Oncological and reproductive outcomes of AH and EC patients undergoing conservative therapy with HR.
| CR | Recurrence | Pregnancy (For all patients) | Pregnancy (For complete responders) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of women/ studies | Estimate (95% CI; %) | I 2 (%) | No. of women/studies | Estimate (95% CI; %) | I 2 (%) | No. of women/ studies | Estimate (95% CI; %) | I 2 (%) | No. of women/ studies | Estimate (95% CI; %) | I 2 (%) | |
| AH | 444/12 | 97.0 [94.7; 98.8] | 48.5% | 419/12 | 10.8 [4.6; 18.6] | 71.7% | ||||||
| EC | 407/21 | 88.6 [84.8; 92.0] | 48.7% | 327/18 | 18.3 [13.7; 23.3] | 44.3% | 252/12 | 32.4 [20.2; 45.9] | 76.2% | 221/12 | 36.7 [23.6; 50.8] | 74.4% |
| Pregnancy (For complete responders who attempted to conceive) | Live birth (For all patients) | Live birth (For complete responders) | Live birth (For complete responders who attempted to conceive) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of women/ studies | Estimate (95% CI; %) | I 2 (%) | No. of women/studies | Estimate (95% CI; %) | I 2 (%) | No. of women/ studies | Estimate (95% CI; %) | I 2 (%) | No. of women /studies | Estimate (95% CI; %) | I 2 (%) | |
| AH | 267/9 | 22.2 [10.8; 35.8] | 76.0% | 250/9 | 23.9 [12.3; 37.4] | 73.1% | ||||||
| EC | 127/11 | 59.6 [43.1; 75.2] | 59.8% | 358/18 | 26.0 [17.3; 35.5] | 65.7% | 307/18 | 30.6 [21.0; 41.0] | 63.0% | 131/12 | 51.9 [37.1; 66.6] | 52.6% |
Abbreviations: AH, atypical hyperplasia; CI, confidence interval; CR, complete response; EC, endometrial cancer.
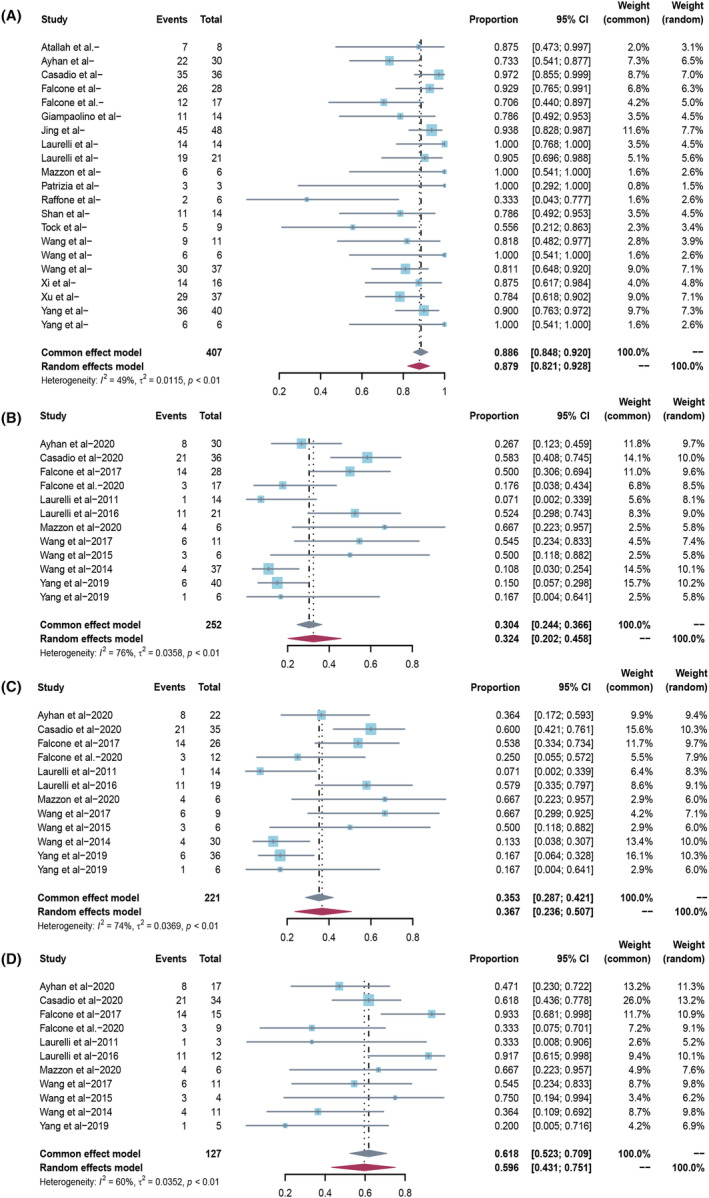
The chance of live birth for all treated patients with EC was 26.0% (95% CI 17.3–35.5; I 2 65.7%) based on a meta‐analysis of 18 studies (358 patients) (Figure 3A). The chance of live birth for complete responders was 30.6% (95% CI 21.0–41.0; I 2 63.0%) based on a meta‐analysis of 18 studies (307 patients) (Figure 3B). The chance of live birth for complete responders who attempted to conceive during follow up was higher at 51.9% (95% CI 37.1–66.6; I 2 52.6%) based on a meta‐analysis of 12 studies (131 patients) (Figure 3C; Table 3).
FIGURE 3.
(A) Overall live birth rate in all endometrial cancer patients treated with hysteroscopic resection‐based conservative therapy. (B) Overall live birth rate in endometrial cancer complete responders treated with hysteroscopic resection‐based conservative therapy. (C) Overall live birth rate in endometrial cancer complete responders who attempted to conceive.
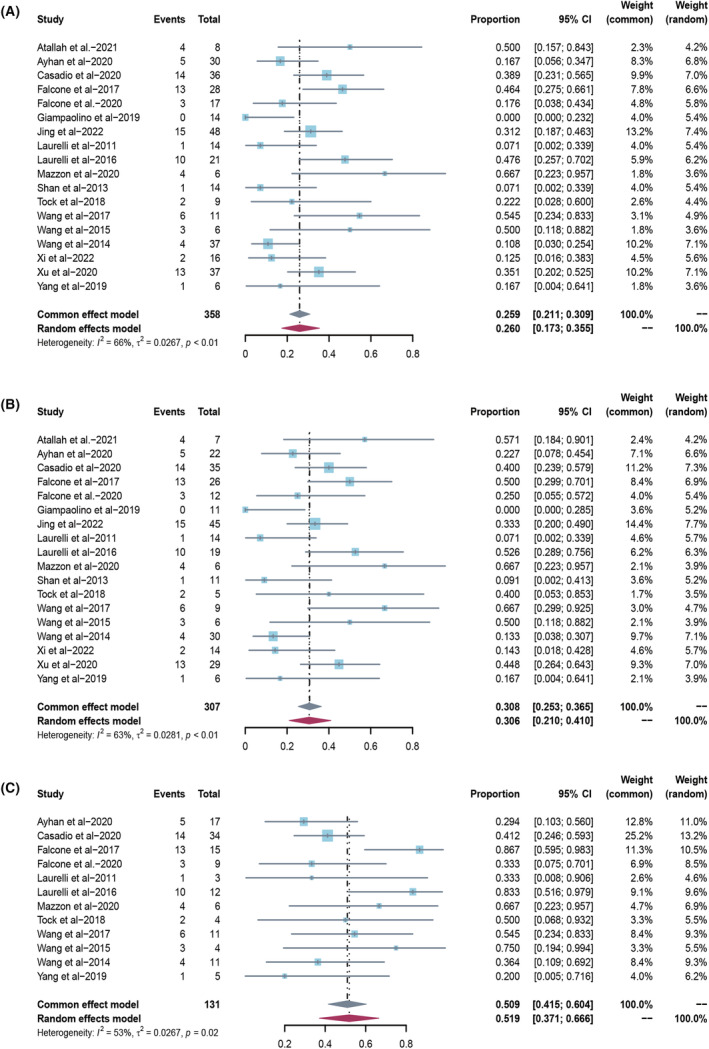
Of the 444 patients with AH, 97.0% (95% CI 94.7–98.8; I 2 48.5%) had a CR (Figure 4A). The chance of live birth for all treated patients with AH was 22.2% (95% CI 10.8–35.8; I 2 76.0%) based on a meta‐analysis of 9 studies (267 patients) (Figure 4B). The chance of live birth for complete responders was 23.9% (95% CI 12.3–37.4; I 2 73.1%) based on a meta‐analysis of 9 studies (250 patients) (Figure 4C; Table 3). Sensitivity analyses using the leave‐one‐out strategy did not significantly affect the results (Figure S1). Funnel plots indicated no significant publication bias (Figure S2).
FIGURE 4.
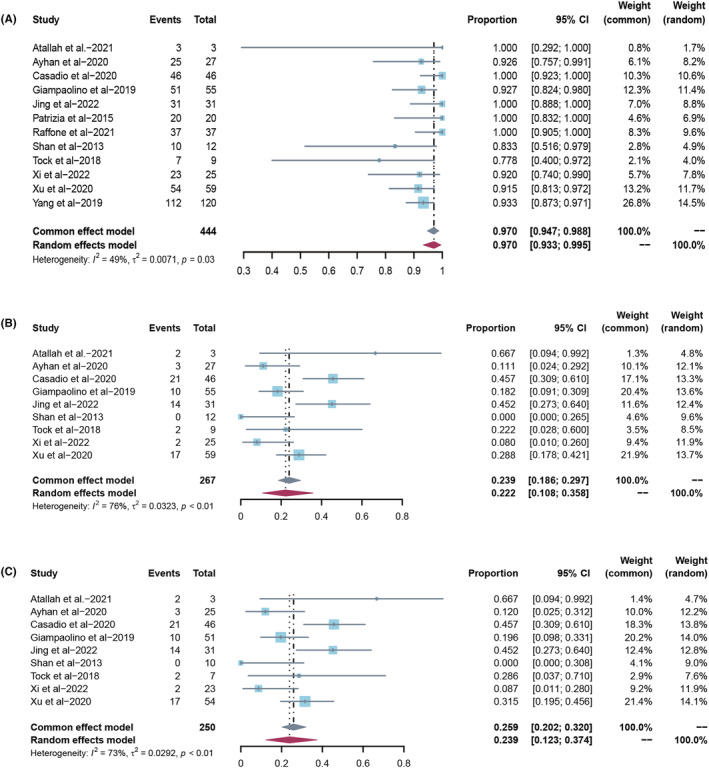
(A) Overall complete response rate in all atypical hyperplasia patients treated with hysteroscopic resection‐based conservative therapy. (B) Overall live birth rate in all atypical hyperplasia patients treated with hysteroscopic resection‐based conservative therapy. (C) Overall live birth rate in atypical hyperplasia complete responders treated with hysteroscopic resection‐based conservative therapy.
3.3. Secondary outcome and subset analyses
The overall pooled disease RR was 18.3% (95% CI 13.7–23.3; I 2 44.3%) in 327 patients with EEC (18 studies) who had a CR. The overall pooled disease RR was 10.8% (95% CI 4.6–18.6; I 2 71.7%) in 419 patients with AH (12 studies) who had a CR (Table 3).
In further subset meta‐analyses of all EEC patients, the CR rates, the chance of pregnancy, and chance of live birth for all treated patients with EEC or complete responders or complete responders who attempted to conceive were higher in patients with BMI ≤28 kg/m2 compared with patients with a BMI >28 kg/m2 (Table 4).
TABLE 4.
Sensitivity analyses in subsets of studies on conservative therapy with HR in EC.
| CR | Recurrence | Pregnancy (For all patients) | Pregnancy (For complete responders) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of women/studies | Estimate (95% CI; %) | I 2 (%) | No. of women/studies | Estimate (95% CI; %) | I 2 (%) | No. of women/studies | Estimate (95% CI; %) | I 2 (%) | No. of women/studies | Estimate (95% CI; %) | I 2 (%) | |
| Type of treatment | ||||||||||||
| HR + OP | 211/12 | 91.8 [86.8; 95.9] | 35.7% | 165/9 | 23.0 [11.4; 36.7] | 61.8% | 149/10 | 36.3 [21.9; 51.9] | 67.2% | 130/10 | 40.6 [25.6; 56.3] | 61.3% |
| HR + L | 83/6 | 82.5 [65.2; 95.4] | 60.3% | 55/4 | 11.9 [3.6; 22.9] | 0.0% | 64/4 | 25.3 [2.8; 57.3] | 81.5% | 55/4 | 29.5 [3.7; 64.0] | 80.9% |
| Mean BMI (kg/m2) | ||||||||||||
| ≤28 | 196/9 | 91.6 [86.6; 95.8] | 0.0% | 160/7 | 22.8 [9.4; 39.2] | 74.8% | 134/7 | 32.9 [15.7; 52.4] | 75.4% | 119/7 | 36.3 [18.0; 56.6] | 74.2% |
| >28 | 124/7 | 86.4 [69.8; 97.8] | 72.2% | 104/7 | 13.4 [6.1; 22.3] | 0.0% | 124/7 | 28.4 [10.8; 49.6] | 76.7% | 104/7 | 32.4 [12.5; 55.6] | 73.7% |
| Pregnancy (For complete responders who attempted to conceive) | Live birth (For all patients) | Live birth (For complete responders) | Live birth (For complete responders who attempted to conceive) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of women/studies | Estimate (95% CI; %) | I 2 (%) | No. of women/studies | Estimate (95% CI; %) | I 2 (%) | No. of women/studies | Estimate (95% CI; %) | I 2 (%) | No. of women/ studies | Estimate (95% CI; %) | I 2 (%) | |
| Type of treatment | ||||||||||||
| HR + OP | 97/10 | 55.5 [44.1; 66.7] | 0.0% | 211/12 | 28.2 [17.5; 40.0] | 55.0% | 186/12 | 29.8 [22.7; 37.2] | 46.1% | 97/10 | 44.0 [32.8; 55.4] | 13.2% |
| HR + L | 30/4 | 67.3 [15.6; 100] | 74.9% | 77/5 | 16.3 [0.5; 42.6] | 83.3% | 66/5 | 19.1 [0.8; 48.3] | 82.5% | 30/4 | 61.7 [18.2; 97.4] | 65.5% |
| Mean BMI (kg/m2) | ||||||||||||
| ≤28 | 49/6 | 66.3 [41.9; 87.6] | 54.6% | 156/8 | 31.1 [16.4; 47.7] | 69.1% | 139/8 | 34.6 [19.2; 51.6] | 65.3% | 49/6 | 65.3 [49.5; 79.9] | 34.1% |
| >28 | 78/6 | 52.4 [26.5; 77.8] | 63.2% | 118/6 | 23.0 [10.0; 38.8] | 63.0% | 102/6 | 26.8 [12.5; 43.6] | 58.7% | 78/6 | 40.3 [18.6; 63.6] | 54.9% |
Abbreviations: BMI, body mass index; CI, confidence interval; CR, complete response; HR + L, combined treatment with HR and levonorgestrel‐releasing intrauterine system; HR + OP, combined treatment with HR and OP; OP, oral progesterone.
In addition, we performed the subset meta‐analyses in different types of HR‐based combination therapy (Table 4). The CR rates and the chances of pregnancy and live birth for all treated patients with EEC or complete responders were higher in patients in the HR + OP subgroups than in patients in the HR + LNG‐IUS subgroups, whereas the chances of pregnancy and live birth for complete responders who attempted to conceive were lower in patients in the HR + OP subgroups than in patients in the HR + LNG‐IUS subgroups.
4. DISCUSSION
Overall, our study showed that HR followed by OP or LNG‐IUS resulted in promising outcomes, which is similar to the findings of previous meta‐analyses or systematic reviews. Herrera Cappelletti et al. reported that pregnancy and live birth rates were 34.0% and 30.7%, respectively, for early‐stage EC patients who underwent HR combined with progestin treatment. 10 Lucchini et al. reported that the CR, RR, and pregnancy rates were 90%, 6.93%, and 34.5%, respectively, in early‐stage EC patients who underwent HR followed by progestins, whereas the CR, RR, and pregnancy rates were 77.7%, 29.17%, and 27.6%, respectively, in patients who only underwent progestin treatment. 9 In our results, RR in patients with EEC was higher compared with the previous study. 9 We speculate that the updated articles published recently influence the results, in addition, we tried to decrease the publication bias as far as possible and case reports reporting patients who achieved successful pregnancies were excluded, which may have exaggerated the success of oncological and reproductive outcomes.
Notably, these previous studies 9 , 10 selected all treated women as the denominators in the calculation of pregnancy and live birth rates, which may have underestimated reproductive outcomes. Of course, choosing only complete responders who attempted to conceive as denominators in the calculation may also overestimate reproductive outcomes. Therefore, we selected treated patients, complete responders, and complete responders who attempted to conceive during follow up separately to obtain comprehensive and objective results showing the reproductive outcomes of patients with AH and EC undergoing conservative therapy. Furthermore, we performed sensitivity analyses to investigate possible effect modifiers and the stability of the findings. Interestingly, the reproductive outcomes among all treated patients with EEC or complete responders were better in the HR + OP subgroups than in the HR + LNG‐IUS subgroups, whereas among complete responders who attempted to conceive they were worse in the HR + OP subgroups than in the HR + LNG‐IUS subgroups. The opposite results show that reproductive outcomes are tightly associated with fertility desire and planning. In addition, the RR was lower in the HR + LNG‐IUS subgroup (11.9%) than in the HR + OP subgroup (23.0%). We anticipate that more patients in the HR + LNG‐IUS subgroup had no short‐term fertility plans, so they chose LNG‐IUS for treatment and maintenance, which may demonstrate the importance of maintenance therapy by using LNG‐IUS.
In previous meta‐analyses, as follow‐up duration increased, the chances of pregnancy and birth increased to some extent, 10 , 37 which can be explained by the fact that reproductive outcomes require a relatively long observation period. However, as follow‐up duration increases, fertility decreases. Not all complete responders attempted to conceive instantly during the limited follow‐up periods in our included studies, and the live birth rate in all treated patients or complete responders was higher in patients with EC compared with patients with AH in this study, which does not conform to the severity of the disease (Table 3). The live birth rate in all treated patients was 26.0% vs. 22.2%, respectively, and the live birth rate in complete responders was 30.6% vs. 23.9%, respectively. We believe that this was associated with unexplored personal or social reasons, including relationship status and short‐term fertility plans, which can explain why there is still room for improvement in pregnancy and live birth rates. Considering the relatively high severity in patients with EC, they were more actively encouraged to try to conceive as soon as CR was achieved, and they may prefer to consider assisted reproductive techniques to improve success rates and reduce the interval to conception with a lower risk of recurrence. However, there is a lack of complete individual data regarding the relationship status and short‐term fertility plans, mode and time of conception, and detailed data during pregnancy and delivery in current studies. Therefore, more detailed evidence is needed for confirmation of these assumptions.
Mazzon et al. first described that HR consisted of three steps: resection of the tumor lesion, the endometrium adjacent to the tumor lesion (4–5 mm outside), and the myometrium under the tumor lesion (3–4 mm). 36 Multiple random endometrial biopsies were obtained. However, in our study, only eight articles performed HR according to the technique described by Mazzon et al. In addition, Giampaolino et al. distinguished AH from EC during HR. They resected the superficial endometrium and preserved the basal layer of the endometrium in AH, whereas they performed HR in EC, according to the three steps described by Mazzon et al. 22 Therefore, the difference in HR procedures between different studies is one of the contributors to heterogeneity in our study, which cannot be overlooked when evaluating the therapeutic effect. In addition, differences in the lesions, including size differences and whether lesions were local or multiple, also influenced the therapeutic effect; therefore, a detailed description of the lesions is needed in future studies to provide more convincing evidence. The adverse effects of repeated and excessive HR, including endometrial destruction, intrauterine adhesion, and dissemination of cancerous cells into the peritoneal cavity, reduce the success rate of pregnancy and live births, which is contrary to the original intention of preserving fertility. Therefore, a standardized HR procedure is necessary to balance therapeutic effect and endometrial protection.
Currently, conservative therapy is considered for patients with AH or G1 EEC without myometrial invasion or genetic risk factors. 38 However, in recent years, an increasing number of young patients have a strong desire to preserve fertility but have EEC with G2 or minimal myometrial infiltration. HR can decrease the tumor burden and improve therapeutic efficacy, thus shortening the therapeutic time to achieve fertility as soon as possible; therefore, some studies have attempted to explore and broaden the indications for conservative therapy, including G2 EECs and minimal myometrial infiltration (less than 3 mm), 18 , 19 , 21 which are also contributors to clinical heterogeneity. However, the data are limited and do not allow us to draw definitive conclusions. Currently, prospective trials exploring broader indications are ongoing (NCT05945407 and NCT05332483), 39 and we believe that it would be more persuasive to include their results in the future.
The risks for early‐stage G2 EECs undergoing conservative therapy are higher because of less responsibility for progestins than for G1. The data for G2 EECs are quite limited; it has been reported that the rates of CR and live birth were 71.4% (35/49) and 28.5% (10/35), respectively, in studies on patients with early‐stage G2 EC receiving conservative therapy. 18 In our review, G2 patients were included in five studies. In four studies, only one patient with G2 EC undergoing HR followed by progestins, with or without GnRHa, was enrolled. None of the patients achieved a CR. 14 , 15 , 16 , 17 Another study reported 17 G2 patients that received HR therapy followed by progestins; 11 achieved CR and three had live births. 18
Casadio et al. reported 5‐year follow‐up outcomes for three patients with G1 EEC with minimal myometrial infiltration treated with HR and hormone therapy. One patient achieved fertility; AH was found during follow up and the patient underwent definitive surgery. One patient did not achieve fertility after 5 years of negative follow up. One patient did not achieve fertility and underwent definitive surgery because AH was found. 19 Overall, evidence on the safety and efficiency of fertility‐sparing treatment in EC with minimal myometrial invasion remains limited.
The molecular profiling of EC has gained attention in recent years. Limited evidence shows that for young patients with low‐grade, early‐stage EC and a desire to preserve fertility, p53 wild‐type EC benefited most from fertility‐sparing therapy, 40 , 41 , 42 whereas p53 abnormal‐type and mismatch repair deficiency EC responded worse, and the therapeutic effect of POLE‐mutated‐type EC is unclear. 40 , 41 , 42 , 43 , 44 The addition of molecular profiling into the process of accurate selection of suitable patients for individual and effective conservative treatments is promising.
The limitations of this study were mainly associated with the available clinical data. Many of the included studies were retrospective with small sample sizes and limited follow‐up lengths. Long‐term follow‐up is required to evaluate recurrence and reproductive outcomes. Large prospective randomized studies are urgently needed to validate the clinical implications of HR. Publication bias is another limitation that may have exaggerated the success of oncological and reproductive outcomes. In addition, conservative therapy includes not only local treatment of lesions but also systemic management of body weight and blood glucose, blood pressure, and blood lipid levels, which have a significant influence on oncological and reproductive outcomes. However, detailed individual data regarding the prognostic factors, including obesity (weight change before and after treatment), type 2 diabetes, polycystic ovary syndrome, and metabolic syndrome, are lacking.
5. CONCLUSION
Our review indicates that HR followed by progestins is a promising choice for fertility‐sparing in young patients with AH and EC with effective and safe responses. The live birth rate remains to be improved by providing medical encouragement and guidance. Large‐scale prospective and randomized studies on HR with long‐term follow ups are urgently needed to gain a better understanding of the reproductive outcomes of young patients with AH and EC undergoing conservative therapy.
AUTHOR CONTRIBUTIONS
Shuangshuang Zhao contributed to methodology, investigation, data curation, writing—original draft, supervision, and project administration. Jingying Zhang and Ye Yan contributed to methodology, investigation, and data curation. Lina Tian contributed to writing—reviewing and editing, and supervision. Lingli Chen and Xingyu Zheng contributed to methodology, software, and data analysis. Yiqing Sun contributed to methodology and investigation. Wenyan Tian, Fengxia Xue and Yingmei Wang contributed to supervision and project administration.
FUNDING INFORMATION
This work was funded by the National Key Technology Research and Developmental Program of China (Program Nos. 2022YFC2704402), the National Natural Science Foundation of China (Program Nos. 82172626; 81972448), the Health Technology Project of Tianjin Municipal Health Commission (Program Nos.TJWJ2022XK009), the Tianjin Key Medical Discipline (Specialty) Construction Project (Program Nos. TJYXZDXK‐031A), the China Anti‐Cancer Association‐Hengrui PARP Nicotinamide Cancer Research Fund (Program Nos. CETSDHRCORP252007).
CONFLICT OF INTEREST STATEMENT
No potential conflicts of interest were disclosed.
Supporting information
Appendix S1.
Figure S1.
Figure S2.
Table S1.
Table S2.
ACKNOWLEDGMENTS
We are thankful to the Key Laboratory of Female Reproductive Health and Eugenics of Tianjin Medical University General Hospital for their support in writing this article.
Zhao S, Zhang J, Yan Y, et al. Oncological and reproductive outcomes of endometrial atypical hyperplasia and endometrial cancer patients undergoing conservative therapy with hysteroscopic resection: A systematic review and meta‐analysis. Acta Obstet Gynecol Scand. 2024;103:1498‐1512. doi: 10.1111/aogs.14815
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. [DOI] [PubMed] [Google Scholar]
- 2. Gu B, Shang X, Yan M, et al. Variations in incidence and mortality rates of endometrial cancer at the global, regional, and national levels, 1990‐2019. Gynecol Oncol. 2021;161(2):573‐580. [DOI] [PubMed] [Google Scholar]
- 3. Crosbie EJ, Kitson SJ, McAlpine JN, Mukhopadhyay A, Powell ME, Singh N. Endometrial cancer. Lancet. 2022;399(10333):1412‐1428. [DOI] [PubMed] [Google Scholar]
- 4. Matsuo K, Mandelbaum RS, Matsuzaki S, Klar M, Roman LD, Wright JD. Ovarian conservation for young women with early‐stage, low‐grade endometrial cancer: a 2‐step schema. Am J Obstet Gynecol. 2021;224(6):574‐584. [DOI] [PubMed] [Google Scholar]
- 5. Gunderson CC, Fader AN, Carson KA, Bristow RE. Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: a systematic review. Gynecol Oncol. 2012;125(2):477‐482. [DOI] [PubMed] [Google Scholar]
- 6. Carugno J, Wong A. Fertility‐sparing approach for endometrial cancer: the role of office hysteroscopy. Minim Invasive Ther Allied Technol. 2021;30(5):296‐303. [DOI] [PubMed] [Google Scholar]
- 7. Zhang Q, Qi G, Kanis MJ, et al. Comparison among fertility‐sparing therapies for well differentiated early‐stage endometrial carcinoma and complex atypical hyperplasia. Oncotarget. 2017;8(34):57642‐57653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fan Z, Li H, Hu R, Liu Y, Liu X, Gu L. Fertility‐preserving treatment in young women with grade 1 presumed stage IA endometrial adenocarcinoma: a meta‐analysis. Int J Gynecol Cancer. 2018;28(2):385‐393. [DOI] [PubMed] [Google Scholar]
- 9. Lucchini SM, Esteban A, Nigra MA, Palacios AT, Alzate‐Granados JP, Borla HF. Updates on conservative management of endometrial cancer in patients younger than 45 years. Gynecol Oncol. 2021;161(3):802‐809. [DOI] [PubMed] [Google Scholar]
- 10. Herrera Cappelletti E, Humann J, Torrejon R, Gambadauro P. Chances of pregnancy and live birth among women undergoing conservative management of early‐stage endometrial cancer: a systematic review and meta‐analysis. Hum Reprod Update. 2022;28(2):282‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Higgins JPT, Thomas J, Chandler J, et al., eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane; 2023. www.training.cochrane.org/handbook [Google Scholar]
- 12. Wells G, Shea B, O'Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta‐Analyses. Accessed 17 October 2023. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 13. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non‐randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712‐716. [DOI] [PubMed] [Google Scholar]
- 14. Atallah D, El Kassis N, Safi J, El Hachem H, Chahine G, Moubarak M. The use of hysteroscopic endometrectomy in the conservative treatment of early endometrial cancer and atypical hyperplasia in fertile women. Arch Gynecol Obstet. 2021;304(5):1299‐1305. [DOI] [PubMed] [Google Scholar]
- 15. Falcone F, Laurelli G, Losito S, Di Napoli M, Granata V, Greggi S. Fertility preserving treatment with hysteroscopic resection followed by progestin therapy in young women with early endometrial cancer. J Gynecol Oncol. 2017;28(1):e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Laurelli G, Falcone F, Gallo MS, et al. Long‐term oncologic and reproductive outcomes in young women with early endometrial cancer conservatively treated: a prospective study and literature update. Int J Gynecol Cancer. 2016;26(9):1650‐1657. [DOI] [PubMed] [Google Scholar]
- 17. Tock S, Jadoul P, Squifflet JL, Marbaix E, Baurain JF, Luyckx M. Fertility sparing treatment in patients with early stage endometrial cancer, using a combination of surgery and GnRH agonist: a monocentric retrospective study and review of the literature. Front Med (Lausanne). 2018;5:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Falcone F, Leone Roberti Maggiore U, Di Donato V, et al. Fertility‐sparing treatment for intramucous, moderately differentiated, endometrioid endometrial cancer: a Gynecologic Cancer Inter‐Group (GCIG) study. J Gynecol Oncol. 2020;31(5):e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Casadio P, Guasina F, Paradisi R, Leggieri C, Caprara G, Seracchioli R. Fertility‐sparing treatment of endometrial cancer with initial infiltration of myometrium by resectoscopic surgery: a pilot study. Oncologist. 2018;23(4):478‐480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ayhan A, Tohma YA, Tunc M. Fertility preservation in early‐stage endometrial cancer and endometrial intraepithelial neoplasia: a single‐center experience. Taiwan J Obstet Gynecol. 2020;59(3):415‐419. [DOI] [PubMed] [Google Scholar]
- 21. Casadio P, La Rosa M, Alletto A, et al. Fertility sparing treatment of endometrial cancer with and without initial infiltration of myometrium: a single center experience. Cancers (Basel). 2020;12(12):3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Giampaolino P, Di Spiezio SA, Mollo A, et al. Hysteroscopic endometrial focal resection followed by levonorgestrel intrauterine device insertion as a fertility‐sparing treatment of atypical endometrial hyperplasia and early endometrial cancer: a retrospective study. J Minim Invasive Gynecol. 2019;26(4):648‐656. [DOI] [PubMed] [Google Scholar]
- 23. Jing CY, Li SN, Shan BE, et al. Hysteroscopic curettage followed by megestrol acetate plus metformin as a fertility‐sparing treatment for women with atypical endometrial hyperplasia or well‐differentiated endometrioid endometrial carcinoma. Clin Med Insights Oncol. 2022;16:11795549221110522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Laurelli G, Di Vagno G, Scaffa C, Losito S, Del Giudice M, Greggi S. Conservative treatment of early endometrial cancer: preliminary results of a pilot study. Gynecol Oncol. 2011;120(1):43‐46. [DOI] [PubMed] [Google Scholar]
- 25. Mazzon I, Masciullo V, Scambia G, Ferrandina G, Corrado G. Long‐term survival of young endometrial cancer patients desiring fertility preservation treated with hysteroscopic resection followed by hormone therapy (NEMO technique). Int J Gynaecol Obstet. 2020;151(2):305‐307. [DOI] [PubMed] [Google Scholar]
- 26. De Marzi P, Bergamini A, Luchini S, et al. Hysteroscopic resection in fertility‐sparing surgery for atypical hyperplasia and endometrial cancer: safety and efficacy. J Minim Invasive Gynecol. 2015;22(7):1178‐1182. [DOI] [PubMed] [Google Scholar]
- 27. Raffone A, Travaglino A, Flacco ME, et al. Clinical predictive factors of response to treatment in patients undergoing conservative management of atypical endometrial hyperplasia and early endometrial cancer. J Adolesc Young Adult Oncol. 2021;10(2):193‐201. [DOI] [PubMed] [Google Scholar]
- 28. Shan BE, Ren YL, Sun JM, et al. A prospective study of fertility‐sparing treatment with megestrol acetate following hysteroscopic curettage for well‐differentiated endometrioid carcinoma and atypical hyperplasia in young women. Arch Gynecol Obstet. 2013;288(5):1115‐1123. [DOI] [PubMed] [Google Scholar]
- 29. Wang F, Yu A, Xu H, et al. Fertility preserved hysteroscopic approach for the treatment of stage Ia endometrioid carcinoma. Int J Gynecol Cancer. 2017;27(9):1919‐1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang Q, Guo Q, Gao S, et al. Fertility‐conservation combined therapy with hysteroscopic resection and oral progesterone for local early stage endometrial carcinoma in young women. Int J Clin Exp Med. 2015;8(8):13804‐13810. [PMC free article] [PubMed] [Google Scholar]
- 31. Wang CJ, Chao A, Yang LY, et al. Fertility‐preserving treatment in young women with endometrial adenocarcinoma: a long‐term cohort study. Int J Gynecol Cancer. 2014;24(4):718‐728. [DOI] [PubMed] [Google Scholar]
- 32. Xi Y, Liu G, Liu D, Jiang J, Gong R. Efficacy and pregnancy outcomes of hysteroscopic surgery combined with progestin as fertility‐sparing therapy in patients with early stage endometrial cancer and atypical hyperplasia. Arch Gynecol Obstet. 2023;307(2):583‐590. [DOI] [PubMed] [Google Scholar]
- 33. Xu Z, Tian Y, Fu J, Xu J, Bao D, Wang G. Efficacy and prognosis of fertility‐preserved hysteroscopic surgery combined with progesterone in the treatment of complex endometrial hyperplasia and early endometrial carcinoma. J BUON. 2020;25(3):1525‐1533. [PubMed] [Google Scholar]
- 34. Yang B, Xu Y, Zhu Q, et al. Treatment efficiency of comprehensive hysteroscopic evaluation and lesion resection combined with progestin therapy in young women with endometrial atypical hyperplasia and endometrial cancer. Gynecol Oncol. 2019;153(1):55‐62. [DOI] [PubMed] [Google Scholar]
- 35. Yang HC, Liu JC, Liu FS. Fertility‐preserving treatment of stage IA, well‐differentiated endometrial carcinoma in young women with hysteroscopic resection and high‐dose progesterone therapy. Taiwan J Obstet Gynecol. 2019;58(1):90‐93. [DOI] [PubMed] [Google Scholar]
- 36. Mazzon I, Corrado G, Morricone D, Scambia G. Reproductive preservation for treatment of stage IA endometrial cancer in a young woman: hysteroscopic resection. Int J Gynecol Cancer. 2005;15(5):974‐978. [DOI] [PubMed] [Google Scholar]
- 37. Wei H, Pan N, Zhang W, et al. Levonorgestrel‐releasing intrauterine system‐based therapies for early‐stage endometrial cancer: a systematic review and meta‐analysis. J Gynecol Oncol. 2023;34(2):e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rodolakis A, Scambia G, Planchamp F, et al. ESGO/ESHRE/ESGE guidelines for the fertility‐sparing treatment of patients with endometrial carcinoma. Hum Reprod Open. 2023;2023(1):hoac057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. ClinicalTrials.gov. 2023. Accessed 17 October 2023: https://www.clinicaltrials.gov/
- 40. Puechl AM, Spinosa D, Berchuck A, et al. Molecular classification to prognosticate response in medically managed endometrial cancers and endometrial intraepithelial neoplasia. Cancers (Basel). 2021;13(11):2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang X, Chen D, Zhao X, et al. Application of molecular classification to guiding fertility‐sparing therapy for patients with endometrial cancer or endometrial intraepithelial neoplasia. Pathol Res Pract. 2023;241:154278. [DOI] [PubMed] [Google Scholar]
- 42. Chung YS, Woo HY, Lee JY, et al. Mismatch repair status influences response to fertility‐sparing treatment of endometrial cancer. Am J Obstet Gynecol. 2021;224:370.e1‐370.e13. [DOI] [PubMed] [Google Scholar]
- 43. Raffone A, Catena U, Travaglino A, et al. Mismatch repair‐deficiency specifically predicts recurrence of atypical endometrial hyperplasia and early endometrial carcinoma after conservative treatment: a multi‐center study. Gynecol Oncol. 2021;161(3):795‐801. [DOI] [PubMed] [Google Scholar]
- 44. Zakhour M, Cohen JG, Gibson A, et al. Abnormal mismatch repair and other clinicopathologic predictors of poor response to progestin treatment in young women with endometrial complex atypical hyperplasia and well‐differentiated endometrial adenocarcinoma: a consecutive case series. BJOG. 2017;124(10):1576‐1583. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.
Figure S1.
Figure S2.
Table S1.
Table S2.


