Abstract
Diminished ovarian reserve (DOR) is associated with reduced fertility and poor reproductive outcomes. The association between dietary patterns and DOR was not well studied. The purpose of this study was to evaluate the relationship between adhering to the healthy eating index (HEI-2015) and the risk of DOR. In this case–control study, 370 Iranian women (120 with DOR and 250 age- and BMI-matched controls) were examined. A reliable semi-quantitative food frequency questionnaire was used to collect diet-related data. We analyzed the HEI-2015 and their dietary intake data to determine major dietary patterns. The multivariable logistic regression was used in order to analyze the association between HEI-2015 and risk of DOR. We found no significant association between HEI-2015 score and risk of DOR in the unadjusted model (OR 0.78; 95%CI 0.59, 1.03). After controlling for physical activity and energy intake, we observed that women in the highest quartile of the HEI-2015 score had 31% decreased odds of DOR (OR 0.69; 95%CI 0.46, 0.93). This association remained significant even after adjusting for all potential confounders. Overall, increased adherence to HEI may lead to a significant reduction in the odds ratio of DOR. Clinical trials and prospective studies are needed to confirm this association.
Keywords: Diminished ovarian reserve, Infertility, Ovarian function, Healthy eating index
Subject terms: Diseases, Health care, Medical research
Introduction
Diminished ovarian reserve (DOR) is characterized as a diminished number or quality of follicles and oocytes, which has a detrimental impact on women’s reproductive health. It is additionally a condition in which the ovary loses its normal reproductive potential, compromising fertility1. In later a long time, the prevalence of DOR has been rising, and the ages of patients are more youthful. The incidence of DOR has been reported to increase from 19 to 26%2,3. DOR is associated with reduced fecundity, an early event of menopause, increased miscarriage rates, poor response to ovarian stimulation in assisted reproduction treatments, recurrent miscarriages, and infertility4–6. Finding effective therapeutic approaches for DOR has become a major focus in the field of reproductive health. In order to find such approaches, it is crucial to explore DOR predisposing risk factors.
Several lifestyle, environmental, and genetic factors contribute to the complex etiology of DOR7,8. More research has emerged in recent years indicating that diet may be associated with ovarian reserve. However, research on the link between dietary factors and the risk of a decreased ovarian reserve is lacking. It seems that consuming dairy products may diminish the rate of AMH decrease in women who menstruate regularly9. Dietary advice as a part of lifestyle adjustment may be considered a preventive modality to slow the rate of ovarian reserve loss9.
Due to the interactive or synergistic effects of nutrients with each other, considering the whole diet as dietary patterns is important in this regard. Several indices, including the healthy eating index (HEI-2015), were developed to assess dietary quality in accordance with the Dietary Guidelines for Americans (DGA)10,11. The HEI-2015 focuses on total fruits, whole fruits, whole grains, total vegetables, total protein foods, greens and beans, dairy, seafood and plant proteins, and fatty acids10,12. Despite ovarian reserve reduction being irreversible and becoming more common3, there are still no accepted dietary recommendations for assessing food habits and their relation to DOR. Therefore, the current case–control study investigated the associations between adherence to HEI-2015 and the risk of DOR. We believe that this is the first study to address such a relationship.
Methods
Participants
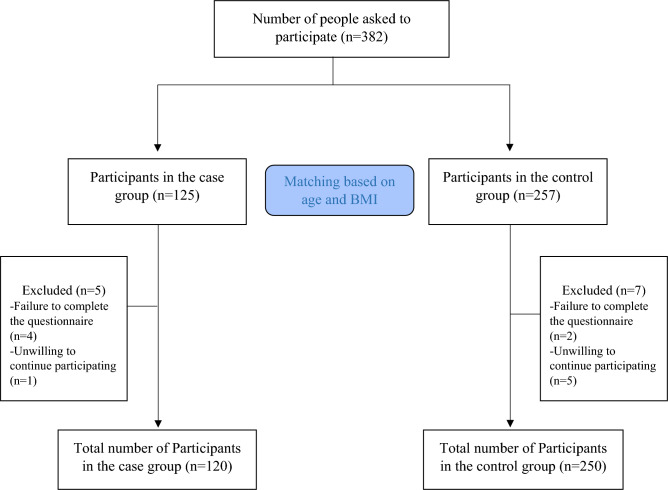
This study conducted on 370 Iranian women (120 with DOR and 250 age- and BMI-matched controls) aged 18 to 45, with a BMI between 20 and 35 kg/m2. In our study, control subjects were frequently matched with cases of DOR according to age and BMI categories. Initially, we organized the DOR cases into nine distinct groups based on their age and BMI. Participants were then subdivided into < 25, 25–30 and over 30 years old and further into BMI fewer than 24.9, 25–30 and over 30 kg/m2 groups. The calculation of an adequate sample size was done based on the appropriate formula for case control studies, considering the 95% confidence interval, 90% power, 30% expected exposure ratio in the control group13 and the Odds ratio equal to 2, sample size was determined as 120 and 250 participants in case and control groups, respectively. Figure 1 shows the flow chart of the study. The subjects were recruited from Shahid-Beheshti women’s hospital, affiliated with Isfahan University of Medical Sciences, Isfahan, Iran. A purposive sampling method is used to select eligible individuals with the exclusion criteria of no surgery history, chemotherapy, radiotherapy, endometriosis, premature ovarian failure, infertility treatment, endocrine and metabolic disorders, chronic or acute inflammation, hormone therapy, special diets, or oral contraceptives. The transvaginal ultrasound and the diagnosis of DOR was made by two qualified gynecologists (H.GHT and FZ.A) based on low AMH (≤ 0.7 ng/mL) or low AFC (≤ 4 in both ovaries) or both14. Written informed consent was obtained from all the participants at the beginning of the study. The study protocol was approved and registered by the Ethics Committee of Isfahan University of Medical Sciences (IR.ARI.MUI.REC.1401.297).
Figure 1.
Flow chart of the study.
Anthropometric and laboratory assessments
Demographic data including age, socioeconomic status (SES) and medical history were completed for each individual in a structured questionnaire by interviewing. Anthropometric assessments including weight, height, waist circumference (WC), and hip circumference (HC) were performed by a trained person. A Seca scale was used to measure the participants’ weight and height while they were in a normal standing position, wearing light clothing and no shoes. By dividing the measured WC and HC the waist to hip ration (WHR) was then computed. Body composition (fat mass (FM) and fat-free mass (FFM)) was measured through body composition analyzer (Inbody 770, Inbody Co, Seoul, Korea). Physical activity level was also evaluated using the validated Iranian version of the international physical activity questionnaire (IPAQ)15. Systolic and diastolic blood pressure (SBP and DBP) were assessed twice (by Microlife Blood Pressure Monitor A100-30, Berneck, Switzerland) in a relaxing position after 15 min-resting and the mean values were recorded. Serum AMH levels were assessed by the enzyme linked immunosorbent assay (ELISA) method (Monobind, California, USA). Antral follicle counts in both ovaries were determined by transvaginal ultrasound on the third day of an unstimulated period.
Dietary assessment
Dietary intake was evaluated by using 80-items validated semi-quantitative food frequency questionnaire (FFQ)16. Portion sizes were changed to grams by using standard Iranian household measurements17. Energy and nutrient intake was estimated using a modified version of Nutritionist IV software for Iranian foods18.
Healthy eating index (HEI)-2015
We calculated HEI-2015 scores using data from the FFQ. HEI-2015 comprises 13 components divided into two main categories: 9 adequacy and 4 moderation components, with a total score of 10012. The lowest and highest consumption of six items of nine adequacy components (total fruit, whole fruit, vegetables, greens and beans, protein foods and seafood and plant proteins) received scores of 0 to 5, respectively. For the lowest and highest consumptions, the other three adequacy items (whole grains, dairy, and fatty acid ratio) were rated 0 to 10. In the moderation section, up to 10 points are awarded for the lowest consumption of the four components, including refined grains, sodium, added sugars, and saturated fat. Higher overall HEI-2015 scores shows greater alignment with dietary guidelines for Americans (DGA) recommendation and better diet quality12.
Statistical analyses
Statistical analyzes were performed using the Statistical Package for the Social Sciences (SPSS, version 21.0., Chicago, Illinois, USA). A two-tailed P-value < 0.05 was considered as statistical significance. Participants were categorized based on quartile of HEI-2015 scores. Higher quartiles demonstrate higher diet quality compared to lower quartiles. One-way analysis of variance (ANOVA) and Chi-square test was used to assess the differences in continuous and categorical variables across quartiles of the HEI-2015 score respectively. Using multivariable logistic regression with multiple covariates in two models, the relationship between the HEI-2015 scores and the odds of DOR was examined. We used analysis of covariance (ANCOVA) to compare adjusted (for FM, BMI, Physical activity, weight, and total energy) means of AMH and AFC across the HEI-2015 scores. Potential confounding variables included in the analyses were chosen based on prior literature19,20 as well as Directed Acyclic Graph (DAG)21. The Model I was adjusted for physical activity and energy and the Model II was adjusted for confounders in Model I plus FM and BMI.
Ethical approval
The study protocol was approved by the local Ethics Committee of Isfahan University of Medical Sciences (IR.ARI.MUI.REC.1401.297).
Results
The present case–control study was conducted on 120 women with DOR (case group) and 250 age- and BMI-matched controls. 382 women were recruited in the first stage. After the interview, 12 participants were excluded from the study due to failure to complete the questionnaire (n = 6) and unwillingness to continue participating in the study (n = 6) (Fig. 1). DOR was determined based on both AMH and AFC assessments in 98 cases (91.8%) and based on only AFC in 22 cases (18.3%). Table 1 presents sociodemographic characteristics, body composition, anthropometric indices, physical activity, and DOR markers between case and control groups. The mean BMI of the cases and controls was 29.85 and 28.75 kg/m2 respectively. A higher mean FM was found among women with DOR compared to women in the control group (38.47 vs 36.47, P = 0.02). Anthropometric measures also revealed that women with DOR had higher WC (102.23 vs 91.7) and WHR (0.9 vs 0.86) than women in control group. According to the results, serum AMH levels (0.56 vs 4.11) and AFC (2.34 vs 9.59) were significantly lower in women with DOR than in controls.
Table 1.
Baseline characteristic of study participants.
| Variable | Case (N = 120) | Control (N = 250) | P-valuea | |
|---|---|---|---|---|
| Age (years) | 33.37 ± 3.24 | 32.91 ± 3.15 | 0.196 | |
| BMI (kg/m2) | 29.85 ± 2.49 | 28.75 ± 3.45 | 0.235 | |
| Weight (kg) | 80.96 ± 4.78 | 79.26 ± 8.41 | 0.487 | |
| FM (kg) | 38.47 ± 7.05 | 36.47 ± 8.91 | 0.020 | |
| FFM (kg) | 57.99 ± 11.33 | 60.12 ± 11.97 | 0.098 | |
| WC (cm) | 102.23 ± 35.95 | 91.70 ± 12.43 | 0.002 | |
| HC (cm) | 109.10 ± 31.59 | 106.10 ± 11.57 | 0.316 | |
| WHR | 0.90 ± 0.12 | 0.86 ± 0.08 | 0.003 | |
| SBP (mmHg) | 122.18 ± 12.77 | 123.58 ± 14.03 | 0.341 | |
| DBP (mmHg) | 79.41 ± 11.67 | 81.85 ± 10.48 | 0.056 | |
| Physical activity (MET/h/day) | 19.05 ± 4.12 | 18.98 ± 4.51 | 0.896 | |
| Socioeconomic status (SES) (%) | Low | 10 (8.3) | 19 (7.6) | 0.252 |
| Middle | 50 (41.7) | 127 (50.8) | ||
| High | 60 (50) | 104 (41.6) | ||
| Education (%) | Illiterate | 14 (11.7) | 34 (13.6) | < 0.001 |
| ≤ High school/diploma | 31(25.8) | 121 (48.4) | ||
| ≥ College degree | 75 (62.5) | 95 (38) | ||
| Occupation (%) | Housewife | 82 (68.3) | 184 (73.6) | < 0.001 |
| Employed | 26 (21.7) | 10 (4) | ||
| Student | 12 (10) | 56 (22.4) | ||
| Pervious pregnancy | Yes | 99 (82.5) | 203 (81.2) | 0.441 |
| No | 21 (17.5) | 47 (18.8) | ||
| AFC count | 2.34 ± 1.19 | 9.59 ± 2.24 | < 0.001 | |
| AMH (ng/ml) | 0.56 ± 0.71 | 4.11 ± 1.18 | < 0.001 | |
Quantitative variables are expressed as mean ± SD and qualitative variables expressed as n (%).
The SES scored was evaluated based on education level of both subjects and the family head, job of both subjects and the family head family size, home status and home type by using self-reported questionnaire.
AFC antral follicle count, BMI body mass index, DBP diastolic blood pressure, DOR duration, diminished or decreased ovarian reserve, FFM fat free mass, FM fat mass, HC hip circumference, SBP systolic blood pressure, WC waist circumference, WHR waist to hip ratio.
aP values resulted from independent t-tests for quantitative and Chi-square for qualitative variables between the two groups.
As illustrated in Table 2, no significant differences were revealed across quartiles of groups in terms of HEI-2015 scores. The between group analysis showed that women with DOR had significantly lower SBP (P = 0.003), higher HC (P = 0.002), and lower serum AMH (P < 0.001).
Table 2.
Characteristic of study participants according to quartiles of healthy eating index-2015 (HEI-2015).
| Variable | Case (120) | Control (250) | P** | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | P* | Q1 | Q2 | Q3 | Q4 | P* | |||
| Age (years) | 33.16 ± 3.74 | 33.84 ± 3.19 | 33.71 ± 2.69 | 32.87 ± 2.98 | 0.670 | 32.71 ± 3.22 | 32.75 ± 2.86 | 33.14 ± 3.04 | 33.15 ± 3.58 | 0.786 | 0.196 | |
| BMI (kg/m2) | 30.04 ± 2.28 | 29.89 ± 2.93 | 30.04 ± 2.62 | 29.22 ± 2.28 | 0.593 | 27.56 ± 3.44 | 28.09 ± 3.50 | 27.52 ± 3.50 | 27.79 ± 3.40 | 0.771 | 0.235 | |
| Weight (kg) | 82.34 ± 3.82 | 81.20 ± 3.31 | 82.32 ± 4.99 | 83.54 ± 4.04 | 0.263 | 77.31 ± 5.61 | 77.87 ± 5.65 | 79.10 ± 3.97 | 78.75 ± 3.26 | 0.157 | 0.487 | |
| FM (kg) | 39.20 ± 6.46 | 40.35 ± 9.39 | 36.73 ± 5.66 | 37.25 ± 6.42 | 0.199 | 36.40 ± 9.29 | 35.71 ± 9.14 | 37.72 ± 7.41 | 36.25 ± 9.65 | 0.653 | 0.020 | |
| FFM (kg) | 58.51 ± 12.07 | 58.67 ± 11.70 | 56.34 ± 10.90 | 58.27 ± 10.54 | 0.855 | 60.12 ± 11.56 | 60.72 ± 12.30 | 58.56 ± 12.16 | 61.01 ± 12.09 | 0.705 | 0.098 | |
| WC (cm) | 108.44 ± 37.54 | 100.72 ± 35.97 | 90.75 ± 24.70 | 106.08 ± 42.38 | 0.217 | 91.67 ± 13.92 | 92.58 ± 11.62 | 90.80 ± 12.31 | 91.52 ± 11.66 | 0.885 | 0.002 | |
| HC (cm) | 111.24 ± 33.07 | 107.24 ± 32.80 | 104.32 ± 28.82 | 112.77 ± 31.86 | 0.745 | 106.56 ± 12.40 | 107.10 ± 10.76 | 105.15 ± 10.42 | 105.13 ± 12.75 | 0.716 | 0.316 | |
| WHR | 0.93 ± 0.13 | 0.89 ± 0.09 | 0.88 ± 0.12 | 0.87 ± 0.11 | 0.226 | 0.86 ± 0.08 | 0.86 ± 0.08 | 0.86 ± 0.07 | 0.87 ± 0.10 | 0.776 | 0.003 | |
| SBP (mmHg) | 120.95 ± 13.04 | 120.68 ± 12.11 | 120.85 ± 12.31 | 127.50 ± 12.85 | 0.157 | 121.78 ± 13.54 | 125.07 ± 14.07 | 121.07 ± 14.63 | 126.86 ± 13.48 | 0.086 | 0.341 | |
| DBP (mmHg) | 77.93 ± 10.00 | 79.84 ± 13.59 | 80.39 ± 11.06 | 80.50 ± 13.38 | 0.774 | 80.80 ± 11.32 | 82.14 ± 12.05 | 80.53 ± 9.56 | 84.41 ± 9.46 | 0.223 | 0.056 | |
| AFC count | 2.48 ± 1.24 | 2.40 ± 0.95 | 2.25 ± 1.32 | 2.12 ± 1.22 | 0.653 | 9.76 ± 2.15 | 9.50 ± 2.30 | 9.60 ± 2.20 | 9.45 ± 2.36 | 0.859 | < 0.001 | |
| AMH (ng/ml) | 0.49 ± 0.23 | 0.75 ± 1.52 | 0.55 ± 0.22 | 0.49 ± 0.16 | 0.518 | 3.94 ± 1.16 | 4.09 ± 1.23 | 4.20 ± 1.22 | 4.28 ± 1.10 | 0.402 | < 0.001 | |
| Physical activity (MET/h/day) | 18.25 ± 4.12 | 18.60 ± 4.15 | 19.75 ± 4.34 | 20.12 ± 3.67 | 0.229 | 18.90 ± 4.59 | 18.98 ± 4.57 | 19.16 ± 4.36 | 18.92 ± 4.60 | 0.990 | 0.896 | |
| Socioeconomic status (SES) (%) | Low | 4 (9.3) | 3 (12) | 1 (3.6) | 2 (8.3) | 0.904 | 5 (6.8) | 7 (10) | 5 (8.9) | 2 (3.9) | 0.053 | 0.252 |
| Middle | 18 (41.9) | 9 (36) | 14 (50) | 9 (37.5) | 47 (64.4) | 47 (64.4) | 30 (53.6) | 22 (43.1) | ||||
| High | 21 (48.8) | 13 (52) | 13 (46.4) | 13 (54.2) | 21 (28.8) | 21 (28.8) | 21 (37.5) | 27 (52.9) | ||||
| Education (%) | Illiterate | 3 (7) | 7 (28) | 3 (10.7) | 1 (4.2) | 0.055 | 13 (17.8) | 7 (10) | 8 (14.3) | 6 (11.8) | 0.497 | < 0.001 |
| ≤ High school/diploma | 14 (32.6) | 7 (28) | 4 (14.3) | 6 (25) | 35 (47.9) | 40 (57.1) | 23 (41.1) | 23 (45.1) | ||||
| ≥ College degree | 26 (60.5) | 11 (44) | 21 (75) | 17 (70.8) | 25 (34.2) | 23 (32.9) | 25 (44.6) | 22 (43.1) | ||||
| Occupation | Housewife | 27 (62.8) | 18 (72) | 18 (64.3) | 19 (79.2) | 0.528 | 55 (75.3) | 50 (71.4) | 41 (73.2) | 38 (74.5) | 0.426 | < 0.001 |
| Employed | 9 (20.9) | 5 (20) | 7 (25) | 5 (20.8) | 1 (1.4) | 3 (4.3) | 5 (8.9) | 1 (2) | ||||
| Student | 7 (16.3) | 2 (8) | 3 (10.7) | 0 (0) | 17 (23.3) | 17 (24.3) | 10 (17.9) | 12 (23.5) | ||||
| Pervious pregnancy | Yes | 36 (83.7) | 18 (72) | 25 (89.3) | 20 (83.3) | 0.414 | 59 (80.8) | 58 (82.9) | 44 (78.6) | 42 (82.4) | 0.934 | 0.441 |
| No | 7 (16.3) | 7 (28) | 3 (10.7) | 4 (16.7) | 14 (19.2) | 12 (17.1) | 12 (21.4) | 9 (17.6) | ||||
Quantitative variables are expressed as mean ± SD and qualitative variables expressed as n (%).
The SES scored was evaluated based on education level of both subjects and the family head, job of both subjects and the family head family size, home status and home type by using self-reported questionnaire.
AFC antral follicle count, BMI body mass index, DBP diastolic blood pressure, DOR duration, diminished or decreased ovarian reserve, FFM fat free mass, FM fat mass, HC hip circumference, SBP systolic blood pressure, WC waist circumference, WHR waist to hip ratio.
Significant values are in bold.
*P values resulted from ANOVA test for quantitative and Chi-square for qualitative variables across quartiles.
**P values resulted from independent t-tests for quantitative and Chi-square for qualitative variables between the two groups.
Multivariable adjusted odds ratios (ORs) for DOR in quartiles of the HEI-2015 scores are provided in Table 3. In the crude model, no significant relationship was found between DOR and HEI-2015 score. After controlling for physical activity and energy intake in Model I, we found that women in the highest quartile of HEI-2015 score had 31% decreased odds of DOR (OR 0.69; 95%CI 0.46, 0.93). This association remained significant even after adjusting for all potential confounders in Model II (OR 0.77; 95%CI 0.69, 0.92).
Table 3.
Odds ratio (95% CI) of DOR according to quartiles of HEI-2015.
| HEI-2015 | |||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | P-trend | |
| DOR/control | 43/73 | 25/70 | 28/56 | 24/51 | |
| Crude | Ref (1.00) | 0.92 (0.71–1.29) | 0.87 (0.72–1.24) | 0.78 (0.59–1.03) | 0.056 |
| Model 1 | Ref (1.00) | 0.91 (0.71–1.28) | 0.87 (0.70–1.21) | 0.69 (0.46–0.93) | 0.01 |
| Model 2 | Ref (1.00) | 0.95 (0.57–1.31) | 0.9 (0.63–1.29) | 0.77 (0.69–0.92) | 0.065 |
Multivariable logistic regression models were used with adjustment of potential confounders.
Model 1: Physical Activity + Energy intake (kcal/day).
Model 2: Model 1 + Fat Mass (kg) and Body mass index.
Discussion
Based on our knowledge and literature review, this study represents the first investigation into the correlation between HEI-2015 and ovarian reserve. Findings from this case–control study indicated that women with DOR exhibited higher values of FM, WC, and WHR compared to the control group. While the initial analysis did not reveal a significant association between HEI and the odds ratio of DOR in the unadjusted model, subsequent adjusted models demonstrated that individuals with stronger adherence to HEI displayed a reduced odds ratio for DOR.
In women, the ovarian follicle pool decreases as they age, which is regarded as a natural occurrence. However, several factors, such as diet, can influence the rate of this process22–24. Over the past decade, mounting evidence suggests that lifestyle factors, including diet, can significantly impact the fertility of women in their reproductive years24–27. Unfortunately, there is a lack of comprehensive studies exploring the specific effects of diet on ovarian reserves, and the existing research yields conflicting results20,24,28.
Most of the previous studies have primarily focused on analyzing the impact of single food items, with limited research conducted on dietary patterns. AMH and AFC serve as the primary indicators of ovarian reserves. By aligning the outcomes of numerous studies investigating single food items with the recent study, it becomes evident that the consumption of monounsaturated and polyunsaturated fatty acids, dairy products, fruits, and antioxidants has a positive effect on ovarian reserves, thereby delaying the onset of menopause. Notably, these recommended foods also form part of the HEI. For instance, one study revealed an inverse relationship between total fat consumption and AMH concentration29. The data obtained from the Nurses’ Health Study II (NHSII) indicated that women who consumed higher levels of trans fats were significantly more susceptible to ovulatory infertility compared to those who consumed monounsaturated or polyunsaturated fats30. Moreover, another study found that increased intake of dairy fat correlated with a reduced annual decline in AMH levels31.
The findings of studies pertaining to dairy consumption have yielded conflicting results. In the EARTH study, which focused on women undergoing in vitro fertilization (IVF), a negative correlation was observed between dairy consumption and AFC28. Moreover, a prospective study indicated that the intake of total dairy, milk, and fermented milk products was linked to annual declines in AMH levels and a reduced probability of rapid AMH decline31. Conversely, a separate study found no significant association between dairy product consumption and menopause occurrence32. However, another study demonstrated that higher dairy product consumption was linked to delayed onset of menopause in women below the age of 5133.
The increased consumption of dietary antioxidants has been proposed as a means to reduce the apoptotic loss of primordial follicles caused by oxidative stress34,35. The study conducted by Moslehi et al. revealed that the intake of various fruits and berries is inversely associated with the annual decrease in AMH levels31. Additionally, research has demonstrated the significance of carbohydrate type in ovarian health. Consumption of carbohydrates with a low glycemic index and dairy-based carbohydrates has been found to enhance ovarian reserves29,31. Furthermore, there is some evidence suggesting the potential benefits of vitamin D supplementation36,37, although the results have varied across studies38–40. In terms of fatty acid supplementation, omega-3 has shown a positive correlation with serum AMH levels41. Conversely, supplementation with mega-6 fatty acids has yielded opposite outcomes29,42.
Studies investigating the association between food intake and ovarian reserves offer valuable insights into the impact of specific nutrients on ovarian reserve. However, their findings may have limitations as nutrients can exhibit mutual or synergistic effects when consumed in combination43. Therefore, it is necessary to conduct more comprehensive studies focusing on dietary patterns. Such studies would provide a better understanding of the overall effects of food consumption on ovarian reserve and facilitate the adoption of healthy eating habits by individuals44. The findings from studies investigating the association between dietary patterns and ovarian reserves have yielded inconsistent results.
One study revealed that adopting a fertility diet (FD), characterized by reduced trans-fat intake and increased consumption of monounsaturated fatty acids, vegetable protein, and high-fat dairy products, and is associated with the lowest risk of ovulatory infertility45. Another study conducted in 2019 demonstrated that women who followed a pro-fertility diet (PFD) prior to undergoing IVF had a higher likelihood of achieving a live birth46. The pro-fertility diet includes whole grains, soy, seafood, dairy products, and low pesticide exposure. In a study investigating the correlation between dietary patterns and markers of ovarian reserve in overweight and obese women, it was discovered that greater adherence to a Prudent Food Diet (PFD) exhibited a linear association with improved markers of ovarian reserve47, which aligns with the findings of the current study. The present study included participants with a body mass index in the overweight range. Overweight and obese women tend to have reduced ovarian response to oral drugs, ovulation induction, and gonadotropin stimulation48–50. Moreover, it has been demonstrated that AMH levels are inversely correlated with BMI51. Additionally, Eskew et al. reported a negative association between markers of ovarian reserve and higher adherence to the Western food pattern, known for its unhealthy characteristics, although this association was not statistically significant47. However, in another investigation comparing the association between three dietary patterns, namely, the FD, PFD, and Mediterranean diet, with AFC in women attending a fertility clinic, no significant association was observed20. Based on the findings of previous studies, it can be inferred that dietary patterns comprising healthier foods tend to have positive effects on ovarian reserves. This conclusion is consistent with the results of a recent study.
This study possesses several strengths. Firstly, the sample size of the study is adequate. Secondly, while most previous studies have explored the relationship between dietary patterns and ovarian reserves by examining specific diets or food patterns that promote fertility and enhance ovarian reserves, the present study focuses on the HEI. Furthermore, the study utilized robust diagnostic criteria for DOR.
However, there were certain limitations to this study. Firstly, Due to the lack of a universally accepted definition for DOR, the participants may incorrectly be classified into DOR and control groups. Secondly, the case–control design employed was a notable limitation, as it inherently carries an increased risk of bias. Third, although the assessment of food intake through the use of a FFQ is a common method in nutritional studies, its reliance on individuals’ memory introduces another potential source of bias. Finally, it is important to note that dietary patterns may not necessarily reflect overall diet intake and often concentrate on specific aspects of the diet. Therefore, the findings of this study cannot be generalized to all population groups.
Conclusion
In summary, the findings of this study indicate that increased adherence to the HEI-2015 may lead to a significant reduction in the risk of DOR in adjusted models. There is a paucity of research on dietary patterns and ovarian reserves. While studies investigating single foods have demonstrated the positive effects of healthier foods on ovarian reserve, most of these beneficial foods are components of the HEI. Considering the synergistic or interactive effects of food combinations, further investigations on dietary patterns are warranted. Additionally, randomized controlled trials with adequate sample sizes and diverse dietary interventions can provide valuable insights into the hypothesis concerning the association between dietary patterns and ovarian reserves.
Author contributions
R.Z. and H.G.-T.; conception and design of the work, H.G.-T. and F.Z.A.; study gynecologists, A.G., M.V. and R.A.K.; analysis and interpretation of data, S.T., G.A., R.Z. and F.A.; draft the work and revise it.
Funding
The present study was supported by a grant from Vice-Chancellor for Research, Isfahan University of Medical Sciences (Grant No: 2401257).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rahele Ziaei, Email: r.ziaei92@gmail.com.
Abed Ghavami, Email: abedghavami@gmail.com.
References
- 1.Cohen, J., Chabbert-Buffet, N. & Darai, E. Diminished ovarian reserve, premature ovarian failure, poor ovarian responder—A plea for universal definitions. J. Assist. Reprod. Genet.32, 1709–1712 (2015). 10.1007/s10815-015-0595-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han, S., Zhai, Y., Guo, Q., Qin, Y. & Liu, P. Maternal and neonatal complications in patients with diminished ovarian reserve in in-vitro fertilization/intracytoplasmic sperm injection cycles. Front. Endocrinol.12, 648287 (2021). 10.3389/fendo.2021.648287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devine, K. et al. Diminished ovarian reserve in the United States assisted reproductive technology population: Diagnostic trends among 181,536 cycles from the society for assisted reproductive technology clinic outcomes reporting system. Fertil. Steril.104(3), 612-619. e613 (2015). 10.1016/j.fertnstert.2015.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bunnewell, S. J. et al. Diminished ovarian reserve in recurrent pregnancy loss: A systematic review and meta-analysis. Fertil. Steril.113, 818-827. e813 (2020). 10.1016/j.fertnstert.2019.11.014 [DOI] [PubMed] [Google Scholar]
- 5.Pastore, L. M. et al. Reproductive ovarian testing and the alphabet soup of diagnoses: DOR, POI, POF, POR, and FOR. J. Assist. Reprod. Genet.35, 17–23 (2018). 10.1007/s10815-017-1058-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Boer, E. J., den Tonkelaar, I., te Velde, E. R., Burger, C. W. & van Leeuwen, F. E. Increased risk of early menopausal transition and natural menopause after poor response at first IVF treatment. Hum. Reprod.18(7), 1544–1552 (2003). 10.1093/humrep/deg278 [DOI] [PubMed] [Google Scholar]
- 7.Rasool, S. & Shah, D. Fertility with early reduction of ovarian reserve: The last straw that breaks the Camel’s back. Fertil. Res. Pract.3(1), 15 (2017). 10.1186/s40738-017-0041-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richardson, M. C., Guo, M., Fauser, B. C. J. M. & Macklon, N. S. Environmental and developmental origins of ovarian reserve. Hum. Reprod. Update20(3), 353–369 (2014). 10.1093/humupd/dmt057 [DOI] [PubMed] [Google Scholar]
- 9.Moslehi, N., Mirmiran, P., Azizi, F. & Tehrani, F. R. Do dietary intakes influence the rate of decline in anti-Mullerian hormone among eumenorrheic women? A population-based prospective investigation. Nutr. J.18, 1–9 (2019). 10.1186/s12937-019-0508-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panizza, C. E. et al. Testing the predictive validity of the healthy eating index-2015 in the multiethnic cohort: Is the score associated with a reduced risk of all-cause and cause-specific mortality?. Nutrients10(4), 452 (2018). 10.3390/nu10040452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasdar, Y. et al. Better muscle strength with healthy eating. Eat. Weight Disord. Stud. Anorex. Bulim. Obes.26, 367–374 (2021). 10.1007/s40519-020-00863-1 [DOI] [PubMed] [Google Scholar]
- 12.Krebs-Smith, S. M. et al. Update of the healthy eating index: HEI-2015. J. Acad. Nutr. Diet.118, 1591–1602 (2018). 10.1016/j.jand.2018.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nesbit, C. B. et al. New perspectives on the genetic causes of diminished ovarian reserve and opportunities for genetic screening: Systematic review and meta-analysis. F&S Rev.1, 1–15 (2020). 10.1016/j.xfnr.2020.06.001 [DOI] [Google Scholar]
- 14.Penzias, A. et al. Testing and interpreting measures of ovarian reserve: A committee opinion. Fertil. Steril.114, 1151–1157 (2020). 10.1016/j.fertnstert.2020.09.134 [DOI] [PubMed] [Google Scholar]
- 15.Moghaddam, M. B. et al. The Iranian version of international physical activity questionnaire (IPAQ) in Iran: Content and construct validity, factor structure, internal consistency and stability. World Appl. Sci. J.18, 1073–1080 (2012). [Google Scholar]
- 16.Nikniaz, L. et al. Reliability and relative validity of short-food frequency questionnaire. Br. Food J.119, 1337–1348 (2017). 10.1108/BFJ-09-2016-0415 [DOI] [Google Scholar]
- 17.Ghaffarpour, M., Houshiar-Rad, A. & Kianfar, H. The manual for household measures, cooking yields factors and edible portion of foods. Tehran Nashre Olume Keshavarzy7, 42–58 (1999). [Google Scholar]
- 18.Azar, M. & Sarkisian, E. Food composition table of Iran. Tehran: National Nutrition and Food Research Institute, Shaheed Beheshti University; 1980. (Farsi).
- 19.Eskew, A. M., Bedrick, B. S., Chavarro, J. E., Riley, J. K. & Jungheim, E. S. Dietary patterns are associated with improved ovarian reserve in overweight and obese women: A cross-sectional study of the lifestyle and ovarian reserve (LORe) cohort. Reprod. Biol. Endocrinol.20, 33 (2022). 10.1186/s12958-022-00907-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maldonado-Cárceles, A. B. et al. Dietary patterns and ovarian reserve among women attending a fertility clinic. Fertil. Steril.114, 610–617 (2020). 10.1016/j.fertnstert.2020.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothman, K. J., Greenland, S. & Lash, T. L. Modern Epidemiology (Wolters Kluwer Health/Lippincott Williams & Wilkins, 2008). [Google Scholar]
- 22.Freeman, E. W., Sammel, M. D., Lin, H., Boorman, D. W. & Gracia, C. R. Contribution of the rate of change of antimüllerian hormone in estimating time to menopause for late reproductive-age women. Fertil. Steril.98, 1254-1259.e1251–1252 (2012). 10.1016/j.fertnstert.2012.07.1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gohari, M. R., Ramezani Tehrani, F., Chenouri, S., Solaymani-Dodaran, M. & Azizi, F. Individualized predictions of time to menopause using multiple measurements of antimüllerian hormone. Menopause (New York, N.Y.)23, 839–845 (2016). 10.1097/GME.0000000000000642 [DOI] [PubMed] [Google Scholar]
- 24.Moslehi, N., Mirmiran, P., Tehrani, F. R. & Azizi, F. Current evidence on associations of nutritional factors with ovarian reserve and timing of menopause: A systematic review. Adv. Nutr. (Bethesda Md.)8, 597–612 (2017). 10.3945/an.116.014647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wise, L. A. et al. Dietary fat intake and fecundability in 2 preconception cohort studies. Am. J. Epidemiol.187, 60–74 (2018). 10.1093/aje/kwx204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hatch, E. E. et al. Intake of sugar-sweetened beverages and fecundability in a North American preconception cohort. Epidemiology (Cambridge, Mass.)29, 369–378 (2018). 10.1097/EDE.0000000000000812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mumford, S. L. et al. Dietary fat intake and reproductive hormone concentrations and ovulation in regularly menstruating women. Am. J. Clin. Nutr.103, 868–877 (2016). 10.3945/ajcn.115.119321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Souter, I. et al. The association of protein intake (amount and type) with ovarian antral follicle counts among infertile women: Results from the EARTH prospective study cohort. BJOG Int. J. Obstet. Gynaecol.124, 1547–1555 (2017). 10.1111/1471-0528.14630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson, C., Mark Park, Y. M., Stanczyk, F. Z., Sandler, D. P. & Nichols, H. B. Dietary factors and serum antimüllerian hormone concentrations in late premenopausal women. Fertil. Steril.110, 1145–1153 (2018). 10.1016/j.fertnstert.2018.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chavarro, J. E., Rich-Edwards, J. W., Rosner, B. A. & Willett, W. C. Dietary fatty acid intakes and the risk of ovulatory infertility. Am. J. Clin. Nutr.85, 231–237 (2007). 10.1093/ajcn/85.1.231 [DOI] [PubMed] [Google Scholar]
- 31.Moslehi, N., Mirmiran, P., Azizi, F. & Tehrani, F. R. Do dietary intakes influence the rate of decline in anti-Mullerian hormone among eumenorrheic women? A population-based prospective investigation. Nutr. J.18, 83 (2019). 10.1186/s12937-019-0508-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagel, G., Altenburg, H. P., Nieters, A., Boffetta, P. & Linseisen, J. Reproductive and dietary determinants of the age at menopause in EPIC-Heidelberg. Maturitas52, 337–347 (2005). 10.1016/j.maturitas.2005.05.013 [DOI] [PubMed] [Google Scholar]
- 33.Carwile, J. L., Willett, W. C. & Michels, K. B. Consumption of low-fat dairy products may delay natural menopause. J. Nutr.143, 1642–1650 (2013). 10.3945/jn.113.179739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagata, C., Takatsuka, N., Kawakami, N. & Shimizu, H. Association of diet with the onset of menopause in Japanese women. Am. J. Epidemiol.152, 863–867 (2000). 10.1093/aje/152.9.863 [DOI] [PubMed] [Google Scholar]
- 35.Pearce, K. & Tremellen, K. Influence of nutrition on the decline of ovarian reserve and subsequent onset of natural menopause. Hum. Fertil. (Cambridge, England)19, 173–179 (2016). 10.1080/14647273.2016.1205759 [DOI] [PubMed] [Google Scholar]
- 36.Dennis, N. A. et al. The level of serum anti-Müllerian hormone correlates with vitamin D status in men and women but not in boys. J. Clin. Endocrinol. Metab.97, 2450–2455 (2012). 10.1210/jc.2012-1213 [DOI] [PubMed] [Google Scholar]
- 37.Naderi, Z., Kashanian, M., Chenari, L. & Sheikhansari, N. Evaluating the effects of administration of 25-hydroxyvitamin D supplement on serum anti-Mullerian hormone (AMH) levels in infertile women. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol.34, 409–412 (2018). 10.1080/09513590.2017.1410785 [DOI] [PubMed] [Google Scholar]
- 38.Chang, E. M., Kim, Y. S., Won, H. J., Yoon, T. K. & Lee, W. S. Association between sex steroids, ovarian reserve, and vitamin D levels in healthy nonobese women. J. Clin. Endocrinol. Metab.99, 2526–2532 (2014). 10.1210/jc.2013-3873 [DOI] [PubMed] [Google Scholar]
- 39.Fabris, A. M. et al. Impact of vitamin D levels on ovarian reserve and ovarian response to ovarian stimulation in oocyte donors. Reprod. Biomed. Online35, 139–144 (2017). 10.1016/j.rbmo.2017.05.009 [DOI] [PubMed] [Google Scholar]
- 40.Irani, M., Minkoff, H., Seifer, D. B. & Merhi, Z. Vitamin D increases serum levels of the soluble receptor for advanced glycation end products in women with PCOS. J. Clin. Endocrinol. Metab.99, E886-890 (2014). 10.1210/jc.2013-4374 [DOI] [PubMed] [Google Scholar]
- 41.Al-Safi, Z. A. et al. Omega-3 fatty acid supplementation lowers serum FSH in normal weight but not obese women. J. Clin. Endocrinol. Metab.101, 324–333 (2016). 10.1210/jc.2015-2913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sjaarda, L. A. et al. Dietary carbohydrate intake does not impact insulin resistance or androgens in healthy, eumenorrheic women. J. Clin. Endocrinol. Metab.100, 2979–2986 (2015). 10.1210/jc.2015-1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu, F. B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol.13, 3–9 (2002). 10.1097/00041433-200202000-00002 [DOI] [PubMed] [Google Scholar]
- 44.Tapsell, L. C., Neale, E. P., Satija, A. & Hu, F. B. Foods, nutrients, and dietary patterns: Interconnections and implications for dietary guidelines. Adv. Nutr. (Bethesda, Md.)7, 445–454 (2016). 10.3945/an.115.011718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chavarro, J. E., Rich-Edwards, J. W., Rosner, B. A. & Willett, W. C. Diet and lifestyle in the prevention of ovulatory disorder infertility. Obstet. Gynecol.110, 1050–1058 (2007). 10.1097/01.AOG.0000287293.25465.e1 [DOI] [PubMed] [Google Scholar]
- 46.Gaskins, A. J. et al. Dietary patterns and outcomes of assisted reproduction. Am. J. Obstet. Gynecol.220(567), e561-567.e518 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eskew, A. M., Bedrick, B. S., Chavarro, J. E., Riley, J. K. & Jungheim, E. S. Dietary patterns are associated with improved ovarian reserve in overweight and obese women: A cross-sectional study of the lifestyle and ovarian reserve (LORe) cohort. Reprod. Biol. Endocrinol. RB&E20, 33 (2022). 10.1186/s12958-022-00907-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Imani, B., Eijkemans, M. J., te Velde, E. R., Habbema, J. D. & Fauser, B. C. A nomogram to predict the probability of live birth after clomiphene citrate induction of ovulation in normogonadotropic oligoamenorrheic infertility. Fertil. Steril.77, 91–97 (2002). 10.1016/S0015-0282(01)02929-6 [DOI] [PubMed] [Google Scholar]
- 49.Mulders, A. G., Laven, J. S., Eijkemans, M. J., Hughes, E. G. & Fauser, B. C. Patient predictors for outcome of gonadotrophin ovulation induction in women with normogonadotrophic anovulatory infertility: A meta-analysis. Hum. Reprod. Update9, 429–449 (2003). 10.1093/humupd/dmg035 [DOI] [PubMed] [Google Scholar]
- 50.Souter, I., Baltagi, L. M., Kuleta, D., Meeker, J. D. & Petrozza, J. C. Women, weight, and fertility: The effect of body mass index on the outcome of superovulation/intrauterine insemination cycles. Fertil. Steril.95, 1042–1047 (2011). 10.1016/j.fertnstert.2010.11.062 [DOI] [PubMed] [Google Scholar]
- 51.Palomaki, G. E. et al. Adjusting antimüllerian hormone levels for age and body mass index improves detection of polycystic ovary syndrome. Fertil. Steril.113, 876-884.e872 (2020). 10.1016/j.fertnstert.2019.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.