Abstract
Human cytomegalovirus UL25 codes for a structural phosphoprotein of 85 kDa (C. J. Baldick and T. Shenk, J. Virol. 70:6097–6105, 1996; M. C. Battista et al., J. Virol. 73:3800–3809, 1999). In this study we analyzed the intracellular and intraviral localization of pUL25 by confocal and immunoelectron microscopy and found that pUL25 is a component of the viral tegument and the dense body matrix, acquired during the late cytoplasmic phase of virus maturation.
The human cytomegalovirus (CMV) virion consists of an icosahedral capsid embedded in an amorphous layer, called the tegument, that fills the space between the capsid and the trilaminar external envelope. The tegument contains approximately 40% of the virion protein mass (7), but little is known about its structure or function. Its protein composition remains incompletely defined, even though the following gene products have been assigned to it: pUL48, pUL47, pUL32, pUL82, pUL83, and pUL99. Other possible tegument components are a subform of pUL69, pUL56, pUL94, and pUL97 (for a review, see references 5 and 14).
CMV UL25 has recently been found to encode a new structural protein, present in both virions and defective virus particles (1). In a more-recent work we defined the main characteristics of pUL25, a phosphoprotein of 85 kDa expressed with true late kinetics (2). Because of its presence both in virions and in the dense bodies and because of its intracellular colocalization with other tegument proteins, such as pUL99, it has been suggested that pUL25 is a new component of the viral tegument (1, 2). In this study confocal microscopy and immunoelectron microscopy were used to define the intracellular and intraviral localization of pUL25. Human embryonic lung (HEL) fibroblasts were grown in Eagle’s minimum essential medium supplemented with 10% fetal calf serum. Infections were made on 70 to 80% confluent monolayers of HEL cells with the CMV strain AD169 at a multiplicity of infection of 1 to 2 for 60 min.
pUL25 expression was determined by indirect immunofluorescence, after fixation with 4% paraformaldehyde for 15 min at 4°C, on CMV-infected glass-adherent HEL cells incubated with polyclonal antibody CK25 in ascitic fluid (2) (dilution, 1:400) for 1 h at 37°C in a humid chamber. A fluorescein-conjugated goat anti-mouse Tec-Fab2 GAM-Ig (Technogenetics, Milan, Italy) was used as a secondary antibody. Fluorescent samples were imaged with a Phoibos 1000 confocal system (Molecular Dynamics, Sunnyvale, Calif.). For three-dimensional reconstruction, 0.3-μm-thick serial optical sections were recorded (512 by 512 pixels) and processed by dedicated software (ImageSpace; Molecular Dynamics) running on an Indigo workstation (Silicon Graphics, Mountain View, Calif.). pUL25 subcellular localization was analyzed by pseudocolor representation of fluorescence intensity on phase-contrast microscopy-imaged cells.
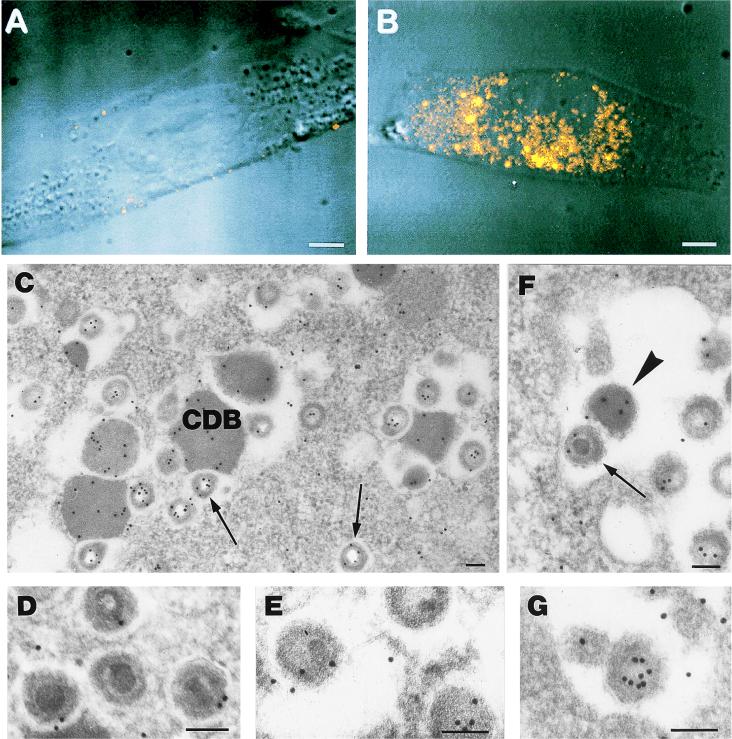
For the immunocytochemical detection of pUL25, the pelleted cells were fixed with 1% glutaraldehyde for 30 min, dehydrated with absolute ethanol, and embedded in Epon. Thin sections were treated for 10 min with 10% H2O2, preincubated with 5% normal goat serum, and incubated with polyclonal antibody CK25 in ascitic fluid (dilution, 1:100). A goat anti-mouse immunoglobulin G conjugated with 10-nm-diameter colloidal gold particles was used as a secondary antibody. Silver enhancement (Silver Enhancer Kit; Amersham, Little Chalfont, Buckinghamshire, United Kingdom) was used to enlarge the gold particle size. Controls consisted of samples not included with the primary antibody. As shown in Fig. 1A, a very low-level background signal was detected in mock-infected HEL cells. In infected HEL cells, starting from 48 h postinfection (p.i.) and increasing thereafter, a strong signal, scattered within the cytoplasm and always localized in that cell compartment, was obtained (Fig. 1B). The signal showed a grainy consistency, similar to that described for the pUL25 murine homologue (4).
FIG. 1.
(A and B) Confocal microscopy of HEL cells labeled with anti-pUL25 antibody. (A) Mock-infected cells show a very faint background signal. (B) At 96 h after infection with CMV, the fluorescence due to the anti-pUL25 antibody in the cytoplasm indicates the presence of the protein in cytoplasmic bodies of different sizes. Bar = 5 μm. (C through G) Electron microscopy of HEL cells stained with anti-pUL25 antibody 120 h after infection. (C) Gold particles are present on CDB and on virion particles generally devoid of a core. The majority of the gold particles are localized along the tegument (arrows). (D and E) High magnification of intracytoplasmic virions in which the core is visible. The labeling is less intense on the tegument than in the virions from which the core has been removed by the section etching, and the tegument is more available to antibody binding (panel C). (F) Extracellular enveloped virions are labeled on the tegument (arrow). An immunolabeled dense body is also present in the extracellular space (arrowhead). (G) Detail of an extracellular virion which is tangentially sectioned, thus not allowing the detection of the core, which presents intense labeling on the tegument. Bar = 0.1 μm.
The results obtained indicate that pUL25 is expressed exclusively at the cytoplasmic level during the late phase of the virus replication cycle, as previously reported for other human CMV tegument proteins, such as pUL99 (10).
In agreement with the data obtained by confocal microscopy, electron microscopy immunolabeling studies revealed completely negative results until 48 h p.i. Thereafter, typical cytoplasmic structures, unique to CMV and called cytoplasmic dense bodies (CDB [17]), were increasingly positive with the anti-pUL25 antibody (Fig. 1C). The HEL cells, at 5 days p.i., presented virions at the different maturation stages, both in the nucleus and in the cytoplasm. The gold labeling due to the anti-pUL25 antibody was present exclusively on cytoplasmic virions, generally localized along the inner surface facing either the core or the empty space in which the core was no longer present (probably because of the “etching effect”) (Fig. 1C through E). Extracellular virus particles showed a labeling pattern identical to that observed on the intracellular particles (Fig. 1F and G). No labeling was detected on the nucleocapsids or on the envelopes of intracellular or extracellular virions (data not shown). Therefore, the presence of the bulk of the observed labeling distribution within the virion particles suggests that CMV pUL25, like its human herpesvirus 7 counterpart U14 (15), is present in the viral tegument during cytoplasmic virion assembly as well as in mature extracellular virions.
Our findings strongly suggest that in addition to tegument proteins, such as pUL82, pUL83, pUL32, and pUL69, which are translocated into the nucleus and probably associate with the nucleocapsid within this cell compartment (6, 8, 9, 11, 12, 17), there are other tegument proteins, such as pUL99 and pUL25, which do not enter the nucleus and associate with the nucleocapsid in the cytoplasm, where envelopment also occurs (8, 13, 16). Envelopment is most likely mediated by a specific recognition between a viral protein(s) residing within the tegument and a viral protein(s) present in the cytoplasmic membranes. We suggest that the tegument proteins which are expressed exclusively in the cytoplasm and during the late phases of virus replication are the most suitable for envelopment, being probably the last to be incorporated. pUL25 seems to be a new candidate for this role.
Acknowledgments
We thank Luisa Bertacchi for excellent technical assistance and Aurelio Valmori for photographic work.
This work was supported by the Italian Ministry of University and Scientific Research and by the University of Bologna (60 and 40%); the work was also supported by the AIDS Projects of the Italian Ministry of Public Health.
REFERENCES
- 1.Baldick C J, Jr, Shenk T. Proteins associated with purified human cytomegalovirus particles. J Virol. 1996;70:6097–6105. doi: 10.1128/jvi.70.9.6097-6105.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Battista M C, Bergamini G, Boccuni M C, Campanini F, Ripalti A, Landini M P. Expression and characterization of a novel structural protein of human cytomegalovirus, pUL25. J Virol. 1999;73:3800–3809. doi: 10.1128/jvi.73.5.3800-3809.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bogner E, Reschke M, Reis B, Mockenhaupt T, Radsak K. Identification of the gene product encoded by ORF UL56 of the human cytomegalovirus genome. Virology. 1993;196:290–293. doi: 10.1006/viro.1993.1477. [DOI] [PubMed] [Google Scholar]
- 4.Dallas P B, Lyons P A, Hudson J B, Scalzo A A, Shellam G R. Identification and characterization of a murine cytomegalovirus gene with homology to the UL25 open reading frame of human cytomegalovirus. Virology. 1994;200:643–650. doi: 10.1006/viro.1994.1227. [DOI] [PubMed] [Google Scholar]
- 5.Gibson W. Structure and assembly of the virion. Intervirology. 1996;39:389–400. doi: 10.1159/000150509. [DOI] [PubMed] [Google Scholar]
- 6.Hensel G, Meyer H, Gartner S, Brand G, Kern H F. Nuclear localization of the human cytomegalovirus tegument protein pp150 (ppUL32) J Gen Virol. 1995;76:1591–1601. doi: 10.1099/0022-1317-76-7-1591. [DOI] [PubMed] [Google Scholar]
- 7.Irmiere A, Gibson W. Isolation and characterization of a noninfectious virion-like particle released from cells infected with human strains of cytomegalovirus. Virology. 1983;130:118–133. doi: 10.1016/0042-6822(83)90122-8. [DOI] [PubMed] [Google Scholar]
- 8.Landini M P, Severi B, Furlini G, Badiali-De Giorgi L. Human cytomegalovirus structural components: intracellular and intraviral localization of p28 and p65-69 by immunoelectron microscopy. Virus Res. 1987;8:15–23. doi: 10.1016/0168-1702(87)90036-0. [DOI] [PubMed] [Google Scholar]
- 9.Liu B, Stinski M F. Human cytomegalovirus contains a tegument protein that enhances transcription from promoters with upstream ATF and AP-1 cis-acting elements. J Virol. 1992;66:4434–4444. doi: 10.1128/jvi.66.7.4434-4444.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyer H, Bankier A T, Landini M P, Brown C M, Barrell B G, Rüger B, Mach M. Identification and procaryotic expression of the gene coding for the highly immunogenic 28-kilodalton structural phosphoprotein (pp28) of human cytomegalovirus. J Virol. 1988;62:2243–2250. doi: 10.1128/jvi.62.7.2243-2250.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanchez V, Angeletti P C, Engler J A, Britt W J. Localization of human cytomegalovirus structural proteins to the nuclear matrix of infected human fibroblasts. J Virol. 1998;72:3321–3329. doi: 10.1128/jvi.72.4.3321-3329.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmolke S, Drescher P, Jahn G, Plachter B. Nuclear targeting of the tegument protein pp65 (UL83) of human cytomegalovirus: an unusual bipartite nuclear localization signal functions with other portions of the protein to mediate its efficient nuclear transport. J Virol. 1995;69:1071–1078. doi: 10.1128/jvi.69.2.1071-1078.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Severi B, Landini M P, Cenacchi G, Zini N, Maraldi N M. Human cytomegalovirus nuclear and cytoplasmic dense bodies. Arch Virol. 1992;123:193–207. doi: 10.1007/BF01317149. [DOI] [PubMed] [Google Scholar]
- 14.Spaete R R, Gehrz R C, Landini M P. Human cytomegalovirus structural proteins. J Gen Virol. 1994;75:3287–3308. doi: 10.1099/0022-1317-75-12-3287. [DOI] [PubMed] [Google Scholar]
- 15.Stefan A, Secchiero P, Baechi T, Kempf W, Campadelli-Fiume G. The 85-kilodalton phosphoprotein (pp85) of human herpesvirus 7 is encoded by open reading frame U14 and localizes to a tegument substructure in virion particles. J Virol. 1997;71:5758–5763. doi: 10.1128/jvi.71.8.5758-5763.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tooze J, Hollinshead M, Reis B, Radsak K, Kern H. Progeny vaccinia and human cytomegalovirus particles utilize early endosomal cisternae for their envelopes. Eur J Cell Biol. 1992;60:163–178. [PubMed] [Google Scholar]
- 17.Winkler M, Stamminger T. A specific subform of the human cytomegalovirus transactivator protein pUL69 is contained within the tegument of virus particles. J Virol. 1996;70:8984–8987. doi: 10.1128/jvi.70.12.8984-8987.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]