Abstract
The fungal genus Fusarium contains many important plant pathogens as well as endophytes of wild and crop plants. Globally, Fusarium toxins in food crops are considered one of the greatest food safety concerns. Their occurrence has become more pronounced in Africa in recent times. Among the major Fusarium mycotoxins with food and feed safety concerns, zearalenone is frequently detected in finished feeds and cereals in Africa. However, the impact of indigenous agricultural practices (pre‐ and postharvest factors) and food processing techniques on the prevalence rate of Fusarium species and zearalenone occurrence in food and feed have not been collated and documented systematically. This review studies and analyzes recent reports on zearalenone contamination in maize and other cereal products from Africa, including its fungi producers, agronomic and climate variables impacting their occurrences, preventive measures, removal/decontamination methods, and legislations regulating their limits. Reports from relevant studies demonstrated a high prevalence of F. verticillioides and F. graminearum as Africa's main producers of zearalenone. Elevated CO2 concentration and high precipitation may carry along an increased risk of zearalenone contamination in maize. African indigenous processing methods may contribute to reduced ZEA levels in agricultural products and foods. Most African countries do not know their zearalenone status in the food supply chain and they have limited regulations that control its occurrence.
Keywords: Africa, gene expression, nonthermal processing, PCR‐ELISA, zearalenone
Although maize has a significant contribution to human nutrition, the grain is susceptible to Fusarium infection and ZEA contamination due to poor pre‐ and post‐harvest factors. However, there are noticeable postharvest measures and processing factors that can reduce ZEA concentration in processed foods. Evidence of high dietary exposure in Africa is limited for use in the establishment of maximum permitted levels in food and feed raw material.
1. INTRODUCTION
Fusarium species are widely distributed and are well known as one of the world's most damaging fungi pathogens. The most harmful groups, zearalenone, trichothecenes, and fumonisin producers, continue to threaten sustainable agriculture and food safety in many regions. Recent reports have shown their common occurrence in African food and feed samples (Biomin, 2018, 2019; Gruber‐Dorninger et al., 2019). Their postharvest occurrence, coupled with pre‐ and postharvest factors such as water activity, temperature, or repeated misuse of mineral fertilizer are causing significant crop yield losses, and accumulation of mycotoxins (Mielniczuk & Skwaryło‐Bednarz, 2020). For example, excess nitrogen favored increased infestation of Fusarium fungi and subsequent production of zearalenone (ZEA) (Blandino et al., 2008; Podolska et al., 2017; Yi et al., 2001). Rapid infestation of grains by Fusarium species may cause a yield reduction of up to 19% (Lobulu et al., 2021), and severe water stress could cause the production of mycotoxins in kernels (Daou et al., 2021). As a result, foods and animal feeds are exposed to multiple mycotoxins simultaneously (Rodrigues et al., 2011). Mycotoxins are toxic metabolites of fungi that have adverse health effects on plants, animals, and humans. Among the identified Fusarium mycotoxins with food and feed safety concerns, ZEA is frequently detected in feeds and cereals in Africa (Gruber‐Dorninger et al., 2018). ZEA and its metabolites are the most studied mycotoxin with endocrine‐disrupting activity (Eze et al., 2018). In Africa, especially South Africa, ZEA has been associated with the clinical diagnosis of gynecomastia with testicular atrophy in males (Shephard, 2008). Its prevalence rate in agri‐food is becoming a prioritized area of research, particularly in Africa.
Limited studies on the natural occurrence of Fusarium species and ZEA in food and feed in Africa are available in literature (Tables 2 and 3). The effect of ZEA on human and animal health has prompted some countries to establish appropriate, permissible levels in foodstuff intended for human and animal use. In the European Union, the maximum level for ZEA ranges between 0.5 and 400 μg/kg in various products intended for human and animal consumption (European Commission, 2006a, 2006b). For example, the maximum acceptable level for ZEA in China is 60 μg/kg (Ward, 2018). Currently, few legislations (South Africa and Morocco) are available for regulating ZEA in food and feed in Africa (Ankwasa et al., 2021; Lahouar et al., 2018).
TABLE 2.
Impact of oxidative stress factors on Fusarium species activities.
| Stress compound | Effect on Fusarium growth, and mycotoxin production | Fusarium strain used | References |
|---|---|---|---|
| Sodium dodecyl sulfate (0.02%) | Decrease FUM gene expression | F. verticillioides | Nagygyörgy et al. (2014) |
| H2O2 (0.5, 2.0 mM) |
Increase fumonisin production by up to 300% Enhance FUM gene expression |
F. verticillioides | Ferrigo et al. (2015) |
| H2O2 |
Increase DON and acetyl‐DON production Enhance TRI gene expression |
F. graminearum | Ponts et al. (2007, 2009) |
| H2O2 (0.05%) | Reduce fungi growth rate | F. graminearum | Zheng et al. (2012) |
| SDS (0.05%) | Enhance the growth of colony diameter | F. graminearum | Zheng et al. (2012) |
| H2O2 (0.5 mM) | Increase DON and NIV production | F. culmorum | Ponts et al. (2009) |
| NaCl | Increased mycelial growth, mycelial biomass, sporulation, and microconidia | F. oxysporum | Maharshi et al. (2021) |
| EC (2–4 dS/m) |
Significantly enhanced fungal growth Increased biomass of fungal up to 90% |
F. oxysporum | Shoaib et al. (2018) |
| Sublethal dose Prothioconazole | Increase DON production | F. graminearum |
TABLE 3.
Summary of zearalenone occurrence in raw food and feed from Africa.
| Country/region | Food crop | Sample size (positive sample) | Detection method | Mean (range) (μg/kg) | References |
|---|---|---|---|---|---|
| Eastern Africa | |||||
| Kenya | Fish feed | 78 (40) | HPLC | 136 (<38–757.9) | Mwihia et al. (2020) |
| Ethiopia | Sorghum | 80 (19) | HPLC | 3.62 (<LOD‐121) | Mohammed, Bekeko, et al. (2022) and Mohammed, Seid, et al. (2022) |
| Kenya | Animal feed | 25 (19) | HPLC | 67 (61–167) | Rodrigues et al. (2011) |
| Kenya | Feed | 10 (6) | LC–MS/MS | NS (11.2–28.2) | Warth et al. (2012) |
| Tanzania | Cassava | 405 (154) | LC–MS/MS | NS (21.4–8493) | Sulyok et al. (2015) |
| Rwanda | Cassava | 222 (104) | LC–MS/MS | NS (100–2826) | Sulyok et al. (2015) |
| Southern Africa | |||||
| South Africa | Chicken feed | 62 (62) | LC–MS/MS | 100 (NS‐610) | Njobeh et al. (2012) |
| South Africa | Cattle feed | 25 (24) | LC–MS/MS | 72 (NS‐123) | Njobeh et al. (2012) |
| South Africa | Horse feed | 3 (3) | LC–MS/MS | 43 (NS‐46) | Njobeh et al. (2012) |
| South Africa | Swine feed | 2 (2) | LC–MS/MS | 148 (NS‐170) | Njobeh et al. (2012) |
| Madagascar | Cassava | 126 (41) | LC–MS/MS | 83.8 (16.3–286.7) | Abass et al. (2019) |
| Namibia | Kalaharituber pfeilii | 8 (8) | ELISA | NS (45–9680) | Hainghumbi et al. (2022) |
| Western Africa | |||||
| Côte d'Ivoire | Cobs | 125 (125) | HPLC | NS (38.61–234.87) | Bamba et al. (2020) |
| Côte d'Ivoire | Spathes | 125 (125) | HPLC | NS (94.54–341.84) | Bamba et al. (2020) |
| Nigeria | Sorghum | 20 (18) | LC–MS/MS | 18 (<LOQ‐20) | Olopade et al. (2021) |
| Nigeria | Millet | 20 (17) | LC–MS/MS | 64 (<LOQ‐396) | Olopade et al. (2021) |
| Nigeria | Granola | 18 (11) | LC–MS/MS | 1.73 (0.81–5.99) | Ezekiel et al. (2020) |
| Nigeria | Popcorn | 19 (1) | LC–MS/MS | 6.40 | Ezekiel et al. (2020) |
| Nigeria | Millet | 87 (14) | LC/MS | 419 (0–1399) | Chilaka et al. (2016) |
| Nigeria | Rice | 41 (33) | HPLC | 203.6 (0.7–570.6) | Egbuta et al. (2015) |
| Togo | Sorghum | 12 (2) | LC–MS/MS | 22 (19–24.6) | Hanvi et al. (2019) |
| Northern Africa | |||||
| Tunisia | Wheat | 155 (123) | HPLC | 110 (0–560) | Zaied et al. (2012) |
| Algeria | Wheat | 30 (19) | UHPLC–MS/MS | 102 (9.6–295) | Mahdjoubi et al. (2020) |
| Algeria | Rice | 30 (6) | UHPLC–MS/MS | 9.9 (8.6–15.5) | Mahdjoubi et al. (2020) |
| Egypt | Wheat | 15 (6) | HPLC | 1.55 (0.53–2.5) | El‐Desouky and Naguib (2013) |
| Egypt | Barley | 15 (4) | 1.5 (0.70–1.77) | El‐Desouky and Naguib (2013) | |
| Egypt | Animal feed | 77 (71) | LC–MS/MS | NS (NS‐791) | Abdallah et al. (2017) |
| Central Africa | |||||
| Cameroon | Edible nontimber products | 210 (194) | ELISA | 62.7 (<15–500) | Djeugap et al. (2019) |
| Cameroon | Peanut | 35 (15) | LC–MS/MS | 4 (<LOQ‐45) | Abia et al. (2013) |
| Cameroon | Soybean | 10 (10) | LC–MS/MS | 15 (12–18) | Abia et al. (2013) |
| Congo | Bean | 30 (27) | TLC | 185.2 (12.5–273.2) | Mulunda et al. (2013) |
Abbreviations: nd, not detected; NS, not stated.
There are few comprehensive databases on the prevalence rate of Fusarium species and ZEA occurrence in Africa. However, these data are limited to natural with no consolidated data on the impact of traditional agronomic practices and climate change on Fusarium and ZEA concentrations. To facilitate data consolidation on the prevalence of Fusarium species and ZEA occurrence in Africa, this article highlights detailed information on ZEA and its associated producing fungi, agronomic practices, and climate change impacting their occurrence, current exposure levels, and regulatory regime in Africa. Furthermore, it summarizes stress levels leading to the production of ZEA in food and feed in Africa.
2. TRENDS IN MAIZE CONSUMPTION AND NUTRIENT SUPPLY IN AFRICA
Although it is clear that maize is a multipurpose crop, maize use as direct human food is repeatedly high in Africa (54%) compared to its global 56% use in feed production and 13% use as a direct human (Erenstein et al., 2022). Maize grain is the first most consumed cereal in Africa with an intake ranging from 50 to >330 g/person/day (Palacios‐Rojas et al., 2020; Ranum & Pe, 2014). The crop and its derived products including infant formulas are widely consumed in Eastern and Southern Africa, contributing to more than 30% of total calorie intake (Nuss & Tanumihardjo, 2010).
The average dietary energy intake per capita in Africa is 399 kcal for maize as food compared to a total intake of 80 kcal for Asia, 301 kcal for America, 59 kcal for Europe, and 38 kcal for Oceania. Within Sub‐Saharan Africa, the energy supply per day from maize is high in eastern and southern Africa (556.0 kcal/capita/day) compared to 287 kcal/capita/day for West and Central Africa and 318.3 kcal/capita/day for northern Africa (Erenstein et al., 2022). Despite these significant contributions of maize to human nutrition in Africa, the grain is highly susceptible to Fusarium infection and ZEA contamination, and their effect on health is extremely unnoticed in developing countries, including Africa.
3. RECENT ADVANCEMENTS IN THE IDENTIFICATION OF ZEARALENONE‐PRODUCING SPECIES
Improved new and emerging technologies are important for optimizing food safety tests. Several rapid tests have been developed and employed to detect Fusarium species and ZEA in food products. Among these methods, quantitative PCR (MitoqPCR, optimized qPCR, multiplex qPCR), loop‐mediated isothermal amplification (LAMP), PCR‐ELISA, metabarcoding, and microarray are frequently used. However, the identification of Fusarium species in Africa mainly depends on morphological growth parameters of the fungi and conventional PCR methods that are time‐consuming, laborious, and require specific expertise and experience (Table 1). Compared to morphological assays and singleplex PCR, multiplex PCR‐based technique has allowed the simultaneous detection of foodborne fungi belonging to distinct genera in a single reaction. This process mainly uses highly conserved and variable sequence regions such as β‐tubulin, elongation factor 1 α, and intergenic spacer region (IGS) of the rDNA that have demonstrated a high throughput and precise technology for characterizing Fusarium species. Although internal transcribed spacer (ITS) has been widely used in Fusarium species identification, it is now commonly accepted that internal transcribed spacer (ITS) is not informative enough to verify species identification within the Fusarium genus (Donnell et al., 2022; Summerell, 2019). Furthermore, conventional PCR assay is disadvantaged by its inability to quantify fungal load, high workload, high cost, and high turnaround time.
TABLE 1.
Summary of recent Fusarium species occurrence in some African countries.
| Country/region | Food crop | AT; time | Target DNA | Primers and probes (5′‐3′) or mode of identification | Identified species | OR | References |
|---|---|---|---|---|---|---|---|
| Eastern Africa | |||||||
| Ethiopia | Maize | 53°C; 50 s | EF‐1α | EF1 ATGGGTAAGGAGGACAAGAC | F. verticillioides | 42 | Tsehaye et al. (2016) |
| EF2 GGAGGTACCAGTCATCATGTT | F. graminearum | 22.6 | |||||
| F. equiseti | 0.5 | ||||||
| Kenya | Wheat | 57°C; 60 s | EF‐1α | EF1 ATGGGTAAGGAAGGACAAGAC | F. verticillioides | 30.0 | Kheseli et al. (2021) |
| EF2 GGAGGTACCAGTCATCATGTT | F. equiseti | 25.3 | |||||
| Maize | 53°C; 30 s | TEF 1‐α |
EF1 GTGGGGCATTTACCCCGCC EF2 ACGAACCCTTACCCACCTC |
F. verticillioides | – | Alaro (2021) | |
| Tanzania | Maize | 62.5°C; 60 s | NS | F. culmorum | 3.8 | Degraeve et al. (2016) | |
| F. graminearum | 75 | ||||||
| F. culmorum | 5 | ||||||
| F. sporotrichioides | 15 | ||||||
| F. verticillioides | 95 | ||||||
| Uganda | Maize, rice, millet | 52°C; 30 s | EF‐1α |
rp32 ACAAGTGTCCTTGGGGTCCAGG rp33 GATGCTCTTGGAAGTGGCCTACG |
F. verticillioides | 20 | Wokorach et al. (2021) |
| Sorghum | TEF 1‐α |
EF1 GTGGGGCATTTACCCCGCC EF2 ACGAACCCTTACCCACCTC |
F. equiseti | 6.9 | |||
| F. proliferatum | 1.3 | ||||||
| Zambia | Maize | – | – | Morphological | F. verticillioides | 37.1 | Kankolongo et al. (2009) |
| Southern Africa | |||||||
| South Africa | Commercial maize | 58°C; 45 s | ITS |
ITS1 TCCGTAGGTGAACCTGCGG ITS4 TCCTCCGCTTATTGATATGC |
F. oxysporum | 20 | |
| F. verticillioides | 88 | Chilaka et al. (2012) | |||||
| F. proliferatum | 73 | ||||||
| F. oxysporum | 65 | ||||||
| F. graminearum | 48 | ||||||
| South Africa | Maize | 58°C; 45 s | ITS |
FF2 GGTTCTATTTTGTTGGTTTCTA FR1 CTCTCAATCTGTCAATCCTTATT |
F. verticillioides | 76 | Ekwomadu et al. (2018) |
| F. oxysporum | 60 | ||||||
| F. graminearum | 18 | ||||||
| F. equiseti | 18 | ||||||
| F. solani | 1 | ||||||
| Angola | Maize | – | – | Morphological | F. verticillioides | 83 | Panzo (2011) |
| F. graminearum | 68 | ||||||
| F. graminearum | 22.5 | ||||||
| F. pseudoanthophilium | 13.4 | ||||||
| Botswana | Maize meal | – | – | Morphological | F. verticillioides | 20.9 | Mokgatlhe et al. (2011) |
| F. proliferatum | 2.5 | ||||||
| Sorghum meal | Morphological | F. verticillioides | 20 | ||||
| F. proliferatum | 3.4 | ||||||
| Western Africa | |||||||
| Burkina Faso | Onion | 52°C; 30 s |
EF1 ATGGGTAAGGARGARGACAAGAC EF2 GGARGTACCAGTSATCATCATGTT |
F. oxysporum F. proliferatum |
44.44 41.66 |
Kintega et al. (2020) | |
| Nigeria | Oil palm | 65°C; 30 s | ITS |
ITS1 TCTGTAGGTGAACCTGCGG ITS4 TCCTCCGCTTATTGATATGC |
F. oxysporum F. equiseti F. verticillioides F. proliferatum |
41.37 20.68 5.17 3.44 |
Chidi et al. (2020) |
| Ivory Coast | Multiple samples | 55°C; 45 s | ITS |
ITS1 TCCGTAGGTGAACCTGCGG ITS4 TCCTCCGCTTATTGATATGC |
F. culmorum F. graminearum F. proliferatum |
7.4 18.5 25.9 |
Aasa et al. (2022) |
| Ghana | Groundnut | – | – | Morphological | F. verticillioides | 20.7 | Korley et al. (2022) |
| F. verticillioides | 31 | ||||||
| Nigeria | Maize | – | – | Morphological | F. sporotrichioides | 96 | Ezekiel et al. (2008) |
| F. verticillioides | 82 | ||||||
| F. graminearum | 50 | ||||||
| F. proliferatum | 40 | ||||||
| F. sporotrichioides | 76 | ||||||
| Northern Africa | |||||||
| Morocco | Wheat |
58°C; 1 min 55°C; 30 s |
TEF 1‐α |
Fu3f GGTATCGACAAGCGAACCAT Fu3f TAGTAGCGGGGAGTCTCGAA |
F. equiseti | 8.82 | Ezrari et al. (2020) |
|
FOF 1 ACATACCACTTGTTG CCTCG FOR1 CGCCAATCAATTTGAGGAACG |
F. oxysporum | 17.65 | |||||
| Egypt | Multiple products | 55°C; 1 min | ITS |
ITS1: TCTGTAGGTGAACCTGCGG ITS4: TCCTCCGCTTATTGATATGC |
F. equiseti F. verticillioides |
El‐Rabbat et al. (2018) | |
| Maize | 58°C; 1 min | TEF |
F1T‐F ATGGGTAAGGAGGACAAGAC EF1T‐R GGAAGTACCAGTGATCATGTT |
F. proliferatum F. culmorum F. oxysporum |
19.0 52.3 14.3 |
Khalil et al. (2013) | |
| Algeria | Wheat | 60°C; 30 s |
Fco1F ATGGTGAACTCGTCGTGGC Fco1R CCCTTCTTACGCCAATCTCG |
F. culmorum | 68 | Abdallah‐Nekache et al. (2019) | |
| 55°C; 30 s |
Fp1 1CGGGGTAGTTTCACATTTCYG Fp1 2 GAGAATGTGATGASGACAATA |
F. pseudograminearum | 10 | ||||
| 57°C; 30 s |
Fum 1–654 CGGTTGTTCATCATCTCTGA Fum 1–654 GCTCCCGATGTAGAGCTTGTT |
F. verticillioides | 3 | ||||
| Algeria | Wheat | 62°C; 5 s |
Fcu‐F GACTATCATTATGCTTGCGAGAG Fcu‐R CTCTCATATAC |
F. culmorum | 40 | Hadjout et al. (2022) | |
| Tunisia/Egypt | Sorghum |
60°C; 53°C |
β‐tubulin, and TEF‐1a |
BT2A GGTAACCAAATCGGTGCTGCTTTC BT2B ACCCTCAGTGTAGTGACCCTTGGC ET1: ATGGGTAAGGARGACAAGAC EF2: GGARGTACCAGTSATCATGTT |
F. equiseta complex F. verticillioides F. proliferatum F. graminearum |
62.7 6.7 3.4 1.7 |
Lahouar et al. (2015) |
| Central Africa | |||||||
| Cameroon | Maize, peanut, poultry feed | 55°C; 20 s | IGS region |
IGSF AAGGAATTCAGGAATTCTCAATTG IGSR GTCCACCGGCAAATCGCCGTGCG |
F. verticillioides | 90 | Kana et al. (2013) |
Abbreviation: OR, occurrence rate.
3.1. Quantitative PCR (qPCR)
Quantitative PCR (qPCR) is a variant of PCR technology that allows the real‐time detection of amplicons as they accumulate. It is important to acknowledge that the widespread adoption of qPCR is not without its challenges. One of the well‐known issues with quantitative PCR is its inability to discriminate between viable and nonviable Fusarium cells. This mostly results in the overestimation of viable Fusarium cells and the overuse of chemicals. To address this limitation, Chen et al. (2022) developed an improved method for quantifying viable Fusarium cells using propidium monoazide (50 mmol/L optimum) coupled with real‐time PCR. This method was able to reliably differentiate between viable Fusarium spores from nonviable spores. Furthermore, the qPCR setup is that each target gene needs its own dye and associated probe with a unique wavelength. Furthermore, qPCR results depend on a calibration curve, involving several calculations, and may not represent the number of Fusarium copies in the sample (Cao et al., 2020). Complex biological molecules such as humic acid significantly inhibit qPCR reactions.
3.2. Digital PCR (dPCR)
Digital PCR (dPCR) is a powerful tool for quantifying the absolute copy number of target DNA or RNA. It plays a crucial role in measuring genetic imbalances that result from an uneven distribution of targets, particularly in mycotoxin analysis. Among the various types of dPCR partitioning methods that have been explored for food safety management, microfluidic chip, and droplets, digital PCR have been examined in fungal biology and mycotoxin detection. With the droplet dPCR system, each DNA sample is mixed with oil, forming a water–oil emulsion. The water–oil emulsion is then divided into many thousands of single nanoliter droplets. During PCR, targets are amplified within each droplet. Recently, a ddPCR assay was developed by Wang et al. (2021) for the simultaneous quantification of 3‐acetyl deoxynivalenol (3ADON) and 15‐acetyl deoxynivalenol (15ADON) chemotypes of DON‐producing Fusarium species. Furthermore, chip digital PCR (cdPCR) was found to accurately track mycotoxigenic Fusarium DNA in wheat and oat plants during the initial phase of infection when symptoms are not visible (Morcia et al., 2020). The technology has not been used in zearalenone detection and quantification. Droplet digital polymerase chain reaction (ddPCR) has demonstrated its power in detecting gene targets differing in one nucleotide. Through not Fusarium, ddPCR was found to accurately distinguish the competitiveness between nonaflatoxigenic and aflatoxigenic Aspergillus flavus strains on maize kernels (Schamann et al., 2022).
3.3. Polymerase chain reaction–enzyme‐linked immunosorbent assay (PCR‐ELISA)
Polymerase chain reaction–enzyme‐linked immunosorbent assay (PCR‐ELISA) is a nucleic acid amplification assay that combines immunodetection method (ELISA) and PCR to quantify specific PCR product directly after immobilization of DNA on a microtiter plate. Compared to qPCR, PCR‐ELISA is sensitive and specific, uses less time, simultaneously detects a large number of samples, and involves fewer carcinogenic chemicals (Tayebeh et al., 2017). Grimm and Geisen (1998) proposed and developed a PCR‐ELISA for the detection of potential Fusarium species using the ribosomal ITS1 sequence as a target. Subsequently, Omori et al. (2018) explored the possibility of using PCR‐ELISA to detect Fusarium verticillioides in corn. The authors targeted the FUM21 gene for Fusarium verticillioides and confirmed that the technique was specific to Fusarium verticillioides isolates, and exhibited 100‐fold more sensitivity than the conventional PCR assay. However, higher DNA concentration (>250 pg) decreases the sensitivity of the detection method (Omori et al., 2018).
3.4. Loop‐mediated isothermal amplification
Loop‐mediated isothermal amplification is a novel amplification for faster detection of nucleic acids. Compared to PCR assays that require three different temperatures (initial temperature, annealing temperature, and the final extension temperature), LAMP technology utilizes a single temperature (Isothermal) and does not require a thermocycler. More recently, few studies have accessed the potential of using LAMP assay to determine Fusarium species and mycotoxin in maize. Wigmann et al. (2020) developed a direct LAMP‐based assay for detecting Fusarium species and mycotoxins in ungrounded maize grains. In their methodology, 1 g of maize grains was soaked with 2.5 mL of sterile tap water containing 1% (v/v) Tween 20. After handshaking, 2 mL of the supernatants was transferred into a reaction vessel and centrifuged at 11,000 g for 2 min. Subsequently, the Tween 20 was removed by washing with sterile deionized water and the pellet was resuspended in 500 μL of sterile deionized water. Five microliters of the suspension was used as a template in LAMP reactions. This method was able to detect Fusarium verticillioides with the potential to produce fumonisin even at lower concentrations.
3.5. Full genome sequencing
Although the full genome of several ZEA‐producing Fusarium isolates from different parts of the world has been sequenced and used in the bioinformatic analysis, there are no similar studies of isolates originating from Africa. The first Fusarium genome to be sequenced completely in Africa was the South Africa's Fusarium circinatum type strain FSP34 (Wingfield et al., 2012). This strain was sequenced on a Roche 454 GS FLX system (Life Sciences, Connecticut, USA) using titanium chemistry and it was found that the strain contains about 44 million bases in size, 15,000 open reading frames with putative gen clusters harboring evidence of the secondary metabolites of fumonisin and fusarin. About 70% of the Fusarium circinatum open read frames were most similar to those of Fusarium verticillioides (Wingfield et al., 2012).
Compared to the current partially sequenced genomes widely used to study Fusarium isolate in Africa, complete genomes provide detailed bioinformatic information including the size, heterozygosity, repetitive sequence content, and interspecific and intraspecific comparison, and facilitate all aspects of biological research. Complete genome sequencing has also shed light on the evolution of the pathogenicity history of various Fusarium fungi and allows the selection of several primer pairs used in PCR reactions for molecular characterization of the specific mycotoxigenic chemotypes. To this end, different primer sets have been designed for rapid and specific detection of ZEA‐producing Fusarium species in contaminated foodstuff such as maize flour. Based on the gene sequence of polyketide synthase PKS4, Meng et al. (2010) developed a low‐cost technique using a specific primer set that was rapid, sensitive, specific, and reliable in the identification and quantification of ZEA‐producing Fusarium species directly in maize flour. Similarly, Atoui et al. (2011) developed a real‐time PCR assay from a polyketide synthase gene PKS13 sequences involved in ZEA biosynthesis and successfully detected and quantified Fusarium graminearum and was used to analyze the occurrence of 32 ZEA‐producing F. graminearum chemotypes on maize.
4. GENES EXPRESSION IN ZEA PRODUCTION
Molecular techniques such as differential display RT‐PCR (DDRT‐PCR) and expressed sequence tags approaches have been used to understand the molecular mechanisms connected with ZEA production in maize genotypes. Two polyketide synthase genes PKS4 and PKS13, transcription factor (ZEB2), and genes similar to isoamyl alcohol oxidase (ZEB1) as well as other regulatory proteins have been identified and reported as genes expressed in ZEA production (Lysøe et al., 2006). Lysøe et al. (2008) used differential display RT‐PCR to identify 54 expressed sequence tags that were upregulated in rice samples with higher ZEA production. Furthermore, the MIPS Functional Catalogue (FunCat) of classifying expressed genes classified these upregulated genes into five major functional categories: 38% unclassified proteins, 15% metabolism, 10% proteins with binding function, 6.5% biogenesis of cellular components, and 5.5% cell rescue, defense, and virulence (Lysøe et al., 2008). Using microarray analysis on F. graminearum strains.
Lee et al. (2011) found significantly elevated ZRA1 gene levels, a putative ATP‐binding cassette transporter gene, in test samples containing 15 μM ZEA compared with those without ZEA supplements. It has been suggested that these genes are expressed between 4 and 14 days after infection with the necessary growth conditions (Lysøe et al., 2006, 2008). This particular finding is significant that plant debris can transfer ZEA and its conjugate metabolites into the soil and subsequently translocate ZEA to other plant parts including leaves, stems, grains, and fruits (Gerling et al., 2022; Jaster et al., 2023; Rolli et al., 2018).
5. ZEARALENONE AND ITS PRODUCING FUNGI IN SUB‐SAHARAN AFRICA
Zearalenone, a metabolite produced by various species of Fusarium fungal, has been observed as a natural contaminant of cereals, in particular maize, in many countries in Africa, Europe, and the USA. Globally, high levels of ZEA production are associated with Fusarium graminearum and Fusarium culmorum. However, F. oxysporum, F. sporotrichioides, F. proliferatum, and F. verticillioides have also been linked to ZEA production (Beev et al., 2013). Although these fungi have been widely reported to colonize a variety of food crops including maize, sorghum, wheat, rice, millet, and other legumes products, recent evidence suggests that ZEA can also contaminate water, meat, fish, and dairy commodities (Alaboudi et al., 2022; Falkauskas et al., 2022; Gonkowski et al., 2018; Jafari‐Nodoushan, 2022).
The chemical structure of ZEA and its five known conjugate metabolites are shown in Figure 1. The toxin is soluble in solvents such as acetonitrile, acetone, methyl chloride, and alcohol. In terms of stability, ZEA is stable to conventional food processing temperatures (EFSA, 2011). Globally, ZEA and its metabolites are well known for their estrogenic activities, resulting in reproductive system dysfunction. Furthermore, ZEA exhibits cytotoxicity by modifying biological macromolecules such as DNA, proteins, and nucleic acid (Eze et al., 2018; Jafari‐Nodoushan, 2022; Rai et al., 2020).
FIGURE 1.
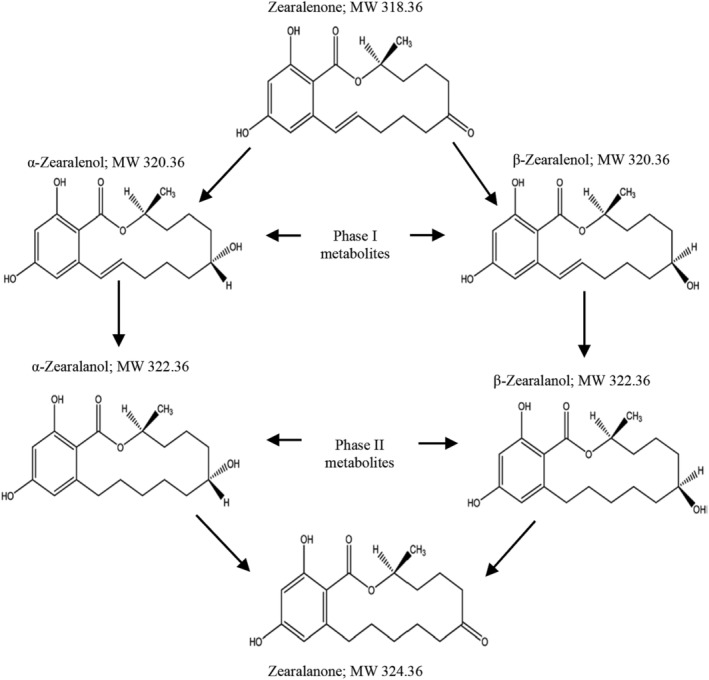
Chemical structure of zearalenone and its associated metabolites.
Over the past decade, morphological and molecular analysis performed on various food crops showed the presence of several fungi genera. Among these fungi genera, Fusarium species are the most dominant fungi frequently isolated from grains in Africa (Akello et al., 2021; Ekwomadu et al., 2018; John et al., 2018). The most common isolated Fusarium species from food crops in Africa are F. graminearum, F. verticillioides, F. culmorum, F. sporotrichioides, and F. equiseti (Table 2). Their occurrence rate ranged from 3% to 100%, showing the ubiquitous presence of potential mycotoxigenic Fusarium species in Africa. These species have been isolated from several grains, including maize, wheat, and sorghum. Table 1 provides a summary of published accounts on the Fusarium species prevalence rate in Africa as well as their detection methods.
6. LEGISLATION ON ZEARALENONE IN AFRICA
As part of managing the exposure risk of animals and humans to different toxic compounds, some countries, including the European Union (European Commission, 2006a, 2006b), Brazil (Corrêa & Ferreira, 2023), and Asia/Oceania (FAO, 2004), have established regulatory limits for mycotoxin concentrations in food and feedstuff. Currently, the UDA has not established regulatory levels for ZEA contamination in grain or food products. The WHO/FAO set 200 g/kg bw/day for pigs and gilts, 17.6 g/kg bw/day for piglets, 56 g/kg bw/day for sheep, and 20 g/kg bw/day for dogs as the lowest observed adverse effect level (LOAEL) for ZEA.
For many African countries, most of these regulations are limited to aflatoxins and fumonisin (Chilaka et al., 2022). Data on ZEA occurrence and evidence of high dietary exposure in individual countries are limited for use in the establishment of maximum permitted levels in food and feed raw material. As a result, many of these countries have adopted the European Commission and Codex Alimentarius Commission standards for ZEA (Ankwasa et al., 2021; Imade et al., 2021; Lahouar et al., 2018). However, only South Africa and Morocco have set regulatory limits for ZEA concentration in food and feed products. In South Africa, the maximum limits for ZEA in feeding stuff for sow/pigs, piglets, and calves/dairy cattle have been pegged at 5000, 3000 μg/kg, and 500 μg/kg, respectively (Government Notice, 2010). In Morocco, Zinedine and Mañes (2009) proposed ZEA limits of 200 μg/kg for all cereals intended for human consumption.
7. CONTRIBUTION OF PREHARVEST AND POSTHARVEST FACTORS TO FUSARIUM INFESTATION AND ZEARALENONE ACCUMULATION IN FOOD
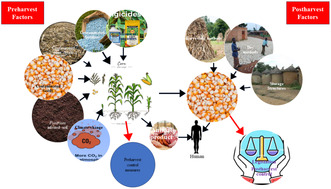
Preharvest practices high in repeated misuse of chemical fertilizer and farmer‐saved seeds, poor harvesting conditions, poor drying conditions, and poor storage structures result in fungi growth and mycotoxin production (Lehmane et al., 2022; Tang et al., 2019). Hence, ZEA concentrations in food are underpinned by three powerful factors—Preharvest factors identified as fertilization levels, fungicides use, and harvest practices; postharvest factors such as drying techniques, storage conditions; and food processing factors including sorting and fermentation. Figure 2 provides a summary of maize exposure routes to Fusarium infestation and ZEA contamination.
FIGURE 2.
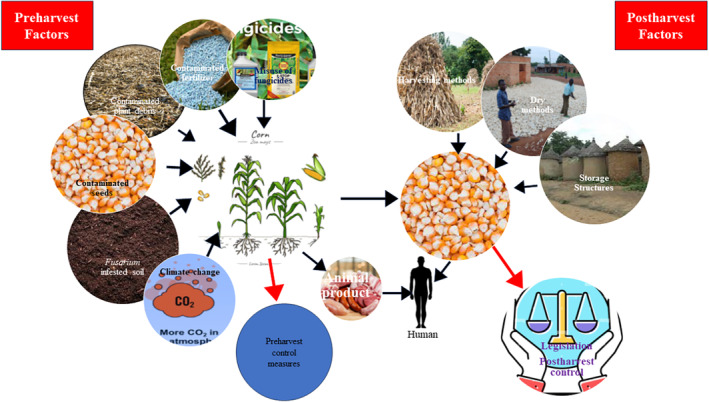
Maize grain and human exposure routes to ZEA contamination in food.
7.1. Nitrogen fertilization
It is well established that nitrogen fertilizer increases maize susceptibility to Fusarium attack and ZEA accumulation through increased oxidative stresses (Borràs‐Vallverdú et al., 2022). The type of stress significantly impacts the kind of mycotoxins observed in maize grains. It has become something of a truism that high nitrogen doses (>200 kg N/ha) increased ZEA accumulation in maize grains (Blandino et al., 2008; Podolska et al., 2017; Scarpino et al., 2022). However, the type of nitrogen fertilizer did not have a significant impact on ZEA accumulation in maize grains (Blandino et al., 2008).
7.2. Use of fungicides
The use of fungicides has been extensively reported in Africa; however, often of over or under (sublethal) applications, a phenomenon that may trigger the production of various mycotoxins. This is so because fungicides have the potential to induce the production of hydrogen peroxide (H2O2), which is a key precursor that influences the biosynthesis pathway of secondary metabolites (Audenaert et al., 2010; Ferrochio et al., 2013). The impact of fungicides on the biosynthesis of mycotoxins using Fusarium species has been reported. Cendoya et al. (2021) studied the impact of commercial fungicides: epoxiconazole + metconazole, tebuconazole, pyra‐clostrobin + epoxiconazole, and prothioconazole on fumonisin accumulation and observed that all the studied fungicides used in sublethal doses enhanced fumonisin production except prothioconazole. The impact of oxidative stress on Fusarium mycotoxins is summarized in Table 2. However, the influence of oxidative stress on ZEA accumulation and ZEA gene expression has not been reported globally.
7.3. Harvesting operations
In Ethiopia and most countries in Africa, farmers use dry and bent‐down kernels that are completely dry and show a black layer at the base to determine harvest time (Mohammed, Bekeko, et al., 2022; Mohammed, Seid, et al., 2022). Other indigenous methods used by farmers to estimate maize moisture content for harvesting and storage are puncturing kernels with their thumbnails, biting kernels with their teeth, or using sound made by kernels when agitated by hand (Joseph et al., 2015; Kagot et al., 2022; Liu et al., 2016). Such harvesting operations may influence Fusarium infestation and ZEA production. A study conducted to evaluate the impact of grain maturation, harvesting time, and late‐season rainfall showed that grains harvested late and exposed to preharvest rainfall had higher ZEA than those harvested early and not subjected to rainfall (Moraes et al., 2022). Recent data indicate that for every 1‐day delay in harvesting, ZEA production is projected to increase by 0.8‐fold in grains (Edwards & Jennings, 2018). It was previously reported that hay dried on the field had more ZEA than those dried under the shed (Taffarel et al., 2013).
7.4. Storage conditions
In Africa, storage methods and structures differ by ethnic groups and agroecological area. Most storage structures in Africa are characterized by high relative humidity and temperatures. These structures and environmental conditions result in high relative humidity (>60%), temperature buildup, and insect propagation which may lead to ZEA accumulation (Tang et al., 2019). A study conducted in three climatic locations in Africa on the influence of storage practices on ZEA contamination in rice showed that storage location (N'diaye in Senegal, Cotonou in Benin, and Yaoundé in Cameroon), processing type (plastic woven bags and pallets), and duration of storage (0, 90, and 180 days) affect ZEA concentration. The authors observed that storage structures with high relative humidity and low temperatures recorded significantly increased levels of ZEA (80% RH; 24.4°C; 400.3 ppm ZEA) for grains stored for 6 months compared to storage structures with low relative humidity (62.8% RH; 27.8°C; 100.2 ppm ZEA).
Similarly, temperature variations affect ZEA levels in grains under storage. High levels of ZEA (51.8–468.6 ppm) were observed in grain spikes exposed to 100% relative humidity at different temperatures. At relative humidity ≤90%, ZEA concentrations were very low (0.1–3.6 ppm) at all tested temperatures. At 100% relative humidity, mean ZEA contamination with significantly higher at 20 and 25°C (235.1 and 278.2 ppm) than at 30°C (104.7 ppm) (Moraes et al., 2022). Additionally, improper cleaning of grains before storage may lead to Fusarium and ZEA contamination of grains. High proportions of plant debris such as cobs and husks may increase ZEA levels in the stored grains due to contaminated plant debris in grains (Bamba et al., 2020; Tang et al., 2019).
7.5. Transboundary trade and zearalenone occurrence
Although reports from Africa are not consistent with levels of ZEA detected in imported and locally produced products, transboundary trade has emerged as one major factor contributing to the spread and distribution of mycotoxigenic fungi. A survey carried out in Nigeria has reported higher concentration levels of deoxynivalenol, and ZEA in imported wheat grains (mean 858.7 μg/kg for deoxynivalenol; 50.1 μg/kg for ZEA), whereas relatively lower levels (mean 517.8 μg/kg for deoxynivalenol; 14.5 μg/kg for ZEA) were detected in samples obtained from local farmers' stores or markets (Egbontan et al., 2017). In a similar study, Manizan et al. (2018) evaluated the occurrence of ZEA in local and imported grains in Cote D'Ivoire and did not detect ZEA in the 41 imported rice samples; however, 44.4% of the local samples being contaminated with ZEA at varying levels.
8. IMPACT OF CLIMATE CHANGE ON ZEARALENONE AND FUSARIUM OCCURRENCE
Climate change has already carried along an increased risk of natural toxins through disruption of plant metabolic and cellular processes. Among these climate variables, elevated atmospheric CO2 concentration, temperature, and precipitation are predicted to affect mycotoxigenic fungi redistribution and their derived mycotoxins (Medina et al., 2017). Recent reports suggest that elevated CO2 had a significant correlation (R 2 = .938; p < .001) with ZEA accumulation (Cui et al., 2022). Atmospheric CO2 concentration has also been shown to influence disease severity caused by Fusarium species. Likewise, higher emission of CO2 increases maize susceptibility to F. verticillioides attack (Vaughan et al., 2014), F. culmorum (Bencze et al., 2017), and F. graminearum (Hay et al., 2021).
Recent projected global climate status showed that Africa will experience extreme precipitation and temperature events like heat waves and drought. Extreme environmental temperatures combined with high precipitation amounts or prolonged droughts increase the level of stress suffered by plants, making all cereals, especially maize, even more, prone to fungi infection and mycotoxin contamination (Kos et al., 2023). For Fusarium mycotoxins, high ZEA concentrations are observed particularly at the flowering and harvesting stage, with extreme levels of precipitations (Han et al., 2022; Janić Hajnal et al., 2023). It is worth mentioning that Fusarium strains isolated from Africa are more resilient to extreme temperatures and lower water potential (Jedidi et al., 2021).
9. INCIDENCES AND LEVELS OF ZEARALENONE CONTAMINATION IN AFRICA
9.1. Zearalenone in cereal and cereal products
Zearalenone is a stable mycotoxin and is not degraded under storage (Krska et al., 2003). Several global surveys found a higher incidence rate of ZEA in Africa, with varying incidence rates and levels among grains. A recent global survey showed a high prevalence rate of ZEA in food and feed samples; for example, a global survey conducted from 2008 to 2017 showed a higher prevalence rate of ZEA prevalence in Sub‐Saharan Africa (52.2%) and South Africa (41.6%) than in Europe, America, and South Asia (Gruber‐Dorninger et al., 2019). Similarly, Lee and Ryu (2017) showed higher incidences of ZEA in unprocessed cereals from Africa (59%) than in Europe and America (48%).
In eastern Africa, Mohammed et al. (2023) profiled multiple mycotoxins in 80 postharvest maize grains from three major producing areas in Ethiopia: of the mycotoxins analyzed, ZEA was detected in 81%, with levels as high as 3750 μg/kg. Previously in Ethiopia, ZEA concentrations were investigated in a total of 100 maize samples collected from smallholder farmers' stores (Getachew et al., 2018). ZEA was detected in 96% of the samples, with mean and maximum concentrations of 92 and 1656 μg/kg, respectively. Of the 96% positive samples, 13.5% contained levels above the European Union (EU) recommended value for unprocessed cereals (100 μg/kg). In another study in south and south‐western Ethiopia, Mesfin et al. (2021) randomly sampled 176 stored maize from various households and analyzed them for Fusarium mycotoxins: ZEA concentration was up to 2447 μg/kg. In Tanzania, Kamala et al. (2015) investigated multiple mycotoxin levels in 300 maize samples collected from various rural households. For the ZEA‐positive samples (10%), 66% contained levels exceeding the EU maximum permitted limits. In a later study by Suleiman et al. (2017), 30 samples of maize purchased from farmers and traders in Tanzania were analyzed for ZEA. All 30 samples tested positive for ZEA with levels ranging from 50 to 189.9 μg/kg. More recently in Kenya, maize samples collected between 2018 and 2020 for multiple mycotoxin profiling revealed that 18% of the 480 maize samples collected from Kenyan households had ZEA levels above 1000 μg/kg (Kagot et al., 2022).
Several studies in southern Africa have also reported maize contamination with ZEA. In a survey conducted between 2001 and 2021 in Eswatini, 892 maize and maize‐based products were sampled and screened for ZEA contamination. In these samples, 7% of maize grain and 17% of maize meal were contaminated with ZEA (Dlamini et al., 2022). A total of 100 maize samples were randomly selected from small‐scale and commercial farmers in South Africa and investigated for ZEA contamination using HPLC. The results showed that more than half of the small‐scale and commercial maize samples were contaminated with ZEA at mean levels below the South African regulatory limits (Ekwomadu et al., 2021). In Zimbabwe, a study conducted to assess the impact of storage duration (0, 90, and 180 days) on ZEA contamination in maize showed an increase in ZEA concentration by 89.6% and 173.6% in 90 and 180 days storage periods, respectively (Hove et al., 2016).
Within the western Africa subregion, higher concentration levels of ZEA have been observed in recent studies. For instance, in Côte d'Ivoire, 125 samples of maize were analyzed for ZEA, and all the samples were contaminated with ZEA with 40% of the samples exceeding the EU regulatory limits (Bamba et al., 2020). In 2019 and 2021, Oyeka et al. (2019) and Olopade et al. (2021), respectively, investigated ZEA concentrations in maize from different agroecological zones in Nigeria. While none of the samples analyzed by Olopade et al. (2021) had levels exceeding the maximum permitted levels, 16.7% of the 36 maize samples analyzed by Oyeka et al. (2019) had ZEA concentrations above the EU maximum level for maize intended for direct human consumption. Additionally, all 20 samples were positive for ZEA phase I metabolites; alpha and beta zearalenol (Olopade et al., 2021). In another study in Togo, ZEA was detected in only one of 52 maize samples in lower concentrations (Hanvi et al., 2019).
Generally, the incidence rate of ZEA is fairly low for maize samples from northern Africa. Mahdjoubi et al. (2020) evaluated Algerian maize kernels from different local markets and reported that only one of seven ZEA‐positive samples exceeded the EU maximum permitted levels. In Egypt, Sebaei et al. (2020) evaluated multiple cereals including 55 maize samples for ZEA contamination and observed that two samples had ZEA at levels 10 and 108 μg/kg. Previous research by Abdallah et al. (2017) also revealed that 10 out of 79 maize samples collected from farms and market centers in Egypt were contaminated with ZEA.
Meanwhile, data on ZEA occurrence in maize grains from Central Africa are quite real. A survey conducted on multiple products including 37 maize samples in Cameroon revealed that about 89% of the maize samples were contaminated with ZEA in levels ranging from 0.2 to 309 μg/kg (Abia et al., 2013). In the Democratic Republic of Congo (Mulunda et al., 2013), 40 maize samples collected from main markets were analyzed for various Fusarium mycotoxins. For ZEA, 92.5% of the samples were positive with concentrations ranging from 24 to 811.2 μg/kg.
9.2. Zearalenone in processed food and beverages
A survey evaluated ZEA levels in 50 traditionally maize fufu collected from households in Bamunka in Cameroon. Forty percent and 90% of the samples contained detectable levels of alpha and beta zearalenol. All 50 traditional maize fufu samples were ZEA positive with levels ranging from 5 to 150 μg/kg (Abia et al., 2017). In another survey, 101 maize porridge samples from households across three rural villages in Tanzania were evaluated for mycotoxins. ZEA was detected in 31% of the samples with levels ranging from 10.20 to 269.9 μg/kg (Geary et al., 2016). In another study in South Africa, Shephard et al. (2013) analyzed maize‐based evening meals and porridge donated by 54 females in Transkei, a region with high esophageal cancer for multiple mycotoxin occurrence. For ZEA, the author observed that all the samples were contaminated with ZEA with values ranging from 0.2 to 239 μg/kg for maize‐based food and 0.44 to 239 μg/kg for porridge samples. Fast forward to Limpopo (South Africa), analyses of 20 maize porridge showed no contamination of ZEA and beta zearalenol. However, alpha zearalenol was detected in 19 of the 20 samples at levels between 10.25 and 61.5 μg/kg (Tebele et al., 2020). A study on the occurrence of ZEA in cooked maize porridge and dietary exposure of Tanzanian households to ZEA was performed in 2016. It was reported that 23% of the processed maize‐based porridge had ZEA concentration above the limit for infant food (ZEA > 20 μg/kg) (Geary et al., 2016).
In Cameroon, a selection of 14 traditional maize beers and eight dagwa—a dry fried snack made from milled maize and groundnuts—samples produced from maize were screened for the presence of ZEA and other mycotoxins. Of these samples, 86% of the traditional beer and 100% of the dagwa samples were confirmed to contain ZEA in levels ranging from 1.6 to 35 μg/kg and 6 to 57 μg/kg, respectively (Abia et al., 2013). The authors further observed that mycotoxin‐related knowledge was low among fermented food sellers. Contrary to this observation, Adekoya, Njobeh, et al. (2017) and Adekoya, Obadina, et al. (2017) evaluated 32 maize‐based beers from South Africa for the cooccurrence of mycotoxins and reported that none of the samples contained detectable levels of ZEA despite the detection of fumonisin in 53% of the samples. Tables 3 and 4 provide a summary of published accounts on ZEA levels in other food products and beverage samples in Africa.
TABLE 4.
Summary of zearalenone occurrence in beverages.
| Country | Product | No. of samples | Detection method | Detection range (μg/L) | References |
|---|---|---|---|---|---|
| Nigeria | Fermented melon | 25 (8) | HPLC | 33 (21–45) | Adekoya, Njobeh, et al. (2017) and Adekoya, Obadina, et al. (2017) |
| Fermented locust bean | 8 (5) | HPLC | 18 (11–33) | Adekoya, Njobeh, et al. (2017) and Adekoya, Obadina, et al. (2017) | |
| Fermented African oil bean | 13 (4) | HPLC | 72 (39–117) | Adekoya, Njobeh, et al. (2017) and Adekoya, Obadina, et al. (2017) | |
| Nigeria | Kumu | – | LC–MS/MS | 0.2 | Ezekiel et al. (2015) |
| Pito | – | LC–MS/MS | 0.2 | ||
| Cameroon | Maize beer | 14 (12) | LC–MS/MS | 17 (1.6–35) | Abia et al. (2013) |
| Dagwa | 8 (8) | LC–MS/MS | 32 (6–57) |
9.3. Occurrence of ZEA in animals and animal products
Apart from grains, a few studies also investigated the occurrence of ZEA in animals and animal products in Africa, where it was reported not to be as frequent as in grains. For instance, in South Africa, the mycotoxin survey in red meat from rural subsistence farmers and registered abattoirs by van Deventer et al. (2021) reported no ZEA contamination in all tested samples (LOD; 20 μg/kg). Meanwhile, in Zambia (another Southern African country), Gonkowski et al. (2018) investigated the presence of ZEA and its analog products in 27 sun‐dried Kapenta fish collected from three cities and found ZEA and α‐ZEA in all samples with concentration levels ranging from 27.2 to 53.9 μg/kg and <3.0 to 71.1 μg/kg, respectively, 66.7% tested positive for β‐zearalenone with levels ranging from <12 to 59.8 μg/kg (Gonkowski et al., 2018).
In the West African subregion, 33% of 108 dried beef samples in Nigeria tested positive for α‐zearalenone with concentration levels ranging from 47.6 to 167.34 μg/kg (Dada et al., 2020). ZEA and β‐zearalenone were, however, absent.
9.4. Zearalenone in water sample
Recently, various studies have quantified ZEA in agricultural and domestic water sources. This may present a potential contamination route for plants through radical uptake and humans through drinking and cooking with ZEA‐contaminated water (Agnieszka et al., 2012; Rolli et al., 2018). An analysis of water samples from Zambia showed the presence of ZEA in three out of four water samples from lakes, with values ranging from <2.0 to 18.0 ng/L (Gonkowski et al., 2018).
10. REPORTED EXPOSURE ASSESSMENT AND RISK CHARACTERIZATION OF ZEARALENONE IN AFRICA
Generally, there are two pathways for measuring human dietary exposure to ZEA. The First and most widely used method is the direct exposure pathway, where dietary exposure evaluation of ZEA combines household consumption data and the concentration of ZEA in food products and expressed as nanogram/kilogram body weight/day. The Food and Agriculture Organization of the United Nations (FAO) and the Joint Expert Committee on Food Additives (JECFA) under the World Health Organization (WHO), as well as the European Food Safety Authority (EFSA), have developed various guidelines for dietary data collection including living standards monitoring survey, household income and expenditure surveys, and National household budget survey.
Using this method, high exposure risks have been reported by some countries in Africa. Consumers in the savannah zone of Nigeria were reported to have a high risk of exposure to ZEA with % tolerable daily intake (TDI) of 395.6, 158.3, and 66 which were 1582, 633, and 264 times higher than the tolerable daily intake of 0.25 μg/kg/bw·day (Adetunji et al., 2017). A study on the occurrence of ZEA in food and dietary exposure of the population to ZEA was assessed in Algeria by Mahdjoubi et al. (2020). Mean ZEA exposure in the adult population through maize was lower (0.08 kg body weight per day; TDI 31.97%) compared to wheat (0.85 kg b.w. per day; TDI 341.4 5).
The second pathway to assessing ZEA exposure is the indirect method. This approach uses biomarkers such as urine, breast milk, and serum. Across the African continent, few studies on the lactational or maternal transfer of ZEA to infant and birth outcomes are available. For Western Africa, Braun et al. (2022) evaluated the association between the transfer of ZEA from food to breastmilk and infants in Nigeria. ZEA was detected in lactating mothers' meals (0.1–33 μg/kg) and urine (11–1142 ng/L) but was not detected in breast milk. However, urine samples from exclusively breastfed infants contained ZEA ranging from 8.6 to 983 ng/L (Braun et al., 2022). Similarly, in Nigeria, Ezekiel et al. (2022) demonstrated that urine samples of exclusively breastfed infants (N = 23) contain ZEA ranging from 17 to 784 ng/L, while ZEA could not be detected in breast milk consumed by exclusively breastfed infants (N = 22).
Recently in Eastern Africa, Mesfin et al. (2023) evaluated mycotoxins in the breast milk of 138 lactating mothers in Ethiopia and reported that ZEA (LOD = 0.66, LOQ = 4.38) could not be detected. However, another study conducted in Ethiopia that determined the association between ZEA in pregnant women and birth outcomes showed that 50.9% (295) out of 579 serum samples of a pregnant woman contained ZEA with a concentration of up to 9 ng/mL (Tesfamariam et al., 2022). Also, 33.9% (196), 42% (243), 65.3% (378), and 64.6% (374) were positive for zearalenone, alpha zearalenol, beta zearalenol, beta zearalanol at concentrations up to 15.6, 10.5, 13.2, and 12.9 ng/mL, respectively; however, the study found no significant association between ZEA, and its derivative compounds exposures, and birth outcomes (Tesfamariam et al., 2022).
In Southern and Central Africa, Shephard et al. (2013) evaluated ZEA exposure levels in 54 adult females from South Africa using urine samples: ZEA was detected in all the urine samples with a mean level of 0.529 ng/mg creatinine, while 92% and 72% of the samples contained alpha and beta zearalenol with mean concentrations of 0.614 ng/mg creatinine and 0.702 ng/mg creatinine, respectively. In Yaoundé, Cameroon, Abia et al. (2020) collected and analyzed 89 Cameroonian adults' urine for various Fusarium mycotoxins; the authors observed that ZEA and its phase I metabolites were the most frequently detected mycotoxins (82% of the sample analyzed contained detectable quantities of ZEA, alpha zearalenol, and beta zearalenol) with two samples exceeding the EU TDI.
Being the major staples of many African communities, cereal and legumes, particularly maize and sorghum, are frequently used as complementary foods for infants and young children. However, the dietary exposure assessment for ZEA reported from different countries in Africa is based on average intake and body weight for adults. This assessment may not represent the true exposure levels for infants and young children.
11. INNOVATIVE STRATEGIES TO REDUCE RISK LEVELS OF ZEARALENONE IN AFRICA
11.1. On‐field preventive methods
There is an urgent need to transform African indigenous food systems to respond to the present increased occurrence of mycotoxins in food grains. It has been established that the first step in mycotoxin management is to prevent fungi infestation, that is, production systems should incorporate elements and activities that will prevent fungi infestation. Providing agricultural education and technologies aimed at changing farmers' pre‐ and postharvest practices, by using demonstrations (e.g., proper fertilization and fungicide use, with an explanation of their impact on agriculture) may help reduce ZEA occurrence and improve mycotoxin levels in the African food supply chain (Njeru et al., 2019; Visser et al., 2020). That is, scale‐up education on the use of resistant cultivars, appropriate soil amendment methods, proper weed management, and harvesting techniques should be facilitated. Unfortunately, commercially available maize cultivars in Africa have not been tested for specific resistance to Fusarium species (Tembo et al., 2022). Previous studies in South Africa, and Nigeria reported potential resistance of some maize‐inbred lines to F. verticillioides and fumonisin accumulation (Olowe et al., 2015; Small et al., 2012). No studies have so far evaluated maize cultivars from Africa for ZEA resistance.
11.1.1. Impact of soil amendments on Fusarium species and zearalenone concentration
Being a typical soil‐borne pathogen, Fusarium species survive on or within infected soil and crop residues, which remains the leading cause of grain contamination before harvest. Consequently, using soil amendments to change the microenvironment of soil should be preferred to direct unamended soil. Biochar though more expensive than common fertilizers (Latawiec et al., 2019) can reduce Fusarium infestation and expression in crops through its potential to fix carbon and increase soil pH and elementary composition (Akhter et al., 2015; Marra et al., 2018). Recently in Nigeria, Akanmu et al. (2020) found biochar as an effective tool in managing resident Fusarium verticillioides and have successfully used biochar from poultry fecal waste and sawdust to reduce disease severity (ear rot) caused by Fusarium verticillioides in maize. Activated carbon would serve as an adsorbent to mycotoxins, capable of binding 100% ZEA at 0.1%, 0.25%, 0.5%, and 1% dose levels (Bueno et al., 2005). This adsorption capacity and increased pH explained that biochar and activated carbon not only delayed conidial germination but also carried a high adsorption capacity for mycotoxin (Li et al., 2022).
11.1.2. Contribution of natural extract in controlling Fusarium species and zearalenone
The utilization of natural extract is emerging as a possible method to suppress mycotoxigenic fungi growth and their production of mycotoxins in grain under storage. Although a higher aw level favors Fusarium growth, antifungal activities of some natural extracts such as essential oils are effective at higher aw levels (Velluti et al., 2004). Olopade et al. (2019) explored the possibility of using montmorillonite clay and/or Cymbopogon citratus to decontaminate ZEA in stored grains and observed that 12% Cymbopogon citratus reduced ZEA contamination by 98.3% while 12% montmorillonite–Cymbopogon citratus mixed showed a 66% reduction of ZEA in for 4 weeks. Ali et al. (2021) suggest that essential oils obtained from Mentha longifolia and Citrus reticulata can inhibit F. culmorum growth at 500 μL/mL. Similarly, Sahab et al. (2014) evaluated the antifungal activities of some essential oils extracted from rocket seeds (Eruca sativa), rosemary (Rosmarinus officinalis) leaves, and tea tree (Melaleuca alternifolia) against Fusarium isolates obtained from maize. Essential oil from rocket seeds and tea trees exhibited high antifungal activity and completely inhibited all Fusarium isolates growth at 0.1% and 0.4% respectively, while rosemary essential oil showed moderated antifungal activity with reduced growth of Fusarium isolates (Sahab et al., 2014). At 0.995 aw, cinnamon oil, clove oil, lemongrass oil, oregano oil, and palmarosa oil exhibited inhibitory effects on F. graminearum growth, and their ability to produce zearalenone (Velluti et al., 2004).
11.1.3. Biological‐driven methods to reduce Fusarium infection and zearalenone
A good number of microbial species have demonstrated their ability to counter the growth and ZEA excretion in toxigenic Fusarium species. A study by Shude et al. (2021) using antagonist yeast species isolated from leaf, flowers, anther, and/or stem of cereals crops, and weed plants exhibited varying inhibitory effects on the mycelial growth of Fusarium graminearum. Most of the yeast antagonists (87.21%) that inhibited the growth could maintain inhibition until 20 days postinoculation (dpi); however, about 58.33% (7 out of 12) of the antagonist yeast increased ZEA concentrations (Shude et al., 2021). Subsequently, acibenzolar‐s‐methyl was combined with yeast antagonist (Papiliotrema flavescens and Pseudozyma sp.) to test against F. graminearum on spring wheat: it was observed that the combination of yeast and acibenzolar‐s‐methyl treatment effectively reduced Fusarium head blight severity, and deoxynivalenol concentration compared to the sole treatments (Nothando et al., 2021; Shude et al., 2022).
Another study conducted by Debbi et al. (2018) explored the potential of using Trichoderma spp. from Algeria as a biocontrol agent against F. oxysporum. f. sp. lycopersici (FOL), and F. oxysporum f. sp. Radices lycopersici (FORL) showed that T. ghanense and T. asperellum might reduce the severity of crown and root rot and Fusarium wilt diseases by 53.1%, and 48.3%, respectively.
11.2. Primary processing methods to reduce zearalenone concentration
11.2.1. Cleaning and sorting
Primary intervention methods such as cleaning and sorting of damaged, discolored, and shriveled kernels reduce Fusarium toxins in grains (Pascale et al., 2022; Schaarschmidt & Fauhl‐Hassek, 2021). In many African countries, such as South Africa, Ghana, Benin, Nigeria, Malawi, and Tanzania, grains are customarily hand‐sorted and cleaned before domestic and industrial use. However, this process is tedious and difficult to apply in medium‐to‐large‐scale food processing industries. For practical purposes involving high production volumes, mechanized tools and ultraviolet light have been used to segregate ZEA‐contaminated products. Aoun et al. (2020) explored and developed a low‐cost sorting tool (Dropsort device) that can be used in Africa for reducing mycotoxin levels in grains. Though not ZEA, the dropsort, a low‐cost sorter that separates grains based on kernel bulk density and 100‐kernel weight, combined with size sorting was more effective in reducing fumonisin (another Fusarium mycotoxin) concentration to under 2 ppm, but could not reduce aflatoxin levels in maize grain to under 20 ppm (Aoun et al., 2020). Although the DropSort accepted fraction had significantly higher 100‐kernel weight and kernel bulk density than the rejected fraction, the technology only or in combination with visual sorting had up to 19% rejection rate, and this may hinder its acceptability in Africa, particularly with commercial traders since volume is the commonly accepted method in trading than weight. Cleaning grains (removal of dust, coarse, small, broken, shriveled, and low‐density kernels) have been tested and proven to be capable of eliminating 67%–87% of ZEA concentration in maize (Pascale et al., 2022).
11.2.2. De‐hulling
De‐hulling is a widespread food processing technique used in Africa and has successfully been used to reduce mycotoxin concentrations in finished food. For zearalenone, the toxin is largely restricted to the outer layers of grain, and therefore, if partitioned into various fractions, including germs, bran, and coarse and fine grits may reduce human and animal exposure to ZEA (Brera et al., 2006; Habschied et al., 2011; Schwake‐Anduschus et al., 2015). In Malawi, Njombwa et al. (2020) evaluated occurrence levels of ZEA in dairy cattle concentrate feed and observed that 75% (83 out of 111) of corn bran tested positive for ZEA with minimum, maximum, and median concentration levels of 100, 2400, and 240 μg/kg, respectively. In South Africa, corn bran, corn flour, corn germ, and corn grits were reported to be contaminated with ZEA at mean levels of 245.6, 31, 29.8, and 8.6 μg/kg, respectively, compared to the mean value of 93.4 μg/kg for whole maize (Burger et al., 2013). The major limiting factor to this control technique in Africa is that the hull is used as a component in animal feeds. This may increase animal exposure risk and invariably human intake of ZEA through the consumption of animal products.
11.3. Secondary processing methods to reduce ZEA concentration in grains
11.3.1. Fermentation
Being water‐soluble mycotoxins, ZEA concentration can be partially reduced during fermentation or in an alkaline solution. Under alkaline conditions, the lactone ring of ZEA (Figure 1) is opened to form water‐soluble salt. The transfer of ZEA into water used for soaking or fermentation is effective at elevated pH. African indigenous fermentation process, mainly as spontaneous or in most cases the addition of yeast, is capable of reducing ZEA levels in traditionally fermented food and beverages. In Nigeria, Ezekiel et al. (2015) assessed the levels of ZEA in Kunu‐zaki—a traditional fermented nonalcoholic maize‐based beverage, and pito—a traditional fermented alcoholic drink made from sorghum. It was observed that the traditional fermentation process used in preparing these drinks can reduce ZEA levels up to 76.2% and 94.8% for kuku‐zaki and pito, respectively. Previously in Botswana, Nkwe et al. (2005) analyzed sorghum malts and their corresponding wort and beer samples for ZEA levels; the traditional wort (90 μg/kg) and beer (92 μg/kg) samples contain less ZEA than their raw malted sorghum samples (485 μg/kg). More recently, an effective reduction in the content of ZEA was observed in bread prepared by baking with the addition of yeast, ranging from 14.3% to 35.4% (Podgórska‐Kryszczuk et al., 2022).
11.3.2. Radiation
Radiation is a powerful tool for decontaminating mycotoxins and improving the storability of food is irradiation (gamma radiation, electron beams, and X‐ray). In recent years, ionizing radiation, as a physical, cold process, has been investigated as a method for the degradation of ZEA and its associated fungi in food grains (Calado et al., 2020). In Egypt, Sebaei et al. (2020) examined the reduction of ZEA in grains using gamma radiation and reported that ZEA was more easily degraded in wheat than in maize. In Tunisia, an irradiation dose (gamma radiation) of 3 and 10 kGy was sufficient to reduce 90% of the natural fungal load and 32% of ochratoxin A in sorghum, respectively (Ben Amara et al., 2022). Similarly, Zhao et al. (2019) demonstrated the 90.24% degradation rate of zearalenone using microwave irradiation for 2 min coupled with activated carbon.
11.3.3. Ozone
Ozonation, an advanced environmentally friendly and generally recognized as safe technology, can attack a wide range of microorganisms and natural compounds (Qi et al., 2016; Ribeiro et al., 2021). The process is highly recognized for its ability to generate ozone gas easily from oxygen (pure or from the air) without leaving residues. Ozone has been approved as a safe antimicrobial agent and has enhanced consumer confidence as well as widespread industrial application in the control of industrial pests such as Fusarium species (Food and Drug Administration, 2001). It is worth mentioning that ozone application allows water reutilization. This technique has been instrumental in managing Fusarium species and ZEA levels in maize products. In a study on maize flour exposure to ozone gas (51.5 mg/L of ozone for up to 60 min), mycotoxin analysis revealed that ZEA concentration was reduced to 62.3% (Alexandre et al., 2019). Similarly, Qi et al. (2016) observed an 86% reduction in naturally contaminated ZEA in maize when exposed to 100 mg/L of ozone for 100 min.
In malt and beer production, Zuluaga‐Calderón et al. (2023) use ozone in the steeping stage to reduce Fusarium graminearum incidence from 100% to 47% without affecting the germinative properties. Although the technology has a superior degradation rate for ZEA than electron beam irradiation (Yang et al., 2020), its application increases monounsaturated fatty acids and decreases polyunsaturated fatty acids such as linoleic, oleic, and α‐linolenic fatty acids (Purar et al., 2022; Qi et al., 2016).
11.3.4. Dielectric barrier discharge
Dielectric barrier discharge is another nonthermal treatment technology employed to degrade mycotoxins in food. The technology has been tested to degrade up to 98.28% of ZEA in food products at 50 KV for 120 s (Huang et al., 2022). Zheng et al. (2023) evaluated the impact of dielectric barrier discharge cold plasma on ZEA degradation in maize and observed a 56.57% degradation of ZEA at 50 KV for 120 s with an increase in the fatty acid composition and reduced crude fiber content. Dielectric barrier discharge treatment for ZEA degradation is more efficient in liquid food products (Feizollahi & Roopesh, 2021). Furthermore, dielectric barrier discharge generated from 85% Ar + 15% O2 resulted in higher degradation of ZEA compared to N2 (Feizollahi & Roopesh, 2021).
12. RECENT PROGRESS IN THE USE OF INDUSTRIAL ADSORBENTS
Different industrial adsorbents have been studied at the laboratory level and have been proposed for use in the elimination of zearalenone at the industrial scale level, with temperature playing a critical role in the quantity and maintenance of ZEA. Activated carbon has proven to be effective in eliminating more than 83% of ZEA during the bleaching process of corn oil at 70°C, although excessive temperature (above 100°C) could cause adsorbed ZEA to desorb from activated carbon (Hu et al., 2023). Du et al. (2023) also reported that the metal–organic framework absorbents exhibited sufficient efficacy in removing more than 83.3% of ZEA in vegetable oil within 30 min. Ghafari et al. (2022) found that silica nanoparticles could remove about 92.1% of ZEA in contaminated sunflower oil (Ghafari et al., 2022).
Commercially available inorganic and organic mycotoxin adsorbents such as S‐Mont, Minazel® plus, and Mycosorb® patented and registered as feed quality enhancers have demonstrated their efficacy in alleviating the harmful effect of ZEA on livestock including pigs (Nesic et al., 2008).
13. CONCLUDING REMARKS AND FUTURE RESEARCH
Although ZEA has a severe impact on grain quality and the economic well‐being of livestock and humans, it is clear that few studies have evaluated its presence in agricultural produce with not much awareness of the toxin in Africa.
Given that climate change patterns are widely evident in Africa with the projected increase in atmospheric CO2 concentration and relative humidity, which flavors Fusarium species growth and ZEA production, little progress is made in understanding the impact of this changing climate with the recently higher levels of ZEA in Africa. Overall, F. verticillioides and F. graminearum were the most commonly isolated species in all the subregional blocks (northern, eastern, western, southern, and central Africa). Understanding plant stress responses, enabling plants to withstand the changing environmental conditions, is essential to address and prevent the Fusarium species activities.
Preventive and mitigation efforts evaluated and applied to control Fusarium species on‐field and in storage as well as ZEA production so far are insufficient and need future investigation (Figure 3).
Most countries in Africa do not know the status of ZEA in their food supply chain, and there are limited regulations (only in South Africa and Morocco) to control ZEA occurrence in maize and other cereal crops.
Some indigenous food processing techniques used in Africa have proven to have an inherent effect in reducing ZEA levels in processed maize foods; however, not much attention has been given to these techniques to further optimize these processing techniques for ZEA elimination (Figure 3).
Although nonthermal food processing technologies have exhibited their power to effectively eliminate a large number of different mycotoxins, including ZEA in processed foods, regulations for its use in mycotoxin management are limited. As such more research is needed to assess the safety of these technologies (ozone, radiation, dielectric barrier discharge) with mycotoxin management in the storage and processing of food, and to enhance consumer acceptability.
FIGURE 3.
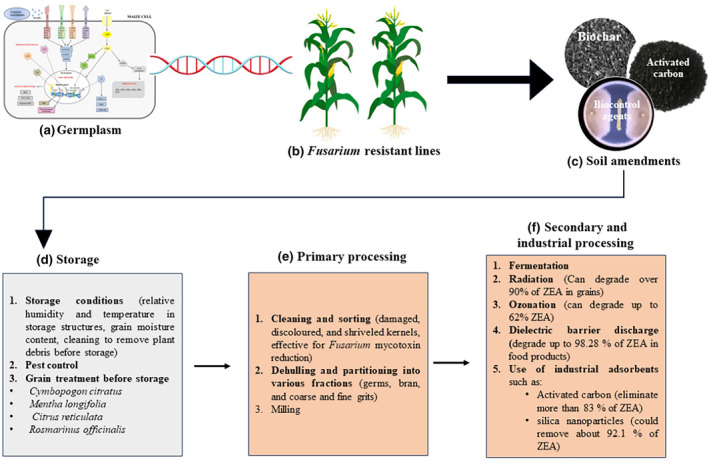
Reported methods used to control Fusarium infestation and ZEA concentration in grains and processed food. (a) Hypothetical model explaining phytohormone‐mediated molecular mechanisms in maize plant defense response against Fusarium verticillioides (Lanubile et al., 2017), (b) Integration of various molecular tools to develop resistant maize line, (c) using soil amendments to change the microenvironment of soil, (d, e, and f) various postharvest methods employed to reduce ZEA levels in grains and processed foods.
AUTHOR CONTRIBUTIONS
Abdul Rashid Hudu: Conceptualization (lead); data curation (equal); methodology (lead); writing – original draft (lead). Francis Addy: Data curation (equal); supervision (equal); writing – review and editing (equal). Gustav Komla Mahunu: Data curation (equal); writing – review and editing (equal). Abdul‐Halim Abubakari: Data curation (equal); writing – review and editing (equal). Nelson Opoku: Data curation (equal); supervision (equal); writing – review and editing (equal).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
This review work is part of a master thesis conducted by the first author. We are grateful to the Islamic Development Bank (IsDB) group for awarding a master's scholarship to Abdul Rashid Hudu.
Hudu, A. R. , Addy, F. , Mahunu, G. K. , Abubakari, A.‐H. , & Opoku, N. (2024). Zearalenone contamination in maize, its associated producing fungi, control strategies, and legislation in Sub‐Saharan Africa. Food Science & Nutrition, 12, 4489–4512. 10.1002/fsn3.4125
DATA AVAILABILITY STATEMENT
Data presented in this study are contained in the article.
REFERENCES
- Aasa, A. O. , Njobeh, P. B. , & Fru, F. F. (2022). Incidence of filamentous fungi in some food commodities from Ivory Coast. Journal of Agriculture and Food Research, 8, 100304. 10.1016/j.jafr.2022.100304 [DOI] [Google Scholar]
- Abass, A. B. , Adegoke, G. O. , Awoyale, W. , Gaspar, A. , Mlingi, N. , Andrianavalona, V. , Randrianarivelo, R. , Sulyok, M. , Mneney, A. , & Ranaivoson, L. R. (2019). Enumeration of the microbiota and microbial metabolites in processed cassava products from Madagascar and Tanzania. Food Control, 99, 164–170. 10.1016/j.foodcont.2018.12.025 [DOI] [Google Scholar]
- Abdallah, M. F. , Girgin, G. , Baydar, T. , Krska, R. , & Sulyok, M. (2017). Occurrence of multiple mycotoxins and other fungal metabolites in animal feed and maize samples from Egypt using LC‐MS/MS. Journal of the Science of Food and Agriculture, 97(13), 4419–4428. 10.1002/jsfa.8293 [DOI] [PubMed] [Google Scholar]
- Abdallah‐Nekache, N. , Laraba, I. , Ducos, C. , Barreau, C. , Bouznad, Z. , & Boureghda, H. (2019). Occurrence of Fusarium head blight and Fusarium crown rot in Algerian wheat: Identification of associated species and assessment of aggressiveness. European Journal of Plant Pathology, 154(3), 499–512. 10.1007/s10658-019-01673-7 [DOI] [Google Scholar]
- Abia, W. A. , Warth, B. , Ezekiel, C. N. , Sarkanj, B. , Turner, P. C. , Marko, D. , Krska, R. , & Sulyok, M. (2017). Uncommon toxic microbial metabolite patterns in traditionally home‐ processed maize dish (fufu) consumed in rural Cameroon. Food and Chemical Toxicology, 107, 10–19. 10.1016/j.fct.2017.06.011 [DOI] [PubMed] [Google Scholar]
- Abia, W. A. , Warth, B. , Sulyok, M. , Krska, R. , Tchana, A. N. , Njobeh, P. B. , Dutton, M. F. , & Moundipa, P. F. (2013). Determination of multi‐mycotoxin occurrence in cereals, nuts and their products in Cameroon by liquid chromatography tandem mass spectrometry (LC‐MS/MS). Food Control, 31(2), 438–453. 10.1016/j.foodcont.2012.10.006 [DOI] [Google Scholar]
- Abia, W. A. , Tchana, A. , Djonabaye, D. , Šarkanj, B. , Mfopou, E. Y. , Ezekiel, C. , Sulyok, M. , Turner, P. , Elliott, C. , Warth, B. , Krska, R. , & Moundipa, P. (2020). Assessment of urinary biomarkers of mycotoxin exposure in adults from Cameroon. Research Square, 1–22. 10.21203/rs.3.rs-31062/v1 [DOI] [Google Scholar]
- Adekoya, I. , Njobeh, P. , Obadina, A. , Chilaka, C. , Okoth, S. , De Boevre, M. , & De Saeger, S. (2017). Awareness and prevalence of mycotoxin contamination in selected Nigerian fermented foods. Toxins, 9(11), 1–16. 10.3390/toxins9110363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adekoya, I. , Obadina, A. , Adaku, C. C. , De Boevre, M. , Okoth, S. , & Saeger, S. D. (2017). Mycobiota and co‐occurrence of mycotoxins in south African maize‐based opaque beer. International Journal of Food Microbiology, 270, 22–30. 10.1016/j.ijfoodmicro.2018.02.001 [DOI] [PubMed] [Google Scholar]
- Adetunji, M. C. , Atanda, O. O. , & Ezekiel, C. N. (2017). Risk assessment of mycotoxins in stored maize grains consumed by infants and young children. Children, 4(58), 1–10. 10.3390/children4070058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnieszka, W. , Karolina, G. , Jan, B. , Paulina, P. , & Piotr, G. (2012). Zearalenone contamination of the aquatic environment as a result of its presence in crops. Mycotoxins in Aquatic Environment, 63, 429–435. 10.2478/10004-1254-63-2012-2229 [DOI] [PubMed] [Google Scholar]
- Akanmu, A. O. , Sobowale, A. A. , Abiala, M. A. , Olawuyi, O. J. , & Odebode, A. C. (2020). Efficacy of biochar in the management of Fusarium verticillioides Sacc. Causing ear rot in Zea mays L. Biotechnology Reports, 26, e00474. 10.1016/j.btre.2020.e00474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akello, J. , Ortega‐beltran, A. , Katati, B. , Atehnkeng, J. , Augusto, J. , Mwila, C. M. , Mahuku, G. , Chikoye, D. , & Bandyopadhyay, R. (2021). Prevalence of aflatoxin‐ and fumonisin‐producing fungi associated with cereal crops grown in Zimbabwe and their associated risks in a climate change scenario. Food, 10(287), 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhter, A. , Hage‐Ahmed, K. , Soja, G. , & Steinkellner, S. (2015). Compost and biochar alter mycorrhization, tomato root exudation, and development of Fusarium oxysporum f. sp. lycopersici . Frontiers in Plant Science, 6, 529. 10.3389/fpls.2015.00529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaboudi, A. R. , Osaili, T. M. , & Otoum, G. (2022). Quantification of mycotoxin residues in domestic and imported chicken muscle, liver, and kidney in Jordan. Food Control, 132, 108511. 10.1016/j.foodcont.2021.108511 [DOI] [Google Scholar]
- Alaro, L. O. (2021). Incidence, morphological and molecular characteterisation of Fusarium species and level of fumonisins in selected maize genotypes in Nakuru County, Kenya . Kenyatta University.
- Alexandre, A. P. S. , Castanha, N. , Costa, N. S. , Santos, A. S. , Badiale‐Furlong, E. , Augusto, P. E. D. , & Calori‐Domingues, M. A. (2019). Ozone technology to reduce zearalenone contamination in whole maize flour: Degradation kinetics and impact on quality. Journal of the Science of Food and Agriculture, 99(15), 6814–6821. 10.1002/jsfa.9966 [DOI] [PubMed] [Google Scholar]
- Ali, H. M. , Elgat, W. A. A. A. , El‐hefny, M. , Salem, M. Z. M. , Taha, A. S. , Al Farraj, D. A. , Elshikh, M. S. , Hatamleh, A. A. , & Abdel‐salam, E. M. (2021). New approach for using Mentha longifolia L. and Citrus reticulata L. essential oils as wood‐biofungicides: GC‐MS, SEM and MNDO quantum chemical studies. Materials, 14(6), 1–18. 10.3390/ma14061361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankwasa, E. M. , Imade, F. , & Tanvir, A. (2021). Update on mycotoxin contamination of maize and peanuts in East African community countries. Journal of Food Science and Nutrition Therapy, 7, 001–010. 10.17352/jfsnt.000026 [DOI] [Google Scholar]
- Aoun, M. , Stafstrom, W. , Priest, P. , Fuchs, J. , Windham, G. L. , Williams, W. P. , & Nelson, R. J. (2020). Low‐cost grain sorting technologies to reduce mycotoxin contamination in maize and groundnut low‐cost grain sorting technologies to reduce mycotoxin contamination in maize and groundnut. Food Control, 118, 107363. 10.1016/j.foodcont.2020.107363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atoui, A. , El Khoury, A. , Kallassy, M. , & Lebrihi, A. (2011). Quantification of Fusarium graminearum and Fusarium culmorum by real‐time PCR system and zearalenone assessment in maize. International Journal of Food Microbiology, 154(1–2), 59–65. 10.1016/J.IJFOODMICRO.2011.12.022 [DOI] [PubMed] [Google Scholar]
- Audenaert, K. , Callewaert, E. , Höfte, M. , De Saeger, S. , & Haesaert, G. (2010). Hydrogen peroxide induced by the fungicide prothioconazole triggers deoxynivalenol (DON) production by Fusarium graminearum . BMC Microbiology, 10(112), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamba, S. , Marius, H. , Biego, G. , & Ake, A. Y. (2020). Assessment of the health quality related to the presence of ochratoxin A, fumonisin B1 and zearalenone in maize (Zea mays L.) produced in Côte D' Ivoire. Asian Journal of Research in Crop Science, 5(3), 41–51. 10.9734/AJRCS/2020/v5i330099 [DOI] [Google Scholar]
- Beev, G. , Denev, S. , & Bakalova, D. (2013). Zearalenone – Producing activity of Fusarium graminearum and Fusarium oxysporum isolated from Bulgarian wheat. Bulgarian Journal of Agricultural Science, 19(2), 255–259. [Google Scholar]
- Ben Amara, A. , Mehrez, A. , Ragoubi, C. , Romero‐González, R. , Garrido Frenich, A. , Landoulsi, A. , & Maatouk, I. (2022). Fungal mycotoxins reduction by gamma irradiation in naturally contaminated sorghum. Journal of Food Processing and Preservation, 46(3), 1–7. 10.1111/jfpp.16345 [DOI] [Google Scholar]
- Bencze, S. , Puskás, K. , Vida, G. , Karsai, I. , & Balla, K. (2017). Rising atmospheric CO2 concentration may imply higher risk of Fusarium mycotoxin contamination of wheat grains. Mycotoxin Research, 33(3), 229–236. 10.1007/s12550-017-0281-2 [DOI] [PubMed] [Google Scholar]
- Biomin . (2018). World mycotoxin survey 2018. Annual Report No. 15. https//www.biomin.net/en/blog‐posts/2018‐biomin‐mycotoxin‐survey‐results/
- Biomin . (2019). World mycotoxin survey 2019. Annual Report No. 16. https//www.biomin.net/en/blog‐posts/2019‐biomin‐mycotoxin‐survey‐results/
- Blandino, M. , Reyneri, A. , & Vanara, F. (2008). Influence of nitrogen fertilization on mycotoxin contamination of maize kernels. Crop Protection, 27(2), 222–230. 10.1016/j.cropro.2007.05.008 [DOI] [Google Scholar]
- Borràs‐Vallverdú, B. , Ramos, A. J. , Cantero‐Martínez, C. , Marín, S. , Sanchis, V. , & Fernández‐Ortega, J. (2022). Influence of agronomic factors on mycotoxin contamination in maize and changes during a 10‐day harvest‐till‐drying simulation period: A different perspective. Toxins, 14, 620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun, D. , Abia, W. A. , Sarkanj, B. , Sulyok, M. , Waldhoer, T. , Erber, A. C. , Krska, R. , Turner, P. C. , Marko, D. , Ezekiel, C. N. , & Warth, B. (2022). Mycotoxin‐mixture assessment in mother‐infant pairs in Nigeria: From mothers' meal to infants' urine. Chemosphere, 287, 132226. [DOI] [PubMed] [Google Scholar]
- Brera, C. , Catano, C. , De Santis, B. , Debegnach, F. , De Giacomo, M. , Pannunzi, E. , & Miraglia, M. (2006). Effect of industrial processing on the distribution of aflatoxins and zearalenone in corn‐milling fractions. Journal of Agricultural and Food Chemistry, 54, 5014–5019. 10.1021/jf060370s [DOI] [PubMed] [Google Scholar]
- Bueno, D. J. , Di Marco, L. , Oliver, G. , & Bardón, A. (2005). In vitro binding of zearalenone to different adsorbents. Journal of Food Protection, 68(3), 613–615. 10.4315/0362-028X-68.3.613 [DOI] [PubMed] [Google Scholar]
- Burger, H. M. , Shephard, G. S. , Louw, W. , Rheeder, J. P. , & Gelderblom, W. C. A. (2013). The mycotoxin distribution in maize milling fractions under experimental conditions. International Journal of Food Microbiology, 165(1), 57–64. [DOI] [PubMed] [Google Scholar]
- Calado, T. , Abrunhosa, L. , Verde, S. C. , Alté, L. , Venâncio, A. , & Fernández‐Cruz, M. L. (2020). Effect of gamma‐radiation on zearalenone‐degradation, cytotoxicity and estrogenicity. Food, 7, 1687. 10.3390/foods9111687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, Y. , Yu, M. , Dong, G. , Chen, B. , & Zhang, B. (2020). Digital PCR as an emerging tool for monitoring of microbial biodegradation. Molecules, 25, 706. 10.3390/molecules25030706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cendoya, E. , Nichea, M. J. , Monge, P. , Zachetti, V. G. L. , Chiacchiera, S. M. , & Ramirez, M. L. (2021). Effect of fungicides commonly used for Fusarium head blight management on growth and fumonisin production by Fusarium proliferatum . Revista Argentina de Microbiología, 53(1), 64–74. 10.1016/j.ram.2019.12.005 [DOI] [PubMed] [Google Scholar]
- Chen, L. , Li, L. , Xie, X. , Chai, A. , Shi, Y. , Fan, T. , Xie, J. , & Li, B. (2022). An improved method for quantification of viable Fusarium cells in infected soil products by propidium monoazide coupled with real‐time PCR. Microorganisms, 10(5), 1037. 10.3390/microorganisms10051037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidi, N. I. , Adekunle, A. A. , Samuel, T. O. , & Eziashi, E. I. (2020). Molecular identification of secreted effector genes involved in African Fusarium oxysporum f. sp. elaeidis strains pathogenesis during screening Nigerian susceptible and tolerant oil palm (Elaeis guineensis Jacq.) genotypes. Frontiers in Cellular and Infection Microbiology, 10, 552394. 10.3389/fcimb.2020.552394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilaka, C. A. , De Boevre, M. , Atanda, O. O. , & Saeger, S. D. (2016). Occurrence of Fusarium mycotoxins in cereal crops and processed products (Ogi) from Nigeria. Toxins, 8, 342. 10.3390/toxins8110342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilaka, C. A. , De Kock, S. , Phoku, J. Z. , Mwanza, M. , Augustina, M. , & Dutton, M. F. (2012). Fungal and mycotoxin contamination of south African commercial maize. Journal of Food, Agriculture and Environment, 10(2), 296–303. [Google Scholar]
- Chilaka, C. A. , Obidiegwu, J. E. , Chilaka, A. C. , Atanda, O. O. , & Mally, A. (2022). Mycotoxin regulatory status in Africa: A decade of weak institutional efforts. Toxins, 14, 442. 10.3390/toxins14070442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrêa, A. N. R. , & Ferreira, C. D. (2023). Mycotoxins in grains and cereals intended for human consumption: Brazilian legislation, occurrence above maximum levels and co‐occurrence. Food Reviews International, 39(8), 5920–5933. 10.1080/87559129.2022.2098318 [DOI] [Google Scholar]
- Cui, H. , Wang, S. , Yang, X. , Zhang, W. , Chen, M. , Wu, Y. , Li, S. , Li, L. , Cai, D. , Guo, B. , Ye, J. , & Wang, S. (2022). Predictive models for assessing the risk of Fusarium pseudograminearum mycotoxin contamination in post‐harvest wheat with multi‐parameter integrated sensors. Food Chemistry: X, 16, 100472. 10.1016/j.fochx.2022.100472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dada, T. A. , Ekwomadu, T. I. , & Mwanza, M. (2020). Multi mycotoxin determination in dried beef using liquid chromatography coupled with triple quadrupole mass spectrometry (LC‐MS/MS). Toxins, 12, 357. 10.3390/toxins12060357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daou, R. , Joubrane, K. , Maroun, R. G. , Khabbaz, L. R. , Ismail, A. , & El Khoury, A. (2021). Mycotoxins: Factors influencing production and control strategies. AIMS Agriculture and Food, 6(1), 416–447. 10.3934/AGRFOOD.2021025 [DOI] [Google Scholar]
- Debbi, A. , Boureghda, H. , Monte, E. , & Hermosa, R. (2018). Distribution and genetic variability of Fusarium oxysporum associated with tomato diseases in Algeria and a biocontrol strategy with indigenous Trichoderma spp. Frontiers in Microbiology, 9, 282. 10.3389/fmicb.2018.00282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degraeve, S. , Madege, R. R. , Audenaert, K. , Kamala, A. , Ortiz, J. , Kimanya, M. , Tiisekwa, B. , De Meulenaer, B. , & Haesaert, G. (2016). Impact of local pre‐harvest management practices in maize on the occurrence of Fusarium species and associated mycotoxins in two agro‐ecosystems in Tanzania. Food Control, 59, 225–233. 10.1016/j.foodcont.2015.05.028 [DOI] [Google Scholar]
- Djeugap, J. F. , Ghimire, S. , Wanjuki, I. , Muiruri, A. , & Harvey, J. (2019). Mycotoxin contamination of edible non‐timber forest products in Cameroon. Toxins, 11(7), 430. 10.3390/toxins11070430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlamini, C. , Earnshaw, D. , & Assefa, Y. (2022). Temporal trends in mycotoxin contaminations in maize supplied to consumers in Eswatini. International Journal of Food Science and Agriculture, 6(2), 128–134. 10.26855/ijfsa.2022.06.001 [DOI] [Google Scholar]
- Donnell, K. O. , Whitaker, B. K. , Laraba, I. , Proctor, R. H. , Brown, D. W. , Broders, K. , Kim, H. S. , McCormick, S. P. , Busman, M. , Aoki, T. , Torres‐Cruz, T. J. , & Geiser, D. M. (2022). DNA sequence‐based identification of Fusarium: A work in progress. Plant Disease, 106, 1597–1609. [DOI] [PubMed] [Google Scholar]
- Du, Q. , Zhang, W. , Xu, N. , Jiang, X. , Cheng, J. , Wang, R. , & Wang, P. (2023). Efficient and simultaneous removal of aflatoxin B1, B2, G1, G2, and zearalenone from vegetable oil by use of a metal–organic framework absorbent. Food Chemistry, 418, 135881. 10.1016/j.foodchem.2023.135881 [DOI] [PubMed] [Google Scholar]
- Edwards, S. G. , & Jennings, P. (2018). Impact of agronomic factors on Fusarium mycotoxins in harvested wheat. Food Additives & Contaminants: Part A, 35(12), 2443–2454. 10.1080/19440049.2018.1543954 [DOI] [PubMed] [Google Scholar]
- EFSA . (2011). Scientific opinion on the risks for public health related to the presence of zearalenone in food. EFSA Journal, 9(6), 17–36. [Google Scholar]
- Egbontan, A. O. , Afolabi, C. G. , Kehinde, I. A. , Enikuomehin, O. A. , Ezekiel, C. N. , Sulyok, M. , Warth, B. , & Krska, R. (2017). A mini‐survey of moulds and mycotoxins in locally grown and imported wheat grains in Nigeria. Mycotoxin Research, 33(1), 59–64. 10.1007/s12550-016-0264-8 [DOI] [PubMed] [Google Scholar]
- Egbuta, M. , Wanza, M. , & Dutton, M. (2015). Evaluation of five major mycotoxins co‐contaminating two cereal grains from Nigeria. International Journal of Biochemistry Research & Review, 6(4), 160–169. 10.9734/ijbcrr/2015/15306 [DOI] [Google Scholar]
- Ekwomadu, T. I. , Gopane, R. E. , & Mwanza, M. (2018). Occurrence of filamentous fungi in maize destined for human consumption in South Africa. Journal of Food Science and Nutrition, 6, 884–890. 10.1002/fsn3.561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekwomadu, T. I. , Dada, T. A. , Akinola, S. A. , Nleya, N. , & Mwanza, M. (2021). Analysis of selected mycotoxins in maize from northwest South Africa using high performance liquid chromatography (HPLC) and other analytical techniques. Separations, 8, 143. 10.3390/separations8090143 [DOI] [Google Scholar]
- El‐Desouky, T. A. , & Naguib, K. (2013). Occurrence of zearalenone contamination in some cereals in Egypt. Journal of Agroalimentary Processes and Technologies, 19(4), 445–450. http://search.ebscohost.com/login.aspx?direct=true&db=ffh&AN=2014‐05‐Ma5610&lang=hu&site=ehost‐liveNS‐ [Google Scholar]
- El‐Rabbat, S. , El‐Maghraby, O. , El‐Debaiky, S. , Haider, H. , & Haider, S. (2018). Isolation and molecular identification of Fusarium species from Egypt. International Journal of Innovative Science, Engineering & Technology, 5(1), 121–132. [Google Scholar]
- Erenstein, O. , Jaleta, M. , Sonder, K. , Mottaleb, K. , & Prasanna, B. M. (2022). Global maize production, consumption and trade: Trends and R&D implications. Food Security, 14, 1295–1319. 10.1007/s12571-022-01288-7 [DOI] [Google Scholar]
- European Commission . (2006a). Commission recommendation of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T‐2 HT‐2 and fumonisins in products intended for animal feeding. Official Journal of the European Union, 299, 7–9. [Google Scholar]
- European Commission . (2006b). Health and food safety; commission regulation (EC) No 118/2006‐ setting maximum levels for certain contaminants in foodstuffs. Official Journal of the European Union, 364, 5–24. [Google Scholar]
- Eze, U. A. , Lewis, S. E. M. , Connolly, L. , & Gong, Y. Y. (2018). Mycotoxins as potential cause of human infertility – A review of evidence from animal and cellular models. Acta Horticulturae, 1225, 513–525. 10.17660/ActaHortic.2018.1225.70 [DOI] [Google Scholar]
- Ezekiel, C. N. , Abia, W. A. , Braun, D. , Sarkanj, B. , Ayeni, K. I. , Oyedele, O. A. , Michael‐chikezie, E. C. , Ezekiel, V. C. , Mark, B. N. , Ahuchaogu, C. P. , Krska, R. , Sulyok, M. , Turner, P. C. , & Warth, B. (2022). Mycotoxin exposure biomonitoring in breastfed and non‐exclusively breastfed Nigerian children. Environment International, 158, 106996. 10.1016/j.envint.2021.106996 [DOI] [PubMed] [Google Scholar]
- Ezekiel, C. N. , Abia, W. A. , Ogara, I. M. , Sulyok, M. , Warth, B. , & Krska, R. (2015). Fate of mycotoxins in two popular traditional cereal‐based beverages (kunu‐zaki and pito) from rural Nigeria. LWT‐ Food Science and Technology, 60(1), 137–141. 10.1016/j.lwt.2014.08.018 [DOI] [Google Scholar]
- Ezekiel, C. N. , Odebode, A. C. , & Fapohunda, S. O. (2008). Zearalenone production by naturally occurring Fusarium species on maize, wheat, and soybeans from Nigeria zearalenone production by naturally occurring Fusarium species on maize, wheat and soybeans from Nigeria. Journal of Biology and Environmental Science, 2(6), 77–82. [Google Scholar]
- Ezekiel, C. N. , Oyedele, O. A. , Kraak, B. , Ayeni, K. I. , Sulyok, M. , Houbraken, J. , & Krska, R. (2020). Fungal diversity and mycotoxins in low moisture content ready‐to‐eat foods in Nigeria. Frontiers in Microbiology, 11, 615. 10.3389/fmicb.2020.00615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezrari, S. , Lahlali, R. , Radouane, N. , Tahiri, A. , & Asfers, A. (2020). Characterization of Fusarium species causing dry root rot disease of citrus trees in Morocco. Journal of Plant Diseases and Protection, 128, 431–447. 10.1007/s41348-020-00392-0 [DOI] [Google Scholar]
- Falkauskas, R. , Bakutis, B. , Jovaišienė, J. , Vaičiulienė, G. , Gerulis, G. , Kerzienė, S. , Jacevičienė, I. , Jacevičius, E. , & Baliukonienė, V. (2022). Zearalenone and its metabolites in blood serum, urine, and milk of dairy cows. Animals, 12(13), 1651. 10.3390/ani12131651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO . (2004). Worldwide regulations for mycotoxins in food and feed in 2003 . Rome, Italy: FAO Food and Nutrition paper No. 81. Food and Agriculture Organization of the United Nations.
- Feizollahi, E. , & Roopesh, M. S. (2021). Degradation of zearalenone by atmospheric cold plasma: Effect of selected process and product factors. Food and Bioprocess Technology, 12, 2107–2119. 10.1007/s11947-021-02692-1 [DOI] [Google Scholar]
- Ferrigo, D. , Raiola, A. , Bogialli, S. , Bortolini, C. , Tapparo, A. , & Causin, R. (2015). In vitro production of fumonisins by Fusarium verticillioides under oxidative stress induced by H2O2 . Journal of Agricultural and Food Chemistry, 63(19), 4879–4885. 10.1021/acs.jafc.5b00113 [DOI] [PubMed] [Google Scholar]
- Ferrochio, L. , Cendoya, E. , Farnochi, M. C. , Massad, W. , & Ramirez, M. L. (2013). Evaluation of the ability of ferulic acid to control growth and fumonisin production of Fusarium verticillioides and Fusarium proliferatum on maize based media. International Journal of Food Microbiology, 167(2), 215–220. 10.1016/j.ijfoodmicro.2013.09.005 [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration . (2001). Rules and regulations. Federal Register, 66(123), 33829–33830. 10.4324/978080473369-10 [DOI] [Google Scholar]
- Geary, P. A. , Chen, G. , Kimanya, M. E. , Shirima, C. P. , Oplatowska‐stachowiak, M. , Elliott, C. T. , Routledge, M. N. , & Yun, Y. (2016). Determination of multi‐mycotoxin occurrence in maize based porridges from selected regions of Tanzania by liquid chromatography tandem mass spectrometry (LC‐MS/MS), a longitudinal study. Food Control, 68, 337–343. 10.1016/j.foodcont.2016.04.018 [DOI] [Google Scholar]
- Gerling, M. , Petry, L. , Barkusky, D. , Büttner, C. , Müller, M. E. H. , & Deoxynivalenol, D. O. N. (2022). Infected grasses as inoculum for Fusarium infestation and mycotoxin accumulation in wheat with and without irrigation. Mycotoxin Research, 39, 19–31. 10.1007/s12550-022-00470-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getachew, A. , Chala, A. , Hofgaard, I. S. , Brurberg, M. B. , Sulyok, M. , & Tronsmo, A. M. (2018). Multimycotoxin and fungal analysis of maize grains from south and southwestern Ethiopia. Food Additives and Contaminants: Part B Surveillance, 11(1), 64–74. 10.1080/19393210.2017.1408698 [DOI] [PubMed] [Google Scholar]
- Ghafari, N. , Paimard, G. , Sadeghi, E. , Choobkar, N. , & Lalabadi, M. A. (2022). Evaluation of nano‐silica, microwave heating, and ultraviolet irradiation effects on zearalenone detoxification in sunflower oils. World Mycotoxin Journal, 14(4), 369–381. [Google Scholar]
- Gonkowski, S. , Obremski, K. , Makowska, K. , Rytel, L. , & Mwaanga, E. S. (2018). Levels of zearalenone and its metabolites in sun‐dried Kapenta fish and water of Lake Kariba in Zambia — A preliminary study. Science of the Total Environment, 637–638, 1046–1050. 10.1016/j.scitotenv.2018.05.091 [DOI] [PubMed] [Google Scholar]
- Government Notice . (2010). Fertilizers, farm feeds, agricultural remedies and stock remedies Act, 1947 (Act No. 36 of 1947) Farm Feed Regulations: Amendment (Vol. 4, Issue 32935).
- Grimm, C. , & Geisen, R. (1998). A PCR‐ELISA for the detection of potential fumonisin producing Fusarium species. Letters in Applied Microbiology, 26(6), 456–462. 10.1046/j.1472-765X.1998.00366.x [DOI] [PubMed] [Google Scholar]
- Gruber‐Dorninger, C. , Jenkins, T. , & Schatzmayr, G. (2018). Multi‐mycotoxin screening of feed and feed raw materials from Africa abstract. World Mycotoxin Journal, 11(3), 369–383. 10.3920/WMJ2017.2292 [DOI] [Google Scholar]
- Gruber‐Dorninger, C. , Jenkins, T. , & Schatzmayr, G. (2019). Global mycotoxin occurrence in feed: A ten‐year survey. Toxins, 11(375), 1–25. 10.3390/toxins11070375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habschied, K. , Šarkanj, B. , Klapec, T. , & Krstanovic, V. (2011). Distribution of zearalenone in malted barley fractions dependent on Fusarium graminearum growing conditions. Food Chemistry, 129, 329–332. 10.1016/j.foodchem.2011.04.064 [DOI] [PubMed] [Google Scholar]
- Hadjout, S. , Chéreau, S. , Mekliche, L. , Marchegay, G. , Ducos, C. , Boureghda, H. , Zouidi, M. , Barreau, C. , Bouznad, Z. , & Richard‐Forget, F. (2022). Molecular identification of some Fusarium isolates and their chemotypes involved in Fusarium head blight on durum wheat in Algeria. Archives of Phytopathology and Plant Protection, 55(4), 499–513. 10.1080/03235408.2022.2034363 [DOI] [Google Scholar]
- Hainghumbi, T. A. , Embashu, W. , Nantanga, K. K. M. , Kadhila, N. P. , & Iipumbu, L. (2022). Assessment of microbiological properties, mycotoxins, and heavy metals in underprized raw Kalahari truffles sold in Namibia. Journal of Food Quality and Hazards Control, 9, 23–31. 10.18502/jfqhc.9.1.9687 [DOI] [Google Scholar]
- Han, X. , Huangfu, B. , Xu, T. , Xu, W. , Asakiya, C. , Huang, K. , & He, X. (2022). Research progress of safety of zearalenone: A review. Toxins, 14(386), 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanvi, D. M. , Lawson‐Evi, P. , De Boevre, M. , Goto, C. E. , De Saeger, S. , & Eklu‐Gadegbeku, K. (2019). Natural occurrence of mycotoxins in maize and sorghum in Togo. Mycotoxin Research, 35, 321–327. [DOI] [PubMed] [Google Scholar]
- Hay, W. T. , Mccormick, S. P. , & Vaughan, M. M. (2021). Effects of atmospheric CO2 and temperature on wheat and corn susceptibility to Fusarium graminearum and deoxynivalenol contamination. Plants, 10, 2582. 10.3390/plants10122582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hove, M. , De Boevre, M. , Lachat, C. , Jacxsens, L. , Nyanga, L. K. , & De Saeger, S. (2016). Occurrence and risk assessment of mycotoxins in subsistence farmed maize from Zimbabwe. Food Control, 69, 36–44. [Google Scholar]
- Hu, Y. , Ma, C. , Huang, W. , Guo, S. , Wang, T. , & Liu, J. (2023). Adsorption behavior of activated carbon for the elimination of zearalenone during bleaching process of corn oil. Grain & Oil Science and Technology, 6(1), 24–33. 10.1016/j.gaost.2022.11.002 [DOI] [Google Scholar]
- Huang, Y. , Liu, L. , & Chen, Y. (2022). Zearalenone degradation by dielectric barrier discharge cold plasma: The kinetics and mechanism. Food, 11, 1494. 10.3390/foods11101494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imade, F. , Ankwasa, E. M. , Geng, H. , Ullah, S. , Wang, G. , Zhang, C. , Dada, O. , & Xing, F. (2021). Updates on food and feed mycotoxin contamination and safety in Africa with special reference to Nigeria. Mycology, 12, 245–260. 10.1080/21501203.2021.1941371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari‐Nodoushan, A. A. (2022). Zearalenone, an abandoned mycoestrogen toxin, and its possible role in human infertility. International Journal of Reproductive BioMedicine, 20(2), 151–153. 10.18502/ijrm.v20i2.10507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janić Hajnal, E. , Kos, J. , Radić, B. , Anić, M. , Radović, R. , Kudumija, N. , Vulić, A. , Đekić, S. , & Pleadin, J. (2023). Impact of climate changes on the natural prevalence of Fusarium mycotoxins in maize harvested in Serbia and Croatia. Food, 12, 1002. 10.3390/foods12051002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaster, J. , Marina, K. , Ahmed, E. H. M. , El Khatib, H. , Lorenz, N. , Bahlmann, A. , Stollin, U. M. , Bunzel, M. , Scheibenzuber, S. , Rychlik, M. , & Von Der Waydbrink, G. (2023). Root uptake and metabolization of Alternaria toxins by winter wheat plants using a hydroponic system. Mycotoxin Research, 39, 109–126. 10.1007/s12550-023-00477-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedidi, I. , Jurado, M. , Cruz, A. , Trabelsi, M. M. , Said, S. , & González‐Jaén, M. T. (2021). Phylogenetic analysis and growth profiles of Fusarium incarnatum‐equiseti species complex strains isolated from Tunisian cereals. International Journal of Food Microbiology, 353, 109297. 10.1016/j.ijfoodmicro.2021.109297 [DOI] [PubMed] [Google Scholar]
- John, W. C. , Ihum, T. A. , Olusolape, O. , & Janfa, N. (2018). Efficacy of turmeric rhizome (Curcuma longa) and moringa leaf (Moringa oleifera) extract in treatment against fungi associated with maize seeds. Asian Plant Research Journal, 1(2), 1–8. 10.9734/aprj/2018/v1i226270 [DOI] [Google Scholar]
- Joseph, O. A. , Lena, D. M. , Chian, C. , & Anthony, R. (2015). Effects of practices of maize farmers and traders in Ghana on contamination of maize by aflatoxins: Case study of Ejura‐Sekyeredumase municipality. African Journal of Microbiology Research, 9(25), 1658–1666. 10.5897/ajmr2014.7293 [DOI] [Google Scholar]
- Kagot, V. , De Boevre, M. , Landschoot, S. , Obiero, G. , Okoth, S. , & De Saeger, S. (2022). Comprehensive analysis of multiple mycotoxins and Aspergillus flavus metabolites in maize from Kenyan households. International Journal of Food Microbiology, 363, 109502. 10.1016/j.ijfoodmicro.2021.109502 [DOI] [PubMed] [Google Scholar]
- Kamala, A. , Ortiz, J. , Kimanya, M. , Haesaert, G. , Donoso, S. , Tiisekwa, B. , & Meulenaer, B. D. (2015). Multiple mycotoxin co‐occurrence in maize grown in three agro‐ecological zones of Tanzania. Food Control, 54, 208–215. 10.1016/j.foodcont.2015.02.002 [DOI] [Google Scholar]
- Kana, J. R. , Gbemenou, B. , Gnonlonfin, J. , Harvey, J. , Wainaina, J. , Wanjuki, I. , Skilton, R. A. , & Teguia, A. (2013). Mycobiota and toxigenicity profile of Aspergillus flavus recovered from food and poultry feed mixtures in Cameroon. Journal of Animal and Poultry Sciences, 2(4), 98–107. [Google Scholar]
- Kankolongo, M. A. , Hellb, K. , & Nawac, I. N. (2009). Assessment for fungal, mycotoxin and insect spoilage in maize stored for human consumption in Zambia. Journal of the Science of Food and Agriculture, 89(8), 1366–1375. 10.1002/jsfa.3596 [DOI] [Google Scholar]
- Khalil, A. A. , Abou‐Gabal, A. E. , Elfaramawy, A. M. , Khaled, A. E. , & Abdellatef, A. A. (2013). Lactic acid bacteria as antimycotic and antimycotoxin agents against toxigenic Fusarium species associated to maize grains stored in Egyptian markets. Journal of Pure and Applied Microbiology, 7, 93–105. [Google Scholar]
- Kheseli, O. P. , Susan, I. S. , Sheila, O. , Otipa, M. , & Wafula, W. V. (2021). Prevalence and phylogenetic diversity of pathogenic Fusarium species in genotypes of wheat seeds in three Rift Valley regions, Kenya. Advances in Agriculture, 2021, 1–13. 10.1155/2021/8839147 [DOI] [Google Scholar]
- Kintega, K. R. , Zida, P. E. , Tarpaga, V. W. , Sankara, P. , & Sereme, P. (2020). Identification of Fusarium species associated with onion (Allium cepa L.) plants in field in Burkina Faso. Advances in Bioscience and Biotechnology, 11(03), 94–110. 10.4236/abb.2020.113008 [DOI] [Google Scholar]
- Korley, N. , Rachel, K. , & Tetteh, A. (2022). Mycobiota profile, phenology, and potential toxicogenic and pathogenic species associated with stored groundnuts (Arachis hypogaea L.) from the Volta region, Ghana. Food Science & Nutrition, 10, 888–902. 10.1002/fsn3.2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kos, J. , Anić, M. , Radić, B. , Zadravec, M. , Janić Hajnal, E. , & Pleadin, J. (2023). Climate change—A global threat resulting in increasing mycotoxin occurrence. Food, 12(14), 2704. 10.3390/foods12142704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krska, R. , Pettersson, H. , & Josephs, R. D. (2003). Zearalenone in maize: Stability testing and matrix characterisation of a certified reference material. Food Additives & Contaminants, 20(12), 1141–1152. 10.1080/02652030310001615203 [DOI] [PubMed] [Google Scholar]
- Lahouar, A. , Jedidi, I. , Said, S. , & Sanchis, V. (2018). Incidence, legislations and strategies of control of mycotoxins in North African countries. International Food Research Journal, 25(6), 2229–2247. [Google Scholar]
- Lahouar, A. , Crespo‐Sempere, A. , Marín, S. , Saïd, S. , & Sanchis, V. (2015). Toxigenic molds in Tunisian and Egyptian sorghum for human consumption. Journal of Stored Products Research, 63, 57–62. 10.1016/j.jspr.2015.07.001 [DOI] [Google Scholar]
- Lanubile, A. , Maschietto, V. , Borrelli, V. M. , Stagnati, L. , Logrieco, A. F. , & Marocco, A. (2017). Molecular basis of resistance to fusarium ear rot in maize. Frontiers in Plant Science, 8, 1–13. 10.3389/fpls.2017.01774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latawiec, A. E. , Strassburg, B. B. N. , Junqueira, A. B. , Araujo, E. , De Moraes, L. F. D. , Pinto, H. A. N. , Castro, A. , Rangel, M. , Malaguti, G. A. , Rodrigues, A. F. , Barioni, L. G. , Novotny, E. H. , Cornelissen, G. , Mendes, M. , & Batista, N. (2019). Biochar amendment improves degraded pasturelands in Brazil: Environmental and cost‐benefit analysis. Scientific Reports, 9, 11993. 10.1038/s41598-019-47647-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H. J. , & Ryu, D. (2017). Worldwide occurrence of mycotoxins in cereals and cereal derived food products: Public health perspectives of their co‐occurrence worldwide occurrence of mycotoxins in cereals and cereal derived food products. Journal of Agriculture and Food Chemistry, 65(33), 7034–7051. 10.1021/acs.jafc.6b04847 [DOI] [PubMed] [Google Scholar]
- Lee, S. , Son, H. , Lee, J. , & Lee, Y. L. Y. (2011). A putative ABC transporter gene, ZRA1, is required for zearalenone production in Gibberella zeae . Current Genetics, 57, 343–351. 10.1007/s00294-011-0352-4 [DOI] [PubMed] [Google Scholar]
- Lehmane, H. , Ba, R. , Dah‐Nouvlessounon, D. , Sina, H. , Roko, G. , Bade, F. T. , Socohou, A. , Adjanohoun, A. , & Baba‐Moussa, L. (2022). Status of techniques used to control moulds in maize storage in Africa. Agricultural Sciences, 13, 49–64. 10.4236/as.2022.131005 [DOI] [Google Scholar]
- Li, T. , Choi, K. , Jung, B. , Ji, S. , Kim, D. , Seo, M. W. , Lee, J. , & Lee, S.‐H. (2022). Biochar inhibits ginseng root rot pathogens and increases soil microbiome diversity. Applied Soil Ecology, 169, 104229. 10.1016/j.apsoil.2021.104229 [DOI] [Google Scholar]
- Liu, Z. , Zhang, G. , Zhang, Y. , Jin, Q. , Zhao, J. , & Li, J. (2016). Factors controlling mycotoxin contamination in maize and food in the Hebei province, China. Agronomy for Sustainable Development, 36(2), 1–10. 10.1007/s13593-016-0374-x [DOI] [Google Scholar]
- Lobulu, J. , Shimelis, H. , Laing, M. D. , Mushongi, A. A. , & Shayanowako, A. I. T. (2021). Characterization of maize genotypes (Zea mays L.) for resistance to striga asiatica and S. hermonthica and compatibility with Fusarium oxysporum f. sp. strigae (FOS) in Tanzania. Agronomy, 11, 1004. [Google Scholar]
- Lysøe, E. , Bone, K. R. , & Klemsdal, S. S. (2008). Identification of up‐regulated genes during zearalenone biosynthesis in Fusarium . European Journal of Plant Pathology, 122, 505–516. 10.1007/s10658-008-9318-x [DOI] [Google Scholar]
- Lysøe, E. , Klemsdal, S. S. , Bone, K. R. , Frandsen, R. J. N. , Johansen, T. , Thrane, U. , & Giese, H. (2006). The PKS4 gene of Fusarium graminearum is essential for zearalenone production. Applied and Environmental Microbiology, 72(6), 3924–3932. 10.1128/AEM.00963-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maharshi, A. , Rashid, M. M. , Teli, B. , Yadav, S. K. , Singh, D. P. , & Sarma, B. K. (2021). Salt stress alters pathogenic behaviour of Fusarium oxysporum f. sp. ciceris and contributes to severity in chickpea wilt incidence. Physiological and Molecular Plant Pathology, 113, 101602. 10.1016/j.pmpp.2021.101602 [DOI] [Google Scholar]
- Mahdjoubi, C. K. , Arroyo‐manzanares, N. , & Hamini‐kadar, N. (2020). Multi‐mycotoxin occurrence and exposure assessment approach in foodstuffs from Algeria. Toxins, 12(194), 1–18. 10.3390/toxins12030194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manizan, A. L. , Oplatowska‐Stachowiak, M. , Piro‐Metayer, I. , Campbell, K. , Koffi‐Nevry, R. , Elliott, C. , Akaki, D. , Montet, D. , & Brabet, C. (2018). Multi‐mycotoxin determination in rice, maize and peanut products most consumed in Côte D'ivoire by UHPLC‐MS/MS. Food Control, 87, 22–30. 10.1016/j.foodcont.2017.11.032 [DOI] [Google Scholar]
- Marra, R. , Vinale, F. , Cesarano, G. , Lombardi, N. , D'Errico, G. , Crasto, A. , Mazzei, P. , Piccolo, A. , Incerti, G. , Woo, S. L. , Scala, F. , & Bonanomi, G. (2018). Biochars from olive mill waste have contrasting effects on plants, fungi and phytoparasitic nematodes. PLoS One, 13(6), 1–24. 10.1371/journal.pone.0198728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina, Á. , González‐Jartín, J. M. , & Sainz, M. J. (2017). Impact of global warming on mycotoxins. Current Opinion in Food Science, 18, 76–81. 10.1016/j.cofs.2017.11.009 [DOI] [Google Scholar]
- Meng, K. , Wang, Y. , Yang, P. , Luo, H. , Bai, Y. , Shi, P. , Yuan, T. , Ma, R. , & Yao, B. (2010). Rapid detection and quantification of zearalenone‐producing Fusarium species by targeting the zearalenone synthase gene PKS4. Food Control, 21(2), 207–211. 10.1016/j.foodcont.2009.05.014 [DOI] [Google Scholar]
- Mesfin, A. , Lachat, C. , Gebreyesus, S. H. , Roro, M. , Tesfamariam, K. , Belachew, T. , De Boevre, M. , & Saeger, S. D. (2023). Mycotoxins exposure of lactating women and its relationship with dietary and pre/post‐harvest practices in rural Ethiopia. Toxins, 15, 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesfin, A. , Tesfamariam, K. , Belachew, T. , De Saeger, S. , Lachat, C. , & De Boevre, M. (2021). Multi‐mycotoxin profiling in maize reveals prevalence of Fusarium mycotoxins in south and west Ethiopia. World Mycotoxin Journal, 15(1), 1–12. [Google Scholar]
- Mielniczuk, E. , & Skwaryło‐bednarz, B. (2020). Fusarium head blight, mycotoxins and strategies for their reduction. Agronomy, 10(509), 1–26. 10.3390/agronomy10040509 [DOI] [Google Scholar]
- Mohammed, A. , Seyoum, C. , Yousuf, J. , Mweetwa, A. , Odera, J. A. , Okello, D. K. , Bekeko, Z. , Tadessa, T. , & Sulyok, M. (2023). Multi‐mycotoxins analysis in post‐harvest maize (Zea mays L.) grain from major producing areas of Ethiopia. World Mycotoxin Journal, 16(3), 1–12. [Google Scholar]
- Mohammed, A. , Bekeko, Z. , Yusufe, M. , Sulyok, M. , & Krska, R. (2022). Fungal species and multi‐mycotoxin associated with post‐harvest sorghum (Sorghum bicolor (L.) Moench) grain in eastern Ethiopia. Toxins, 14, 473. 10.3390/toxins14070473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed, A. , Seid, A. , Terefe, H. , Cervini, C. , & Verheecke‐vaessen, C. (2022). Harvest and post – Harvest handling practices associated with fumonisin – B1 contamination in maize (Zea mays L.): Dietary exposure and risk characterization in eastern Ethiopia. Mycotoxin Research, 38(4), 275–287. 10.1007/s12550-022-00468-w [DOI] [PubMed] [Google Scholar]
- Mokgatlhe, T. M. , Siame, A. B. , & Taylor, J. E. (2011). Fungi and Fusarium mycotoxins associated with maize (Zea mays) and sorghum (Sorghum bicolor) in Botswana. African Journal of Plant Science and Biotechnology, 5(1), 26–32. [Google Scholar]
- Moraes, W. B. , Madden, L. V. , Gillespie, J. , & Paul, P. A. (2022). Environment, grain development, and harvesting strategy effects on zearalenone contamination of grain from Fusarium head blight‐affected wheat spikes. Phytopathology, 113, 225–238. 10.1094/PHYTO-05-22-0190-R [DOI] [PubMed] [Google Scholar]
- Morcia, C. , Tumino, G. , Gasparo, G. , Ceresoli, C. , Fattorini, C. , Ghizzoni, R. , Carnevali, P. , & Terzi, V. (2020). Moving from qPCR to chip digital PCR assays for tracking of some Fusarium species causing Fusarium head blight in cereals. Microorganisms, 8(9), 1–12. 10.3390/microorganisms8091307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulunda, M. , Dzoma, B. , Nyirenda, M. , & Bakunzi, F. (2013). Mycotoxins occurrence in selected staple food in main markets from Lubumbashi, Democratic Republic of Congo. Journal of Food, Agriculture and Environment, 11(3–4), 51–54. [Google Scholar]
- Mwihia, E. W. , Lyche, J. L. , Mbuthia, P. G. , Ivanova, L. , Uhlig, S. , Gathumbi, J. K. , Maina, J. G. , Eshitera, E. E. , & Eriksen, G. S. (2020). Co‐occurrence and levels of mycotoxins in fish feeds in Kenya. Toxins, 12(10), 1–31. 10.3390/toxins12100627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagygyörgy, E. D. , Kovács, B. , Leiter, É. , Miskei, M. , Pócsi, I. , Hornok, L. , & Ádám, A. L. (2014). Toxicity of abiotic stressors to Fusarium species: Differences in hydrogen peroxide and fungicide tolerance. Acta Microbiologica et Immunologica Hungarica, 61(2), 189–208. 10.1556/AMicr.61.2014.2.9 [DOI] [PubMed] [Google Scholar]
- Nesic, K. , Resanovic, R. , & Nesic, V. (2008). Efficacy of mineral and organic adsorbent in alleviating harmful effects of zearalenone on pigs performance and health. Acta Veterinaria, 58(2–3), 211–219. 10.2298/AVB0803211N [DOI] [Google Scholar]
- Njeru, N. K. , Midega, C. A. O. , Muthomi, J. W. , Wagacha, J. M. , & Khan, Z. R. (2019). Influence of socio‐economic and agronomic factors on aflatoxin and fumonisin contamination of maize in western Kenya. Food Science & Nutrition, 7, 2291–2301. 10.1002/fsn3.1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njobeh, P. B. , Dutton, M. F. , Åberg, A. T. , & Haggblom, P. (2012). Estimation of multi‐mycotoxin contamination in south African compound feeds. Toxins, 4, 836–848. 10.3390/toxins4100836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njombwa, C. A. , Hamie, J. C. , & Banda, M. (2020). Occurrence of total aflatoxin and zearalenone in dairy cattle concentrate feeds in Malawi. Research Square, 14, 1–15. 10.21203/rs.3.rs-30295/v1 [DOI] [Google Scholar]
- Nkwe, D. O. , Taylor, J. E. , & Siame, B. A. (2005). Fungi, aflatoxins, fumonisin Bl and zearalenone contaminating sorghum‐based traditional malt, wort and beer in Botswana. Mycopathologia, 160, 177–186. 10.1007/s11046-005-6867-9 [DOI] [PubMed] [Google Scholar]
- Nothando, S. S. P. , Mbili, N. C. , & Yobo, K. S. (2021). Combination of yeast antagonists and Acibenzolar‐S‐methyl reduced the severity of Fusarium head blight of wheat incited by Fusarium graminearum sensu stricto. Plant Science Today, 9(1), 198–205. 10.14719/pst.1438 [DOI] [Google Scholar]
- Nuss, E. T. , & Tanumihardjo, S. A. (2010). Maize: A paramount staple crop in the context of global nutrition. Comprehensive Reviews in Food Science and Food Safety, 9(4), 417–436. 10.1111/j.1541-4337.2010.00117.x [DOI] [PubMed] [Google Scholar]
- Olopade, B. K. , Oranusi, S. U. , Nwinyi, O. C. , Gbashi, S. , & Njobeh, P. B. (2021). Occurrences of deoxynivalenol, zearalenone and some of their masked forms in selected cereals from Southwest Nigeria. Nutrition and Food Science Journal, 23, 24–29. 10.1016/j.nfs.2021.03.001 [DOI] [Google Scholar]
- Olopade, B. K. , Oranusi, S. U. , Nwinyi, O. C. , Njobeh, P. B. , & Lawal, I. A. (2019). Modification of montmorillonite clay with Cymbopogon citratus for the decontamination of zearalenone in millet. AIMS Agriculture and Food, 4(3), 643–657. 10.3934/agrfood.2019.3.643 [DOI] [Google Scholar]
- Olowe, O. , Olawuyi, O. , & Odebode, A. (2015). Response of maize genotypes to Fusarium verticillioides strains from two agro ecological zones in Southwest Nigeria. International Journal of Pure and Applied Sciences and Technology, 27(2), 77–86. [Google Scholar]
- Omori, A. M. , Ono, E. Y. S. , Bordini, J. G. , Hirozawa, M. T. , Fungaro, M. H. P. , & Ono, M. A. (2018). Detection of Fusarium verticillioides by PCR‐ELISA based on FUM21 gene. Food Microbiology, 73, 160–167. 10.1016/j.fm.2018.01.020 [DOI] [PubMed] [Google Scholar]
- Oyeka, C. A. , Amasiani, R. N. , & Ekwealor, C. C. (2019). Mycotoxins contamination of maize in Anambra state, Nigeria. Food Additives & Contaminants: Part B, 12, 280–288. 10.1080/19393210.2019.1661528 [DOI] [Google Scholar]
- Palacios‐Rojas, N. , McCulley, L. , Kaeppler, M. , Titcomb, T. J. , Gunaratna, N. S. , Lopez‐Ridaura, S. , & Tanumihardjo, S. A. (2020). Mining maize diversity and improving its nutritional aspects within agro‐food systems. Comprehensive Reviews in Food Science and Food Safety, 19, 1809–1834. 10.1111/1541-4337.12552 [DOI] [PubMed] [Google Scholar]
- Panzo, J. D. (2011). The incidence of fungi and their mycotoxins in angolan food and crops with particular reference to maize . University of Johannesburg.
- Pascale, M. , Logrieco, A. F. , Lippolis, V. , De Girolamo, A. , Cervellieri, S. , Lattanzio, V. M. T. , Ciasca, B. , Vega, A. , Reichel, M. , Graeber, M. , & Slettengren, K. (2022). Industrial – Scale cleaning solutions for the reduction of Fusarium toxins in maize. Toxins, 14, 728. 10.3390/toxins14110728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podgórska‐Kryszczuk, I. , Solarska, E. , & Kordowska‐wiater, M. (2022). Nivalenol and zearalenone by selected non‐conventional. Molecules, 27, 1578. 10.3390/molecules27051578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolska, G. , Bryła, M. , Sulek, A. , Waskiewicz, A. , Szymczyk, K. , & Jedrzejczak, R. (2017). Influence of the cultivar and nitrogen fertilisation level on the mycotoxin contamination in winter wheat. Quality Assurance & Safety of Crops and Food, 9(4), 451–461. 10.3920/QAS2016.1064 [DOI] [Google Scholar]
- Ponts, N. , Couedelo, L. , Pinson‐Gadais, L. , Verdal‐Bonnin, M. N. , Barreau, C. , & Richard‐Forget, F. (2009). Fusarium response to oxidative stress by H2O2 is trichothecene chemotype‐dependent. FEMS Microbiology Letters, 293(2), 255–262. 10.1111/j.1574-6968.2009.01521.x [DOI] [PubMed] [Google Scholar]
- Ponts, N. , Pinson‐Gadais, L. , Barreau, C. , Richard‐Forget, F. , & Ouellet, T. (2007). Exogenous H2O2 and catalase treatments interfere with tri genes expression in liquid cultures of Fusarium graminearum . Federation of European Biochemical Societies, 581(3), 443–447. 10.1016/j.febslet.2007.01.003 [DOI] [PubMed] [Google Scholar]
- Purar, B. , Djalovic, I. , Bekavac, G. , Grahovac, N. , Krstović, S. , Latković, D. , Janić Hajnal, E. , & Živančev, D. (2022). Changes in Fusarium and aspergillus mycotoxin content and different maize hybrids. Food, 11, 2877. 10.3390/foods11182877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi, L. , Li, Y. , Luo, X. , Wang, R. , Zheng, R. , Wang, L. , Li, Y. , Yang, D. , Fang, W. , & Chen, Z. (2016). Detoxification of zearalenone and ochratoxin A by ozone and quality evaluation of ozonised corn. Food Additives and Contaminants – Part A, 33(11), 1700–1710. 10.1080/19440049.2016.1232863 [DOI] [PubMed] [Google Scholar]
- Rai, A. , Das, M. , & Tripathi, A. (2020). Occurrence and toxicity of a fusarium mycotoxin, zearalenone. Critical Reviews in Food Science and Nutrition, 60(16), 2710–2729. 10.1080/10408398.2019.1655388 [DOI] [PubMed] [Google Scholar]
- Ranum, P. , & Pe, J. P. (2014). Global maize production, utilization, and consumption. Annals of the New York Academy of Sciences, 1312, 105–112. 10.1111/nyas.12396 [DOI] [PubMed] [Google Scholar]
- Ribeiro, D. F. , Faroni, L. R. D. A. , Pimentel, M. A. G. , Prates, L. H. F. , Heleno, F. F. , & De Alencar, E. R. (2021). Ozone as a fungicidal and detoxifying agent to maize contaminated with fumonisins. Ozone: Science and Engineering, 44(1), 38–49. 10.1080/01919512.2021.1924616 [DOI] [Google Scholar]
- Rodrigues, I. , Handl, J. , & Binder, E. M. (2011). Mycotoxin occurrence in commodities, feeds and feed ingredients sourced in the middle east and Africa. Food Additives and Contaminants: Part B Surveillance, 4(3), 168–179. 10.1080/19393210.2011.589034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolli, E. , Righetti, L. , Galaverna, G. , Suman, M. , Asta, C. D. , & Bruni, R. (2018). Zearalenone uptake and biotransformation in micropropagated Triticum durum Desf. plants: A xenobolomic approach. Journal of Agricultural and Food Chemistry, 66, 1523–1532. 10.1021/acs.jafc.7b04717 [DOI] [PubMed] [Google Scholar]
- Sahab, A. F. , Aly, S. , Hathout, A. S. , Ziedan, E. H. , & Sabry, B. A. (2014). Application of some plant essential oils to control Fusarium isolates associated with freshly harvested maize in Egypt. Journal of Essential Oil Bearing Plants, 17(6), 1146–1155. 10.1080/0972060X.2014.891447 [DOI] [Google Scholar]
- Scarpino, V. , Sulyok, M. , Krska, R. , Reyneri, A. , & Blandino, M. (2022). The role of nitrogen fertilization on the occurrence of regulated, modified and emerging mycotoxins and fungal metabolites in maize kernels. Toxins, 14, 448. 10.3390/toxins14070448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaarschmidt, S. , & Fauhl‐Hassek, C. (2021). The fate of mycotoxins during the primary food processing of maize. Food Control, 121, 107651. 10.1016/j.foodcont.2020.107651 [DOI] [PubMed] [Google Scholar]
- Schamann, A. , Schmidt‐Heydt, M. , & Geisen, R. (2022). Analysis of the competitiveness between a non‐aflatoxigenic and an aflatoxigenic Aspergillus flavus strain on maize kernels by droplet digital PCR. Mycotoxin Research, 38(1), 27–36. 10.1007/s12550-021-00447-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwake‐Anduschus, C. , Proske, M. , Sciurba, E. , Muenzing, K. , Koch, M. , & Maul, R. (2015). Distribution of deoxynivalenol, zearalenone, and their respective modified analogues in milling fractions of naturally contaminated wheat grains. World Mycotoxin Journal, 8(4), 433–443. 10.3920/WMJ2014.1818 [DOI] [Google Scholar]
- Sebaei, A. S. , Sobhy, H. M. , Fouzy, A. S. M. , & Hussain, O. A. (2020). Occurrence of zearalenone in grains and its reduction by gamma radiation. International Journal of Environmental Analytical Chemistry, 102(11), 2503–2511. 10.1080/03067319.2020.1756282 [DOI] [Google Scholar]
- Shephard, G. S. (2008). Impact of mycotoxins on human health in developing countries. Food Additives and Contaminants – Part A, 25(2), 146–151. 10.1080/02652030701567442 [DOI] [PubMed] [Google Scholar]
- Shephard, G. S. , Burger, H. , Gambacorta, L. , Yun, Y. , Krska, R. , Rheeder, J. P. , Solfrizzo, M. , Srey, C. , Sulyok, M. , Visconti, A. , Warth, B. , & Van Der Westhuizen, L. (2013). Multiple mycotoxin exposure determined by urinary biomarkers in rural subsistence farmers in the former Transkei, South Africa. Food and Chemical Toxicology, 62, 217–225. 10.1016/j.fct.2013.08.040 [DOI] [PubMed] [Google Scholar]
- Shoaib, A. , Meraj, S. , Nafisa , Khan, K. A. , & Javaid, M. A. (2018). Influence of salinity and Fusarium oxysporum as the stress factors on morpho‐physiological and yield attributes in onion. Physiology and Molecular Biology of Plants, 24(6), 1093–1101. 10.1007/s12298-018-0570-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shude, S. P. N. , Mbili, N. C. , & Yobo, K. S. (2021). Epiphytic yeasts as potential antagonists against Fusarium head blight of wheat (Triticum aestivum L.) caused by Fusarium graminearum sensu stricto. Journal of the Saudi Society of Agricultural Sciences, 21, 404–411. 10.1016/j.jssas.2021.11.001 [DOI] [Google Scholar]
- Shude, S. P. N. , Mbili, N. C. , & Yobo, K. S. (2022). Combination of yeast antagonists and Acibenzolar‐S‐Methyl reduced the severity of Fusarium head blight of wheat incited by Fusarium graminearum sensu stricto. Plant Science Today, 9(1), 198–205. 10.14719/pst.1438 [DOI] [Google Scholar]
- Small, I. M. , Flett, B. C. , Marasas, W. F. O. , Mcleod, A. , Stander, M. A. , & Viljoen, A. (2012). Resistance in maize inbred lines to Fusarium verticillioides and fumonisin accumulation in South Africa. Plant Disease, 96(6), 881–888. 10.1094/PDIS-08-11-0695 [DOI] [PubMed] [Google Scholar]
- Suleiman, R. A. , Rosentrater, K. A. , & Chove, B. (2017). Understanding postharvest practices, knowledge, and actual mycotoxin levels in maize in three agro‐ ecological zones in Tanzania. Journal of Stored Products and Postharvest Research, 8(7), 73–84. 10.5897/JSPPR2017.0243 [DOI] [Google Scholar]
- Sulyok, M. , Beed, F. , Boni, S. , Abass, A. , Mukunzi, A. , & Krska, R. (2015). Quantitation of multiple mycotoxins and cyanogenic glucosides in cassava samples from Tanzania and Rwanda by an LC‐MS/MS‐based multi‐toxin method. Food Additives and Contaminants – Part A Chemistry, Analysis, Control, Exposure and Risk Assessment, 32(4), 488–502. 10.1080/19440049.2014.975752 [DOI] [PubMed] [Google Scholar]
- Summerell, B. A. (2019). Resolving Fusarium: Current status of the genus. Annual Review of Microbiology, 57, 323–339. [DOI] [PubMed] [Google Scholar]
- Taffarel, L. E. , Mesquita, E. E. , Castagnara, D. D. , Costa, P. B. , Abbado Neres, M. , Bottoni Horn, M. , Rabellodeoliveira, P. S. , & Meinerz, C. C. (2013). Dehydration curve, fungi and mycotoxins in tifton 85 hay dehydrated in the field and in shed. Revista Brasileira de Zootecnia, 42(6), 395–403. 10.1590/S1516-35982013000600003 [DOI] [Google Scholar]
- Tang, E. N. , Ndindeng, S. A. , Bigoga, J. , Traore, K. , Silue, D. , & Futakuchi, K. (2019). Mycotoxin concentrations in rice from three climatic locations in Africa as affected by grain quality, production site, and storage duration. Food Science & Nutrition, 7(4), 1274–1287. 10.1002/fsn3.959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayebeh, F. , Nazarian, S. , Mirhosseini, S. A. , & Amani, J. (2017). Novel PCR‐ELISA technique as a good substitute in molecular assay. Journal Of Applied Biotechnology Reports, 4(2), 567–572. [Google Scholar]
- Tebele, S. M. , Gbashi, S. , Adebo, O. , Changwa, R. , Naidu, K. , & Njobeh, P. B. (2020). Quantification of multi‐mycotoxin in cereals (maize, maize porridge, sorghum and wheat) from Limpopo province of South Africa. Food Additives & Contaminants: Part A, 1–17, 1922–1938. 10.1080/19440049.2020.1808715 [DOI] [PubMed] [Google Scholar]
- Tembo, E. , Minnaar‐Ontong, A. , Menkir, A. , Marais, G. , Magorokosho, C. , & Labuschagne, M. T. (2022). Inheritance of resistance to Fusarium verticillioides ear rot in maize inbred lines of southern, west and Central Africa origin. Crop Science, 1–16, 1818–1833. 10.1002/csc2.20776 [DOI] [Google Scholar]
- Tesfamariam, K. , Argaw, A. , Hanley‐cook, G. T. , Gebreyesus, S. H. , Kolsteren, P. , Belachew, T. , Van de Velde, M. , De Saeger, S. , De Boevre, M. , & Lachat, C. (2022). Multiple mycotoxin exposure during pregnancy and risks of adverse birth outcomes: A prospective cohort study in rural Ethiopia. Environment International, 160, 107052. 10.1016/j.envint.2021.107052 [DOI] [PubMed] [Google Scholar]
- Tsehaye, H. , Brurberg, M. B. , Sundheim, L. , Assefa, D. , & Tronsmo, A. (2016). Natural occurrence of Fusarium species and fumonisin on maize grains in Ethiopia. European Journal of Plant Pathology, 147(1), 141–155. 10.1007/s10658-016-0987-6 [DOI] [Google Scholar]
- van Deventer, M. M. , Pretorius, B. , & Schönfeldt, H. C. (2021). A preliminary study on mycotoxin contamination in red meat from registered abattoirs in South Africa. Mycotoxin Research, 37(1), 105–108. 10.1007/s12550-020-00420-w [DOI] [PubMed] [Google Scholar]
- Vaughan, M. M. , Huffaker, A. , Schmelz, E. A. , Dafoe, N. J. , Christensen, S. , Sims, J. , Martins, V. F. , Swerbilow, J. , Romero, M. , Alborn, H. T. , Allen, L. H. , & Teal, P. E. A. (2014). Effects of elevated CO2 on maize defence against mycotoxigenic Fusarium verticillioides . Plant, Cell and Environment, 37(12), 2691–2706. 10.1111/pce.12337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velluti, A. , Sanchis, V. , Ramos, A. J. , Turon, C. , & Marín, S. (2004). Impact of essential oils on growth rate, zearalenone, and deoxynivalenol production by Fusarium graminearum under different temperature and water activity conditions in maize grain. Journal of Applied Microbiology, 96(4), 716–724. 10.1111/j.1365-2672.2004.02212.x [DOI] [PubMed] [Google Scholar]
- Visser, M. E. , Schoonees, A. , Ezekiel, C. N. , Randall, N. P. , & Naude, C. E. (2020). Agricultural and nutritional education interventions for reducing aflatoxin exposure to improve infant and child growth in low‐ and middle‐income countries. Cochrane Database of Systematic Reviews, 4, CD013376. 10.1002/14651858.CD013376.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S. S. , Cui, H. , Chen, M. Z. , Li, L. , Wu, Y. , & Wang, X. (2021). Simultaneous quantitation of 3ADON and 15ADON chemotypes of DON‐producing Fusarium species in Chinese wheat based on duplex droplet digital PCR assay. Journal of Microbiological Methods, 190, 106319. 10.1016/j.mimet.2021.106319 [DOI] [PubMed] [Google Scholar]
- Ward, M. (2018). China – Peoples Republic of China Releases Standard for Maximum Levels of Mycotoxins in Food. Global Agricultural Information Network (GAIN), 1–10.
- Warth, B. , Parich, A. , Atehnkeng, J. , Bandyopadhyay, R. , Schuhmacher, R. , Sulyok, M. , & Krska, R. (2012). Quantitation of mycotoxins in food and feed from Burkina Faso and Mozambique using a modern LC‐MS/MS multitoxin method. Journal of Agricultural and Food Chemistry, 60, 9352–9363. [DOI] [PubMed] [Google Scholar]
- Wigmann, É. F. , Meyer, K. , Cendoya, E. , Maul, R. , Vogel, R. F. , & Niessen, L. (2020). A loop‐mediated isothermal amplification (LAMP) based assay for the rapid and sensitive group‐specific detection of fumonisin producing Fusarium spp. International Journal of Food Microbiology, 325, 108627. 10.1016/j.ijfoodmicro.2020.108627 [DOI] [PubMed] [Google Scholar]
- Wingfield, B. D. , Steenkamp, E. T. , Santana, Q. C. , Coetzee, M. P. A. , Bam, S. , Barnes, I. , Beukes, C. W. , Chan, W. Y. , De Vos, L. , Fourie, G. , Friend, M. , Gordon, T. R. , Herron, D. A. , Holt, C. , Korf, I. , Kvas, M. , Martin, S. H. , Mlonyeni, X. O. , Naidoo, K. , … Wingfield, M. J. (2012). First fungal genome sequence from Africa: A preliminary analysis. South African Journal of Science, 108(1/2), 1–9. 10.4102/sajs.v108i1/2.537 [DOI] [Google Scholar]
- Wokorach, G. , Landschoot, S. , Audenaert, K. , Echodu, R. , & Haesaert, G. (2021). Genetic characterization of fungal biodiversity in storage grains: Towards enhancing food safety in northern Uganda. Microorganisms, 9(2), 1–18. 10.3390/microorganisms9020383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, K. , Li, K. , Pan, L. , Luo, X. , Xing, J. , Wang, J. , Wang, L. , Wang, R. , Zhai, Y. , & Chen, Z. (2020). Effect of ozone and electron beam irradiation on degradation of zearalenone and ochratoxin A. Toxins, 12(138), 1–10. 10.3390/toxins12020138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi, C. , Kaul, H. P. , Schwadorf, K. K. , & Aufhammer, W. (2001). Head blight (Fusarium graminearum) and deoxynivalenol concentration in winter wheat as affected by pre‐crop, soil tillage and nitrogen fertilization. Journal of Plant Diseases and Protection, 108(3), 217–230. https://www.jstor.org/stable/45154853 [Google Scholar]
- Zaied, C. , Zouaoui, N. , Bacha, H. , & Abid, S. (2012). Natural occurrence of zearalenone in Tunisian wheat grains. Food Control, 25(2), 773–777. 10.1016/j.foodcont.2011.12.012 [DOI] [Google Scholar]
- Zhao, R. , Sun, S. , & Xie, Y. (2019). Removal of zearalenone in corn oil by microwave‐induced activated carbon. Journal of Henan University of Technology (Natural Science Edition), 40(2), 34–40. [Google Scholar]
- Zheng, D. , Zhang, S. , Zhou, X. , Wang, C. , Xiang, P. , Zheng, Q. , & Xu, J. R. (2012). The FgHOG1 pathway regulates hyphal growth, stress responses, and plant infection in Fusarium graminearum . PLoS One, 7(11), 1–12. 10.1371/journal.pone.0049495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, Z. , Niu, L. , Yang, W. , Chen, Y. , Huang, Y. , & Li, C. (2023). Degradation of zearalenone by dielectric barrier discharge cold plasma and its effect on maize quality. Food, 12, 1129. 10.3390/foods12061129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinedine, A. , & Mañes, J. (2009). Occurrence and legislation of mycotoxins in food and feed from Morocco. Food Control, 20(4), 334–344. 10.1016/j.foodcont.2008.07.002 [DOI] [Google Scholar]
- Zuluaga‐Calderón, B. , González, H. H. L. , Alzamora, S. M. , & Coronel, M. B. (2023). Multi‐step ozone treatments of malting barley: Effect on the incidence of Fusarium graminearum and grain germination parameters. Innovative Food Science and Emerging Technologies, 83, 103219. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data presented in this study are contained in the article.


