Abstract
Progressive multifocal leukoencephalopathy (PML) is a fatal demyelinating disease caused by cytolytic destruction of oligodendrocytes, the myelin-producing cells of the central nervous system, by the human neurotropic JC virus (JCV). The early protein of JCV, T antigen, which is produced at the early stage of infection, is important for orchestrating the events leading to viral lytic infection and cytolytic destruction of oligodendrocytes. Results from transgenic mouse studies, however, have revealed that, in the absence of lytic infection, this protein can induce brain hypomyelination and suppression of myelin gene expression. Since expression of the gene encoding myelin basic protein, the major component of myelin, can be regulated by a DNA-binding transcription factor, MEF-1/Purα, (Purα), we have examined the level of this protein in transgenic mouse brains. Results from immunoprecipitation and Western blots showed that while there was no drastic decrease in the level of MEF-1/Purα in transgenic mouse brains, JCV T antigen was found in a complex with MEF-1/Purα. Immunohistological studies revealed abnormal oligodendrocytes in white matter, where MEF-1/Purα and T antigen were detected. Furthermore, immunogold electron microscopic studies revealed that Purα and T antigen are colocalized in the nucleus of the oligodendrocytes and in hippocampal neurons. Interestingly, results from cell culture studies revealed that incubation of oligodendrocytes with JCV led to a drastic decrease in the level of MEF-1/Purα protein. These observations provide insight into the molecular pathogenesis of PML and support a model for a dual effect of JCV on inducing hypomyelination by (i) affecting myelin gene expression via interaction of JCV T antigen and the myelin gene transcription factor, MEF-1/Purα, and (ii) causing a decline in the level of the host regulatory proteins, including MEF-1/Purα, which are involved in myelin gene expression.
Replication of JC virus (JCV) in oligodendrocytes, the myelin-producing cells of the central nervous system (CNS), results in the development of the human demyelinating disease progressive multifocal leukoencephalopathy (PML) (3). JCV is a polyomavirus which, like other members of this family, possesses a circular genome of double-stranded DNA within an icosahedral capsid (21). The prototype strain of JCV contains 5,130 bp which can be functionally divided into three regions: an early coding region, a late coding region, and a regulatory (noncoding) region (8). The regulatory region, which contains the promoters-enhancers for early and late gene transcription, as well as the origin of DNA replication, is located between the early and the late coding regions. The viral early gene encodes the viral regulatory proteins, large T antigen and small t antigen, whereas the late genes encode the structural capsid proteins, VP1, VP2, and VP3, as well as agnoprotein (20). The viral lytic cycle begins with expression of the viral early protein, T antigen, which occurs before replication of the viral DNA. T antigen is a multifunctional protein which interacts with several host regulatory proteins and, by manipulating host gene expression and/or function, orchestrates subsequent steps of the viral life cycle including viral DNA replication (4) and activation of late gene transcription (17). The products of the late genes, the capsid proteins, accumulate in the nucleus and associate with the replicated viral DNA-forming virions which, in turn, lyse the host oligodendrocytes. Thus, T antigen acts as the central regulator of the viral lytic cycle. Further insights into the mechanism of JCV-induced oligodendrocyte dysfunction have been obtained from transgenic mice containing the JCV early genome under the control of the JCV early promoter. Some transgenic mouse lines display a characteristic shaking (23) similar to the phenotype in myelin-deficient jimpy and quaking mice (13, 22, 24). Neuropathological analysis has shown hypomyelination in the CNS, high levels of JCV large T antigen in oligodendrocytes, immature oligodendrocytes with abnormal morphology, and hyperproliferating astrocytes with abnormal morphology (25). These observations demonstrate that dysmyelination in the CNS of transgenic animals, and perhaps demyelination in PML patients, is related at least partially to CNS T-antigen expression (7). Expression of the myelin-specific genes including that encoding myelin basic protein (MBP) in the brains of these transgenic mice is decreased at the mRNA level, suggesting that T antigen may impair transcriptional regulation of the MBP gene promoter (12). Similar to that of other cellular genes, transcription of the MBP gene is regulated by upstream promoter sequences which have the ability to interact with specific DNA-binding transcription factors (5, 18). Previous studies from our laboratory have identified a cellular protein, named MEF-1, from mouse brain nuclear extract that binds to a specific GC-rich region of the MBP gene promoter and stimulates its expression both in vivo and in vitro (10, 12). Results from amino acid analysis of MEF-1 along with several of its characteristics have led us to believe that this protein is a single-stranded DNA-binding protein named Purα (1, 2). MEF-1, hereafter designated MEF-1/Purα, interacts preferentially with single-stranded DNA containing the GGN motif. This protein has also been shown to interact with several viral and cellular proteins including the human immunodeficiency virus type 1 Tat protein (16), the JCV early protein T antigen (9), and the product of the retinoblastoma gene, Rb (14). Since protein-protein interaction may play a major role in the activity of this regulatory protein, in this study we have examined the level of expression and the association of MEF-1/Purα with JCV T antigen in brains of dysmyelinated mice transgenically expressing T antigen under the control of the JCV early promoter. Our results indicate that MEF-1/Purα in extracts from transgenic mouse brains is associated with JCV T antigen. Interestingly, infection of oligodendrocytes with JCV in cell culture results in the inhibition of MEF-1/Purα gene expression, suggesting that at least two distinct mechanisms which involve interaction of the viral early protein with MEF-1/Purα and suppression of MEF-1/Purα expression may participate in JCV-induced hypomyelination of the CNS.
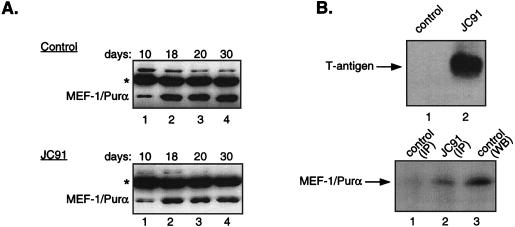
MEF-1/Purα is a DNA-binding transcription factor first purified from mouse brains based on its ability to bind to the MBP gene promoter sequence and enhance its activity in oligodendrocytic cells (10, 12). To assess the levels of MEF-1/Purα in the developing JCV transgenic mouse, JC91, which experiences CNS hypomyelination due to JCV T-antigen expression (11, 23), and to compare Purα levels with those from control littermates, protein extracts from mouse brains at various stages after birth were prepared and examined by immunoprecipitation-Western blot analysis. As illustrated in Fig. 1A (top), the level of MEF-1/Purα was low in control mouse brain tissue at 10 days of age, increased at day 18, and remained fairly constant thereafter. The pattern of MEF-1/Purα expression in the JC91 mouse brain during the course of brain development and its levels were similar to those from control animals (Fig. 1A, bottom). The levels of MEF-1/Purα in JC91, however, were marginally lower than those seen in control animals. These differences, however, may not account for the observed decrease in MBP gene expression. Since the activity of transcription factors may also be dictated by their association with other proteins, we sought to determine if MEF-1/Purα, which is produced in transgenic mouse brain, is in a complex with JCV T antigen. Toward this end, protein extracts prepared from the brains of adult JC91 mice and the control age-matched littermates were prepared and used in coimmunoprecipitation-Western blot assays. Figure 1B (top) shows the presence of JCV T antigen in JC91 mouse brain, but not in the control animals, as determined by immunoprecipitation with antibody to T antigen followed by Western blotting with the same antibody. Next, the immunocomplexes precipitated upon incubation of the extracts from control and transgenic mouse brains by anti-T-antigen antibody were analyzed by Western blotting with anti-Purα antibody. A band corresponding to MEF-1/Purα was detected in immunocomplexes obtained from JC91 but not control animals (Fig. 1B, bottom, compare lane 1 to lane 2), suggesting that MEF-1/Purα and T antigen are in a complex with each other in the protein extracts from the brains of transgenic animals. A band corresponding to MEF-1/Purα was detected when brain extract of control animals was incubated with anti-Purα antibody and analyzed by Western blotting (Fig. 1B, bottom, lane 3). These observations corroborate our earlier results, suggesting that MEF-1/Purα and JCV T antigen interact with one another in both in vitro and in vivo systems (9).
FIG. 1.
Level of MEF-1/Purα in brains of JC91 and control age-matched mice during development. (A) Nuclear extracts from mouse brains at different stages of development (as indicated above the panels) were prepared according to the method described by Dignam et al. (6). Approximately 100 μg of extract from the controls (top) and JC91 (bottom) was incubated with 1 μg of monoclonal 9C12 anti-Purα antibody, and the immunocomplexes were analyzed by Western blotting with the anti-Purα antibody as described previously (26). The position of the 39-kDa MEF-1/Purα protein is shown. The asterisks depict the position of the immunoglobulin G heavy chain. (B) (Top) Approximately 250 μg of brain nuclear extract from control animals (lane 1) or the JC91 transgenic animals (lane 2) was reacted with monoclonal antibody 2000 against JCV T antigen (kindly provided by R. Frisque, Pennsylvania State University, State College), and the immunocomplexes were analyzed by Western blotting with the same antibody. The position of T antigen produced in the brains of JC91 is shown. (Bottom) Western blot analysis of the immunocomplex pulled down by anti-T-antigen antibody from control (lane 1) and JC91 (lane 2) with anti-Purα antibody. In lane 3, extract from control mice was immunoprecipitated (IP) with anti-Purα antibody and analyzed by Western blotting (WB) with anti-Purα antibody.
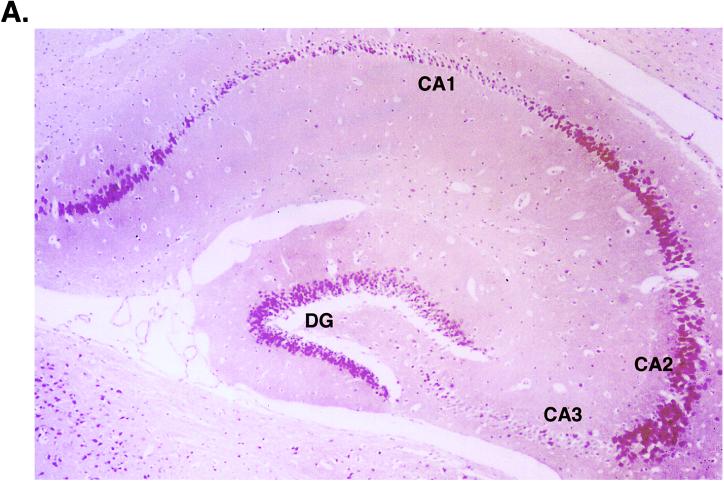
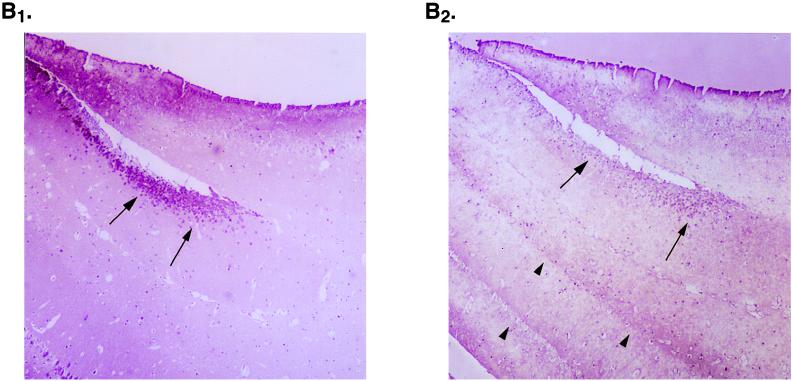
To compare levels of expression of the transgene and MEF-1/Purα anatomically, we performed immunohistochemistry with monoclonal antibodies to T antigen and MEF-1/Purα. As illustrated in Fig. 2A, JCV T antigen was detected quite prominently in neurons, both in the dentate gyrus and in the pyramidal layer. Figure 2 also illustrates, at medium power, T-antigen-positive neurons in the hippocampus from JC91 mouse brain (B1) and the parallel staining of the same cells with anti-MEF-1/Purα antibody (B2). As shown in this figure, MEF-1/Purα-positive staining overlaps with T antigen (compare panels B1 and B2). Also, it was noted that striations seen in white matter stained with MEF-1/Purα (see arrowhead in Fig. 2B2) are absent with T-antigen staining. Examination at high power of the white matter in close proximity to the hippocampus where oligodendrocytes are located for expression of T antigen and MEF-1/Purα by immunostaining revealed production of T antigen in nuclei of oligodendrocytes. Figure 2C1 demonstrates staining of JC91 brain sections with anti-T-antigen antibody. Figure 2 also shows staining of similar regions of the control age-matched mouse brain with anti-T antigen (Fig. 2C1, inset). As expected, no T-antigen-positive cells were detected in the control samples. Immunostaining of the JC91 brain with anti-MEF-1/Purα antibody showed nuclear and cytoplasmic staining of oligodendrocytes. As before, striation in the white matter along with oligodendrocytes was observed in JC91 brain staining (Fig. 2C2). Immunostaining of the control age-matched tissue revealed less nuclear staining of oligodendrocytes (Fig. 2C2, inset).
FIG. 2.
Histological analysis of JC91 mouse brain and production of MEF-1/Purα and T antigen. (A) View of the hippocampus of JC91 mice illustrating positive staining for T antigen in the pyramidal-layer neurons (CA1, CA2, and CA3) and in granule neurons of the dentate gyrus (DG). While strong staining is seen in CA2, both CA1 and CA3 exhibit weak staining with anti-T-antigen antibody. (B) MEF-1/Purα localization forms striations in white matter of JC91 mice. Staining of mouse hippocampus for large T antigen (B1) and MEF-1/Purα (B2) at medium power (20×) visualizes neurons (arrows). Note striations in white matter with MEF-1/Purα (arrowheads) that are absent with T-antigen staining. (C) Staining of JC91 mouse brain sections with antibodies for large T antigen (Lee Biomolecular, San Diego, Calif.) (C1) or MEF-1/Purα (monoclonal antibody 9C12) at high power (40×) (C2). A section of white matter near the hippocampus is shown. Staining was done with Vecta Red, and counterstaining was done with hematoxylin. In panel C1, the arrows point to the oligodendrocytes in the white matter, which are atypical and reduced in number in comparison to those in normal mice and show light nuclear staining for T antigen. Control sections stained for T antigen show no nuclear positivity (inset). In panel C2, MEF-1/Purα staining is prominent in the atypical oligodendrocytes, forming a red striation in the white matter. A control section also stained for MEF-1/Purα (inset) shows less nuclear staining.
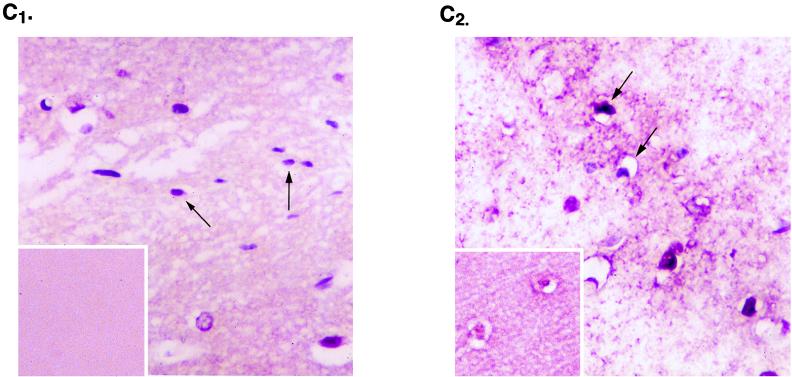
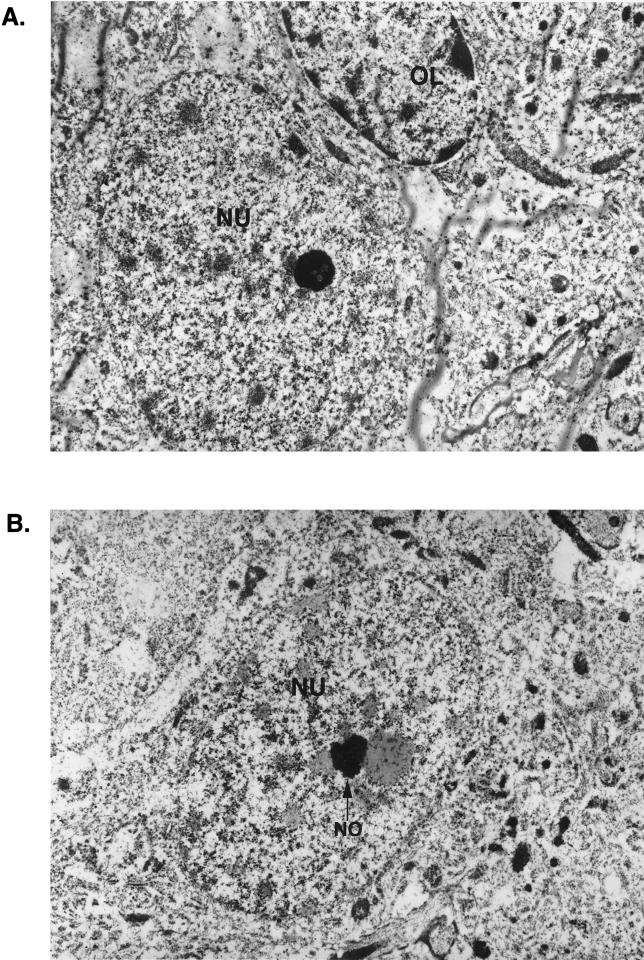
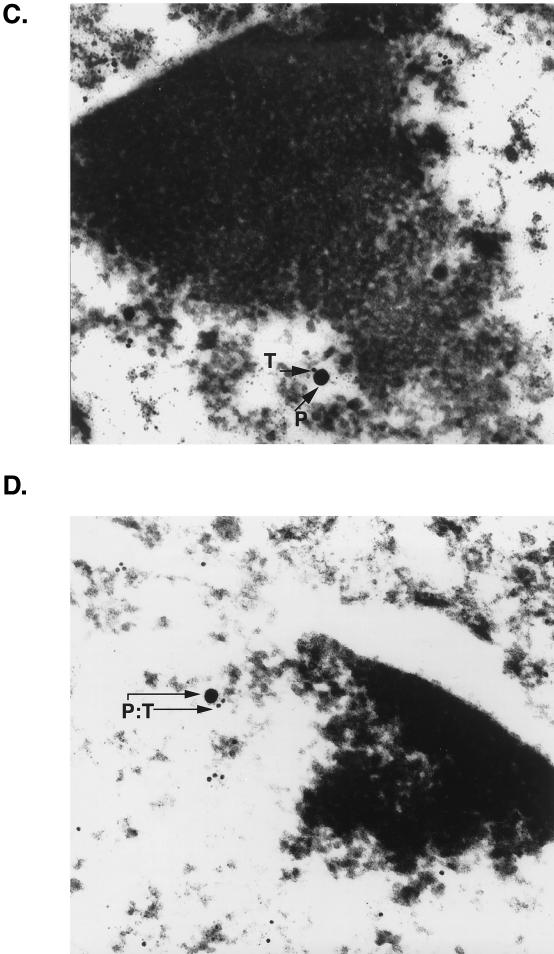
To investigate the association of MEF-1/Purα and T antigen at the subcellular level, we performed immunogold electron microscopy. Brains were perfusion fixed in 3.2% paraformaldehyde in phosphate-buffered saline and embedded in Unicryl. Ultrathin sections were cut, placed on Formvar-coated nickel grids, and treated sequentially for colocalization of T antigen and MEF-1/Purα. Tissues were initially stained with rabbit polyclonal anti-simian virus 40 T antigen, which recognizes JCV T antigen, followed by goat anti-rabbit antibody coupled to 10-nm gold beads. Sections were then incubated with anti-Purα mouse monoclonal antibody followed by protein A coupled to 30-nm gold beads. The grids were examined with a JEOL JEM100CX electron microscope. Diameters of the gold beads indicate the magnification. For each pair of primary antibodies, the antibodies were tested alone and in both forward and reverse order to determine whether there was a difference in labeling patterns and efficiencies of labeling. Under the conditions described, no such differences were noted. Additional controls, employing either no first antibody or a glutathione S-transferase–Purα blocking agent, were also performed to ensure specificity and the lack of cross-reactivity of the antibodies. The data were essentially negative (data not shown). Figure 3 illustrates, in a view with lower magnification, an oligodendrocyte (OL) with dense perinuclear chromatin positioned adjacent to a neuron (NU) (Fig. 3A). Also in Fig. 3, a low-magnification view of a nucleus of a large neuron with a single nucleolus is shown (Fig. 3B). Evaluation of the printed photographs for colocalization of MEF-1/Purα and T antigen revealed that many T-antigen (T) and MEF-1/Purα (P) molecules in the nuclei of oligodendrocytes are adjacent to dense chromatin at the nuclear membrane (Fig. 3C and D). In many instances, the different molecules, MEF-1/Purα (P) and T antigen (T), are detected within 10 nm of each other (PT). These data indicate that JCV T antigen is juxtaposed with MEF-1/Purα in the nuclei of oligodendrocytes of these transgenic mice.
FIG. 3.
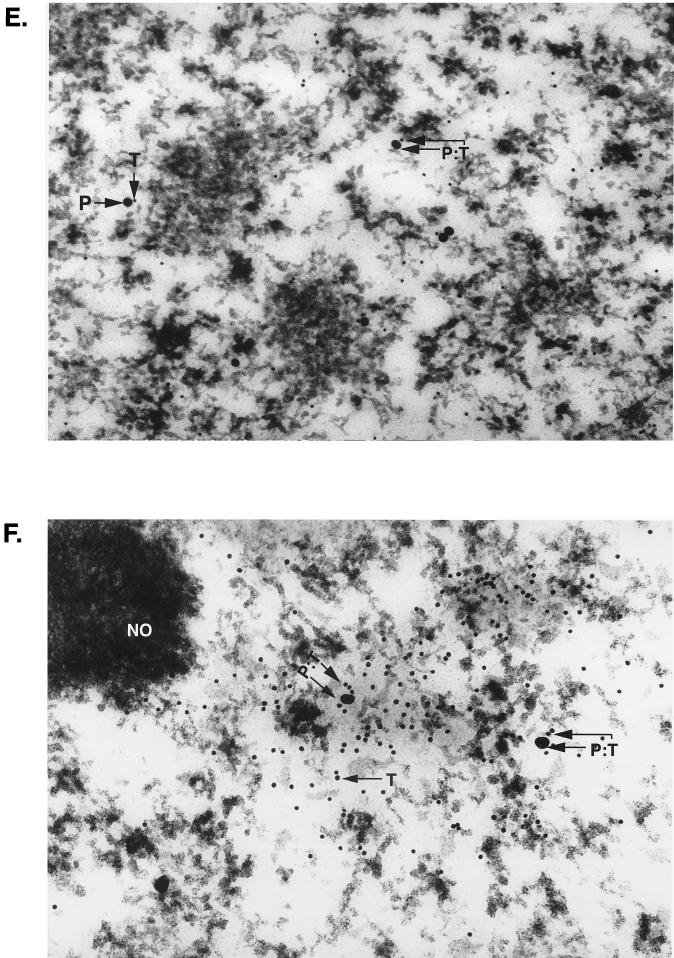
Detection of T antigen and MEF-1/Purα in both oligodendrocytes and neurons of the hippocampus of a JC91 transgenic mouse. (A) Low-magnification view of an oligodendrocyte (OL) with dense clumps of nuclear chromatin which is seen adjacent to a neuron (NU) to the lower left of it. Gold particles are barely visible in this view. (B) Low-magnification view of a large neuron with a nucleolus (NO). (C and D) Colocalization of MEF-1/Purα and T antigen in the nucleus of an oligodendrocyte of a JC91 mouse by immunogold electron microscopy. Two views of the nucleus of a single oligodendrocyte in the vicinity of the hippocampus are shown. Many T-antigen (T) and MEF-1/Purα (P) molecules are detected adjacent to dense chromatin at the nuclear membrane. Large (30-nm) gold beads represent MEF-1/Purα (P), whereas the smaller (10-nm) beads indicate T antigen (T). In many areas, the different beads are detected within 10 nm of one another (P:T). (E) High-magnification view of neuronal nucleus illustrating several T-antigen (10-nm beads) and MEF-1/Purα (30-nm beads) molecules which can be detected within 1 cm of one another. (F) High concentration of T antigen in diffuse chromatin adjacent to the nucleolus. A high concentration of T antigen (10-nm beads) is visualized in association with strands of diffuse chromatin just external to the nucleolus (NO) of an atypical neuron in white matter. Homogeneous chromatin adjacent to the nucleolus may represent a pathological effect of T antigen. Again, several T-antigen molecules are detected in strand-like structures associated with MEF-1/Purα.
Examination of hippocampal neurons by immunogold electron microscopy demonstrated T antigen in association with strands of diffuse chromatin just external to the nucleolus (Fig. 3E and F). In addition, several T-antigen molecules were detected in strand-like structures in juxtaposition with MEF-1/Purα. It should be noted that, under similar conditions, immunogold staining of control age-matched mouse brain with anti-T-antigen antibody showed no specific signal to indicate that the T-antigen immunogold staining of the transgenic animal brain is specific. Furthermore, in corroboration with immunohistochemical data, particles corresponding to Purα in control and transgenic samples were detected in the cytoplasm and nuclei (data not shown). These observations suggest that the JCV promoter can also be activated in neurons and that activation of the viral early promoter can result in expression of the viral early protein, T antigen, in these cells. Although there has been no report on neuronal expression of the JCV early genome in the demyelinating lesions from brains of PML patients, where active replication of the virus destroys oligodendrocytes, one may question whether, at the earlier stages of the disease, when demyelination has not been fully developed, the JCV early gene is expressed in the neurons. Currently, studies are in progress to assess the level of JCV early gene expression in the normal and less affected areas of brain from PML patients.
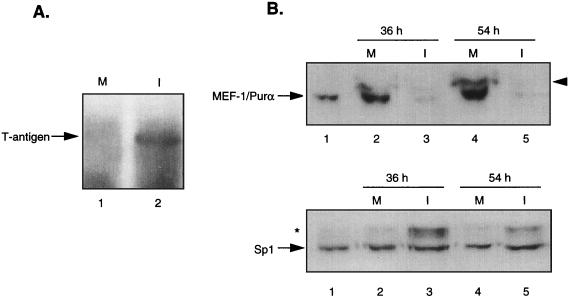
The association of MEF-1/Purα with JCV T antigen might also serve to modulate the level of JCV transcription and replication during the course of the lytic cycle. To examine the level of MEF-1/Purα interaction with JCV T antigen during viral infection, oligodendrocytic cells were prepared from CG-4 cells (19) and incubated with the Mad-1 strain of JCV according to the procedure described earlier (15). At 36 and 54 h postinfection, protein extracts were prepared and analyzed for expression of T antigen. As shown in Fig. 4A, a band corresponding to T antigen was detected in extract from JCV-infected (lane 2) but not mock-infected (lane 1) cells. Interestingly, examination of MEF-1/Purα expression in mock- and JCV-infected cells revealed that infection of the cells with JCV results in a substantial decrease in the level of MEF-1/Purα (Fig. 4B, compare lanes 2 and 4 with lanes 3 and 5, respectively). Figure 4B also shows the level of MEF-1/Purα in the control oligodendrocytes (lane 1). The observed inhibition of MEF-1/Purα expression in the infected cells is not a general event, as the level of Sp1, a GC-rich DNA-binding transcription factor, was not decreased (Fig. 4B, bottom). In fact, we noticed the appearance of a larger band in the infected cells which may represent a modified form of Sp1 (see asterisk for lanes 3 and 5). The absence of MEF-1/Purα in the infected oligodendrocytes may not be due to the expression of T antigen in these cells, since in earlier studies we demonstrated the presence of a significant amount of MEF-1/Purα in glial cells which express JCV T antigen (4). Interestingly, MEF-1/Purα was associated with T antigen in these cells. Thus, it is likely that the observed inhibition of MEF-1/Purα in JCV-infected oligodendrocytes is dependent on events involved in viral infection and not on T-antigen production.
FIG. 4.
The level of MEF-1/Purα during the infection of oligodendrocytes with JCV. A permanent cell line, CG-4, was induced to differentiate toward oligodendrocytes according to the method established previously (19, 27). Approximately 105 cells were infected with the Mad-4 strain of JCV (hemagglutinin units ≅ 100), and after 36 and 54 h, cells were harvested and protein extracts were prepared. (A) Approximately 200 μg of protein from mock-infected (M) and JCV-infected (I) cells at 36 h postinfection was analyzed by Western blotting with anti-T-antigen antibody. Low, but detectable, levels of T antigen were observed in the lane corresponding to the infected extracts. (B) Approximately 100 μg of protein extracts from mock (M)- and JCV (I)-infected cells harvested at 36 and 54 h was analyzed by Western blotting with anti-Purα antibody (top) or anti-Sp1 antibody (bottom). The arrow in the top panel points to the position of the 39-kDa MEF-1/Purα protein, and the arrowhead points to the nonspecific background signals which appeared on top of the band corresponding to MEF-1/Purα. In the bottom panel, the arrow points to Sp1. For lane 1, an extract from growing oligodendrocytic CG-4 cells was analyzed for production of MEF-1/Purα and Sp1.
The observed suppression of MEF-1/Purα expression during JCV lytic infection of oligodendrocytes, which is in contrast to the findings for transgenic animals expressing JCV T antigen (shown in Fig. 1A), suggests that at least two distinct events may lead to JCV-induced hypomyelination of the CNS. Prior to active viral infection, expression of JCV T antigen and its association with MEF-1/Purα may decrease the level of MBP gene expression and myelination. We have previously demonstrated that association of MEF-1/Purα with T antigen also decreases the ability of T antigen to transactivate the JCV late promoter, an event which is important for a productive JCV lytic cycle. Thus, one can envision a model in which, at the earlier stage of the disease when the level of T antigen is low, interaction of T antigen with MEF-1/Purα may prevent MEF-1/Purα from exerting its regulatory activity in oligodendrocytes. This interaction, which also blocks T antigen from stimulating the late stage of the viral infection cycle, prolongs the early stage and permits continuous production of T antigen in the affected cells. As the level of T antigen is increased, the unoccupied protein eventually guides the virus through the lytic cycle, causing cytolytic destruction of oligodendrocytes. Consistent with a number of animal viruses, the lytic infection of JCV seems to be associated with inhibited expression of some cellular genes including MEF-1/Purα. While JC91 transgenic mice are an important tool for studying the interaction of viral and host factors in the CNS, they do not provide a perfect animal model for the human demyelinating disease, PML. A complete understanding of PML progression in the human CNS must await studies with systems which allow virus expression in a more natural setting.
Acknowledgments
We express our appreciation to Judy Small (NINDS/NIH, Bethesda, Md.) for her generosity in providing the JC91 transgenic line and Susan Morgello (Mt. Sinai School of Medicine, New York, N.Y.) for advice and technical assistance. We also thank past and present members of the Center for NeuroVirology and NeuroOncology for sharing of reagents and insightful discussion and Cynthia Schriver for editorial assistance and preparation of the manuscript. We thank Luis DelValle for assistance in histological studies. We thank Ronald E. Gordon for microscopic and electron microscopic experiments.
This work was made possible by grants awarded by the NIH to S.A., K.K., and E.M.J.
REFERENCES
- 1.Bergemann A D, Johnson E M. The HeLa Pur factor binds single-stranded DNA at a specific element conserved in gene flanking regions and origins of DNA replication. Mol Cell Biol. 1992;12:1257–1265. doi: 10.1128/mcb.12.3.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergemann A D, Ma Z W, Johnson E M. Sequence of cDNA comprising the human pur gene and sequence-specific single-stranded-DNA-binding properties of the encoded protein. Mol Cell Biol. 1992;12:5673–5682. doi: 10.1128/mcb.12.12.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger J R, Concha M. Progressive multifocal leukoencephalopathy: the evolution of a disease once considered rare. J Neurovirol. 1995;1:5–18. doi: 10.3109/13550289509111006. [DOI] [PubMed] [Google Scholar]
- 4.Chang C F, Gallia G L, Muralidharan V, Chen N N, Zoltick P, Johnson E M, Khalili K. Evidence that replication of human neurotropic JC virus DNA in glial cells is regulated by the sequence-specific single-stranded DNA-binding protein Purα. J Virol. 1996;70:4150–4156. doi: 10.1128/jvi.70.6.4150-4156.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devine-Beach K, Lashgari M, Khalili K. Proximal and distal upstream activating sequences regulate transcription of the myelin basic protein gene in glial cells. J Biol Chem. 1990;265:13830–13835. [PubMed] [Google Scholar]
- 6.Dignam J D, Lebovitz R M, Roeder R G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feigenbaum L, Khalili K, Major E, Khoury G. Regulation of the host range of human papovavirus JCV. Proc Natl Acad Sci USA. 1987;84:3695–3698. doi: 10.1073/pnas.84.11.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frisque R J, Bream G L, Cannella M T. Human polyomavirus JC virus genome. J Virol. 1984;51:458–469. doi: 10.1128/jvi.51.2.458-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallia G L, Safak M, Khalili K. Interaction of single-stranded DNA binding protein, Purα, with human polyomavirus, JCV, early protein, T-antigen. J Biol Chem. 1998;273:32662–32669. doi: 10.1074/jbc.273.49.32662. [DOI] [PubMed] [Google Scholar]
- 10.Haas S, Gordon J, Khalili K. A developmentally regulated DNA-binding protein from mouse brain stimulates myelin basic protein gene expression. Mol Cell Biol. 1993;13:3103–3112. doi: 10.1128/mcb.13.5.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haas S, Haque N, Beggs A, Khalili K, Small J. Expression of myelin basic protein gene in transgenic mice expressing human neurotropic virus early protein: T-Ag. Virology. 1994;202:89–96. doi: 10.1006/viro.1994.1325. [DOI] [PubMed] [Google Scholar]
- 12.Haas S, Thatikunta P, Steplewski A, Johnson E M, Khalili K, Amini S. A 39-kD DNA-binding protein from mouse brain stimulates transcription of myelin basic protein gene in oligodendrocytes. J Cell Biol. 1995;130:1171–1179. doi: 10.1083/jcb.130.5.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogan E L, Greenfield S. Animal models of genetic myelin disorders. In: Morrell P, editor. Myelin. New York, N.Y: Plenum Press; 1982. pp. 489–534. [Google Scholar]
- 14.Johnson E M, Chen P L, Krachmarov C P, Barr S M, Kanovsky M, Ma Z W, Lee W H. Association of human Purα with the retinoblastoma protein, Rb, regulates binding to the single-stranded DNA Purα recognition element. J Biol Chem. 1995;270:24352–24360. doi: 10.1074/jbc.270.41.24352. [DOI] [PubMed] [Google Scholar]
- 15.Khalili K, Feigenbaum L, Khoury G. Early region of a human papovavirus contains multiple 5′-termini: evidence for a shift in 5′-termini during the lytic cycle of JC virus. Virology. 1987;158:496–472. doi: 10.1016/0042-6822(87)90224-8. [DOI] [PubMed] [Google Scholar]
- 16.Krachmarov C P, Chepenik L G, Barr-Vagell S, Khalili K, Johnson E M. Activation of the JC virus Tat-responsive transcriptional control element by association of the Tat protein of human immunodeficiency virus 1 with cellular protein Pur alpha. Proc Natl Acad Sci USA. 1996;93:14112–14117. doi: 10.1073/pnas.93.24.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lashgari M, Tada H, Amini S, Khalili K. Regulation of JCV late promoter function. Virology. 1989;170:292–295. doi: 10.1016/0042-6822(89)90381-4. [DOI] [PubMed] [Google Scholar]
- 18.Lashgari M S, Devine-Beach K, Haas S, Khalili K. Nuclear proteins in mouse brain cells bind specifically to the myelin basic protein regulatory regions. J Clin Investig. 1990;86:1671–1677. doi: 10.1172/JCI114890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Louis J C, Magal E, Muir D, Manthorpe M, Varon S. CG-4, a new bipotential glial cell line from rat brain, is capable of differentiating in vitro into either mature oligodendrocytes or type 2 astrocytes. J Neurosci Res. 1992;31:193–204. doi: 10.1002/jnr.490310125. [DOI] [PubMed] [Google Scholar]
- 20.Raj G V, Khalili K. Transcriptional regulation: lessons from the human neurotropic polyomavirus, JCV. Virology. 1995;213:283–291. doi: 10.1006/viro.1995.0001. [DOI] [PubMed] [Google Scholar]
- 21.Shah K V. Polyomaviruses. In: Fields B, Knipe D M, editors. Fields virology. Vol. 2. New York, N.Y: Raven Press; 1990. pp. 1609–1625. [Google Scholar]
- 22.Sidman R L, Dickie M M, Appel S H. Mutant mice (quaking and jimpy) with deficient myelination in the central nervous system. Science. 1964;144:309–311. doi: 10.1126/science.144.3616.309. [DOI] [PubMed] [Google Scholar]
- 23.Small J A, Scangos G A, Cork L, Jay G, Khoury G. The early region of human papovavirus JC induces dysmyelination in transgenic mice. Cell. 1986;46:13–18. doi: 10.1016/0092-8674(86)90855-x. [DOI] [PubMed] [Google Scholar]
- 24.Sorg B A, Smith M M, Campagnoni A T. Developmental expression of the myelin proteolipid protein and basic protein mRNAs in normal and dysmyelinating mutant mice. J Neurochem. 1987;49:1146–1154. doi: 10.1111/j.1471-4159.1987.tb10005.x. [DOI] [PubMed] [Google Scholar]
- 25.Trapp B D, Small J A, Pulley M, Khoury G, Scangos G A. Dysmyelination in transgenic mice containing JC virus early region. Ann Neurol. 1988;23:38–48. doi: 10.1002/ana.410230108. [DOI] [PubMed] [Google Scholar]
- 26.Tretiakova A, Gallia G L, Shcherbik N, Jameson B A, Johnson E M, Amini S, Khalili K. Association of Purα with RNAs homologous to 7SL determines its binding ability to the myelin basic protein promoter DNA sequence. J Biol Chem. 1998;273:22241–22247. doi: 10.1074/jbc.273.35.22241. [DOI] [PubMed] [Google Scholar]
- 27.Tretiakova, A., B. Krynska, J. Gordon, and K. Khalili. Human neurotropic JC virus early protein de-regulates glial cell cycle pathway and impairs cell differentiation. J. Neurosci. Res., in press. [DOI] [PubMed]