Abstract
The limitations of adeno-associated virus (AAV)-mediated vectors for lung-directed gene transfer were investigated by using differentiated human respiratory epithelium in air-liquid interface cultures. Transduction efficiency was high in undifferentiated cells and was enhanced in well-differentiated cells after basolateral application of the vector or after apical application following disruption of tight junctions or pretreatment of the cultures with glycosidases. These results indicate that transduction of airway epithelia by AAV vectors is limited by entry and reinforce the importance of a physical barrier on the airway surface.
Cystic fibrosis is a common, lethal genetic disease caused by inherited defects of the cystic fibrosis transmembrane conductance regulator (CFTR) and is characterized by chronic lung infection and inflammation (2). Adeno-associated virus (AAV) is currently being considered as a vector for gene transfer into airway epithelium (7). Recent phase I clinical trials with AAV vectors have also shown efficient and safe delivery of the CFTR gene into epithelial cells with low levels of gene transfer (18). AAV vectors offer several potential advantages over adenoviral vectors, including more stable gene expression and diminished cellular immunity to transgene products (10, 21). However, the transduction efficiency of AAV in vivo is rather low in the lung (15, 18). In other organs or tissues, such as muscle, brain, and liver, infection with AAV vectors resulted in efficient and stable gene expression (6, 11, 20, 21). The reason for the low performance of this vector system in lungs is not fully understood.
It is the goal of this study to investigate barriers to efficient transduction with AAV into airway epithelium. Our hypothesis is that a physical barrier of negatively charged molecules, such as mucins or glycosaminoglycans, or limited virus receptors at the apical surfaces of epithelial cells limit viral entry, resulting in inefficient transduction. Studies were performed with AAV vectors containing enhanced green fluorescent protein (eGFP) as a reporter gene driven from the 5′ flanking region of the immediate-early gene of cytomegalovirus (CMV) and an air-liquid interface culture system of well-differentiated human bronchial epithelium. Recombinant AAV was created by a transfection approach that does not require coinfection with adenovirus (20). Cultures of human tracheobronchial epithelial cells in transwells were established and maintained as described by Wang et al. (19) by using a medium consisting of a 50:50 mixture of Ham’s F-12 medium and Dulbecco modified Eagle medium as the base, supplemented with 2% Ultroser G (BioSepra, Villeneuve-la-Garenne Cedex, France). Differentiated cultures were selected for experiments by measuring the transepithelial resistance (Rt) with an ohmmeter (EVOM; World Precision Instruments, Inc., Sarasota, Fla.). Cultures were considered confluent and differentiated if the Rt was greater than 1,300 Ω/cm2 (19).
Initial experiments evaluated the impact of cell differentiation on transduction. At different time points after seeding onto transwells, cultivated cells were infected with 1011 genomic particles of AAV-CMV-eGFP in 200 μl of medium applied to the upper or lower buffer compartment of the transwell. After 2 h the cultures were washed and assayed over time for gene expression. Numbers of positive cells were determined by counting ten randomly selected fields with a Nikon Diaphot inverted microscope under UV illumination. A microscopic field usually contained 800 to 900 cells as determined by counting of nuclei under phase contrast. Cultures used had an Rt of >1,000 Ω/cm2.
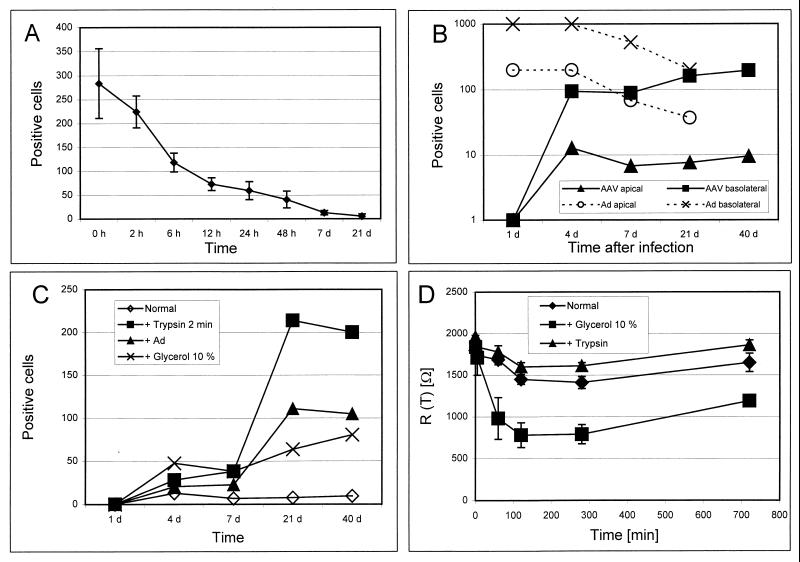
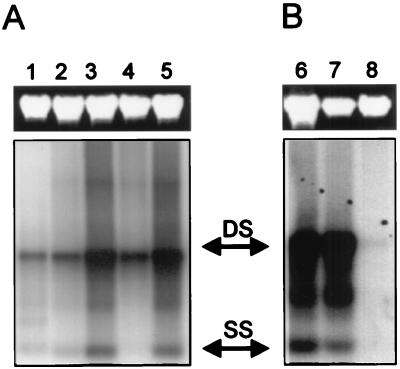
Transduction was much more efficient in undifferentiated cells, as determined by GFP expression (Fig. 1A). Total cellular DNA analyzed by DNA hybridization demonstrated significant levels of both single- and double-stranded monomers of the viral genome under most conditions (Fig. 2). This analysis does not specifically evaluate randomly integrated proviral DNA that would migrate with high-molecular-weight DNA as a smear. Cells infected in an undifferentiated state (Fig. 2, lane 1) contained amounts of vector DNA equal to those in cells that were infected in a differentiated state (lane 2). The number of transgene-positive cells, however, was much higher in the cells infected in an undifferentiated state (Fig. 1A), indicating that postentry processes may contribute to differences in transduction efficiency. This suggests that cellular factors present in nondifferentiated cells or absent in mature cells are necessary for efficient gene expression. The relationship between transduction and the state of differentiation has been described for adenoviral and retroviral vectors (8, 13, 19) and proposed for AAV vectors (17); for integrating viruses, postentry processes related to vector genome replication have been proposed (4, 5).
FIG. 1.
Levels of transgene expression after infection of human airway cultures in transwell cultures. (A) The status of differentiation is an important determinant of transduction efficiency. Cells were infected from the apical side at different time points after seeding onto the transwells, and numbers of transgene-expressing cells were determined 7 days after infection. (B) In polarized epithelium, transduction efficiency is dependent on the site of vector application. AAV was applied to fully differentiated epithelium (after 3 weeks of culture) from the apical or basolateral side, and the expression of the transgene was assayed over 40 days. Recombinant adenovirus (rAd) coding for GFP (Ad5.010–CMV–eGFP) was applied to the apical or basolateral side of the cultures (106 particles/200 μl). This treatment did not result in a change in the Rt (data not shown). (C) Treatment with glycerol (10%) or pretreatment with 0.05 g of trypsin/liter–0.05 mM EDTA for 2 min, followed by three washes, increased the transduction efficiency of AAV vectors. Coinfection with wild-type adenovirus (serotype 5) (Ad) resulted in increased numbers of positive cells compared to no treatment (normal). (D) To determine whether the apical application of glycerol or trypsin to differentiated cultures resulted in temporal disruption of the apical tight junctions, Rt was measured up to 24 h after the application of the agent. Trypsinization did not decrease the Rt compared to the medium control (normal); however, glycerol resulted in a significant decrease in Rt.
FIG. 2.
Southern blots of total cellular DNA extracted after 40 days of culture (except for lane 1, where cells were harvested 14 days after seeding and infection) were hybridized to radiolabeled eGFP cDNA as a probe. Shown are amounts of viral DNA in cells after infection immediately after seeding of the cells into the transwells (lane 1); after infection of differentiated cells by apical application with no further treatment (lane 2), apical application together with 10% glycerol (lane 3), basolateral application (lane 4), or apical application after predigestion with trypsin for 2 min (lane 5); and after infection of differentiated cells that had been pretreated with neuraminidase (lane 6) or endoglycosidase H (lane 7) compared to no treatment (lane 8). DS, double-stranded viral DNA; SS, single-stranded viral DNA.
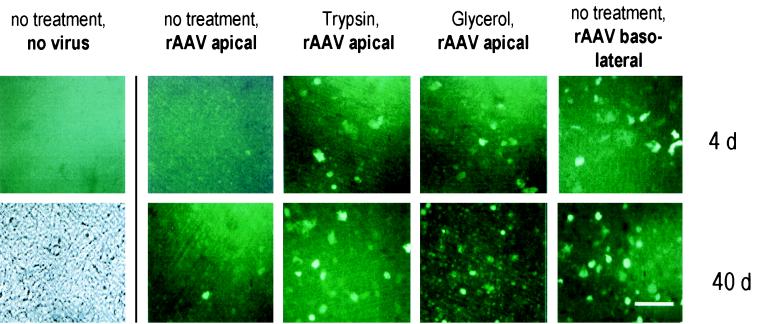
We then investigated whether entry limitations contribute to the low transduction efficiency of differentiated, polarized epithelium. Application of AAV vector to the basolateral compartment resulted in substantially enhanced transduction compared to transduction from apically applied vector (Fig. 1B and 3). Disruption of tight junctions by glycerol increased the numbers of positive cells when vector was applied to the apical surface (Fig. 1C and D and Fig. 3). Southern blots of total cellular DNA at day 40 of the culture revealed increased vector DNA in cells where virus was applied from the basolateral side (Fig. 2, lane 4) or in cultures treated with glycerol (Fig. 2, lane 3) compared to cultures infected from the apical side without any further treatment (Fig. 2, lane 2). The increased transduction efficiency following basolateral administration of vector may be due to the enhanced permissivity of basally located cells, higher concentrations of viral receptors, or the absence of a physical barrier. Putative AAV receptor structures, such as heparan sulfate (12, 16) and fibroblast growth factor receptor 1 (14), have been localized to the basolateral compartment of the respiratory epithelium (1, 3, 9).
FIG. 3.
Micrographs showing representative areas of the epithelial layer using UV illumination. Cells were infected with a standard dose of 1011 genome particles of AAV vector coding for eGFP. All cultures were fully differentiated at the time of infection. Different treatments are indicated at the top; the times after infection are indicated on the right. Bar, 100 μm.
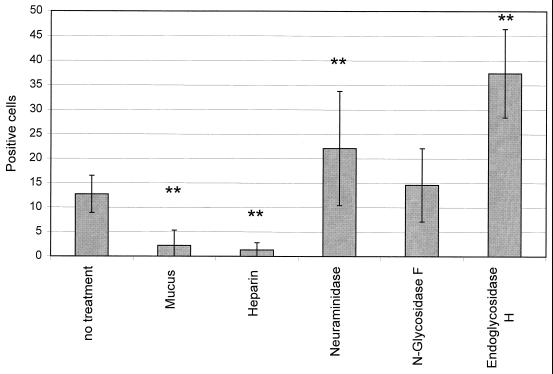
To determine whether a physical barrier at the apical surface might hinder AAV-mediated gene transfer to the airways, we analyzed the impact of enzymatic modification of surface structures on transduction frequency. Pretreatment of the culture surface with trypsin (Fig. 1C and D and Fig. 3) and several glycosidases (Fig. 4) resulted in increased transduction, whereas application of the vector together with anionic macromolecules, such as heparin or mucin, resulted in decreased numbers of positive cells (Fig. 4). Exposure of cultures to trypsin or glycerol 14 days after infection of the cells with AAV vector did not impact on transgene expression, indicating that treatment did not induce changes in postentry steps (data not shown). Capsid proteins of AAV contain many basic amino acid residues (16), making entrapment of the viral particles by negatively charged macromolecules likely. Southern blots of total cellular DNA at day 40 of the culture showed that viral DNA was more abundant in cultures treated with trypsin (Fig. 2, lane 5) or certain glycosidases (Fig. 2, lanes 6 and 7) than in cultures that received the AAV vector apically without any further treatment (Fig. 2, lane 2 or 8). This indicates that apical structures or secretions interact with the virus and limit entry, contributing to reduced transduction efficiency.
FIG. 4.
Negatively charged carbohydrates inhibit the transduction efficiency of AAV vector added to the apical side of differentiated cultures. The addition of mucin (pig stomach mucin; final concentration, 1 mg/ml) or heparin (5 μg/ml) (both from Sigma) resulted in inhibition of transduction, whereas pretreatment with several glycosidases for 1 h enhanced transduction. N-glycosidase F (final concentration, 5 U/ml), neuraminidase (0.01 mg/ml), N-glycosidase A (1 mU/ml), or endoglycosidase H (0.2 U/ml) (all from Boehringer Mannheim) was diluted in medium and applied to the apical surface of the epithelium for 1 h. After three washes, the medium containing the virus was added. ∗∗, statistically significant difference between the specified group and the “no treatment” group (P < 0.005).
In conclusion, the results of the present study indicate that AAV-based gene transfer to respiratory epithelium is limited by entry restrictions and postentry events, which are a function of the biology of the target tissue. Differentiated cells are far less transducible than nondifferentiated cells for reasons that go beyond differences in entry. This indicates complex interactions of cellular components with vector DNA in postentry steps. Further characterization of differentiated cells confirmed that entry is a rate-limiting step in transduction. The physical barrier created by the mucous layer clearly contributes to this block. Strategies to improve the performance of AAV vectors for lung-directed gene transfer should consider impediments to transduction at each level.
Acknowledgments
R. Bals and W. Xiao contributed equally to this work.
This work was supported by the Cystic Fibrosis Foundation and the NIH (P30 DK 47757 and R01 HL 49040) as well as Genovo, Inc., a biotechnology company that J. M. Wilson founded and in which he has equity. R. Bals was a recipient of a fellowship of the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Daugaard S, Strange L, Schiodt T. Immunohistochemical staining for chondroitin sulfate and keratin sulfate. An evaluation of two monoclonal antibodies. Histochemistry. 1991;95:585–589. doi: 10.1007/BF00266746. [DOI] [PubMed] [Google Scholar]
- 2.Davis P B, Drumm M, Konstan M W. Cystic fibrosis. Am J Respir Crit Care Med. 1996;154:1229–1256. doi: 10.1164/ajrccm.154.5.8912731. [DOI] [PubMed] [Google Scholar]
- 3.Erlinger R. Glycosaminoglycans in the porcine lung: an ultrastructural study using cupromeronic blue. Cell Tissue Res. 1995;281:473–483. doi: 10.1007/BF00417864. [DOI] [PubMed] [Google Scholar]
- 4.Ferrari F K, Samulski T, Shenk T, Samulski R J. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J Virol. 1996;70:3227–3234. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher K J, Gao G P, Weitzman M D, DeMatteo R, Burda J F, Wilson J M. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J Virol. 1996;70:520–532. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher K J, Jooss K, Alston J, Yang Y, Haecker S E, High K, Pathak R, Raper S E, Wilson J M. Recombinant adeno-associated virus for muscle directed gene therapy. Nat Med. 1997;3:306–312. doi: 10.1038/nm0397-306. [DOI] [PubMed] [Google Scholar]
- 7.Flotte T, Carter B. Adeno-associated virus vectors for gene therapy of cystic fibrosis. Methods Enzymol. 1998;292:717–732. doi: 10.1016/s0076-6879(98)92055-9. [DOI] [PubMed] [Google Scholar]
- 8.Goldman M J, Lee P S, Yang J S, Wilson J M. Lentiviral vectors for gene therapy of cystic fibrosis. Hum Gene Ther. 1997;8:2261–2268. doi: 10.1089/hum.1997.8.18-2261. [DOI] [PubMed] [Google Scholar]
- 9.Hughes S E, Hall P A. Immunolocalization of fibroblast growth factor receptor 1 and its ligands in human tissues. Lab Investig. 1993;69:173–182. [PubMed] [Google Scholar]
- 10.Jooss K, Yang Y, Fisher K J, Wilson J M. Transduction of dendritic cells by DNA viral vectors directs the immune response to transgene products in muscle fibers. J Virol. 1998;72:4212–4223. doi: 10.1128/jvi.72.5.4212-4223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplitt M G, Leone P, Samulski R J, Xiao X, Pfaff D W, O’Malley K L, During M J. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat Genet. 1994;8:148–154. doi: 10.1038/ng1094-148. [DOI] [PubMed] [Google Scholar]
- 12.Mizukami H, Young N S, Brown K E. Adeno-associated virus type 2 binds to a 150-kilodalton cell membrane glycoprotein. Virology. 1996;217:124–130. doi: 10.1006/viro.1996.0099. [DOI] [PubMed] [Google Scholar]
- 13.Pickles R J, McCarty D, Matsui H, Hart P J, Randell S H, Boucher R C. Limited entry of adenovirus vectors into well-differentiated airway epithelium is responsible for inefficient gene transfer. J Virol. 1998;72:6014–6023. doi: 10.1128/jvi.72.7.6014-6023.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qing K, Mah C, Hansen J, Zhou S, Dwarki V, Srivastava A. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat Med. 1999;5:71–77. doi: 10.1038/4758. [DOI] [PubMed] [Google Scholar]
- 15.Rubenstein R C, McVeigh U, Flotte T R, Guggino W B, Zeitlin P L. CFTR gene transduction in neonatal rabbits using an adeno-associated virus (AAV) vector. Gene Ther. 1997;4:384–392. doi: 10.1038/sj.gt.3300417. [DOI] [PubMed] [Google Scholar]
- 16.Summerford C, Samulski R J. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teramoto S, Bartlett J S, McCarty D, Xiao X, Samulski R J, Boucher R C. Factors influencing adeno-associated virus-mediated gene transfer to human cystic fibrosis airway epithelial cells: comparison with adenovirus vectors. J Virol. 1998;72:8904–8912. doi: 10.1128/jvi.72.11.8904-8912.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagner J A, Reynolds T, Moran M L, Moss R B, Wine J J, Flotte T R, Gardner P. Efficient and persistent gene transfer of AAV-CFTR in maxillary sinus. Lancet. 1998;351:1702–1703. doi: 10.1016/S0140-6736(05)77740-0. [DOI] [PubMed] [Google Scholar]
- 19.Wang G, Davidson B L, Melchert P, Slepushkin V A, van Es H H G, Bodner M, Jolly D J, McCray P B., Jr Influence of cell polarity on retrovirus-mediated gene transfer to differentiated human airway epithelia. J Virol. 1998;72:9818–9826. doi: 10.1128/jvi.72.12.9818-9826.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao W, Berta S C, Lu M M, Moscioni A D, Tazelaar J, Wilson J M. Adeno-associated virus as a vector for liver-directed gene therapy. J Virol. 1998;72:10222–10226. doi: 10.1128/jvi.72.12.10222-10226.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao X, Li J, Samulski R J. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]